Abstract
Objective:
Examine relationships between adverse childhood experiences (ACEs) and related life events and allostatic load (AL) – “wear and tear” from chronic stress – in a pediatric population.
Methods:
Children were screened with the PEARLS tool, a 17-item questionnaire capturing experiences of abuse, neglect, household challenges, and related life events. Biologic data was available for 207 participants and AL was operationalized using clinical or empirical cutoff points across 4 physiologic systems (i.e., cardiac, metabolic, inflammatory, neurologic). Covariate-adjusted multivariable regression models were used to examine associations between AL with adversity and health.
Results:
Children (Mean age= 6.5 years, range= 1–11 years) had an average AL score of 1.9 (SD 1.7), and a U-shaped relationship was observed with child’s age. Continuous PEARLS and original ACE scores were not associated with AL. However, children with a reported PEARLS score of 1–2 or original ACEs score of 1–3 had 1.5 (IRR 1.50; 95% CI 1.09, 2.08) and 1.4 (IRR 1.41; 95% CI 1.08, 1.84) times greater AL, respectively, compared to participants with none reported. In secondary analyses, caregiver mental illness was associated with higher child AL (adjusted IRR 1.27; 95% CI 1.01, 1.58). AL was also associated with poorer perceived child general health (aß = −0.87, 95% CI: −1.58, −0.15) and greater odds of child obesity (aOR 1.51; 95% CI: 1.23, 1.89).
Conclusions:
Measuring AL in a pediatric population requires careful consideration of age. Higher AL was associated with a greater number of reported adversities and worse child health.
Keywords: adversity, allostatic load, pediatric, mental illness, health, obesity
Introduction
Nearly half of all children in the United States report current exposure to at least one adverse childhood experience (ACE) (1). It is well established that ACEs are associated with poor health outcomes over the life course (2–6). The relationship between ACEs and health is complex, with evidence pointing towards direct effects on the stress response that, overtime, contribute to poor health (7,8).
Chronic exposure to psychosocial stressors, such as ACEs, can lead to dysregulation of multiple physiological systems. This cumulative “wear and tear” across systems is referred to as allostatic load (AL) and is postulated to increase risk of disease (9). The AL framework proposes that psychosocial stressors elicit the secretion of primary mediators (e.g. glucocorticoids and catecholamines), which have downstream effects and eventually disrupt secondary mediators across immune, metabolic, and cardiovascular systems (10). Previous studies have demonstrated childhood adversity to be associated with increased AL in adulthood (11,12). Yet, no studies have examined if this association between ACEs and AL can be detected earlier in life (e.g., early and mid-childhood). Cumulative risk has been associated with increased AL as early as 9 years old and this association persisted to age 17 (13,14), suggesting that biological embedding of adversity occurs during childhood. In addition to the majority of studies of AL being predominantly focused in adult populations, there is a lack of consistency in biological markers used to construct an index (15). Measuring AL in children requires careful consideration of which biomarkers are most relevant during different stages of the developmental period (16). By examining stress-related biomarkers during early and mid-childhood, we can begin to reach consensus on how to operationalize AL in pediatric populations. Lastly, research on AL as a predictor of health outcomes in children is limited, with some evidence suggesting that higher AL may be associated with increased obesity (17) and asthma (18,19) risk.
We previously developed and validated the PEdiatric ACEs and Related Life Event Screener (PEARLS) tool to collect information on ACEs and related adversities as part of pediatric primary care (20,21). We found that high PEARLS scores were associated with stomachaches and asthma, poorer perceived general health, and lower global executive functioning in children (21). Consequently, in the present study, we hypothesized that exposure to ACEs may become biologically embedded during early- and mid-childhood and detectable in a range of biological markers of health. The main objectives of this study were to: 1) examine how age and AL relate in early and mid-childhood, 2) estimate and compare associations between the original ACE items and PEARLS scores with AL, and 3) assess whether AL was an independent predictor of adverse health outcomes in the Pediatric ACEs and Resiliency Study, a predominantly Black and Latinx population receiving primary care through a Federally Qualified Health Center (Figure 1). To our knowledge, this work is the first to simultaneously investigate relationships between AL, ACEs, and health outcomes during early to mid-childhood.
Figure 1.
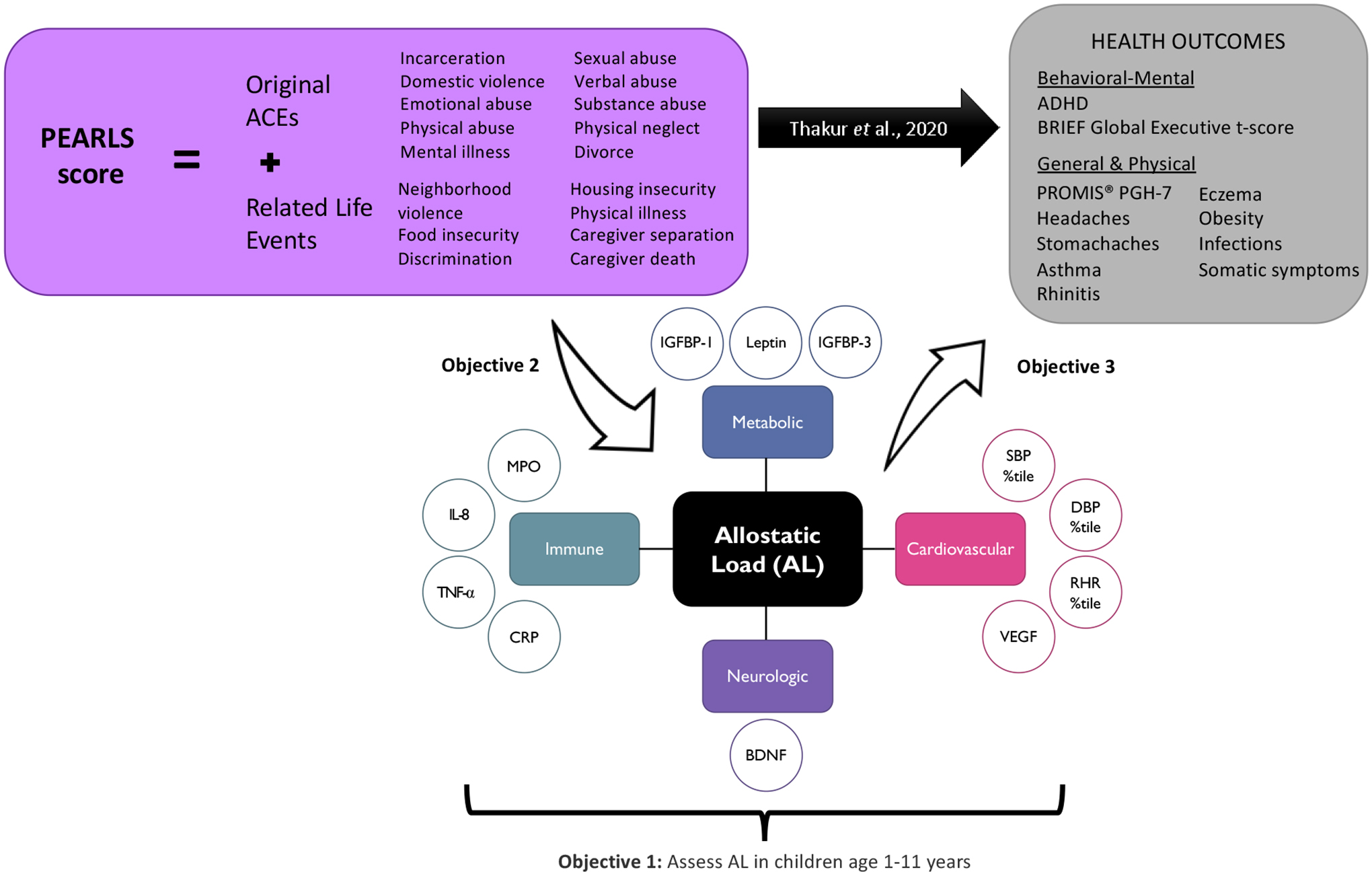
Schematic of research design. The first objective of this project was to operationalize allostatic load (AL) during early and mid-childhood (1–11 years) using stress-related biomarkers that represent cardiovascular [systolic blood pressure (SBP) percentile, diastolic blood pressure (DBP) percentile, resting heart rate (RHR) percentile, vascular endothelial growth factor (VEGF)], metabolic [insulin-like growth factor-binding protein (IGFBP)-1, IGFBP-3, leptin], inflammatory [tumor necrosis factor alpha (TNF-α), interleukin (IL)-1ß, IL-6, IL-8, and IL-10], and neurologic [brain-derived neurotrophic factor (BDNF)] system function. We then examined relationships between reported adverse childhood experiences (ACEs) and related life events measured using the PEdiatric ACEs and Related Life Event Screener (PEARLS) tool with AL (Objective 2). Lastly, the final objective was to assess if higher AL was associated with greater odds of behavioral, mental, and physical health outcomes previously associated with higher reported PEARLS scores (Thakur et. al 2022).
