Abstract
Objective
Cerebral circulation time (CCT) and collateral score (CS) are associated with functional outcomes in acute ischemic stroke (AIS) patients after endovascular treatment (EVT), and may be related to each other. We aim to determine the relationship between CS and CCT on functional outcomes.
Methods
We retrospectively enrolled consecutive patients with anterior circulation large vessel occlusion (LVO) AIS who received EVT. CS and CCT were measured based on digital subtraction angiography (DSA). We defined CS 0–2 and 3–4 as poor and good collateral status, respectively, and used change of CCT (cCCT), which was defined as the change of stroke side CCT (sCCT) versus healthy side CCT (hCCT). Mediating analysis was used to evaluate the influence of cCCT on the association between CS and functional outcomes, and ROC curves were further used to explore the predictive ability of the interaction between cCCT and CS for functional outcomes.
Results
A total of 100 patients were enrolled in the final analysis. A higher cCCT (r = −0.239; p = 0.017) was associated with lower CS, and cCCT mediated the association of CS with functional outcome. Logistic regression analysis found that CS, cCCT and cCCT‐CS interactions were independently associated with functional outcome, and cCCT‐CS interaction has better predictive performance, with a higher area under curve value than CS or cCCT alone (0.79 vs. 0.75 or 0.75).
Interpretation
To our knowledge, this study provides the first report of the association of collateral status with cCCT, and their interaction effect on functional outcome in AIS‐LVO patients receiving EVT.
Introduction
Endovascular treatment (EVT) has become the main treatment for acute ischemic stroke (AIS) with large vessel occlusion (LVO) within a 24‐h time window. 1 , 2 However, despite successful recanalization, half of patients fail to regain functional independence at 3 months. 3 Patients with similar large vessel occlusion sites, baseline characteristics, and successful reperfusion can have a wide range of outcomes. The individual variability of collateral circulation has been considered to contribute to the differing outcomes, because it has been found to be one of the most important factors in predicting stroke outcome, 4 , 5 , 6 regardless of a fixed time window. As demonstrated in the ESCAPE trial, 7 the assessment of collateral status through a series of neuroimaging techniques has become a reliable decision‐making tool in the selection, triage, and management of patients with acute ischemic stroke. Although good collateral status is known to increase the probability of good outcome in patients by reducing infarct volume and increasing the likelihood of recanalization, 8 , 9 the mechanism underlying the role of the collateral circulation on the outcome of patients after successful recanalization is not completely understood.
Previous studies have shown that good collateral circulation may help reduce cerebral circulation time (CCT) in some patients with high‐grade carotid artery stenosis, 10 which may be related to the fact that the presence of good collateral status is associated with a higher degree of stenosis. Our recent study found that for patients with successful reperfusion, CCT and collateral status based on DSA data were highly correlated with functional outcomes. 11 In this context, we hypothesize that change in CCT may be one of the mechanisms by which CS improves outcomes. Therefore, our aim was to investigate whether CCT changed the relationship between collateral status and functional outcomes, and whether the interaction between collateral status and CCT was a better predictor of functional outcomes in AIS‐LVO patients after EVT.
Methods
Patient selection
This retrospective study was approved by our institutional review board (IRB: Y(2020)087). Patient informed consent was waived given the retrospective nature of the analysis and minimal risk to subjects. We used the same AIS‐LVO patient cohort, which has been reported in detail in our recent study. 11 Briefly, we enrolled all unilateral, anterior circulation AIS‐LVO consecutive patients who were treated with EVT and achieved successful reperfusion (modified Thrombolysis in Cerebral Infarction (mTICI) score of 2b‐3) from May 2019 to May 2021 at the General Hospital of the Northern Theater Command. We excluded the following patients: (1) posterior circulation infarction, (2) intra‐arterial thrombolysis, (3) pre‐stroke disability (mRS ≥ 2), (4) poor quality or incomplete DSA imaging, and (5) incomplete clinical data. The demographic characteristics, stroke risk factors, clinical scale scores [National Institutes of National Institutes of Health Stroke Scale (NIHSS) and modified Rankin Scale (mRS)], intracerebral hemorrhage, and some critical clinical data such as onset‐to‐reperfusion time (ORT) were reviewed and analyzed.
Endovascular treatment protocol, collateral and cerebral circulation time assessment
In our stroke center, the EVT protocol has been recently described in detail. 11 Briefly, a stroke patient with a highly suspected LVO was directly transferred to the angiographic suite without additional CTA or/and CTP after head Computed Tomography was performed. Digital subtraction angiography (DSA) was performed on the normal side before proceeding to thrombectomy, in order to evaluate the arterial occlusion site and collateral compensation of the circle of Willis as a basis for endovascular treatment. All subjects were examined by a biplane angiography unit (PHILIPS. UNIQ Clarity FD20/20, Holland) with a power injector (8 mL ioversol injection with 6 mL/s speed, 200 psi/kg pressure) with a uniform and standard protocol in the General Hospital of the Northern Theater Command, Shenyang, China. The catheters were placed at the distal end of the common carotid artery during angiography of the healthy side. The location of catheter positioning during angiography on the stroke side was determined by the interventionalist. The American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology collateral flow grading system was used to evaluate the collateral score (CS) based on DSA. 12 This grading system is on a 5‐ point scale (0–4). In the current study, we defined 0 to 2 as poor and 3 to 4 as good collateral status, respectively. As shown in Figure 1, CCT was defined as the time from the beginning of contrast opacification of the carotid siphon segment of the internal carotid artery to the end of the arterial phase on DSA images, while the later was defined as the last frame of late arterial phase (the vascular tree is complete). 13 Two types of CCT were measured: (1) the CCT of the healthy side (hCCT), which was based on DSA performed on the normal side before EVT and (2) the CCT of the stroke side (sCCT), which was based on DSA confirming successful recanalization. The final index of CCT was the change of CCT (cCCT), which was calculated with the following formula: cCCT = (sCCT − hCCT)/hCCT. All images were evaluated by two experienced individuals (W.L., J.Q.) who were blind to the clinical data. A third adjudicator (X.H.S) who was unaware of the clinical information participated in evaluation in case of disagreement. Specially, CCT of the same patient was measured three times to reduce error in data acquisition, and the final CCT was determined by the average of the three sets of data.
