Abstract
Background and purpose
Nontuberculous mycobacteria (NTM) disease is an important infection disease throughout the world. Mycobacterium xenopi (M. xenopi) is a common NTM. Extrapulmonary infections due to M. xenopi, particularly spine infections, are a rare occurrence, but lack of research is cited as a constraint for implementing NTM control in such patients. The purposes of this paper are to describe a case of spondylodiscitis, to review the published literature on cases of M. xenopi spine infections, and to summarize the predisposing factors, diagnosis, and treatment of infection.
Methods
A case of spondylodiscitis was caused by M. xenopi in a patient with systemic lupus erythematosus (SLE). Research was conducted using the PubMed, ScienceDirect, Embase, Wiley Online Library, and Scopus databases using the following search terms: “Mycobacterium xenopi”, “vertebral”, “spinal”, “spondylodiscitis”, “infection”, and “osteomyelitis”.
Results
We retrieved 14 cases published before August 2022. The risk factors for infection were iatrogenic infections (3/14, 21.43%), SLE (4/14, 28.57%), AIDS (4/14, 28.57%), and immunocompetence without any comorbidities (3/14, 21.43%). The most common sites of infection were thoracic vertebrae (10/14, 71.43%) and lumbar vertebrae (4/14, 28.57%). A total of 14 cases were isolated and identified as M. xenopi from a toad by mycobacterial culture. The identification time was 55.00 ± 7.55 days (the present report identification time of metagenomic next generation sequencing (mNGS) was only 2 days). All patients were treated with antibiotic therapy, and the duration of treatment was 13.18 ± 2.13 months. Clarithromycin-based therapy showed a higher improvement rate (5/6, 83.33%). Surgical intervention was performed in 5 patients. Only 1 patient did not show any improvement after surgical treatment.
Conclusion
M. xenopi spine infection in humans presents with atypical clinical symptoms. mNGS identification may be a good choice. M. xenopi may be considered in immunocompromised patients with spinal infection. We recommend a clarithromycin-containing regimen and prolonging the duration of treatment to ensure effectiveness.
Keywords: Mycobacterium xenopi, Spine infections, Predisposing factors, Treatment
1. Introduction
Mycobacterium xenopi (M. xenopi) is a slow-growing, nonphotochromogenic, nontuberculous mycobacterium [1]. It was isolated from the South African toad for the first time in 1959, and then it was found in the natural waters and hospital ‘s hot water system. It was thought that patients were mainly infected by exposure to contaminated soil or water, or by infectious aerosols [2]. Data of species identification of nontuberculous mycobacteria (NTM) from clinical specimens in 14 countries, including Europe and Asia, have shown an increasing trend of the isolation rate of M. xenopi [3]. Pneumonia is the most common infection caused by M. xenopi. Extrapulmonary localizations, particularly spine infections, are a rare occurrence [4]. The clinical characteristics and iconography of M. xenopi infection are similar to those of Mycobacterium tuberculosis. The identification of M. xenopi is difficult, and it can be easily overlooked. In this study, we report a case of spine infection caused by M. xenopi and review the published literature on cases of spine infections caused by M. xenopi, with a focus on the predisposing factors, diagnostic and therapeutic difficulties to assist future research on this priority disease.
2. Case description
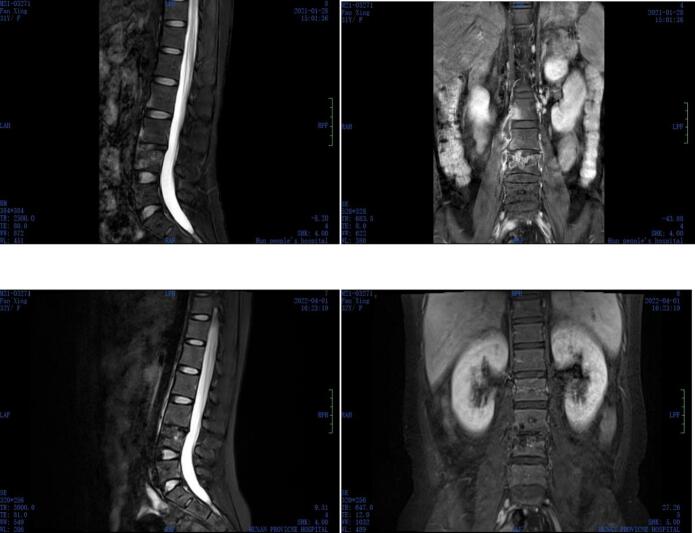