Methods
Study Participants
For the present analysis, we included 207 participants from the Pediatric ACEs and Resiliency Study, a predominantly non-Hispanic Black and low-income population. The study was designed to examine associations between adversities identified with the PEARLS tool and stress-related biomarkers in a high-burdened community to develop potential mitigation strategies. Details on subject recruitment and study design are described elsewhere (21). Briefly, 555 participants between the ages of 3 months to 11 years were enrolled in the study from March 2017-October 2018 during well-child checks at the University of California San Francisco (UCSF) Benioff’s Children Hospital Oakland (Benioff Oakland) Primary Care Clinic. Eligible participants were: English and/or Spanish speaking, had a primary caregiver ≥18 years who spoke English and/or Spanish, and not in foster care. At baseline, participants were consented and randomized to one of three screening formats: no PEARLS screening (n=188), single item-level response screening (n=185), and aggregate-level response (e.g., how many in total has your child experienced?) screening (n=182). For the present analysis, item-level and aggregate level responses were combined as we previously observed no significant differences in associations between PEARLS scores and health outcomes by screening format (21). Participants randomized to the aggregate-level PEARLS tool were later asked to specify the individual items that contributed to their aggregate PEARLS score, Single item-level responses from both PEARLS screened groups were combined in an exploratory analysis investigating which specific experiences may potentially influence AL. Trained, bilingual research staff also administered comprehensive questionnaires to collect sociodemographic, psychosocial stress, and health data at baseline. During a separate study visit scheduled within one month after baseline, all participants underwent an abbreviated clinical exam and biospecimen collection. All participant caregivers provided written informed consent and, where appropriate, children provided oral assent. The study was approved by the UCSF Benioff Oakland institutional review board.
Eligibility criteria for this analysis are presented in Figure S1, Supplemental Digital Content. The present study limited analysis to those randomized to the two screening arms (n=367) and measures that occurred at baseline or one-month follow-up, which we considered in tandem. Children younger than 1 year were excluded from the analysis as venous blood was not collected from this age group. Of the 243 participants with blood collected, we only included individuals with complete data for the 12 biomarkers described below (n=207).
ACEs and Related Life Events
ACEs and Related Life Events were measured using the PEdiatric ACEs and Related Life Event Screener (PEARLS), a validated pediatric ACEs screen developed with patient families and providers for use in clinical practice (21,22). The 17-item screen includes the ten original ACE items (23), plus Related Life Events including exposure to discrimination, food insecurity, housing instability, community violence, physical illness/disability of a caregiver, death of a caregiver, and forced separation from caregiver. Item responses were summed and analyzed as a continuous variable (total PEARLS Score, possible range 0 to 17) and as sample-specific quartile categories. Additionally, the original ten ACEs items (possible range 0 to 10) and seven Related Life Events items (possible range 0 to 7) were analyzed separately as continuous variables. To remain consistent with previous literature, we also examined associations with the ten original ACEs items categorized as “no ACEs,” “1–3 ACEs,” and “≥4 ACEs” (23). Individual item-level responses were binary (yes/no). Associations were also examined with caregiver stress, assessed by the widely used 10-item Perceived Stress Scale (PSS-10) instrument for measuring stress perception (24), since higher levels of parenting stress has previously been associated with ACEs (25) and may also impact biologic processes in children.
Biospecimen Collection
Whole venous blood samples were collected at the clinic visit in BD Vacutainer tubes with K2-EDTA anticoagulant (BD, Franklin Lakes, NJ; cat. #367861) and shipped overnight to our laboratory at the University of California, San Francisco for processing. Blood samples were centrifuged upon arrival and plasma was immediately stored at −80 C. Collection-to-processing time was less than 24 hours.
Measuring AL Biomarkers
The AL framework was operationalized by constructing an index from biological markers that captured multi-system dysregulation. Prior to the start of recruitment, we pre-identified 16 candidate biomarkers from the literature for measurement (26–39). Systolic blood pressure (SBP), diastolic blood pressure (DBP), and resting heart rate (RHR) were collected during the clinical exam visit along with height and weight to calculate percentile values. The remaining biomarkers were measured from frozen plasma samples prior to analysis. Candidate biomarkers and corresponding references are listed by physiological system in Table S1 (Supplemental Digital Content).
SBP, DBP, and RHR were measured with a Welch Allyn Connex® Vital Signs Monitor 6000 (cat# 901060) after having participants sit on their caregiver’s lap if <5 years or lay on the exam table (≥5 years) quietly for five minutes; all measurements were repeated three times and averaged. SBP and DBP percentiles were SBP and DBP percentiles were assigned using the American Academy of Pediatrics guidelines for children aged 1–13 years since these measures change substantially throughout childhood and development (40,41). Percentile values were also calculated for RHR using reference data from the National Health and Nutrition Examination Survey collected between 1999 and 2008 for all ages (1–80 years) of the U.S. population (42). Vascular endothelial growth factor (VEGF), tumor necrosis factor alpha (TNF-α), interleukin (IL)-1ß, IL-6, IL-8, and IL-10 were measured with the Human Magnetic Luminex® Assay High Sensitivity Cytokine Panel A (R&D Systems, Minneapolis, MN; cat. #FCSTM09). Custom multiplex Luminex® assays (cat. #LXSAHM) measured insulin-like growth factor-binding protein (IGFBP)-1, IGFBP-3, leptin, C-reactive protein (CRP), brain-derived neurotrophic factor (BDNF), and myeloperoxidase (MPO). Analytes were detected by a MAGPIX® instrument and analyzed with the xPONENT 4.2 software (Luminex Corp. Austin, TX). Concentrations were extrapolated by the software using a logistic 5-parameter model fit to standard curves included on each plate. Endothelial-1 (ET-1) plasma concentrations were measured by enzyme-linked immunosorbent assay (ELISA, R&D Systems, Minneapolis, MN; cat# QET00B). ET-1 concentrations were extrapolated from standard curves fit using a logistic 4-parameter model. Samples, standards, and controls were assayed in duplicate for all Luminex and ELISA assays. Plasma analyte concentrations were log-transformed since distributions were not normal.
Biomarkers with >10% of measurements below the lowest standard curve calibrator (ET-1, IL-1ß, IL-6, and IL-10) were excluded from the analysis to avoid introducing additional bias when assigning values below the limit of quantification (LOQ) (43). Other studies have also reported similar challenges with detecting these cytokines in healthy children (44,45). Analyte concentrations below the lowest standard curve calibrator were assigned a value of LOQ/2 multiplied by the sample dilution factor. Similarly, concentrations above the highest calibrator were substituted with the maximum standard value multiplied by the dilution factor. All included biomarker measurements had a coefficient of variation <20%, the acceptable limit for accuracy and precision of immunoassays (46). There were 207 participants with complete data for the 12 included biomarkers: SBP percentile, DBP percentile, RHR percentile, VEGF, IGFBP-1, IGFBP-3, Leptin, CRP, TNF-α, IL-8, MPO, and BDNF.