Figure 1.
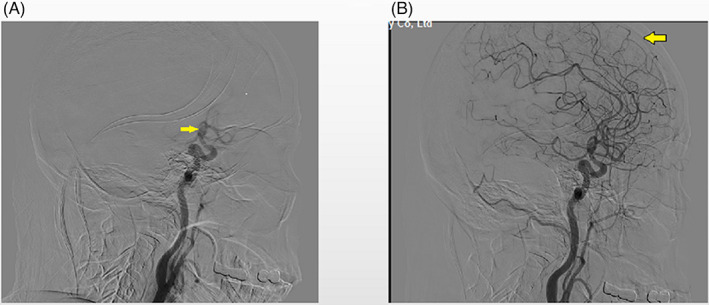
Schematic diagram of the measurement of cerebral circulation time (CCT). (A) shows the appearance of the siphon segment of internal carotid. (B) shows the end of the arterial phase. The CCT was defined as the time from the appearance of the siphon segment of internal carotid to the end of the arterial phase during digital subtraction angiography.
Final infarct volume measures
The infarct volume was measured at 48 h after EVT. The CT data by Digital Imaging and Communications in Medicine format were imported into 3D Slicer software (3D Slicer 4.11). After adjusting the window width to CT‐brain, run “Editor” to set “Threshold” range according to the HU value of infarct volume, and mark the pixels of infarct volume manually. Then, the three‐dimensional reconstruction of the infarct volume was realized, and the precise volume was calculated automatically.
Outcome measures
Functional outcome was assessed using the modified Rankin Scale (mRS) score at 90 days performed by two evaluators who were blinded to endovascular treatment and DSA data. The primary outcomes were good and poor outcome, which was defined as mRS 0–2 and mRS 3–6 at 90 days after the index stroke.
Statistical analysis
Categorical variables are presented as absolute values and percentages, and continuous variables as mean with standard deviation if normally distributed or median with interquartile range if not normally distributed. Continuous or categorical variables were compared between the two groups by independent t tests, Mann–Whitney tests, or Chi‐square tests. The correlation between CS and cCCT was analyzed using Spearman correlation coefficients.
The mediation analysis 14 , 15 , 16 was used to assess whether the relationship between collateral circulation and functional outcome could be secondary to the influence of CCT. Mediation analysis attempts to explain a relation between the independent variable (CS) and the dependent variable (mRS score) by the third hypothetical variable (cCCT). To evaluate the intermediary, the first step is to demonstrate that the independent variable (X: CS) is related to the outcome (Y: mRS score). The second step is to explore the relationship between X (CS) and the mediator (M: cCCT). The third step is to demonstrate the relationship between M (cCCT) and Y (mRS score), using X (CS) and M (cCCT) as independent variables. The mediation model proposes that X (CS) influences M (cCCT), and M (cCCT) indirectly affects Y (mRS score).
Binary logistic regression was employed to examine the relationship between the functional outcomes and the interaction between CS and cCCT, as well as to identify the impact of other indicators on the functional outcomes in our data. With favorable outcome (mRS ≤ 2) as the dependent variable, the multivariable receiver operating characteristic (ROC) curve was used to analyze the predictive effect of the interaction between CS and cCCT on functional outcome. p < 0.05 was considered statistically significant. All analyses were performed using SPSS Statistics version 25 (IBM Corp, Armonk, NY).
Results
From May 2019 to May 2021, 230 consecutive patients were enrolled in our study. After excluding 58 patients with posterior circulation stroke, 45 patients without successful recanalization, 18 patients who received intra‐arterial thrombolysis, 4 patients with incomplete DSA images, 3 patients with incomplete clinical data and 2 patients with mRS ≥ 2 before onset, 100 patients were included in the final analysis. 11
Overall, 26 patients (26.0%) were in the good collateral group and 74 patients (74.0%) in the poor collateral group. A total of 43 patients (43.0%) had favorable functional outcome at 90 days (mRS score 0–2). In the good collateral group, the median cCCT was lower than that in the poor collateral group (−0.068 vs. 0.046, p = 0.048). Baseline characteristics were summarized in Table 1. For the 90‐day outcome, there was shorter sCCT and cCCT, higher CS and NIHSS, and smaller infarct volume in the good outcome group (Table S1, all p < 0.05). In addition, shorter OPT, DPT and ORT were found in the good outcome group although it was not significant (Table S1).
Table 1.