In January 2021, a 32-year-old woman was referred to our hospital because of fever since six days and rash since five days. The patient had a history of systemic lupus erythematosus (SLE) for about 15 years, and it was being treated with prednisolone 10–15 mg daily. In December 2019, the patient was diagnosed with M. tuberculosis-related spine infection at another center, and she was treated with quadruple anti-tuberculosis treatment (isoniazid, rifampicin, pyrazinamide, and ethambutol) for 1 year without any improvement. Skin testing revealed anergy to tuberculin. On examination, she was found to be febrile with a body temperature of 39.4 °C. Assessment of her spine showed tenderness in the L2–4 lumbar vertebrae, and a full neurologic assessment revealed no abnormality. The basic laboratory indices showed the following findings: White blood cell (WBC) count 1.77*109/L, erythrocyte sedimentation rate (ESR) 26 mm/h, C-reactive protein (CRP) 67.80 mg/L, and Interferon-γassays <1.0 (negative). Magnetic resonance imaging (MRI) of the spinal column revealed destruction of the L2–4 discs, with associated abnormal paravertebral soft tissue (Fig. 1a). On the 9th day of hospitalization, piperacillin/tazobactam therapy was added to the patient's regimen on the basis of quadruple anti-tuberculosis treatment. After the procedures, her fever and rash improved. But the pain was not reduced. On the 13th day of hospitalization, the patient underwent abscess drainage. The result of metagenome next-generation sequencing (mNGS) of the tissue specimen showed M. xenopi two days later. Based on the above results, treatment was initiated with a combination of amikacin (0.4 g ivgtt q12 h), rifampicin (0.6 g po Qd), ethambutol (0.75 g po Qd), and clarithromycin (0.5 g po Bid). After 1 month, amikacin therapy was discontinued by the patient herself. Nonetheless, 5 months after the initiation of treatment, the cultures were negative, and the patient's condition had greatly improved. But MRI of the spinal column revealed that the abscess had increased in size. The patient's drug regimen was subsequently modified to rifampicin (0.6 g po Qd), ethambutol (0.75 g po Qd), clarithromycin (0.5 g po Bid), and levofloxacin (0.5 g po qd). After 1 month, MRI of the spinal column still showed that the abscess had further slightly increased in size further. Linezolid (0.6 g po q12 h) was added to the original drug regimen. After 4 months, linezolid therapy was discontinued because of numbness in both lower limbs and chills. After another 7 months, MRI of the spinal column revealed that the abscess had significantly reduced in size, and the degree of enhancement of vertebral lesions was significantly lower than that on the earlier MRI (Fig. 1b). Her pain was also markedly improved. We plan to continue a 2-year course of antimycobacterial therapy, and then to monitor the relapse risk in the patient.
Fig. 1.
A T2-weighted sagittal and coronal magnetic resonance image of the lumbar spine.
a: At admission: Image showing L2, L3, and L4 bone destruction and a paravertebral soft tissue mass.
b: After anti-M. xenopi treatment: Image showing a significantly reduced degree of enhancement in L2, L3, and L4 vertebrae and abscess shrinkage.
3. Materials and methods
We conducted a review of case reports and case series published before August 2022. No restrictions based on the language or year of publication were applied.
3.1. Search strategy
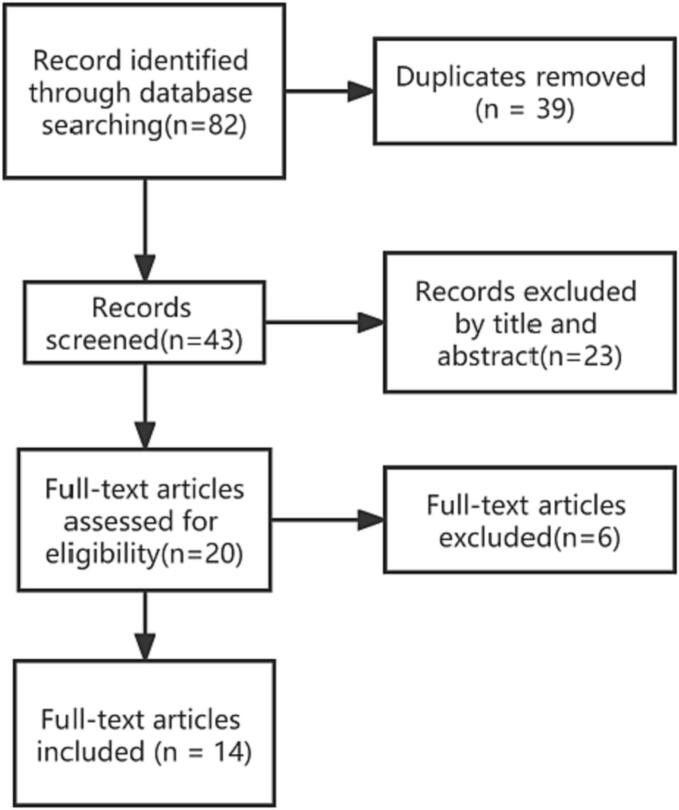
The following databases were searched for relevant studies: PubMed, ScienceDirect, Embase, Wiley Online Library, and Scopus using the following search terms: “Mycobacterium xenopi”, “vertebral”, “spinal”, “spondylodiscitis”, “infection”, and “osteomyelitis”. The study flow is described in Fig. 2.
Fig. 2.
Study flow chart.