Constructing AL score
Participants were each assigned an AL score based on the sum of biomarkers that exceeded high-risk values listed in SDC Table S2 (possible range 0 to 12). Clinical cutoff values were used for SBP (≥ 95th percentile), DBP (≥ 95th percentile), and RHR (≥ 90th percentile). For plasma biomarkers, empirical cutoffs were calculated separately for males and females as one standard deviation (SD) above the sample mean of log-transformed values, except for IGFBP-1 where having <1 SD was defined as high risk. Other studies have used a similar approach to estimate empirical cutoffs (47,48). AL scores were examined as a continuous variable to account for the cumulative nature of this measurement.
Sociodemographic covariates
Covariates were identified based on existing literature on the relationship between childhood adversity and AL in adults (11,49). Race/ethnicity was categorized as Non-Hispanic Black (reference group based on sample size), Non-Hispanic White, Hispanic, or other (this category combined groups with small sample sizes and included American Indian/Alaska Native, Asian, Middle Eastern/North African, Native Hawaiian/Pacific Islander, individuals with two or more races, and those who chose “Other”); caregiver’s educational level was categorized as some high school or less, high school graduate, some college, and college or greater; family income was categorized as <100%, 100–200%, and >200% of the federal poverty level (FPL) based on the sample distribution of children per household and federal poverty guidelines provided by the U.S. Department of Health and Human Services for a family of four in 2018 (50).
Health Outcomes
ACEs have been associated with proximal outcomes that occur during childhood, including behavioral challenges (51–53), mental health (ADHD) (52,54), and physical health (obesity, respiratory, and recurrent infections) (51,55–57). Similarly, high allostatic load has been associated with greater behavioral difficulties and poorer physical health in children (47). Health outcomes included in this study are in agreement with Thakur et. al 2022 that previously examined relationships between adversities identified by PEARLS with behavioral, mental, and physical health measures (21).
Child’s general health was assessed using the Patient-Reported Outcomes Measurement Information System (PROMIS®) Parent-Proxy Pediatric Global Health Measure (PGH-7), a 7-item caregiver questionnaire that assessed general, physical, mental, and social health of their child (58). Continuous raw scores were converted into T-scores and norm-referenced. Attention Deficit Hyperactivity Disorder (ADHD) diagnosis was based on ICD-10 codes with current disease. Behavioral health was assessed using the Behavior Rating Inventory of Executive Function (BRIEF 2/P versions administered to appropriate age group) tool (59), reporting on Global Executive Composite scale T-score, in which scores ≥ 65 are considered clinically significant. Obesity was defined by a body mass index (BMI) in the 95th percentile or greater. The International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire (60) was used to obtain history of asthma, rhinitis, and eczema. ICD-10 codes from EHR records were retrieved to create binary measures of the presence of acute infections (upper and lower respiratory infection, sinusitis, bronchiolitis, pneumonia, influenza and other viral infections, scarlet fever, otitis media, conjunctivitis, and urinary tract infections) in the 12 months prior to recruitment. Data were also collected on self-reported headaches/dizziness and stomachaches in the previous 12 months (yes/no).
Statistical Analysis
Wilcoxon-rank sum was used to test for differences in median AL by sociodemographic factors. The U-shaped relationship between age and AL was assessed by the two-lines approach (61). Associations between the PEARLS tool (total score, original ACEs, Related Life Events) with AL (outcome) were evaluated by multivariable negative binominal regressions adjusted for child’s age, sex, race/ethnicity, and caregiver’s education. Covariate-adjusted negative binomial regressions were also used to examine associations between individual item-level PEARLS data (yes/no) and AL. Analyses of individual ACEs were limited to items that were experienced by at least 25 percent of children. Associations between AL (predictor) and health outcomes were examined by either multivariable linear or logistic regression adjusted for covariates (child’s age, sex, race/ethnicity, and caregiver’s education). All analyses were performed with R version 4.1.0. and statistical significance was defined by two-sided p-values <0.05.
Results
Distribution of AL scores are shown in Figure S2 (Supplemental Digital Content). Study participants had an average AL score of 1.93 (SD 1.67), with a range from 0 to 9. The sample was predominantly non-Hispanic Black (59.4%). In addition, 82.6% of participants reported an annual household family income at or below 200% FPL. Sociodemographic differences in median AL scores are presented in Table 1.
Table 1.
Study characteristics and median AL scores
| N (%) | Median AL (IQR) | P-value1 | |
|---|---|---|---|
| All | 207 (100) | 2.0 (1.0–3.0) | |
| Age (years) | |||
| 1–4 | 75 (36.2) | 2.0 (1.0–3.0) | Ref |
| 5–8 | 71 (34.3) | 1.0 (0.0–2.0) | 0.048 |
| 9–11 | 61 (29.5) | 2.0 (1.0–3.0) | 0.85 |
| Sex | |||
| Male | 108 (52.2) | 2.0 (1.0–3.0) | Ref |
| Female | 99 (47.8) | 1.0 (1.0–2.0) | 0.12 |
| Race/ethnicity | |||
| Non-Hispanic Black | 123 (59.4) | 2.0 (1.0–3.0) | Ref |
| Non-Hispanic White | 8 (3.9) | 1.5 (0.8–2.3) | 0.86 |
| Hispanic | 40 (19.3) | 2.0 (0.8–3.3) | 0.66 |
| Other | 36 (17.4) | 2.0 (1.0–3.0) | 0.57 |
| Caregiver Education | |||
| College | 63 (30.4) | 2.0 (1.0–3.0) | Ref |
| Some college | 78 (37.7) | 2.0 (1.0–3.0) | 0.99 |
| High school | 47 (22.7) | 2.0 (0.0–3.0) | 0.90 |
| Some high school or less | 19 (9.2) | 3.0 (1.5–4.0) | 0.028 |
| Income | |||
| <100% FPL | 115 (55.6) | 2.0 (1.0–3.0) | Ref |
| 100–200% FPL | 56 (27.1) | 2.0 (1.0–3.0) | 0.94 |
| >200% FPL | 17 (8.2) | 1.0 (0.0–2.0) | 0.25 |
| Missing | 19 (9.2) |
P-values are from Wilcoxon rank sum test
Child Age and AL.
There was evidence of a non-linear relationship between age and AL. Children in the 1–4 years age group had a median AL significantly greater than the 5–8 years age group (median AL 2 vs 1, p=0.048). No differences were observed between the youngest (1–4 years) and oldest (9–11 years) age groups (p=0.85). The two-lines test was used to evaluate if the relationship between age and AL was U-shaped (Figure 2). Age and AL were inversely associated with each other (slope 1: −0.59, p=0.002) until the breakpoint of 5.02 years, after which a positive association was observed (slope 2: 0.14, p=0.016), indicating a U-shaped relationship between both variables. The U-shape relationship between age and AL persisted after sequential exclusion of each biological measurement, except for when leptin (slope 1Leptin excluded: −0.64, p=0.003 & slope 2Leptin excluded: 0.07, p=0.14) and IGFBP-1 (slope 1IGFBP-1 excluded: −0.61, p=0.001, slope 2IGFBP-1 excluded: 0.06, p=0.27) were removed, highlighting that these two metabolic biomarkers may contribute to observed dynamics with age. To account for this U-shaped association with age, all subsequent analyses included an interaction term between age and age-5-years-and-above (age*≥5years).
Figure 2.
