Abstract
Background
SARS-CoV-2 infection rates are likely to be underestimated in children because of asymptomatic or mild infections. We aim to estimate national and regional prevalence of SARS-CoV-2 antibodies in primary (4–11 years old) and secondary (11–18 years old) school children between 10 November and 10 December 2021.
Methods
Cross-sectional surveillance in England using two-stage sampling, firstly stratifying into regions and selecting local authorities, then selecting schools according to a stratified sample within selected local authorities. Participants were sampled using a novel oral fluid-validated assay for SARS-CoV-2 spike and nucleocapsid IgG antibodies.
Results
4980 students from 117 state-funded schools (2706 from 83 primary schools, 2274 from 34 secondary schools) provided a valid sample. After weighting for age, sex, and ethnicity, and adjusting for assay accuracy, the national prevalence of SARS-CoV-2 antibodies in primary school students, who were all unvaccinated, was 40.1% (95% CI 37.3–43.0). Antibody prevalence increased with age (p < 0.001) and was higher in urban than rural schools (p = 0.01). In secondary school students, the adjusted, weighted national prevalence of SARS-CoV-2 antibodies was 82.4% (95% CI 79.5–85.1); including 71.5% (95% CI 65.7–76.8) in unvaccinated and 97.5% (95% CI 96.1–98.5) in vaccinated students. Antibody prevalence increased with age (p < 0.001), and was not significantly different in urban versus rural students (p = 0.1).
Conclusions
In November 2021, using a validated oral fluid assay, national SARS-CoV-2 seroprevalence was estimated to be 40.1% in primary school students and 82.4% in secondary school students. In unvaccinated children, this was approximately threefold higher than confirmed infections highlighting the importance of seroprevalence studies to estimate prior exposure.
Data availability
Deidentified study data are available for access by accredited researchers in the ONS Secure Research Service (SRS) for accredited research purposes under part 5, chapter 5 of the Digital Economy Act 2017. For further information about accreditation, contact Research.support@ons.gov.uk or visit the SRS website.
Keywords: Seroprevalence, SARS-CoV-2, Antibodies, Oral fluid, School pupils, COVID-19 vaccination
Introduction
Children have a very low risk of severe or fatal COVID-19 compared to adults and typically remain asymptomatic or develop mild, transient symptoms when infected with SARS-CoV-2, the virus responsible for COVID-19.1 Early pandemic investigations using PCR-testing in symptomatic individuals suggested that children may be less susceptible to SARS-CoV-2 infection,1 but subsequent studies, importantly using SARS-CoV-2 antibodies to measure both symptomatic and asymptomatic infections, indicated a bigger role for children in infection and transmission of the virus.2 Additionally, we and others have shown that children develop robust humoral and cellular immune responses against SARS-CoV-2 irrespective of symptomatic or asymptomatic infection.3
In the UK, 16–17 years old were offered the vaccine from 4 August 2021, after adults and those with comorbidities.4 On 3 September 2021, the UK Joint Committee on Vaccination and Immunisation (JCVI) recommended against vaccinating healthy 12–15 years old because of marginal risk-benefits and concerns about the risk of vaccine-induced myocarditis in young men.5 This decision was, however, overturned by UK ministers on 14 September 2021 and the vaccine was subsequently offered to this age group to reduce educational disruption.6
The UK has been proactive in keeping schools open throughout the pandemic, ensuring that they were the last settings to close and first to reopen. All schools were closed following the first national lockdown in March 2020, and then partially reopened in June 2020 with extensive mitigations in place.7 In-school studies showed limited infection and transmission among staff or students, facilitating the full reopening of all school years for in-person learning from September 2020.8 In school-aged children, case rates increased after returning to school during September-October 2020, with further increases in December 2020 following the emergence of the Alpha variant in November 2020, during June-July 2021 following the emergence of the Delta variant in April/May 2020, and during September-December 2021, when all mitigations were removed from schools.9, 10, 11
As part of national surveillance, the UK Health Security Agency (UKHSA) developed and validated an oral fluid (OF) assay to facilitate large-scale community SARS-CoV-2 seroprevalence studies.12 This assay was initially used in a large national Schools Infection Survey (SIS1), an open cohort study of SARS-CoV-2 infection and transmission in state-funded educational settings during the 2020/21 academic year.13 For the 2021/22 academic year (SIS2), the methodology was revised and extended to include a nationally and regionally representative sample of primary and secondary school-aged children.14 An additional assay was used to distinguish between infection-(nucleocapsid and spike protein) and vaccine-induced (spike protein only) antibodies. Here, we report the first results of SIS-2 with national and regional prevalence of SARS-CoV-2 antibodies in primary and secondary school aged children during November/December 2021 in England.
Methods
Sampling methodology
The schools were identified from a list of state-funded schools held by the Department for Education (DfE) and were equally distributed across nine English regions. Independent schools, special education schools, pupil referral units, further education colleges and home-schooled children were excluded. Only sixth forms (16–18 years old) attached to or part of secondary schools were included.
In participating schools, parents were invited to enroll their children on a voluntary basis. An online consent and survey form were sent to all parents/students using the Smart Survey platform. Detailed methodology of the sampling frame and approach can be found in Supplement 1.14 Parents of children from Reception to Year 11 (4–16 years old) gave consent and enrolled on behalf of their children, while students in Years 12 and 13 (16–18 years old) consented and enrolled themselves. Trained Office for National Statistics (ONS) field staff attended participating schools on pre-specified testing days and provided written and verbal instruction on self-sampling for SARS-CoV-2 antibodies using the Oracol OF collection device (Malvern Medical Developments Ltd, UK).
