Abstract
The surface of cells is coated with a dense layer of glycans, known as the cell glycocalyx. The complex glycans in the glycocalyx are involved in various biological events, such as bacterial pathogenesis, protection of bacteria from environmental stresses, etc. Polysaccharides on the bacterial cell surface are the highly conserved and accessible molecules, and thus are excellent immunological targets. Consequently, bacterial polysaccharides and their repeating units have been extensively studied as antigens for the development of antibacterial vaccines. This review surveys the recent development in the synthetic and immunological investigations of bacterial polysaccharide repeating unit-based conjugate vaccines against several human pathogenic bacteria. The major challenges associated with the development of functional carbohydrate-based antibacterial conjugate vaccines are also considered.
Keywords: Polysaccharide, Oligosaccharide, Glycoconjugate, Immunogenicity, Conjugate Vaccine, Antibacterial Vaccine
Graphical abstract

1. Introduction
Pathogens, such as bacteria, viruses, parasites, fungi, etc., are the biggest threat to human life.1 The recent COVID-19 pandemic is a clear example. Currently, there are two main strategies to address pathogenic infections. One strategy is to treat patients with antibiotics or antiviral and antiparasitic drugs. The other strategy is to immunize the human population against pathogens via vaccination. Of the two strategies, vaccination is not only effective but also economical and long lasting, and has helped control and even eliminate many infectious diseases.2, 3 Moreover, the rapid emergence of drug resistance to various antibiotics in the past few decades has made the vaccination strategy even more attractive.
The discovery by Edward Jenner in 1796 that humans could be protected against smallpox after immunization with cowpox was a historical milestone during the development of vaccine concept (Figure 1).4 Since then, different types of vaccines, such as attenuated or inactivated vaccines,5, 6 subunit vaccines7–9 and DNA or RNA vaccines,10–12 against various human pathogens have emerged. This review is focused on antibacterial vaccines, especially the subunit vaccines derived from bacterial polysaccharides and their repeating units.
Figure 1:
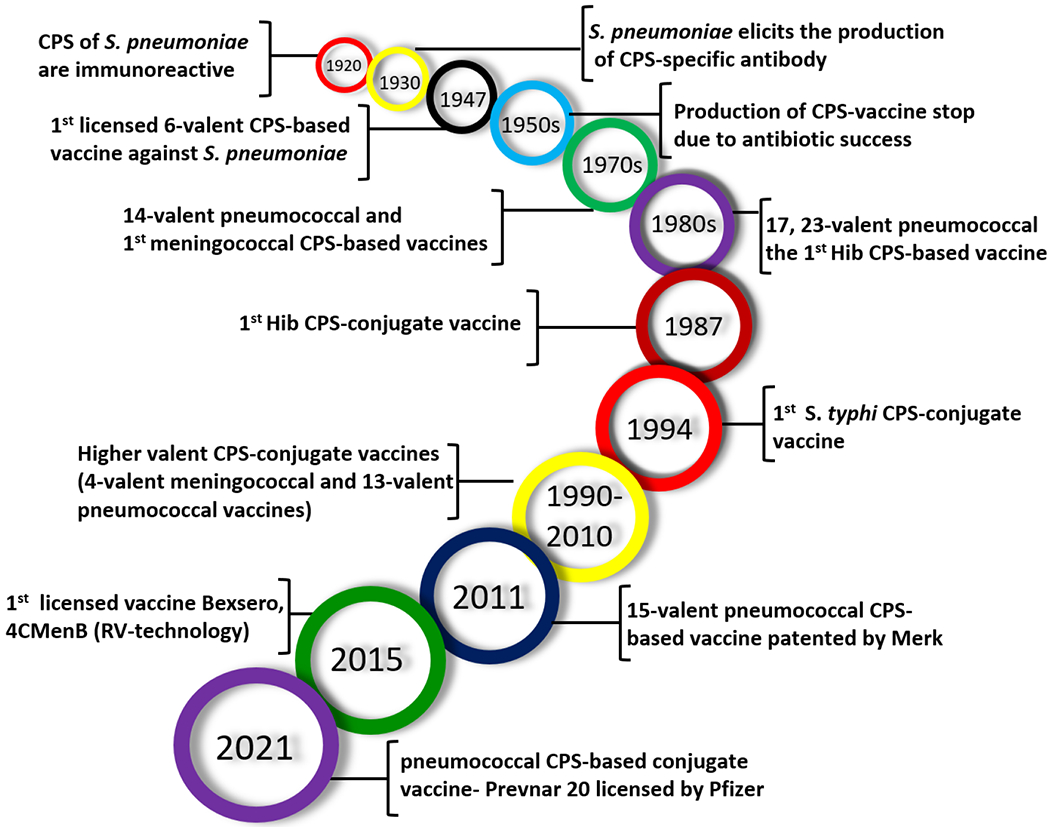
Major milestones in the history of carbohydrate-based antibacterial vaccine development.
The concept of carbohydrate-based antibacterial vaccines was first introduced by Avery and Heidelberger in the 1920s,13 when they found that the capsular polysaccharides (CPSs) of the Gram-positive bacterium Streptococcus pneumoniae were immunogenic.14 In addition, it was discovered that CPSs also played a critical role in the serotype specificity of different S. pneumoniae strains.15,16 Francis and Tillett further observed that intravenous injections of the CPSs derived from various pneumoniae serotypes elicited respective CPS-specific antibodies in patients.17 In 1935, after studying the antibody responses induced by type III and type VIII pneumococcal CPSs, Finland and Ruegsegger developed the first CPS-based pneumococcal vaccine.18 A 6-valent CPS-based pneumococcal vaccine was developed by Heidelberger et al. in 1942-1945, which was used to immunize the US air force.19 However, the development of antibacterial vaccines, including carbohydrate-based vaccines, stopped in the early 1950s due to the great success of antibiotics. It was soon discovered that antibiotics were not the panacea for all bacterial infections. Thus, the interest in antibacterial vaccines resurged. In particular, the ever-growing bacterial resistance to antibiotics in the past few decades has boosted the interest in developing antibacterial vaccines. In 1983, Merck introduced the first carbohydrate-based pneumococcal vaccine, PneumoVax, made up of purified CPSs from 14 pneumococcal serotypes.20 Presently, the second-generation pneumococcal vaccine is composed of 90 pneumococcal CPSs from 23 serotypes.21 Overall, carbohydrate-based antibacterial vaccines have contributed significantly to human health and welfare.
However, stand-alone polysaccharide vaccines are effective mainly in adult populations. They remain deficient in high-risk groups (e.g., children under five years of age) and virtually ineffective in infants (below two years of age), mainly because polysaccharides alone cannot elicit strong T-cell dependent immune responses in these people.22 To address this problem, carbohydrate-based conjugate (glycoconjugate) vaccines have emerged. Its basic concept is to covalently link a highly immunogenic carrier molecule (e.g., a protein) to carbohydrate antigens to improve their immunogenicity and T-cell dependent immune responses.23, 24 The first bacterial polysaccharide-protein conjugate vaccine against Haemophilus influenzae type b (Hib) was approved for clinical use in 1987.25 Since then, a series of other bacterial polysaccharide-protein conjugate vaccines have been developed. These vaccines have been proved effective both in adults and in children and thus are widely used.
Despite the great advancement of conjugate vaccines made up of bacterial polysaccharides, these vaccines have their limitations mainly because the polysaccharides produced by bacteria are complex mixtures that are difficult to control in quality and in the conjugation with carrier proteins (CPs). Consequently, the resultant conjugates are complex and ill-defined cross-linked lattices.26–28 To address this issue, currently, great effort has been devoted to the synthesis of oligosaccharides containing the repeating units of bacterial polysaccharides and applying them to the development of oligosaccharide-based antibacterial conjugate vaccines.29 This type of conjugate vaccines that contain synthetic and structurally defined carbohydrate antigens can have several advantages, such as controlled conjugation chemistry and product quality, reduced contamination, and feasibility to perform structural and structure-activity relationship studies and vaccine optimization.30,31 A successful example in this field is the vaccine made up of fully synthetic oligosaccharides of Hib polysaccharides approved by Cuba and other countries for clinical use in 1999.25 Recently, an oligosaccharide-based vaccine for shigellosis has reached phase II clinical trials.32,33 In the literature, there are several reviews about carbohydrate-based vaccines and therapeutics,34,35,36,37,38 including a recent review by Adamo et al.39 The present review is unique in that it focuses mainly on antibacterial conjugate vaccines made of synthetic oligosaccharides and their structure–immunogenicity relationships.
2. Development of Carbohydrate-Based Antibacterial Conjugate Vaccines
2.1. The general structure of carbohydrate-based conjugate (i.e., glycoconjugate) vaccines:
A glycoconjugate vaccine is usually composed of three components, a carbohydrate antigen, a carrier molecule, and a linker. In the process of vaccine development, the first step is to identify the target antigen, which, theoretically, should be antigenic, conserved, and exposed on the cell surface to facilitate immune recognition. The next step is to select the proper carrier molecule, which should be an immunostimulator to help enhance the immunogenicity of the carbohydrate antigen. Finally, a linker is identified for carbohydrate antigen and carrier molecule coupling, which should be governed by the conjugation chemistry and related reaction conditions.
Based on the properties of the carbohydrate antigens, carrier molecules, and the preparation methods of glycoconjugate vaccines, they are divided into three different types (Figure 2). One type of glycoconjugate vaccines has polysaccharides derived from natural sources, e.g., bacterial cultures, directly conjugated with CPs (Figure 2a).40 Most FDA-approved carbohydrate-based antibacterial conjugate vaccines belong to this type. In this case, the polysaccharide antigens are complex mixtures and, more importantly, need to be activated by methods to enable their conjugation with CPs,41 which further increases the degree of complexity of the polysaccharide mixtures. In addition, the conjugation sites for both the carbohydrate antigens and the CPs are uncontrollable. As a result, this type of glycoconjugate vaccines is complex and batch-to-batch different. Another type of glycoconjugate vaccines has synthetic oligosaccharide antigens linked to CPs, which are called semi-synthetic conjugate vaccines (Figure 2b).42–44 In these vaccines, the structure of carbohydrate antigens and their linkage forms to CPs are well defined, although the protein conjugation sites are usually undefined. The third type of glycoconjugate vaccines has synthetic carbohydrate antigens conjugated with synthetic carrier molecules, such as lipids, glycolipids,45 glycopeptides,46 oligosaccharides or polysaccharides23 and synthetic polymers,47 as well as other synthetic materials,48 which are called fully synthetic glycoconjugate vaccines (Figure 2c).49 These vaccines have well-defined structures that can be fully characterized by modern analytical techniques, including NMR and MS. This review is mainly focused on semi- and fully synthetic antibacterial glycoconjugate vaccines.
Figure 2.
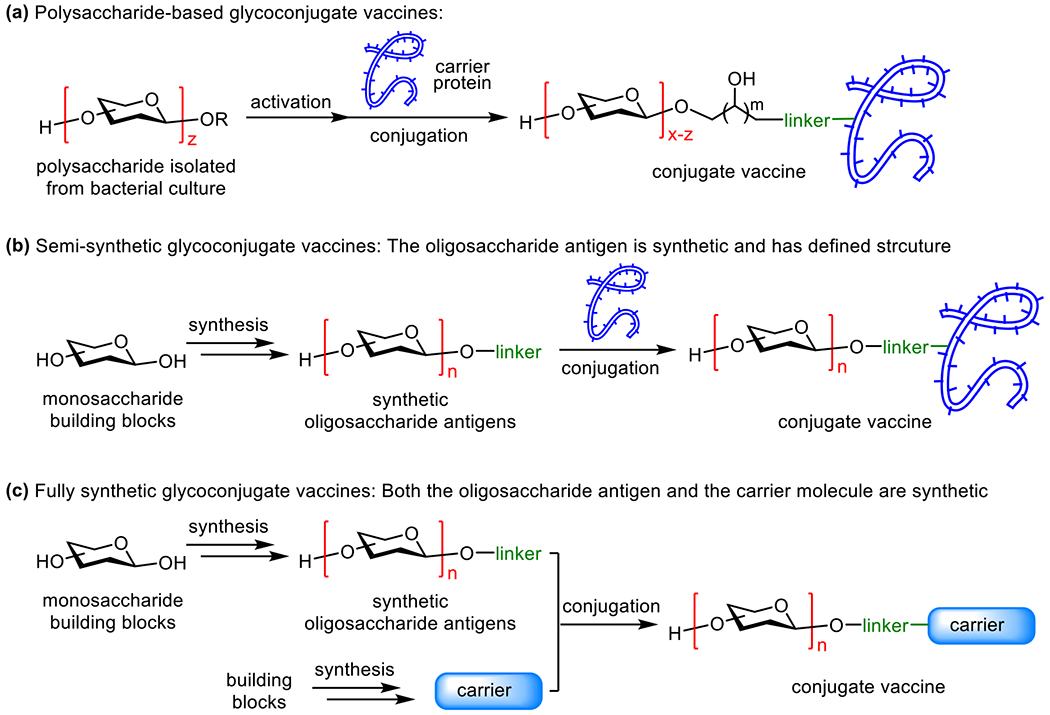
Three major classes of glycconjugate vaccines. (a) The traditional polysaccharide-based glycoconjugate vaccines; (b) semi-synthetic glycoconjugate vaccines consisting of synthetic, strcuturally well-defined oligosaccharide antigens; (c) structurally defined fully synthetic glycoconjugate vaccines consisting of synthetic oligosaccharide antigens and synthetic carrier molecules.
2.2. Bacterial carbohydrate antigen:
Bacteria express diverse polysaccharides, such as CPSs, lipopolysaccharides (LPSs) and exopolysaccharides (EPSs). CPSs and LPSs are conserved and exposed on the bacterial cell surface, making them useful immunological targets for vaccine development. However, bacterial polysaccharides are not only species-specific but also strain-specific. For instance, the antigenic diversities of bacterial polysaccharides have resulted in 13 meningococci serotypes50 and 90 pneumococcal serotypes.51 Therefore, it should be noted that a vaccine derived from a specific bacterial polysaccharide is only functional for that specific bacterium and strain, and an effective vaccine against an infectious disease may need to be of multivalent property, i.e., vaccines derived from the polysaccharide antigens of multiple strains of the disease-causing bacterium.
Although bacterial polysaccharides are useful for the development of vaccines, they are not necessarily the optimal immunogens, because large polysaccharides may affect the interactions of carrier molecules in the resultant glycoconjugates with immune cells. Furthermore, although bacterial polysaccharides are large and complex, they are usually composed of small repeating units of monosaccharides or oligosaccharides. Most likely, the immune system only recognizes some unique epitopes of a polysaccharide, often the non-reducing end epitope52 or the epitope containing one to several repeating units,53 instead of the full glycan. Thus, the glycan length is always one of the concerns in the design of carbohydrate-based vaccines. Deciphering the optimal number of repeating units of a polysaccharide or glycan length required for immune recognition and vaccine development is not easy, because this needs in-depth analysis of the structure-activity relationships of carbohydrate antigens, which is challenging. Nevertheless, there is sufficient information to suggest that it may not be necessarily “the longer glycans, the better” for vaccine development. Moreover, it seems that there is not a golden standard about the optimal glycan length as antigens, thus the best oligosaccharide substitute for each specific polysaccharide antigen may need to be determined individually. For example, in semi-synthetic Hib vaccine Quimi-Hib, the optimal oligosaccharide epitope ranges from six to eight repeating units of the Hib polysaccharide,54 but in other cases, the oligosaccharide length varies.55,56,57 In addition, other factors, such as the structural uniqueness, frameshift58 and functionalization of carbohydrate antigens59,60,61,62 and the epitope stability that may affect the immunogenicity and antigenicity,63 should also be taken into consideration during the design and development of carbohydrate-based antibacterial vaccines.
2.3. Vaccine carrier:
In glycoconjugate vaccines, carrier molecules also play a critical role in stimulating the immune system and enhancing immune responses, especially T-cell dependent immune responses, to the targeted carbohydrate antigens. Thus, the carrier molecule needs to contain T-cell helper epitopes and be immunologically active. Other properties that a vaccine carrier should possess are that it should be safe and easily produced in low cost and consistent quality. Among various vaccine carriers, proteins are the most commonly used for antibacterial vaccine development. In addition to the necessary properties for carrier molecules as mentioned above, proteins contain many and different functional groups, such as amino, thiol, carboxylic and hydroxyl groups, which enables various conjugation methods. Currently, there are several common CPs, such as mutant cross-reacting material (CRM) from diphtheria toxin, diphtheria toxoid (DT), tetanus toxoid (TT), meningococcal outer membrane protein complex (OMPC), and H. influenzae protein D (HiD), which are all used in licensed bacterial vaccines. Besides these established CPs, many other CPs are in preclinical studies or clinical trials.48 For example, recombinant Pseudomonas aeruginosa exotoxin A (rEPA) is utilized as a carrier for Shigella O-antigens.64 Clinical results have demonstrated the effectiveness of glycoconjugate vaccines, as well as the long-term and protective immune responses that they induce. However, it should be noted that CPs can also induce specific antibody responses that may supress the immune response to the conjugated carbohydrate antigens.65–67 Repeated immunizations using vaccines containing the same CP can reduce the effectiveness of vaccination as well. Thus, research to discover new CPs or non-protein carrier molecules is important.
2.4. Glycoconjugate vaccine linker:
It has been well established that carbohydrate antigens must be covalently linked to carrier molecules to exhibit enhanced immunogenicity. The choice of a proper linker between carbohydrate antigens and CPs depends on several factors. First, it should be non-immunogenic so that it will not cause unwanted antibodies or immune response. It was also shown that strong immune reactions to the linker can supress immune responses to the target carbohydrate antigen.68–70 Second, it needs to be bi- or multi-functional, as it has to bridge two distinctive biomolecules, i.e., the carbohydrate antigen and the CP. Furthermore, the conjugation reactions should be highly efficient and selective, which can help reduce the complexity and heterogeneity and achieve desired and consistent antigen loading of resultant glycoconjugates. It has been demonstrated that the antigen loading of glycoconjugate vaccines can have a big impact on their immunological properties.71–73 Many linkers and the associated conjugation methods have been developed for the preparation of glycan-protein conjugates.74,75 The selection of a proper linker and conjugation method for a vaccine is largely decided by the structure of carbohydrates and sometimes by the CP as well. For polysaccharide antigens, their activation method and the resulting functional groups dominate the conjugation method since the functionalized sugar units in the polysaccharide antigen usually serve as the linker directly. For synthetic oligosaccharide antigens, it is flexible to install various functional groups in the synthetic process to facilitate specific linkage forms and conjugation methods. In the literature, there are several well-established methods developed for carbohydrate-protein conjugation, and each method has its advantages and limitations.76 Generally, the amino and thiol groups in CPs are frequently used for the attachment of carbohydrate antigens. Activated acyl groups, aldehydes or alkenes are also introduced to the carbohydrate antigens to enable their coupling with protein via chemoselective N-acylation, reductive animation, Michael addition, etc. Other conjugation methods involving click reaction and other chemistry are also reported.76
In summary, in the development of antibacterial glycoconjugate vaccines, the selection of proper carbohydrate antigens is critical. With polysaccharide antigens, their activation method to facilitate the conjugation with CPs is also important, as it will not only decide the properties (e.g., the structural integrity and molecular size) of the to-be-conjugated carbohydrate antigens but also the linker and linkage form, conjugation chemistry, antigen loading, chemical stability, product quality and biological activity consistence and, thereby, the immunological properties of the resultant glycoconjugate vaccines. Furthermore, the properties of linker and CP also have a significant impact on the immunological properties of glycoconjugate vaccines. Therefore, all these factors should be considered and optimized during the design and development of new glycoconjugate vaccines.
3. Immunology of Carbohydrate-Based Antibacterial Vaccines
Bacterial polysaccharides are promising targets for the development of glycoconjugate vaccines for infectious diseases. In as early as 1930’s, antibodies against pneumococcal polysaccharides were already observed,75 and the antibody-mediated immunity was found to last at least several months. Ultimately, bacterial CPSs have been developed into antibacterial vaccines. However, a major problem for polysaccharide vaccines, or carbohydrate antigens in general, is that they are not highly immunogenic and induce only poor quality of antibody responses, especially in children, largely due to their inability to be properly presented to T cells77 and provoke T-cell dependent immune responses and immune memory.78, 79 As shown in Figure 3a, polysaccharide vaccines mainly interact with B-cells to elicit quick and arbitrary innate immune responses and generate T-cell independent non-specific immunoglobulin M (IgM) antibodies, which have a low affinity toward the carbohydrate antigens.80 T-cell independent responses are less robust than adaptive immune responses and short-lived. Moreover, even repeated vaccination will not establish strong enough immune responses to fight pathogens.81
Figure 3.
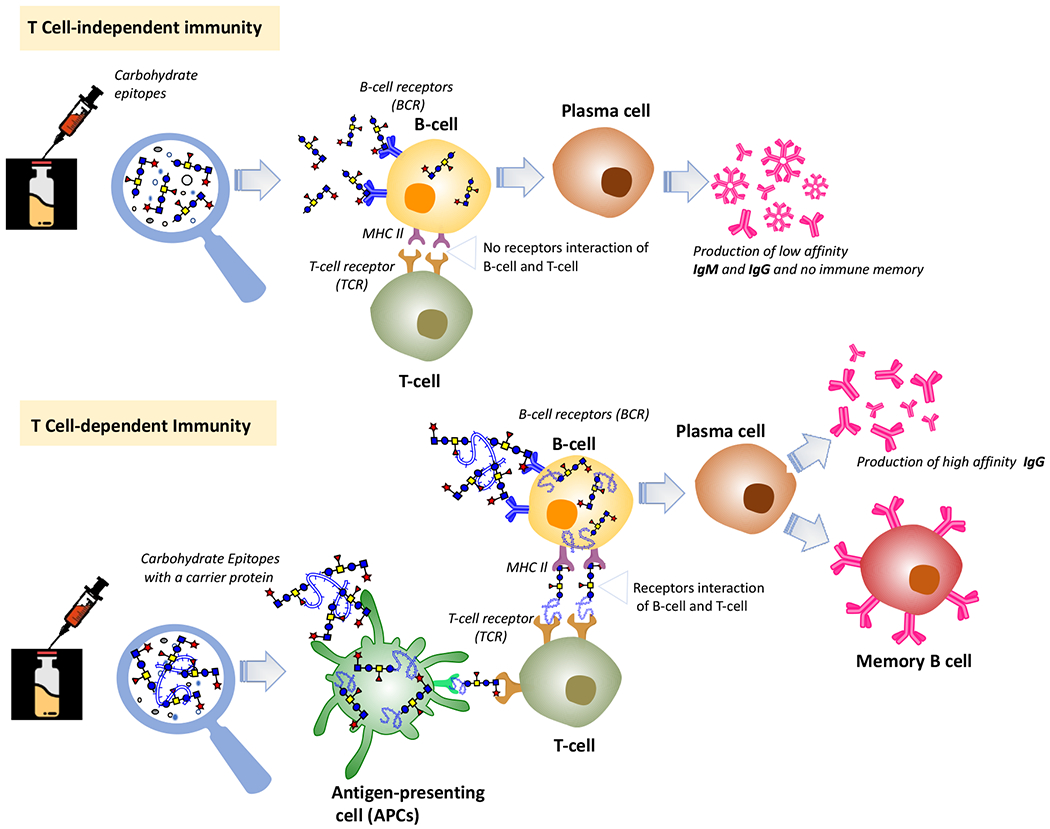
The general immunology of carbohydrate-based antibacterial vaccines in human.
On the other hand, the covalent coupling of carbohydrates with immunogenic CPs, which contain T-cell epitopes, can convert them from T-cell independent to T-cell dependent antigens to induce adaptive immune responses involving both B- and T-cells (Figure 3b).82 Adaptive immunities are antigen-specific, robust, and persistent but take longer time to establish, when compared to innate immunities. In the development of adaptive immunity, antigen-presenting cells (APCs), such as dendritic cells (DCs)—the most important professional APCs, play a key role. APC presentation of the antigenic epitopes of glycoproteins to T-cell enables the cross-linkage of T-cell immunogens with B cell receptors (BCRs) to initiate the signalling processes of adaptive immune responses.83 The co-stimulatory signals from DCs and macrophages help stimulate B-cell activation and maturation into plasma cells and release specific antibodies.84,85 Other B-cells reproduce independently, and marginal zone B-cells and non-circulating mature B-cells are segregated in the spleen and other lymphoid tissues. Specifically, glycoconjugate vaccines are internalized by APCs and processed in endosomes, and the resulting carbohydrate epitopes are presented to T-cells along with peptides generated from CP digestion.71,86 These epitopes bind to major histocompatibility complex class II (MHC-II) ligand present on the T-lymphocyte surface through the peptide portion with the carbohydrate epitopes recognized by T-cell receptors (TCRs). The recognition of the glycan epitope with MHC-II ligand by CD4+ T-cell receptors and the secreted signaling cytokines help B-cell maturation and secretion of carbohydrate-specific high-affinity IgG antibodies and generation of memory B cells (MBCs). However, the role of B- and T-cell co-signaling is unclear. A study suggests that glycoproteins are processed in B-cells into small glycopeptides that bind MHC-II ligand with the hydrophilic glycan exposed to TCRs to interact specifically with carbohydrate-specific CD4+ cells, elicit T-cell responses, and promote immunoglobulin switching from IgM to IgG.87
Of course, there are exceptions to the above scenarios. For example, the meningococcal A polysaccharide was observed to elicit immune responses in children and stimulate MBCs.88 In addition, zwitterionic polysaccharides (ZPS) also induce T-cell dependent immune responses with the zwitterionic epitopes involved in the activation of T-cells. In the activation step, MHC-II ligand is needed to present the processed antigens. Subsequently, they are recognized by the APCs and B-cells, macrophages, and TCRs.89,90
4. Bacterial Polysaccharide-Protein Conjugate Vaccines
Due to the advantages of conjugate vaccines over polysaccharide vaccines, great efforts have been devoted to developing antibacterial glycoconjugate vaccines with natural polysaccharides as antigens. As a result, notable advancements have been achieved in this field. For example, many antibacterial glycoconjugate vaccines, e.g., Hiberix® (Glaxo Smith Kline), Menjugate® (Chiron), PedvaxHIB® (Merck), Prevenar 13® (Wyeth Pharmaceuticals), etc., as listed in Table 1, have been approved for clinic use.91 In the meantime, many new antibacterial glycoconjugate vaccines are presently at various stages of clinical studies.
Table 1.
Licensed or in-clinical-trial carbohydrate-based antibacterial conjugate vaccines
| Bacteria/Pathogens | Carbohydrate antigens | Carrier proteins | Clinical phases | Trade names, Manufactures | Targeted age groups |
|---|---|---|---|---|---|
| Haemophilus influenzae type B | Natural polysaccharides | TT | Licensed | ActHIb®, Sanofi Pasteur | Children (2 months to 5 years) |
| Natural polysaccharides | TT | Licensed | Hiberix®, GSK | Children (15 months to 4 years) | |
| Medium-length capsular polysaccharides | OMP | Licensed | PedaxHib®, Merck | Children (2 to 71 months) | |
| Capsular oligosaccharides | CRM197 | Licensed | HibTiter®, Pfizer | Children (2 to 71 months) | |
| Capsular oligosaccharides | CRM197 | Licensed | VaxemHib®, Novartis | Children (2 months to 4 years) | |
| Synthetic oligosaccharides of capsular polysaccharides (eight repeating units in average) | TT | Licensed | Quimi-Hib®, CIGB, Cuba | Children | |
| Neisseria meningitidis | Capsular oligosaccharides of MenA/C/W/Y | CRM197 | Licensed | Menveo®, GSK | Children (from 2 months), adults (up to 55 years) |
| Capsular polysaccharides of MenA/C/W/Y | DT | Licensed | Menactra®, Sanofi Pasteur | Children (from 9 months), adults (up to 55 years) | |
| Medium-length capsular polysaccharides of MenA/C/W/Y | TT | Licensed | Nimenrix®, Pfizer | Children (from 12 months), adults (up to 55 years) | |
| Capsular oligosaccharides of MenC strain C11 | CRM197 | Licensed | Menjugate®, | Children (from 2 months), adults, seniors (up to 64 years) | |
| Capsular oligosaccharides of MenC | CRM197 | Licensed | Meningite®, Pfizer | Children (from 6 weeks), adults, seniors (up to 64 years) | |
| De-O-acetylated polysaccharides of MenC strain C11 | TT | Licensed | NeisVac-C®, Pfizer | Children (from 2 months), adults, seniors (up to 64 years) | |
| Medium-length (100–200 kDa) capsular polysaccharides of MenA |
TT | Licensed | MenAfriVac®, Ser. Ins. India | Children (from 1 year), adults (up to 29 years) | |
| Streptococcus pneumoniae | Native capsular polysaccharides of pneumococci serotypes 1, 3, 4, 5, 6A, 6B, 7F, 8, 9 V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, and 33F |
CRM197 | Licensed | Prevnar 20®, Pfizer | Adults (from 18 year or older) |
| Native capsular polysaccharides of pneumococci serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9 V, 14, 18C, 19A, 19F and 23F | CRM197 | Licensed | Prevnar 13®, Wyeth/Pfizer | Children (6 weeks to 17 years), adults (50 years or older) | |
| Native capsular polysaccharides of pneumococci serotypes 4, 6B, 9 V, 14, 18C, 19F, and 23F | CRM197 | Licensed | Prevnar®, Wyeth/Pfizer |
Children (up to 9 years) | |
| Native capsular polysaccharides of pneumococci serotypes 1, 4, 5, 6B, 7F, 9 V, 14, 18C, 19F and 23F | Protein D, TT, DT | Licensed | Synflorix®, GSK | Children (up to 5 years) | |
| Natural polysaccharides of serogroups 1, 2, 3, 4, 5, 6A, 6B, 7F, 8, 9N, 9 V, 10A, 11A, 12F, 14, 15B, 177, 18C, 19A, 19F, 20B, 22F, 23F, 33F | Rhizavidin | Phase II | Affinia | Children | |
| Natural polysaccharides of serogroups 1, 5, 14, 6B, 18C, 19F, 23F | TT | Phase III | QuimiVio/CIGB | Children | |
| Group B Streptococcus | Natural polysaccharides of serotypes Ia, Ib, II, III, IV, V | CRM197 | Phase II | Pfizer | Children |
| Klebsiella 4V | Oligosaccharides of O-antigen | EPA | Phase I | LMTB-GSK | Children |
| Shigella 2a and 4V | Oligosaccharides | EPA | Phase I | LMTB-GSK | Children |
| Shigella 2a | Synthetic oligosaccharides | TT | Phase I | Pasteur Institute | Children |
| Extraintestinal pathogenic Escherichia Coli | Oligosaccharides of serogroups O1A, O6A, O18A, O25B, O2, O4, O8, O15, O16, and O75 | EPA | Phase III | Johnson & Johnson | Children |
| PNAG (Staphylococcus aureus, E. coli, Klebsiella pneumoniae, N. gonorrhea, N. meningitidis, S. pneumoniae) | Synthetic oligosaccharides | TT | Phase II | Alopex | Children |
TT: tetanus toxoid; DT: diphtheria toxoid; CRM197: tetanus toxin mutant; OMP: outer membrane protein derived from Neisseria meningitides serotype B1; Protein D: conserved surface protein from non-typed Haemophilus influenzae (NTHi); MenA/C/W/Y: group A, C, W and Y meningococci; rEPA: Pseudomonas aeruginosa exotoxin A
5. Semi- and Fully Synthetic Carbohydrate-Based Antibacterial Conjugate Vaccines
Despite the great success of bacterial polysaccharide-protein conjugate vaccines, they still have limitations as noted above.42, 92, 93 As a result, the interest in glycoconjugate vaccines containing structurally well-defined carbohydrate antigens, which possess several potential advantages, is growing rapidly. For example, since the first oligosaccharide-protein conjugate vaccine against Hib was licensed in the late 1990’s,94 several oligosaccharide-based conjugate vaccines against different infectious diseases have been developed or are evaluated in clinical trials (Table 1).68 Moreover, fully synthetic glycoconjugate vaccines, such as that against group C meningitis using monophosphoryl lipid A (MPLA) as the vaccine carrier, were demonstrated to exhibit promising immunological properties.49 This section is devoted to the review of the progresses made in this specific area.