Baseline characteristics of 100 patients.
| Total (n = 100) | Good collaterals (n = 26) | Poor collaterals (n = 74) | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Sex (female), n (%) | 20 (20.0) | 8 (30.8) | 12 (16.2) | 0.153 |
| Age, y; mean (SD) | 63.0 ± 10.7 | 63.4 ± 11.6 | 62.9 ± 10.5 | 0.834 |
| Hypertension, n (%) | 65 (65.0) | 16 (61.5) | 49 (66.2) | 0.811 |
| Diabetes mellitus, n (%) | 28 (28.0) | 3 (11.5) | 25 (33.8) | 0.041 |
| Atrial fibrillation, n (%) | 32 (32.0) | 7 (26.9) | 25 (33.8) | 0.628 |
| Stroke, n (%) | 27 (27.0) | 6 (23.1) | 21 (28.4) | 0.798 |
| Smoking, n (%) | 58 (58.0) | 13 (50.0) | 45 (60.8) | 0.364 |
| Clinical and imaging features | ||||
| NIHSS, mean (SD) | 15.7 ± 4.5 | 14.5 ± 2.8 | 16.1 ± 4.9 | 0.104 |
| ASPECT, mean (SD) | 9.0 (7.0–10.0) | 9.0 (7.8–10.0) | 9.0 (7.0–10.0) | 0.805 |
| SBP after EVT, mean (SD) | 139.7 ± 23.5 | 140.3 ± 21.2 | 139.5 ± 24.4 | 0.891 |
| DBP after EVT, mean (SD) | 82.6 ± 14.7 | 84.7 ± 18.7 | 81.8 ± 13.1 | 0.383 |
| sCCT, median (IQR) | 2.170 (1.830–3.000) | 2.000 (1.830–2.668) | 2.193 (1.830–3.000) | 0.521 |
| hCCT, median (IQR) | 2.250 (1.670–2.830) | 2.330 (2.000–3.270) | 2.170 (1.670–2.670) | 0.161 |
| cCCT, median (IQR) | 0.000 (−0.153–0.333) | −0.068 (−0.341–0.228) | 0.046 (−0.132–0.376) | 0.048 |
| Number of passes, median (IQR) | 1 (1 2) | 1 (1 2) | 2 (1 2) | 0.088 |
| NRV stenosis, n (%) | 52 (52.0) | 10 (38.5) | 42 (56.8) | 0.118 |
| OPT, min, median (IQR) | 337.5 (232.3–535.8) | 483.5 (315.5–633.3) | 314.5 (229.8–468.3) | 0.024 |
| OCT, min, median (IQR) | 324.5 (243.5–549.5) | 508.0 (322.3–473.5) | 322.5 (235.8–473.5) | 0.022 |
| DPT, min, median (IQR) | 50.0 (34.8–88.5) | 72.0 (47.8–108.0) | 46.5 (31.8–79.3) | 0.014 |
| ORT, min, median (IQR) | 410.0 (319.8–615.8) | 548.5 (360.8–788.8) | 386.5 (309.8–575.3) | 0.024 |
| TOAST 0.212 | ||||
| LAA, n (%) | 60 (60.0) | 18 (69.2) | 42 (56.8) | |
| Cardioemblism, n (%) | 26 (26.0) | 7 (26.9) | 19 (25.7) | |
| Undetermined, n (%) | 14 (14.0) | 1 (3.8) | 13 (17.6) | |
| Intravenous thrombolysis | 0.720 | |||
| NO | 76 (76.0) | 21 (80.8) | 55 (74.3) | |
| rt‐PA | 17 (17.0) | 4 (15.4) | 13 (17.6) | |
| UK | 7 (7.0) | 1 (3.8) | 6 (8.1) | |
| Final mTICI | 0.402 | |||
| Final mTICI 2B, n (%) | 43 (43.0) | 13 (50.0) | 30 (40.5) | |
| Final mTICI 3, n (%) | 57 (57.0) | 13 (50.0) | 44 (59.5) | |
| ICH, n (%) | 41 (41.0) | 8 (30.8) | 33 (44.6) | 0.218 |
| Infarct volume, cm3, median (IQR) | 49.9 (17.3–128.8) | 32.7 (4.6–105.9) | 56.8 (21.1–132.1) | 0.063 |
| 90‐d mRS ≤ 2 | 43 (43.0) | 16 (61.5) | 27 (36.5) | 0.038 |
ASPECTS, Alberta Stroke Program Early CT Score; cCCT, change of CCT; DBP, diastolic blood pressure; DPT, door‐to‐puncture time; hCCT, CCT of healthy side; ICH, intracerebral hemorrhage; mTICI, modified thrombolysis in cerebral ischemia; NIHSS, National Institutes of Health Stroke Scale; NRV stenosis, non‐responsible vessel stenosis; OCT, onset‐to‐collateral assessment time; OPT, onset‐to‐puncture time; ORT, onset‐to‐reperfusion time; rt‐PA, Recombinant Tissue Plasminogen Activator; SBP, systolic blood pressure; sCCT, CCT of stoke side; UK, Urokinase.
Association between cCCT and CS
We analyzed the correlation of CS with the independent parameter and observed a significant correlation with cCCT (r = −0.239; p = 0.017), indicating that patients with good collateral status usually have a lower cCCT.
Association between CS and mRS score mediated by cCCT
As shown in Figure 2, cCCT mediated the association between CS and mRS score and the significance of the indirect pathway (test coefficient c') was 0.035. After adding cCCT as an independent variable, the regression coefficient of CS with mRS score was reduced from 0.45 to 0.37 (Table 2). The contribution rate of the mediating effect to the total effect was 27.1%, meaning that 27.1% of the effect of CS on mRS was realized by cCCT.