Laboratory testing
OF samples were processed and tested for antibodies by UKHSA as described previously.12 Samples were anonymised using barcodes, so laboratory staff were unaware of the infection and vaccination status of participants. Briefly, OF was extracted by adding the elution buffer and agitating the swab. The resulting eluate was centrifuged, collected, and stored at −30 °C until testing. OF samples were tested for SARS-CoV-2 antibodies using an in-house, Immunoglobulin G antibody capture enzyme immunoassay (EIA) based on a solid-phase anti-human IgG with either an HRP-conjugated Nucleoprotein (N) antigen probe,12 or an HRP-conjugated Spike (S) S1 subunit antigen probe. The SARS-CoV-2 S1 antigen was produced and gifted by The Francis Crick Institute.15 In unvaccinated children and adults, the N assay had a sensitivity and specificity of 80% and 99%, respectively,12 and 75% and 98%, respectively, for the S1 assay (unpublished data).
Sample size and sampling design
The original SIS1 study had oversampled schools in high SARS-CoV-2 prevalence areas in September 2020 to understand virus transmission risks and dynamics in educational settings.13 Sampled schools were expanded for SIS2, to produce robust estimates of SARS-CoV-2 antibody prevalence in school children at a regional and national level. We calculated the sample size for the most statistically conservative scenario of 50% antibodies prevalence, resulting in the widest confidence intervals, and aimed for±5% precision with 95% confidence at a regional level for primary and secondary schools. Assumptions on school’s average population and school-level response rate were based on administrative data and SIS1, respectively. After accounting for clustering within schools (design effect 2.3 based on SIS1), the estimated sample size was 13 primary and 7 secondary schools in each of the nine regions, for a total of 180 schools nationally (further detail in Supplement 1).
Schools were selected using a two-stage self-weighted sampling scheme, stratified by region and primary versus secondary schools. The primary sampling unit (PSU) in each region was the local authority area (LA). LAs were sorted using the percentage of primary schools located in urban areas within them, to ensure sampled LAs were representative of the urban/rural split in the region, and five LAs were selected with probability proportional to size (number of primary schools) to increase likelihood of sampling LAs with more schools (Supplement 1).
The second sampling unit was the school, stratified by regions and by primary versus secondary schools, and selected using systematic random sampling. Schools’ sampling was implicitly stratified on percentage of students eligible for free school meals (FSM – a proxy-measure of socioeconomic deprivation) and presence of Sixth Form (for secondary schools), by sorting on these criteria prior to sampling. All students in selected schools were eligible to participate. Schools already participating in SIS1 replaced any newly sampled schools with similar characteristics (Supplement 2).
Statistical analysis
We calculated antibody prevalence overall and by groups. Those with inconclusive or no results (e.g., missing or poor sample quality) were excluded. All estimates were weighted to be representative at regional and national levels, accounting for sampling design, school-level response rate, age, sex, ethnicity, and free-school meals (Supplement 1). The 95% CIs were calculated using robust standard error to account for the cluster sampling design. In unvaccinated children, the presence of N-antibody and/or S-antibody was considered as evidence of past infection; antibody prevalence was adjusted to reflect the sensitivity (80%) and specificity (99%) of the OF assay compared to serum (Supplement 1). In vaccinated participants, only N-antibodies were considered as evidence of past infection; no adjustments were made to estimate prevalence in vaccinated children because of the very high S-antibody levels achieved by mRNA vaccines and the consequent high correlation between serum and OF antibody levels (data not shown).
Direct deterministic linkage using personal identifying information was used to link participant data to national laboratory reports of SARS-CoV-2 PCR and lateral flow device (LFD) tests (Second Generation Surveillance System, SGSS) to ascertain prior SARS-CoV-2 infection and to the National Immunisation Management Service (NIMS) database to identify COVID-19 vaccination status and date of vaccination, both held at UKHSA. Students were grouped as unvaccinated or vaccinated if they had received ≥ 1 COVID-19 vaccine dose ≥14 days before their antibody test. SARS-CoV-2 antibody prevalence is reported for primary and secondary school-aged students nationally, by region and by vaccination status. Non-overlapping 95% CIs were used to assess statistically significant differences between groups. Where shown, P-values were estimated using the Chi-squared test.
For comparison with community SARS-CoV-2 antibody seroprevalence in young adults, regional seroprevalence data from NHS Blood and Transplant (NHSBT) serosurveillance of blood donors were obtained for 17–30 years old from November 1 to December 3, 2021. Donor sera are tested for N- and S-antibodies on Roche Elecsys Anti-SARSCoV-2N assay and seroprevalence results are weighted using age, sex, and region. Data were managed in Microsoft Access and analyzed in Stata SE (version 15.1).