5.1. Hib vaccine.
Hib is a Gram-negative bacterium in both encapsulated and unencapsulated forms. It causes severe infections such as meningitis. Hib used to be a leading cause of bacterial meningitis in younger children (up to five years of age) in the USA before the introduction of Hib vaccines. Presently, several carbohydrate-based Hib vaccines are commercially available in the single form or in combination with other vaccines (Table 1). These vaccines are derived from the poly-ribosyl ribitol phosphate (PRP) epitope. Vaccines made up of natural PRPs are inconsistent in carbohydrate antigen size and linkage to CPs, and thus exhibit different immune responses in the population40,95 Nonetheless, these glycoconjugate vaccines have been adopted by many countries and various immunization programs to successfully and drastically reduce the number of Hib infections. For example, in the USA, by 1997 the number of reported Hib cases in children under five years of age had decreased by 99%.96 Similarly, in the UK, the frequency of Hib infections in children under five years of age reduced from over 20/100,000 to below 1.0/100,000 within three years after vaccines were introduced.97 The first synthetic oligosaccharide antigen-based conjugate Hib vaccine, Quimi-Hib, developed in Cuba was able to help reduce the cost of Hib vaccine significantly.94 Its efficiency is proved to be about 99.7% in children, and it shows an excellent safety profile.
Quimi-Hib (1, Figure 4a) comprises of synthetic oligosaccharide antigens with an average of seven repeating units of PRP conjugated to TT through a maleimido linker.94 To further investigate the appropriate length of PRP oligosaccharides as antigens for the development of Hib conjugate vaccines, the Seeberger group synthesized various sizes of PRP oligosaccharides by a [2 + 2], [4 + 2], [6 + 2], and [8 + 2] elongation strategy and conjugated them with CRM197. Immunological evolutions of the resultant glycoconjugates 2-5 (Figure 1b) with the Zika rabbit model showed that 2 containing a tetramer of the PRP repeating unit was an excellent antigen for developing semisynthetic glycoconjugate Hib vaccines.98 On the other hand, the Pozsgay group reported the first total synthesis of PRP oligosaccharides up to twelve repeating units,99 among which the octamer and dodecamer were linked to bovine serum albumin (BSA) but no immunological results of these conjugates were reported.
Figure 4.
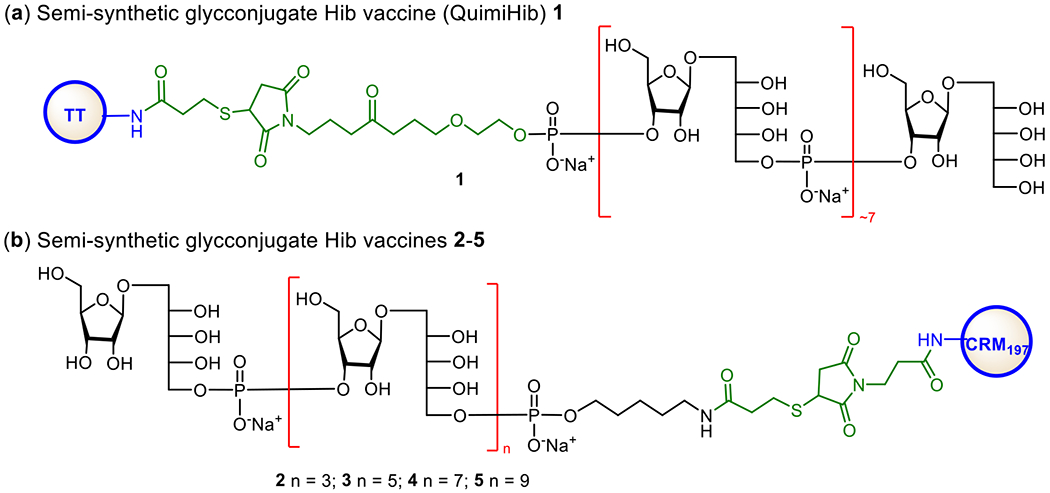
Structures of commercialized semi-synthetic glycoconjugate Hib vaccine Quimi-Hib 1 (a) and semi-synthetic glycoconjugates 2–5 explored as Hib vaccines by the Seeberger group (b).
5.2. Meningitis vaccine.
Meningococci are commensal bacteria in humans and observed in diversity. Some of the bacteria cause invasive diseases. For example, Neisseria meningitidis is a Gram-negative bacterium and the cause of various infections, predominantly meningococcal meningitis, in young children and older adults worldwide.100 One of the significant difficulties in diagnosing meningococcal diseases is that its clinical manifestations are hard to differentiate from common and less severe illnesses. To date, 13 serogroups of N. meningitidis have been identified based on the chemical composition of their CPSs.101 Among them, six serogroups (A, B, C, W135, X, Y) are mainly responsible for invasive meningococcal infections.102 The global picture of these serogroups is: serogroup W135 (MenW) is mainly found in African and South American regions; serogroup X (MenX) is found in some areas of Africa; serogroup A (MenA) is found in Asia and Africa, while serogroup B (MenB), C (MenC), and Y (MenY) are usually observed in Europe and North America.103
Currently, several vaccines are available for the disease-causing serogroups A, B, C, W135, Y of N. meningitidis, but there is no vaccine for serogroup X yet. These vaccines have helped dramatically decrease invasive meningococcal infections and associated diseases. They are all glycoconjugate vaccines made up of purified polysaccharides derived from pathogens.104 There are three licensed quadrivalent meningococcal glycoconjugate vaccines for serotypes A, C, Y, and W135 marketed by different manufacturers, i.e., Menveo® (MenA/C/W135/Y-CRM197) by GSK, Nimenirix® (MenA/C/W135/Y-TT) by Pfizer, and Menactra® (MenA/C/W135/Y-DT) by Sanofi Pasteur. One monovalent glycoconjugate vaccine (MenAfriVac) is available for serotype A and three for serotype C. Among the monovalent vaccines for serotype C, NeisVac-C® (Pfizer) employs TT as the CP, whereas the other two, Menjugate® (GlaxoSmithKline) and Meningtec® (Pfizer), use CRM197.105 Because natural polysaccharides are used to produce these vaccines, they are heterogenic in respect to antigen, linkage, and conjugation site. However, they are uniform in immunogenicity against bacteria of specific serotypes and eligible for all age groups from 2 months to 55 years. In addition, other licensed monovalent conjugate vaccines against serogroups A and C are also available.
As shown in Figure 5, the MenA CPS is composed of repeating →6)-2-acetamido-2-deoxy-α-D-mannopyranosyl phosphate(→ with about 70–80% O-acetylation of the 3-OH group.106 The Pozsgay and Oscarson groups independently reported the synthesis of oligosaccharides of MenA CPS.106,107 It was demonstrated that oligosaccharides higher than trisaccharide of MenA CPS repeat were unstable due to the hydrolysis of anomeric phosphates, which is enhanced by the adjacent NHAc group.108 This explains the poor stability of innate MenA CPS in aqueous medium. To alleviate this problem and develop stable glycoconjugate vaccines, several groups explored the mimics of MenA CPS oligosaccharides. Replacing the ring oxygen atom of the pyranose and/or the anomeric oxygen atom with methylene groups led to the stable carbocyclic and 1-C-phosphono analogs, which were conjugated with CPs to obtain respective conjugates (Figure 5).109,110 In vivo studies of CRM197 conjugates 6-8 containing the carbocyclic analogs of MenA CPS oligosaccharides revealed that only antibodies induced by the trimer conjugate 8 could recognize MenA polysaccharide and exhibit in vitro antibacterial activity, although all other conjugates elicited specific antibodies against the synthetic antigens.111 In vitro biological studies of the 1-C-phosphono analogs 9-11, which can mimic MenA CPS oligosaccharides more closely, showed that these unnatural antigens were recognized by human polyclonal anti-MenA CPS antibodies and inhibited the binding of antibodies to MenA polysaccharide. This activity was independent of the anomeric configuration of the N-acetyl mannosamine in 9-11 but affected by the carbohydrate chain length.112 Similarly, it was shown that the human serum albumin (HSA) conjugates 12-14 of oligosaccharides 9-11 induced T-cell proliferation in vitro by 40% at a concentration of 10 μM, in contrast to 28% induced by phosphonodisaccharide conjugate at the same concentration, and stimulated specific IgG production in vivo.113 These results suggested that the above synthetic analogues of MenA CPS oligosaccharides might be useful for developing anti-MenA vaccines.
Figure 5.
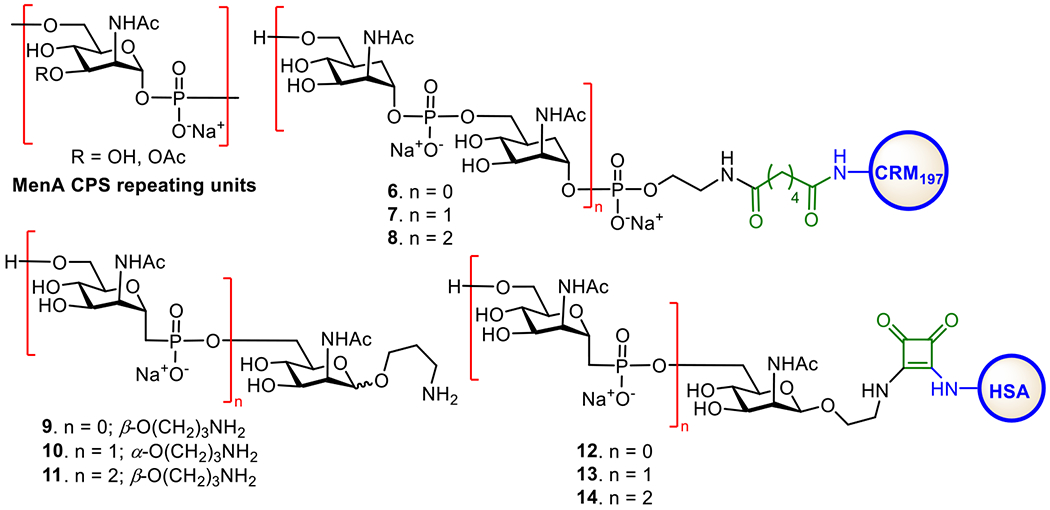
Structures of MenA CPS and its synthetic 1-C-phosphono and carbocyclic analogues, as well as their protein conjugates explored as MenA vaccines.
The MenC CPS is a homopolymer of α-(2→9)-linked sialic acids with irregular 7/8-O-acetylations (Figure 6). Deacetylated MenC CPS is more immunogenic than the natural glycan, but both can elicit protective antibodies with bactericidal activities.114 The Wu and Wong group developed a convergent and effective route to synthesize a series of α-(2→9)-linked sialic acid oligomers 15-20 (Figure 6).115 The Guo group also prepared several α-(2→9)-linked oligosialic acids and conjugated them with keyhole limpet hemocyanin (KLH).116 In vivo studies showed that the resultant glycoconjugates 21-24 elicited strong T cell-mediated immune responses that recognized α-2,9-polysialic acid. Furthermore, the synthetic oligosialic acids were found to be more immunogenic than natural polysialic acid.
Figure 6.
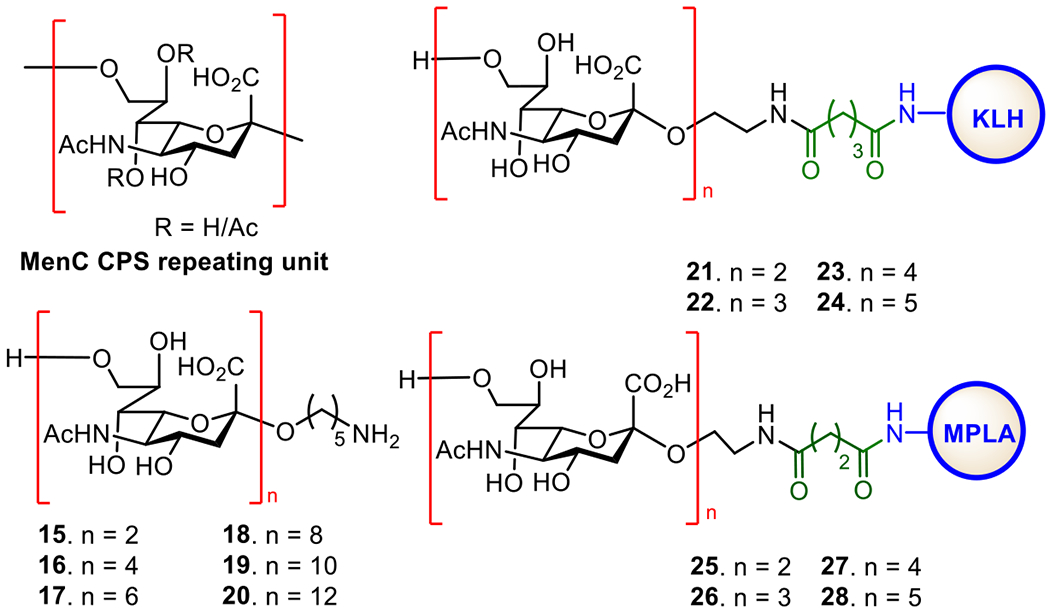
Structures of MenC CPS, its synthetic oligosaccharides 15-20, and the protein conjugates 21-28 of oligosaccharides 15-20.
The synthetic α-(2→9)-oligosialic acids were also coupled with synthetic monophosphoryl lipid A (MPLA) to generate glycoconjugates 25-28, which represent the first fully synthetic antibacterial vaccines.49 Immunological studies in mice showed that conjugates 25-28 alone provoked robust T cell-dependent immune responses comparable to the corresponding KLH conjugates. Out of these conjugates, tri- and tetrasaccharide conjugates 26 and 27 induced a higher titer of antibodies. These results suggested that MPLA was not only an excellent carrier molecule for antibacterial glycoconjugate vaccines but also a potent adjuvant to construct self-adjuvanting vaccines. The elicited antibodies were able to bind to N. meningitidis cells that expressed α-(2→9)-polysialic acid, suggesting the potentials of this type of fully synthetic glycoconjugate vaccines.
The MenW CPS is composed of a disaccharide repeating unit, →6)-α-D-Galp-(1→4)-α-d-Neup5Ac(7/9OAc)-(2→ (Figure 7). To facilitate the development of oligosaccharide-based conjugate vaccines against MenW, the Wu group first developed a [2 + n] synthetic strategy to obtain oligomers (e.g., monomer to pentamer 29-33a) of the disaccharide repeating unit of MenW CPS via stereoselective glycosylation using a disaccharide donor.117 The synthesized oligosaccharides was conjugated with CRM197, and the immunological properties of resultant glycoconjugates 29-33b were evaluated in mice. It was demonstrated that conjugates 30-33b induced robust immune responses and the induced antibodies could recognize both the di- and the tetrasaccharide epitopes. However, antibodies induced by conjugate 29b recognized only the disaccharide epitope but not the tetrasaccharide and other longer oligosaccharides. Among these glycoconjugates, antibodies elicited by 32b exhibited more potent antibacterial activity. These results imply that the minimum length of an oligosaccharide required to represent the characteristic antigenic epitopes of MenW CPS is a tetrasaccharide and that the oligosaccharide containing four disaccharide repeating units of MenW CPS is a promising candidate antigen for the development of anti-MenW vaccines.
Figure 7.
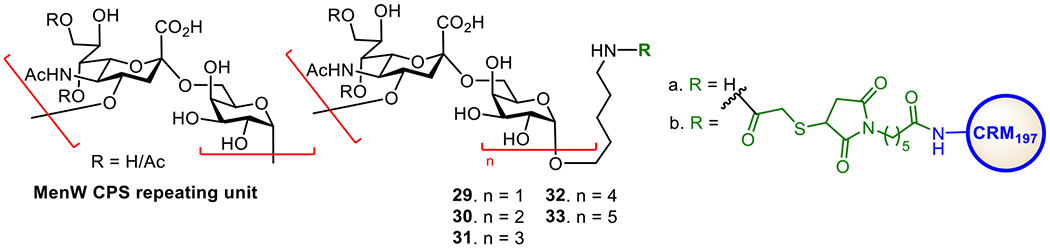
Structures of MenW CPS, its synthetic oligosaccharides 29-33a, and the CRM197 conjugates 29-33b of oligosaccharides 29-33a.
MenX infections spread worldwide, but a significant prevalence is observed in the region of sub-Saharan Africa known as the “meningitis belt”.118 For example, the outbreaks of MenX infections occur frequently in African counties such as Uganda,119 Niger,120 Kenya,119 Togo,50 etc. In the past few decades, its occurrence in America, Europe,121 and China122 is also raising. However, currently, there is no vaccine to prevent MenX. Therefore, many studies are focused on developing anti-MenX vaccines.
The MenX CPS is a homopolymer of the →4)-2-acetamido-2-deoxy-α-D-glucopyranosyl phosphate-(→ repeating unit (Figure 8). MenX CPSs of varied chain length were coupled with CRM197 to form glycoconjugate vaccines (Figure 8). The conjugates induced high titers of IgG antibodies in mice, and the elicited antibodies exhibited significant bactericidal activities.123 Chhikara et al. developed a synthetic route for MenX CPS oligosaccharides for the preparation of semi-synthetic glycoconjugate vaccines.124 Both the MenX tetrasaccharide-TT conjugate 34 and trisaccharide-CRM197 conjugate 35 exhibited lower immunogenicity than the conjugates of natural MenX CPSs.125 However, when the length of the oligosaccharide increased further, the resultant conjugates were able to induce immune responses comparable to that induced by the conjugates of natural CPSs. Recently, Adamo et al. developed a one-pot chemoenzymatic strategy for the synthesis of MenX oligosaccharides.126 This strategy allows for rapid access to complex bacterial oligosaccharides or CPS fragments of various lengths under pathogen-free conditions. The oligosaccharides were conjugated to CRM197, and the resultant conjugates were evaluated in mice. Glycoconjugate 36 induced antibodies comparable to those induced by the conjugates of natural CPSs.
Figure 8.
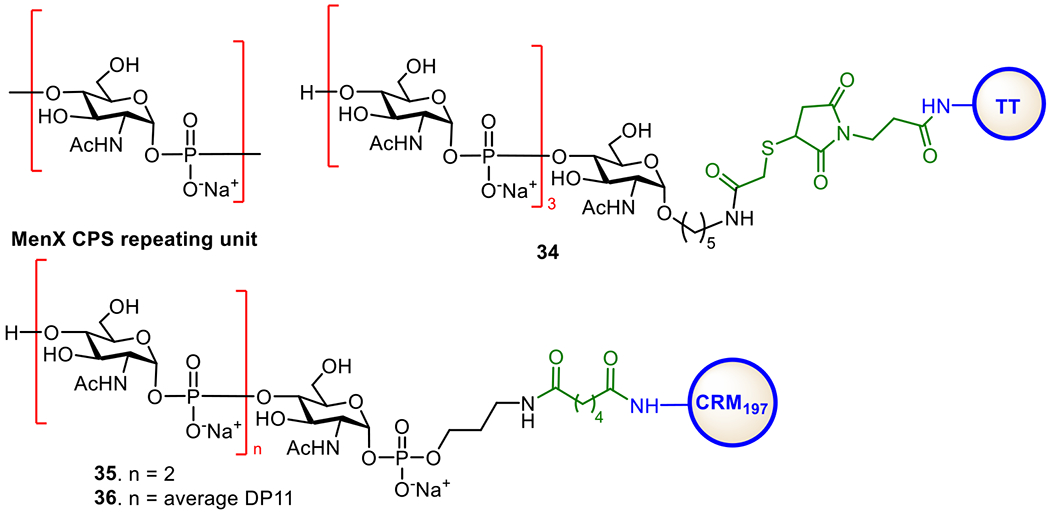
Structures of the MenX CPS and protein conjugates 34–36 of MenX CPS oligosaccharides.
5.3. Vaccine against Acinetobacter baumannii.
A. baumannii is a pathogenic aerobic Gramnegative bacterium discovered in 2003 during the Iraq War.127 It belongs to the Neisseriaceae family. Its outbreak occurred in Germany in 2013, and it is multi-drug resistant (MDR), thus it is becoming a global threat. In literature, twenty structures128 and >90 serotypes129, 130 of A. baumannii are reported. The major component of the EPS of A. baumannii strain 54149 has →3)-[Pse5NAc7NAc-α-(2→6)-Glcp-β-(1→6)]-Galp-β-(1→3)-GalNAcp-β-(1→ (Figure 9, 37a) as its repeating unit.131 A. baumannii strain ATCC17978 expresses mainly 37c, consisting of a trisaccharide core →3)-α-d-Gal(1→6)-β-d-Glc(1→3)-β-d-GalNAc-(1→ and a triacetylated 2,3-diamino-d-glucuronate and a GlcNAc residues as branches β-linked to the Gal 4-O- and 6-O-positions of the core, respectively. The core trisaccharide is also observed in the EPSs of other A. baumannii strains, such as ATCC NIPH146,132 17961,133 SMAL,134 LUH5537,128 KL22 and PSgc9.135 Several groups are engaged in synthesizing and using the oligosaccharides of A. baumannii EPSs as antigens for the design of new glycoconjugate vaccines. For example, Li et al. prepared the conjugates (Figure 9) of pseudaminose (Pse) that mimics the side chain non-reducing end monosaccharide epitope of the polysaccharide.136 These conjugates had different sugar/protein ratios (4.76, 8.27, and 14.34 for conjugates 38-40, respectively), aiming to disclose the impact of antigen loading on the immunological properties of glycoconjugate vaccines. Pse-BSA conjugate 41 was used as a coating antigen to detect anti-Pse-antibodies during immunological studies. All the three conjugates elicited glycan-specific IgG antibodies in mice and protected mice from A. baumannii infection. These results suggested that a partial structure of a bacterial polysaccharide antigen, so long as its epitope is sufficiently unique, may be sufficient to induce antibacterial immune responses and useful for vaccine development.
Figure 9.
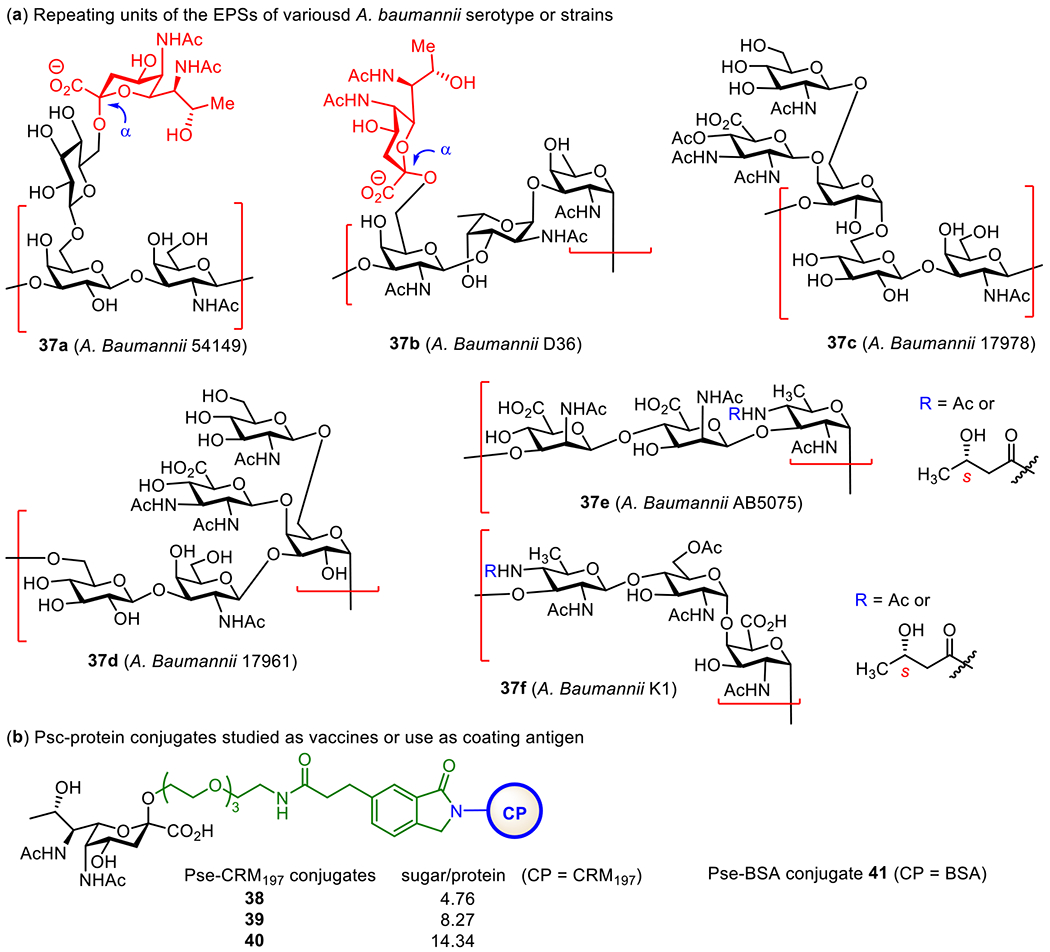
Structures of the repeating units of A. baumannii EPSs and their protein glycoconjugates.
Recently, the Seeberger group designed and synthesized a series of well-defined mono- and oligosaccharide units of A. baumannii ATCC17978 EPS, including four pentasaccharides, three tetrasaccharides, four trisaccharides, four disaccharides and one monosaccharides, with an aminopentyl linker at the reducing end for microarray printing and protein conjugation.137 The study aimed to define the key epitopes and better understand the role of the acetyl groups in the tri-acetylated glucuronic fragment of 37c in antibody binding. The synthetic glycans were printed onto microarray slides and screened with diseased patient sera and a monoclonal antibody (mAb) C8. Among the sixteen glycans, acetylated oligosaccharides were specifically recognized by antibodies and constituted the promising vaccine candidates. Furthermore, they have also proposed the study of conjugates of these synthetic glycans in vivo for development of vaccines against A. baumannii infection.
5.4. Pneumonia vaccine.
S. pneumoniae infections remain a major challenge globally. These Gram-positive bacteria mainly cause invasive diseases like pneumonia, septicemia, meningitis, and otitis media in newborns or infants. S. pneumoniae bacteria are classified into 97 serotypes based upon their CPSs. Among them, the most virulent 20 serotypes cause more than 90% of pneumococcal infections.138 In 2016, a worldwide survey showed that S. pneumoniae was the leading cause of lower respiratory infection morbidity and mortality, contributing to more than one million deaths (1,189,937 deaths) in all age groups.139 About 36% of the global burden of pneumonia and 27% of the global burden of otitis media are due to S. pneumonia.
To reduce the disease burden globally, pneumococcal vaccination is crucial, especially in underdeveloped countries. Currently, two types of vaccines are available on the market, i.e., the polyvalent polysaccharide vaccine PPV23 and the glycoconjugate vaccines Prevnar13® and Synflorix®. The most used PPV23 vaccine (Pneumovax®23) is composed of 23 native CPSs, and it is used to immunize people above 50 years of age. Pneumococcal glycoconjugate vaccine (PCV) Prevnar13® contains 13 glycoconjugate vaccines with CRM197 as the CP. It is approved for all age groups (infant, children, and adults over 65 years of age), while Synflorix® (PCV10) contains 10 glycoconjugate vaccines with three different CPs, and it is permitted for children under five years old.140 Recently, Merk has developed two new 15-valent glycoconjugate PCVs (PCV15-A and PCV15-B), which have been verified in phase I and II clinical trials and should be available for vaccination soon.141 PVC15 uses CRM197 as the CP, and is formulated with aluminum phosphate adjuvant.
A primary concern about these vaccines is that they do not provide protections against all serogroups of S. pneumoniae.142 For example, PCV7 is a 7-valent vaccine that only protects serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, while PCV13 protects serotypes 1, 3, 5, 6A, 7F, and 19A in addition.143 Another concern about the multivalent vaccines is that some conjugates have low immunogenicity in the formulation, e.g., the vaccine against serotype ST3, or cannot protect some strains with structurally similar polysaccharides, e.g., ST19A and 19F.
To develop more effective PVCs, many research groups have been engaged in establishing effective synthetic methods for oligosaccharide segments of S. pneumoniae polysaccharides and using them as antigens for the design of novel glycoconjugate vaccines (Figure 10). In this respect, serotype 1 (ST1) ZPS is an attractive target due to its unique immunological properties. For instance, it is the first known carbohydrate antigen that induces T-cell dependent immune responses without conjugation with a CP.144 Several research groups have developed synthetic methods for ST1 zwitterionic oligosaccharides for vaccine design.66, 145,146,147 To gain a better understanding of the immunomodulatory activity of ZPSs, the Seeberger group prepared an ST1 zwitterionic trisaccharide with a thioether spacer and conjugated it with CRM197 to obtain glycoconjugate 42.148 It was revealed that 42 induced a robust antibody response in rabbits comparable to the control vaccine PCV13. In addition, the synthetic trisaccharide in 42 was recognized by an antiserum against the native polysaccharide.
