Abstract
Vasculogenesis and angiogenesis are the major forms of blood vessel formation. Angiogenesis is the process where new vessels grow from pre-existing blood vessels, and is very important in the functional recovery of pathological conditions, such as wound healing and ischemic heart diseases. The development of better animal model and imaging technologies in past decades has greatly enriched our understanding on vasculogenesis and angiogenesis processes. Hypoxia turned out to be an important driving force for angiogenesis in various ischemic conditions. It stimulates expression of many growth factors like vascular endothelial growth factor, platelet-derived growth factor, insulin-like growth factor, and fibroblast growth factor, which play critical role in induction of angiogenesis. Other cellular components like monocytes, T cells, neutrophils, and platelets also play significant role in induction and regulation of angiogenesis. Various stem/progenitor cells also being recruited to the ischemic sites play crucial role in the angiogenesis process. Pre-clinical studies showed that stem/progenitor cells with/without combination of growth factors induce neovascularization in the ischemic tissues in various animal models. In this review, we will discuss about the fundamental factors that regulate the angiogenesis process and the use of stem cells as therapeutic regime for the treatment of ischemic diseases.
Keywords: Neovascularization, Hematopoietic stem cells, Hypoxia, Neutrophils, Notch signaling
Introduction
One of the major causes of human mortality in the United States as well as in the world is the ischemic heart disease (IHD, coronary heart disease). American Heart Association Statistics Committee and Stroke Statistics Subcommittee estimated that in 2006 nearly 17 million American adults suffered from IHD [1]. Myocardial ischemia (MI) is generally caused by occlusion of coronary artery because of the cholesterol fat deposition into the arterial lumen, and results in shortage of oxygen and nutrition. It will lead to cellular mortality, ischemia, and eventually heart failure. Due to the enormous development in pharmacological therapy and revascularization procedures during the last decades, the life expectation of patients has been significantly improved [2]. However, a significant number of patients were not considered for coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) treatment [3]. The problems in these patients involved a deficiency in the blood supply to the myocardial and lower extremity muscle beds [4]. For those patients, an alternative strategy for revascularization would be beneficial to increase the quality of life and maximize the efficacy of therapy. The concept of therapeutic angiogenesis has brought enormous attentions to the healthcare, in which induced neovascularization is to be generated into the ischemic tissue to improve blood flow and subsequently reduce symptoms of those suboptimal patients. Various angiogenic growth factors, such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), insulin-like growth factors (IGF), and fibroblast growth factors (FGF), have been widely studied and successfully induced angiogenesis in a number of animal models [5–8]. In this review, we will focus on the basic mechanism and signaling pathway of angiogenesis and various factors that could potentially regulate the process, such as hypoxia, growth factors, inflammatory cells, and endothelial progenitor cells, which will shed light on the future development direction of therapeutic angiogenesis.
Angiogenesis
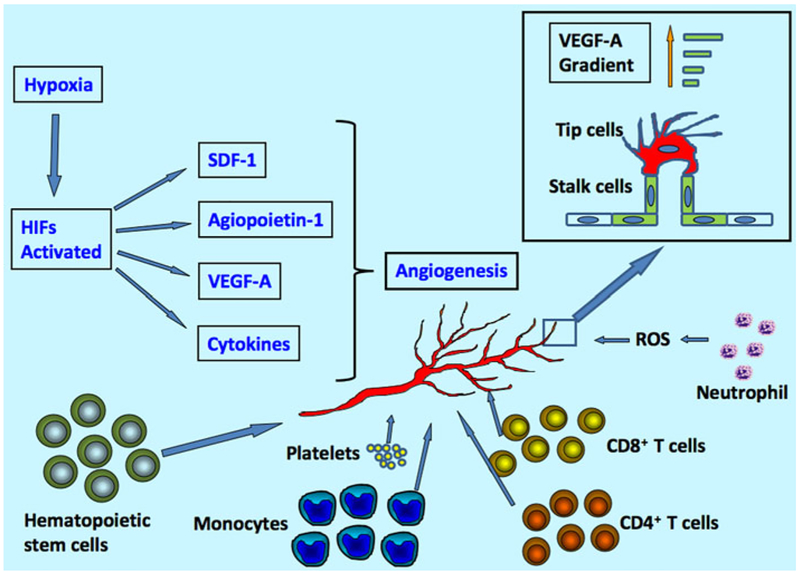
There are two major pathways for generation of blood vessel, angiogenesis, and vasculogenesis. Angiogenesis mainly describes about the growth of new blood vessels from pre-existing vessels, and is the main form of blood vessel formation in pathological conditions [9]. Vasculogenesis, on the other hand, describes about the spontaneous blood-vessel formation, and involves generation of blood vessel from blood islands. In this review, we will mainly focus on angiogenesis, the signaling pathways of which may recapture many molecular events occurring during vascular development [10]. The major cause of angiogenesis is hypoxia, deprivation of adequate oxygen supply [11]. Hypoxia triggers a series molecular event, which leads to release of angiogenic growth factors, such as VEGF [11]. Inflammatory cells release cytokines and chemokines in response to ischemia, which also play a prominent role in angiogenesis [12]. These environmental signals activate endothelial cells, which line along blood vessels, and promote sprouting of new vessels. The tip of these sprouts are formed by specialized type of endothelial cells called tip cells, while another specialized endothelial stalk cells follow tip cells, and proliferate to form the trunk of new blood vessel [13].
It is of immense interest to dissect out the signaling pathways of the micro-environmental factors, which regulate endothelial cells for angiogenesis and specify the nature of tip and stalk cells, and also direct the migration of tip cell and proliferation of stalk cells during the process of sprout formation [14].
Vascular endothelial growth factor-A (VEGF-A) is one of the most important regulators of angiogenesis. VEGF-A has been shown to guide the filopodial extension of tip endothelial cells, while at the same time stimulate the proliferation of stalk cells through VEGF receptor 2 (VEGFR2)/ Flk-1. While tip cells are directed by the gradient of VEGF-A, and proliferation is regulated by its concentration [15]. The vascular lumen formation accompany with the invasion of vascular sprouts by stalk cells. The lumen formation starts with the presence of large intracellular vesicles. Upon fusion, the vesicles form an incipient lumen. The integrity and polarity of the newly formed lumen are established by intercellular junctional adherents of the stalk cells and cell-extracellular matrix (ECM) interactions [16]. The intracellular vesicles are then exported into a common intercellular space that is bounded by endothelial cells, which are joined together by junctional contacts. This leads to the formation of a common intercellular luminal space. The newly formed sprouts were connected with each other through the tip cells and eventually form a continuous lumen [17]. Physical factors generated by blood flow, like shear stress, continuously regulate the property of lumen, such as diameter [16]. Finally, enrichment of oxygen concentration following the flow down regulated VEGF-A production, and lead to re-establish quiescent state of the new vessels (Fig. 1) [14].
Fig. 1.