Results
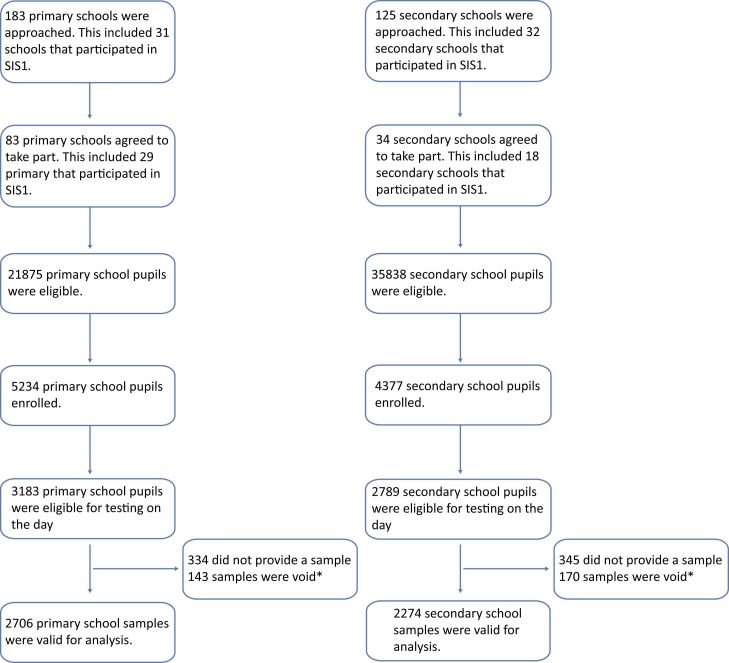
In this first round of testing (10 November to 10 December 2021), 183 primary and 125 secondary schools were approached to participate ( Fig. 1). Overall, 117 schools (83 primary, 34 secondary) enrolled, and 5972 students (3183 primary, 2789 secondary) were eligible for testing on the day and, after excluding 313 samples which were void, 4980 results were included (2706 primary, 2274 secondary). Participant response rate was 10% (15% primary, 8% secondary) ( Table 1). Comparison of this cohort with national data showed some differences in characteristics, including proportion White (89.3% vs 71.7%) and proportion on FSM (9.7% vs 18.9%) although age groups and sex were similar. These differences were accounted for using weighting methodology. Linkage with SGSS showed that 13.3% (359/2706) of primary and 19.5% (443/2276) of secondary school students had ≥1 confirmed SARS-CoV-2 infection>2 weeks prior to OF sampling.
Fig. 1.
Flow chart of recruitment numbers achieved in the School Infection Survey (SIS2) by school type and participants sampled during 10 November–10 December 2021 in England. *Void tests are oral fluid samples that were rejected at the processing stage prior to antibody testing, for a number of reasons, most commonly where>2 mL volume was recovered after processing (resulting in a dilution of antibody levels) or samples were incorrectly taken by participant or incorrectly packaged or transported.
Table 1.
Characteristics of primary and secondary school students in England participating in Round 1 (10 November–10 December 2021) oral fluid sampling of the School Infection Survey (SIS2) and comparison with national demographics in England.
| SIS2 Cohort |
National Data* |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total |
Primary |
Secondary |
Total |
Primary |
Secondary |
||||
| n | % | n | % | n | % | % | % | % | |
| Sex | |||||||||
| Female | 2503 | 50.3 | 1337 | 49.4 | 1166 | 51.3 | 49.4 | 49.1 | 49.8 |
| Male | 2447 | 49.7 | 1369 | 50.1 | 1108 | 48.7 | 50.6 | 50.9 | 50.2 |
| Age group (years) | |||||||||
| 4–7 | 1333 | 26.8 | 1333 | 49.3 | - | - | 31.8 | 56.6 | - |
| 8–11 | 1867 | 37.5 | 1367 | 50.5 | 500 | 22.0 | 32.6 | 43.4 | 18.7 |
| 12–15 | 1648 | 33.1 | - | - | 1648 | 72.5 | 30.2 | - | 69.1 |
| 16–17 | 118 | 2.4 | - | - | 118 | 5.2 | 5.4 | - | 12.3 |
| Missing | 14 | 0.3 | 6 | 0.2 | 8 | 0.4 | - | - | - |
| Ethnicity | |||||||||
| White | 4361 | 87.6 | 2330 | 86.1 | 2031 | 89.3 | 72.4 | 72.8 | 71.7 |
| Black | 56 | 1.1 | 33 | 1.2 | 23 | 1.0 | 5.4 | 5.4 | 6.2 |
| Asian | 196 | 3.9 | 130 | 4.8 | 66 | 2.9 | 12.0 | 11.8 | 12.1 |
| Mixed | 312 | 6.3 | 182 | 6.7 | 130 | 5.7 | 6.4 | 6.6 | 6.0 |
| Other | 55 | 1.1 | 31 | 1.2 | 24 | 1.1 | 3.7 | 3.4 | 4.0 |
| Region | |||||||||
| East Midlands | 386 | 7.8 | 225 | 8.3 | 161 | 7.1 | 4.6 | 4.8 | 4.6 |
| East of England | 563 | 11.3 | 382 | 14.1 | 181 | 8.0 | 13.2 | 14.0 | 12.9 |
| London | 511 | 10.3 | 366 | 13.5 | 145 | 6.4 | 9.8 | 10.3 | 9.9 |
| North East | 737 | 14.8 | 383 | 14.2 | 354 | 15.6 | 8.4 | 8.6 | 8.6 |
| North West | 666 | 13.4 | 418 | 15.4 | 248 | 10.9 | 11.0 | 11.1 | 11.3 |
| South East | 536 | 10.8 | 297 | 11.0 | 239 | 10.5 | 11.3 | 11.2 | 11.5 |
| South West | 707 | 14.2 | 254 | 9.4 | 453 | 19.9 | 16.3 | 15.4 | 16.0 |
| West Midlands | 201 | 4.0 | 134 | 5.0 | 67 | 2.9 | 16.4 | 15.6 | 15.8 |
| Yorkshire and the Humber | 673 | 13.4 | 247 | 9.1 | 426 | 18.7 | 9.2 | 9.0 | 9.4 |
| Location type | |||||||||
| Urban | 4199 | 84.3 | 2182 | 80.6 | 2017 | 88.7 | 85.5 | 84.2 | 88.1 |
| Rural | 781 | 15.7 | 524 | 19.4 | 257 | 11.3 | 14.5 | 15.8 | 11.9 |
| Vaccination status | |||||||||
| Unvaccinated | 3977 | 79.9 | 2706 | 100 | 1271 | 55.9 | - | - | - |
| Vaccinated** | 1003 | 20.1 | - | 0 | 1003 | 44.1 | - | - | 37.8 |
| Free school meals (FSM) | |||||||||
| Yes | 848 | 17.0 | 627 | 23.2 | 221 | 9.7 | 20.8 | 21.6 | 18.9 |
| No | 3927 | 78.9 | 1931 | 71.4 | 1996 | 87.8 | - | - | - |
| Don't know | 151 | 3.0 | 104 | 3.8 | 47 | 2.1 | - | - | - |
| Missing | 54 | 1.1 | 44 | 1.6 | 10 | 0.4 | - | - | - |
| Total | 4980 | 2706 | 2274 | N/A | N/A | N/A | |||
National data for each of the categories except for vaccination status was found online here: Schools, pupils and their characteristics, Academic Year 2020/21 – Explore education statistics – GOV.UK (explore-education-statistics.service.gov.uk). Data for vaccination status was found online here: National flu and COVID-19 surveillance reports: 2021–2022 season - GOV.UK (www.gov.uk).