3.2. Inclusion and exclusion criteria
Inclusion criteria: all case reports of adult patients diagnosed with spine infections caused by M. xenopi for whom the treatment and outcome were specified. Exclusion criteria: (1) A case report on other sites of infection (pulmonary or extrapulmonary); (2) Lack of clinical data of individual patients; (3) Unpublished reports, book paragraph, and meeting abstracts.
3.3. Data collection
Clinical data and medication were entered into a pre-designed Excel file. The following information was extracted from each paper: (1) Characteristics of patients (sex and age); (2) Infection characteristics, site of infection, predisposing factors, underlying diseases, symptoms, and laboratory examination results; (3) Treatment; (4) Outcome after treatment.
4. Results
As shown in Fig. 2, a total of 14 articles were included in the study, accounting for 14 patients (Table 1). Both sexes were equally affected, with 7 females (including 4 patients with SLE) and 7 males (including 4 HIV patients). The mean patient age was 50.57 years. A total of 10 cases of infection in the thoracic vertebrae were identified. All patients had clinical symptoms. A total of 7 cases had purified protein derivative (PPD) skin test records, and only 1 case was positive. The results of pathological examination were recorded in 11 cases, and 10 cases showed granulomatous inflammatory changes. The results of staining smear examintation were recorded in 8 cases, and acid-fast bacilli (AFB) were found in 3 cases. All 14 cases were identified as M. xenopi by microbial culture, and the identification time was 55.00 ± 7.55 days. All patients were treated with drug combinations (i.e., at least two antibiotics), but the regimens varied widely (Table 1). A rifamycin (rifampin) regimen was used in 13 patients; and ethambutol was used in 13 cases, isoniazid was used in 9 cases, pyrazinamide was used in 6 cases, clarithromycin was used in 6 cases, fluoroquinolones were used in 5 cases (ciprofloxacin, moxifloxacin, pefloxacin, and levofloxacin), and azithromycin was used in 1 case. A total of 11 cases showed improvement in clinical symptoms and/or imaging, of which 1 case recurred and was cured by surgery combined with drug therapy. Among the cases treated with clarithromycin-based regimen, 5 cases improved and 1 case did not improve. Among them, One case each including isoniazid or quinolones was improved. In the non-clarithromycin or azithromycin treatment, the combination of isoniazid and rifampicin was mainly used; with 6 cases showing improvement and 3 cases not showing any improvement. A total of 5 cases received surgical treatment. The duration of treatment was 13.18 ± 2.13 months. The results are shown in Table 1, Table 2.
Table 1.
Features of the 14 reported cases of M. xenopi spinal infections.
| Ref | Age(y)/sex | Site of infection | Predisposing factors | Underlying diseases | Presenting symptoms | biomarker | MT | QFT | Histopathologic | Acid-fast staining | Time to species identification |
Medical therapy (duration in months) |
surgery | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [5] | 44/M | T9/10 | HIV, Kaposi's sarcoma (not on HAART) | coronary artery bypass graf |
Chest pain and notalgia(1d) | / | / | − | / | / | / | CCR, R, E, MXF | No | unkown |
| [6] | 55/F | L3 | systemic lupus erythematosus (15y,prednisolone 5 mg daily.) |
None | left loin pain, nausea and weight loss. 1y) |
ESR:55 mm/h | − | / | Granuloma | − | 4 m |
|
No |
|
| [7] | 28/M | T10 | HIV | None | back pain(10y), nocturnal awakening, sweating, weight loss, fever (3y) |
CRP:38 mg/L, WBC:13.7/nL, ESR:40 mm/h, CD4T-cell:400cells/mm3 CD4/CD8:0.5 |
/ | / | Granuloma | − | / | Rfb, I, E, CCR(6 m) | Yes | Clinical and radiographic improvement |
| [8] | 75/F | T6/7 | None | None | back pain and night sweats.(6w) | ESR:79 mm/h | − | / | Granuloma | + | 8w |
|
Yes | 1.Clinical improvement 2.Clinical and radiographic improvement |
| [9] | 33/M | T11/12 | HIV, CD4 count was 20 cells/mm3 |
primary Pneumocystis carinii pneumonia | back pain and night sweats(2 m) | WBC:14.86*109/L; NE:11.56*109/L; ESR:18 mm/h; CD4:490cells/mm3 |
/ | / | Granuloma | + | / | R, E, I(6 m) | No | Clinical and radiographic improvement |
| [10] | 56/F | T8/9 | SLE(20y);low-dose corticosteroids and azathio prine. |
/ | Left shoulder pain and notalgia(2 m) | ESR:31 mm/h | / | / | without granuloma | / | 7 W | I, CCR, C(9 m) | No | Clinical and radiographic improvement |
| [11] | 70/F | T3/4 | / | Hypertensie cardiovascular disease; II diabetes mellitus; Radical left mastectomy |
Back pain; weakness of both legs(6w) |
WBC:5700/mm | − | / | Granuloma | − | / |
|
Yes |
|