Figure 2.
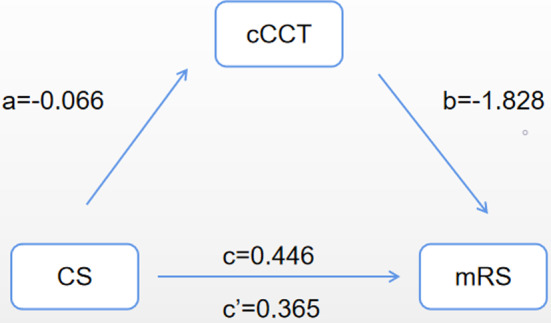
The effect of change of cerebral circulation time (cCCT) on the association between collateral score (CS) and modified Rankin Scale (mRS) score. a, regression coefficient of the association between collateral score (CS) and cCCT; b, regression coefficient of the association between cCCT and mRS score, using CS and cCCT as independent variables; c, regression coefficient of the association between CS and mRS score; ć, regression coefficient of the association between CS and mRS score, using CS and cCCT as independent variables.
Table 2.
Mediation analysis by cCCT on the association of collateral score with functional outcome (mRS score).
| Effect | B | OR | CI 95% | p value | |
|---|---|---|---|---|---|
| a | −0.066 | – | – | – | 0.011 |
| b | −1.828 | 0.161 | 0.038 | 0.684 | 0.013 |
| c | 0.446 | 1.562 | 1.129 | 2.160 | 0.007 |
| c' | 0.365 | 1.441 | 1.026 | 2.023 | 0.035 |
B is unstandardized regression coefficient; a, regression coefficient of the association between collateral score (CS) and cCCT; b, regression coefficient of the association between cCCT and mRS score, using CS and cCCT as independent variables; c, regression coefficient of the association between CS and mRS score; c', regression coefficient of the association between CS and mRS score, using CS and cCCT as independent variables.
CI 95%, 95% confidence interval; cCCT, change of CCT; mRS, modified Rankin Scale; OR, odds ratio.
In the overall analyses, the outcome of patients in the good collaterals group was better than that of the poor collaterals group (Table 1). When the collateral status was poor, the proportion of good functional outcomes was higher when the cCCT was lower (p = 0.017, Fig. 3). The same trend was found in the good collaterals group but there was no statistical significance (p = 0.221, Fig. 3).
Figure 3.
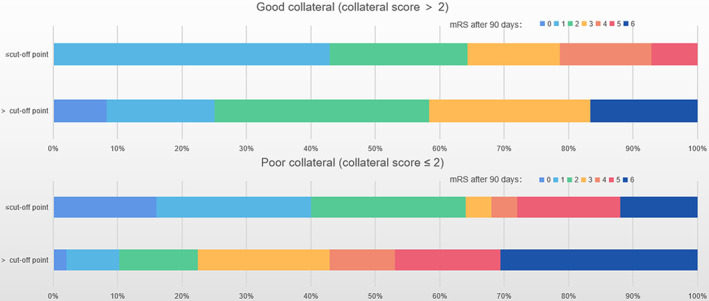
Displaying the impact of collateral status and cCCT on clinical outcome. A collateral score of 2 and the cut‐off value of cCCT was used to distinguish patients according to modified Ranking Scale (mRS) score after 90 days. Good collateral status (p for χ 2 tests is 0.221). Poor collateral status (p for χ 2 tests is 0.017). Cut‐off value was derived from the ROC curve with 90mRs ≤ 2 as the end point, which is −0.062.
The interaction between CS and cCCT in predicting functional outcomes
As shown in Table 3, univariable logistic regression analyses of the entire cohort showed that good outcome was significantly associated with lower NIHSS, shorter cCCT, and higher CS. Multivariable logistic regression analysis revealed that higher CS and shorter cCCT remained positive prognostic factors after adjusting for potential confounders (age, NIHSS, SBP after EVT, non‐responsible vessel stenosis (NRV stenosis), number of passes, mTICI, and ORT). It is noteworthy that an interaction was found between cCCT and CS on favorable outcome, which reached significance (Table 3).
Table 3.
The interaction between CS and cCCT in predicting functional outcomes.
| Clinical outcome (mRS 0–2) | |||
|---|---|---|---|
| Univariable analysis | |||
| Parameter | OR | 95%CI | p value |
| NIHSS | 0.882 | 0.797–0.978 | 0.017 |
| SBP after EVT | 0.991 | 0.974–1.009 | 0.315 |
| NRV stenosis | 1.110 | 0.502–2.454 | 0.796 |
| number of passes | 0.748 | 0.454–1.233 | 0.255 |
| mTICI | 2.145 | 0.943–4.883 | 0.069 |
| ORT | 0.999 | 0.998–1.001 | 0.240 |
| cCCT | 0.124 | 0.030–0.504 | 0.004 |
| CS | 1.562 | 1.129–2.160 | 0.007 |
| cCCT–CS | 5.982 | 1.473–24.291 | 0.012 |
| Multivariable analysis | |||
|---|---|---|---|
| Parameter | Adjusted OR | 95%CI | p value |
| CS | 1.650 | 1.101–2.472 | 0.015 |
| cCCT | 0.003 | 0.000–0.147 | 0.003 |
| cCCT–CS | 8.145 | 1.663–39.880 | 0.010 |
Adjusted for age, NIHSS, SBP after EVT, NRV stenosis, number of passes, mTICI, and ORT.
cCCT, change of cerebral circulation time; cCCT–CS, the interaction between cCCT and CS; CS, Collateral Score; mTICI, modified thrombolysis in cerebral ischemia; NIHSS, National Institutes of Health Stroke Scale; NRV stenosis, non‐responsible vessel stenosis; ORT, onset‐to‐reperfusion time; SBP, systolic blood pressure.