Factors regulating angiogenesis. Angiogenesis is mainly regulated by hypoxia, growth factors (VEGF-A, angiopoiein-1, and other cytokines), immune cells (monocytes, T cells, Neutrophil and platelets), and stem/ progenitor cells. Tip cells and stalk cells of the vessel are regulated by the gradient of VEGF-A molecule
Angiogenesis and Hypoxia
Since the main function of blood vessel is to transport oxygen and nutrients to different tissues, oxygen concentration and metabolic factors might play a critical role in angiogenesis process. Hypoxia, or inadequate of oxygenation, is the driving force of angiogenesis. In normal tissue, oxygen concentration varies between 30 and 50 mmHg and the blood vessels do not undergo significant growth [18–20]. In rapidly expanding embryonic tissues, oxygen consumption outpaces the oxygen supply, and thus creates hypoxia conditions, which promote the development of the vascular system. In adult tissues, especially in pathological condition like ischemia, reduction of oxygen tension may also trigger angiogenesis. Down regulation of oxygen concentration inactivates some of the enzymatic factors and leads to the accumulation of hypoxia inducible factors (HIFs), which composite two subunits alpha and beta. Vascular cells express various oxygen-sensing enzymes, including prolyl hydroxylase domain proteins (PHD1–3) and factor inhibiting HIFs (FIH), which are important in HIFs degradation and inhibition [21]. Oxygen molecule is a substrate to PHDs, and thus HIF-alpha accumulation is achieved in reduced oxygen concentration environment because of poor hydroxylation reactions [20]. HIFs, in turn, regulate hundreds of genes encoding proteins, such as VEGF-A, glycolytic enzyme, erythropoietin and etc. HIF-β can stimulate the production of angiogenic cytokines releasing by hematopoietic cells, such as VEGF and angiopoietin-1 [22]. It has also been shown that hypoxia can promote vascular progenitors expressing endothelial markers like CD31, VEGFR2, and endothelial nitric oxide synthase (eNOS) [23, 24]. HIF-1 alpha up-regulates chemo-attractant stromal cell-derived factor (SDF)-1 in the ischemia site and thus help recruiting circulating endothelial and pericyte progenitors, and angiocompetent CD45+ myeloid cells expressing SDF-1 receptor CXCR4 [24, 25]. HIF-1 also induces the expression of inter-cellular adhesion molecule 1 (ICAM-1), which serves as a docking site for mobilized progenitor cells in ischemic tissue [26, 27].
Angiogenesis and Growth Factors
A variety of growth factors have been used to induce angiogenesis in pre-clinical or clinical studies [28–44] (Table 1). Of these growth factors, VEGF is one of the main factors that regulate angiogenesis. VEGF-A plays a critical role in promoting endothelial cell differentiation, migration, and proliferation. It interacts through different pathways including Angiopoietin/Tie2, Notch, Wnt, and transforming growth factor (TGF)-β [14]. VEGFR and angiopoietin/Tie mutant mice failed to develop different phases of normal vasculature in embryonic stage [45]. The major receptor for VEGF-A is Flk1/VEGFR2, and is the earliest marker for angioblast precursor cells. Flk1+ cells give rise to blood islands, which is composed of endothelial cells and hematopoietic progenitor cells (HPCs). In the mouse model, it has been shown that the dorsal aorta and cardinal veins develop directly from aggregated angioblasts. Flk−/− embryos die as early as 9 days of development and show no growth of blood vessels or hematopoietic cells [46, 47]. It has been shown that Flk1+ cells isolated from differentiating embryonic stem cells can give rise to single-cell-derived blast colonies called blast colony forming cells (BL-CFCs). BL-CFCs can give rise to both endothelial and hematopoietic cells [48, 49]. It is presumed that endothelial and hematopoietic cells develops from a common progenitor, hemangioblast [50]. In angiogenesis, endothelial cells stimulated by VEGF-A compete for the tip position via Delta-like 4 (Dll4)/Notch pathway. Genetic mosaic analysis in Drosophila melanogaster has shown that by competing in activation of the Notch signaling in neighbor cells, the cell that produces more Dll4 will eventually remain the tip cells [51]. VEGF-A triggers endothelial cells expressing Dll4, which activates Notch signaling in adjacent cells and leads to the down regulation of VEGFR and thus, of the VEGF response. The surrounding cells, which were activated by Notch signaling, differentiated into stalk cells. Computational modeling also confirmed the VEGF-A concentration with Dll4/Notch-mediated lateral inhibition and indicated that VEGF-concentration, VEGF-A gradient, and filopodia extension is critical in determining the tip/stalk pattern. [52]. VEGF-A can also promote endothelial proliferation through activation of extracellular signal-regulated kinases (Erk)1/2 signaling pathway [53].
Table 1.
Therapeutic angiogenesis targeting growth factors
| Targeted growth factors | Year | Number of patients | Disease model | Results | References |
|---|---|---|---|---|---|
| FGF1 | 1998 | 20 | CAD | Formation of capillaries around the injection site | [28] |
| FGF2 | 1999 2002 | 24 | CABG | Reduced ischemic zone size Effect sustained at 3 years | [29, 30] |
| FGF2 | 2000 | 52 | CABG | Increased regional wall thickness reduction in ischemic area | [31] |
| FGF2 | 2000 | 30 | CAD not amenable for CABG or PTCA | Hypotension at high dosages improved resting perfusion | [32, 33] |
| FGF2 | 2002 | 337 | CAD | Safe short-term improvement in symptoms | [34] |
| VEGF-A165 | 2000 | 15 | CAD | Improved rest perfusion | [35] |
| VEGF-A165 | 2001 | 14 | CAD not amenable for CABG or PTCA | Improved collateral density score | [36] |
| VEGF-A165 | 2003 | 165 | CAD not amenable for CABG or PTCA | No improvement in ETT or SPECT compare to controls | [37] |
| GM-CSF | 2001 | 21 | CAD not amenable for CABG or PTCA | Improved collateral flow index | [38] |
| FGF | 2000 | 40 | CABG | Improved capillary network increased LVEF | [39] |
| VEGF | 2001 | 6 | CAD | Reduced angina and ischemia improved myocardial perfusion | [40] |
| VEGF | 2002 | 19 | CAD with class III or IV angina | Improved angina improvement of other functionality | [41] |
| FGF4 | 2002 | 79 | CAD with angina | Improved exercise time | [42] |
| FGF4 | 2003 | 52 | CAD with angina | No significant improvement | [43] |
| VEGF ± arginine-1 | 2008 | 19 | CAD | Improved anterior wall perfusion better anterior wall contractility | [44] |
FGF Fibroblast growth factor, VEGF vascular endothelial growth factor, GM-CSF granulocyte macrophage colony-stimulating factor, CAD coronary artery disease, CABG coronary artery bypass graft, PTCA percutaneous transluminal coronary angioplasty, ETT exercise treadmill test, SPECT single photon emission computed tomography, LVEF left ventricular ejection fraction
Angiogenesis and Notch Signaling
Notch signaling plays an important role in many aspects of angiogenesis including angiogenic growth of the blood vessel network, proliferation of endothelial cells, and differentiation of arteries and veins [54]. Inhibition of Notch signaling promotes the number of tip cells. Activation of Notch by Jagged 1 peptide, on the contrary, leads to fewer tip cells and vessel branches [55–57]. In Dll4-Notch defective zebrafish, higher migration and proliferation was observed in endothelial cells, while over activation the Notch pathway inhibited these functions. It was further shown that Dll4 signals through Notch1b in an rbpja-dependent manner to limiting the number of endothelial tip cells formation [14, 58, 59]. It was shown that endothelial cells autonomously turn to stalk cells rather than tip cells in Notch deficient zebrafish and mouse using mosaic technique. Activated Notch signaling pathway turns endothelial cells into stalk cell by suppressing the tip cell phenotype. Injection of Jag 1 peptide, which activates Notch signaling in the mouse retina, leads to reduced tip cell formation and filopodia extension [55]. These experiments showed that Notch signaling pathway plays an important role in endothelial cells specification, and tip and stalk cells are undergoing dynamic regulation by Notch signaling. In the mRNA level, tip cells express higher levels of Pdgfb, Dll4, Unc5b, Kdr, Flt4 etc. while stalk cells have an increased expression of Robo4, Jag1, Flt1 and etc. It was believed that by regulating the expression of Flt1, Kdr, Nrp1, and Flt4, Notch signaling modulates the output of VEGF-VEGFR signaling in endothelial cells [14, 60].