Figure 10.
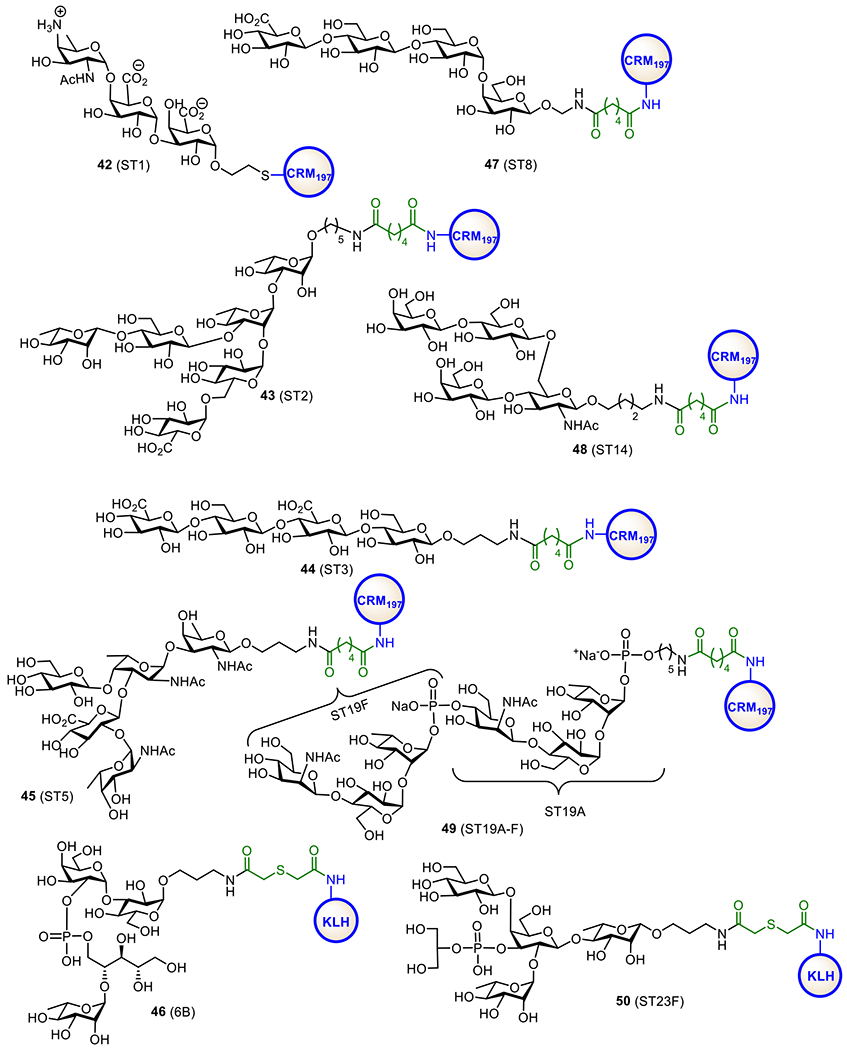
Structures of the synthetic oligosaccharide-protein conjugates 42-50 as vaccine candidates for S. pneumoniae serotypes ST1, ST2, ST3, ST5, ST8, ST6B, ST14, ST19A-F, and ST23F.
The Seeberger group also synthesized and explored oligosaccharide-protein conjugates as vaccines for S. pneumoniae serotypes ST2 (43), ST3 (44), ST5 (45), ST8 (47), and ST14 (48) (Figure 10).149 All these glycoconjugates induced high levels of protective antibodies against specific S. pneumoniae serotypes. Furthermore, combining these glycoconjugate vaccines with commercial PCV13 and PCV10 resulted in robust protective immunities in mice. It provides an example of a fully synthetic carbohydrate-based multivalent vaccine in combination with conjugates made up of native polysaccharides.
The Kamerling group synthesized a series of protein conjugates, such as 46 (Figure 10), of S. pneumoniae serotype ST6B CPS oligosaccharides.150 Immunological evaluations of these conjugates in rabbits revealed that they induced high levels of pneumococcal ST6B antibodies that could accelerate type-specific phagocytosis. Moreover, the rabbit antisera could passively protect mice against pneumococcal ST6B. In addition, tetrasaccharide conjugate 46 provoked phagocytic and protective anti-ST6 B antibodies in mice as well.
S. pneumoniae serotypes 19A (ST19A) and 19F (ST19F) can cause pneumococcal diseases even after immunization with PCV13. To address this problem, the Morelli151 and Seeberger152 groups designed chimeric oligosaccharide-containing protein conjugates as candidate vaccines against ST19A and ST19F. Glycoconjugate 49 synthesized by the Seeberger group comprised of the repeating units of ST19A and ST19F CPSs (Figure 10).152 Immunological studies of 49 showed that it induced high titers of antibodies in rabbits that were bactericidal to both ST19A and ST19F strains in vitro and protected animals from infection in vivo.
In addition, other vaccine carriers or antigen delivery methods are explored for developing new anti-S.pneumoniae conjugate vaccines as well. For example, gold nanoparticles have been used to construct multivalent vaccines that carried the CPS motifs of different serotypes (e.g., ST14 and ST19F).153 Similarly, virus-like particles (VLP) have been utilized as carriers along with the carbohydrate antigens of ST3 and ST14 to construct vaccines that initiate both B- and T-cell dependent immune responses.154 These new vaccine carriers or antigen delivery methods gave positive results and may be further exploited in vaccine design.
5.5. Vaccines against Shigella group bacteria.
In underdeveloped countries, Shigellosis is a common and devastating diarrheal disease in the paediatric population—children under the age of five years old. A survey of the mortality and morbidity rate caused by Shigella showed that this pathogen was responsible for 212,438 deaths and the second leading cause of diarrhea in all age groups (2.69 million people) worldwide in 2016.155 Shigella bacteria are Gram-negative and have 50 serotypes, falling into four distinct groups, namely, S. dysenteriae (15 serotypes), S. flexneri (15 serotypes), S. boydii (19 serotypes), and S. sonnei (1 serotype). The strains S. dysenteriae and S. flexineri are more infectious and invasive, while S. sonnei is less virulent than others.156 S. flexneri 2a (SF2a) is the most virulent and a primary cause of Shigellosis.157
Shigella infections result in the expression of Shiga toxins that can cause severe intestinal symptomatology with many complications in humans. However, currently, there is no vaccine against Shigella. Most vaccine candidates are still in developmental or clinical trial stages.157 SF2a, the most virulent strain, expresses a LPS with O-polysaccharide consisting of a branched pentasaccharide repeating unit: →2)-α-d-Glc-(1→4)-[α-l-Rha-(1→2)-α-l-Rha-(1→3)]-α-l-Rha-(1→3)-β-d-GlcpNAc-(1→.158 To develop anti-Shigella glycoconjugate vaccines, Mulard et al. synthesized a series of protein conjugates 51-53 having one, two, and three repeating units of the SF2a O-antigen linked to TT (Figure 11). Immunological studies of these conjugates in mice showed that they elicited robust IgG responses and the immune responses increased with the size of oligosaccharide antigens from 51 to 53. Glycoconjugate 53 containing a trimer of the pentasaccharide repeating unit was the best vaccine candidate, since it induced specific and long-lasting anti-SF2a O-polysaccharide antibodies and protected mice from Shigella infection. Currently, 53 is in Phase II clinical trials (NCT04078022).159
Figure 11.
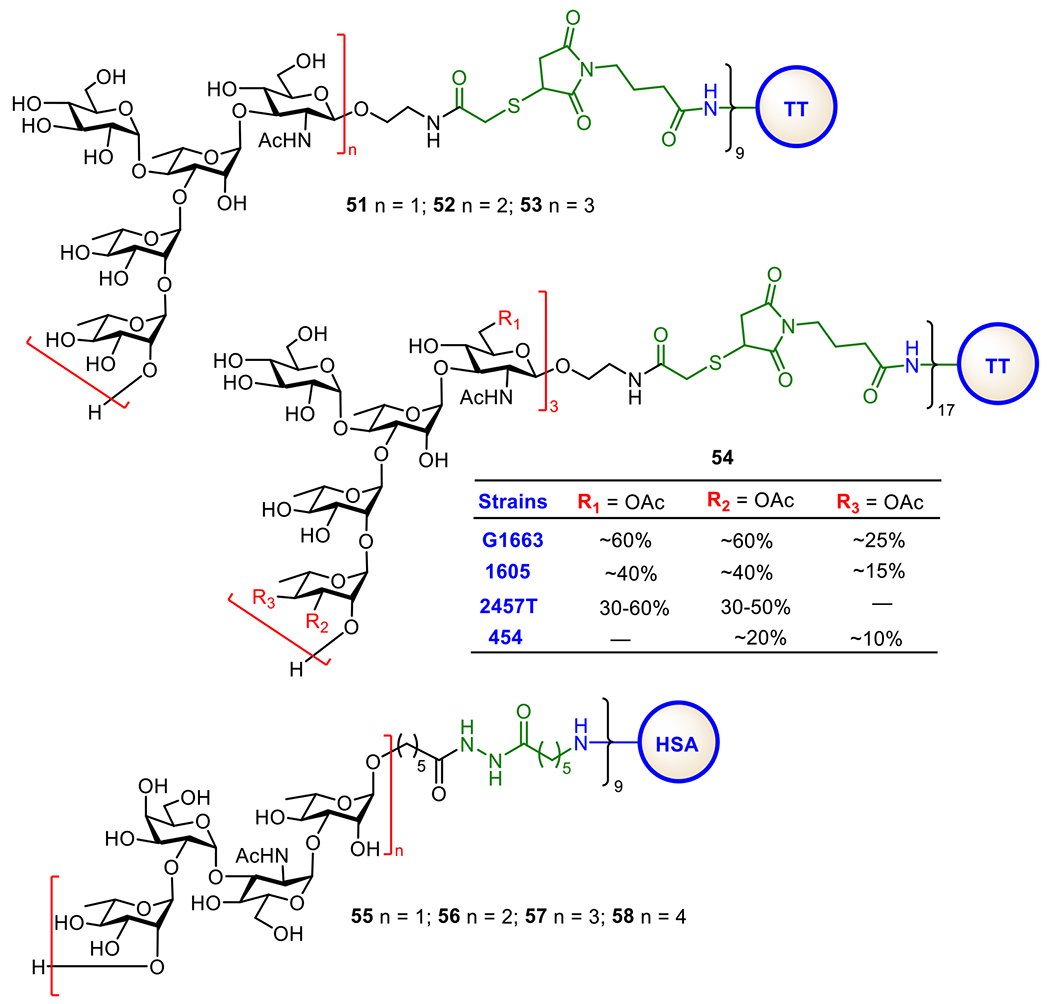
Structures of synthetic conjugate vaccines 51–53 for S. flexneri 2a made of oligosaccharides of its O-antigen and 54 made of S. flexneri 2a O-antigen oligosaccharides with nonstoichiometric O-acetylation, as well as glycoconjugate vaccines 55–58 for S. dysenteriae type-1.
The Mulard group has recently reported a scalable process for the preparation of another synthetic glycan-based anti-Shigella conjugate vaccine 54 with TT as its CP (Figure 11).33 The oligosaccharide antigen in 54 is a trimer of the pentasaccharide repeating unit of SF2a O-antigen featuring with nonstoichiometric O-acetylation, which is observed in various strains of SF2a.160,161 This synthetic procedure has enabled the scale-up GMP production of the conjugate vaccine with uniform glycan/protein ratio. Conjugate 58 has been shown to induce bactericidal antibody responses in mice and cleared all toxicity-related criteria. Furthermore, the synthetic glycoconjugate vaccine was proved to be stable for at least 66 months.
S. dysenteriae type 1 (or Shiga Bacillus) is mainly responsible for epidemic dysentery and diarrhea diseases in developing countries.162 The LPS expressed by S. dysenteriae type 1 is a virulence factor and protective antigen, and its O-specific polysaccharide antigen consists of a tetrasaccharide repeating unit: →2)-α-l-Rha-(1→2)-α-d-Gal-(1→3)-α-d-GlcNAc-(1→3)-α-l-Rha-(1→. Pozsgay and co-workers reported the first experimental glycoconjugate vaccines 55-58 (Figure 11) for S. dysenteriae type 1, which were made of synthetic oligosaccharide antigens carrying a monomer, dimer, trimer, and tetramer, respectively, of the tetrasaccharide repeating unit of the S. dysenteriae type 1 (O-antigen, Immunological studies of these glycoconjugates in mice showed that 55 induced a weaker immune response than other conjugates. Conjugate 58 containing the tetrameric oligosaccharide antigen was the most immunogenic and provoked high levels of antigen-specific IgG antibodies that were cross-reactive with S. dysenteriae type 1 LPS. To study the impact of the glycan non-reducing end sequence on the immunological properties of oligosaccharide antigens and glycoconjugate vaccines, they also synthesized a series of oligosaccharides (hexa- to tridecasaccharides) with different non-reducing ends and their protein conjugates.163 It was shown that the protein conjugates of glycans with GlcNAc or Gal at the non-reducing end elicited the highest levels of anti-LPS antibodies, but still, their antigenicity was lower than that of native (O-antigen-protein conjugates.164 Very recently, Yin and co-workers accomplished the first total synthesis of the tetrasaccharide of S. dysenteriae serotype 10 (O-antigen in a stereoselective manner.165 Additionally, corresponding (R)-4,6-O-pyruvylated and non-pyruvylated tetrasaccharides, as well as (S)-4,6-O-pyruvylated mannose, were also synthesized and analyzed for their antigenicity through glycan microarray screening. The results indicated that the tetrasachharide repeating unit is a crucial antigenic epitope of the S. dysenteriae serotype 10 O-antigen. Importantly, the direct comparison of native (S)-4,6-O-pyruvylated mannose with its analog (R)-4,6-O-pyruvylated mannose revealed that this motif was responsible for the antibody-binding of the O-antigen. This finding is helpful to understand the biological significance of this rare pyruvyl ketal functional group in the existing pathogenic bacterial surface glycans.
5.6. Anthrax vaccine.
Anthrax is caused by a highly lethal Gram-positive bacterium Bacillus anthracis that has three well-known strains, Ames, Sterne, and Vollum, among which Amens is the most virulent.166, 167 The main virulent factors of B. anthracis are its CPSs and anthrax toxins.168 B. anthracis spores are refractive to extreme conditions, such as stresses, desiccation solvents, high temperature, pressure and pH, UV light and even ionizing radiation.169 These properties of B. anthracis ensure its perseverance in the environments. For example, it can survive thousands of years in the wet state, and when favourable conditions return, the spores become active and undergo germination and growth processes. As a result, B. anthracis is a choice for biological weapons. To address the threat of B. anthracis, significant efforts have been made to diagnose the disease early and develop anti-anthrax vaccines.
Cell-free vaccines containing the anthrax toxin protective antigen (PA) components have been proven safe and effective. On the other hand, glycans in the exosporium nap layer of B. anthracis are also extensively explored to identify new antigenic targets. It was found that the non-reducing end of its poly-β-l-rhamnose (Rha) constituent is capped with a rare sugar, 2-O-methyl-4-(3-hydroxy-3-methylbutana-mido)-4,6-dideoxy-d-glucopyranose, known as anthrose (Figure 12). The unique structure of anthrose makes it a useful target for anti-anthrax vaccine development. The Seeberger group synthesized an anthrose-containing tetrasaccharide and conjugated it with KLH to get conjugate 59 (Figure 12). Its immune-easy adjuvant (QIAGEN) formulation elicited tetrasaccharide-binding IgG antibodies that recognize B. anthracis.170 This work has proven the feasibility of anthrose-containing oligosaccharides as antigens for anti-B. anthracis glycoconjugate vaccine development. In the meantime, the Boons group prepared several KLH conjugates 60a-d (Figure 12) of the anthrax trisaccharide carrying differently acylated anthrose and analysed their structure-activity relationships. This study had disclosed that even the simple N-acetylated trisaccharide in conjugate 60 was sufficient to elicit a desired immune response in rabbits. More importantly, structure-activity studies demonstrated the significance of the N-(3-methylbutyryl) moiety as an antigenic epitope in immune recognition of the bacterial spores.171 Djedaïni-Pilard et al. synthesized a simple anthrose-KLH conjugate 61 (Figure 12) and found that it induced specific immune responses against anthrax spores in rabbits.172 Other studies gave additional information about the immunological functions of the 2-O-Me, 4-N-(3-methylbutyryl), and 6-Me groups in anthrose and the poly-Rha moiety.173,174 It was shown that all the substituents on anthrose, except for 2-O-Me, were necessary for the oligosaccharide antigenicity and anti-spore antibody recognition. Moreover, without anthrose, the poly-Rha moiety also induced immune responses against the spores of B. anthracis.
Figure 12.
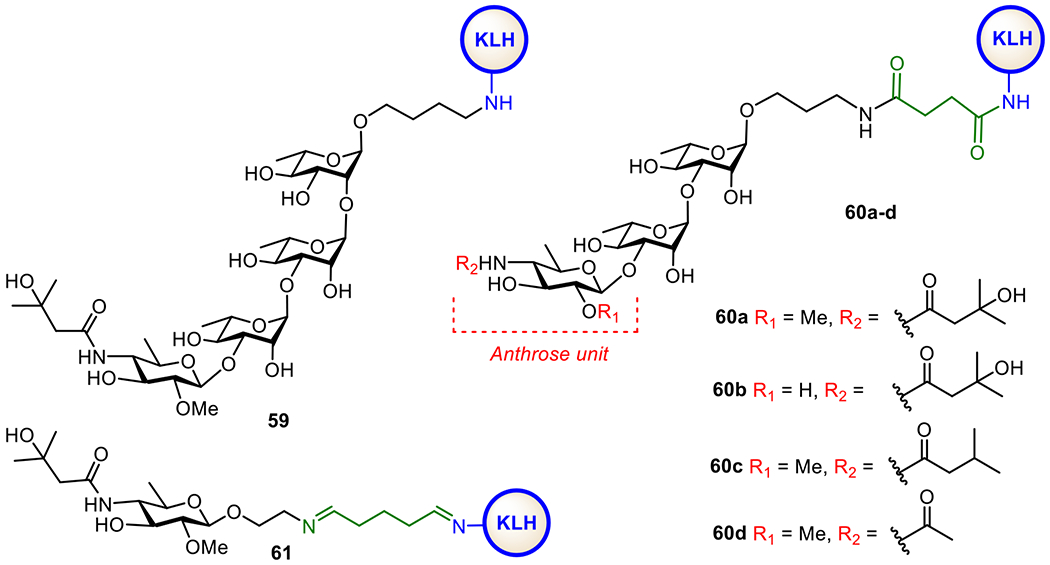
Structures of synthetic glycoconjugates 59, 60a-d, and 61 explored as anti-anthrax vaccines
5.7. Vaccine against Clostridium difficile.
C. difficile is an anaerobic Gram-positive pathogen. Its spores are essential to cause infection and gastrointestinal diseases,175 such as hypervirulent antibiotic-associated diarrhea, pseudomembranous colitis, and toxic megacolon in humans.176 Like B. anthracis, the spores of C. difficile are robust and dormant, and can survive indefinitely outside their hosts. The asymptomatic persistence and resistance of C. difficile to disinfectants have made C. difficile infections (CDIs) a serious public health problem.177 C. difficile secretes two potent toxins, TcdA and TcdB, which can enormously damage intestinal epithelium to lead to diarrhea and colitis.178 In addition, both toxins induce the release of various chemokines and cytokines, which causes acute inflammation of the large intestine.179 The exact strains of C. difficile are difficult to diagnose; more importantly, they are resistant to antibiotics. Therefore, many efforts are directed toward the development of effective vaccines against CDI.
In this regard, toxoid-based anti-CDI vaccines are extensively explored, and several are in various clinical stages. These vaccines are mainly made of formalin detoxified TcdA and TcdB derived from the bacterial culture. Safety concerns and the low yields in large-scale production of toxins, stability issues, and other limitations related to storage and so on compel people to look for alternatives. Thus, their unique glycans have become targets for developing anti-CDI glycoconjugate vaccines. Structural studies on the glycocalyx of C. difficile cell revealed three types of important glycans, PS-I (62), PS-II (63), and PS-III (64) (Figure 13a).180 Particularly, PS-II is a highly conserved polysaccharide on the cell surface of different strains of C. difficile and hence represents a useful target for vaccine development.181 Several PS-II oligosaccharides with or without a phosphate group at their non-reducing end have been synthesized and coupled with CRM197 via different linkers.182, 183 In vivo studies on the resultant glycoconjugates 65-67 (Figure 13b) in mice revealed that they were highly immunogenic. Especially, the Seeberger group found that 66 induced high levels of specific IgG antibodies.183 A comparison between conjugates 65, 67, and 66 containing oligosaccharides without and with the phosphate group, respectively, showed that the negatively charged phosphate was vital for the elicitation of IgG antibodies and the recognition of the whole polysaccharide of PS-II. A comparison between conjugates 66 and 68 containing only one and multiple repeating units of PS-II, respectively, revealed that they induced comparable antibody responses. Therefore, it was concluded that the negatively charged hexasaccharide epitope in glycoconjugate 66 should be sufficient for the design and development of conjugate vaccines against C. difficile.
Figure. 13.
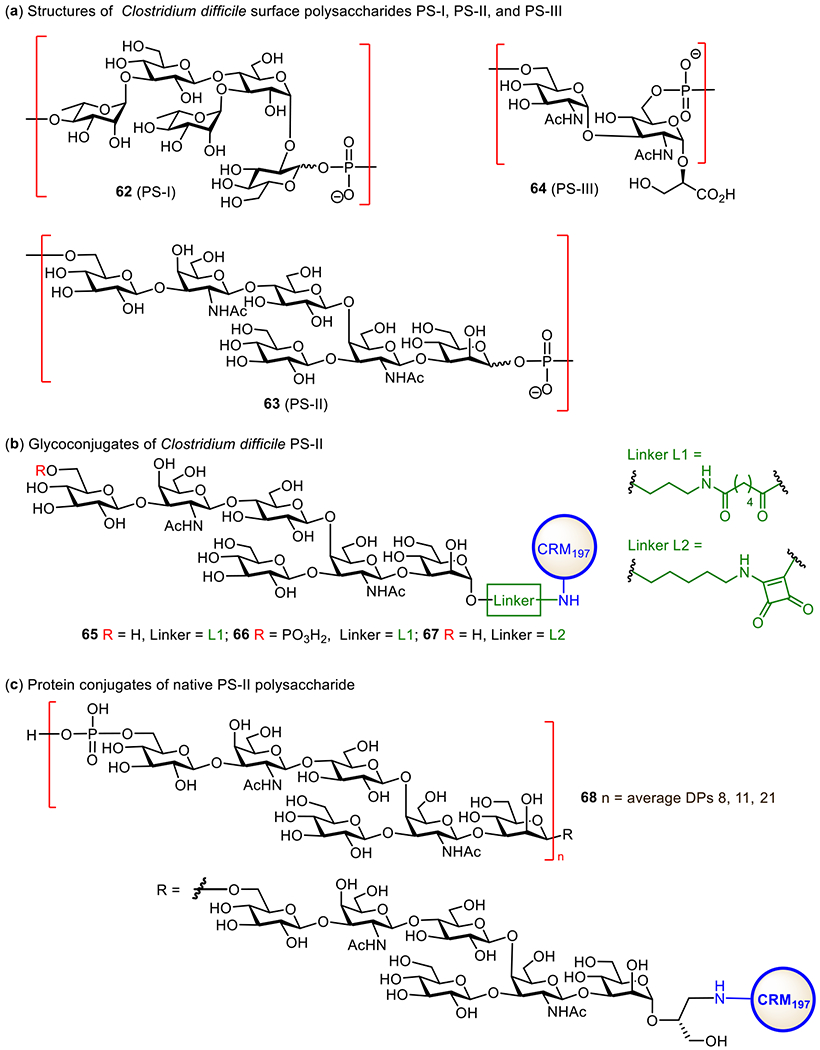
Structures of the surface polysaccharides of C. difficile PS-I, PS-II, and PS-III 62–64 (a), synthetic glycoconjugates 65–67 consisting of PS-II oligosaccharides (b), and native polysaccharide PS-II-CRM197 glycoconjugate 68 (c) investigated as vaccines for C. difficile.
In addition, Monteiro et al. discovered that horse sera that contained natural anti-PS-I IgG antibodies could detect both the synthetic non-phosphorylated repeating unit of PS-I and the native PS-I with a slightly higher affinity for the latter.184 These results further demonstrated the potential of phosphorylated glycans in the development of anti-CDI vaccines.
5.8. Brucella vaccine.
The Brucella genus contains ten Gram-negative proteobacterial species, Brucella abortus, melitensis, suis, ovis, canis, neotome, microti, inopinata, pinnipedialis, and ceti. Five of them cause infections in both animals and human.185 They are facultative intra-cellular pathogens that can cause severe zoonotic bacterial diseases like brucellosis.186 Brucella species are genetically similar to each other, which makes the diagnosis of human brucellosis challenging in the laboratory and clinic.187 Many efforts have been devoted to developing new tools and methods to identify brucella infections. Furthermore, treating human brucellosis by antibiotics is a lengthy and costly process. Thus, vaccination is considered the best alternative to fight this disease, although currently no anti-brucellosis vaccine is available.
To identify proper antigens for the design of vaccines against brucella, its DNA sequences and surface protein and carbohydrate epitopes have been extensively analyzed. It was found that the brucella cell wall contains a unique LPS with the O-antigen composed of a rare sugar, 4,6-dideoxy-4-formamido-α-D-mannopyranose (Rha4NFo), in two distinctive linking patterns, known as antigen A and antigen M (Figure 14).188 Antigen A is composed of only a-1,2-linked Rha4NFo, which forms the long inner sequence of O-antigens, and the shorter antigen M has an α-1,3-linked d-Rha4NFo after every four α-1,2-linked d-Rha4NFo units, which forms the cap of O-antigens.189 It was found that both antigen A and antigen M were virulent.190
Figure 14.
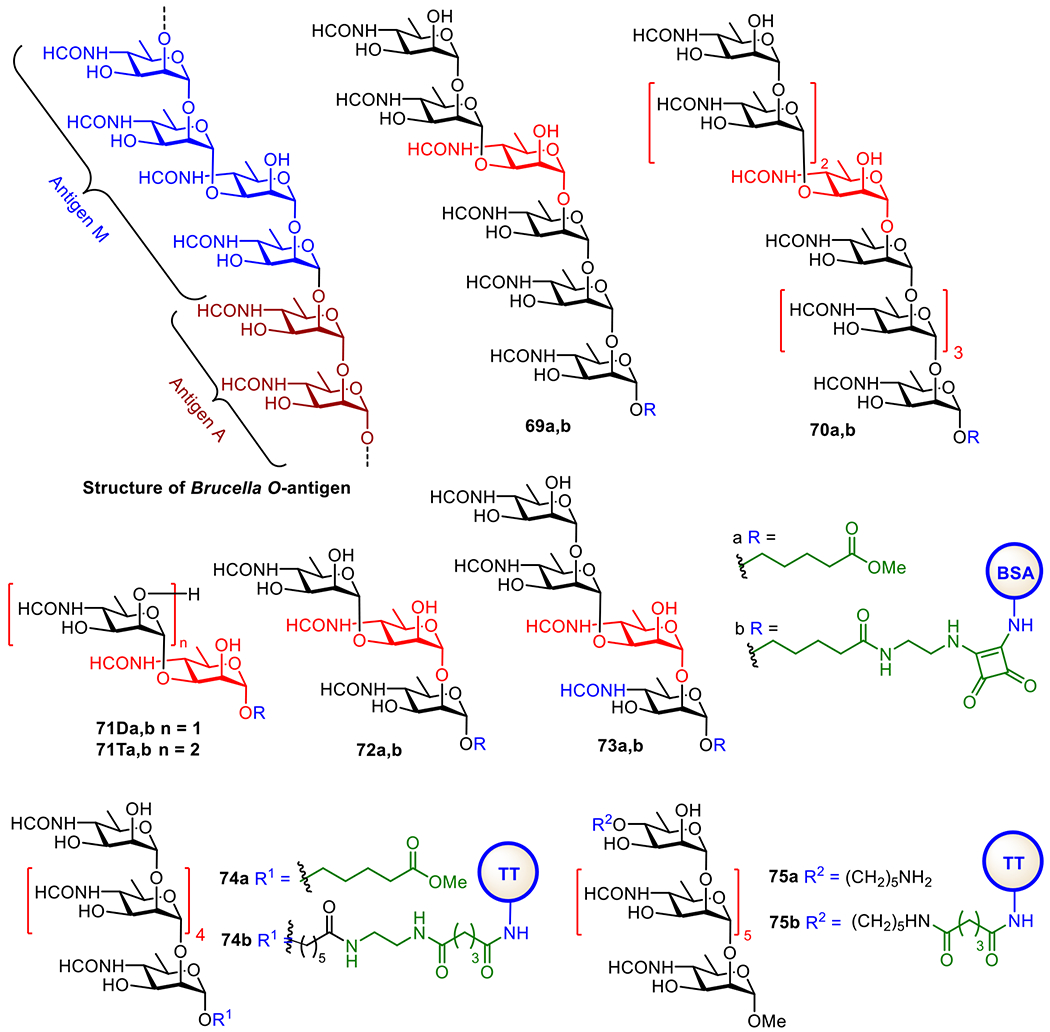
Structures of Brucella O-antigen and its synthetic oligosaccharide analogs 69-75a, as well as their protein conjugates 69-75b.
The O-antigens of Brucella are structurally unique, and this results in its complex serology. Since the antibodies to the Brucella O-antigens are useful for the diagnosis of infections, the ideal vaccine candidates should be built upon a protective epitope that does not interfere with the diagnosis. Early structural studies established that two epitopes, A and M, exist in the O-polysaccharide. The A epitope is a 1,2-linked homopolymer of 4,6-dideoxy-4-foramido-α-d-mannopyranose,191 and the M epitope is 1,3-linked.191, 192 It was later realized that, in fact, the O-polysaccharide consists of α–1,2-linked polysaccharide capped by a 1,2-/1.3-/1,2-linked tetrasaccharide sequence.189 Prior to these definitive structural studies, a series of well-defined oligosaccharides 69-75a (Figure 14) were designed and synthesized to represent the structural epitopes of antigens A and M in different settings.193 For example, 69a was considered as a mimic of mainly antigen M, while 70a contained the epitopes of both antigens A and M.