In multivariable ROC analysis, CS, cCCT, and cCCT‐CS interaction were used to construct the models by adjusting for age, NIHSS, SBP after EVT, NRV stenosis, number of passes, mTICI, and ORT to predict favorable outcome. The AUC for the adjusted Model 1 (CS, age, NIHSS, SBP after EVT, NRV stenosis, number of passes, mTICI, and ORT) was 0.75 (95% CI 0.66–0.85), Model 2 (cCCT, age, NIHSS, SBP after EVT, NRV stenosis, number of passes, mTICI, and ORT) was 0.75 (95% CI 0.65–0.84), while the AUC of the adjusted Model 3 (cCCT by CS, age, NIHSS, SBP after EVT, NRV stenosis, number of passes, mTICI, and ORT)was 0.79 (95%CI 0.71–0.88) (Fig. 4). As shown in Figure 5, the probability of good outcome increased with an increase of CS, or decrease of cCCT. Furthermore, for patients with similar collateral status, decreased cCCT led to higher probability of good outcome, while for patients with the same cCCT, the better the collateral status, the higher the probability of good outcome.
Figure 4.
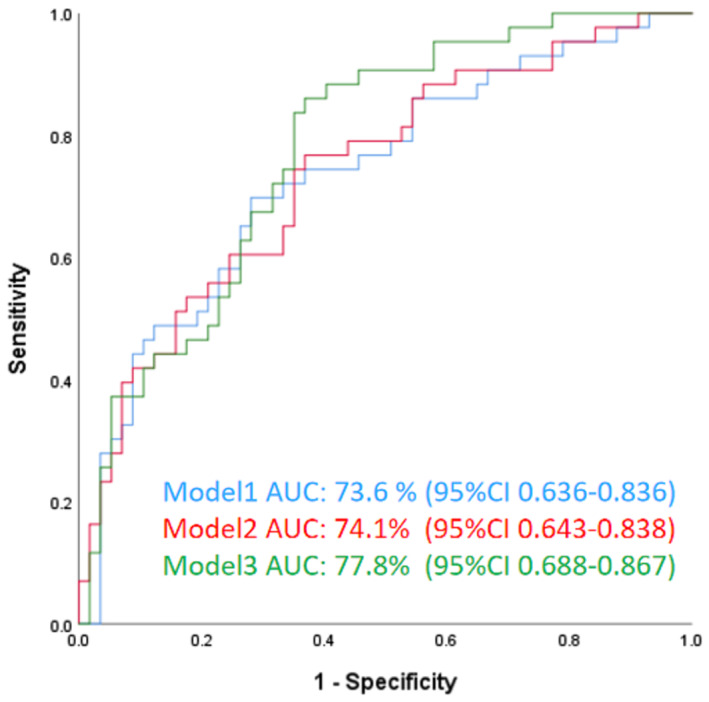
Receiver operating characteristics (ROC) analysis. Model 1: adjusted by CS, age, NIHSS, SBP after EVT, NRV stenosis, number of passes, mTICI, and ORT; Model 2: adjusted by cCCT, age, NIHSS, SBP after EVT, NRV stenosis, number of passes, mTICI, and ORT; Model 3: adjusted by cCCT‐CS, age, NIHSS, SBP after EVT, NRV stenosis, number of passes, mTICI, and ORT. cCCT, change of cerebral circulation time; CS, Collateral Score; mTICI, modified thrombolysis in cerebral ischemia; NIHSS, National Institutes of Health Stroke Scale; NRV stenosis, non‐responsible vessel stenosis; ORT, onset‐to‐reperfusion time; SBP, systolic blood pressure.
Figure 5.
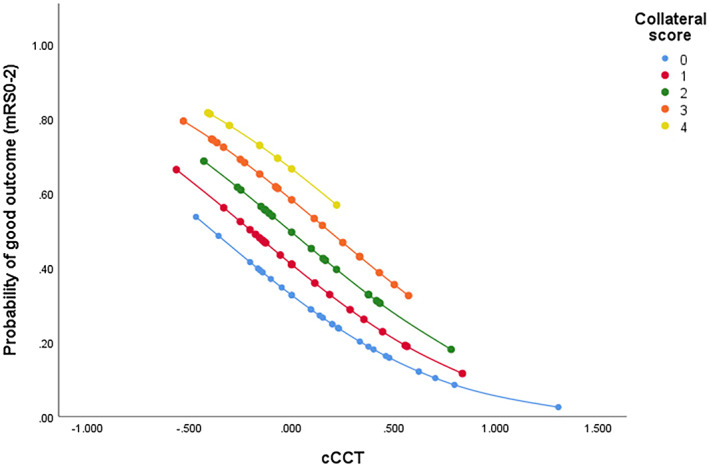
The scatter plot showing the probability of good functional outcome with change of collateral score (CS) and change of cerebral circulation time (cCCT). The association between cCCT and favorable outcomes varied according to CS. The probabilities were calculated using the ordinal regression equation with multiplicative interaction terms. mRS indicates modified Rankin Scale.