Notch signaling not only play an important role in endothelial cells specification, but also are important in regulating endothelial cell proliferation, which coordinates sprout growth in length and diameter. Notch signaling may play an essential role in the maintenance of vascular homeostasis by repressing endothelial cell proliferation through transcription factor recombination signal-binding protein J kappa (RBP-J) [61]. In mouse, inhibition of Notch signaling may lead to increased proliferation of tip, which contributes to increased vessel branching [55]. Activation of Notch pathway can suppresses endothelial cell proliferation through mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/Akt pathways [62, 63] and conditional overexpression of Dll4 in mice results in enlarged dorsal aorta [64, 65]. Further study has shown that Notch signaling acts through transcriptional regulation of the Notch intracellular domain (NICD)/CSL/Mastermind (MAML) complex, which leads to the down regulation of endothelial cell proliferation [62].
Angiogenesis and Immune Cells
In angiogenesis, CD8+ T cells and CD4+ T cells infiltrate the ischemic site and secrete cytokines that recruit monocytes. The recruitment of monocytes triggers the synthesis of angiogenic cytokines and promotes the development of vessels [66].
Monocytes were shown to have a crucial role during arteriogenesis. Different types of inflammatory cells were recruited to the ischemia area through up-regulation of chemo-attractant molecules. Expression of integrin receptors like macrophage-1 antigen (Mac-1) and lymphocyte function-associated antigen 1 (LFA-1) were up-regulated by VEGF, PDGF, and other growth factors [67]. The recruitment of monocytes correlated with the intensity of neovascularization [66]. It was shown that monocyte depletion resulted in a reduction of blood flow reconstitution [68]. Treating with granulocyte colony-stimulating factor (G-CSF) can significantly stimulate angiogenesis in murine model of acute hind limb ischemia mediated by monocytes [69].
T lymphocytes, important in host defense against infection and wound healing, are also involved in angiogenesis process. CD4+ T lymphocyte-deficient mice showed reduced collateral flow induction, macrophage number, and VEGF level in ischemic muscle. CD4+ T lymphocytes may be important in recruiting macrophages, which influence the arteriogenic response [70, 71]. CD8+ T cells also infiltrated the perivascular space after acute cerebral ischemia. The secreted interleukin-16 (IL-16) of CD8+ T cells is a potent chemo-attractant for monocytes and CD4+ cells, which indicates a possible role for CD8+ T cells in angiogenesis. Indeed, reduced blood flow recovery were observed in CD8+ T cells deficient mice, which may be because of decreased recruiting of CD4+ T cells an monocytes [72].
Nature killer (NK) cells has also been shown to play a role in angiogenesis. It was reported that arteriogenesis was impaired in mice depleted for NK cells by anti-NK1.1 antibodies and in NK cell deficient transgenic mice. However, artreiogenesis was unaffected in J alpha 281-knockout mice that lack NK1.1+ nature killer T (NKT) cells, indicating that NK cells rather than NKT cells are involved in arteriogenesis [73]. It was suggested NK cells play a role in the initiation of collateral growth. In the contrary, polymorphonuclear leukocytes (PMN) showed anti-angiogenic properties. In the experiment, unilateral hind limb ischemia was surgically induced in athymic nude rats. Addition of PMN to peripheral blood (PB) mononuclear cells and platelets attenuated blood perfusion and capillary formation [74].
Neutrophils adhere to vascular endothelium and regulate angiogenesis process. There are mainly two ways that neutrophils mediate with endothelial injury, generating high levels of reactive oxygen species (ROS) and releasing lysosomal proteinases. ROS are chemically reactive molecules because of the presence of unpaired valence shell electrons. Examples include superoxide, hydrogen peroxide, hydroxyl anion and etc. Generation of ROS was suggested to play an important role in vascular biology through redox signaling [75–77]. Adding low concentration of hydrogen peroxide (H2O2) was shown to stimulate cell migration and proliferation in endothelial cells, possibly through transcription factor ets-1 [78]. Nitric oxide has diverse effects on blood vessels, which include simulation of angiogenesis and vasodilation to vessel normalization [27]. Accumulation of H2O2 in junD−/− cells reduces the activity of PHDs, which target hypoxia inducible factor-alpha (HIF-α). Accumulation of HIF-α enhances the transcription of VEGF-A and promotes angiogenesis [79, 80]. Moreover, ROS regulate angiogenesis in a dose dependent manner. At low concentration ROS stimulate post-ischemic revascularization but inhibit it at high concentrations [81]. Nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidases are suggested to be the dominant source of ROS in neutrophil and endothelial cells in normal vessel [75, 76, 82]. In ischemic tissue, administration of neutrophil NADPH oxidase inhibitors and antibody against neutrophil CD18 adhesion molecules could significantly reduce radicals production, which showed that ROS in the damaged tissue mainly comes from neutrophil rather than other resources [83, 84]. Neutrophils-derived neutral proteases, such as elastase, have been shown to mediate endothelial cell detachment in vitro [85]. Neutrophil elastase inhibitor reversed the inhibitory effect of PMN in hindlimb ischemia model indicating that neutrophil and elastases are responsible for anti-angiogenic effect [74].
Platelets interact with leukocytes, endothelial cells, and circulating progenitor cells. The activation of platelet occurs in acute ischemic events, which promote leukocyte arrest on the vascular endothelium. [86]. Platelets can express both angiogenic (VEGF-A, angiopoietin-1, PDGF, bFGF, thrombin) and anti-angiogenic growth factors (thrombospondin, PF-4, endostatin). However, the net effect of platelets is pro-angiogenic [87]. Thrombin, blood-clotting factors, has been shown to promote the growth of blood vessel. Anticoagulant drugs, which suppress thrombin generation, immediately hamper in vivo angiogenic response after tissue ischemia in a rodent hindlimb ischemia model [88].