Defined as vaccinated if participant received ≥1 COVID-19 vaccine dose ≥14 days before sample date.
Primary school students
Overall, 34.1% (924/2706) of primary school students tested positive for SARS-CoV-2 antibodies, reflecting previous infection only as all were unvaccinated. After weighting and adjusting for test sensitivity and specificity, national prevalence of SARS-CoV-2 antibodies in primary school-aged children was estimated at 40.1% (95% CI 37.3–43.0%) ( Table 2), regional estimates ranged from 21.9% (95% CI 13.9–31.8) in the South West to 53.8% (95% CI 43.1–64.3) in the West Midlands ( Table 3). Antibody prevalence increased with age (p < 0.001) (Supplement 3) and was higher in urban schools than rural (p = 0.01).
Table 2.
Unweighted totals and weighted SARS-CoV-2 spike protein (S) and nucleocapsid (N) antibody positivity in unvaccinated primary school children and by COVID-19 vaccination status in secondary school children during Round 1 (10 November–10 December 2021) in the SIS2 study.
| Primary |
Secondary |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total |
Total |
Vaccinated |
Unvaccinated |
|||||
| n* (%*) | Weighted (% (95% CI))† | n* (%) | Weighted (% (95% CI))† | n* (%) | Weighted (% (95% CI))† | n* (%) | Weighted (% (95% CI))† | |
| Negative | 1782 (65.9) | - | 620 (27.3) | - | 35 (3.5) | - | 585 (46.0) | - |
| Total Positive | 924 (34.1) | 40.1 (37.3–43.0) | 1654 (72.7) | 82.4 (79.5–85.1) | 968 (96.5) | 97.5 (96.1–98.5) | 686 (54.0) | 71.5 (65.7–76.8) |
| S positive / N positive | 627 (23.1) | 26.7 (24.2–29.3) | 690 (30.3) | 30.7 (27.4–34.1) | 276 (27.5) | 23.2 (19.3–27.4) | 414 (32.6) | 40.4 (34.5–46.5) |
| S positive / N negative | 205 (7.6) | 8.3 (6.8–10.0) | 888 (39.0) | 46.9 (43.3–50.5) | 692 (69.0) | 74.3 (70.0–78.4) | 196 (15.4) | 23.5 (18.6–29.0) |
| S negative / N positive | 92 (3.4) | 2.5 (1.7–3.6) | 76 (3.3) | 3.0 (1.9–4.5) | 0 (0.0) | 0.0 (0.0–0.8) | 76 (6.0) | 5.2 (2.9–8.6) |
| Unweighted total | 2706 | 2274 | 1003 | 1271 | ||||
Secondary total is the combined result of vaccinated and unvaccinated students whereas the primary school cohort only includes unvaccinated students therefore is presented as the total
n denotes the number of samples, percentages are unweighted and unadjusted.
Weighted by age, sex, ethnicity, and sampling design.
Table 3.
Weighted and adjusted regional SARS-CoV-2 antibody positivity in unvaccinated primary school children and by COVID-19 vaccination status in secondary school children during Round 1 (10 November–10 December 2021) in the SIS2 study.
| Primary |
Secondary |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total |
Total |
Vaccinated |
Unvaccinated |
|||||
| Region | n* | Antibody positive† (% (95CIs)) | n* | Antibody positive† (% (95CIs)) | n* | Antibody positive† (% (95CIs)) | n* | Antibody positive† (% (95CIs)) |
| East Midlands | 225 | 39.5 (30.1–49.5) | 161 | 72.4 (62.3–81.1) | 110 | 92.7 (78.7–98.7) | 51 | 65.1 (52–76.8) |
| East of England | 382 | 50.3 (40.6–59.9) | 181 | 82.1 (73.8–88.7) | 101 | 100 (94.3–100) | 80 | 66.4 (53.7–77.5) |
| London | 366 | 34.8 (27.9–42.1) | 145 | 88.7 (78.1–95.3) | 66 | 100.0 (96.8 – 100.0) | 79 | 79.1 (59.8–92) |
| North East | 383 | 46.5 (40.7–52.4) | 354 | 75.1 (69.3–80.3) | 195 | 92.9 (86.7–96.8) | 159 | 59.9 (51–68.3) |
| North West | 418 | 49.3 (43.7–54.8) | 248 | 87.5 (81.7–91.9) | 126 | 98.1 (92.9–99.8) | 122 | 79.3 (69.4–87.2) |
| South East | 297 | 30.2 (22.6–38.8) | 239 | 79.7 (72.6–85.6) | 122 | 96.8 (90.3–99.4) | 117 | 59.9 (48.2–70.8) |
| South West | 254 | 21.9 (13.9–31.8) | 453 | 70.2 (64.7–75.3) | 262 | 97.3 (93–99.3) | 191 | 56.0 (48.2–63.5) |
| West Midlands | 134 | 53.8 (43.1–64.3) | 67 | 88.3 (70.4–97.3) | 28 | 100 (81–100) | 39 | 78.6 (46.1–96.3) |
| Yorkshire and the Humber | 247 | 39.1 (31.7–46.8) | 426 | 89.0 (83.6–93.1) | 261 | 94.7 (86.4–98.7) | 165 | 87.8 (80.9–92.9) |
| Total | 2706 | 2274 | 1003 | 1271 | ||||
n denotes the number of samples tested.