The interaction between CS and cCCT in infarct volume
The ROC curve was used to calculate the final infarct volume to predict good outcome at 3 months, and the threshold of the infarct volume below which good outcome was present was 32.5 cm3. Patients were divided into two groups according to the cut‐off value of infarct volume, and binary logistic regression analysis was performed. As shown in Table S2, univariable logistic regression analyses of the entire cohort showed that smaller infarct volume was significantly associated with shorter cCCT, and higher CS. After adjusting for potential confounders (age, NRV stenosis, mTICI, and ORT), multivariable regression showed that cCCT and the interaction between cCCT and CS were closely related to smaller infarct volume.
Discussion
In the present study of patients with anterior circulation large vessel occlusion stroke, we identified several interesting findings. First, cCCT was inversely associated with collateral status. Second, the association between collateral status and functional outcome varied with cCCT: 27.1% of the effect of collateral score on mRS was realized by cCCT. Third, the interaction between CS and cCCT was significantly associated with 90‐day outcome: the predictive ability for outcome was improved by their interaction. Patients with good collateral status and lower cCCT were more likely to have a good functional outcome. Fourth, The optimal cutoff point of cCCT (−0.062) predicted good outcome at 3 months with 75% sensitivity and 58% specificity. 11 The current findings suggest the combination of CS with cCCT based on DSA may provide a novel and promising prediction model for clinical outcome in AIS‐LVO patients after EVT.
It is well known that the collateral circulation is associated with the clinical outcome of AIS patients receiving acute reperfusion therapy. 17 , 18 , 19 , 20 For example, patients with good collaterals were more likely to have a smaller infarct volume, 21 , 22 , 23 increased the probability of recanalization, and decreased distal embolization of fragments separated from the main retrieved thrombus. 24 In concordance with these studies, we also found that good collateral was associated with good outcome. Moreover, CCT has been found to play a role in predicting hyperperfusion phenomena after carotid stenting or angioplasty. 25 Our recent study identified cCCT as an independent predictor of clinical outcomes in AIS patients after EVT,and lower cCCT associated with good clinical outcome. 11 Several studies have assessed the relation between CS and CCT, and found that (1) good collateral circulation may help reduce CCT in some patients with higher grade carotid stenosis 10 ; (2) the difference in CCT between occluded and non‐occluded hemispheres is related to secondary collateral circulation through the ophthalmic artery. 26 Taken together with the present results and our recent results, 11 these studies provided support for the interaction effect of CCT and CS on clinical outcome, as also shown in the current study.
In contrast to previous studies, the present study used cCCT as the main imaging metric of interest. cCCT reflects the change of stroke side CCT (sCCT) relative to the healthy side (hCCT) after recanalization, thus reducing the potential impact of individual differences. We found that cCCT is negatively correlated with CS, which may be related to the following points: (1) Patients with good collateral status usually have lower resistance to venous return, 27 thus possibly shortening cCCT; (2) Several studies 28 , 29 have shown that collateral status is usually related to the impairment of dynamic cerebral autoregulation. After recanalization, the vasodilation response of the stroke side may slightly change in patients with good versus poor collateral status, resulting in lower cCCT in patients with good collateral status. In addition, we found that after adding cCCT as an independent variable, the regression coefficient of CS score and mRS score dropped from 0.45 to 0.37, which suggested that cCCT changed the relationship between CS and functional outcome. AUC analysis showed that the interaction between CS and cCCT is a better predictor of clinical outcome than traditional collateral or CCT assessments. We argue that CCT reflects an important physiological aspect of the circulation, which could be related to cerebrovascular reserve, and cCCT reflects the changes of cerebral circulation of the stroke versus healthy side in different individuals, while the collateral state is closely associated with cerebral circulation. Patients with higher CS and lower cCCT were more likely to have a good clinical outcome after EVT. In addition, we found that cCCT and the interaction between cCCT and CS were closely related to smaller infarct volume, which is in agreement with their association with good functional outcome.
The time from onset to recanalization has been confirmed by many studies to be closely associated with patient outcome. 30 The same trend was found in our study, although the differences did not reach statistical significance, which may be attributed to the small sample size. However, it was unexpected that patients with shorter OPT, DPT and ORT were found in patients with poor compared to good collateral, yet the shorter time did not result in a good outcome. This finding may be due to the greater importance of the collateral circulation on the outcome of patients with successful reperfusion than ORT. On the other hand, this phenomenon could be due to the fact that the patients with good collateral were more likely to undergo endovascular treatment in a longer time window than patients with poor collateral.
The strength of this study is that it is the first to assess the relationship between CCT and CS after EVT recanalization in AIS patients, and found that the relationship between CS and functional outcome is affected by cCCT. DSA, the gold standard for the evaluation of collateral circulation, 31 , 32 , 33 can immediately and accurately provide CCT and collateral status of patients with large vessel occlusion during EVT, provide insights to a more complete (dynamic and spatial) information of cerebral circulation, and potentially inform on clinical outcome. These findings may provide a substrate of information for clinical decision‐making and trial design in this population.
Limitation
Our study has several limitations. First, this study is a single‐center retrospective study with a relatively small sample size. Selection bias is inevitable, although cCCT was used to control for potential confounding factors. Second, the measurement of CCT may be affected by differing manual injection versus machine injection rate and volume settings. To attenuate this possible variability, our patient images were routinely obtained with a power injector with a standardized rate. Third, the catheter position may present a bias, but in our study the effect is likely minimal due to lack of effect of the catheter position on sCCT, and the consistency for both hCCT and sCCT between interobservers or different catheter locations in the same patient, as discussed by our recent study. 11 Forth, the triage of patient selection to perform EVT was not standard in the current study, which may limit the generalizability of our results. Finally, the small number of cases, under‐representation of female patients, and the imbalance in the number of cases between groups may lead to confounding factors that were not observed in our study. In the future, it will be important to confirm our results in prospective, large sample studies.