Angiogenesis and Stem Cells
Following ischemia, angiogenic factors, such as VEGF-A, were up-regulated and promoted migration of endothelial progenitor cells (EPC), circulating EPCs, hematopoietic stem cells (HSCs), and HPCs to the site of injury by interacting with VEGFR2 and VEGFR1 expressed on these cells [89]. It has been shown that circulating EPCs were able to incorporate into neovascular within the ischemic tissue and differentiate into endothelial cells, even though the contribution varies from organ to organ [90–93]. The incorporation has been shown to be proportional to the degree of tissue ischemia, which indicated that the gradient of hypoxia is important in directing circulating EPCs to the injury site [94]. Those circulating EPCs may possibly come from bone marrow. Reports have shown that bone marrow derived cells can contribute to angiogenesis during wound healing and limb ischemia [95–97]. By analysis, the blood samples from bone marrow transplant recipients using in situ hybridization, it was proposed that most circulating endothelial cells in fresh blood originate from vessel walls and have limited growth capability, while bone-marrow derived cells originated endothelial cells showed a delayed outgrowth but a greater proliferation rate [98]. Non-bone marrow-derived cells may also contribute for neovascularization. In a model by combining parabiosis with reverse bone marrow transplantation followed by hindlimb ischemia, it has been shown that non-bone marrow-derived c-kit+ CD45− progenitors contributed to post-natal neo-vascularization to an extent that is similar to that of bone marrow-derived progenitor cells [99]. Progenitor cells can also differentiate into supporting cells, which deliver growth factors to ischemia tissue and promote angiogenesis through paracrine effects. These cells include fibroblasts, pericytes, and primarily leukocytes [91, 100–102]. Pro-genitors from human peripheral blood have been shown to differentiate into both early EPCs, which function through paracrine effect, and late EPC, which function directly through vasculogenesis [66, 102, 103]. Hematopoietic cells (HSCs, HPCs, platelets, monocytes, erythroblasts and etc.) could release angiogenic factors like VEGF-A, angio-poietins, and matrix metalloproteinases (MMPs), which facilitate angiogenesis [104–106]. Self-renewing adult HSCs were shown to have functional hemangioblast activity, which clonally differentiated into all hematopoietic cell lineages and endothelial cells that revascularize adult retina [107].
Angiogenesis and HSCs
It is now well known that HSCs and EPCs derive from the same precursor hemangioblasts, and transplanted adult HSCs can be differentiated into functional endothelial cells [107, 108]. During embryo development, HSCs and leukocytes also stimulate angiogenesis by transdifferentiating into endothelial cells or by releasing angiogenic factors [109, 110]. Using specific markers for HSCs, such as CD133, it has shown that HSCs were able to differentiate to endothelial cells in vitro [111]. And ex vivo expanded adult EPCs and hematopoietic stem/progenitor cells may also follow the similar pathways to differentiate into endothelial cells in the ischemic tissues [112]. CD34+ cells isolated from umbilical cord blood (UCB), when cultured in conditioned medium with IL-2, express mature endothelial markers von-Willebrand factor (VWF), ICAM-1 (CD54), E-selectin (CD62E), and platelet endothelial cell adhesion molecule (PECAM,CD31) [113]. By using a 3-dimensional matrix model together with human micro-vascular endothelial cells, it was observed that CD34+ cells were able to home and proliferate around the capillary sprouts and co-culturing with CD34+ cells were able to lead to 68% enhancement of neovascularization [114]. Subsets of CD34+ cells isolated from UCB, and PB can participated in capillary network formation in vivo in the ischemic tissues of immunodeficient nude rats after local transplantation [115]. Injection of human UCB mononuclear cells into non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice reduced the scar size and increased capillary density following surgical ligation of the left anterior descending (LAD) coronary artery [116]. CD133+ cells from human cord blood and bone marrow were injected in the necrosis border zone of NOD/SCID mice with left ventricular cryo-injury. Capillary density in ischemic area were found higher after both BM and UCB CD133+ cell treated mice compare to controls, which indicated that both cord blood- and marrow- derived CD133+ cells have beneficial effects on post-injury angiogenesis [117]. It has also been shown that HSCs also express markers of pericytes and play a role in shaping the angio-architecture in the vascular niche of brain tumors [118]. Besides freshly isolated HSCs, we recently used polyethersulfone (PES) nanofiber-expanded CD133+/ CD34+ cells from freshly isolated cord bloods [119, 120] for ischemic therapies in animal models [8, 121]. The nanofiber-expanded cells were shown to be more efficient in promoting neovascularization in ischemic tissues possibly because of the higher angiogenic effects [8, 119].
It is now well documented the transplantation of HSCs provides beneficial effect for the treatment of IHDs. Detailed study showed that transplanted HSCs incorporate into the vasculature in a limited number [122] after homing to the injury sites. These transplanted cells mainly secrete pro-angiogenic growth factors, which are responsible for the initiation and maintenance of the neovaslularization process [123, 124]. Further study is needed to enhance the therapeutic potential of HSC transplantation along with additional factors.
Future Directions
Recent advancement in research has dramatically enriched our understanding on the process of angiogenesis in vivo using sophisticated animal models and better imaging techniques [125]. Although several signaling pathway has been revealed, however, the mechanisms by which transcriptional factors are being regulated by signaling pathways are yet to be clearly defined. Eventual findings will lead us to regulate angiogenesis by targeting these transcriptional factors and signaling pathways for the therapeutic angiogenesis. Moreover, the mechanism by which stem cells regulate angiogenesis process is yet to be defined. Therapeutic angiogenesis, either inducing or reducing, is a rapidly emerging field and stem cell therapy shows the promise for enhancement of angiogenesis. Mechanistic investigation in future might be appropriate to make the stem cell therapy more acceptable regiment for the therapeutic angiogenesis to treat degenerative and ischemic diseases.