| [12] | 46//F | T8/9 | SLE(15); prednisolone (10 mg daily) | pyelonephritis of the right kidney,mitral valve insufficiency and aphasia | Skelasthenia, walking disorder and notalgia(10 m) | ESR: 27 mm/h; CRP:49.7 mg/L | / | − | Granuloma | − | 37d | E, Rfb, A(5 m) | Yes | Clinical and radiographic improvement |
| [13] | 77/F | L1/2 | Her husband had tuberculosis 40 years previously. |
hypertension | lower back pain radiating to the right buttock. (5y) | ESR:54 mm/h | / | / | / | − | 2 m |
|
No |
|
| [14] | 42/M | T7/8 | HIV | Pneumocystis carinii pneumonia | flflaccid paralysis of both legs(2w) | CD4:41/mm3 | − | / | without granuloma | − | 6w |
|
No |
|
| [15] | 61/M | L3/4 | a history of L3-L4 and L4-L5 percutaneous nucleotomy performed 15 years earlier (in 1992) | None | pain in the low back and left buttock. (6 m) |
Normal | − | / | Granuloma | + | 8w |
|
No | 1.Clinical and radiographic improvement2.Recovery |
| [16] | 28/M | L4/5 | percutaneous nucleotomy as treatment of sciatica at the L5 level due to a herniated disk at the L4-L5 level before a month | lumbar spine pain | ESR:32 mm/h CRP:10 mg/L WBC:7500/mm |
+ | / | Granuloma | / | 2 m |
|
No | 1.Persistence of infection; 2.Recovery |
|
| [17] | 30/F | T10/11 | SLE(15);prednisone(15 mg daily) | None | Back pain | ESR:30 mm/h | + | / | / | + | 4w | I, R, E, P(18 m) | Yes | 1.Clinical and radiographic improvement; 2. relapsed after 9 mo off treatment; Clinical improvementwith debridement and antituberculous medication。 |
| [18] | 63/M | T12/L1 | A needle biopsywas performed at T12-L1 before 10 years | T12-L1nondisplaced fracture; myocardial infarction. |
back pain (20y) |
/ | − | / | without ganuloma | / | 6w | Le, R, E, CCR(10.5 m) | Yes | Persistence of infection; |
Note:clarithromycin(CCR), rifabutin(Rfb), rifampicin(R), isoniazid(I), ethambutol(E), pyrazinamide(P), streptomycin(S), ciprofloxacin(C), pefloxacin(Pe), moxifloxacin(MXF), levofloxacin(Le), azithromycin(A).
MT: Mantoux test; QFT:Quantiferon test for TB.
-: Negative; +: positive; /:no record.
d: day; m: month; y:year.
Table 2.
Characteristics of the 14 cases of spine infection due to M. xenopi.
| Parameter | Total | immunocompetent | Cases with iatrogenic infections | Cases with SLE | Cases with HIV | |
|---|---|---|---|---|---|---|
| Sex | Male | 7 | 0 | 3 | 0 | 4 |
| Female | 7 | 3 | 0 | 4 | 0 | |
| Age(y) | 50.57 ± 4.45 | 74.00 ± 1.70 | 50.67 ± 9.27 | 46.75 ± 5.21 | 36.75 ± 3.27 | |
| Site of infection | Thoracic vertebra | 10 | 2 | 1 | 3 | 4 |
| Lumbar vertebra | 4 | 1 | 2 | 1 | 0 | |
| Pre-treatment biomarkers (↑/normal/no record) |
WBC | 1/7/6 | 0/2/1 | 0/2/1 | 0/2/2 | 1/1/2 |
| ESR | 6/2/5 | 2/0/1 | 1/1/1 | 3/0/1 | 0/1/3 | |
| CRP | 1/1/12 | 0/0/3 | 0/1/2 | 1/0/3 | 0/0/4 | |
| Biomarkers after treatment (↓/normal/no record) |
WBC | 1/7/6 | 0/2/1 | 0/2/1 | 0/2/2 | 1/1/2 |
| ESR | 6/2/5 | 2/0/1 | 1/1/1 | 3/0/1 | 0/1/3 | |
| CRP | 1/1/12 | 0/0/3 | 0/1/2 | 1/0/3 | 0/0/4 | |
| Tuberculosis test (+/−/no record) |
MT | 4/4/6 | 2/1/0 | 1/1/1 | 0/2/2 | 1/0/3 |
| QFT | 0/2/12 | 0/0/3 | 0/0/3 | 0/1/3 | 0/1/3 | |
| Histopathologic | Granuloma | 11 | 2 | 2 | 2 | 3 |
| without granuloma | 2 | 0 | 1 | 1 | 0 | |
| no record | 2 | 1 | 0 | 1 | 1 | |
| microorganism | Acid-fast staining(+/−/no record) |
3/5/6 | 0/1/2 | 1/0/2 | 1/2/1 | 1/2/1 |
| Identification time(m) | 55.00 ± 7.55 | 58 ± 2 | 58.50 ± 4.46 | 36.28 ± 18.14 | 42 | |
| treatment | Antibiotic | 9 | 1 | 3 | 2 | 3 |
| Antibiotic+surgery | 5 | 2 | 0 | 2 | 1 | |
| outcome | Improvement | 4/7 | 1/2 | 1/1 | 2/1 | 0/3 |
| Relapse | 2 | / | 1 | 1 | / | |
| Unkown | 1 | / | / | / | 1 | |
5. Discussion
NTM are ubiquitous in the environment. NTM infections are rarely transmissible between humans and epidemic cases [19], and they are often associated with contaminated water, soil, or aerosols [2]. It is postulated that susceptible individuals acquire disseminated infections through respiratory and digestive tract colonization [19]. M. xenopi is the third most common NTM isolated from pulmonary samples in Europe, and it is less commonly isolated in Asia, USA, and Australia [20]. M. xenopi is widely isolated from environmental sources, such as water, soil, and tap water systems. The most common clinical manifestation of M. xenopi infection is lung disease. However, spine infection caused by M. xenopi is a rare occurrence [[21], [22], [23]]. The pathogenic factors underlying NTM disease have not been fully understood. Current studies indicate that patients, and environmental and bacteriological factors interact to create a ‘susceptible host’ [24]. Immunodeficiency is a nonnegligible risk factor, especially in female SLE patients and HIV patients [25,26]. Immunocompetent patients usually acquire infection by direct penetration of the pathogen as a result of a penetrating injury or contamination during surgical procedures [27,28]. In the present study, there were 11 (78.57%) cases of SLE, HIV, and surgical contamination. In addition, the mean age of immunocompetent patients (non-surgical contamination) was 74.00 ± 1.70 years. It should be noted that increasing age may be an independent risk factor for spinal infection in elderly patients [8].