Conclusions
This is the first report about the association of collateral status with cCCT, and the effect of the interaction between collateral status and cCCT on functional outcome in AIS‐LVO patients receiving EVT. We found that higher CS had lower cCCT, and the interaction between collateral state and cCCT improved the predictive power of functional outcomes in AIS‐LVO patients after EVT. These findings potentially provide new insight into the interpretation of angiographic collaterals and CCT as it relates to patient outcome.
Acknowledgements
None.
Author Contributions
Y.J.W., J.Q.W., J.Q., W.L., X.H.S., Y.G.Z., X.L., and Z.A.Z. contributed to the acquisition of data; Y.J.W., J.Q.W., conducted data analysis; Y.J.W. drafted the manuscript; T.N.N. critically revised the manuscript; H.S.C. contributed to the study design and critically edited the manuscript.
Conflict of Interest
None declared.
Supporting information
Table S1. The baseline characteristics in the patients with poor vs. good outcome.
Table S2. The interaction between CS and cCCT in predicting infarct volume.
Funding Information
The work was supported by grants from the Science and Technology Plan of Shen Yang (20‐205‐4‐007) and the Science and Technology Project Plan of Liao Ning Province (2019JH2/10300027, 2020JH1/10300002).
Funding Statement
This work was funded by the Science and Technology Plan of Shen Yang grant 20‐205‐4‐007; the Science and Technology Project Plan of Liao Ning Province grants 2019JH2/10300027 and 2020JH1/10300002.
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.
References
- 1. Lin Y, Schulze V, Brockmeyer M, et al. Endovascular thrombectomy as a means to improve survival in acute ischemic stroke: a meta‐analysis. JAMA Neurol. 2019;76:850‐854. doi: 10.1001/jamaneurol.2019.0525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Powers W, Rabinstein A, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344‐e418. doi: 10.1161/STROKEAHA.119.026917 [DOI] [PubMed] [Google Scholar]
- 3. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large‐vessel ischaemic stroke: a meta‐analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723‐1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 4. Nelson PK, Setton A, Choi IS, Ransohoff J, Berenstein A. Current status of interventional neuroradiology in the management of meningiomas. Neurosurg Clin N Am. 1994;5:235‐259. doi: 10.1016/s1042-3680(18)30529-1 [DOI] [PubMed] [Google Scholar]
- 5. Ginsberg MJN. The cerebral collateral circulation: relevance to pathophysiology and treatment of stroke. Neuropharmacology. 2018;134:280‐292. doi: 10.1016/j.neuropharm.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 6. Romero JR, Pikula A, Nguyen TN, Nien YL, Norbash A, Babikian VL. Cerebral collateral circulation in carotid artery disease. Curr Cardiol Rev. 2009;5:279‐288. doi: 10.2174/157340309789317887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019‐1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 8. Jung S, Gilgen M, Slotboom J, et al. Factors that determine penumbral tissue loss in acute ischaemic stroke. Brain. 2013;136:3554‐3560. [DOI] [PubMed] [Google Scholar]
- 9. Yeo L, Kong W, Paliwal P, et al. Intravenous thrombolysis for acute ischemic stroke due to cervical internal carotid artery occlusion. J Stroke Cerebrovasc Dis. 2016;25:2423‐2429. doi: 10.1016/j.jstrokecerebrovasdis.2016.06.014 [DOI] [PubMed] [Google Scholar]
- 10. Hu Y, Guo W, Lee I, et al. Prolonged cerebral circulation time is more associated with symptomatic carotid stenosis than stenosis degree or collateral circulation. J Neurointerv Surg. 2018;10:476‐480. doi: 10.1136/neurintsurg-2017-013293 [DOI] [PubMed] [Google Scholar]
- 11. Wang JQ, Wang YJ, Qiu J, et al. Cerebral circulation time after thrombectomy: a potential predictor of outcome after recanalization in acute stroke. J Am Heart Assoc. 2022;11(11):e025853. doi: 10.1161/JAHA.122.025853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fujita K, Tanaka K, Yamagami H, et al. Detrimental effect of chronic hypertension on leptomeningeal collateral flow in acute ischemic stroke. Stroke. 2019;50:1751‐1757. doi: 10.1161/STROKEAHA.119.025142 [DOI] [PubMed] [Google Scholar]
- 13. Shi K, Xiao W, Wu G, et al. Temporal‐spatial feature extraction of DSA video and its application in AVM diagnosis. Front Neurol. 2021;12:655523. doi: 10.3389/fneur.2021.655523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saarinen J, Rusanen H, N S. Collateral score complements clot location in predicting the outcome of intravenous thrombolysis. AJNR Am J Neuroradiol. 2014;35:1892‐1896. doi: 10.3174/ajnr.A3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frolich AM, Schrader D, Klotz E, et al. 4d ct angiography more closely defines intracranial thrombus burden than single‐phase ct angiography. AJNR Am J Neuroradiol. 2013;34:1908‐1913. doi: 10.3174/ajnr.A3533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alves H, Treurniet K, Dutra B, et al. Associations between collateral status and thrombus characteristics and their impact in anterior circulation stroke. Stroke. 2018;49:391‐396. doi: 10.1161/STROKEAHA.117.019509 [DOI] [PubMed] [Google Scholar]