Acknowledgments
This study was supported in part by National Institutes of Health grants, K01 AR054114 (NIAMS), SBIR R44 HL092706–01 (NHLBI), Third Frontier Projects, Ohio Technology BRCP Grant, and The Ohio State University start-up fund for stem cell research. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. (2009). Heart disease and stroke statistics–2009 update: a report from the American heart association statistics committee and stroke statistics subcommittee. Circulation, 119(3), 480–486. [DOI] [PubMed] [Google Scholar]
- 2.Cha KS, Schwartz RS, & Henry TD (2005). Myocardial angiogenesis protein growth factors. In Laham RJ & Baim DS (Eds.), Contemporary cardiology: Angiogenesis and direct myocardial revasularization. Totowa, NJ: Humana Press Inc. [Google Scholar]
- 3.Mukherjee D, Bhatt DL, Roe MT, Patel V, & Ellis SG (1999). Direct myocardial revascularization and angiogenesis- how many patients might be eligible? American Journal of Cardiology, 84(5), 598–600. A8. [DOI] [PubMed] [Google Scholar]
- 4.Shah PB, Lotun K, & Losordo DW (2005). Gene therapy for angiogenesis in the treatment of cardiovascular and peripheral arterial disease. In Laham RJ & Baim DS (Eds.), Contemporary cardiology: angiogenesis and direct myocardial revasularization. Totowa: Humana Press Inc. [Google Scholar]
- 5.Schaper W, & Ito WD (1996). Molecular mechanisms of coronary collateral vessel growth. Circulation Research, 79(5), 911–919. [DOI] [PubMed] [Google Scholar]
- 6.Ware JA, & Simons M (1997). Angiogenesis in ischemic heart disease. Nature Medicine, 3(2), 158–164. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J (1998). Angiogenic therapy of the human heart. Circulation, 97(7), 628–629. [DOI] [PubMed] [Google Scholar]
- 8.Das H, George JC, Joseph M, Das M, Abdulhameed N, Blitz A, et al. (2009). Stem cell therapy with overexpressed VEGF and PDGF genes improves cardiac function in a rat infarct model. Plos One, 4(10), e7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmeliet P (2005). Angiogenesis in life, disease and medicine. Nature, 438(7070), 932–936. [DOI] [PubMed] [Google Scholar]
- 10.Byrd N, & Gradel L (2004). Hedgehog signaling in murine vasculogenesis and angiogenesis. Trends in Cardiovascular Medicine, 14(8), 308–313. [DOI] [PubMed] [Google Scholar]
- 11.Pugh CW, & Ratcliffe PJ (2003). Regulation of angiogenesis by hypoxia: role of the HIF system. Nature Medicine, 9(6), 677–684. [DOI] [PubMed] [Google Scholar]
- 12.Egami K, Murohara T, Aoki M, & Matsuishi T (2006). Ischemia-induced angiogenesis: role of inflammatory response mediated by P-selectin. Journal of Leukocyte Biology, 79(5), 971–976. [DOI] [PubMed] [Google Scholar]
- 13.Suchting S, & Eichmann A (2009). Jagged gives endothelial tip cells an edge. Cell, 137(6), 988–990. [DOI] [PubMed] [Google Scholar]
- 14.Phng LK, & Gerhardt H (2009). Angiogenesis: a team effort coordinated by notch. Developmental Cell, 16(2), 196–208. [DOI] [PubMed] [Google Scholar]
- 15.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, et al. (2003). VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. Journal of Cell Biology, 161(6), 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iruela-Arispe ML, & Davis GE (2009). Cellular and molecular mechanisms of vascular lumen formation. Developmental Cell, 16(2), 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blum Y, Belting HG, Ellertsdottir E, Herwig L, Luders F, & Affolter M (2008). Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Developmental Biology, 316(2), 312–322. [DOI] [PubMed] [Google Scholar]
- 18.Goetze JP, Gore A, Moller CH, Steinbruchel DA, Rehfeld JF, & Nielsen LB, (2004). Acute myocardial hypoxia increases BNP gene expression. FASEB Journal, 18(12), 1928–1930. [DOI] [PubMed] [Google Scholar]
- 19.Smith LM, Golub AS, & Pittman RN (2002). Interstitial Po-2 determination by phosphorescence quenching microscopy. Microcirculation, 9(5), 389–395. [DOI] [PubMed] [Google Scholar]
- 20.Fong GH (2009). Regulation of angiogenesis by oxygen sensing mechanisms. Journal of Molecular Medicine, 87(6), 549–560. [DOI] [PubMed] [Google Scholar]
- 21.Kaelin WG, & Ratcliffe PJ (2008). Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Molecular Cell, 30(4), 393–402. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez-Bergeron DL, Runge A, Dahl KDC, Fehling HJ, Keller G, & Simon MC (2004). Hypoxia affects mesoderm and enhances hemangioblast specification during early development. Development, 131(18), 4623–4634. [DOI] [PubMed] [Google Scholar]
- 23.Tillmanns J, Rota M, Hosoda T, Misao Y, Esposito G, Gonzalez A, et al. (2008). Formation of large coronary arteries by cardiac progenitor cells. Proceedings of the National Academy of Sciences of the United States of America, 105(5), 1668–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, et al. (2008). HIF1 alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell, 13(3), 206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. (2004). Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nature Medicine, 10(8), 858–864. [DOI] [PubMed] [Google Scholar]
- 26.Lee SP, Youn SW, Cho HJ, Li L, Kim TY, Yook HS, et al. (2006). Integrin-linked kinase, a hypoxia-responsive molecule, controls postnatal vasculogenesis by recruitment of endothelial progenitor cells to ischemic tissue. Circulation, 114(2), 150–159. [DOI] [PubMed] [Google Scholar]
- 27.Fraisl P, Mazzone M, Schmidt T, & Carmeliet P (2009). Regulation of angiogenesis by oxygen and metabolism. Dev Cell, 16(2), 167–179. [DOI] [PubMed] [Google Scholar]
- 28.Schumacher B, Pecher P, von Specht BU, & Stegmann T (1998). Induction of neoangiogenesis in ischemic myocardium by human growth factors: first clinical results of a new treatment of coronary heart disease. Circulation, 97(7), 645–650. [DOI] [PubMed] [Google Scholar]
- 29.Laham RJ, Sellke FW, Edelman ER, Pearlman JD, Ware JA, Brown DL, et al. (1999). Local perivascular delivery of basic fibroblast growth factor in patients undergoing coronary bypass surgery: results of a phase I randomized, double-blind, placebo-controlled trial. Circulation, 100(18), 1865–1871. [DOI] [PubMed] [Google Scholar]
- 30.Ruel M, Laham RJ, Parker JA, Post MJ, Ware JA, Simons M, et al. (2002). Long-term effects of surgical angiogenic therapy with fibroblast growth factor 2 protein. Journal of Thoracic and Cardiovascular Surgery, 124(1), 28–34. [DOI] [PubMed] [Google Scholar]
- 31.Laham RJ, Chronos NA, Pike M, Leimbach ME, Udelson JE, Pearlman JD, et al. (2000). Intracoronary basic fibroblast growth factor (FGF-2) in patients with severe ischemic heart disease: results of a phase I open-label dose escalation study. Journal of the American College of Cardiology, 36(7), 2132–2139. [DOI] [PubMed] [Google Scholar]
- 32.Unger EF, Goncalves L, Epstein SE, Chew EY, Trapnell CB, Cannon RO 3rd, et al. (2000). Effects of a single intracoronary injection of basic fibroblast growth factor in stable angina pectoris. American Journal of Cardiology, 85(12), 1414–1419. [DOI] [PubMed] [Google Scholar]