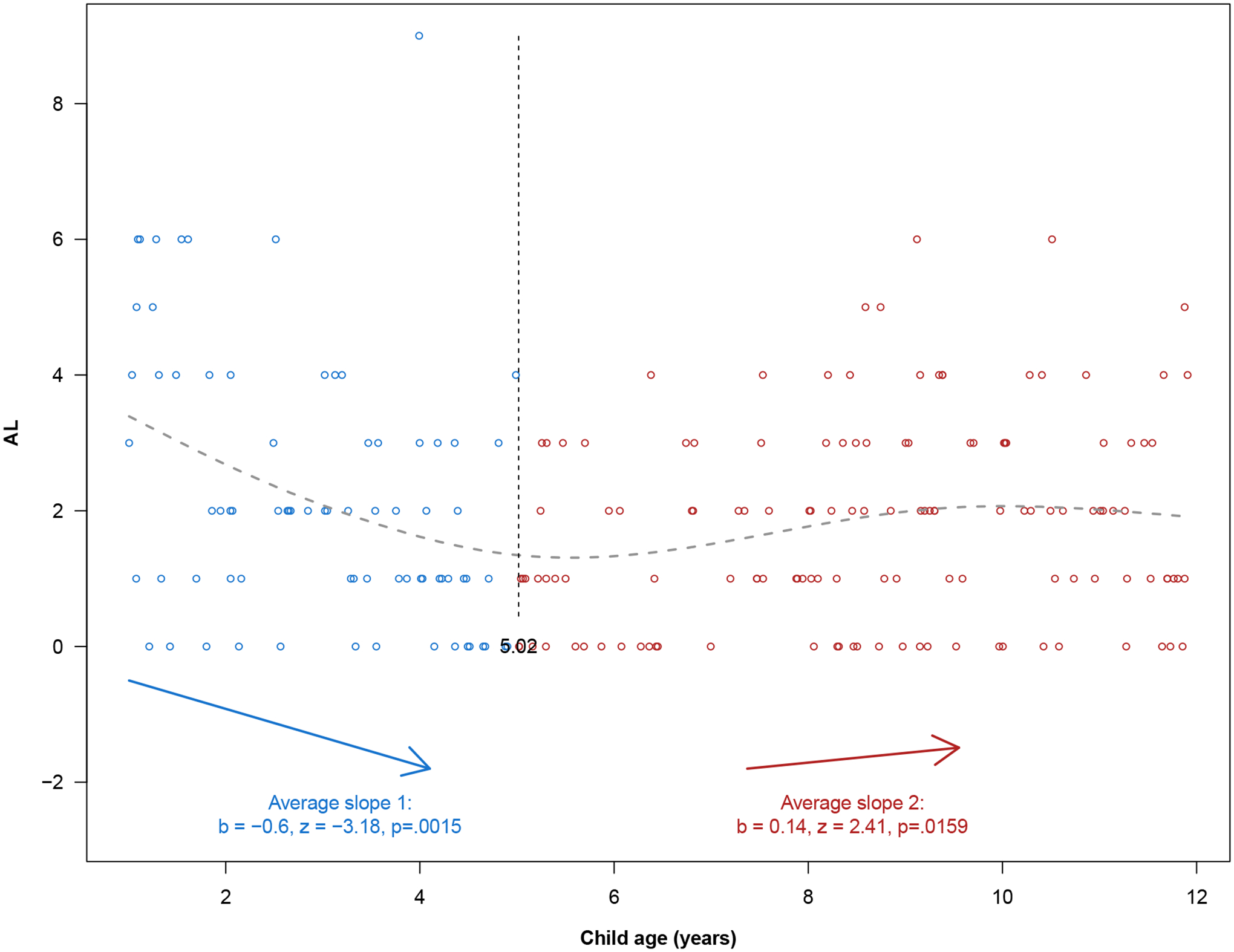
Two Lines Test evaluating the U-shaped relationship between child age and allostatic load. Each circle represents a data point. Fitted values (grey dashed line) were obtained by smoothing cubic splines. The two lines are represented by blue and red arrows with a midpoint of 5.02 years (black dashed vertical line).
Other family demographics and AL.
Median AL did not differ by sex, race/ethnicity, or family income. Children with a caregiver that reported completing college had median AL scores comparable to the “high school” (p=0.90) and “some college” (p=0.99) education groups. However, children whose caregiver reported less than a high school education completed had higher AL scores than children with a college educated caregiver (median AL 3 vs 2, p=0.028).
PEARLS score and AL.
Table 2 lists associations between PEARLS scores and AL. Children in the second quartile of total PEARLS scores (1–2 items) had 1.5 times greater AL than individuals in the reference group (PEARLS score = 0). There were no statistically significant differences between the reference group and the two highest PEARLS score quartiles (3–4 items and ≥5 items). When examined as a continuous variable, total PEARLS score was not associated with AL. Similar results were observed for original ACEs and Related Life Events analyzed separately as continuous variables. To assist with comparison with the published literature on ACEs, we also report associations limited to categories of number of original ACEs (0, 1–3, 4 or more). Participants that reported experiencing 1–3 original ACEs had 1.4 times greater AL compared to participants with no ACEs reported (IRR 1.41; 95% CI 1.08, 1.84). No association was observed with 4 or more original ACEs. Perceived caregiver stress was not associated with AL in children (IRR 1.01; 95% CI 0.98, 1.04).
Table 2.
Associations between ACEs and caregiver stress with AL
| N (%) | IRR (95% CI) | |
|---|---|---|
| Total PEARLS Score | ||
| Categorical | ||
| 0 | 46 (22.2) | Ref |
| 1–2 | 47 (22.7) | 1.50 (1.09, 2.08) |
| 3–4 | 49 (23.7) | 1.29 (0.91, 1.82) |
| ≥5 | 65 (31.4) | 1.32 (0.94, 1.85) |
| p-trend | 0.31 | |
| Continuous | 1.01 (0.97, 1.05) | |
| Original ACEs | ||
| Categorical | ||
| 0 | 61 (29.5) | Ref |
| 1–3 | 96 (46.4) | 1.41 (1.08, 1.84) |
| ≥4 | 50 (24.2) | 1.20 (0.86, 1.67) |
| p-trend | 0.20 | |
| Continuous, mean (SD) | 2.2 (2.1) | 1.01 (0.96, 1.07) |
| Related Life Events | ||
| Continuous, mean (SD) | 1.3 (1.4) | 1.03 (0.94, 1.11) |
| Caregiver Stress | ||
| Continuous, mean (SD) | 21.6 (4.6) | 1.01 (0.98, 1.04) |
Models were adjusted for child’s age × above 5 years, sex, race/ethnicity, and caregiver’s educational level
Individual PEARLS items and AL.
There were 198 participants with individual-level PEARLS item data and AL scores. Associations between individual PEARLS items and AL were explored (Table S3, Supplemental Digital Content), since certain ACEs may have differential effects on biological processes. Report of caregiver mental illness (41.4%), domestic violence (37.9%), divorce (31.3%), and neighborhood violence (27.3%) were the most prevalent ACEs in this study population. Caregiver mental illness was also associated with higher AL (IRR 1.27; 95% CI 1.01, 1.58). Associations were not observed with domestic violence, divorce, or neighborhood violence.
AL and child health outcomes.
Lastly, we examined AL as a predictor of child health outcomes (Table 3). Higher AL scores were associated with lower caregiver ratings of child’s general health as assessed by PROMIS® PGH-7 T-scores (β −0.87; −1.58, −0.15). The odds of obesity also increased 1.5-fold with each unit increase in AL score (OR 1.51; 95% CI 1.23, 1.89). No associations were observed with other examined child health outcomes.
Table 3.