Oligosaccharides 69-75a (Figure 14) and other structures were used to probe mAbs raised against the whole bacteria.194 With BSA conjugates 69b and 70b as the probes, two mAbs, YsT9-1 and Bm 10, were discovered in animals. It was demonstrated that the hexasaccharide antigen 69a preferentially bound the M antibody while the nonasaccharide antigen 70a bound A- and M-antibodies in equal affinity. Further studies using conjugates 71-74b as the probes showed that the M-type disaccharide 71Da and tetrasaccharide 73a could detect antibodies in the sera of human and animals infected with B. suis and B. abortus. Both conjugates 71Db and 73b had strong binding to M-specific mAbs and weak to A-specific mAbs. Glycoconjugate 75 with an α-1,2-linked hexasaccharide was prepared and shown to elicit A-specific antibodies that also recognized M-type oligosaccharides 71a and 73a.193 This result suggested that a-1,2-Rha4NFo may represent the epitopes of both A- and M-antigenic serotypes and, thus, be useful for the development of broadly applicable anti-Brucella glycoconjugate vaccines.
However, these reports preceded the correct structural elucidation of the Brucella A and M epitopes by Vinogradov and co-worker189 and illustrated the hazard of conducting synthesis without full knowledge of the complete structure.189,193 Subsequent publications employed the structure identified by Kubler-Kielb and Vinogradov and established the structural basis for understanding the fine specificity of mAbs and polyclonal antibodies (pAbs) that bind the M antigen. This resulted in the discovery of a disaccharide that shows considerable potential as a diagnostic antigen for the detection of brucellosis in human and animals.195 The ability to create discrete O-polysaccharide antigens identified an O-polysaccharide epitope equally common to all Brucella abortus and Brucella melitensis strains but unique to Brucella. The previously untapped diagnostic potential within this key diagnostic structure also holds significance for the design of brucellosis vaccines and diagnostics that enable the differentiation of infections from vaccinated animals.196
Currently available whole cell vaccines induce anti-A and M antibodies and thus infected animals cannot be differentiated from vaccinated animals as both produce A- and M-specific antibodies. It was hypothesized that chemical synthesis of a pure A vaccine would offer unique identification of infected animals by a synthetic M diagnostic antigen that would not react with antibodies elicited by the vaccine. TT conjugates of two forms of the A antigen, hexasaccharide 74a and heptasaccharide 75b linked to TT via the reducing and non-reducing terminal sugars, respectively, were synthesized and explored as vaccine candidates. Mouse antibody profiles to these immunogens showed that, to avoid reaction with diagnostic M antigen, it was essential to maximize the induction of anti-A antibodies that bind internal oligosaccharide sequences and minimize production of antibodies directed toward the non-reducing monosaccharide. This objective was achieved by conjugation of Brucella O-polysaccharide to tetanus toxoid via its periodate-oxidized non-reducing monosaccharide moiety, thereby destroying terminal epitopes to focus the antibody response to internal A epitopes. This established a method to resolve the decade-long challenge of how to create effective brucellosis vaccines without compromising diagnosis of infected animals.188, 197
5.9. Vaccines against Burkholderia pseudomallei and B. mallei.
B. pseudomallei (Bp) and B. mallei (Bm) are two highly virulent Gram-negative bacteria, which are serious threat to human and animal lives.198 Bp and Bm are sporadically found in tropical and subtropical regions of the world, and if infected patients are left untreated, the fatal rate is up to 50%.199 Bp causes serious melioidosis, and Bm causes glanders in solipeds, both leading to death. These diseases are difficult to diagnose as their clinic symptoms are multifaceted, from skin abscess to acute pulmonary infection and fulminating septicemia. Moreover, both bacteria are CDC “Tier 1” select agents because of their high infectivity via inhalation, low infectious doses, and potential for misuse as biothreat agents. Antibiotics can be used to control these diseases, but not entirely due to drug resistance. However, currently, there is no approved prophylactic vaccine for these infections. Therefore, the development of effective countermeasures to combat these diseases, including vaccines, is of utmost importance.
The attractive outer surface LPS O-antigens of Bp and Bm (76a and 76b, respectively) as targets for the development of vaccines are depicted in Figure 15. Both O-antigens are linear polysaccharides with the backbone composed of a disaccharide repeating unit →3)-6-deoxy-α-l-Tap-(1→3)-β-d-Glc-(1→, with 2-O-acetylation of the talose (Tal) residue. However, their non-reducing end capping disaccharide is distinctive since it contains a unique methylation and acetylation pattern with the terminal Tal residue and this pattern varies from species to species. It has been shown that, in Bm, the terminal Tal residue is 3-O-methylated and 2-O-acetylated, whereas it is also O-4-acetylated in Bp.200
Figure 15.
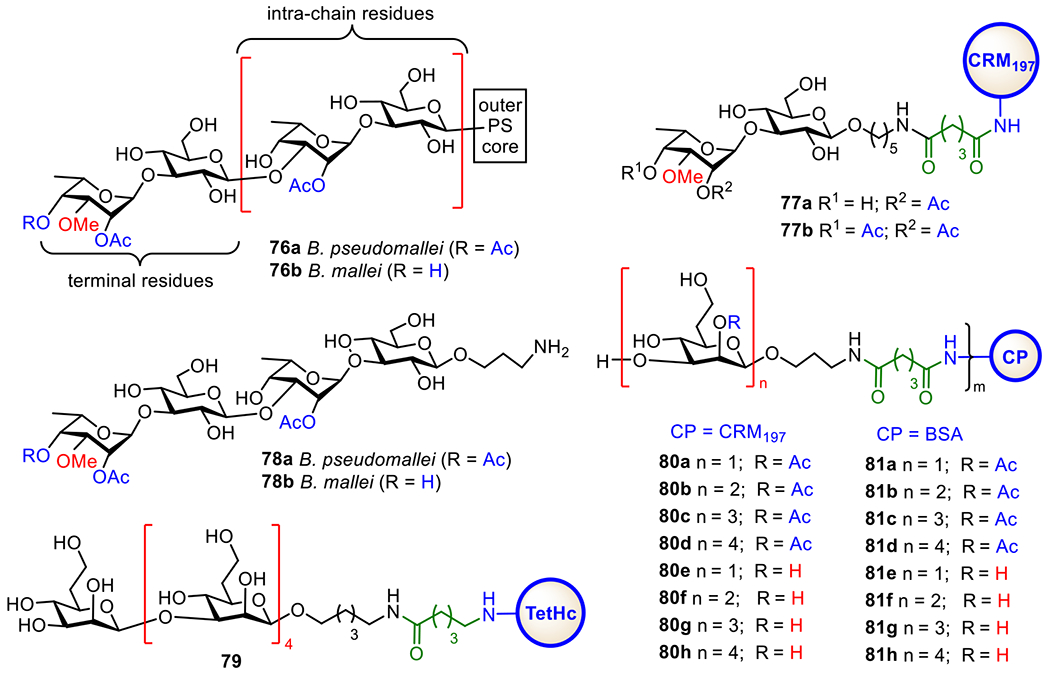
Structures of the type I O-PS 76a and 76b of B. pseudomallei and B. mallei and the protein conjugates 77a-b, 78a-b, 79, 80a-f, and 81a-f of B. pseudomallei and B. mallei CPS oligosaccharides.
To study the significance of O-acetyl and O-methyl modifications for the immunology of Bp and Bm LPSs and for the recognition of Bp and Bm by mAbs obtained with vaccinations, Gauthier et al. synthesized a library of di- and trisaccharides containing minimal structures of all reported acetylation and methylation patterns associated with Bp and Bm LPS O-antigens and coupled them with CRM197 to afford glycoconjugates 77a and 77b (Figure 15).201 They used the synthetic conjugates to characterize the minimal epitopes required for binding with a series of Bp and Bm LPS-specific mAbs, which were passively protective in mice models of melioidosis and glanders. It was demonstrated that Bp and Bm LPS-specific mAbs could bind to the terminal sugar residues of the O-antigens. Furthermore, immunizing mice with conjugate 77a containing a Bm-like disaccharide elicited a high level of antibody response that protected mice from the infection. In contrast, conjugate 77b with a Bp-like disaccharide could not induce a strong antibody response in BALB/c and C57BL/6 mice. Recently, the same group synthesized two larger oligosaccharides, 78a and 78b (Figure 15), comprising both the internal and terminal epitopes of Bp and Bm O-antigens.202 These Bp and Bm-related tetrasaccharides exhibited high reactivity towards the serum of Thai melioidosis patients and the similar antigenicity as native Bp O-antigen. These findings suggested that tetrasaccharides 78a and 78b could mimic the epitopes of Bp and Bm O-antigens and be useful antigens for the development of functional oligosaccharide-based conjugate vaccines against melioidosis and glanders.
On the other hand, Scott et al. synthesized a Bm CPS-associated linear pentasaccharide of β-(1→3)-linked 2-O-acetyl-6-deoxy-d-manno-heptopyranose.203 Its conjugate with the nontoxic Hc-domain of tetanus toxin (HcTT), namely 79 (Figure 15), was shown to elicit IgM and IgG antibodies, which recognized the native CPS and effectively protect against B. pseudomallei (strain K96243) in a mouse model. In addition, recently, Gu et al. developed an efficient strategy to access various oligosaccharide analogs of Bp and Bm CPS in a large scale. Using this strategy, they prepared a series of mono-, di-, tri-, and tetrasaccharides of Bp and Bm O-antigens with and without 2-O-acetylation and coupled these oligosaccharides with CPs to afford glycoconjugates 80a-h and 81a-h.204 The CRM197 conjugates 80a-f were found to elicit robust antigen-specific T-cell dependent IgG antibody responses, demonstrating its desirability as a new vaccine candidate. Comparing the immunological properties of 80a-d and 80e-f containing 2-O-acetylated and deacetylated oligosaccharides, respectively, led to a conclusion that the acetyl groups at the oligosaccharide 2-O-position had a rather small impact on their immunogenicity. The study further reveals that gly coconjugates 80c and 80g are the most immunogenic among all synthetic conjugates and are promising vaccine candidates for BP and BM.
5.10. Cholera vaccine.
Cholera is an acute watery diarrheal disease caused by the pathogenic Gram-negative bacterium Vibrio cholerae.205 Cholera causes widely publicized epidemics and have an especially large burden in sub-Saharan Africa and South Asia countries. Thus, it is a challenge to the public health in many parts of the world.206, 207 According to the World Health Organization (WHO), in 2018, 34 countries reported a total of 1,227,391 cholera cases and 5654 deaths, with case-fatality rate (CFR) of 0.6%.208 Current cholera vaccines are oral, either attenuated or killed whole cell vaccines with or without the cholera toxins B subunit (CtxB).209 Although the existing cholera vaccines are able to control the pandemic of infection but they have limitations. For example, they cannot effectively protect younger populations, the major health tension in many cholera-endemic countries.210 Therefore, it is necessary to develop new and effective vaccines that can provide high-level and long-term immunity.
Over 200 serogroups of V. cholerae have been identified, and toxigenic strains of O1 and O139 are the major causes of epidemic diseases. V. cholerae O1 strain has two serotypes, i.e., Ogawa and Inaba, which are classified based on the structures of their LPS O-antigens, which are linear homopolymers of α-1,2-linked 3-deoxy-l-glycero-tetronamido-d-perosamine with or without a 2-O-methyl group on the non-reducing end perosamine moiety (Figure 16). The Ogawa serotype LPS O-antigen has been exploited for the design and development of cholera O1 vaccines. For example, the Kovac group has been engaged in developing synthetic methods to access the O-antigen oligosaccharides of V. cholera equipped with a squaramide linker.211,212 The BSA conjugates (Figure 16) of these synthetic antigens were immunologically studied to reveal that only the hexasaccharide conjugate 83c showed the vibriocidal humoral response, suggesting that shorter oligosaccharides lacked the required epitope. It has also been found that conjugate 83c with the lowest carbohydrate loading provided a higher protective capability in mice.213 Furthermore, they compared immunologically the P. aeruginosa rEPA conjugates of Ogawa and Inaba serotype O-antigen oligosaccharides and found that the conjugates of Ogawa oligosaccharides boosted Inaba-primed mice, whereas the conjugates of Inaba oligosaccharides could not boost Ogawa-primed mice. This result implies that Ogawa and Inaba LPSs contain different immunodominant epitopes.214
Figure 16.
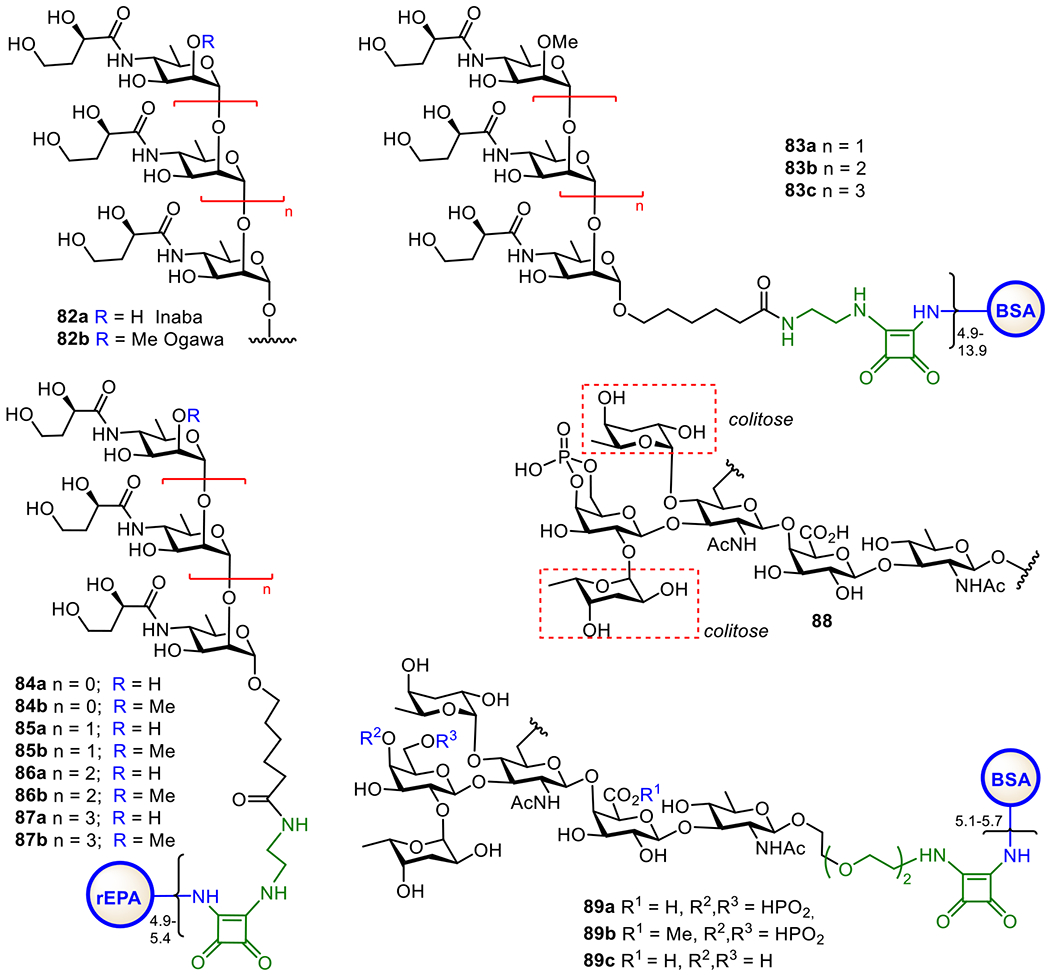
Structures of the O-antigens 82a and 82b of Vibrio cholerae O1 Inaba and Ogawa serotypes, respectively, the BSA-Ogawa serotype oligosaccharide conjugates 83a-c, and rEPA-Inaba and Ogawa serotype oligosaccharide 84-87a,b, as well as structures of the V. cholerae O139 O-antigen 88 and its protein conjugates 99a-c.
The first emergence of a new V. cholerae serotype O139 was noticed in the southern part of India in 1992 and becomes the non-O1 V. cholera serotype to cause epidemic cholera.215 V. cholerae O139 possess a high molecular weight CPS, in distinctive composition from the LPS antigen.216 This O139 CPS consists of uniquely branched hexasaccharide 88 (Figure 16), which features two units of a rare deoxysugar, 3,6-dideoxy-l-xylohexose (colitose), and a 4,6-cyclic phosphate group on the Gal unit. The Kovác group completed the first chemical synthesis of the fully protected oligosaccharide that is equipped with linker, making it ready for conjugation to CPs.217 Later on, an alternative synthesis of the hexasaccharide of O139 antigen and its ester form was reported, which were then coupled with BSA via squaric acid chemistry to generate glycoconjugates 89a-c that were immunologically investigated.218
5.11. Tuberculosis vaccine.
Tuberculosis (TB) is a contagious, fatal, and devastating disease caused by bacillus Mycobacterium tuberculosis (Mtb). It is one of the leading causes of human death. The Global Tuberculosis Report 2021 states that a total of 1.5 million people died from TB in 2020 worldwide.219 For TB treatment, antibiotic drugs, such as rifampin, pyrazinamide etc., have been proven to be effective, but prolonged regimens of multiple antibiotic treatments result in multiple-drug resistance.220 Moreover, TB and HIV co-infection is a huge problem.221 Therefore, TB is an important burden of the health care system worldwide.
To control TB, a safe and effective vaccine is highly desired. Currently, the most broadly used vaccine for the protection against TB, especially in children, is Bacilli Calmette-Guerin (BCG). However, the efficacy of this vaccine is wildly variable (from 0 to 80%).222 Therefore, other approaches have also been explored for vaccine development. For example, attenuated auxotrophic strains of M. tuberculosis,223 DNA vaccines,224, 225 and sub-cellular (protein and peptide antigens) vaccines,226 have been investigated. Recent advancement in glycomics and accumulated information on the carbohydrate antigens of M. tuberculosis bacilli allow a new view on the development of carbohydrate-based anti-TB vaccines.
The cell envelope of M. tuberculosis consists of mainly three distinguished components, the plasma membrane, the cell wall core, and the outer layer, while its outer surface is covered by a thick layer of complex carbohydrates.227,228 Within the bacterial wall envelope, mycolic-arabinogalactan (mAG) complexes and lipoarabinomannan (LAM)-related glycolipids are the two major constituents,229 which protect the bacteria and assist their survival.230 Furthermore, LAMs are also implicated in the immunogenicity of M. tuberculosis and have been shown to inhibit T-cell activation231 and various microbicidal activity.232 Therefore, LAMs are excellent targets for vaccine development.
As shown in Figure 17, LAMs contain a phosphatidyl myo-inositol (PI) anchor that is non-covalently associated with the plasma membrane of M. tuberculosis.229 The PI unit is extended at the inositol 2-O-position with an α-mannose (Man) residue to generate phosphatidylinositol mannose known as PIM1. Adding another Man unit to the inositol 6-O-position of PIM1 leads to PIM2, and adding more α1,6-linked Man residues to the 6-O-Man of PIM2 gives PIM3 and PIM4. Further extension of the sugar chain from PIM4 is diverse. For examples, a-1,2-linked Man residues are added to PIM4 to form PIM5 and PIM6. PIM6 and more complex PIMs serve as key intermediates for the biosynthesis of polysaccharides, such as LAMs and ManLAMs, through a series of enzymatic mannosylation and arabinosylation.233, 234 The exact structures of the mannan and arabinan polymer of the LAM backbone still remain unclear but the glycan structures of PIMs are well characterized.235, 236 Earlier studies have confirmed the crucial role of α-1,3, α-1,5, and β-1,2-linked oligoarabinofuranosyl motifs of LAMs in the immunological events related to mycobacterial infections.237–239 Owing to the interesting biological properties of LAMs, significant efforts have been devoted to the synthesis of related structures, such as mycobacterial PIMs, lipomannan (LMs), and LAM.240,241
Figure 17.
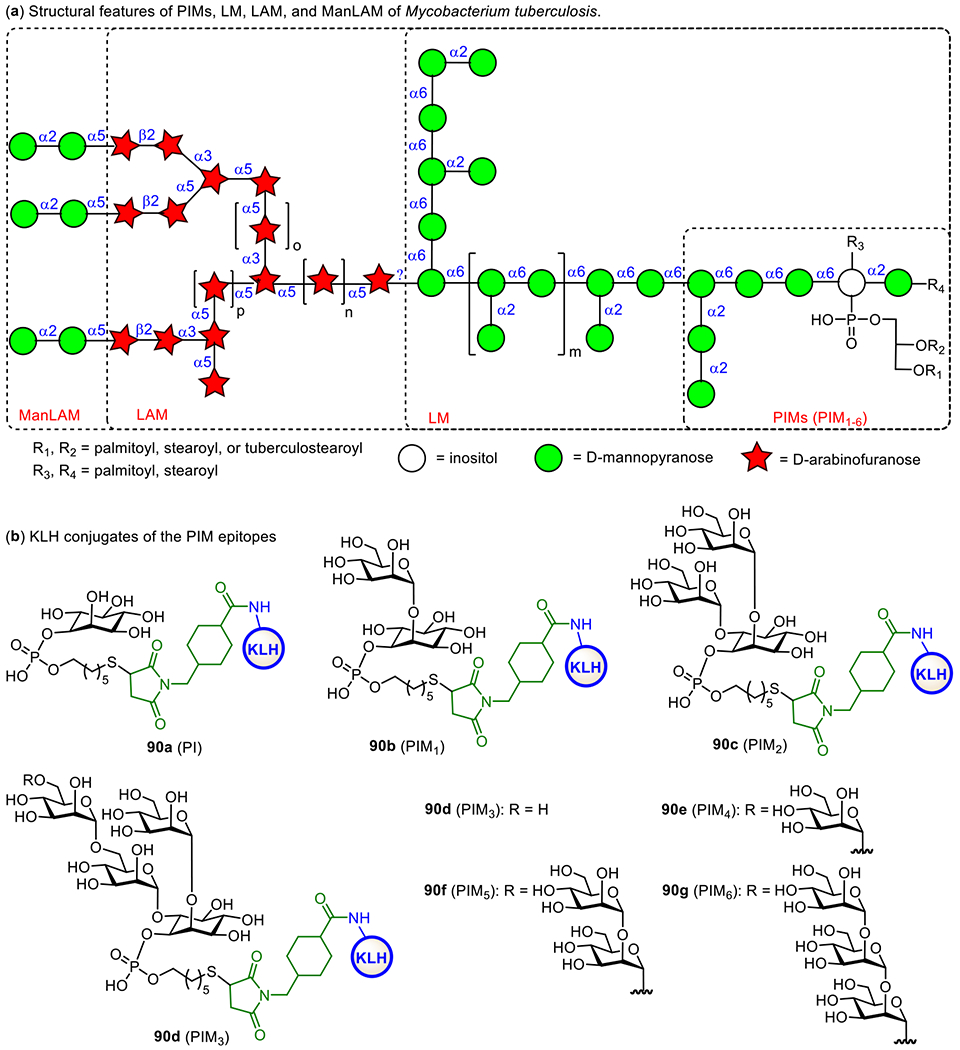
Structures of mycobacterial LAMs and related molecules (a) and the KLH conjugates 90a-f of synthetic PIM derivatives (b).
Here, we will only cover the synthetic studies on LAM-related oligosaccharides and their conjugates that have been subjected to immunological studies as TB vaccines. The Seeberger group has chemically synthesized several PIM derivatives and investigated their activities as potential adjuvants or antigens for vaccine development.242 All of the synthetic PIMs contained a thiol group that enabled their immobilization onto microarray chips and/or conjugation with carrier molecules. Conjugation of the synthesized oligosaccharides with KLH through the thio-linker gave conjugates 90b-g (Figure 17). Biological studies showed that PIM5 and PIM6 has the highest binding affinity to dendritic cell-specific intercellular adhesion molecule-grabbing nonintegrin (DC-SIGN) receptor. Moreover, the PIM-KLH conjugates were proved as strong immunostimulators during the immunization of mice. PIM6 conjugate 90g induced a marked increase in anti-KLH antibodies compared to KLH alone. The adjuvant property of PIM6 was also confirmed by measurement of the cytokine production.
To facilitate development of LAM-based TB vaccines, Guo and Gu established an efficient method to synthesize LAM arabinofuranosyl oligosaccharides, and then conjugated them with BSA and KLH via a dicarboxyl linker (Figure 18).243 The resultant glycoconjugates 91-93a were immunologically evaluated with conjugates 91-93b as coating antigens. It was found that all the synthetic glycoconjugates could elicit carbohydrate antigen-specific immune responses. Remarkably, 92a induced higher antibody titers than 91a and 93a. Overall, these studies have validated that LAM oligosaccharides are useful epitopes for TB vaccine development.
Figure 18.
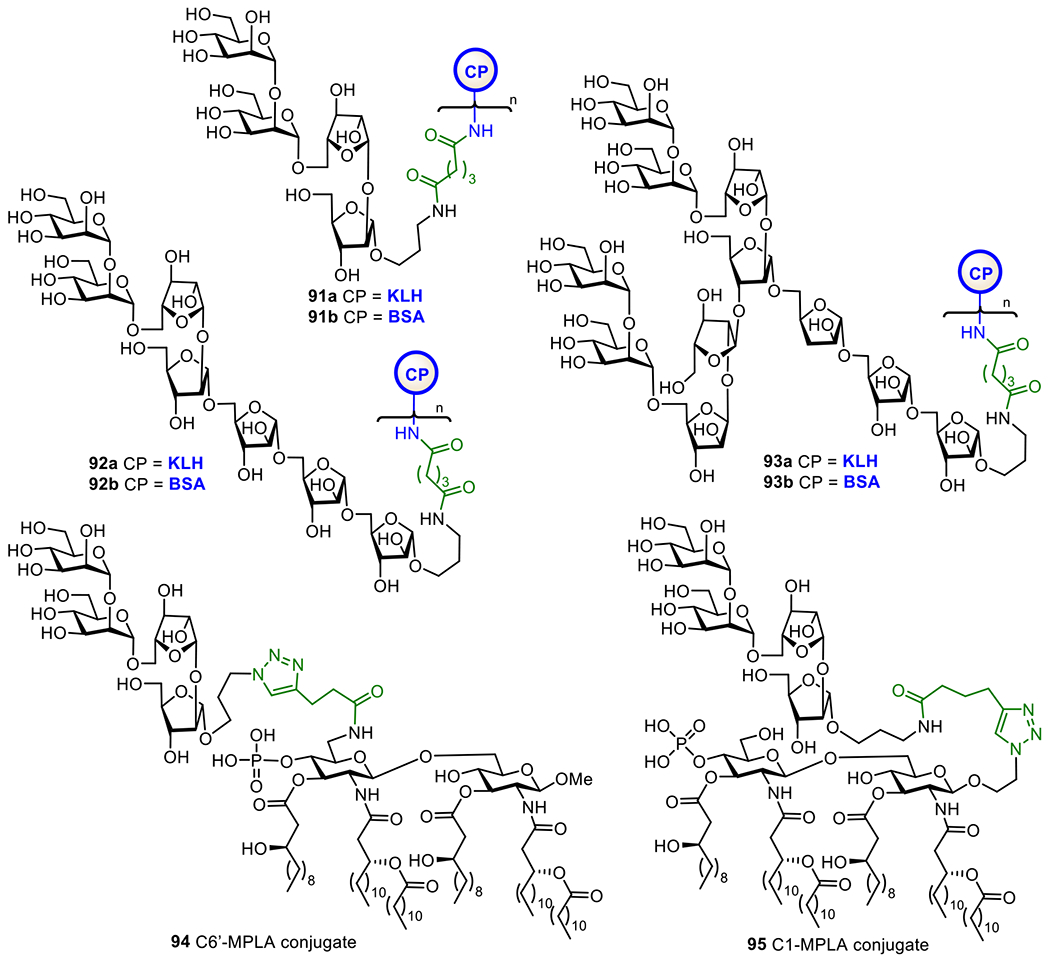
Structures of KLH and BSA glycoconjugates 91-93a and 91-93b of the synthetic LAM oligosaccharides and MPLA conjugates 94 and 95 of LAM tetrasaccharide
The Guo and Gu group also conjugated a LAM tetrasaccharide antigen with MPLA at its 6’-O- and 1-O-positions to get conjugates 94 and 95, respectively (Figure 18), as fully synthetic glycoconjugate vaccines. They anticipated these conjugates to have self-adjuvanting activity, and immunological comparison of the two conjugates was expected to also reveal the impact of conjugation sites to MPLA on the immunological properties resultant conjugate vaccines.244 It was found that both conjugates induced strong antibody responses against LAM compared to the mixture of tetrasaccharide and MPLA. Furthermore, 6’-N-linked MPLA-conjugate 94 induced significantly higher IgG titer than corresponding 1-O-linked MPLA-glycoconjugate 95. The outcome of this study proved the vital role of MPLA as a vaccine carrier molecule and as a self-adjuvant and the influence of linkage site between carbohydrate antigen and MPLA on their immunological properties.
In addition to vaccine development, early and easy diagnosis of TB is also a curial step in the control of TB spread. This relies on the specificity and sensitivity of tests. Previous studies on TB diagnosis have shown that anti-LAM IgG antibodies are present in most patients and LAM-related arabinofuranosyl oligosaccharides can selectively bind to anti-LAM antibodies. Therefore, anti-LAM antibodies are considered as useful tools for TB diagnosis.245 As a result, several groups have synthesized protein conjugates of LAM oligosaccharide to investigate their application to TB diagnosis.246
For example, the Kononov group synthesized two LAM arabinofuranosyl hexasaccharide conjugates 96a and 96b (Figure 19) employing MPB-64 and Rv0934 recombinant protein as carriers, respectively, and examined them as antigens for serological assays of TB.246 These conjugates bound to the targeted antibodies. A series of α-linked linear oligoarabinofuranosides with a 4-(2-chloroethoxy)phenyl aglycon were also synthesized and coupled with BSA to get glycoconjugates 97a-c, which are useful for biological study and application in TB diagnosis and vaccine development.247 The Bundle group has developed the biocompatible Copovidone polymer as a non-protein carrier for conjugation with carbohydrate epitopes and investigation of antibody binding.248 It was discovered that, the polymer-based glycoconjugate 96d showed similar binding properties as BSA conjugate 96c. Some important features of the copovindone conjugates are that the glycan is stable to acid and the assay plate can be reused up to 3 times without any degradation in quality. In addition, Derda et al. linked this LAM hexasaccharide to several peptide carriers derived from phage display library.249 The resultant glycoconjugates 96e-h were utilized as ligands for selective detection of anti-LAM antibodies generated from mycobacterial infection. This study has demonstrated the potential of structurally defined LAM oligosaccharide-peptide conjugates as diagnostic reagents for active and latent tuberculosis.