This study suggested that the most common sites of M. xenopi infection are the thoracic and lumbar vertebrae. Walid Osman proposed that tuberculosis of the spine occurs by hematogenous spread of infection from a pulmonary or extrapulmonary site. The infection may begin in the subchondral bone and spread slowly to the intervertebral disc space and adjacent vertebral bodies, usually in the lower dorsal and upper lumbar spines in the absence of pulmonary infection [29,30]. The most common site of infection for spinal tuberculosis is also the thoracolumbar spine. Therefore, it is considered that M. xenopi may have a similar infection pathway as M. tuberculosis.
Previous reports have indicated that the ESR or CRP level is increased in spinal tuberculosis and it tends to decrease after initiation of treatment, and that patients with poor prognosis tend to have a higher ESR during treatment than those with good prognosis [31]. Among the cases with recorded biomarkers in this study, mainly ESR, most of the biomarker levels were increased and decreased significantly after treatment. It can be considered that the inflammatory state caused by NTM infection may be similar to that of M. tuberculosis, and the level of inflammation is affected by the disease, although a study found a significant difference in the CRP and procalcitonin (PCT) levels between SLE patients and SLE patients with an infection [32]. Nevertheless, some studies do not support the widespread use of ESR, CRP, or PCT to diagnose infection in SLE inpatients due to the increased risk of heterogeneity and bias [33,34], which requires further investigation.
This study showed that it should be carefully considered whether PPD skin test results can be used as exclusion criteria for M. xenopi spinal infection, with a positive to negative ratio of 1:1 in PPD skin test results. The microbial culture and identification time of M. xenopi is long, with an average time period of 55.00 ± 7.55 days; thus, the best treatment time can be easily delayed. Metagenome next-generation sequencing analysis of BALF samples has high accuracy in the differential diagnosis of MTB and NTM [35]. According to the mNGS results interpretation guidelines [36], high-grade pathogenic agents, such as M. tuberculosis and cryptococcus neoformans, may be pathogens in most types of samples. This article reported that mNGS identification of M. xenopi only took only two days and was accurate. Therefore, on the basis of absence of microbial culture results, mNGS may be a good choice for timely and effective treatment of patients.
The treatment plan for spinal infection caused by M. xenopi mainly refers to pulmonary infection, and the best treatment regimen and duration have not yet been determined. An in vitro drug susceptibility test showed that clarithromycin and moxifloxacin had the lowest MIC values and ethambutol/rifampicin combined with clarithromycin or moxifloxacin had significant bactericidal activity against M. xenopi.
[37,38]. Nevertheless, the response of this organism to treatment is variable and not always well correlated with the results of in vitro susceptibility. Amikacin-containing regimens are most effective in rat models of M. xenopi infection [39]. The guidelines [40] pointed out that reasonable treatment options may include isoniazid, rifabutin or rifampicin, ethambutol, and clarithromycin. Streptomycin may or may not be added in the initial regimen. The duration of treatment is 12 months after a negative culture. But the recurrence rate is high, even if a regimen containing macrolides is used. Experts also supported the claim that [41] reasonable treatment options include amikacin (the first three months of treatment), clarithromycin/azithromycin, rifabutin/rifampicin, moxifloxacin/linezolid, and ethambutol. The duration of treatment lasts at least 1 year after a negative culture. The difference between the two options is the recommendation of amikacin, isoniazid, and linezolid. The latter is recommended based on in vitro susceptibility. The vast majority of M. xenopi pathogens are sensitive to rifabutin, macrolides, moxifloxacin, and linezolid, and they are moderately sensitive to isoflurane, rifampicin, ethambutol, and ciprofloxacin [[42], [43], [44]].