Associations between AL and child health outcomes
| Health Outcome | N (%) | OR1 (95% CI) |
|---|---|---|
| PROMIS® PGH-7 T-score | −0.87 (−1.58, −0.15) | |
| BRIEF Global Executive T-score | ||
| ≥65 | 127 (61.4) | Ref |
| <65 | 44 (21.3) | 0.99 (0.76, 1.26) |
| Missing | 36 (17.4) | |
| ADHD | ||
| No | 182 (87.9) | Ref |
| Yes | 25 (12.1) | 0.99 (0.73, 1.33) |
| Headaches/dizziness | ||
| No | 175 (84.5) | Ref |
| Yes | 32 (15.5) | 1.12 (0.86, 1.43) |
| Stomachaches | ||
| No | 172 (83.1) | Ref |
| Yes | 35 (16.9) | 1.09 (0.86, 1.37) |
| Asthma | ||
| No | 104 (50.2) | Ref |
| Yes | 103 (49.8) | 0.86 (0.69, 1.06) |
| Rhinitis | ||
| No | 125 (60.4) | Ref |
| Yes | 82 (39.6) | 1.11 (0.92, 1.34) |
| Eczema | ||
| No | 109 (52.7) | Ref |
| Yes | 98 (47.3) | 1.07 (0.89, 1.27) |
| Obesity | ||
| No | 152 (73.4) | Ref |
| Yes | 55 (26.6) | 1.51 (1.23, 1.89) |
| Infections | ||
| No | 102 (49.3) | Ref |
| Yes | 105 (50.7) | 1.01 (0.85, 1.20) |
| Somatic symptoms | ||
| No | 170 (82.1) | Ref |
| Yes | 37 (17.9) | 1.00 (0.79, 1.25) |
Models were adjusted for child’s age, sex, race/ethnicity, and caregiver’s educational level
ß coefficient and not an OR
Discussion
This study reports on the biological response to ACEs in early to mid-childhood using an AL framework. In our pediatric population, the relationship between age and AL was a U-shaped relationship, such that AL decreased until age 5 and then began to increase. Although the PEARLS score was not associated with AL when examined as a continuous variable, children with a PEARLS score of 1–2 had higher AL compared to children with a score of 0. When examining individual PEARLS items, we found that report of caregiver mental illness was associated with greater AL. Lastly, AL was also associated with poorer perceived child general health and greater odds of obesity. This work provides critical evidence informing early-to-middle childhood stress-related health processes as it is the first to simultaneously investigate relationships between ACEs, AL, and health outcomes during early to mid-childhood.
Operationalizing AL in children has proven problematic since the measurement of dysregulation related to timing of exposures need to be accounted for, as well as selection of clinically important biomarkers during childhood (16,62). Differences in AL as a result of cumulative risk have been detected as early as 9 years old (13), but the majority of our population was below this age (median age 6.7 years; IQR 4.0–9.3). Therefore, our results contribute to the limited data on levels and patterns of these stress-related biomarkers during earlier developmental years. Average AL (mean: 1.9) in our study population was only slightly above the sample means of two other pediatric studies (mean AL range: 1–1.19) that used different biomarkers but similar methods to estimate empirical cut points (47,48). Notably, we observed that AL declined with age until 5 years and this decrease could not be attributed to any specific biomarker. AL also increased with age in children older than 5 years. In sensitivity analyses, we identified leptin and IGFBP-1 as biomarkers that contributed to the association between age and greater AL in older children, suggesting that these metabolic markers may drive the U-shaped relationship between AL and age. A longitudinal prospective study conducted on Mexican-American children from Salinas Valley, CA observed a similar age-trajectory with leptin, where levels were highest at birth, declined at year 2, and then gradually increased at years 5 and 9 (63). The U-shaped relationship between age and AL may also mirror hypothalamic-pituitary-adrenal (HPA) axis development during early childhood. The HPA axis is under strong social regulation over the first year of life and continues to mature until around age 4 (64). Maturation and myelinization of the prefrontal cortex may also reduce HPA activity during this developmental period by establishing daytime napping periods and self-regulation (65). Lastly, a breakpoint, or change in direction, in the association between age and AL was observed at 5 years old. The switch from an inverse to direct correlation between age and AL at age 5 may align with the start of kindergarten or related adaptational challenges (e.g., peer rejection) that have been previous associated with higher HPA activity (66,67). Additionally, it is important to consider the relevance of the individual biomarkers used to operationalize AL during childhood. We only considered biomarkers previously quantified in other pediatric populations with similar ages to participants in this study (Table S1). AL measures may be less useful to characterize risk during early development than in adulthood (16). For example, biomarkers serving as proxies for clinical outcomes, such as blood pressure, may not show sufficient variability or reach thresholds for increased disease risk during early childhood (68). However, we used age-based normative values for SBP, DBP, and RHR and found that approximately 15 percent of child participants had levels above clinically relevant risk thresholds for each of these measures, suggesting that increased cardiovascular risk can be detected in this population. For all other biomarkers, since age-based normative values were not available, population-based empirical cut points were used to define high-risk groups. To advance this work, large population studies are needed to establish normal values for these biomarkers during early- and mid- childhood. Lastly, the cross-sectional study design only allowed for consideration of biomarkers as static measures rather than considering how their trajectories and sensitivity to adversity might change throughout childhood. Future research should longitudinally examine AL and the individual biological indicators to better understand the trajectory of these measures over the life course and their association with health at various stages of development.
AL is a cumulative measure of multi-system physiological dysregulation frequently used to capture the biological response to chronic stress. We did not observe an association between continuous PEARLS scores and AL, which is contrary to previous studies conducted in adult populations (11,12).
One possible explanation for this inconsistency is that a longer duration of adversity exposure may have been required prior to biomarker assessment given the cumulative nature of AL. Previous work demonstrated that the duration of poverty, but not concurrent poverty, was predictive of elevated overnight cortisol and cardiovascular function in adolescents (69). There is also evidence that the stress response is related to pubertal changes and that the impact of early life experiences on stress regulation may not be observable until after puberty (64). Additionally, several studies have suggested that the relationship between early life adversity and stress responsivity may be curvilinear in children of our study median age (70,71). Although the curvilinear hypothesis was not specifically tested in this study, the reporting of 1–2 PEARLS [and 1–3 original ACE items] was associated with an approximate 1.5-fold increase in AL and this association was slightly lower for children that experienced higher levels of adversity (1.3-fold increase in AL for PEARLS scores of 3–4 and 5+). The non-linear relationship between reported levels of adversity and AL may reflect differences in the type of or magnitude of physiological stress-response between children that experience low versus high adversity. For example, experiencing higher levels of adversity may have down-regulated HPA axis activity through negative feedback mechanisms (8). Alternatively, higher AL scores observed among children with lower PEARLS scores may reflect a more recent or acute exposure. Unfortunately, information on when (at what age) the first adversity occurred, and its duration of stress or impact, is not captured by the PEARLS screening tool. Although it is intentionally focused and brief, a resulting limitation of the PEARLS screening tool is that it does not capture information on frequency of exposure to certain event types, when exposures first occurred, or the chronicity of the impact of events on children’s lives, which could be used to gain better understanding about how timing, frequency, and duration of the influence of ACEs on AL. Furthermore, this study is cross-sectional and does not allow us to examine how PEARLS scores influence AL over the life course and during sensitive periods of development. Lastly, the curvilinear results may reflect a “steeling effect” such that that children with higher PEARLS scores have acquired experience and coping skills that decrease the negative biologic impact of subsequent adversities (72).
Activation of stress response pathways may also differ by ACE type. For instance, we observed that reporting a caregiver with mental illness was associated with increased AL. Maternal depression has been associated with increased allostatic load in their adolescent offspring (73). Living with a mentally ill parent may be stressful and impede positive relationships with the child, resulting in increases of AL. Positive child-caregiver dyad relationship has been shown to buffer the association between stress and cortisol reactivity in children (74). Future work should further examine this child-caregiver relationship on biologic stress. In addition, caregivers play a critical role in developing their child’s self-regulation and health-related behaviors, including feeding practices (75) and sleep behaviors (76). Therefore, caregiver mental illness may have disruptive effects on their ability to establish positive health-related behaviors for their children. The mediating role of these health-related behaviors in the relationship between caregiver mental illness and increased AL should be explored further.
