Abstract
Stem cell therapy is currently considered as an important regime for repairing, replacing or enhancing the biological functions of the damaged tissues. Among adult stem cells, hematopoietic stem cells (HSCs) are commonly used for cure of hematological disorders. However, the number of HSCs obtained from sources like bone marrow, peripheral or umbilical cord blood is not sufficient for routine clinical application. Thus, ex-vivo expansion of HSCs becomes critically important. Ex-vivo culture and expansion of stem cells are challenging, as stem cells differentiate in culture rather than self-renew. Lack of clarity about the factors responsible for quiescence and differentiation of HSCs, investigators struggled to optimize conditions for ex vivo expansion. As we understand better, various strategies can be incorporated to mimic in vivo conditions for successful expansion of stem cells. However, characterization and biological functionality should also be tested for expanded stem cells prior to clinical application. To treat ischemia by enhancing therapeutic angiogenesis and neo-vascularization, the role of genetic modification of HSCs with pro-angiogenic factors is the focus of this review.
Keywords: Hematopoietic Stem Cells, Niche, Genetic Modification, Virus, Transduction, Non-Viral, Ex-Vivo, Expansion, Ischemia, Pro-Angiogenic Factors, Neo-vascularization, Review
2. INTRODUCTION
Stem cells can be defined as cells, which possess the capability of self-renewal and are able to produce a terminally differentiated cell of at least one lineage (1). Stem cells are broadly categorized into embryonic stem cells and adult stem cells, and these cells originate from the embryonic tissues de-novo. Embryonic stem cells are derived from inner mass cells of the blastocyst of embryo and are capable of forming all germ layer of the embryo except the extra-embryonic organs such as placenta (2). However, adult stem cells are found in adult tissue and are capable of forming cells of specific lineage only.
Stem cells are characterized as totipotent, pluripotent and multipotent according to their developmental potential. Totipotent stem cells have the ability to form all cell lineages of an organism, although only zygote and the first cleavage blastomeres retain that ability. Next in the hierarchy are pluripotent stem cells such as embryonic stem cells that have the ability to form all cell lineages of the body. Coming down the hierarchy are the multipotent stem cells, which retain the ability to form multiple cell types of one lineage, such as hematopoietic stem cells (HSCs), and is our interest of discussion in the current review.
There is another class of stem cells called induced pluripotent cells (iPS), which are generated by reprogramming of the somatic cells by induction of genes (eg. adult mouse or human fibroblasts cells), as a result, induced cells transformed into pluripotent stem cells (3, 4). This induced pluripotency required viral vector-mediated integration procedures of genes such as Klf4, c-Myc, Oct3/4, Sox-2 to re-program the somatic cells towards ES-like functionality and phenotype. However, long-term effects and clinical applicability of these iPS cells are yet to be defined. Teratomas (cancerous growth) generated by the implantation of iPS cells are a proof of their pluripotency or an overexpression of the putative oncogenes such as c-Myc and Klf-4.As using viral vectors reprograms iPS cells, in vivo effects of viral vectors are yet to be determined. Viral transduction procedure poses more complications and is potentially bio-hazardous as discussed later in this review (5). However, recently non-viral methods were employed to generate iPS cells. Plasmids instead of the lentivirus or retroviruses were used to generate viral-free iPS cells which had the expression level of the genes similar to the ES cells and also gave rise to adult chimeric mice (6). However, the long-term biological efficacy and safety of iPS cells are under critical investigation and yet to be proven for therapeutic use. Therefore, further investigations are required to prove iPS cells as a “bio-safe” for the therapeutic purposes.
The current review provides an overview of the stem cells focusing on regulation of HSC in vivo, to provide a better understanding for successful ex-vivo expansion without the induction of terminal differentiation. The review also outlines some of the technological advances used to genetically modify HSCs for their effective use in pre-clinical studies, especially in the context of ischemic diseases.