Previous studies have suggested that M. xenopi pulmonary infection can achieve good results after standard treatment [45,46]. In this study, most of the cases of spinal infection were significantly improved after treatment, but the combination of drugs and the duration of treatment were quite different. The improvement rate of clarithromycin-based treatment was 83.33%, while the success rate of non-clarithromycin-based treatment was 66.67% (excluding azithromycin). It can be suggested that the success rate of clarithromycin treatment for spinal infection caused by M. xenopi may be higher. The efficacy gap was not obvious for the regimen included isoniazid or quinolones. Infection in the case reported in this article was aggravated by the quadruple anti-tuberculosis regimen. The abscess continued to increase in size after isoniazid was replaced with clarithromycin, but infection was improved after clarithromycin was combined with levofloxacin. This confirms the treatment variability of spinal infection caused by M. xenopi. The evaluation and adjustment of clinical treatment are based on the actual treatment effect. In addition, some cases with poor drug treatment achieved cure or improvement after surgical treatment and postoperative anti-NTM treatment; thus, suggesting that surgical debridement may also be an important factor in the success of treatment.
In conclusion, spinal infections caused by M. xenopi are still a challenging issue due to the rarity of occurrence and complexity of treatment. We have summarized and updated the predisposing factors, diagnosis, and treatment of 14 cases of M. xenopi spinal infection through a systematic review. We have suggested that prolonged antibiotic therapy (≥ 12 months, according to the efficacy can be extended to 24 months, possibly including clarithromycin-based combination with rifampicin/rifabutin, ethambutol, isoniazid, or quinolones) is indicated for the treatment of M. xenopi spinal infections. Nevertheless, this study has several shortcomings. The first limitation is the small number of cases, especially long-term follow-up cases. Secondly, there may be some differences in the collected information of different case reports. Although there are some limitations, this study has a certain reference value for the clinicians to help identify, diagnose, and treat spinal infection caused by M. xenopi.
Ethics statement
Consent was provided by the patient and her parents to enable anonymized reporting of the results of this study.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
CRediT authorship contribution statement
Min Peng: Conceptualization, Software, Validation, Investigation, Resources, Writing – original draft. Wei Li: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – review & editing, Supervision. Fengyi Li: Methodology. Bo Tang: Methodology, Software. Yinhua Deng: Validation. Shuai Peng: Resources. Li Chen: Visualization. Yingchun Dai: Visualization.
Declaration of Competing Interest
None.
Acknowledgments
None to declare.
Data availability
Data will be made available on request.
References
- 1.Rodari P., Marocco S., Buonfrate D., et al. Prosthetic joint infection due to mycobacterium xenopi: a review of the literature with a new case report infection. Infection. 2020;48(2):165–171. doi: 10.1007/s15010-019-01318-1. [DOI] [PubMed] [Google Scholar]
- 2.Wu M.L., Aziz D.B., Dartois V., et al. NTM drug discovery: status, gaps and the way forward. Drug Discov Today. 2018;23(8):1502–1519. doi: 10.1016/j.drudis.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martín-Casabona N., Bahrmand A.R., Bennedsen J., et al. Non-tuberculous mycobacteria: patterns of isolation. A multi-country retrospective survey. Int. J. Tubercul. Lung Dis. Off. J. Int. Union Against Tubercul. Lung Dis. 2004;8(10):1186–1193. [PubMed] [Google Scholar]
- 4.Griffith D.E., Aksamit T., Brown-Elliott B.A., et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 5.Alfreijat M., Ononiwu C., Sexton C. Pott’s disease: a case of mycobacterium xenopi infection of the spine. J Comm Hosp Internal Med Perspect. 2012;2(4):45–49. doi: 10.3402/jchimp.v2i4.20150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prosser A.J. Spinal infection with mycobacterium xenopi. Tubercle. 1986;67(3):229–232. doi: 10.1016/s0041-3879(86)80030-7. [DOI] [PubMed] [Google Scholar]
- 7.Sobottke R., Zarghooni K., Seifert H., et al. Spondylodiscitis caused by mycobacterium xenopi. Arch Orthop Trauma Surg. 2008;128(10):1047–1053. doi: 10.1007/s00402-007-0553-y. [DOI] [PubMed] [Google Scholar]
- 8.Danesh-Clough T., Theis J.C., Linden A.V.D. Mycobacterium xenopi infection of the spine: a case report and literature review. Spine. 2000;25(5):626–628. doi: 10.1097/00007632-200003010-00015. [DOI] [PubMed] [Google Scholar]