Figure 19:
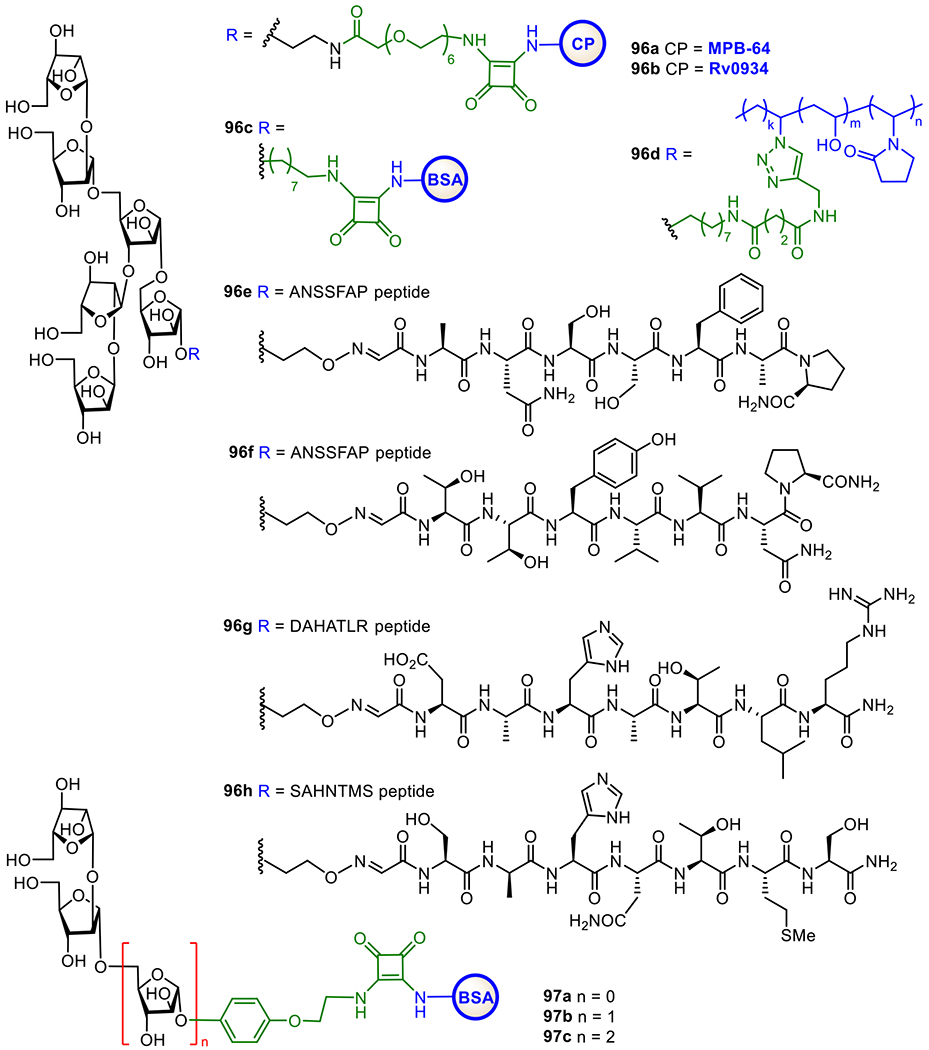
LAM-related arabinofuranosyl oligosaccharides conjugates 96a-h and 97a-c studied as TB diagnostic agents.
5.12. Group A streptococcus vaccine.
Group A streptococcus (GAS) is a Gram-positive bacterium that can cause a wide spectrum of clinical syndromes.250 The severe infections range from pharyngitis, cellulitis, and pyoderma to necrotizing fasciitis, sepsis, pneumonia, and streptococcal toxic shock syndrome.251 Annually, over half a million people die from GAS infections.252 The economic burden from these infections in both developed and developing countries is significant. GAS infections are usually treated with antibiotics, but drug resistance has become a major issue. To develop GAS vaccines, many virulent components of GAS have been identified as potential targets. Among them, the cell wall M protein is one of the leading candidates and several M-protein vaccine candidates have entered clinical trials.253 However, a major concern about these vaccines is immunological safety due to the cross-immune reaction with human tissues.254, 255 Therefore, other GAS cell surface components, such as other surface proteins like C5a peptidase,256, 257 toxins and polysaccharides,56, 258 have been considered as attractive alternatives for GAS vaccine development.
One of the major polysaccharides on the cell surface of GAS is 98 (Figure 20), composed of a branched trisaccharide repeating unit. The Pinto group has explored the potential of this polysaccharide for vaccine development. Accordingly, they prepared several oligosaccharides and linked them to CPs to get glycoconjugates that were evaluated as GAS vaccines.259 Their results indicated that the branch and size of the oligosaccharide antigens are central elements of the epitope recognized by both rabbit polyclonal and mouse monoclonal antibodies that bind to native polysaccharide antigens.259–262 Moreover, synthetic hexasaccharide-TT conjugate 99 containing in average 30 hexasaccharide haptens per TT molecule elicited specific antibodies in mice and response surge with booster immunization, suggesting immunological memory.263 This study validated glycoconjugate 99 as a promising GAS vaccine candidate.
Figure 20.
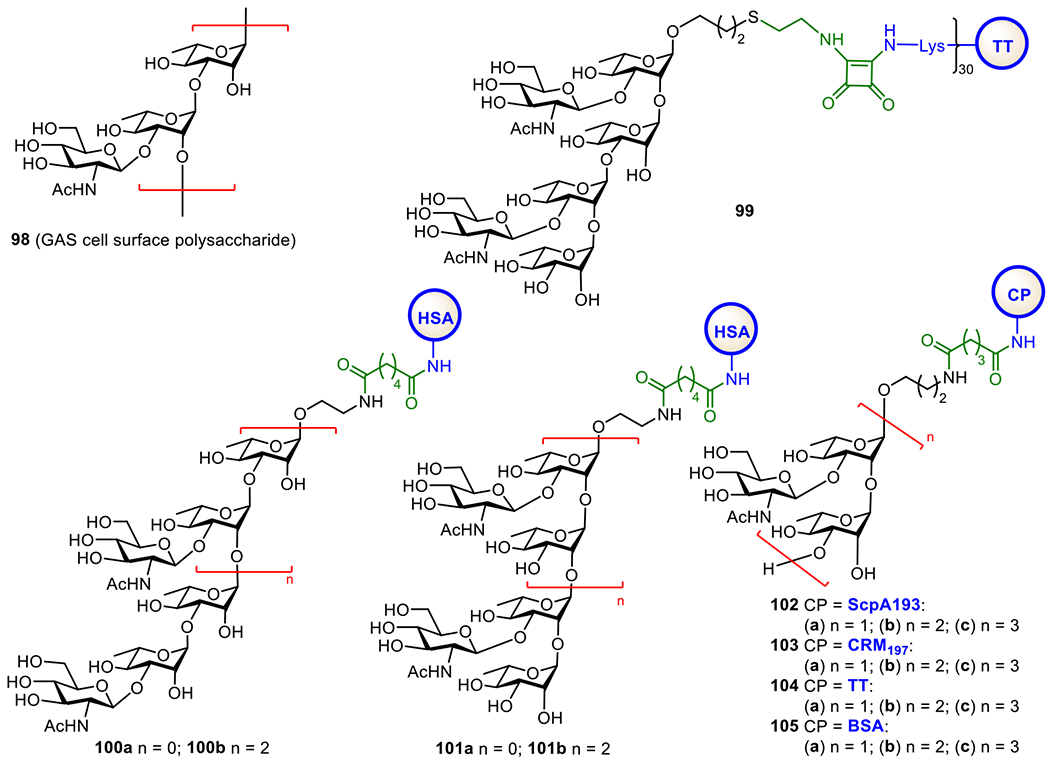
Structures of the conserved GAS cell wall polysaccharide 101 and GAS oligosaccharide–protein conjugates 102-108 investigated as anti-GAS vaccines.
Costantino and co-workers264 synthesized a series of oligosaccharides of this cell wall polysaccharide of different chain lengths (hexa- vs nonasaccharides) and non-reducing end terminal sequences, and coupled them with CRM197 to get conjugates 100a,b and 101a,b (Figure 20). The immunoprotective efficacies of these semi-synthetic conjugate vaccines were evaluated by in vitro opspnophagocytosis assays. It was disclosed that conjugates 100 and 101 displayed equal protections in mice. Their studies further indicated the potential of oligosaccharide-based anti-GAS conjugate vaccines.
The Guo and Gu group69, 265 designed and synthesized a series of GAS oligosaccharide-protein conjugates 102-104a-c (Figure 20) to systematically examine the structure-activity relationships of GAS oligosaccharide-based conjugate vaccines. The streptococcal C5a peptidase (ScpA)-193 conjugates 102a-c of GAS oligosaccharides were specially designed as bivalent vaccines to boost immune responses against both the cell wall polysaccharide and ScpA. Immunological studies in mice revealed that Scp193 conjugates 102a-c elicited robust antibody responses specific to not only the carbohydrate but also ScpA, suggesting ScpA as a promising target antigen for the development of anti-GAS vaccines. Their results indicate that nanasaccharide-ScpA193 conjugate 102c is a hopeful GAS vaccine candidate. In addition, the CRM197 conjugates 103a-c and the TT conjugates 104a-c induced even higher titers of antibodies, suggesting the influence of CPs on the immunological properties of glycoconjugate vaccines.
5.13. Vaccine against Group B streptococcus:
Since the early 1970’s, group B Streptococcus (GBS), a Gram-positive bacterium, has been one of the major concerns of bacterial infections in neonates and pregnant women. It causes many severe diseases, such as sepsis, meningitides, pneumonia, abortion, etc.,266–268 and S. agalactiae is one of the strains mainly responsible for morbidity.269, 270 Globally, about 18% of pregnant women are colonized by GBS,271 which has remarkably increased the infection risk of pregnant women and babies. In 2015, GBS-caused diseases affected 319,000 newborns and resulted in 90,000 deaths, as well as up to 3.5 million preterm births and around 57,000 fetality.272 The preventive measures aimed to reduce the risk of invasive GBS diseases in infants have been focused on intrapartum antibiotic prophylaxis (IAP).273 However, IAP is effective for early-onset GBS diseases but cannot prevent late-onset GBS diseases. Consequently, effective vaccines against GBS are highly desirable.
GBS isolates from humans express unique CPSs as their major virulence factors to support their evasion of the host defense mechanisms.274 Based on the structures of their specific CPSs, GBS is classified into ten serotypes, including Ia, Ib, II, III, IV, V, VI, VII, VIII, and IX (Figure 21).275,276 Meta-analysis data revealed that five serotypes, including Ia, Ib, II, III and V, account for the vast majority of GBS infections.277 Most GBS CPSs are composed of Glc, Gal, GlcNAc, and Neu5Ac, which are differently arranged with diverse branches that are typically terminated with a Neu5Ac residue. These structurally unique CPSs can be useful immunotargets for the development of GBS vaccines. However, the oligosaccharide repeating units of GBS CPSs, especially their densely branched oligomers, are challenging synthetic targets. Therefore, many research groups have been focused on the synthesis of GBS oligosaccharides,39 as well as their application to vaccine developmemt.268, 273, 277, 278 This is a huge subject, hence we have to limit our discussion on only a few examples to demonstrate the recent development in the synthesis and immunological evaluations of oligosaccharide-based conjugate vaccines against GBS.
Figure 21.
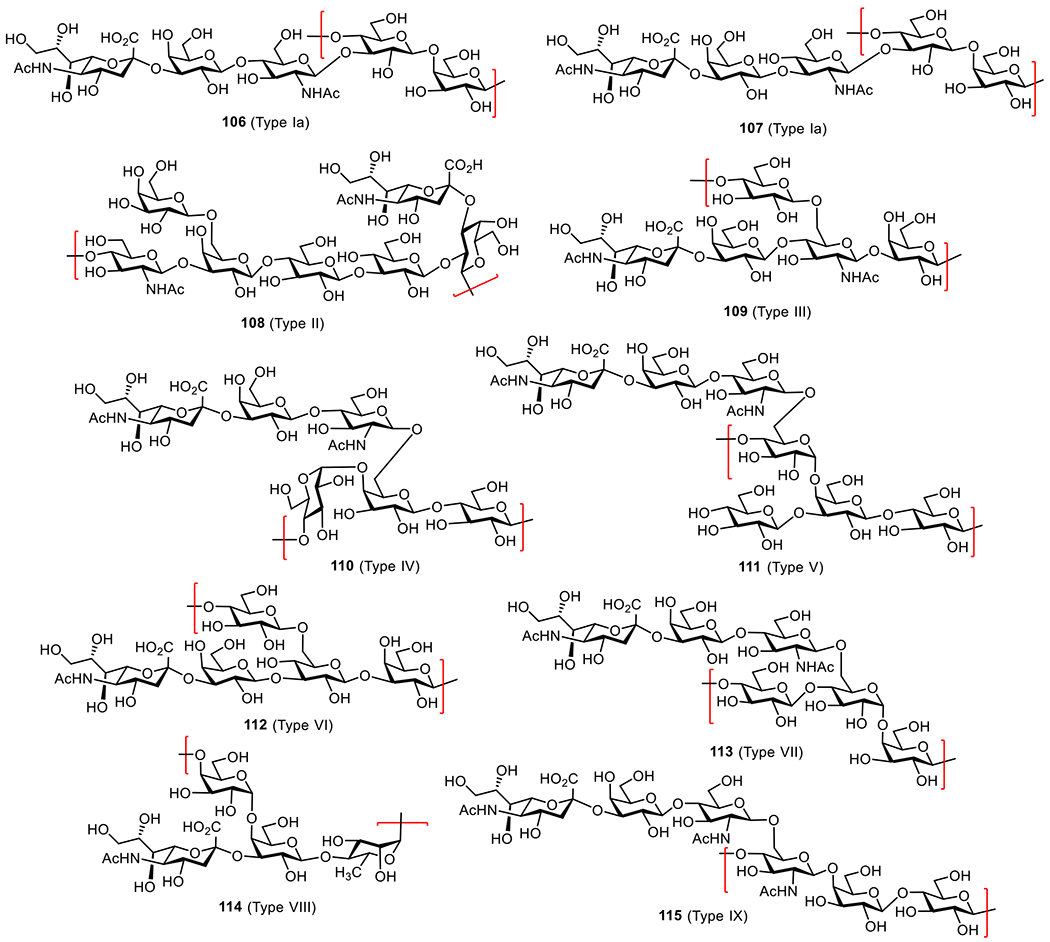
Structures of the conserved CPSs on the cell surface of GBS serotypes Ia, Ib, II, III, IV, V, VI, VII, VIII, and IX.
As depicted in Figure 22, Adamo et al. synthesized glycoconjugates 116-119 containing oligosaccharides of different lengths and frameshifts of the repeating units of GBS serotype III CPS, with CRM197 as the CP.279 Both glycoconjugates 117 and 119, but not 116, could bind to polyclonal antibodies derived from anti-GBS serotype III murine sera. This result suggests that the branching motif β-linked to the GlcNAc 6-O-position is important for antibody recognition. STD-NMR and X-ray studies further implicate that a hexasaccharide (shown in 119) spanning two repeating units of the native CPS is responsible for interactions with a protective mAb.280 Studies on CRM197-conjugates carrying 2 to 6 repeating units of GBS CPS III, obtained from degradation of natural polysaccharides, suggest that an oligosaccharide containing 2 repeating units is enough to represent the antigenic epitope of GBS CPS III.281 Moreover, hexasaccharide conjugate 119 was able to elicit functional immune responses comparable to that induced by the native CPS conjugates. These results have confirmed that the hexasaccharide is the minimal GBS III CPS epitope as a functional antigen for synthetic conjugate vaccine development.282 In addition, they found that the oligosaccharide loading degree of glycoconjugate vaccines also has a significant impact on their immunological properties, and a high loading is required to elicit robust immune responses probably due to multivalent effects.281
Figure 22.
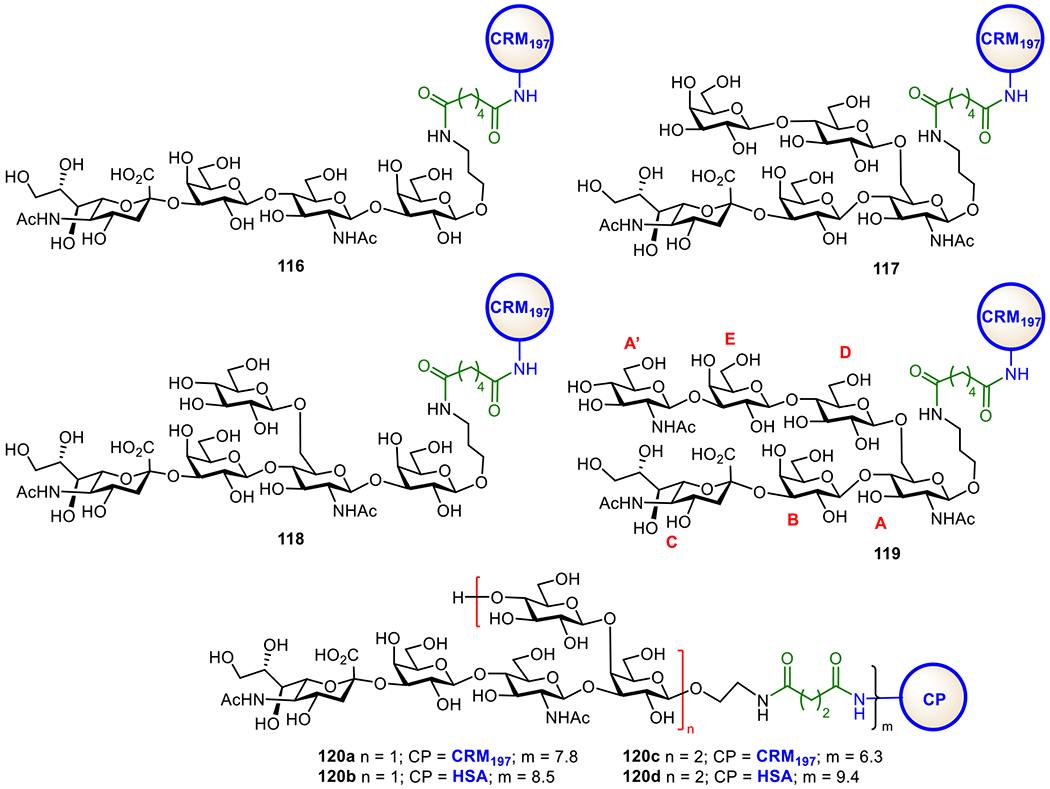
Structure of serotype III GBS oligosaccharide-CRM197 conjugates 116-119 and serotype Ia GBS oligosaccharide-protein conjugates 120a-d.
Recently, the Guo group reported a chemical synthesis of the pentasaccharide repeating unit of GBS serotype Ia CPS,283 as well as a decasaccharide representing a dimer of the repeating units of GBS Ia CPS. Subsequently, the synthetic oligosaccharides, both monomer and dimer, were conjugated with CRM197 and HSA to generate glycoconjugates 120a-d (Figure 22). The CRM197 conjugates 120 and 120c were immunologically investigated in mice, both induced robust IgG and IgM antibody responses. In addition, the antibodies elicited by these conjugates were cross-reactive, suggesting the potential of the synthetic oligosaccharides as antigens for vaccine development.
The cell glycocalyx of GBS is complex. Another unique and group-specific polysaccharide on GBS cell surface shared by all serotypes is comprised of a rhamnose-rich oligosaccharide interspaced with a glucitol phosphate.284, 285 Each repeating unit is made up of five L-rhamnose, one D-N-acetylglucosamine, one D-galactose, and one D-glucitol residues (Figure 23). The repeating units are linked through a phosphate diester group between the glucitol residue and the 6-O-position of the galactose residue. This unique polysaccharide plays a key role in GBS cell growth and immunological property. However, this polysaccharide is difficult to isolate from natural sources in homogeneous form and sufficient quantity, which hinders its potential application.286, 287 Recently, the Codee group reported the synthesis of several oligosaccharides of this polysaccharide and coupled them with CPs to generate conjugates 121-123a,b (Figure 23).288 CRM197 conjugates 121-123a were immunologically evaluated, and the results showed that each conjugate could elicit a specific immune response and the induced antibodies were cross-reactive with the other oligosaccharides. Immunization of mice with conjugate 123a that contained a large oligosaccharide elicited antibodies that recognized and bound the bacterium. It was also demonstrated that the glycoconjugates elicited functional immune responses against different GBS serotypes. Therefore, these oligosaccharides are identified as promising antigens for the development of multivalent vaccines against all clinically relevant GBS serotypes such as Ia, Ib, II, III, IV, and V.
Figure 23.
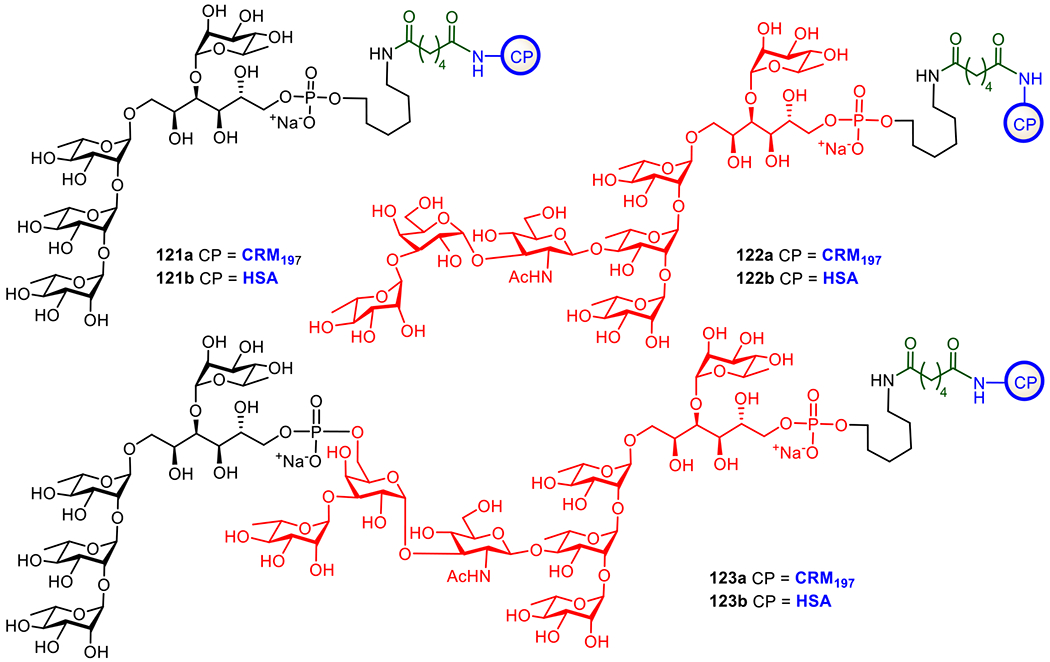
Structures of the protein conjugates of oligosaccharide epitopes of a group-specific cell surface polysaccharide shared by different GBS serotypes.
6. Concluding Remarks
Prophylactic vaccines are useful tools to control infectious diseases and prepare people against epidemic situations.289 In history, vaccines have helped successively restrain and exterminate many fatal infectious diseases, such as smallpox, polio, rubella, etc.290 The power of vaccine has been demonstrated again during the recent pandemic of COVID-19. Therefore, vaccination is considered the most effective and cost-efficient platform against infectious diseases. Among various technologies for vaccine development, glycoconjugate vaccines are attractive because carbohydrates as antigens are abundant and exposed on the cell surface for the recognition by the host immune system, highly conserved on bacterial cells, and essential virulence factors for bacterial survival. In this regard, the unique polysaccharides on the bacterial cell surface are particularly useful. As a result, many bacterial polysaccharides and their protein conjugates have been successfully developed into vaccines and used in clinic to control various bacterial infections. However, as discussed earlier, these vaccines still have their limitations, and hence conjugate vaccines based on oligosaccharide analogues of bacterial polysaccharides have been actively pursued in the past decades. Some of the oligosaccharide-based vaccines have been approved for clinical use and more are in clinical trial stages.
Despite these great advancements, there are still a number of hurdles remaining in the field. Particularly, to design and develop effective glycoconjugate vaccines, it is necessary to know the structural epitopes of bacterial polysaccharides required for inducing protective immune responses to targeted pathogens. This is not easily achievable as it needs extensive structural studies and structure-activity relationship analysis of the polysaccharide antigens. In addition, the immunology and the exact functional mechanisms of carbohydrate-based vaccines are still poorly understood. Another problem is related to the synthesis of complex oligosaccharides, especially oligomers of the repeating units of bacterial polysaccharides, which remains challenging and therefore has hindered the access to these molecules in large quantity and to their derivatives. Furthermore, the carrier molecules for conjugate vaccines also need new alternatives during the development of glycoconjugate vaccines because repeated uses of the same CPs in various glycoconjugates can cause concerns, such as immunotolerance and decreased efficiency of vaccines.
On the other hand, we believe that, currently, the rapid and continuous progress and expansion of new methods for carbohydrate synthesis, technologies for structural study of carbohydrates, and in-depth understanding of the interactions between carbohydrate antigens and antibodies or the host immune system and of the functional mechanisms of carbohydrate-based vaccines, will provide more effective platforms for the design of new vaccines, e.g., through rational design, and speed up the research and development process and result in more and efficacious vaccines. In the meantime, new biotechnologies are emerging as attractive alternatives to the currently chemical strategies for more flexible and cost-effective vaccine production.291 For example, new enzymatic strategies for protein glycosylation have been employed to access glycoconjugates. One of the strategies exploited oligosaccharyltransferase-dependent N-linked glycosylation process to produce glycoconjugate vaccines against bacterial pathogens and humanized glycoproteins. A recent application of this highly innovative glycoengineering method is to produce a Shigella dysenteriae O1 conjugate vaccine from E. coli,292 and the vaccine has cleared Phase 1 clinical trials. Vaccines against other pathogens, e.g., Francisella tularensis, Staphylococcus aureus, etc. are currently under development.293,294 Although the bacterial glycosylation systems are attractive and have advantage over chemical methods, there are still some technical limitations associated with it, e.g., (i) the assignment of a glycosylation sequon to a particular site within the target protein is sometimes difficult, (ii) complications were observed while working with mammalian cell lines; (iii) the products generated by this approach has the concern of spreading viral diseases. Therefore, more glycosylation systems remain to be explored. Nonetheless, the new technologies will promote vaccine development and help fight various infections that are threatening human life and further reduce the social and economic burdens caused by infectious diseases, thereby strengthening the health and welfare of humankind.
Finally, we want to point out that the topic that this article tries to review is very broad, and the research area has been developing rapidly in the past few decades and is still growing. For example, in the process of preparing this article, Pereira and co-workers reported the synthesis and evaluation of some new glycoconjugate vaccine candidates for Escherichia coli O25B.295 Therefore, it is challenging to cover the conjugate vaccines against all bacterial pathogens, and we choose to review only some common bacteria and their vaccine development. Furthermore, even among the few bacterial pathogens selected, it is impossible to include all the researches related to glycoconjugate vaccine development. Here, we try to include the reports that contain biological evaluation of the conjugates as many as we can but still, some of the excellent works may be uncited. Additionally, in the literature, there are many excellent synthetic works about bacterial oligosaccharides and their conjugates that are included herein due to the limited scope of this article. We sincerely apologize for all those authors.
Acknolwedgement
Our research on carbohydrate-based vaccines and adjuvants is supported by NIH/NIAID (R21 AI170129). ZG is also grateful to Steven and Rebecca Scott for the endowment of our research.
References
- (1).Doron S; Gorbach SL Bacterial Infections: Overview. In International Encyclopedia of Public Health, Heggenhougen HK Ed.; Academic Press, 2008; pp 273–282. [Google Scholar]
- (2).Baicus A History of polio vaccination. World J. Virol 2012, 1 (4), 108–114. DOI: 10.5501/wjv.v1.i4.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Smallpox overview. 2004, Pamphlet (or booklet).