- 33.Udelson JE, Dilsizian V, Laham RJ, Chronos N, Vansant J, Blais M, et al. (2000). Therapeutic angiogenesis with recombinant fibroblast growth factor-2 improves stress and rest myocardial perfusion abnormalities in patients with severe symptomatic chronic coronary artery disease. Circulation, 102(14), 1605–1610. [DOI] [PubMed] [Google Scholar]
- 34.Simons M, Annex BH, Laham RJ, Kleiman N, Henry T, Dauerman H, et al. (2002). Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation, 105(7), 788–793. [DOI] [PubMed] [Google Scholar]
- 35.Hendel RC, Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordano FJ, et al. (2000). Effect of intracoronary recombinant human vascular endothelial growth factor on myocardial perfusion: evidence for a dose-dependent effect. Circulation, 101(2), 118–121. [DOI] [PubMed] [Google Scholar]
- 36.Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordano FJ, Simons M, et al. (2001). Intracoronary administration of recombinant human vascular endothelial growth factor to patients with coronary artery disease. American Heart Journal, 142(5), 872–880. [DOI] [PubMed] [Google Scholar]
- 37.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, et al. (2003). The VIVA trial: vascular endothelial growth factor in Ischemia for vascular angiogenesis. Circulation, 107(10), 1359–1365. [DOI] [PubMed] [Google Scholar]
- 38.Seiler C, Pohl T, Wustmann K, Hutter D, Nicolet PA, Windecker S, et al. (2001). Promotion of collateral growth by granulocyte-macrophage colony-stimulating factor in patients with coronary artery disease: a randomized, double-blind, placebo-controlled study. Circulation, 104(17), 2012–2017. [DOI] [PubMed] [Google Scholar]
- 39.Pecher P, & Schumacher BA (2000). Angiogenesis in ischemic human myocardium: clinical results after 3 years. Annals of Thoracic Surgery, 69(5), 1414–1419. [DOI] [PubMed] [Google Scholar]
- 40.Vale PR, Losordo DW, Milliken CE, McDonald MC, Gravelin LM, Curry CM, et al. (2001). Randomized, single-blind, placebo-controlled pilot study of catheter-based myocardial gene transfer for therapeutic angiogenesis using left ventricular electromechanical mapping in patients with chronic myocardial ischemia. Circulation, 103(17), 2138–2143. [DOI] [PubMed] [Google Scholar]
- 41.Losordo DW, Vale PR, Hendel RC, Milliken CE, Fortuin FD, Cummings N, et al. (2002). Phase 1/2 placebo-controlled, double-blind, dose-escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia. Circulation, 105(17), 2012–2018. [DOI] [PubMed] [Google Scholar]
- 42.Grines CL, Watkins MW, Helmer G, Penny W, Brinker J, Marmur JD, et al. (2002). Angiogenic gene therapy (AGENT) trial in patients with stable angina pectoris. Circulation, 105(11), 1291–1297. [DOI] [PubMed] [Google Scholar]
- 43.Grines CL, Watkins MW, Mahmarian JJ, Iskandrian AE, Rade JJ, Marrott P, et al. (2003). A randomized double-blind, placebo-controlled trial of Ad5FGF-4 gene therapy and its effect on myocardial perfusion in patients with stable angina. Journal of the American College of Cardiology, 42(8), 1339–1347. [DOI] [PubMed] [Google Scholar]
- 44.Ruel M, Beanlands RS, Lortie M, Chan V, Camack N, deKemp RA, Suuronen EJ, Rubens FD, DaSilva JN, Sellke FW, Stewart DJ, & Mesana TG, (2008). Concomitant treatment with oral L-arginine improves the efficacy of surgical angiogenesis in patients with severe diffuse coronary artery disease: the endothelial modulation in angiogenic therapy randomized controlled trial. Journal of Thoracic and Cardiovascular Surgery, 135(4), 762–770, 770 e1. [DOI] [PubMed] [Google Scholar]
- 45.Bikfalvi A, & Bicknell R (2002). Recent advances in angiogenesis, anti-angiogenesis and vascular targeting. Trends in Pharmacological Sciences, 23(12), 576–582. [DOI] [PubMed] [Google Scholar]
- 46.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, et al. (1995). Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature, 376(6535), 62–66. [DOI] [PubMed] [Google Scholar]
- 47.Shalaby F, Ho J, Stanford WL, Fischer KD, Schuh AC, Schwartz L, et al. (1997). A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell, 89(6), 981–990. [DOI] [PubMed] [Google Scholar]
- 48.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, & Keller G (1998). A common precursor for hematopoietic and endothelial cells. Development, 125(4), 725–732. [DOI] [PubMed] [Google Scholar]
- 49.Chung YS, Zhang WJ, Arentson E, Kingsley PD, Palis J, & Choi K (2002). Lineage analysis of the hemangioblast as defined by FLK1 and SCL expression. Development, 129(23), 5511–5520. [DOI] [PubMed] [Google Scholar]
- 50.Ema M, & Rossant J (2003). Cell fate decisions in early blood vessel formation. Trends in Cardiovascular Medicine, 13(6), 254–259. [DOI] [PubMed] [Google Scholar]
- 51.Ghabrial AS, & Krasnow MA (2006). Social interactions among epithelial cells during tracheal branching morphogenesis. Nature, 441(7094), 746–749. [DOI] [PubMed] [Google Scholar]
- 52.Bentley K, Gerhardt H, & Bates PA (2008). Agent-based simulation of Notch-mediated tip cell selection in angiogenic sprout initialisation. Journal of Theoretical Biology, 250(1), 25–36. [DOI] [PubMed] [Google Scholar]
- 53.Gille H, Kowalski J, Li B, LeCouter J, Moffat B, Zioncheck TF, et al. (2001). Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2)—a reassessment using novel receptor-specific vascular endothelial growth factor mutants. Journal of Biological Chemistry, 276(5), 3222–3230. [DOI] [PubMed] [Google Scholar]
- 54.Roca C, & Adams RH (2007). Regulation of vascular morphogenesis by Notch signaling. Genes and Development, 21(20), 2511–2524. [DOI] [PubMed] [Google Scholar]
- 55.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, et al. (2007). Dll4 signaling through Notch1 regulates formation of tip cells during angiogenesis. Nature, 445(7129), 776–780. [DOI] [PubMed] [Google Scholar]
- 56.Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, et al. (2007). The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proceedings of the National Academy of Sciences of the United States of America, 104(9), 3225–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, et al. (2008). Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature, 454(7204), 656–660. [DOI] [PubMed] [Google Scholar]
- 58.Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, & Lewis J (2007). Endothelial signaling by the Notch ligand Delta-like 4 restricts angiogenesis. Development, 134(5), 839–844. [DOI] [PubMed] [Google Scholar]
- 59.Siekmann AF, & Lawson ND (2007). Notch signaling limits angiogenic cell behaviour in developing zebrafish arteries. Nature, 445(7129), 781–784. [DOI] [PubMed] [Google Scholar]
- 60.Siekmann AF, Covassin L, & Lawson ND (2008). Modulation of VEGF signaling output by the Notch pathway. Bioessays, 30(4), 303–313. [DOI] [PubMed] [Google Scholar]
- 61.Dou GR, Wang YC, Hu XB, Hou LH, Wang CM, Xu JF, et al. (2008). RBPJ the transcription factor downstream of Notch receptors, is essential for the maintenance of vascular homeostasis in adult mice. FASEB Journal, 22(5), 1606–1617. [DOI] [PubMed] [Google Scholar]