Weighted by age, sex, ethnicity and sampling design.
Secondary school-aged children
The overall antibody positivity rate in secondary school students was 72.7% (1654/2274) and includes 44.1% (1003/2274) who had received ≥1 COVID-19 vaccine dose. After weighting and adjusting for test sensitivity, the national prevalence of SARS-CoV-2 antibodies was 82.4% (95% CI 79.5–85.1) including 71.5% (95% CI 65.7–76.8) in unvaccinated and 97.5% (95% CI 96.1–98.5) in vaccinated students (Table 2). A higher proportion of unvaccinated students were S and N positive (40.4%; 95% CI 34.5–46.5) compared to vaccinated 23.2% (95% CI 19.3–27.4). In unvaccinated students, antibody prevalence ranged from 56.0% (95% CI 48.2–63.5) in the South West to 87.8% (95CI; 80.9–92.9) in Yorkshire and the Humber region while, in vaccinated students, antibody prevalence ranged from 92.7% (95% CI 78.7–98.7) in the East Midlands to 100% (95% CI 81.0–100.0) in the West Midlands (Table 3). Antibody prevalence increased with age (p < 0.001) (Supplement 3) and was higher in urban versus rural, although not significant (p = 0.1).
Comparison with seroprevalence in young adults
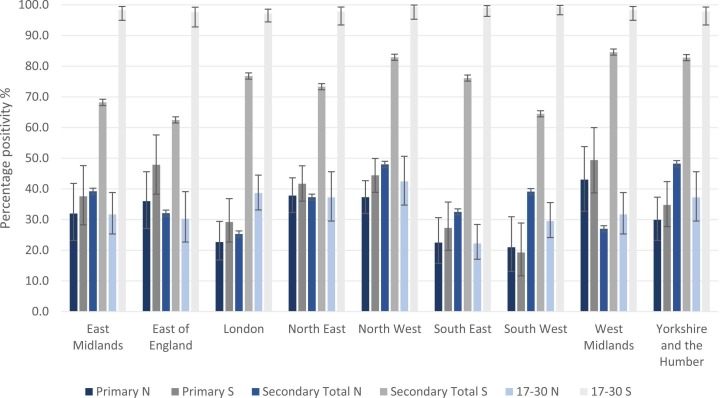
Adults aged 17–30 years were eligible for COVID-19 vaccination earlier and had higher vaccine uptake than secondary school students. Adults had significantly higher S-antibodies than primary and secondary school students through a combination of natural infection and vaccination in all regions. ( Fig. 2). There was some variability of N-antibodies between regions, between primary and secondary school students and compared to adults.
Fig. 2.
Regional weighted, adjusted total antibody prevalence in primary and secondary students participating in Round 1 (10 November–10 December 2021) of SIS2 and N and S antibody positivity in 17–30 years old in NHSBT serosurveillance data. NB: NHSBT data use NHS regions; therefore, data for the Midlands were used for comparison with our cohorts in the East and West Midlands. North East data were used for comparison with our cohort in Yorkshire and the Humber. NHSBT data are weighted by age and sex.
Discussion
We used an in-house OF assay developed and validated by UKHSA to estimate for the first time regional and national SARS-CoV-2 antibody prevalence after natural infection and vaccination in primary and secondary school-aged children in England.12 In November 2021, an estimated 40.1% of primary school-aged students had infection-induced antibodies, while 82.4% of secondary school-aged children had SARS-CoV-2 antibodies through a combination of infection and vaccination, with 44% of our secondary school students having received ≥1 dose of COVID-19 mRNA vaccine ≥14 days prior to their antibody test. Across all regions, young adults, who became eligible for COVID-19 vaccination earlier than adolescents, had higher S-antibody prevalence than the students with variations in N-antibody prevalence. These findings provide a baseline for monitoring national and regional trends in natural and vaccine-induced antibodies with increasing vaccine uptake and emergence of new variants in children and adolescents.
Antibody prevalence increased with age in primary and secondary school pupils. In primary school pupils, these data are in line with previous UK data reporting increasing seroprevalence with age,16 and other regional and national seroprevalence studies reporting higher antibody prevalence in>10-year-old than<10-year-old.17, 18 Trends in secondary school students are harder to interpret because the characteristics of the vaccinated and unvaccinated are likely to be different. A higher proportion of unvaccinated secondary school pupils, however, were N-positive compared to vaccinated, either because vaccination helped protect against infection or the previously infected were less likely to get vaccinated. Primary school students were more likely to have antibodies in urban over rural areas, consistent with higher infection rates in urban areas.19 A similar trend was observed in our secondary school students, but the difference was not significant, possibly because of vaccination in this cohort.20
OFs have multiple advantages over serum antibody testing; they are easy to use on a large-scale, are non-invasive, painless, more acceptable to participants and can be used in challenging populations, such as young children, allowing higher recruitment rates across geographical areas because specialist expertise is not needed to collect the OF sample compared to blood.12 Although the sensitivity of our OF assay is lower than serum antibody assays, population estimates can be adjusted for its sensitivity and specificity. Adjustment was, however, not required for the vaccinated cohort because of higher sensitivity and specificity of the OF assay compared to serum achieved through the high S-antibody levels after vaccination, even with a single dose.