Studies have also shown that high AL is predictive of adult morbidity and mortality (77), as well as poorer health outcomes and behavioral problems in children (18,47). Although disease development is often not yet detectable in childhood, it is important to ascertain whether and when AL biomarker indicators associate with disease in childhood. Our findings provide initial evidence in this realm, showing that higher AL was associated with worse general health in children, as measured by the PROMIS® PGH-7 T-score. We previously reported that higher PEARLS scores were associated with lower PROMIS® T-scores (21). Therefore, AL may mediate the relationship between PEARLS and poorer child health. We were unable to conduct mediation analyses because of limited sample size and lack of an association between continuous PEARLS scores and AL, but this should be further investigated in a larger study population. AL was also associated with obesity. Our results support results from a previous study conducted in a multiethnic population of 7–12 years old children where higher AL was associated with higher BMI (17). There were several other adverse health outcomes previously associated with higher PEARLS scores (21) that were not associated with AL in this study (e.g., headaches/dizziness, stomachaches, rhinitis, and eczema). Low prevalence of these health problems in this young sample may have left us underpowered to detect statistically significant differences in AL. One study previously reported an association between AL and asthma prevalence in adolescent boys (18), but in the present study, we were unable to conduct stratified analyses by sex because of small sample size. It is also possible that relationships between PEARLS and these health outcomes may be mediated by biologic mechanisms not captured by our AL index. Longitudinal studies are needed to assess whether AL during early- and mid-childhood is predictive of adverse health outcomes over the life course.
A clear strength of this study is that the population was composed predominately of Black and Latinx children, a population with disproportionate exposure to ACEs and high disease burden. For example, 76% of children in this study population experienced at least one original ACE, nearly double the prevalence for children in the rest of California (78). Recruitment of this underrepresented study population was intentional, with the goal of examining health impacts of ACEs in a high-burdened community to develop potential mitigation strategies; however, this intentionality in recruitment may limit generalizability of study results. For example, the income range for this population was narrow and low (only 8.2% had a family income above $50,000) since recruitment occurred in an urban, primary care center where most patients (>95%) are on state-sponsored Medicaid. Higher socioeconomic status has consistently been associated with lower AL in adult populations (15,79).The lack of income variability in our study population may have limited our ability to detect an association between AL and this variable. However, participants with a caregiver that reported having less than a high school education had higher AL than participants with a college educated caregiver (median AL 3 vs 2, p=0.028), suggesting that caregiver education may be a more useful measure of SES than income in this study population. This work contributes to the limited number of studies examining the biological burden of childhood adversity in the context of under-resourced and burdened families that may be under greater allostatic load because of economic insufficiency, poor housing quality, and other related stressors (80). The PEARLS screener tool also includes items such as discrimination and community violence that are more prevalent in communities of color due to long-standing social inequities, including residential segregation, redlining, and discriminatory policing practices (81). Thus, controlling for race/ethnicity may have limited our ability to estimate the impact of reported PEARLS items on AL. However, results were comparable between models with and without adjustment for race/ethnicity.
Our study has several limitations. The cross-sectional study design limits our ability to predict outcomes since temporality between ACEs, AL, and health cannot be established. A recent study of two large prospective cohorts reported that ACEs poorly predicted mental and physical health outcomes at age 18 and at 45 years (6). Biomarkers and contextual factors may shed light on the heterogeneity of response to ACEs in individual-level analyses. For example, social buffering and cultural context not assessed in this study may produce individual differences in the physiologic response to ACEs (74). Future work examining all these in tandem are needed to identify a predictive risk profile and identify promising buffering factors to inform intervention. Additionally, we did not account for timing and chronicity of PEARLS items which may have differential effects on the induced biological response. While only 6.1% of recruited individuals declined participation because of biospecimen collection, this may have potentially produced volunteer and selection bias. Lastly, we also excluded several cytokines below the LOD. These biological markers were most likely undetectable because they have low circulatory levels in healthy children. For example, a different study reported that >69% of blood samples had IL-6 and IL-10 levels below the LOD (44). Despite these detection issues, inclusion of these biomarkers would not have drastically changed our results since IL-1β and IL-6 were highly correlated with IL-8 (ρ=0.84) and TNF-alpha (ρ=0.67), respectively, in our study.
Conclusion:
The relationship between child AL and age appears to be U-shaped between 1–11 years. There was also some evidence that AL was associated with ACEs and poor health outcomes during early and mid- childhood.
Supplementary Material
Acknowledgements:
We would like to acknowledge the contributions of the project manager, Mindy Benson.
Conflicts of Interest and Source of Funding:
The authors have no conflicts of interest to declare. This work was supported in part by the TARA Health Foundation, Genentech Corporate Giving, and the California Initiative to Advance Precision Medicine. RD was supported by the University of California’s Presidential Postdoctoral Fellowship Program and NT was supported by a career development award from the NHLBI (K23- HL125551-01A1) and NB was supported by the Lisa Stone Pritzker Family Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the State of California or the National Institutes of Health.
Acronyms
- ACE
Adverse childhood experience
- ADHD
Attention deficit hyperactivity disorder
- AL
Allostatic load
- BDNF
Brain derived neurotrophic factor
- BMI
Body mass index
- BRIEF 2/P
Behavior rating inventory of executive function
- CRP
C-reactive protein
- DBP
Diastolic blood pressure
- ELISA
Enzyme-linked immunoassay
- ICD-10
International classification of diseases, Tenth Revision
- IGFBP-1
Insulin like growth factor binding protein 1
- IGFBP-3
Insulin like growth factor binding protein 3
- IL-1ß
Interleukin 1 beta
- IL-6
Interleukin 6
- IL-8
Interleukin 8
- IL-10
Interleukin 10
- IRR
Incidence rate ratio
- ISAAC
The International Study of Asthma and Allergies in Childhood
- LOQ
Limit of quantification
- MPO
Myeloperoxidase
- PEARLS
PEdiatric ACEs and Related Life Event Screener
- PROMIS
Patient-Reported Outcomes Measurement Information System Global 10-item questionnaire
- PSS-10
Perceived Stress Scale (10-item)
- RHR
Resting heart rate
- SBP
Systolic blood pressure
- SD
Standard deviation
- TNF-α
Tumor necrosis factor alpha
- VEGF
Vascular endothelial growth factor
References
- 1.Bethell CD, Carle A, Hudziak J, Gombojav N, Powers K, Wade R, Braveman P. Methods to Assess Adverse Childhood Experiences of Children and Families: Toward Approaches to Promote Child Well-being in Policy and Practice. Academic Pediatrics. 2017;17:S51–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felitti, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine. 1998;14:245–58. [DOI] [PubMed] [Google Scholar]