3. HSC REGULATION AND MICROENVIRONMENT
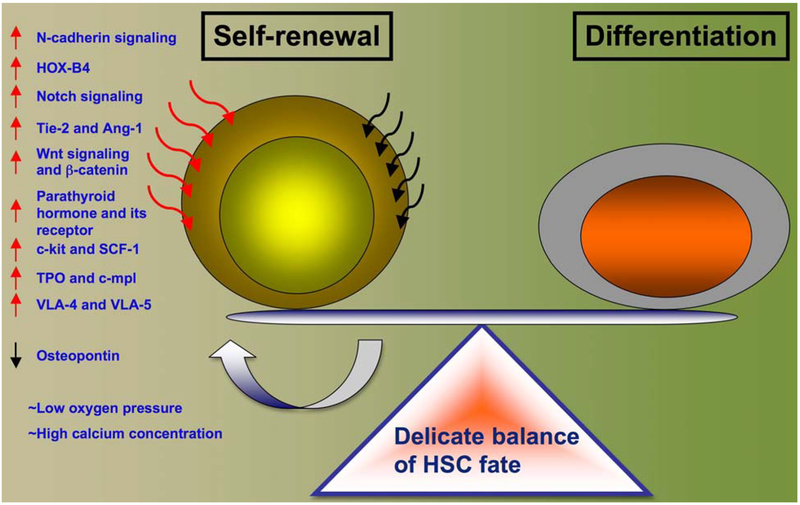
Stem cells are maintained in a specialized microenvironment called niche. Niche is specialized anatomic location composed of supporting cells and extra-cellular matrices that regulate the basic functions of stem cells and maintain homeostasis (7, 8). Microenvironment saves the stem cell population from depletion and regulates uncontrolled malignant proliferation (9). Such spatiotemporal relationship is maintained lifelong in various organs of the body including bone marrow niche where HSC resides. Bone marrow niche has unique cellular composition, which is intrinsically linked with the HSC cell cycling and functioning. Of those, osteoblasts, the bone lining cells (10); stromal cells such as vascular endothelial cells, extra-cellular matrix proteins, high mineral content and low oxygen content were shown to participate in regulation of stem cell micro-environment (11) (Figure 1).
Figure 1.
HSC regulation within bone marrow. Factors responsible for maintenance of stem cell self-renewal versus differentiation are indicated with arrowheads.
3.1. Niche cells
Osteoblast and vascular endothelial cells play major role in regulation of the HSC niche. Based on the anatomical considerations, trabecular bone fragments are embedded in stromal rich reticular tissues that form the endo-osteal or osteoblastic niche (12). Fenestrated sinusoidal endothelial cells and perivascular space forms the vascular niche (13). Several reports suggested an osteoblast (0B) mediated regulation of HSCs. Concomitant increase in the number of trabecular osteoblast (OB) cells caused an significant increase in the Lin− Sca-1+c-Kit+ subpopulation of HSCs in the transgenic mice, whose OB cells constitutively expressed PPR (parathyroid hormone receptor). Parathyroid hormone is known to activate the osteoblast cell activity. Primitive HSCs were also increased and superior engraftment in the irradiated recipients was observed. However, more mature colony forming unit cells were not increased indicating that the effect was more pronounced on early primitive HSCs (14). Endo-osteal sites have been identified as the sites for quiescent stem cell localization (15). Interestingly, ablation of osteoblast cells in vivo caused re-location of HSCs to sites outside bone marrow such as liver and spleen indicating an intricate link between HSCs and osteoblasts (16).