- (4).Hilleman MR Vaccines in historic evolution and perspective: A narrative of vaccine discoveries. Vaccine 2000, 18 (15), 1436–1447. DOI: 10.1016/s0264-410x(99)00434-x. [DOI] [PubMed] [Google Scholar]
- (5).Khoshnood S; Arshadi M; Akrami S; Koupaei M; Ghahramanpour H; Shariati A; Sadeghifard N; Heidary M An overview on inactivated and live-attenuated SARS-CoV-2 vaccines. J. Clin. Lab. Anal 2022, 36 (5), e24418. DOI: 10.1002/jcla.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Pollard AJ; Bijker EM A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol 2021, 21 (2), 83–100. DOI: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Bill RM Recombinant protein subunit vaccine synthesis in microbes: a role for yeast? J. Pharm. Pharmacol 2014, 67 (3), 319–328. DOI: 10.1111/jphp.12353. [DOI] [PubMed] [Google Scholar]
- (8).Heidary M; Kaviar VH; Shirani M; Ghanavati R; Motahar M; Sholeh M; Ghahramanpour H; Khoshnood S A comprehensive review of the protein subunit vaccines against COVID-19. Front. Microbio 2022, 13, 927306. DOI: 10.3389/fmicb.2022.927306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Moyle PM; Toth I Modern subunit vaccines: Development, components, and research opportunities. ChemMedChem 2013, 8 (3), 360–376. DOI: 10.1002/cmdc.201200487. [DOI] [PubMed] [Google Scholar]
- (10).Leitner WW; Ying H; Restifo NP DNA and RNA-based vaccines: Principles, progress and prospects. Vaccine 1999, 18 (9), 765–777. DOI: 10.1016/S0264-410X(99)00271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Pardi N; Hogan MJ; Porter FW; Weissman D mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discovery 2018, 17 (4), 261–279. DOI: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Jawalagatti V; Kirthika P; Lee JH Oral mRNA vaccines against infectious diseases-A bacterial perspective. Front. Immunol 2022, 13, 884862. DOI: 10.3389/fimmu.2022.884862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Heidelberger M; Avery OT The soluble specific substance of pneumococcus. J. Exp. Med 1923, 38 (1), 73–79. DOI: 10.1084/jem.38.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Heidelberger M; Avery OT The soluble specific substance of pneumococcus: Second paper. J. Exp. Med 1924, 40 (3), 301–317. DOI: 10.1084/jem.40.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Dubos R; Avery OT Decomposition of the capsular polysaccharide of pneumococcus Type I (ii) by a bacterial enzyme. J. Exp. Med 1931, 54 (1), 51–71. DOI: 10.1084/jem.54.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Avery OT; Dubos R The protective action of a specific enzyme against Type I (ii) pneumococcus infection in mice. J. Exp. Med 1931, 54 (1), 73–89. DOI: 10.1084/jem.54.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Francis T; Tillett WS Cutaneous reactions in pneumonia. The development of antibodies following the intradermal injection of type-specific polysaccharide. J. Exp. Med 1930, 52 (4), 573–585. DOI: 10.1084/jem.52.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Finland M; Ruegsegger JM Immunization of human subjects with the specific carbohydrates of type-III and the related type VIII pneumococcus. J. Clin. Invest 1935, 14 (6), 829–832. DOI: 10.1172/jci100731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Macleod CM; Hodges RG; Heidelberger M; Bernhard WG Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J. Exp. Med 1945, 82 (6), 445–465. [PMC free article] [PubMed] [Google Scholar]
- (20).Merck & Co., Inc. Pneumovax 23 (pneumococcal vaccine polyvalent). Merck website:. 2021. https://www.merck.com/news/cdc-acip-unanimously-votes-to-provisionally-recommend-mercks-vaxneuvance-pneumococcal-15-valent-conjugate-vaccine-in-series-with-pneumovax-23-pneumococcal-vaccine-polyvalen/
- (21).Robbins JB; Austrian R; Lee CJ; Rastogi SC; Schiffman G; Henrichsen J; Mäkelä PH; Broome CV; Facklam RR; Tiesjema RH; Parke JC Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J. Infect. Dis 1983, 148 (6), 1136–1159. DOI: 10.1093/infdis/148.6.1136. [DOI] [PubMed] [Google Scholar]
- (22).Cobb BA; Wang Q; Tzianabos AO; Kasper DL Polysaccharide processing and presentation by the MHCII pathway. Cell 2004, 117 (5), 677–687. DOI: 10.1016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Nishat S; Andreana PR Entirely carbohydrate-based vaccines: An emerging field for specific and selective immune responses. Vaccines 2016, 4 (2), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Sun L; Middleton DR; Wantuch PL; Ozdilek A; Avci FY Carbohydrates as T-cell antigens with implications in health and disease. Glycobiology 2016, 26 (10), 1029–1040. DOI: 10.1093/glycob/cww062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Kaiser J Synthetic vaccine is a sweet victory for Cuban science. Science 2004, 305 (5683), 460–460. DOI: doi: 10.1126/science.305.5683.460. [DOI] [PubMed] [Google Scholar]
- (26).Jones C The Regulatory Framework for Glycoconjugate Vaccines. In Carbohydrate-Based Vaccines, ACS Symposium Series, Vol. 989; American Chemical Society, 2008; pp 21–35. [Google Scholar]
- (27).Buskas T; Thompson P; Boons G-J Semisynthetic and Fully Synthetic Carbohydrate-Based Cancer Vaccines. In Carbohydrate-Based Vaccines and Immunotherapies, 2009; pp 263–311. [Google Scholar]
- (28).Zou W; Jennings HJ Preparation of Glycoconjugate Vaccines. In Carbohydrate-Based Vaccines and Immunotherapies, 2009; pp 55–88. [Google Scholar]
- (29).Micoli F; Del Bino L; Alfini R; Carboni F; Romano MR; Adamo R Glycoconjugate vaccines: Current approaches towards faster vaccine design. Expert Rev. Vaccines 2019, 18 (9), 881–895. DOI: 10.1080/14760584.2019.1657012. [DOI] [PubMed] [Google Scholar]
- (30).Soliman C; Pier GB; Ramsland PA Antibody recognition of bacterial surfaces and extracellular polysaccharides. Curr. Opin. Struct. Biol 2020, 62, 48–55. DOI: 10.1016/j.sbi.2019.12.001. [DOI] [PubMed] [Google Scholar]
- (31).Anish C; Schumann B; Pereira CL; Seeberger PH Chemical biology approaches to designing defined carbohydrate vaccines. Chem Biol. 2014, 21 (1), 38–50. DOI: 10.1016/j.chembiol.2014.01.002. [DOI] [PubMed] [Google Scholar]
- (32).Cohen D; Atsmon J; Artaud C; Meron-Sudai S; Gougeon ML; Bialik A; Goren S; Asato V; Ariel-Cohen O; Reizis A; Dorman A; Hoitink CWG; Westdijk J; Ashkenazi S; Sansonetti PJ; Mulard LA; Phalipon A Safety and immunogenicity of a synthetic carbohydrate conjugate vaccine against Shigella flexneri 2a in healthy adult volunteers: A phase 1, dose-escalating, single-blind, randomised, placebo-controlled study. Lancet Infect. Dis 2021, 21, 546–558. [DOI] [PubMed] [Google Scholar]
- (33).van der Put RMF; Smitsman C; de Haan A; Hamzink M; Timmermans H; Uittenbogaard J; Westdijk J; Stork M; Ophorst O; Thouron F; Guerreiro C; Sansonetti PJ; Phalipon A; Mulard LA The first-in-human synthetic glycan-based conjugate vaccine candidate against Shigella. ACS Cent. Sci 2022, 8 (4), 449–460. DOI: 10.1021/acscentsci.1c01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Morelli L; Poletti L; Lay L Carbohydrates and immunology: Synthetic oligosaccharide antigens for vaccine formulation. Eur. J. Org. Chem 2011, 2011 (29), 5723–5777. DOI: 10.1002/ejoc.201100296. [DOI] [Google Scholar]
- (35).Fernández-Tejada A; Cañada FJ; Jiménez-Barbero J Recent developments in synthetic carbohydrate-based diagnostics, vaccines, and therapeutics. Chem. - Eur. J 2015, 21 (30), 10616–10628. DOI: 10.1002/chem.201500831. [DOI] [PubMed] [Google Scholar]
- (36).Khatun F; Stephenson RJ; Toth I An overview of structural features of antibacterial glycoconjugate vaccines that influence their immunogenicity. Chemistry 2017, 23 (18), 4233–4254. DOI: 10.1002/chem.201603599. [DOI] [PubMed] [Google Scholar]
- (37).Seeberger PH; Werz DB Synthesis and medical applications of oligosaccharides. Nature 2007, 446 (7139), 1046–1051. DOI: 10.1038/nature05819. [DOI] [PubMed] [Google Scholar]
- (38).Colombo C; Pitirollo O; Lay L Recent advances in the synthesis of glycoconjugates for vaccine development. Molecules 2018, 23 (7), 1712. DOI: 10.3390/molecules23071712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Del Bino L; Østerlid KE; Wu D-Y; Nonne F; Romano MR; Codée J; Adamo R Synthetic glycans to improve current glycoconjugate vaccines and fight antimicrobial resistance. Chem. Rev 2022, 122, 15672–15716. DOI: 10.1021/acs.chemrev.2c00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Jones C Vaccines based on the cell surface carbohydrates of pathogenic bacteria. An. Acad. Bras. Cienc 2005, 77 (2), 293–324. DOI: 10.1590/s0001-37652005000200009. [DOI] [PubMed] [Google Scholar]
- (41).Jennings HJ; Lugowski C Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. The Journal of Immunology 1981, 127 (3), 1011–1018. DOI: 10.4049/jimmunol.127.3.1011. [DOI] [PubMed] [Google Scholar]
- (42).Adamo R; Nilo A; Castagner B; Boutureira O; Berti F; Bernardes GJL Synthetically defined glycoprotein vaccines: Current status and future directions. Chem. Sci 2013, 4 (8), 2995–3008. DOI: 10.1039/C3SC50862E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Zasłona ME; Downey AM; Seeberger PH; Moscovitz O Semi- and fully synthetic carbohydrate vaccines against pathogenic bacteria: Recent developments. Biochem. Soc. Trans 2021, 49 (5), 2411–2429. DOI: 10.1042/bst20210766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Seeberger PH Discovery of semi- and fully-synthetic carbohydrate vaccines against bacterial infections using a medicinal chemistry approach. Chem. Rev 2021, 121 (7), 3598–3626. DOI: 10.1021/acs.chemrev.0c01210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Huang Y-L; Hung J-T; Cheung SKC; Lee H-Y; Chu K-C; Li S-T; Lin Y-C; Ren C-T; Cheng T-JR; Hsu T-L; Yu AL; Wu C-Y; Wong C-H Carbohydrate-based vaccines with a glycolipid adjuvant for breast cancer. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (7), 2517–2522. DOI: doi: 10.1073/pnas.1222649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Brown S Jr., J. P. S. M; Walker S Wall teichoic acids of Gram-positive bacteria. Anna. Rev. Microbiol 2013, 67 (1), 313–336. DOI: 10.1146/annurev-micro-092412-155620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Mammen M; Dahmann G; Whitesides GM Effective inhibitors of hemagglutination by influenza virus synthesized from polymers having active ester groups. Insight into mechanism of inhibition. J. Med. Chem 1995, 38 (21), 4179–4190. DOI: 10.1021/jm00021a007. [DOI] [PubMed] [Google Scholar]
- (48).Micoli F; Adamo R; Costantino P Protein Carriers for Glycoconjugate Vaccines: History, Selection Criteria, Characterization and New Trends. Molecules 2018, 23 (6), 1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Liao G; Zhou Z; Suryawanshi S; Mondal MA; Guo Z Fully synthetic self-adjuvanting α-2,9-oligosialic acid based conjugate vaccines against Group C Meningitis. ACS Cent. Sci 2016, 2 (4), 210–218. DOI: 10.1021/acscentsci.5b00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Delrieu I; Yaro S; Tamekloé TAS; Njanpop-Lafourcade B-M; Tall H; Jaillard P; Ouedraogo MS; Badziklou K; Sanou O; Drabo A; Gessner BD; Kambou JL; Mueller JE Emergence of Epidemic Neisseria meningitidis Serogroup X Meningitis in Togo and Burkina Faso. PLOS ONE 2011, 6 (5), e19513. DOI: 10.1371/journal.pone.0019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Calix JJ; Porambo RJ; Brady AM; Larson TR; Yother J; Abeygunwardana C; Nahm MH Biochemical, genetic, and serological characterization of two capsule subtypes among Streptococcus pneumoniae serotype 20 strains: Discovery of a new pneumococcal serotype. J. Biol. Chem 2012, 287 (33), 27885–27894. DOI: 10.1074/jbc.M112.380451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Villeneuve S; Souchon H; Riottot M-M; Mazié J-C; Lei P.-s.; Glaudemans CPJ; Kováč P; Fournier J-M; Alzari PM Crystal structure of an anti-carbohydrate antibody directed against Vibrio cholerae O1 in complex with antigen: Molecular basis for serotype specificity. Proceedings of the National Academy of Sciences 2000, 97(15), 8433–8438. DOI: doi: 10.1073/pnas.060022997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Jörbeck HJ; Svenson SB; Lindberg AA Artificial Salmonella vaccines: Salmonella typhimurium O-antigen-specific oligosaccharide-protein conjugates elicit opsonizing antibodies that enhance phagocytosis. Infection and Immunity 1981, 32 (2), 497–502. DOI: doi: 10.1128/iai.32.2.497-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Recommendations for the production and control of Haemophilus influenzae type b conjugate vaccines, https://www.who.int/publications/m/item/hib-conjugate-vaccines-annex-1-trs-no-897
- (55).Martin CE; Broecker F; Oberli MA; Komor J; Mattner J; Anish C; Seeberger PH Immunological evaluation of a synthetic Clostridium difficile oligosaccharide conjugate vaccine candidate and identification of a minimal epitope. J. Am. Chem. Soc 2013, 135 (26), 9713–9722. DOI: 10.1021/ja401410y. [DOI] [PubMed] [Google Scholar]
- (56).Sabharwal H; Michon F; Nelson D; Dong W; Fuchs K; Manjarrez RC; Sarkar A; Uitz C; Viteri-Jackson A; Suarez RS; Blake M; Zabriskie JB Group A streptococcus (GAS) carbohydrate as an immunogen for protection against GAS infection. J. Infect. Dis 2006, 193 (1), 129–135. DOI: 10.1086/498618. [DOI] [PubMed] [Google Scholar]
- (57).Broecker F; Hanske J; Martin CE; Baek JY; Wahlbrink A; Wojcik F; Hartmann L; Rademacher C; Anish C; Seeberger PH Multivalent display of minimal Clostridium difficile glycan epitopes mimics antigenic properties of larger glycans. Nat. Commun 2016, 7, 11224. DOI: 10.1038/ncomms11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Schumann B; Hahm HS; Parameswarappa SG; Reppe K; Wahlbrink A; Govindan S; Kaplonek P; Pirofski LA; Witzenrath M; Anish C; Pereira CL; Seeberger PH A semisynthetic Streptococcus pneumoniae serotype 8 glycoconjugate vaccine. Sci. Transl. Med 2017, 9 (380), eaaf5347. DOI: 10.1126/scitranslmed.aaf5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Pereira CL; Geissner A; Anish C; Seeberger PH Chemical synthesis elucidates the immunological importance of a pyruvate modification in the capsular polysaccharide of Streptococcus pneumoniae serotype 4. Angew. Chem., Int. Ed. Engl 2015, 54 (34), 10016–10019. DOI: 10.1002/anie.201504847. [DOI] [PubMed] [Google Scholar]
- (60).Geissner A; Pereira CL; Leddermann M; Anish C; Seeberger PH Deciphering antigenic determinants of Streptococcus pneumoniae serotype 4 capsular polysaccharide using synthetic oligosaccharides. ACS Chem. Biol 2016, 11 (2), 335–344. DOI: 10.1021/acschembio.5b00768. [DOI] [PubMed] [Google Scholar]
- (61).McKenney D; Pouliot KL; Wang Y; Murthy V; Ulrich M; Döring G; Lee JC; Goldmann DA; Pier GB Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 1999, 284 (5419), 1523–1527. DOI: 10.1126/science.284.5419.1523. [DOI] [PubMed] [Google Scholar]
- (62).Gening ML; Maira-Litrán T; Kropec A; Skurnik D; Grout M; Tsvetkov YE; Nifantiev NE; Pier GB Synthetic beta-(1-6)-linked N-acetylated and nonacetylated oligoglucosamines used to produce conjugate vaccines for bacterial pathogens. Infect. Immun 2010, 78(2), 764–772. DOI: 10.1128/iai.01093-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Jansson PE; Lindberg B; Lindquist U Structural studies of the capsular polysaccharide from Streptococcus pneumoniae type 5. Carbohydr. Res 1985, 140 (1), 101–110. DOI: 10.1016/0008-6215(85)85053-9. [DOI] [PubMed] [Google Scholar]
- (64).Cohen D; Ashkenazi S; Green MS; Gdalevich M; Robin G; Slepon R; Yavzori M; Orr N; Block C; Ashkenazi I; Shemer J; Taylor DN; Hale TL; Sadoff JC; Pavliakova D; Schneerson R; Robbins JB Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet 1997, 349 (9046), 155–159. DOI: 10.1016/s0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- (65).Tzianabos A; Wang JY; Kasper DL Biological chemistry of immunomodulation by zwitterionic polysaccharides. Carbohydr. Res 2003, 338 (23), 2531–2538. DOI: 10.1016/j.carres.2003.06.005. [DOI] [PubMed] [Google Scholar]
- (66).Gattoji VK; Valentin G; Geremia ML; Andreana PR Zwitterionic Polysaccharides in Immunity. In Comprehensive Glycoscience (Second Edition), Barchi JJ Ed.; Elsevier, 2021; pp 454–469. [Google Scholar]
- (67).Zhang Q; Overkleeft HS; van der Marel GA; Codée JDC Synthetic zwitterionic polysaccharides. Curr. Opin. Chem. Biol 2017, 40, 95–101. DOI: 10.1016/j.cbpa.2017.07.010. [DOI] [PubMed] [Google Scholar]
- (68).Micoli F; Costantino P; Adamo R Potential targets for next generation antimicrobial glycoconjugate vaccines. FEMS Microbiol. Rev 2018, 42 (3), 388–423. DOI: 10.1093/femsre/fuy011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Zhao Y; Wang S; Wang G; Li H; Guo Z; Gu G Synthesis and immunological studies of group A Streptococcus cell-wall oligosaccharide–streptococcal C5a peptidase conjugates as bivalent vaccines. Org. Chem. Front 2019, 6 (20), 3589–3596. DOI: 10.1039/C9Q000651F. [DOI] [Google Scholar]
- (70).Anish C; Beurret M; Poolman J Combined effects of glycan chain length and linkage type on the immunogenicity of glycoconjugate vaccines. npj Vaccines 2021, 6 (1), 150. DOI: 10.1038/s41541-021-00409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Avci FY; Li X; Tsuji M; Kasper DL A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat. Med 2011, 17(12), 1602–1609. DOI: 10.1038/nm.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).MacCalman TE; Phillips-Jones MK; Harding SE Glycoconjugate vaccines: Some observations on carrier and production methods. Biotechnol. Genet. Eng. Rev 2019, 35 (2), 93–125. DOI: 10.1080/02648725.2019.1703614. [DOI] [PubMed] [Google Scholar]
- (73).Rappuoli R; De Gregorio E A sweet T cell response. Nat. Med 2011, 17 (12), 1551–1552. DOI: 10.1038/nm.2587. [DOI] [PubMed] [Google Scholar]
- (74).Dondoni A; Marra A Recent applications of thiol–ene coupling as a click process for glycoconjugation. Chem. Soc. Rev 2012, 41 (2), 573–586. DOI: 10.1039/C1CS15157F. [DOI] [PubMed] [Google Scholar]
- (75).Huang YL; Wu CY Carbohydrate-based vaccines: Challenges and opportunities. Expert Rev. Vaccines 2010, 9 (11), 1257–1274. DOI: 10.1586/erv.10.120. [DOI] [PubMed] [Google Scholar]
- (76).Lu L; Duong VT; Shalash AO; Skwarczynski M; Toth I Chemical conjugation strategies for the development of protein-based subunit nanovaccines. Vaccines (Basel) 2021, 9 (6), 563. DOI: 10.3390/vaccines9060563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Astronomo RD; Burton DR Carbohydrate vaccines: Developing sweet solutions to sticky situations? Nat. Rev. Drug Discovery 2010, 9 (4), 308–324. DOI: 10.1038/nrd3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Mond JJ; Lees A; Snapper CM T cell-independent antigens type 2. Annu. Rev. Immunol 1995, 13, 655–692. DOI: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- (79).Khatun F; Toth I; Stephenson RJ Immunology of carbohydrate-based vaccines. Adv. Drug Delivery Rev 2020, 165-166, 117–126. DOI: 10.1016/j.addr.2020.04.006. [DOI] [PubMed] [Google Scholar]
- (80).Beuvery EC; van Rossum F; Nagel J Comparison of the induction of immunoglobulin M and G antibodies in mice with purified pneumococcal type 3 and meningococcal group C polysaccharides and their protein conjugates. Infect. Immun 1982, 37 (1), 15–22. DOI: 10.1128/iai.37.1.15-22.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Richmond P; Kaczmarski E; Borrow R; Findlow J; Clark S; McCann R; Hill J; Barker M; Miller E Meningococcal C polysaccharide vaccine induces immunologic hyporesponsiveness in adults that is overcome by meningococcal C conjugate vaccine. J. Infect. Dis 2000, 181 (2), 761–764. DOI: 10.1086/315284. [DOI] [PubMed] [Google Scholar]
- (82).Rappuoli R Glycoconjugate vaccines: Principles and mechanisms. Sci. Transl. Med 2018, 10 (456), eaat4615. DOI: 10.1126/scitranslmed.aat4615. [DOI] [PubMed] [Google Scholar]
- (83).Snapper CM; Mond JJ A model for induction of T cell-independent humoral immunity in response to polysaccharide antigens. J. Immunol 1996, 157 (6), 2229–2233. [PubMed] [Google Scholar]
- (84).Balázs M; Martin F; Zhou T; Kearney J Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity 2002, 17 (3), 341–352. DOI: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- (85).MacLennan I; Vinuesa C Dendritic cells, BAFF, and APRIL: Innate players in adaptive antibody responses. Immunity 2002, 17 (3), 235–238. DOI: 10.1016/s1074-7613(02)00398-9. [DOI] [PubMed] [Google Scholar]
- (86).Sun X; Stefanetti G; Berti F; Kasper DL Polysaccharide structure dictates mechanism of adaptive immune response to glycoconjugate vaccines. Proc. Natl. Acad. Sci. U. S. A 2019, 116 (1), 193–198. DOI: doi: 10.1073/pnas.1816401115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Avci FY; Li X; Tsuji M; Kasper DL Isolation of carbohydrate-specific CD4+ T cell clones from mice after stimulation by two model glycoconjugate vaccines. Nat. Protoc 2012, 7 (12), 2180–2192. DOI: 10.1038/nprot.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Gold R; Lepow ML; Goldschneider I; Gotschlich EC Immune Response of human infants of polysaccharide vaccines of group A and C Neisseria meningitidis. J. Infect. Dis 1977, 136 Suppl, S31–S35. DOI: 10.1093/infdis/136.supplement.s31. [DOI] [PubMed] [Google Scholar]
- (89).Tzianabos AO; Onderdonk AB; Rosner B; Cisneros RL; Kasper DL Structural features of polysaccharides that induce intra-abdominal abscesses. Science 1993, 262 (5132), 416–419. DOI: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- (90).Kalka-Moll WM; Tzianabos AO; Bryant PW; Niemeyer M; Ploegh HL; Kasper DL Zwitterionic polysaccharides stimulate T cells by MHC class II-dependent interactions. J. Immunol 2002, 169 (11), 6149–6153. DOI: 10.4049/jimmunol.169.11.6149. [DOI] [PubMed] [Google Scholar]
- (91).Finn A Bacterial polysaccharide-protein conjugate vaccines. Br. Med. Bull 2004, 70, 1–14. DOI: 10.1093/bmb/ldh021. [DOI] [PubMed] [Google Scholar]
- (92).Hütter J; Lepenies B Carbohydrate-based vaccines: An overview. Methods Mol. Biol 2015, 1331, 1–10. DOI: 10.1007/978-1-4939-2874-3_1. [DOI] [PubMed] [Google Scholar]
- (93).Costantino P; Rappuoli R; Berti F The design of semi-synthetic and synthetic glycoconjugate vaccines. Expert Opin. Drug Discov 2011, 6 (10), 1045–1066. DOI: 10.1517/17460441.2011.609554. [DOI] [PubMed] [Google Scholar]
- (94).Verez-Bencomo V; Fernández-Santana V; Hardy E; Toledo ME; Rodríguez MC; Heynngnezz L; Rodriguez A; Baly A; Herrera L; Izquierdo M; Villar A; Valdés Y; Cosme K; Deler ML; Montane M; Garcia E; Ramos A; Aguilar A; Medina E; Toraño G; Sosa I; Hernandez I; Martínez R; Muzachio A; Carmenates A; Costa L; Cardoso F; Campa C; Diaz M; Roy R A synthetic conjugate polysaccharide vaccine against Haemophilus influenzae type b. Science 2004, 305 (5683), 522–525. DOI: 10.1126/science.1095209. [DOI] [PubMed] [Google Scholar]
- (95).Zarei AE; Almehdar HA; Redwan EM Hib vaccines: Past, present, and future perspectives. J. Immunol. Res 2016, 2016, 7203587. DOI: 10.1155/2016/7203587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Roberge JY; Beebe X; Danishefsky SJ Convergent synthesis of N-linked glycopeptides on a solid support. J. Am. Chem. Soc 1998, 120, 3915–3927. [DOI] [PubMed] [Google Scholar]
- (97).Ramsay ME; McVernon J; Andrews NJ; Heath PT; Slack MP Estimating Haemophilus influenzae type b vaccine effectiveness in England and Wales by use of the screening method. J. Infect. Dis 2003, 188 (4), 481–485. DOI: 10.1086/376997. [DOI] [PubMed] [Google Scholar]
- (98).Baek JY; Geissner A; Rathwell DCK; Meierhofer D; Pereira CL; Seeberger PH A modular synthetic route to size-defined immunogenic Haemophilus influenzae b antigens is key to the identification of an octasaccharide lead vaccine candidate. Chem. Sci 2018, 9 (5), 1279–1288. DOI: 10.1039/C7SC04521B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Fekete A; Hoogerhout P; Zomer G; Kubler-Kielb J; Schneerson R; Robbins JB; Pozsgay V Synthesis of octa- and dodecamers of D-ribitol-1-phosphate and their protein conjugates. Carbohydr. Res 2006, 341 (12), 2037–2048. DOI: 10.1016/j.carres.2005.10.023. [DOI] [PubMed] [Google Scholar]
- (100).Rosenstein NE; Perkins BA; Stephens DS; Popovic T; Hughes JM Meningococcal disease. N. Engl. J. Med 2001, 344 (18), 1378–1388. DOI: 10.1056/nejm200105033441807. [DOI] [PubMed] [Google Scholar]
- (101).Stephens DS Conquering the meningococcus. FEMS Microbiol. Rev 2007, 31 (1), 3–14. DOI: 10.1111/j.1574-6976.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- (102).Harrison OB; Claus H; Jiang Y; Bennett JS; Bratcher HB; Jolley KA; Corton C; Care R; Poolman JT; Zollinger WD; Frasch CE; Stephens DS; Feavers I; Frosch M; Parkhill J; Vogel U; Quail MA; Bentley SD; Maiden MC Description and nomenclature of Neisseria meningitidis capsule locus. Emerg. Infect. Dis 2013, 19 (4), 566–573. DOI: 10.3201/eid1904.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Borrow R; Alarcón P; Carlos J; Caugant DA; Christensen H; Debbag R; De Wals P; Echániz-Aviles G; Findlow J; Head C; Holt D; Kamiya H; Saha SK; Sidorenko S; Taha MK; Trotter C; Vázquez Moreno JA; von Gottberg A; Sáfadi MA The global meningococcal initiative: Global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev. Vaccines 2017, 16 (4), 313–328. DOI: 10.1080/14760584.2017.1258308. [DOI] [PubMed] [Google Scholar]
- (104).McCarthy PC; Sharyan A; Sheikhi Moghaddam L Meningococcal vaccines: Current status and emerging strategies. Vaccines 2018, 6 (1), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Bröker M; Berti F; Costantino P Factors contributing to the immunogenicity of meningococcal conjugate vaccines. Hum. Vaccines Immunother 2016, 12 (7), 1808–1824. DOI: 10.1080/21645515.2016.1153206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Berkin A; Coxon B; Pozsgay V Towards a synthetic glycoconjugate vaccine against Neisseria meningitidis A. Chemistry 2002, 8 (19), 4424–4433. DOI: . [DOI] [PubMed] [Google Scholar]
- (107).Slättegård R; Teodorovic P; Hadgu Kinfe H; Ravenscroft N; Gammon DW; Oscarson S Synthesis of structures corresponding to the capsular polysaccharide of Neisseria meningitidis group A. Org. Biomol. Chem 2005, 3 (20), 3782–3787. DOI: 10.1039/B507898A. [DOI] [PubMed] [Google Scholar]
- (108).Berti F; Romano MR; Micoli F; Pinto V; Cappelletti E; Gavini M; Proietti D; Pluschke G; MacLennan CA; Costantino P Relative stability of meningococcal serogroup A and X polysaccharides. Vaccine 2012, 30 (45), 6409–6415. DOI: 10.1016/j.vaccine.2012.08.021. [DOI] [PubMed] [Google Scholar]
- (109).Gao Q; Zaccaria C; Tontini M; Poletti L; Costantino P; Lay L Synthesis and preliminary biological evaluation of carba analogues from Neisseria meningitidis A capsular polysaccharide. Org. Biomol. Chem 2012, 10 (33), 6673–6681. DOI: 10.1039/C2OB25222H. [DOI] [PubMed] [Google Scholar]
- (110).Teodorović P; Slättegård R; Oscarson S Synthesis of stable C-phosphonate analogues of Neisseria meningitidis group A capsular polysaccharide structures using modified Mitsunobu reaction conditions. Org. Biomol. Chem 2006, 4 (24), 4485–4490. DOI: 10.1039/B614038F. [DOI] [PubMed] [Google Scholar]