- 9.Kulasegaram R., et al. Mycobacterium xenopi osteomyelitis in a patient on highly active antiretroviral therapy (HAART) Int J STD AIDS. 2001;12(6):404–406. doi: 10.1258/0956462011923219. [DOI] [PubMed] [Google Scholar]
- 10.Telgt D.S., Van H.F.H., Meis J.F., et al. Arthritis and spondylodiscitis caused by mycobacterium xenopi in a patient with systemic lupus erythematosus. Br J Rheumatol. 1997;36(9):1025–1026. doi: 10.1093/rheumatology/36.9.1025. [DOI] [PubMed] [Google Scholar]
- 11.William C., Mark D., William J., et al. Pott’s disease caused by mycobacterium xenopi: case report and review. Clin Infect Dis. 1994;19(6):1024–1028. doi: 10.1093/clinids/19.6.1024. [DOI] [PubMed] [Google Scholar]
- 12.Smimmo A., Perna A., Fantoni M., et al. Non tuberculous mycobacteria related spondylodiscitis: a case report and systematic literature review. Le Infezioni in Medicina. 2020;28(3):425–435. [PubMed] [Google Scholar]
- 13.Rahman M.A., Phongsathorn V., Hughes T., et al. Spinal infection by Mycobacterium xenopi in a non-immunosuppressed patient. Tuber Lung Dis. 1992;73(6):392–395. doi: 10.1016/0962-8479(92)90047-N. [DOI] [PubMed] [Google Scholar]
- 14.Meybeck A., Fortin C., Abgrall S., et al. Spondylitis due to mycobacterium xenopi in a human immunodeficiency virus type 1-infected patient: case report and review of the literature. J Clin Microbiol. 2005;43(3):1465–1466. doi: 10.1128/JCM.43.3.1465-1466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jean-Hugues S., Guillaume D., Jean-Marc Z., et al. Discitis and sacroiliitis diagnosed 15 years after iatrogenic mycobacterium xenopi inoculation. Joint Bone Spine. 2012;79(4):409–411. doi: 10.1016/j.jbspin.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Froideveaux D., Claudepierre P., Brugieres P., et al. Iatrogenically induced spondylodiskitis due to mycobacterium xenopi in an immunocompetent patient. Clin Infect. Dis. Off. Publicat. Infect. Dis. Soc. America. 1996;22(4):723–724. doi: 10.1093/clinids/22.4.723. [DOI] [PubMed] [Google Scholar]
- 17.Paula G., Mark A. Vertebral osteomyelitis with paravertebral abscess due to mycobacterium xenopi. Clin Microbiol Newsl. 1990;12:94–95. [Google Scholar]
- 18.Hulten E.A., Hartzell J., Conrath S.M., et al. Mycobacterium xenopi: a case report of a rare cause of Pott disease. Infect Dis Clin Pract. 2006;14(3):177–180. [Google Scholar]
- 19.Guglielmetti L., Mougari F., Lopes A., et al. Human infections due to nontuberculous mycobacteria: the infectious diseases and clinical microbiology specialists’ point of view. Future Microbiol. 2015;10(9):1467–1483. doi: 10.2217/fmb.15.64. [DOI] [PubMed] [Google Scholar]
- 20.The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J. 2013;42(6):1604–1613. doi: 10.1183/09031936.00149212. [DOI] [PubMed] [Google Scholar]
- 21.Haworth C.S., Banks J., Capstick T., et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease(NTM-PD) Thorax. 2017;72(Suppl. 2):il–ii64. doi: 10.1136/thoraxjnl-2017-210927. [DOI] [PubMed] [Google Scholar]
- 22.Adzic-Vukicevic T., Barac A., Blanka-Protic A., et al. Clinical features of infection caused by non-tuberculous mycobacteria:7 years’ experience. Infection. 2018;46(3):357–363. doi: 10.1007/s15010-018-1128-2. [DOI] [PubMed] [Google Scholar]
- 23.Rodari P., Marocco S., Buonfrate D., et al. Prosthetic joint infection due to mycobacterium xenopi:a review of the literature with a new case report. Infection. 2020;48(2):165–171. doi: 10.1007/s15010-019-01318-1. [DOI] [PubMed] [Google Scholar]
- 24.Ratnatunga C.N., Tungatt K., Proietti C., et al. Characterizing and correcting immune dysfunction in non-tuberculous mycobacterial disease. Front Immunol. 2022;13:1047781. doi: 10.3389/fimmu.2022.1047781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juffermans N.P., Verbon A., Danner S.A., et al. Mycobacterium xenopi in HIV-infected patients: an emerging pathogen. AIDS. 1998;12(13):1661–1666. doi: 10.1097/00002030-199813000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Kim Chung-Jong, Uh-Jin, et al. Vertebral osteomyelitis caused by non-tuberculous mycobacteria: predisposing conditions and clinical characteristics of six cases and a review of 63 cases in the literature. Infect Dis Ther. 2016;48(7):509–516. doi: 10.3109/23744235.2016.1158418. [DOI] [PubMed] [Google Scholar]