- 3.Flaherty EG, Thompson R, Litrownik AJ, Theodore A, English DJ, Black MM, Wike T, Whimper L, Runyan DK, Dubowitz H. Effect of early childhood adversity on child health. Archives of Pediatrics & Adolescent Medicine. 2006;160:1232–38. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty EG, Thompson R, Dubowitz H, Harvey EM, English DJ, Proctor LJ, Runyan DK. Adverse childhood experiences and child health in early adolescence. JAMA pediatrics. 2013;167:622–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, Jones L, Dunne MP. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. The Lancet. Public Health. 2017;2:e356–66. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin JR, Caspi A, Meehan AJ, Ambler A, Arseneault L, Fisher HL, Harrington H, Matthews T, Odgers CL, Poulton R, Ramrakha S, Moffitt TE, Danese A. Population vs Individual Prediction of Poor Health From Results of Adverse Childhood Experiences Screening. JAMA Pediatrics. 2021;175:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bucci M, Marques SS, Oh D, Harris NB. Toxic Stress in Children and Adolescents. Advances in Pediatrics. 2016;63:403–28. [DOI] [PubMed] [Google Scholar]
- 8.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior. 2012;106:29–39. [DOI] [PubMed] [Google Scholar]
- 9.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 10.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999;896:30–47. [DOI] [PubMed] [Google Scholar]
- 11.Barboza Solís C, Kelly-Irving M, Fantin R, Darnaudéry M, Torrisani J, Lang T, Delpierre C. Adverse childhood experiences and physiological wear-and-tear in midlife: Findings from the 1958 British birth cohort. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gustafsson PE, Janlert U, Theorell T, Westerlund H, Hammarström A. Social and material adversity from adolescence to adulthood and allostatic load in middle-aged women and men: results from the Northern Swedish Cohort. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine. 2012;43:117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology. 2003;39:924–33. [DOI] [PubMed] [Google Scholar]
- 14.Doan SN, Dich N, Evans GW. Childhood cumulative risk and later allostatic load: mediating role of substance use. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association. 2014;33:1402–9. [DOI] [PubMed] [Google Scholar]
- 15.Johnson SC, Cavallaro FL, Leon DA. A systematic review of allostatic load in relation to socioeconomic position: Poor fidelity and major inconsistencies in biomarkers employed. Social Science & Medicine (1982). 2017;192:66–73. [DOI] [PubMed] [Google Scholar]
- 16.Doan SN. Allostatic load: Developmental and conceptual considerations in a multi-system physiological indicator of chronic stress exposure. Developmental Psychobiology. 2021; [DOI] [PubMed] [Google Scholar]
- 17.Cedillo YE, Murillo AL, Fernández JR. The association between allostatic load and anthropometric measurements among a multiethnic cohort of children. Pediatric obesity. 2019;14:e12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bahreinian S, Ball GDC, Vander Leek TK, Colman I, McNeil BJ, Becker AB, Kozyrskyj AL. Allostatic Load Biomarkers and Asthma in Adolescents. American Journal of Respiratory and Critical Care Medicine. 2013;187:144–52. [DOI] [PubMed] [Google Scholar]
- 19.Barry LE, O’Neill C, Heaney LG. Association between asthma, corticosteroids and allostatic load biomarkers: a cross-sectional study. Thorax. 2020;75:835–41. [DOI] [PubMed] [Google Scholar]
- 20.Koita K, Long D, Hessler D, Benson M, Daley K, Bucci M, Thakur N, Burke Harris N. Development and implementation of a pediatric adverse childhood experiences (ACEs) and other determinants of health questionnaire in the pediatric medical home: A pilot study. PloS One. 2018;13:e0208088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thakur N, Hessler D, Koita K, Ye M, Benson M, Gilgoff R, Bucci M, Long D, Burke Harris N. Pediatrics adverse childhood experiences and related life events screener (PEARLS) and health in a safety-net practice. Child Abuse & Neglect. 2020;108:104685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koita K, Long D, Hessler D, Benson M, Daley K, Bucci M, Thakur N, Burke Harris N. Development and implementation of a pediatric adverse childhood experiences (ACEs) and other determinants of health questionnaire in the pediatric medical home: A pilot study. PLOS ONE. 2018;13:e0208088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 1998/06/23 ed. 1998;14:245–58. [DOI] [PubMed] [Google Scholar]
- 24.Cole SR. Assessment of differential item functioning in the Perceived Stress Scale-10. Journal of Epidemiology and Community Health. 1999;53:319–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crouch E, Radcliff E, Brown M, Hung P. Exploring the association between parenting stress and a child’s exposure to adverse childhood experiences (ACEs). Children and youth services review. 2019;102:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pretty C, O’Leary DD, Cairney J, Wade TJ. Adverse childhood experiences and the cardiovascular health of children: a cross-sectional study. BMC Pediatrics. 2013;13:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerr DM, McDonald J, Minnis H. The association of child maltreatment and systemic inflammation in adulthood: A systematic review. PloS One. 2021;16:e0243685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su S, Wang X, Pollock JS, Treiber FA, Xu X, Snieder H, McCall WV, Stefanek M, Harshfield GA. Adverse Childhood Experiences and Blood Pressure Trajectories from Childhood to Young Adulthood: The Georgia Stress and Heart Study. Circulation. 2015;131:1674–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danese A, Dove R, Belsky DW, Henchy J, Williams B, Ambler A, Arseneault L. Leptin deficiency in maltreated children. Translational Psychiatry. 2014;4:e446–e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joung KE, Park K-H, Zaichenko L, Sahin-Efe A, Thakkar B, Brinkoetter M, Usher N, Warner D, Davis CR, Crowell JA, Mantzoros CS. Early life adversity is associated with elevated levels of circulating leptin, irisin, and decreased levels of adiponectin in midlife adults. The Journal of Clinical Endocrinology and Metabolism. 2014;99:E1055–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heard-Garris N, Davis MM, Estabrook R, Burns J, Briggs-Gowan M, Allen N, Carnethon M, Aguayo L, Wakschlag L, Penedo F. Adverse childhood experiences and biomarkers of inflammation in a diverse cohort of early school-aged children. Brain, Behavior, & Immunity - Health. 2020;1:100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartwell KJ, Moran-Santa Maria MM, Twal WO, Shaftman S, DeSantis SM, McRae-Clark AL, Brady KT. Association of elevated cytokines with childhood adversity in a sample of healthy adults. Journal of psychiatric research. 2013;47:604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slopen N, Kubzansky LD, McLaughlin KA, Koenen KC. Childhood adversity and inflammatory processes in youth: A prospective study. Psychoneuroendocrinology. 2013;38:188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biological Psychiatry. 2012;72:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bücker J, Fries GR, Kapczinski F, Post RM, Yatham LN, Vianna P, Bogo Chies JA, Gama CS, Magalhães PV, Aguiar BW, Pfaffenseller B, Kauer-Sant’Anna M. Brain-derived neurotrophic factor and inflammatory markers in school-aged children with early trauma. Acta Psychiatrica Scandinavica. 2015;131:360–68. [DOI] [PubMed] [Google Scholar]
- 36.Leviton A, Allred EN, Dammann O, Joseph RM, Fichorova RN, O’Shea TM, Kuban KCK. Socioeconomic status and early blood concentrations of inflammation-related and neurotrophic proteins among extremely preterm newborns. PLoS ONE. 2019;14:e0214154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsotsoros C, Stout M, Hawkins M. Associations of Adverse Childhood Experiences with Executive Function and Brain-Derived Neurotrophic Factor. Innovation in Aging. 2021;5:1034–35. [Google Scholar]
- 38.Kim S, Watt T, Ceballos N, Sharma S. Adverse childhood experiences and neuroinflammatory biomarkers-The role of sex. Stress and Health: Journal of the International Society for the Investigation of Stress. 2019;35:432–40. [DOI] [PubMed] [Google Scholar]
- 39.Şimşek Ş, Yüksel T, Kaplan İ, Uysal C, Alaca R. Examining the levels of BDNF and cortisol in children and adolescent victims of sexual abuse--a preliminary study. Comprehensive Psychiatry. 2015;61:23–27. [DOI] [PubMed] [Google Scholar]
- 40.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM, Children S on S and M of HBPI. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics [Internet]. 2017. [cited 2020 Jul 29];140. Available from: https://pediatrics.aappublications.org/content/140/3/e20171904 [DOI] [PubMed] [Google Scholar]
- 41.Daniels SR. How to Define Hypertension in Children and Adolescents. Circulation. 2016;133:350–51. [DOI] [PubMed] [Google Scholar]
- 42.Ostchega Y, Porter KS, Hughes J, Dillon CF, Nwankwo T. Resting pulse rate reference data for children, adolescents, and adults: United States, 1999–2008. National Health Statistics Reports. 2011;1–16. [PubMed] [Google Scholar]