- 62.Liu ZJ, Xiao M, Balint K, Soma A, Pinnix CC, Capobianco AJ, Velazquez OC, & Herlyn M (2006). Inhibition of endothelial cell proliferation by Notch1 signaling is mediated by repressing MAPK and PI3 K/Akt pathways and requires MAML1. FASEB Journal, 20(7), 1009–1011. [DOI] [PubMed] [Google Scholar]
- 63.Noseda M, Chang L, McLean G, Grim JE, Clurman BE, Smith LL, et al. (2004). Notch activation induces endothelial cell cycle arrest and participates in contact inhibition: role of p21Cip1 repression. Molecular and Cellular Biology, 24(20), 8813–8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harrington LS, Sainson RC, Williams CK, Taylor JM, Shi W, Li JL, et al. (2008). Regulation of multiple angiogenic pathways by Dll4 and Notch in human umbilical vein endothelial cells. Microvascular Research, 75(2), 144–154. [DOI] [PubMed] [Google Scholar]
- 65.Trindade A, Kumar SR, Scehnet JS, Lopes-da-Costa L, Becker J, Jiang W, et al. (2008). Overexpression of delta-like 4 induces arterialization and attenuates vessel formation in developing mouse embryos. Blood, 112(5), 1720–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silvestre JS, Mallat Z, Tedgui A, & Levy BI (2008). Post-ischaemic neovascularization and inflammation. Cardiovascular Research, 78(2), 242–249. [DOI] [PubMed] [Google Scholar]
- 67.Heil M, & Schaper W (2007). Insights into pathways of arteriogenesis. Current Pharmaceutical Biotechnology, 8(1), 35–42. [DOI] [PubMed] [Google Scholar]
- 68.Heil M, Ziegelhoeffer T, Pipp F, Kostin S, Martin S, Clauss M, et al. (2002). Blood monocyte concentration is critical for enhancement of collateral artery growth. American Journal of Physiology-Heart and Circulatory Physiology, 283(6), H2411–H2419. [DOI] [PubMed] [Google Scholar]
- 69.Capoccia BJ, Shepherd RM, & Link DC (2006). G-CSF and AMD3100 mobilize monocytes into the blood that stimulate angiogenesis in vivo through a paracrine mechanism. Blood, 108(7), 2438–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stabile E, Burnett MS, Watkins C, Kinnaird T, Bachis A, la Sala A, et al. (2003). Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation, 108(2), 205–210. [DOI] [PubMed] [Google Scholar]
- 71.Couffinhal T, Silver M, Kearney M, Sullivan A, Witzenbichler B, Magner M, et al. (1999). Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in ApoE (−/−) mice. Circulation, 99(24), 3188–3198. [DOI] [PubMed] [Google Scholar]
- 72.Stabile E, Kinnaird T, la Sala A, Hanson SK, Watkins C, Campia U, et al. (2006). CD8(+) T lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessel development and recruiting CD4(+) mononuclear cells through the expression of interleukin-16. Circulation, 113(1), 118–124. [DOI] [PubMed] [Google Scholar]
- 73.van Weel V, Toes REM, Seghers L, Deckers MML, de Vries MR, Eilers PH, et al. (2007). Natural killer cells and CD4(+) T-Cells modulate collateral artery development. Arteriosclerosis, Thrombosis, and Vascular Biology, 27(11), 2310–2318. [DOI] [PubMed] [Google Scholar]
- 74.Iba O, Matsubara H, Nozawa Y, Fujiyama S, Amano K, Mori Y, et al. (2002). Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation, 106(15), 2019–2025. [DOI] [PubMed] [Google Scholar]
- 75.Griendling KK, & FitzGerald GA (2003). Oxidative stress and cardiovascular injury—Part I: basic mechanisms and in vivo monitoring of ROS. Circulation, 108(16), 1912–1916. [DOI] [PubMed] [Google Scholar]
- 76.Griendling KK, & FitzGerald GA (2003). Oxidative stress and cardiovascular injury—Part II: animal and human studies. Circulation, 108(17), 2034–2040. [DOI] [PubMed] [Google Scholar]
- 77.Li JM, & Shah AM (2004). Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. American Journal of Physiology-Regulatory Integrative and Comparative Physiology, 287(5), R1014–R1030. [DOI] [PubMed] [Google Scholar]
- 78.Yasuda M, Ohzeki Y, Shimizu S, Naito S, Ohtsuru A, Yamamoto T, et al. (1998). Stimulation of in vitro angiogenesis by hydrogen peroxide and the relation with ETS-1 in endothelial cells. Life Sciences, 64(4), 249–258. [DOI] [PubMed] [Google Scholar]
- 79.Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D, et al. (2004). JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell, 118(6), 781–794. [DOI] [PubMed] [Google Scholar]
- 80.Guzy RD, & Schumacker PT (2006). Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Experimental Physiology, 91(5), 807–819. [DOI] [PubMed] [Google Scholar]
- 81.Ebrahimian TG, Heymes C, You D, Blanc-Brude O, Mees B, Waeckel L, et al. (2006). NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. American Journal of Pathology, 169(2), 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bayraktutan U, Blayney L, & Shah AM (2000). Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology, 20(8), 1903–1911. [DOI] [PubMed] [Google Scholar]
- 83.Duilio C, Ambrosio G, Kuppusamy P, DiPaula A, Becker LC, & Zweier JL (2001). Neutrophils are primary source of O2 radicals during reperfusion after prolonged myocardial ischemia. American Journal of Physiology–Heart and Circulatory Physiolology, 280(6), H2649–H2657. [DOI] [PubMed] [Google Scholar]
- 84.Golino P, Ragni M, Cirillo P, Avvedimento VE, Feliciello A, Esposito N, et al. (1996). Effects of tissue factor induced by oxygen free radicals on coronary flow during reperfusion. Nature Medicine, 2(1), 35–40. [DOI] [PubMed] [Google Scholar]
- 85.Harlan JM, Killen PD, Harker LA, Striker GE, & Wright DG (1981). Neutrophil-mediated endothelial injury in vitro mechanisms of cell detachment. Journal of Clinical Investigation, 68(6), 1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.May AE, Seizer P, & Gawaz M (2008). Platelets: inflammatory firebugs of vascular walls. Arteriosclerosis, Thrombosis, and Vascular Biology, 28(3), S5–S10. [DOI] [PubMed] [Google Scholar]
- 87.Nierodzik ML, & Karpatkin S (2006). Thrombin induces tumor growth, metastasis, and angiogenesis: evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell, 10(5), 355–362. [DOI] [PubMed] [Google Scholar]
- 88.De Paula EV, Nascimento MCF, Ramos CD, Ozelo MC, Machado TF, Guillaumon AT, et al. (2006). Early in vivo anticoagulation inhibits the angiogenic response following hindlimb ischemia in a rodent model. Thrombosis and Haemostasis, 96(1), 68–72. [DOI] [PubMed] [Google Scholar]
- 89.Rafii S, & Lyden D (2003). Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nature Medicine, 9(6), 702–712. [DOI] [PubMed] [Google Scholar]
- 90.Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, & Salven P (2004). Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood, 104(7), 2084–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, et al. (2004). Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circulation Research, 94(2), 230–238. [DOI] [PubMed] [Google Scholar]
- 92.Peters BA, Diaz LA, Polyak K, Meszler L, Romans K, Guinan EC, et al. (2005). Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nature Medicine, 11(3), 261–262. [DOI] [PubMed] [Google Scholar]