Multiple reports showed that cytokines secreted from osteoblasts regulate the stem cell function and viability. Among the cytokines, interleukin 3 and 6 (IL-3 and -6), leukemia inhibitory factor (LIF), granulocytecolony stimulating factor (G-CSF), macrophage stimulating factor (M-CSF), osteoprotegrin (OPG), vascular endothelial growth factor (VEGF), stromal derived factor (SDF), receptor activator of NF-□B ligand (RANK-L) and many others were indicated to play an important roles (10, 17–20). G-CSF plays a significant role for maintenance and differentiation of HSC towards the hematopoietic lineage (21), while osteoprotegrin showed inhibitory effects on the HSC function and proliferation (20). Also, it was suggested that osteoblasts secrete soluble factors that were not detected in absence of HSCs, thus may play a major role in quiescence of HSCs (22).
Vascular endothelial cells also provided regulatory cues for HSC fate. About two-third of the HSCs were found localized to sinusoids in bone marrow and spleen (23). The reticular cells around the sinusoids secreted high levels of SDF-1, chemokine required for HSC maintenance and homing (24) interacting with SDF-1 receptor called CXC chemokine receptor 4 (CXCR4) expressed on HSCs (25). HSCs were consistently found in contact with vascular endothelial cells and SDF-1 was implicated in regulation in the above-discussed niche (26). Cumulative contributions to HSC fate from lesser-studied bone marrow cells such as MSCs, osteoclast, adipocytes, megakarocytes was also suspected (26, 27).
3.2. Niche signaling
Stem cells renewal is broadly dependent on the signaling pathways regulated via Notch, Wnt and other factors. Notch signaling was reported to be a gatekeeper that maintains the undifferentiated state of HSC and inhibits commitment to any lineage (28). Hematological malignancies demonstrated the importance of Notch signaling in the HSC niche. Notch signaling was responsible for increasing the number of stem cells (29). This signaling involves receptors-Notch 1, 2, 3 and 4 whose ligands belong to either Jagged or Delta family of ligands. Notch ligand, Jagged-1 is expressed on bone marrow stromal and osteoblastic cells (30, 31). Activated Notch cytoplasmic domain was detected in the HSCs and increased Notch ligands were detected on osteoblastic cells (14). Wnt signaling was also shown to play a significant role in HSC self-renewal. Inhibition of Wnt signaling reduced the numbers of primitive HSC and their viability in vitro and decreased their expansion in vivo (32, 33). Wnt signaling is interdependent on Notch signaling as inhibition of Notch signaling disrupted the ability of Wnt signaling to maintain HSCs in undifferentiated state (28). Previous report showed that Wnt signaling caused an increase in immature colony forming cells in human and mouse progenitors alike (32).
Along with the osteoblasts, other cells such as stromal cells were also implicated to cause Notch signaling and maintain HSC population. HSC expansion is also regulated via bone morphogenetic protein receptor (BMPR1A) signaling mediated through osteoblastic cells (15). Quiescent HSC state, maintained by activation of p21 signaling, and in its absence increased stem cell cycling lead to stem cell exhaustion in irradiated p21 deficient mice (34).
3.3. Niche adhesion molecules
Survival of the HSCs was shown to depend on the expression and its engagement with the osteoblast cells through integrin family called very late antigens (VLA-4 and VLA-5) (10). Stem cell receptor tyrosine kinase (Tie-2) and its ligand angiopoietin (Ang1) also contributed to the expansion of the long-term HSCs and were found to promote tight adhesion of HSC to their niche (35). Increase in the osteoblastic population caused simultaneous augmentation of the long-term HSCs and interaction through Tie-2 / Ang1, crucial for maintenance of quiescence of HSCs (35). Ang-1 was shown to express on osteocalcin producing cells (marker for mature osteoblast) and the expression level was much higher than the HSC, confirming that Ang-1 was mainly produced by osteoblasts. Furthermore, it was shown that most of the side-population HSCs (SP-HSCs) expressed Tie-2 receptor and Tie-2 expressing cells adhered to the Ang-1 expressing cells confirming that HSC niche was strongly regulated by osteoblastic interactions (35).