- 43.Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L, Hartge P. Epidemiologic Evaluation of Measurement Data in the Presence of Detection Limits. Environmental Health Perspectives. 2004;112:1691–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Decker M-L, Gotta V, Wellmann S, Ritz N. Cytokine profiling in healthy children shows association of age with cytokine concentrations. Scientific Reports. 2017;7:17842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kleiner G, Marcuzzi A, Zanin V, Monasta L, Zauli G. Cytokine Levels in the Serum of Healthy Subjects. Mediators of Inflammation. 2013;2013:e434010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Findlay JWA, Smith WC, Lee JW, Nordblom GD, Das I, DeSilva BS, Khan MN, Bowsher RR. Validation of immunoassays for bioanalysis: a pharmaceutical industry perspective. Journal of Pharmaceutical and Biomedical Analysis. 2000;21:1249–73. [DOI] [PubMed] [Google Scholar]
- 47.Rogosch FA, Dackis MN, Cicchetti D. Child maltreatment and allostatic load: Consequences for physical and mental health in children from low-income families. Development and Psychopathology. 2011;23:1107–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Theall KP, Drury SS, Shirtcliff EA. Cumulative Neighborhood Risk of Psychosocial Stress and Allostatic Load in Adolescents. American Journal of Epidemiology. 2012;176:S164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Misiak B, Stańczykiewicz B, Pawlak A, Szewczuk-Bogusławska M, Samochowiec J, Samochowiec A, Tyburski E, Juster R-P. Adverse childhood experiences and low socioeconomic status with respect to allostatic load in adulthood: A systematic review. Psychoneuroendocrinology. 2022;136:105602. [DOI] [PubMed] [Google Scholar]
- 50.2018 Poverty Guidelines. ASPE. [cited 2021 Sep 8]. Available from: https://aspe.hhs.gov/topics/poverty-economic-mobility/poverty-guidelines/prior-hhs-poverty-guidelines-federal-register-references/2018-poverty-guidelines
- 51.Burke NJ, Hellman JL, Scott BG, Weems CF, Carrion VG. The impact of adverse childhood experiences on an urban pediatric population. Child Abuse & Neglect. 2011;35:408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunt TKA, Slack KS, Berger LM. Adverse childhood experiences and behavioral problems in middle childhood. Child Abuse & Neglect. 2017;67:391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McKelvey LM, Edge NC, Mesman GR, Whiteside-Mansell L, Bradley RH. Adverse experiences in infancy and toddlerhood: Relations to adaptive behavior and academic status in middle childhood. Child Abuse & Neglect. 2018;82:168–77. [DOI] [PubMed] [Google Scholar]
- 54.Jimenez ME, Roy W, Schwartz-Soicher O, Lin Y, Reichman NE. Adverse Childhood Experiences and ADHD Diagnosis at Age 9 Years in a National Urban Sample. Academic pediatrics. 2017;17:356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McKelvey LM, Saccente JE, Swindle TM. Adverse Childhood Experiences in Infancy and Toddlerhood Predict Obesity and Health Outcomes in Middle Childhood. Childhood Obesity. 2019;15:206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wing R, Gjelsvik A, Nocera M, McQuaid EL. Association between adverse childhood experiences in the home and pediatric asthma. Annals of Allergy, Asthma & Immunology: Official Publication of the American College of Allergy, Asthma, & Immunology. 2015;114:379–84. [DOI] [PubMed] [Google Scholar]
- 57.Oh DL, Jerman P, Silvério Marques S, Koita K, Purewal Boparai SK, Burke Harris N, Bucci M. Systematic review of pediatric health outcomes associated with childhood adversity. BMC pediatrics. 2018;18:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forrest CB, Bevans KB, Pratiwadi R, Moon J, Teneralli RE, Minton JM, Tucker C. Development of the PROMIS® Pediatric Global Health (PGH-7) Measure. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2014;23:1221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sherman EMS, Brooks BL. Behavior rating inventory of executive function - Preschool version (BRIEF-P): Test review and clinical guidelines for use. Child Neuropsychology. 2010;16:503–19. [Google Scholar]
- 60.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, Mitchell EA, Pearce N, Sibbald B, Stewart AW, Strachan D, Weiland SK, Williams HC. International study of asthma and allergies in childhood (ISAAC): Rationale and methods. In: European Respiratory Journal. European Respiratory Society; 1995. p. 483–91. [DOI] [PubMed] [Google Scholar]
- 61.Simonsohn U. Two Lines: A Valid Alternative to the Invalid Testing of U-Shaped Relationships With Quadratic Regressions. Advances in Methods and Practices in Psychological Science. 2018;1:538–55. [Google Scholar]
- 62.Whelan E, O’Shea J, Hunt E, Dockray S. Evaluating measures of allostatic load in adolescents: A systematic review. Psychoneuroendocrinology. 2021;131:105324. [DOI] [PubMed] [Google Scholar]
- 63.Volberg V, Heggeseth B, Harley K, Huen K, Yousefi P, Davé V, Tyler K, Vedar M, Eskenazi B, Holland N. Adiponectin and Leptin Trajectories in Mexican-American Children from Birth to 9 Years of Age. PLOS ONE. 2013;8:e77964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gunnar MR, Quevedo KM. Early care experiences and HPA axis regulation in children: a mechanism for later trauma vulnerability. Progress in brain research. 2008;167:137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. [DOI] [PubMed] [Google Scholar]
- 66.Smider NA, Essex MJ, Kalin NH, Buss KA, Klein MH, Davidson RJ, Goldsmith HH. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: a prospective study. Child Development. 2002;73:75–92. [DOI] [PubMed] [Google Scholar]
- 67.Gunnar MR, Sebanc AM, Tout K, Donzella B, van Dulmen MMH. Peer rejection, temperament, and cortisol activity in preschoolers. Developmental Psychobiology. 2003;43:346–68. [DOI] [PubMed] [Google Scholar]
- 68.Repetti RL, Robles TF, Reynolds B. Allostatic processes in the family. Development and Psychopathology. 2011;23:921–38. [DOI] [PubMed] [Google Scholar]
- 69.Evans GW, Kim P. Childhood poverty and health: cumulative risk exposure and stress dysregulation. Psychological Science. 2007;18:953–57. [DOI] [PubMed] [Google Scholar]
- 70.Shakiba N, Ellis BJ, Bush NR, Boyce WT. Biological sensitivity to context: A test of the hypothesized U-shaped relation between early adversity and stress responsivity. Development and Psychopathology. 2020;32:641–60. [DOI] [PubMed] [Google Scholar]
- 71.Hosseini-Kamkar N, Lowe C, Morton JB. The differential calibration of the HPA axis as a function of trauma versus adversity: A systematic review and p-curve meta-analyses. Neuroscience & Biobehavioral Reviews. 2021;127:54–135. [DOI] [PubMed] [Google Scholar]
- 72.Rutter M Resilience as a dynamic concept. Development and Psychopathology. 2012;24:335–44. [DOI] [PubMed] [Google Scholar]
- 73.Nelson BW, Sheeber L, Pfeifer J, Allen NB. Psychobiological markers of allostatic load in depressed and nondepressed mothers and their adolescent offspring. Journal of Child Psychology and Psychiatry. 2021;62:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hostinar CE, Johnson AE, Gunnar MR. Early Social Deprivation and the Social Buffering of Cortisol Stress Responses in Late Childhood: An Experimental Study. Developmental psychology. 2015;51:1597–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wood AC, Blissett JM, Brunstrom JM, Carnell S, Faith MS, Fisher JO, Hayman LL, Khalsa AS, Hughes SO, Miller AL, Momin SR, Welsh JA, Woo JG, Haycraft E, null null. Caregiver Influences on Eating Behaviors in Young Children. Journal of the American Heart Association. 2020;9:e014520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hash JB, Oxford ML, Fleming CB, Ward TM, Spieker SJ, Lohr MJ. Impact of a Home-Visiting Program on Sleep Problems Among Young Children Experiencing Adversity. Child abuse & neglect. 2019;89:143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic Load as a Marker of Cumulative Biological Risk: MacArthur Studies of Successful Aging. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4770–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Slone L. Adverse Childhood Experiences. Let’s Get Healthy California. [cited 2022 Jan 14]. Available from: https://letsgethealthy.ca.gov/goals/healthy-beginnings/adverse-childhood-experiences/
- 79.Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Annals of the New York Academy of Sciences. 2010;1186:223–39. [DOI] [PubMed] [Google Scholar]
- 80.Blair C, Raver C, Granger D, Mills-Koonce R, Hibel L. Allostasis and Allostatic Load in the Context of Poverty in Early Childhood. Development and psychopathology. 2011;23:845–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martinez A, de la Rosa R, Mujahid M, Thakur N. Structural racism and its pathways to asthma and atopic dermatitis. The Journal of Allergy and Clinical Immunology. 2021;148:1112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