- 93.Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, et al. (2001). Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. Journal of Experimental Medicine, 193(9), 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, et al. (2002). Human endothelial progenitor exhibit impaired proliferation, cells from type II diabetics adhesion, and incorporation into vascular structures. Circulation, 106(22), 2781–2786. [DOI] [PubMed] [Google Scholar]
- 95.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. (1999). Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circulation Research, 85(3), 221–228. [DOI] [PubMed] [Google Scholar]
- 96.Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Seifert RA, et al. (2000). Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circulation Research, 87(9), 728–730. [DOI] [PubMed] [Google Scholar]
- 97.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. (1999). Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nature Medicine, 5(4), 434–438. [DOI] [PubMed] [Google Scholar]
- 98.Lin Y, Weisdorf DJ, Solovey A, & Hebbel RP (2000). Origins of circulating endothelial cells and endothelial out-growth from blood. Journal of Clinical Investigation, 105(1), 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fandrich F, Siebert R, et al. (2007). Non bonemarrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circulation Research, 100(4), 581–589. [DOI] [PubMed] [Google Scholar]
- 100.You D, Waeckel L, Ebrahimian TG, Blanc-Brude O, Foubert P, Barateau V, et al. (2006). Increase in vascular permeability and vasodilation are critical for proangiogenic effects of stem cell therapy. Circulation, 114(4), 328–338. [DOI] [PubMed] [Google Scholar]
- 101.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, et al. (2005). Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. Journal of Molecular and Cellular Cardiology, 39(5), 733–742. [DOI] [PubMed] [Google Scholar]
- 102.Rehman J, Li J, Orschell CM, & March KL (2003). Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation, 107(8), 1164–1169. [DOI] [PubMed] [Google Scholar]
- 103.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, et al. (2004). Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology, 24(2), 288–293. [DOI] [PubMed] [Google Scholar]
- 104.Tordjman R, Delaire S, Plouet J, Ting S, Gaulard P, Fichelson S, et al. (2001). Erythroblasts are a source of angiogenic factors. Blood, 97(7), 1968–1974. [DOI] [PubMed] [Google Scholar]
- 105.Huang YQ, Li JJ, & Karpatkin S (2000). Identification of a family of alternatively spliced mRNA species of angiopoietin-1. Blood, 95(6), 1993–1999. [PubMed] [Google Scholar]
- 106.Coussens LM, Tinkle CL, Hanahan D, & Werb Z (2000). MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell, 103(3), 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, Mames RN, et al. (2002). Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nature Medicine, 8(6), 607–612. [DOI] [PubMed] [Google Scholar]
- 108.Bailey AS, Jiang SG, Afentoulis M, Baumann CI, Schroeder DA, Olson SB, et al. (2004). Transplanted adult hematopoietic stems cells differentiate into functional endothelial cells. Blood, 103(1), 13–19. [DOI] [PubMed] [Google Scholar]
- 109.Takakura N, Watanabe T, Suenobu S, Yamada Y, Noda T, Ito Y, et al. (2000). A role for hematopoietic stem cells in promoting angiogenesis. Cell, 102(2), 199–209. [DOI] [PubMed] [Google Scholar]
- 110.Carmeliet P (2003). Angiogenesis in health and disease. Nature Medicine, 9(6), 653–660. [DOI] [PubMed] [Google Scholar]
- 111.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, et al. (2000). In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood, 95(10), 3106–3112. [PubMed] [Google Scholar]
- 112.Urbich C, & Dimmeler S (2004). Endothelial progenitor cells—characterization and role in vascular biology. Circulation Research, 95(4), 343–353. [DOI] [PubMed] [Google Scholar]
- 113.Nieda M, Nicol A, Denning-Kendall P, Sweetenham J, Bradley B, & Hows J (1997). Endothelial cell precursors are normal components of human umbilical cord blood. British Journal Haematology, 98(3), 775–777. [DOI] [PubMed] [Google Scholar]
- 114.Rookmaaker MB, Verhaar MC, Loomans CJ, Verloop R, Peters E, Westerweel PE, et al. (2005). CD34+ cells home, proliferate, and participate in capillary formation, and in combination with CD34− cells enhance tube formation in a 3-dimensional matrix. Arteriosclerosis, Thrombosis, and Vascular Biology, 25(9), 1843–1850. [DOI] [PubMed] [Google Scholar]
- 115.Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, et al. (2000). Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. Journal of Clinical Investigation, 105(11), 1527–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ma N, Stamm C, Kaminski A, Li W, Kleine HD, Muller-Hilke B, et al. (2005). Human cord blood cells induce angiogenesis following myocardial infarction in NOD/scid-mice. Cardiovascular Research, 66(1), 45–54. [DOI] [PubMed] [Google Scholar]
- 117.Ma N, Ladilov Y, Moebius JM, Ong L, Piechaczek C, David A, et al. (2006). Intramyocardial delivery of human CD133 + cells in a SCID mouse cryoinjury model: bone marrow vs. cord blood-derived cells. Cardiovascular Research, 71(1), 158–169. [DOI] [PubMed] [Google Scholar]
- 118.Bababeygy SR, Cheshier SH, Hou LC, Higgins DM, Weissman IL, & Tse VC (2008). Hematopoietic stem cell-derived pericytic cells in brain tumor angio-architecture. Stem Cells and Development, 17(1), 11–18. [DOI] [PubMed] [Google Scholar]
- 119.Das H, Abdulhameed N, Joseph M, Sakthivel R, Mao HQ, & Pompili VJ (2009). Ex vivo nanofiber expansion and genetic modification of human cord blood-derived progenitor/ stem cells enhances vasculogenesis. Cell Transplantation, 18(3), 305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lu J, Aggarwal R, Pompili VJ, & Das H (2010). A novel technology for hematopoietic stem cell expansion using combination of nanofiber and growth factors. Recent Patents on Nanotechnology, 4(2), 125–135. [DOI] [PubMed] [Google Scholar]
- 121.Aggarwal R, Pompili VJ, & Das H (2010). Genetic modification of ex vivo expanded stem cells for clinical application. Frontiers in Bioscience, 15, 854–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, et al. (2001). Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. Journal of Clinical Investigation, 107(11), 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rohde D, Wickenhauser C, Denecke S, Stach A, Lorenzen J, Hansmann ML, et al. (1994). Cytokine release by human bone marrow cells: analysis at the single cell level. Virchows Archiv, 424(4), 389–395. [DOI] [PubMed] [Google Scholar]
- 124.Bikfalvi A, & Han ZC (1994). Angiogenic factors are hematopoietic growth factors and vice versa. Leukemia, 8(3), 523–529. [PubMed] [Google Scholar]
- 125.Adams RH, & Alitalo K (2007). Molecular regulation of angiogenesis and lymphangiogenesis. Nature Reviews Molecular Cell Biology, 8(6), 464–478. [DOI] [PubMed] [Google Scholar]