Our study provides the first snapshot of national and regional seroprevalence of SARS-CoV-2 antibodies in English children, when 12–15 years old became eligible for COVID-19 vaccination and highlights the importance of seroprevalence studies to assess population immunity and trends over time. Because of difficulties in sampling large numbers of children, there are few other large-scale, childhood national seroprevalence studies for comparison. In England, comparison with regional NHSBT seroprevalence data in adults shows SARS-CoV-2 antibody prevalence in children cannot be inferred from adult studies. By week 42 (beginning 17 October) 2022, COVID-19 vaccine uptake in young adults was 63–68%, for the first dose and 55–57% for the second dose.21 Consequently, S-antibody prevalence rates were higher in young adults compared to secondary or primary school children. There were, however, wide regional differences in infection-induced and vaccine-induced SARS-CoV-2 antibody prevalence rates between these cohorts. Moreover, since most children have asymptomatic or mild, transient infection, they may not get tested for the virus and, therefore, extrapolating from community infection rates will likely grossly underestimate population prevalence of SARS-Cov-2 antibodies,22 as evidenced by the three-fold lower confirmed infection rates in our cohort.
In England, the ONS COVID-19 Infection Survey (CIS) extended its national seroprevalence surveillance, using finger prick tests to measure serum antibodies in adults, to include a small cohort of children as young as 8 years. Sampling between 29 November 2021 and 5 December 2021 estimated 33% antibody seropositivity for 8–11 years old and 81% for 12–15 years old, which is consistent with our national surveillance estimates. However, the small sample size of children in ONS SIS precluded regional estimates and a high threshold used to confirm SARS-CoV-2 antibody positivity may underestimate seroprevalence rates in children.23 The availability of regional data on infection- and vaccine-induced antibody prevalence may be particularly useful for immunizers and policymakers for targeting vaccine uptake in more susceptible regions and populations across England.
In England and elsewhere, opportunistic testing of residual sera from children having blood tests, in hospital, for example, has been used to assess SARS-CoV-2 antibody prevalence in children but these are prone to multiple biases, especially representativeness to the general population.24, 25, 26, 27, 28
Internationally, one Mexican study did include a nationally representative sample of children along with adults to estimate an overall SARS-CoV-2 antibody seroprevalence of 24.9% during August-November 2020, including 22.5% in 1891 children; notably, two-thirds of all seropositive participants reported being asymptomatic with their infection.29 In Portugal, national seroprevalence using 8463 participants was estimated to be 15.5% across all age groups during February–March 2021, including 14.3% among 721 children aged 1–9 years and 12.9% among 955 10–19 years old.22
Interpretation of findings
The UK strategy of closing schools last and opening schools first compared to other settings meant that children could benefit from in-person schooling throughout most of the pandemic, with closures only during national lockdown periods in March–May 2020 (first pandemic wave) and January–February 2021 (alpha variant wave). Confirmed cases in school-aged children generally fluctuated with community infection rates.30 As more adults were vaccinated as part of the national rollout, childhood infection rates became more disconnected from adult infection rates. In September 2021, for example, when the Delta variant was dominant in England, higher SARS-CoV-2 infection rates were observed in children compared to adults, who were protected from infection and severe disease through vaccination.11 This trend, however, became less apparent with the emergence of the Omicron variant, which was able to evade both natural and vaccine-induced immunity, and infect both previously-infected and previously-vaccinated children and adults.11
Contrary to other countries, the UK adopted a cautious approach to vaccinating children and adolescents because of their low risk of severe COVID-19 and initial concerns about vaccine-induced myocarditis after the second mRNA vaccine dose in young males.5 Consequently, roll-out of the Pfizer-BioNTech mRNA vaccine in adolescents (the only vaccine authorized in this age group) has been slow and, after a rapid early uptake through a school-based program for 12–15 year-olds, vaccine uptake reached 32% for one dose on 01 November 2021 and then plateaued. The vaccine has been shown to provide high protection against the low risk of severe COVID-19 from the Delta variant in adolescents a month after dose one,31 but provides moderate short-term protection against symptomatic disease due to the Omicron variant,32 which can evade both natural and vaccine-induced immunity.33, 34
Strengths and limitations
Our large, representative cohort allowed regional and national estimation of SARS-CoV-2 antibodies in primary and secondary school children across England. The establishment of this cohort has allowed other assessments, including vaccine sentiment, long-COVID and well-being surveys, while longitudinal follow-up will allow monitoring of changes over time, especially given the ongoing vaccination program and emergence of new variants.
There are some limitations. We were unable to recruit the required 180 schools and student participant rates were lower than the predicted 25% in primary and 15% in secondary schools; however, we were able to estimate regional and national prevalence with adequate precision with the sample sizes achieved. Additionally, SARS-CoV-2 antibodies wane over time, especially N-antibodies which decline quicker than S-antibodies after natural infection.3 This may underestimate SARS-CoV-2 antibody prevalence in our cohort because some children may have been infected up to 18 months prior to their antibody test, particularly because OF antibody levels may be 10- to 100-fold lower than in serum. We partly mitigated this by measuring both N- and S-antibodies in all participants. The high S-antibodies achieved after even a single vaccine dose substantially increases the sensitivity of the OF assay (unpublished data). Previous infection and vaccination help protect against re-infection but neither antibody positivity status or antibody thresholds can yet predict the risk of infection or re-infection, especially with new variants that can evade prior immunity. Certain biases such as an individual’s likelihood of taking part cannot be controlled for; the demographics of the study compared to the national population, however, were controlled for therefore less likely to influence results. Also, we have only presented qualitative results; further work is required to assess the use of quantitative antibody levels with the OF assay to understand antibody protection against infection and waning over time. Finally, we compared our cohort with data from NHSBT, with under-representation of minority ethnic groups, and may therefore not be representative of the young adult population nationally.