- 17. Anadani M, Finitsis S, Clarençon F, et al. Collateral status reperfusion and outcomes after endovascular therapy: insight from the Endovascular Treatment In Ischemic Stroke (ETIS) Registry. J Neurointerv Surg. 2022;14:551‐557. doi: 10.1136/neurintsurg-2021-017553 [DOI] [PubMed] [Google Scholar]
- 18. Gui X, Wang L, Wu C, Wang H, J K. Prognosis of subtypes of acute large artery atherosclerotic cerebral infarction by evaluation of established collateral circulation. J Stroke Cerebrovasc Dis. 2020;29:105232. doi: 10.1016/j.jstrokecerebrovasdis.2020.105232 [DOI] [PubMed] [Google Scholar]
- 19. Wang Q, Zhang S, Zhang M, Chen Z, M L. Collateral score based on ct perfusion can predict the prognosis of patients with anterior circulation ischemic stroke after thrombectomy. Med Sci. 2017;46:377‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sheth S, Sanossian N, Hao Q, et al. Collateral flow as causative of good outcomes in endovascular stroke therapy. J Neurointerv Surg. 2016;8:2‐7. doi: 10.1136/neurintsurg-2014-011438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Al‐Dasuqi K, Payabvash S, Torres‐Flores G, et al. Effects of collateral status on infarct distribution following endovascular therapy in large vessel occlusion stroke. Stroke. 2020;51:e193‐e202. doi: 10.1161/STROKEAHA.120.029892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ribo M, Flores A, Rubiera M, et al. Extending the time window for endovascular procedures according to collateral pial circulation. Stroke. 2011;42:3465‐3469. doi: 10.1161/STROKEAHA.111.623827 [DOI] [PubMed] [Google Scholar]
- 23. Lin L, Yang J, Chen C, et al. Association of collateral status and ischemic core growth in patients with acute ischemic stroke. Neurology. 2021;96:e161‐e170. doi: 10.1212/WNL.0000000000011258 [DOI] [PubMed] [Google Scholar]
- 24. García‐Tornel Á, Ciolli L, Rubiera M, et al. Leptomeningeal collateral flow modifies endovascular treatment efficacy on large‐vessel occlusion strokes. Stroke. 2021;52:299‐303. doi: 10.1161/STROKEAHA.120.031338 [DOI] [PubMed] [Google Scholar]
- 25. Yamauchi K, Enomoto Y, Otani K, Egashira Y, TJJons I. Prediction of hyperperfusion phenomenon after carotid artery stenting and carotid angioplasty using quantitative dsa with cerebral circulation time imaging. J Neurointerv Surg. 2018;10:576‐579. doi: 10.1136/neurintsurg-2017-013259 [DOI] [PubMed] [Google Scholar]
- 26. Sato K, Shimizu H, Inoue T, et al. Angiographic circulation time and cerebral blood flow during balloon test occlusion of the internal carotid artery. J Cereb Blood Flow Metab. 2014;34:136‐143. doi: 10.1038/jcbfm.2013.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Faizy TD, Kabiri R, Christensen S, et al. Association of venous outflow profiles and successful vessel reperfusion after thrombectomy. Neurology. 2021;96:e2903‐e2911. doi: 10.1212/WNL.0000000000012106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reinhard M, Muller T, Guschlbauer B, Timmer J, Hetzel A. Dynamic cerebral autoregulation and collateral flow patterns in patients with severe carotid stenosis or occlusion. Ultrasound Med Biol. 2003;29:1105‐1113. doi: 10.1016/s0301-5629(03)00954-2 [DOI] [PubMed] [Google Scholar]
- 29. Vernieri F, Pasqualetti P, Matteis M, et al. Effect of collateral blood flow and cerebral vasomotor reactivity on the outcome of carotid artery occlusion. Stroke. 2001;32:1552‐1558. doi: 10.1161/01.str.32.7.1552 [DOI] [PubMed] [Google Scholar]
- 30. Kim JT, Goyal M, Levy EI, et al. Onset to reperfusion time as a determinant of outcomes across a wide range of ASPECTS in endovascular thrombectomy: pooled analysis of the SWIFT, SWIFT PRIME, and STAR studies. J Neurointerv Surg. 2020;12(3):240‐245. [DOI] [PubMed] [Google Scholar]
- 31. Cross DT 3rd, Moran CJ, Akins PT, Angtuaco EE, Derdeyn CP, Diringer MN. Collateral circulation and outcome after basilar artery thrombolysis. AJNR Am J Neuroradiol. 1998;19:1557‐1563. [PMC free article] [PubMed] [Google Scholar]
- 32. Liebeskind DS, Cotsonis GA, Saver JL, et al. Collateral circulation in symptomatic intracranial atherosclerosis. J Cereb Blood Flow Metab. 2011;31:1293‐1301. doi: 10.1038/jcbfm.2010.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee WJ, Jung KH, Ryu YJ, et al. Utility of digital subtraction angiography‐based collateral evaluation in medically treated acute symptomatic basilar artery stenosis. Eur J Neurol. 2017;24:1148‐1155. doi: 10.1111/ene.13351 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The baseline characteristics in the patients with poor vs. good outcome.
Table S2. The interaction between CS and cCCT in predicting infarct volume.
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.