- 27.Griffith D.E., Aksamit T., Brown-Elliott B.A., et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 28.Daley C.L., Iaccarino J.M., Lange C.G., et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. 2020;56(1):2000535. doi: 10.1183/13993003.00535-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walid O., Meriem B., Zeineb A., et al. A rare case of tuberculosis with Sacrococcygeal involvement miming a neoplasm. Case Rep Orthoped. 2016;2016:7286806. doi: 10.1155/2016/7286806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rafael D., Rory C., Nancy Abu-Bonsrah B., et al. The epidemiology of spinal tuberculosis in the United States: an analysis of 2002–2011 data. J Neurosurg. 2017;26(4):507–512. doi: 10.3171/2016.9.SPINE16174. [DOI] [PubMed] [Google Scholar]
- 31.Kim J.H., Jin Y.A., Su J.J., et al. Prognostic factors for unfavourable outcomes of patients with spinal tuberculosis in a country with an intermediate tuberculosis burden: a multicentre cohort study. Bone Joint J. 2019;101:1542–1549. doi: 10.1302/0301-620X.101B12.BJJ-2019-0558.R1. B(12) [DOI] [PubMed] [Google Scholar]
- 32.Aljarhi U.M., Sadek K.M., Darwish E.M., et al. Evaluation of serum presepsin, procalcitonin, copeptin, and high-sensitivity C-reactive protein for differentiating bacterial infection from disease activity in Egyptian patients with systemic lupus erythematosus. Clin Rheumatol. 2021;40(5):1861–1869. doi: 10.1007/s10067-020-05471-z. [DOI] [PubMed] [Google Scholar]
- 33.Bruera S., Ventura M.J., Agarwal S.K., Krause K.J., et al. The utility of erythrocyte sedimentation rate, C-reactive protein, and procalcitonin in detecting infections in patients with systemic lupus erythematosus: A systematic review. 2022;31(10):1163–1174. doi: 10.1177/09612033221106157. [DOI] [PubMed] [Google Scholar]
- 34.Liu L.-N., Wang P., Guan S.-Y., et al. Comparison of plasma/serum levels of procalcitonin between infection and febrilediseaseflare in patients with systemic lupus erythematosus: a meta-analysis. Rheumatol Int. 2017;37:1991–1998. doi: 10.1007/s00296-017-3827-x. [DOI] [PubMed] [Google Scholar]
- 35.Xu P., Yang K., Yang L., et al. Next-generation metagenome sequencing shows superior diagnostic performance in acid-fast staining sputum smear-negative pulmonary tuberculosis and non-tuberculous mycobacterial pulmonary disease. Front Microbiol. 2022;1(13) doi: 10.3389/fmicb.2022.898195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jun H. Jiangsu expert consensus on detection of infectious pathogens by metagenomic sequencing technology.Chinese journal of. Clin Lab Sci. 2020;38(9):641–645. [Google Scholar]
- 37.LiG Pang H., Guo Q., et al. Antimicrobial susceptibility and MIC distribution of 41 drugs against clinical isolates from China and reference strains of nontuberculous mycobacteria. Int J Antimicrob Agents. 2017;49(3):364–374. doi: 10.1016/j.ijantimicag.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 38.Litvinov V., Makarova M., Galkina K., et al. Drug susceptibility testing of slowly growing non-tuberculous mycobacteria using slomyco test-system. PLoS One. 2018;13(9) doi: 10.1371/journal.pone.0203108. e0203108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claire A., Deepak V.A., Sandeep T., et al. Improving existing tools for mycobacterium xenopi treatment: assessment of drug combinations and characterization of mouse models of infection and chemotherapy. J Antimicrob Chemother. 2013;68(3):659–665. doi: 10.1093/jac/dks421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Society A T An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 41.Chinese Society for Tuberculosis, Chinese Medical Association Nontuberculous mycobacterial disease diagnosis and treatment guidelines. Chinese J. Tubercul. Respirat. Dis. 2020;43(11):918–946. [Google Scholar]
- 42.Litvinov V., Makarova M., Galkina K., et al. Drug susceptibility testing of slowly growing non-tuberculous mycobacteria using slomyco test-system. PLoS One. 2018;13(9) doi: 10.1371/journal.pone.0203108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heidarieh Mirsaeidi M., Hashemzadeh M., et al. In vitro antimicrobial susceptibility of nontuberculous mycobacteria in Iran. Microb Drug Resist. 2016;22(2):172–178. doi: 10.1089/mdr.2015.0134. [DOI] [PubMed] [Google Scholar]
- 44.Olivier K.N., Griffith D.E., Eagle G., et al. Randomized trial of liposomal amikacin for inhalation in nontuberculous mycobacterial lung disease. Am J Respir Crit Care Med. 2017;195(6):814–823. doi: 10.1164/rccm.201604-0700OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yong-Soo K., Won-Jung K. Diagnosis and treatment of nontuberculous mycobacterial lung disease. J Korean Med Sci. 2016;31(5):649–659. doi: 10.3346/jkms.2016.31.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Damien Basille, Vincent, et al. Treatment of other nontuberculous mycobacteria. Sem Resp Crit Care Med. 2018;39(3):377–382. doi: 10.1055/s-0038-1660473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.