- (111).Gao Q; Tontini M; Brogioni G; Nilo A; Filippini S; Harfouche C; Polito L; Romano MR; Costantino P; Berti F; Adamo R; Lay L Immunoactivity of protein conjugates of carba analogues from Neisseria meningitidis a capsular polysaccharide. ACS Chem. Biol 2013, 8 (11), 2561–2567. DOI: 10.1021/cb400463u. [DOI] [PubMed] [Google Scholar]
- (112).Torres-Sanchez MI; Zaccaria C; Buzzi B; Miglio G; Lombardi G; Polito L; Russo G; Lay L Synthesis and biological evaluation of phosphono analogues of capsular polysaccharide fragments from Neisseria meningitidis A. Chemistry 2007, 13 (23), 6623–6635. DOI: 10.1002/chem.200601743. [DOI] [PubMed] [Google Scholar]
- (113).Fallarini S; Buzzi B; Giovarruscio S; Polito L; Brogioni G; Tontini M; Berti F; Adamo R; Lay L; Lombardi G A synthetic disaccharide analogue from Neisseria meningitidis a capsular polysaccharide stimulates immune cell responses and induces immunoglobulin G (IgG) production in mice when protein-conjugated. ACS Infect. Dis 2015, 1 (10), 487–496. DOI: 10.1021/acsinfecdis.5b00071. [DOI] [PubMed] [Google Scholar]
- (114).Richmond P; Borrow R; Findlow J; Martin S; Thornton C; Cartwright K; Miller E Evaluation of De-O-acetylated meningococcal C polysaccharide-tetanus toxoid conjugate vaccine in infancy: reactogenicity, immunogenicity, immunologic priming, and bactericidal activity against O-acetylated and De-O-acetylated serogroup C strains. Infect. Immun 2001, 69 (4), 2378–2382. DOI: 10.1128/iai.69.4.2378-2382.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Chu K-C; Ren C-T; Lu C-P; Hsu C-H; Sun T-H; Han J-L; Pal B; Chao T-A; Lin Y-F; Wu S-H; Wong C-H; Wu C-Y Efficient and stereoselective synthesis of α(2→9) oligosialic acids: From monomers to dodecamers. Angew. Chem., Int. Ed 2011, 50 (40), 9391–9395. DOI: 10.1002/anie.201101794. [DOI] [PubMed] [Google Scholar]
- (116).Liao G; Zhou Z; Guo Z Synthesis and immunological study of α-2,9-oligosialic acid conjugates as anti-group C meningitis vaccines. Chem. Commun 2015, 51 (47), 9647–9650. DOI: 10.1039/C5CC01794G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Wang C-H; Li S-T; Lin T-L; Cheng Y-Y; Sun T-H; Wang J-T; Cheng T-JR; Mong KKT; Wong C-H; Wu C-Y Synthesis of Neisseria meningitidis serogroup W135 capsular oligosaccharides for immunogenicity comparison and vaccine development. Angew. Chem., Int. Ed 2013, 52 (35), 9157–9161. DOI: 10.1002/anie.201302540. [DOI] [PubMed] [Google Scholar]
- (118).Stephens DS; Greenwood B; Brandtzaeg P Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 2007, 369 (9580), 2196–2210. DOI: 10.1016/s0140-6736(07)61016-2. [DOI] [PubMed] [Google Scholar]
- (119).Mutonga DM; Pimentel G; Muindi J; Nzioka C; Mutiso J; Klena JD; Morcos M; Ogaro T; Materu S; Tetteh C; Messonnier NE; Breiman RF; Feikin DR Epidemiology and risk factors for serogroup X Meningococcal Meningitis during an outbreak in Western Kenya, 2005–2006. Am. J. Trop. Med. Hyg 2009, 80 (4), 619–624. DOI: 10.4269/ajtmh.2009.80.619. [DOI] [PubMed] [Google Scholar]
- (120).Boisier P; Nicolas P; Djibo S; Taha MK; Jeanne I; Maiïnassara HB; Tenebray B; Kairo KK; Giorgini D; Chanteau S Meningococcal meningitis: Unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clin. Infect. Dis 2007, 44 (5), 657–663. DOI: 10.1086/511646. [DOI] [PubMed] [Google Scholar]
- (121).del Castillo CM; Vázquez JA; Romero J; Pascual A Infections by Neisseria meningitidis serogroup X in Spain. Clin. Microbiol. Infect 2003, 9 (9), 964–965. DOI: 10.1046/j.1469-0691.2003.00685.x. [DOI] [PubMed] [Google Scholar]
- (122).Chen C; Zhang TG; He JG; Wu J; Chen LJ; Liu JF; Pang XH; Yang J; Shao ZJ; Huang YC A first meningococcal meningitis case caused by serogroup X Neisseria meningitidis strains in China. Chin. Med. J. (Engl) 2008, 121 (7), 664–666. [PubMed] [Google Scholar]
- (123).Micoli F; Romano MR; Tontini M; Cappelletti E; Gavini M; Proietti D; Rondini S; Swennen E; Santini L; Filippini S; Balocchi C; Adamo R; Pluschke G; Norheim G; Pollard A; Saul A; Rappuoli R; MacLennan CA; Berti F; Costantino P Development of a glycoconjugate vaccine to prevent meningitis in Africa caused by meningococcal serogroup X. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (47), 19077–19082. DOI: doi: 10.1073/pnas.1314476110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Harale KR; Dumare NB; Singh D; Misra AK; Chhikara MK Synthesis of a tetrasaccharide and its glycoconjugate corresponding to the capsular polysaccharide of Neisseria meningitidis serogroup X and its immunochemical studies. RSC Adv. 2015, 5 (52), 41332–41340. DOI: 10.1039/C5RA02993G. [DOI] [Google Scholar]
- (125).Morelli L; Cancogni D; Tontini M; Nilo A; Filippini S; Costantino P; Romano MR; Berti F; Adamo R; Lay L Synthesis and immunological evaluation of protein conjugates of Neisseria meningitidis X capsular polysaccharide fragments. Beilstein J. Org. Chem 2014, 10, 2367–2376. DOI: 10.3762/bjoc.10.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Oldrini D; Fiebig T; Romano MR; Proietti D; Berger M; Tontini M; De Ricco R; Santini L; Morelli L; Lay L; Gerardy-Schahn R; Berti F; Adamo R Combined chemical synthesis and tailored enzymatic elongation provide fully synthetic and conjugation-ready Neisseria meningitidis serogroup X vaccine antigens. ACS Chem. Biol 2018, 13 (4), 984–994. DOI: 10.1021/acschembio.7b01057. [DOI] [PubMed] [Google Scholar]
- (127).Scott P; Deye G; Srinivasan A; Murray C; Moran K; Hulten E; Fishbain J; Craft D; Riddell S; Lindler L; Mancuso J; Milstrey E; Bautista CT; Patel J; Ewell A; Hamilton T; Gaddy C; Tenney M; Christopher G; Petersen K; Endy T; Petruccelli B An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin. Infect. Dis 2007, 44 (12), 1577–1584. DOI: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- (128).Giguère D. Surface polysaccharides from Acinetobacter baumannii: Structures and syntheses. Carbohydr. Res 2015, 418, 29–43. DOI: 10.1016/j.carres.2015.10.001. [DOI] [PubMed] [Google Scholar]
- (129).Hu D; Liu B; Dijkshoorn L; Wang L; Reeves PR Diversity in the major polysaccharide antigen of Acinetobacter Baumannii assessed by DNA sequencing, and development of a molecular serotyping scheme. PLoS One 2013, 8 (7), e70329. DOI: 10.1371/journal.pone.0070329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Kenyon JJ; Hall RM Variation in the complex carbohydrate biosynthesis loci of Acinetobacter baumannii Genomes. PLoS One 2013, 8 (4), e62160. DOI: 10.1371/journal.pone.0062160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Lee IM; Yang FL; Chen TL; Liao KS; Ren CT; Lin NT; Chang YP; Wu CY; Wu SH Pseudaminic acid on exopolysaccharide of Acinetobacter baumannii plays a critical role in phage-assisted preparation of glycoconjugate vaccine with high antigenicity. J. Am. Chem. Soc 2018, 140 (28), 8639–8643. DOI: 10.1021/jacs.8b04078. [DOI] [PubMed] [Google Scholar]
- (132).Arbatsky NP; Shneider MM; Kenyon JJ; Shashkov AS; Popova AV; Miroshnikov KA; Volozhantsev NV; Knirel YA Structure of the neutral capsular polysaccharide of Acinetobacter baumannii NIPH146 that carries the KL37 capsule gene cluster. Carbohydr. Res 2015, 413, 12–15. DOI: 10.1016/j.carres.2015.05.003. [DOI] [PubMed] [Google Scholar]
- (133).MacLean LL; Perry MB; Chen W; Vinogradov E The structure of the polysaccharide O-chain of the LPS from Acinetobacter baumannii strain ATCC 17961. Carbohydr. Res 2009, 344 (4), 474–478. DOI: 10.1016/j.carres.2008.12.026. [DOI] [PubMed] [Google Scholar]
- (134).Fregolino E; Gargiulo V; Lanzetta R; Parrilli M; Holst O; Castro CD Identification and structural determination of the capsular polysaccharides from two Acinetobacter baumannii clinical isolates, MG1 and SMAL. Carbohydr. Res 2011, 346 (7), 973–977. DOI: 10.1016/j.carres.2011.03.024. [DOI] [PubMed] [Google Scholar]
- (135).Shashkov AS; Kenyon JJ; Arbatsky NP; Shneider MM; Popova AV; Miroshnikov KA; Hall RM; Knirel YA Related structures of neutral capsular polysaccharides of Acinetobacter baumannii isolates that carry related capsule gene clusters KL43, KL47, and KL88. Carbohydr. Res 2016, 435, 173–179. DOI: 10.1016/j.carres.2016.10.007. [DOI] [PubMed] [Google Scholar]
- (136).Wei R; Yang X; Liu H; Wei T; Chen S; Li X Synthetic pseudaminic-acid-based antibacterial vaccine confers effective protection against Acinetobacter baumannii infection. ACS Cent. Sci 2021, 7 (9), 1535–1542. DOI: 10.1021/acscentsci.1c00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Sianturi J; Priegue P; Hu J; Yin J; Seeberger PH Semi-synthetic glycoconjugate vaccine lead against Acinetobacter baumannii 17978. Angew. Chem., Int. Ed 2022, 61 (41), e202209556 ( 202209551–202209558). DOI: 10.1002/anie.202209556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Geno KA; Gilbert GL; Song JY; Skovsted IC; Klugman KP; Jones C; Konradsen HB; Nahm MH Pneumococcal capsules and their types: Past, present, and future. Clin. Microbiol. Rev. 2015, 28 (3), 871–899. DOI: 10.1128/cmr.00024-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: A systematic analysis for the global burden of disease study 2016. Lancet Infect. Dis 2018, 18 (11), 1191–1210. DOI: 10.1016/s1473-3099(18)30310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Feldman C; Anderson R Review: Current and new generation pneumococcal vaccines. J. Infect 2014, 69 (4), 309–325. DOI: 10.1016/j.jinf.2014.06.006. [DOI] [PubMed] [Google Scholar]
- (141).Rupp R; Hurley D; Grayson S; Li J; Nolan K; McFetridge RD; Hartzel J; Abeygunawardana C; Winters M; Pujar H; Benner P; Musey L A dose ranging study of 2 different formulations of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants. Hum. Vaccines Immunother 2019, 15 (3), 549–559. DOI: 10.1080/21645515.2019.1568159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Wijmenga-Monsuur AJ; van Westen E; Knol MJ; Jongerius RM; Zancolli M; Goldblatt D; van Gageldonk PG; Tcherniaeva I; Berbers GA; Rots NY Direct comparison of immunogenicity induced by 10- or 13-valent pneumococcal conjugate vaccine around the 11-month booster in dutch infants. PLoS One 2015, 10 (12), e0144739. DOI: 10.1371/journal.pone.0144739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (143).Gruber WC; Scott DA; Emini EA Development and clinical evaluation of Prevnar 13, a 13-valent pneumocococcal CRM197 conjugate vaccine. Ann. N. Y. Acad. Sci 2012, 1263, 15–26. DOI: 10.1111/j.1749-6632.2012.06673.x. [DOI] [PubMed] [Google Scholar]
- (144).Cobb BA; Kasper DL Zwitterionic capsular polysaccharides: The new MHCII-dependent antigens. Cell. Microbiol 2005, 7 (10), 1398–1403. DOI: 10.1111/j.1462-5822.2005.00591.x. [DOI] [PubMed] [Google Scholar]
- (145).Wu X; Cui L; Lipinski T; Bundle DR Synthesis of monomeric and dimeric repeating units of the zwitterionic Type 1 capsular polysaccharide from Streptococcus pneumoniae. Chem. - Eur. J 2010, 16 (11), 3476–3488. DOI: 10.1002/chem.200902460. [DOI] [PubMed] [Google Scholar]
- (146).Christina AE; van den Bos LJ; Overkleeft HS; van der Marel GA; Codée JDC Galacturonic acid lactones in the synthesis of all trisaccharide repeating units of the zwitterionic polysaccharide Sp1. J. Org. Chem 2011, 76 (6), 1692–1706. DOI: 10.1021/jo102363d. [DOI] [PubMed] [Google Scholar]
- (147).Schumann B; Pragani R; Anish C; Pereira CL; Seeberger PH Synthesis of conjugation-ready zwitterionic oligosaccharides by chemoselective thioglycoside activation. Chem. Sci 2014, 5 (5), 1992–2002. DOI: 10.1039/C3SC53362J. [DOI] [Google Scholar]
- (148).Schumann B; Reppe K; Kaplonek P; Wahlbrink A; Anish C; Witzenrath M; Pereira CL; Seeberger PH Development of an efficacious, semisynthetic glycoconjugate vaccine candidate against Streptococcus pneumoniae serotype 1. ACS Cent. Sci 2018, 4 (3), 357–361. DOI: 10.1021/acscentsci.7b00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Kaplonek P; Khan N; Reppe K; Schumann B; Emmadi M; Lisboa MP; Xu FF; Calow ADJ; Parameswarappa SG; Witzenrath M; Pereira CL; Seeberger PH Improving vaccines against Streptococcus pneumoniae using synthetic glycans. Proc. Natl. Acad. Sci. U. S. A 2018, 115 (52), 13353–13358. DOI: 10.1073/pnas.1811862115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).Jansen WT; Hogenboom S; Thijssen MJ; Kamerling JP; Vliegenthart JF; Verhoef J; Snippe H; Verheul AF Synthetic 6B di-, tri-, and tetrasaccharide-protein conjugates contain pneumococcal type 6A and 6B common and 6B-specific epitopes that elicit protective antibodies in mice. Infect. Immun 2001, 69 (2), 787–793. DOI: 10.1128/iai.69.2.787-793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Morelli L; Fallarini S; Lombardi G; Colombo C; Lay L; Compostella F Synthesis and biological evaluation of a trisaccharide repeating unit derivative of Streptococcus pneumoniae 19A capsular polysaccharide. Bioorg. Med. Chem 2018, 26 (21), 5682–5690. DOI: 10.1016/j.bmc.2018.10.016. [DOI] [PubMed] [Google Scholar]
- (152).Sanapala SR; Seco BMS; Baek JY; Awan SI; Pereira CL; Seeberger PH Chimeric oligosaccharide conjugate induces opsonic antibodies against Streptococcus pneumoniae serotypes 19A and 19F. Chem. Sci 2020, 11 (28), 7401–7407. DOI: 10.1039/D0SC02230F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Vetro M; Safari D; Fallarini S; Salsabila K; Lahmann M; Penadés S; Lay L; Marradi M; Compostella F Preparation and immunogenicity of gold glyconanoparticles as antipneumococcal vaccine model. Nanomedicine (Lond) 2017, 12 (1), 13–23. DOI: 10.2217/nnm-2016-0306. [DOI] [PubMed] [Google Scholar]
- (154).Polonskaya Z; Deng S; Sarkar A; Kain L; Comellas-Aragones M; McKay CS; Kaczanowska K; Holt M; McBride R; Palomo V; Self KM; Taylor S; Irimia A; Mehta SR; Dan JM; Brigger M; Crotty S; Schoenberger SP; Paulson JC; Wilson IA; Savage PB; Finn MG; Teyton L T cells control the generation of nanomolar-affinity anti-glycan antibodies. J. Clin. Invest 2017, 127 (4), 1491–1504. DOI: 10.1172/jci91192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Khalil IA; Troeger C; Blacker BF; Rao PC; Brown A; Atherly DE; Brewer TG; Engmann CM; Houpt ER; Kang G; Kotloff KL; Levine MM; Luby SP; MacLennan CA; Pan WK; Pavlinac PB; Platts-Mills JA; Qadri F; Riddle MS; Ryan ET; Shoultz DA; Steele AD; Walson JL; Sanders JW; Mokdad AH; Murray CJL; Hay SI; Reiner RC Jr. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: The global burden of disease study 1990-2016. Lancet Infect. Dis 2018, 18 (11), 1229–1240. DOI: 10.1016/s1473-3099(18)30475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Muthuirulandi Sethuvel DP; Devanga Ragupathi NK; Anandan S; Veeraraghavan B Update on: Shigella new serogroups/serotypes and their antimicrobial resistance. Lett. Appl. Microbiol 2017, 64 (1), 8–18. DOI: 10.1111/lam.12690. [DOI] [PubMed] [Google Scholar]
- (157).Mani S; Wierzba T; Walker RI Status of vaccine research and development for Shigella. Vaccine 2016, 34 (26), 2887–2894. DOI: 10.1016/j.vaccine.2016.02.075. [DOI] [PubMed] [Google Scholar]
- (158).Perepelov AV; Shekht ME; Liu B; Shevelev SD; Ledov VA; Senchenkova S. y. N.; L’vov VL; Shashkov AS; Feng L; Aparin PG; Wang L; Knirel YA Shigella flexneri O-antigens revisited: Final elucidation of the O-acetylation profiles and a survey of the O-antigen structure diversity. FEMS Immunol. Med. Microbiol 2012, 66 (2), 201–210. DOI: 10.1111/j.1574-695X.2012.01000.x. [DOI] [PubMed] [Google Scholar]
- (159).van der Put RM; Kim TH; Guerreiro C; Thouron F; Hoogerhout P; Sansonetti PJ; Westdijk J; Stork M; Phalipon A; Mulard LA A synthetic carbohydrate conjugate vaccine candidate against shigellosis: Improved bioconjugation and impact of alum on immunogenicity. Bioconjugate Chem. 2016, 27 (4), 883–892. DOI: 10.1021/acs.bioconjchem.5b00617. [DOI] [PubMed] [Google Scholar]
- (160).Perepelov AV; L’Vov V L; Liu B; Senchenkova SN; Shekht ME; Shashkov AS; Feng L; Aparin PG; Wang L; Knirel YA A similarity in the O-acetylation pattern of the O-antigens of Shigella flexneri types 1a, 1b, and 2a. Carbohydr. Res 2009, 344 (5), 687–692. DOI: 10.1016/j.carres.2009.01.004. [DOI] [PubMed] [Google Scholar]
- (161).Theillet F-X; Simenel C; Guerreiro C; Phalipon A; Mulard LA; Delepierre M Effects of backbone substitutions on the conformational behavior of Shigella flexneri O-antigens: Implications for vaccine strategy. Glycobiology 2010, 21 (1), 109–121. DOI: 10.1093/glycob/cwq136. [DOI] [PubMed] [Google Scholar]
- (162).Phalipon A; Mulard LA; Sansonetti PJ Vaccination against shigellosis: Is it the path that is difficult or is it the difficult that is the path? Microbes Infect. 2008, 10 (9), 1057–1062. DOI: 10.1016/j.micinf.2008.07.016. [DOI] [PubMed] [Google Scholar]
- (163).Vince Pozsgay BC, Glaudemans Cornelis P. J., Schneerson Rachel, Robbins John B.. Towards an oligosaccharide-based glycoconjugate vaccine against Shigella dysenteriae Type 1. Synlett 2003, 6, 743–767. DOI: 10.1055/s-2003-38724. [DOI] [Google Scholar]
- (164).Pozsgay V; Kubler-Kielb J; Schneerson R; Robbins JB Effect of the nonreducing end of Shigella dysenteriae type 1 O-specific oligosaccharides on their immunogenicity as conjugates in mice. Proc. Natl. Acad. Sci. U. S. A 2007, 104 (36), 14478–14482. DOI: 10.1073/pnas.0706969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (165).Qin C; Li L; Tian G; Ding M; Zhu S; Song W; Hu J; Seeberger PH; Yin J Chemical Synthesis and Antigenicity Evaluation of Shigella dysenteriae Serotype 10 O-Antigen Tetrasaccharide Containing a (S)-4,6-O-Pyruvyl Ketal. Journal of the American Chemical Society 2022, 144 (46), 21068–21079. DOI: 10.1021/jacs.2c05953. [DOI] [PubMed] [Google Scholar]
- (166).Ross JM The pathogenesis of anthrax following the administration of spores by the respiratory route. J. Pathol. Bacteriol 1957, 73 (2), 485–494. DOI: 10.1002/path.1700730219. [DOI] [Google Scholar]
- (167).Guidi-Rontani C; Weber-Levy M; Labruyère E; Mock M Germination of Bacillus anthracis spores within alveolar macrophages. Mol. Microbiol 1999, 31 (1), 9–17. DOI: 10.1046/j.1365-2958.1999.01137.x. [DOI] [PubMed] [Google Scholar]
- (168).Goel AK Anthrax: A disease of biowarfare and public health importance. World J. Clin. Cases 2015, 3 (1), 20–33. DOI: 10.12998/wjcc.v3.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Gould GW Recent advances in the understanding of resistance and dormancy in bacterial spores. J. Appl. Bacteriol 1977, 42 (3), 297–309. DOI: 10.1111/j.1365-2672.1977.tb00697.x. [DOI] [PubMed] [Google Scholar]
- (170).Tamborrini M; Werz DB; Frey J; Pluschke G; Seeberger PH Anti-carbohydrate antibodies for the detection of anthrax spores. Angew. Chem., Int. Ed. Engl 2006, 45 (39), 6581–6582. DOI: 10.1002/anie.200602048. [DOI] [PubMed] [Google Scholar]
- (171).Mehta AS; Saile E; Zhong W; Buskas T; Carlson R; Kannenberg E; Reed Y; Quinn CP; Boons G-J Synthesis and antigenic analysis of the BclA glycoprotein oligosaccharide from the Bacillus anthracis exosporium. Chem. - Eur. J 2006, 12 (36), 9136–9149. DOI: 10.1002/chem.200601245. [DOI] [PubMed] [Google Scholar]
- (172).Dhénin SG; Moreau V; Morel N; Nevers MC; Volland H; Créminon C; Djedaïni-Pilard F Synthesis of an anthrose derivative and production of polyclonal antibodies for the detection of anthrax spores. Carbohydr. Res 2008, 343 (12), 2101–2110. DOI: 10.1016/j.carres.2007.11.030. [DOI] [PubMed] [Google Scholar]
- (173).Adamo R. Glycan surface antigens from Bacillus anthracis as vaccine targets: Current status and future perspectives. Expert Rev. Vaccines 2014, 13 (7), 895–907. DOI: 10.1586/14760584.2014.924404. [DOI] [PubMed] [Google Scholar]
- (174).Chitlaru T; Altboum Z; Reuveny S; Shafferman A Progress and novel strategies in vaccine development and treatment of anthrax. Immunol. Rev 2011, 239 (1), 221–236. DOI: 10.1111/j.1600-065X.2010.00969.x. [DOI] [PubMed] [Google Scholar]
- (175).Rupnik M; Wilcox MH; Gerding DN Clostridium difficile infection: New developments in epidemiology and pathogenesis. Nat. Rev. Microbiol 2009, 7 (7), 526–536. DOI: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- (176).Denève C; Janoir C; Poilane I; Fantinato C; Collignon A New trends in Clostridium difficile virulence and pathogenesis. Int. J. Antimicrob. Agents 2009, 33 Suppl 1, 524–528. DOI: 10.1016/s0924-8579(09)70012-3. [DOI] [PubMed] [Google Scholar]
- (177).Gerding DN; Muto CA; Owens RC Jr. Measures to control and prevent Clostridium difficile infection. Clin. Infect. Dis 2008, 46 Suppl 1, S43–S49. DOI: 10.1086/521861. [DOI] [PubMed] [Google Scholar]
- (178).Martin JS; Monaghan TM; Wilcox MH Clostridium difficile infection: Epidemiology, diagnosis and understanding transmission. Nat. Rev. Gastroenterol Hepatol 2016, 13 (4), 206–216. DOI: 10.1038/nrgastro.2016.25. [DOI] [PubMed] [Google Scholar]
- (179).Solomon K. The host immune response to Clostridium difficile infection. Ther. Adv. Infect. Dis 2013, 1 (1), 19–35. DOI: 10.1177/2049936112472173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Reid CW; Vinogradov E; Li J; Jarrell HC; Logan SM; Brisson JR Structural characterization of surface glycans from Clostridium difficile. Carbohydr. Res 2012, 354, 65–73. DOI: 10.1016/j.carres.2012.02.002. [DOI] [PubMed] [Google Scholar]
- (181).Monteiro MA; Ma Z; Bertolo L; Jiao Y; Arroyo L; Hodgins D; Mallozzi M; Vedantam G; Sagermann M; Sundsmo J; Chow H Carbohydrate-based Clostridium difficile vaccines. Expert Rev. Vaccines 2013, 12 (4), 421–431. DOI: 10.1586/erv.l3.9. [DOI] [PubMed] [Google Scholar]
- (182).Adamo R; Romano MR; Berti F; Leuzzi R; Tontini M; Danieli E; Cappelletti E; Cakici OS; Swennen E; Pinto V; Brogioni B; Proietti D; Galeotti CL; Lay L; Monteiro MA; Scarselli M; Costantino P Phosphorylation of the synthetic hexasaccharide repeating unit is essential for the induction of antibodies to Clostridium difficile PSII cell wall polysaccharide. ACS Chem. Biol 2012, 7 (8), 1420–1428. DOI: 10.1021/cb300221f. [DOI] [PubMed] [Google Scholar]
- (183).Oberli MA; Hecht ML; Bindschädler P; Adibekian A; Adam T; Seeberger PH A possible oligosaccharide-conjugate vaccine candidate for Clostridium difficile is antigenic and immunogenic. Chem Biol. 2011, 18 (5), 580–588. DOI: 10.1016/j.chembiol.2011.03.009. [DOI] [PubMed] [Google Scholar]
- (184).Jiao Y; Ma Z; Hodgins D; Pequegnat B; Bertolo L; Arroyo L; Monteiro MA Clostridium difficile PSI polysaccharide: Synthesis of pentasaccharide repeating block, conjugation to exotoxin B subunit, and detection of natural anti-PSI IgG antibodies in horse serum. Carbohydr. Res 2013, 378, 15–25. DOI: 10.1016/j.carres.2013.03.018. [DOI] [PubMed] [Google Scholar]
- (185).Haag AF; Myka KK; Arnold MF; Caro-Hernández P; Ferguson GP Importance of lipopolysaccharide and cyclic β-1,2-Glucans in Brucella-mammalian infections. Int. J. Microbiol 2010, 2010, 124509. DOI: 10.1155/2010/124509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (186).Boschiroli ML; Foulongne V; O’Callaghan D Brucellosis: A worldwide zoonosis. Curr. Opin. Microbiol 2001, 4 (1), 58–64. DOI: 10.1016/s1369-5274(00)00165-x. [DOI] [PubMed] [Google Scholar]
- (187).Colmenero JD; Reguera JM; Cabrera FP; Cisneros JM; Orjuela DL; Fernández-Crehuet J Serology, clinical manifestations and treatment of brucellosis in different age groups. Infection 1990, 18 (3), 152–156. DOI: 10.1007/BF01642103. [DOI] [PubMed] [Google Scholar]
- (188).Bundle DR; McGiven J Brucellosis: Improved diagnostics and vaccine insights from synthetic glycans. Acc. Chem. Res 2017, 50 (12), 2958–2967. DOI: 10.1021/acs.accounts.7b00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (189).Kubler-Kielb J; Vinogradov E Reinvestigation of the structure of Brucella O-antigens. Carbohydr. Res 2013, 378, 144–147. DOI: 10.1016/j.carres.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (190).Zaccheus MV; Ali T; Cloeckaert A; Zygmunt MS; Weintraub A; Iriarte M; Moriyón I; Widmalm G The epitopic and structural characterization of brucella suis biovar 2 O-polysaccharide demonstrates the existence of a new M-negative C-negative smooth brucella serovar. PLoS One 2013, 8 (1), e53941. DOI: 10.1371/journal.pone.0053941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (191).Cherwonogrodzky JW; Perry MB; Bundle DR Identification of the A and M antigens of Brucella as the O-polysaccharides of smooth lipopolysaccharides. Canadian Journal of Microbiology 1987, 33 (11), 979–981. DOI: 10.1139/m87-172%M 3129169. [DOI] [PubMed] [Google Scholar]
- (192).Meikle PJ; Perry MB; Cherwonogrodzky JW; Bundle DR Fine structure of A and M antigens from Brucella biovars. Infection and Immunity 1989, 57 (9), 2820–2828. DOI: doi: 10.1128/iai.57.9.2820-2828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (193).Guiard J; Paszkiewicz E; Sadowska J; Bundle DR Design and synthesis of a universal antigen to detect brucellosis. Angew. Chem., Int. Ed. Engl 2013, 52 (28), 7181–7185. DOI: 10.1002/anie.201302303. [DOI] [PubMed] [Google Scholar]
- (194).Bundle DR; Cherwonogrodzky JW; Gidney MA; Meikle PJ; Perry MB; Peters T Definition of Brucella A and M epitopes by monoclonal typing reagents and synthetic oligosaccharides. Infection and Immunity 1989, 57 (9), 2829–2836. DOI: doi: 10.1128/iai.57.9.2829-2836.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (195).Ganesh NV; Sadowska JM; Sarkar S; Howells L; McGiven J; Bundle DR Molecular Recognition of Brucella A and M Antigens Dissected by Synthetic Oligosaccharide Glycoconjugates Leads to a Disaccharide Diagnostic for Brucellosis. Journal of the American Chemical Society 2014, 136 (46), 16260–16269. DOI: 10.1021/ja5081184. [DOI] [PubMed] [Google Scholar]
- (196).McGiven J; Howells L; Duncombe L; Stack J; Ganesh NV; Guiard J; Bundle DR Improved Serodiagnosis of Bovine Brucellosis by Novel Synthetic Oligosaccharide Antigens Representing the Capping M Epitope Elements of Brucella O-Polysaccharide. Journal of Clinical Microbiology 2015, 53 (4), 1204–1210. DOI: doi: 10.1128/JCM.03185-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Mandal SS; Duncombe L; Ganesh NV; Sarkar S; Howells L; Hogarth PJ; Bundle DR; McGiven J Novel Solutions for Vaccines and Diagnostics To Combat Brucellosis. ACS Central Science 2017, 3 (3), 224–231. DOI: 10.1021/acscentsci.7b00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (198).Titball RW; Burtnick MN; Bancroft GJ; Brett P Burkholderia pseudomallei and Burkholderia mallei vaccines: Are we close to clinical trials? Vaccine 2017, 35 (44), 5981–5989. DOI: 10.1016/j.vaccine.2017.03.022. [DOI] [PubMed] [Google Scholar]