In conclusion, we developed and implemented a novel, non-invasive OF test on a large scale to estimate national and regional SARS-CoV-2 antibody prevalence in children across England. In November 2021, 40.1% of primary school children and 82.4% of secondary school children had antibodies through natural infection and/or vaccination in England. In unvaccinated children, this was approximately threefold higher than confirmed infections highlighting the importance of seroprevalence studies to estimate prior exposure. Ongoing surveillance will be important to monitor the effects of vaccination and future SARS-CoV-2 variants in children.
Ethical approval
This study was granted approval by UKHSA Research Ethics and Governance Group on 2/11/2021, R&D ref: 474.
Funding information
This study was funded by the UK Department of Health and Social Care. Work in PC’s laboratory was supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001061), the Wellcome Trust (FC001061), and the UK Medical Research Council (FC001061). The concept of capture assays was developed in collaboration with Imperial College London, UKRI CV220–11: Serological detection of past SARS-CoV-2 infection by non-invasive sampling for field epidemiology and quantitative antibody detection.
Conflict of interest
PC and RT have a patent on SARS-CoV-2 antibody detection assay; PC has a patent on modified SARS virus spike protein subunit.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jinf.2023.02.016.
Contributor Information
on behalf of the COVID-19 Schools Infection Survey Group:
Shazaad Ahmad, Frances Baawuah, Joanne Beckmann, Andrew Brent, Bernadette Brent, Joanna Garstang, Ifeanyichukwu O. Okike, Kevin Brown, Mary Ramsay, Chris Bonell, Sarah Cook, Charlotte Warren-Gash, Jody Phelan, James Hargreaves, Sinead Langan, Neisha Sundaram, Elliot McClenaghan, Gillian McKay, John Edmunds, and Paul Fine
Appendix A. . Supplementary material
Supplementary material
.
References
- 1.Viner R.M., Mytton O.T., Bonell C., Melendez-Torres G.J., Ward J., Hudson L., et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2021;175(2):143–156. doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viner R., Waddington C., Mytton O., Booy R., Cruz J., Ward J., et al. Transmission of SARS-CoV-2 by children and young people in households and schools: ameta-analysis of population-based and contact-tracing studies. J Infect. 2022;84(3):361–382. doi: 10.1016/j.jinf.2021.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowell A.C., Butler M.S., Jinks E., Tut G., Lancaster T., Sylla P., et al. Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection. Nature Immunol. 2022;23(1):40–49. doi: 10.1038/s41590-021-01089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 vaccination in children and young people aged 16 to 17 years: JCVI statement, November 2021 [press release]. 15th November 2021.
- 5.Department of Health and Social Care. JCVI statement on COVID-19 vaccination of children aged 12 to 15 years: 3 September 2021 2021. Available from: 〈https://www.gov.uk/government/publications/jcvi-statement-september-2021-covid-19-vaccination-of-children-aged-12-to-15-years/jcvi-statement-on-covid-19-vaccination-of-children-aged-12-to-15-years-3-september-2021#annex-a-jcvi-advice-on-vaccination-of-children-aged-12-to-15-years-with-underlying-health-conditions-31-august-2021〉.
- 6.Department of Health and Social Care. Universal vaccination of children and young people aged 12 to 15 years against COVID-19 2021. Available from: 〈https://www.gov.uk/government/publications/universal-vaccination-of-children-and-young-people-aged-12-to-15-years-against-covid-19/universal-vaccination-of-children-and-young-people-aged-12-to-15-years-against-covid-19〉.
- 7.Ismail S.A., Saliba V., Lopez Bernal J., Ramsay M.E., Ladhani S.N., et al. SARS-CoV-2 infection and transmission in educational settings: a prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect Dis. 2021;21(3):344–353. doi: 10.1016/S1473-3099(20)30882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ladhani S.N., Baawuah F., Beckmann J., Okike I.O., Ahmad S., Garstang J. SARS-CoV-2 infection and transmission in primary schools in England in June-December, 2020 (sKIDs): an active, prospective surveillance study. Lancet Child Adolesc Health. 2021;5(6):417–427. doi: 10.1016/S2352-4642(21)00061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Public Health England SARS-CoV-2 variants of concern and variants under investigation in England. Tech Briefing. 2021;17 [Google Scholar]
- 10.Public Health England Investigation of novel SARS-CoV-2 variant. Tech Briefing. 2020;5 [Google Scholar]
- 11.UK Health Security Agency. Weekly national Influenza and COVID-19 surveillance report. 2022.
- 12.Hoschler K., Ijaz S., Andrews N., Ho S., Dicks S., Jegatheesan K., et al. SARS antibody testing in children: development of oral fluid assays for IgG measurements. Microbiol Spectr. 2022;10(1) doi: 10.1128/spectrum.00786-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Public Health England. COVID-19 Schools Infection Survey, 2020.Available from: 〈https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/949467/SIS_protocol_v1.1_13102020.pdf〉.
- 14.Office for National Statistics. COVID-19 Schools Infection Survey, 2021 to 2022: methods and further information 2022. Available from: 〈https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/methodologies/covid19schoolsinfectionsurvey2021to2022methodsandfurtherinformation〉.