- (199).Cheng AC; Currie BJ Melioidosis: Epidemiology, pathophysiology, and management. Clin. Microbiol. Rev 2005, 18 (2), 383–416. DOI: 10.1128/cmr.l8.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (200).Heiss C; Burtnick MN; Roberts RA; Black I; Azadi P; Brett PJ Revised structures for the predominant O-polysaccharides expressed by Burkholderia pseudomallei and Burkholderia mallei. Carbohydr. Res 2013, 381, 6–11. DOI: 10.1016/j.carres.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (201).Tamigney Kenfack M; Mazur M; Nualnoi T; Shaffer TL; Ngassimou A; Blériot Y; Marrot J; Marchetti R; Sintiprungrat K; Chantratita N; Silipo A; Molinaro A; AuCoin DP; Burtnick MN; Brett PJ; Gauthier C Deciphering minimal antigenic epitopes associated with Burkholderia pseudomallei and Burkholderia mallei lipopolysaccharide O-antigens. Nat. Commun 2017, 8 (1), 115. DOI: 10.1038/s41467-017-00173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (202).Cloutier M; Delar E; Muru K; Ndong S; Hoyeck RR; Kaewarpai T; Chantratita N; Burtnick MN; Brett PJ; Gauthier C Melioidosis patient serum-reactive synthetic tetrasaccharides bearing the predominant epitopes of Burkholderia pseudomallei and Burkholderia mallei O-antigens. Org. Biomol. Chem 2019, 17 (39), 8878–8901. DOI: 10.1039/c9ob0171la. [DOI] [PubMed] [Google Scholar]
- (203).Scott AE; Christ WJ; George AJ; Stokes MGM; Lohman GJS; Guo Y; Jones M; Titball RW; Atkins TP; Campbell AS; Prior JL Protection against experimental melioidosis with a synthetic manno-heptopyranose hexasaccharide glycoconjugate. Bioconjugate Chem. 2016, 27 (6), 1435–1446. DOI: 10.1021/acs.bioconjchem.5b00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (204).Geng X; Wang G; Guo Z; Gu G Synthesis of the oligosaccharides of Burkholderia pseudomallei and B. mallei capsular polysaccharide and preliminary immunological studies of their protein conjugates. J. Org. Chem 2020, 85 (4), 2369–2384. DOI: 10.1021/acs.joc.9b03085. [DOI] [PubMed] [Google Scholar]
- (205).Sack DA; Sack RB; Nair GB; Siddique AK Cholera. Lancet 2004, 363 (9404), 223–233. DOI: 10.1016/S0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- (206).Mintz ED; Guerrant RL A lion in our village—The unconscionable tragedy of cholera in Africa. N. Engl. J. Med 2009, 360 (11), 1060–1063. DOI: 10.1056/NEJMp0810559. [DOI] [PubMed] [Google Scholar]
- (207).Glass RI; Becker S; Huq MI; Stoll BJ; Khan MU; Merson MH; Lee JV; Black RE Endemic cholera in rural Bangaldesh, 1966–1980. Am. J. Epidemiol 1982, 116(6), 959–970. DOI: 10.1093/oxfordjoumals.aje.al13498. [DOI] [PubMed] [Google Scholar]
- (208).Organization, W. H. Cholera, 2018. Weekly epidemiological record 2019, 94 (48), 561–568. [Google Scholar]
- (209).Harris JB Cholera: Immunity and prospects in vaccine development. J. Infect. Dis 2018, 218 (suppl_3), S141–S146. DOI: 10.1093/infdis/jiy414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (210).Shaikh H; Lynch J; Kim J; Excler J-L Current and future cholera vaccines. Vaccine 2020, 38, A118–A126. DOI: 10.1016/j.vaccine.2019.12.011. [DOI] [PubMed] [Google Scholar]
- (211).Saksena R; Ma X; Kováč P One-pot preparation of a series of glycoconjugates with predetermined antigen–carrier ratio from oligosaccharides that mimic the O-PS of Vibrio cholerae O:1, serotype Ogawa. Carbohydr. Res 2003, 338 (23), 2591–2603. DOI: 10.1016/S0008-6215(03)00273-8. [DOI] [PubMed] [Google Scholar]
- (212).Saksena R; Ma X; Wade TK; Kováč P; Wade WF Effect of saccharide length on the immunogenicity of neoglycoconjugates from synthetic fragments of the O-SP of Vibrio cholerae O1, serotype Ogawa. Carbohydr. Res 2005, 340 (14), 2256–2269. DOI: 10.1016/j.carres.2005.07.017. [DOI] [PubMed] [Google Scholar]
- (213).Chernyak A; Kondo S; Wade TK; Meeks MD; Alzari PM; Fournier J-M; Taylor RK; Kovaáč P; Wade WF Induction of protective immunity by synthetic Vibrio cholerae hexasaccharide derived from V. cholerae O1 Ogawa lipopolysaccharide bound to a protein carrier. J. Infect. Dis 2002, 185 (7), 950–962. DOI: 10.1086/339583. [DOI] [PubMed] [Google Scholar]
- (214).Wade TK; Saksena R; Shiloach J; Kováč P; Wade WF Immunogenicity of synthetic saccharide fragments of Vibrio cholerae O1 (Ogawa and Inaba) bound to exotoxin A. FEMS Immunol. Med. Microbiol 2006, 48 (2), 237–251. DOI: 10.1111/j.1574-695X.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- (215).Albert MJ Vibrio cholerae O139 Bengal. J. Clin. Microbiol 1994, 32 (10), 2345–2349. DOI: doi: 10.1128/jcm.32.10.2345-2349.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (216).Weintraub A; Widmalm G; Jansson P-E; Jansson M; Hultenby K; Albert MJ Vibrio cholerae O139 Bengal possesses a capsular polysaccharide which may confer increased virulence. Microb. Pathog 1994, 16 (3), 235–241. DOI: 10.1006/mpat.1994.1024. [DOI] [PubMed] [Google Scholar]
- (217).Soliman SE; Kováč P Total synthesis of the complete protective antigen of Vibrio cholerae O139. Angew. Chem., Int. Ed 2016, 55 (41), 12850–12853. DOI: 10.1002/anie.201606116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (218).Pfister HB; Kelly M; Qadri F; Ryan ET; Kováč P Synthesis of glycocluster-containing conjugates for a vaccine against cholera. Org. Biomol. Chem 2019, 17 (16), 4049–4060. DOI: 10.1039/C90B00368A. [DOI] [PubMed] [Google Scholar]
- (219).Global tuberculosis report 2021. Geneva: World Health Organization; 2021. Geneva: World Health Organization, 2021. https://www.who.int/publications/i/item/9789240037021 (accessed. [Google Scholar]
- (220).Gandhi NR; Nunn P; Dheda K; Schaaf HS; Zignol M; van Soolingen D; Jensen P; Bayona J Multidrug-resistant and extensively drug-resistant tuberculosis: A threat to global control of tuberculosis. Lancet 2010, 375 (9728), 1830–1843. DOI: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- (221).Egelund EF; Dupree L; Huesgen E; Peloquin CA The pharmacological challenges of treating tuberculosis and HIV coinfections. Expert Rev. Clin. Pharmacol 2017, 10 (2), 213–223. DOI: 10.1080/17512433.2017.1259066. [DOI] [PubMed] [Google Scholar]
- (222).Fine PEM Variation in protection by BCG: Implications of and for heterologous immunity. Lancet 1995, 346 (8986), 1339–1345. DOI: 10.1016/S0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- (223).Guleria I; Teitelbaum R; McAdam RA; Kalpana G; Jacobs WR; Bloom BR Auxotrophic vaccines for tuberculosis. Nat. Med 1996, 2 (3), 334–337. DOI: 10.1038/nm0396-334. [DOI] [PubMed] [Google Scholar]
- (224).Tascon RE; Colston MJ; Ragno S; Stavropoulos E; Gregory D; Lowrie DB Vaccination against tuberculosis by DNA injection. Nat. Med 1996, 2 (8), 888–892. DOI: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- (225).Zhu X; Venkataprasad N; Thangaraj HS; Hill M; Singh M; Ivanyi J; Vordermeier HM Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J. Immunol 1997, 158 (12), 5921–5926. [PubMed] [Google Scholar]
- (226).Pal PG; Horwitz MA Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect. Immun 1992, 60 (11), 4781–4792. DOI: doi: 10.1128/iai.60.11.4781-4792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (227).Kaur D; Guerin ME; škovierová H; Brennan PJ; Jackson M Biogenesis of the cell wall and other glycoconjugates of Mycobacterium tuberculosis. In Adv. Appl. Microbiol, Vol. 69; Academic Press, 2009; pp 23–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (228).Angala SK; Belardinelli JM; Huc-Claustre E; Wheat WH; Jackson M The cell envelope glycoconjugates of Mycobacterium tuberculosis. Crit. Rev. Biochem. Mol. Biol 2014, 49 (5), 361–399. DOI: 10.3109/10409238.2014.925420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (229).Bai B; Chu C.-j.; Lowary TL Lipooligosaccharides from mycobacteria: Structure, function, and synthesis. Isr. J. Chem 2015, 55 (3-4), 360–372. DOI: 10.1002/ijch.201400194. [DOI] [Google Scholar]
- (230).Lee A; Wu S-W; Scherman MS; Torrelles JB; Chatterjee D; McNeil MR; Khoo K-H Sequencing of oligoarabinosyl units released from mycobacterial arabinogalactan by endogenous arabinanase: Identification of distinctive and novel structural motifs. Biochemistry 2006, 45 (51), 15817–15828. DOI: 10.1021/bi060688d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (231).Kaplan G; Gandhi RR; Weinstein DE; Levis WR; Patarroyo ME; Brennan PJ; Cohn ZA Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J. Immunol 1987, 138 (9), 3028–3034. [PubMed] [Google Scholar]
- (232).Chan J; Lan XD; Hunter SW; Brennan PJ; Bloom BR Lipoarabinomannan. a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect. Immun 1991, 59, 1755–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (233).Seidel M; Alderwick LJ; Birch HL; Sahm H; Eggeling L; Besra GS Identification of a novel arabinofuranosyltransferase AftB involved in a terminal step of cell wall arabinan biosynthesis in corynebacterianeae, such as Corynebacterium glutamicum and Mycobacterium tuberculosis. J. Biol. Chem 2007, 282 (20), 14729–14740. DOI: 10.1074/jbc.M700271200. [DOI] [PubMed] [Google Scholar]
- (234).Birch HL; Alderwick LJ; Bhatt A; Rittmann D; Krumbach K; Singh A; Bai Y; Lowary TL; Eggeling L; Besra GS Biosynthesis of mycobacterial arabinogalactan: Identification of a novel alpha(1-3) arabinofuranosyltransferase. Mol. Microbiol 2008, 69 (5), 1191–1206. DOI: 10.1111/j.l365-2958.2008.06354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (235).Brennan P; Ballou CE Biosynthesis of annophosphoinositides by Mycobacterium phlei: The family of dimannophosphoinositides. J. Biol. Chem 1967, 242 (13), 3046–3056. DOI: 10.1016/S0021-9258(18)95931-4. [DOI] [PubMed] [Google Scholar]
- (236).Hill DL; Ballou CE Biosynthesis of mannophospholipids by Mycobacterium phlei. J. Biol. Chem 1966, 241 (4), 895–902. DOI: 10.1016/S0021-9258(18)96849-3. [DOI] [PubMed] [Google Scholar]
- (237).Jankute M; Cox JAG; Harrison J; Besra GS Assembly of the mycobacterial cell wall. Annu. Rev. Microbiol 2015, 69 (1), 405–423. DOI: 10.1146/annurev-micro-091014-104121. [DOI] [PubMed] [Google Scholar]
- (238).Jankute M; Alderwick LJ; Noack S; Veerapen N; Nigou J; Besra GS Disruption of mycobacterial AftB results in complete loss of terminal β(1→2) arabinofuranose residues of lipoarabinomannan. ACS Chem. Biol 2017, 12 (1), 183–190. DOI: 10.1021/acschembio.6b00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (239).Alderwick LJ; Birch HL; Mishra AK; Eggeling L; Besra GS Structure, function and biosynthesis of the Mycobacterium tuberculosis cell wall: Arabinogalactan and lipoarabinomannan assembly with a view to discovering new drug targets. Biochem. Soc. Trans 2007, 35 (5), 1325–1328. DOI: 10.1042/bst0351325. [DOI] [PubMed] [Google Scholar]
- (240).Cao B; Williams SJ Chemical approaches for the study of the mycobacterial glycolipids phosphatidylinositol mannosides, lipomannan and lipoarabinomannan. Nat. Prod. Rep 2010, 27 (6), 919–947. DOI: 10.1039/C000604A. [DOI] [PubMed] [Google Scholar]
- (241).Holzheimer M; Buter J; Minnaard AJ Chemical synthesis of cell wall constituents of Mycobacterium tuberculosis. Chem. Rev 2021, 121 (15), 9554–9643. DOI: 10.1021/acs.chemrev.1c00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (242).Boonyarattanakalin S; Liu X; Michieletti M; Lepenies B; Seeberger PH Chemical synthesis of all phosphatidylinositol mannoside (PIM) glycans from Mycobacterium tuberculosis. J. Am. Chem. Soc 2008, 130 (49), 16791–16799. DOI: 10.1021/ja806283e. [DOI] [PubMed] [Google Scholar]
- (243).Wang L; Feng S; An L; Gu G; Guo Z Synthetic and immunological studies of mycobacterial lipoarabinomannan oligosaccharides and their protein conjugates. J. Org. Chem 2015, 80(20), 10060–10075. DOI: 10.1021/acs.joc.5b01686. [DOI] [PubMed] [Google Scholar]
- (244).Wang L; Feng S; Wang S; Li H; Guo Z; Gu G Synthesis and immunological comparison of differently linked lipoarabinomannan oligosaccharide–Monophosphoryl Lipid A conjugates as antituberculosis vaccines. J. Org. Chem 2017, 82 (23), 12085–12096. DOI: 10.1021/acs.joc.7b01817. [DOI] [PubMed] [Google Scholar]
- (245).Chan ED; Reves R; Belisle JT; Brennan PJ; Hahn WE Diagnosis of tuberculosis by a visually detectable immunoassay for lipoarabinomannan. Am. J. Respir. Crit. Care Med 2000, 161 (5), 1713–1719. DOI: 10.1164/ajrccm.161.5.9908125. [DOI] [PubMed] [Google Scholar]
- (246).Abronina PI; Podvalnyy NM; Mel’nikova TM; Zinin AI; Fedina KG; Kachala VV; Torgov VI; Kononov LO; Panfertsev EA; Baranova EV; Mochalov VV; Dyatlova VI; Biketov SF Synthesis of covalent conjugates of hexaarabinofuranoside with proteins and their testing as antigens for serodiagnosis of tuberculosis. Russ. Chem. Bull 2010, 59 (12), 2333–2337. DOI: 10.1007/sl1172-010-0397-4. [DOI] [Google Scholar]
- (247).Podvalnyy NM; Chizhov AO; Zinin AI; Kononov LO Rapid synthesis of linear homologous oligoarabinofuranosides related to mycobacterial lipoarabinomannan and a neoglycoconjugate thereof. Carbohydr. Res 2016, 431, 25–32. DOI: 10.1016/j.carres.2016.05.009. [DOI] [PubMed] [Google Scholar]
- (248).Bundle DR; Tam P-H; Tran H-A; Paszkiewicz E; Cartmell J; Sadowska JM; Sarkar S; Joe M; Kitov PI Oligosaccharides and peptide displayed on an amphiphilic polymer enable solid phase assay of hapten specific antibodies. Bioconjugate Chem. 2014, 25 (4), 685–697. DOI: 10.1021/bc400486w. [DOI] [PubMed] [Google Scholar]
- (249).Chou Y; Kitova EN; Joe M; Brunton R; Lowary TL; Klassen JS; Derda R Genetically-encoded fragment-based discovery (GE-FBD) of glycopeptide ligands with differential selectivity for antibodies related to mycobacterial infections. Org. Biomol. Chem 2018, 16(2), 223–227. DOI: 10.1039/C70B02783D. [DOI] [PubMed] [Google Scholar]
- (250).Cunningham MW Pathogenesis of Group A streptococcal infections and their sequelae. New York, NY, 2008; Springer New York: pp 29–42. [DOI] [PubMed] [Google Scholar]
- (251).Walker MJ; Barnett TC; McArthur JD; Cole JN; Gillen CM; Henningham A; Sriprakash KS; Sanderson-Smith ML; Nizet V Disease manifestations and pathogenic mechanisms of Group A Streptococcus. Clin. Microbiol. Rev 2014, 27 (2), 264–301. DOI: doi: 10.1128/CMR.00101-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (252).Carapetis JR; Steer AC; Mulholland EK; Weber M The global burden of group A streptococcal diseases. Lancet Infect. Dis 2005, 5 (11), 685–694. DOI: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- (253).Dale JB; Penfound TA; Chiang EY; Walton WJ New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci. Vaccine 2011, 29 (46), 8175–8178. DOI: 10.1016/j.vaccine.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (254).Cunningham MW; Hall NK; Krisher KK; Spanier AM A study of anti-group A streptococcal monoclonal antibodies cross-reactive with myosin. J. Immunol 1986, 136 (1), 293–298. [PubMed] [Google Scholar]
- (255).Cunningham MW Pathogenesis of Group A streptococcal infections. Clin. Microbiol. Rev 2000, 13 (3), 470–511. DOI: doi: 10.1128/CMR.13.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (256).Cleary PP; Matsuka YV; Huynh T; Lam H; Olmsted SB Immunization with C5a peptidase from either group A or B streptococci enhances clearance of group A streptococci from intranasally infected mice. Vaccine 2004, 22 (31), 4332–4341. DOI: 10.1016/j.vaccine.2004.04.030. [DOI] [PubMed] [Google Scholar]
- (257).Shet A; Kaplan EL; Johnson DR; Cleary PP Immune response to group A Streptococcal C5a peptidase in children: Implications for vaccine development. J. Infect. Dis 2003, 188 (6), 809–817. DOI: 10.1086/377700. [DOI] [PubMed] [Google Scholar]
- (258).van Sorge Nina M.; Cole Jason N.; Kuipers K; Henningham A; Aziz Ramy K.; Kasirer-Friede A; Lin L; Berends Evelien T. M.; Davies Mark R.; Dougan G; Zhang F; Dahesh S; Shaw L; Gin J; Cunningham M; Merriman Joseph A.; Hütter J; Lepenies B; Rooijakkers Suzan H. M.; Malley R; Walker Mark J.; Shattil Sanford J.; Schlievert Patrick M.; Choudhury B; Nizet V The classical lancefield antigen of Group A Streptococcus is a virulence determinant with implications for vaccine design. Cell Host Microbe 2014, 15 (6), 729–740. DOI: 10.1016/j.chom.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (259).Reimer KB; Gidney MAJ; Bundle DR; Pinto BM Immunochemical characterization of polyclonal and monoclonal Streptococcus group A antibodies by chemically defined glycoconjugates and synthetic oligosaccharides. Carbohydr. Res 1992, 232 (1), 131–142. DOI: 10.1016/S0008-6215(00)91000-0. [DOI] [PubMed] [Google Scholar]
- (260).Michon F; Moore SL; Kim J; Blake MS; Auzanneau F-I; Johnston BD; Johnson MA; Pinto BM Doubly branched hexasaccharide epitope on the cell wall polysaccharide of Group A Streptococci recognized by human and rabbit antisera. Infect. Immun 2005, 73 (10), 6383–6389. DOI: doi: 10.1128/IAI.73.10.6383-6389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (261).Harris SL; Craig L; Mehroke JS; Rashed M; Zwick MB; Kenar K; Toone EJ; Greenspan N; Auzanneau F-I; Marino-Albernas J-R; Pinto BM; Scott JK Exploring the basis of peptide-carbohydrate crossreactivity: Evidence for discrimination by peptides between closely related anti-carbohydrate antibodies. Proc. Natl. Acad. Sci. U. S. A 1997, 94 (6), 2454–2459. DOI: doi: 10.1073/pnas.94.6.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (262).Pitner JB; Beyer WF; Venetta TM; Nycz C; Mitchell MJ; Harris SL; Mariño-Albernas JR; Auzanneau F-I; Forooghian F; Mario Pinto B Bivalency and epitope specificity of a high-affinity IgG3 monoclonal antibody to the Streptococcus Group A carbohydrate antigen. Molecular modeling of a Fv fragment. Carbohydr. Res 2000, 324 (1), 17–29. DOI: 10.1016/S0008-6215(99)00279-7. [DOI] [PubMed] [Google Scholar]
- (263).Auzanneau F-I; Borrelli S; Pinto BM Synthesis and immunological activity of an oligosaccharide-conjugate as a vaccine candidate against Group A Streptococcus. Bioorg. Med. Chem. Lett 2013, 23 (22), 6038–6042. DOI: 10.1016/j.bmcl.2013.09.042. [DOI] [PubMed] [Google Scholar]
- (264).Kabanova A; Margarit I; Berti F; Romano MR; Grandi G; Bensi G; Chiarot E; Proietti D; Swennen E; Cappelletti E; Fontani P; Casini D; Adamo R; Pinto V; Skibinski D; Capo S; Buffi G; Gallotta M; Christ WJ; Stewart Campbell A; Pena J; Seeberger PH; Rappuoli R; Costantino P Evaluation of a Group A Streptococcus synthetic oligosaccharide as vaccine candidate. Vaccine 2010, 29 (1), 104–114. DOI: 10.1016/j.vaccine.2010.09.018. [DOI] [PubMed] [Google Scholar]
- (265).Wang S; Zhao Y; Wang G; Feng S; Guo Z; Gu G Group A Streptococcus cell wall oligosaccharide-streptococcal C5a peptidase conjugates as effective antibacterial vaccines. ACS Infect. Dis 2020, 6 (2), 281–290. DOI: 10.1021/acsinfecdis.9b00347. [DOI] [PubMed] [Google Scholar]
- (266).Le Doare K; Heath PT An overview of global GBS epidemiology. Vaccine 2013, 31, D7–D12. DOI: 10.1016/j.vaccine.2013.01.009. [DOI] [PubMed] [Google Scholar]
- (267).Heath PT; Feldman RG Vaccination against Group B streptococcus. Expert Rev. Vaccines 2005, 4 (2), 207–218. DOI: 10.1586/14760584.4.2.207. [DOI] [PubMed] [Google Scholar]
- (268).Johri AK; Paoletti LC; Glaser P; Dua M; Sharma PK; Grandi G; Rappuoli R Group B Streptococcus: Global incidence and vaccine development. Nat. Rev. Microbiol 2006, 4 (12), 932–942. DOI: 10.1038/nrmicro1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (269).Dermer P; Lee C; Eggert J; Few B A history of neonatal group B streptococcus with its related morbidity and mortality rates in the United States. J. Ped. Nur 2004, 19 (5), 357–363. DOI: 10.1016/j.pedn.2004.05.012. [DOI] [PubMed] [Google Scholar]
- (270).High KP; Edwards MS; Baker CJ Group B streptococcal infections in elderly adults. Clin. Infect. Dis 2005, 41 (6), 839–847. DOI: 10.1086/432804. [DOI] [PubMed] [Google Scholar]
- (271).Bianchi-Jassir F; Paul P; To K-N; Carreras-Abad C; Seale AC; Jauneikaite E; Madhi SA; Russell NJ; Hall J; Madrid L; Bassat Q; Kwatra G; Le Doare K; Lawn JE Systematic review of Group B streptococcal capsular types, sequence types and surface proteins as potential vaccine candidates. Vaccine 2020, 38 (43), 6682–6694. DOI: 10.1016/j.vaccine.2020.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (272).Seale AC; Bianchi-Jassir F; Russell NJ; Kohli-Lynch M; Tann CJ; Hall J; Madrid L; Blencowe H; Cousens S; Baker CJ; Bartlett L; Cutland C; Gravett MG; Heath PT; Ip M; Le Doare K; Madhi SA; Rubens CE; Saha SK; Schrag SJ; Sobanjo-ter Meulen A; Vekemans J; Lawn JE Estimates of the burden of group B streptococcal disease worldwide for pregnant women, stillbirths, and children. Clin. Infect. Dis 2017, 65 (suppl_2), S200–S219. DOI: 10.1093/cid/cix664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (273).Lin SM; Zhi Y; Ahn KB; Lim S; Seo HS Status of group B streptococcal vaccine development. Clin. Exp. Vaccine Res 2018, 7 (1), 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (274).Rubens CE; Wessels MR; Heggen LM; Kasper DL Transposon mutagenesis of type III group B Streptococcus: Correlation of capsule expression with virulence. Proc. Natl. Acad. Sci. U. S. A 1987, 84 (20), 7208–7212. DOI: doi: 10.1073/pnas.84.20.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (275).Cieslewicz MJ; Chaffin D; Glusman G; Kasper D; Madan A; Rodrigues S; Fahey J; Wessels MR; Rubens CE Structural and genetic diversity of Group B Streptococcus capsular polysaccharides. Infect. Immun 2005, 73 (5), 3096–3103. DOI: doi: 10.1128/IAI.73.5.3096-3103.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (276).Berti F; Campisi E; Toniolo C; Morelli L; Crotti S; Rosini R; Romano MR; Pinto V; Brogioni B; Torricelli G; Janulczyk R; Grandi G; Margarit I Structure of the Type IX Group B Streptococcus Capsular Polysaccharide and Its Evolutionary Relationship with Types V and VII. J. Biol. Chem 2014, 289 (34), 23437–23448. DOI: 10.1074/jbc.M114.567974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (277).Nuccitelli A; Rinaudo CD; Maione D Group B Streptococcus vaccine: State of the art. Ther. Adv. Vaccines 2015, 3 (3), 76–90. DOI: 10.1177/2051013615579869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (278).Chen VL; Avci FY; Kasper DL A maternal vaccine against group B Streptococcus: Past, present, and future. Vaccine 2013, 31, D13–D19. DOI: 10.1016/j.vaccine.2012.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (279).Cattaneo V; Carboni F; Oldrini D; Ricco RD; Donadio N; Ros IMY; Berti F; Adamo R Synthesis of Group B Streptococcus type III polysaccharide fragments for evaluation of their interactions with monoclonal antibodies. Pure Appl. Chem 2017, 89 (7), 855–875. DOI: doi: 10.1515/pac-2016-0918. [DOI] [Google Scholar]
- (280).Carboni F; Adamo R; Fabbrini M; De Ricco R; Cattaneo V; Brogioni B; Veggi D; Pinto V; Passalacqua I; Oldrini D; Rappuoli R; Malito E; Margarit I. y. R.; Berti F Structure of a protective epitope of group B Streptococcus type III capsular polysaccharide. Proc. Natl. Acad. Sci. U. S. A 2017, 114 (19), 5017–5022. DOI: doi: 10.1073/pnas.1701885114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (281).Carboni F; Angiolini F; Fabbrini M; Brogioni B; Corrado A; Berti F; Adamo R; Margarit I Evaluation of immune responses to Group B Streptococcus Type III oligosaccharides containing a minimal protective epitope. J. Infect. Dis 2019, 221 (6), 943–947. DOI: 10.1093/infdis/jiz551. [DOI] [PubMed] [Google Scholar]
- (282).Oldrini D; del Bino L; Arda A; Carboni F; Henriques P; Angiolini F; Quintana JI; Calloni I; Romano MR; Berti F; Jimenez-Barbero J; Margarit I; Adamo R Structure-guided design of a Group B Streptococcus Type III synthetic glycan–conjugate vaccine. Chem. - Eur. J 2020, 26 (31), 7018–7025. DOI: 10.1002/chem.202000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (283).Mondal PK; Liao G; Mondal MA; Guo Z Chemical synthesis of the repeating unit of Type Ia Group B Streptococcus capsular polysaccharide. Org. Lett 2015, 17 (5), 1102–1105. DOI: 10.1021/ol5036563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (284).Michon F; Brisson JR; Dell A; Kasper DL; Jennings HJ Multiantennary group-specific polysaccharide of group B streptococcus. Biochemistry 1988, 27 (14), 5341–5351. DOI: 10.1021/bi00414a059. [DOI] [PubMed] [Google Scholar]
- (285).Michon F; Katzenellenbogen E; Kasper DL; Jennings HJ Structure of the complex group-specific polysaccharide of group B Streptococcus. Biochemistry 1987, 26 (2), 476–486. DOI: 10.1021/bi00376a020. [DOI] [PubMed] [Google Scholar]
- (286).Marques MB; Kasper DL; Shroff A; Michon F; Jennings HJ; Wessels MR Functional activity of antibodies to the group B polysaccharide of group B Streptococci elicited by a polysaccharide-protein conjugate vaccine. Infect. Immun 1994, 62 (5), 1593–1599. DOI: doi: 10.1128/iai.62.5.1593-1599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (287).Caliot É; Dramsi S; Chapot-Chartier MP; Courtin P; Kulakauskas S; Péchoux C; Trieu-Cuot P; Mistou MY Role of the Group B antigen of Streptococcus agalactiae: A peptidoglycan-anchored polysaccharide involved in cell wall biogenesis. PLoS Pathog. 2012, 8 (6), e1002756. DOI: 10.1371/joumal.ppat.l002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (288).Wang Z; Enotarpi J; Buffi G; Pezzicoli A; Gstöttner CJ; Nicolardi S; Balducci E; Fabbrini M; Romano MR; van der Marel GA; del Bino L; Adamo R; Codée JDC Chemical synthesis and immunological evaluation of fragments of the multiantennary group-specific polysaccharide of Group B Streptococcus. In JACS Au, 2022; Vol. 2, pp 1724–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (289).Aschwanden C. Five reasons why COVID herd immunity is probably impossible. Nature 2021, 591, 520–522, News Feature. DOI: 10.1038/d41586-021-00728-2. [DOI] [PubMed] [Google Scholar]
- (290).Minor PD Live attenuated vaccines: Historical successes and current challenges. Virology 2015, 479-480, 379–392. DOI: 10.1016/j.virol.2015.03.032. [DOI] [PubMed] [Google Scholar]
- (291).Cuccui J; Wren B Hijacking bacterial glycosylation for the production of glycoconjugates, from vaccines to humanised glycoproteins. Journal of Pharmacy and Pharmacology 2015, 67 (3), 338–350. DOI: 10.11n/jphp.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (292).Ravenscroft N; Haeuptle MA; Kowarik M; Fernandez FS; Carranza P; Brunner A; Steffen M; Wetter M; Keller S; Ruch C; Wacker M Purification and characterization of a Shigella conjugate vaccine, produced by glycoengineering Escherichia coli. Glycobiology 2015, 26 (1), 51–62. DOI: 10.1093/glycob/cwv077. [DOI] [PubMed] [Google Scholar]
- (293).Cuccui J; Thomas RM; Moule MG; D’Elia RV; Laws TR; Mills DC; Williamson D; Atkins TP; Prior JL; Wren BW Exploitation of bacterial N-linked glycosylation to develop a novel recombinant glycoconjugate vaccine against Francisella tularensis. Open Biology 2013, 3 (5), 130002. DOI: doi: 10.1098/rsob.130002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (294).Wacker M; Wang L; Kowarik M; Dowd M; Lipowsky G; Faridmoayer A; Shields K; Park S; Alaimo C; Kelley KA; Braun M; Quebatte J; Gambillara V; Carranza P; Steffen M; Lee JC Prevention of Staphylococcus aureus Infections by Glycoprotein Vaccines Synthesized in Escherichia coli. The Journal of Infectious Diseases 2013, 209 (10), 1551–1561. DOI: 10.1093/infdis/jit800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (295).Naini A; Bartetzko MP; Sanapala SR; Broecker F; Wirtz V; Lisboa MP; Parameswarappa SG; Knopp D; Przygodda J; Hakelberg M; Pan R; Patel A; Chorro L; Illenberger A; Ponce C; Kodali S; Lypowy J; Anderson AS; Donald RGK; von Bonin A; Pereira CL Semisynthetic Glycoconjugate Vaccine Candidates against Escherichia coli O25B Induce Functional IgG Antibodies in Mice. JACS Au 2022, 2 (9), 2135–2151. DOI: 10.1021/jacsau.2c00401. [DOI] [PMC free article] [PubMed] [Google Scholar]