- 15.Rosa A., Pye V.E., Graham C., Muir L., Seow J., Ng K.W., et al. SARS-CoV-2 can recruit a heme metabolite to evade antibody immunity. Sci Adv. 2021;7(22) doi: 10.1126/sciadv.abg7607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratcliffe H., Tiley K.S., Andrews N., Amirthalingam G., Vichos I., Morey E., et al. Community seroprevalence of SARS-CoV-2 in children and adolescents in England, 2019–2021. Arch Dis Childhood. 2022;108(2):123–130. doi: 10.1136/archdischild-2022-324375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stringhini S., Wisniak A., Piumatti G., Azman A.S., Lauer S.A., Baysson H., et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396(10247):313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reicher S., Ratzon R., Ben-Sahar S., Hermoni-Alon S., Mossinson D., Shenhar Y., et al. Nationwide seroprevalence of antibodies against SARS-CoV-2 in Israel. Eur J Epidemiol. 2021;36(7):727–734. doi: 10.1007/s10654-021-00749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Office for National Statistics. Coronavirus (COVID-19) case rates by socio-demographic characteristics, England: 1 September 2020 to 25 July 2021 2021. Available from: 〈https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19caseratesbysociodemographiccharacteristicsengland/1september2020to25july2021〉.
- 20.The Office for National Statistics. Coronavirus (COVID-19) vaccination uptake in school pupils, England: up to 9 January 2022 2022. Available from: 〈https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandwellbeing/articles/coronaviruscovid19vaccinationuptakeinschoolpupilsengland/upto9january2022〉.
- 21.UK Health Security Agency. COVID-19 vaccine surveillance report. 2022 31 March 2022.
- 22.Kislaya I., Gonçalves P., Gómez V., Gaio V., Roquette R., Barreto M., et al. SARS-CoV-2 seroprevalence in Portugal following the third epidemic wave: results of the second National Serological Survey (ISN2COVID-19) Infect Dis. 2022:1–7. doi: 10.1080/23744235.2021.2025421. [DOI] [PubMed] [Google Scholar]
- 23.Office for National Statistics. Coronavirus (COVID-19) latest insights: Antibodies 2022.Available from: 〈https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19latestinsights/antibodies〉.
- 24.Wachter F., Regensburger A.P., Antonia Sophia P., Knieling F., Wagner A.L., Simon D., et al. Continuous monitoring of SARS-CoV-2 seroprevalence in children using residual blood samples from routine clinical chemistry. Clin Chem Lab Med. 2022;60(6):941–951. doi: 10.1515/cclm-2022-0037. [DOI] [PubMed] [Google Scholar]
- 25.Rotee I.L.M., Ong D.S.Y., Koeleman J.G.M., Vos E.R.A., Tramper-Stranders G.A. Trends in SARS-CoV-2 seroprevalence amongst urban paediatric patients compared with a nationwide cohort in the Netherlands. J Clin Virol Plus. 2021;1(4) doi: 10.1016/j.jcvp.2021.100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazzerini M., Benvenuto S., Mariani I., Fedele G., Leone P., Stefanelli P., et al. Evolution of SARS-CoV-2 IgG seroprevalence in children and factors associated with seroconversion: results from a multiple time-points study in Friuli-Venezia Giulia Region, Italy. Children. 2022;9(2) doi: 10.3390/children9020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vette K.M., Machalek D.A., Gidding H.F., Nicholson S., O'Sullivan M.V.N., Carlin J.B., et al. Seroprevalence of severe acute respiratory syndrome coronavirus 2-specific antibodies in Australia after the first epidemic wave in 2020: a national survey. Open Forum Infect Dis. 2022;9(3):ofac002. doi: 10.1093/ofid/ofac002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oeser C., Whitaker H., Linley E., Borrow R., Tonge S., Brown C.S., et al. Large increases in SARS-CoV-2 seropositivity in children in England: effects of the delta wave and vaccination. J Infect. 2022;84(3):418–467. doi: 10.1016/j.jinf.2021.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basto-Abreu A., Carnalla M., Torres-Ibarra L., Romero-Martínez M., Martínez-Barnetche J., López-Martínez I., et al. Nationally representative SARS-CoV-2 antibody prevalence estimates after the first epidemic wave in Mexico. Nat Commun. 2022;13(1):589. doi: 10.1038/s41467-022-28232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mensah A.A., Sinnathamby M., Zaidi A., Coughlan L., Simmons R., Ismail S.A. SARS-CoV-2 infections in children following the full re-opening of schools and the impact of national lockdown: prospective, national observational cohort surveillance, July-December 2020, England. J Infect. 2021;82(4):67–74. doi: 10.1016/j.jinf.2021.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powell A.A., Kirsebom F., Stowe J., McOwat K., Saliba V., Ramsay M.E., et al. Effectiveness of BNT162b2 against COVID-19 in adolescents. Lancet Infect Dis. 2022;22(5):581–583. doi: 10.1016/S1473-3099(22)00177-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powell A.A., Kirsebom F., Stowe J., Ramsay M.E., Lopez-Bernal J., Andrews N., et al. Protection against symptomatic infection with delta (B.1.617.2) and omicron (B.1.1.529) BA.1 and BA.2 SARS-CoV-2 variants after previous infection and vaccination in adolescents in England, August, 2021-March, 2022: a national, observational, test-negative, case-control study. The Lancet Infectious Diseases. DOI: 10.1016/S1473-3099(22)00729-0. [DOI] [PMC free article] [PubMed]
- 33.Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. New England JMed. 2022 doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilhelm A., Widera M., Grikscheit K., Toptan T., Schenk B., Pallas C., et al. Limited neutralisation of the SARS-CoV-2 Omicron subvariants BA.1 and BA.2 by convalescent and vaccine serum and monoclonal antibodies. EBioMedicine. 2022;82 doi: 10.1016/j.ebiom.2022.104158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Deidentified study data are available for access by accredited researchers in the ONS Secure Research Service (SRS) for accredited research purposes under part 5, chapter 5 of the Digital Economy Act 2017. For further information about accreditation, contact Research.support@ons.gov.uk or visit the SRS website.