3.4. Chemical regulation of niche
Emerging studies reveal that extremely high concentrations of calcium (40 mmol/L) in endo-osteal sites play a crucial role in HSC functions. HSCs were found to express calcium-sensing receptor (CaR) (36) and responded to extra-cellular ionic calcium concentrations. Preferential localization of the HSCs was found to be dictated via the CaR signaling during mammalian ontogeny. As hematopoiesis shifts from fetal liver to bone marrow and continues to occur throughout the post-natal life, HSCs migrate and home to the bone marrow niche. CaR−/- HSCs were shown to have defects in the ability to adhere to collagen I, an extra-cellular matrix protein of bone secreted by osteobalsts. CaR knockout studies also revealed that circulating HSCs home to bone marrow but failed to lodge to their endo-osteal niche (11) and as a result reduced number of cells were found in the bone marrow.
Partial pressure of oxygen also showed a significant role in maintenance of hematopoietic progenitor cells. Endo-osteum has the lowest partial pressure of oxygen as it is located distal to bone vasculature (37) and has highest number of osteoblasts compared to other locations of the bone marrow. Thus, maintenance of the primitiveness of HSCs could be related to interplay between these factors. Low oxygen culture for HSCs was shown to maintain the multi-lineage engraftment potential, when transplanted into an irradiated mouse (38). Another report showed that oxygen pressure is crucial for HSCs survival, when umbilical cord blood derived HSCs (UCB-HSCs) were cultured in the oxygen concentrations, relevant to the bone marrow microenvironment, failed to survive, however, when supplemented with bone marrow relevant cytokines and adhesion molecules, they survived. These findings were explained by stating the fact that UCB microenvironment is fundamentally different from the bone marrow, because osteoblasts are absent and partial oxygen pressure is higher (5%) in UCB (39). It was also found that survival signals were activated via AKT phosphorylation downstream to the VLA-4 and VCAM-1 interactions (40) in low oxygen pressures, only when proteins and cytokines were supplemented. Thus, low oxygen pressure in bone marrow may compensate for paracrine effects of the surrounding cells. However clinically, cord blood derived stem cells have been shown to be more safe for allogenic transplantation in the patients with the hematopoietic diseases than those derived from bone marrow and peripheral blood. Also, since UCB derived HSC allows for greater HLA disparity thus having a higher chance of getting a donor for minority population.
4. HEMATOPOIETIC STEM CELL EXPANSION
HSCs can be harvested from multiple sources, such as bone marrow, peripheral blood or from the umbilical cord blood. Recently, human umbilical cord blood was implicated to be a potential source for variety of stem cells such as hematopoietic stem cells, unrestricted somatic stem cells, mesenchymal stem cells and endothelial progenitors (41, 42). Previously, umbilical cord blood was discarded after the delivery of fetus and placenta but recently it has been collected and used as a source of progenitor cells (43). The fetal blood, including umbilical cord or placental blood obtained after the birth, was also shown to be a significant source of haematopoietic stem cells (44) and can be used as an alternative source to bone marrow for clinical transplantations (45). Clinically, use of cord blood is advantageous as it is easy to harvest, harmless to donor, ethical with negligible viral or bacterial contamination, ontogentically primitive and can be stored in cord blood bank for years. UCB transplantation was associated with reduced risk of developing graft versus host disease (46). All the above reasons made researchers to be interested in the cord blood for isolation of stem cells, and expansion as well as genetic modification.
4.1. Factors regulating ex-vivo expansion
Self-renewal, proliferation and differentiation of HSCs are tightly regulated in-vivo by variety of autocrine and paracrine signaling. Ex-vivo expansion of the stem cell strategies mimicked the in-vivo environment by use of cocktail of growth factors, cytokines, biomaterials that simulate extra-cellular matrix, feeder stromal layers but with limited success. Delicate balance of self-renewal versus commitment towards differentiation has been the major challenge of ex-vivo expansion (Figure 1). Use of feeder layer for expansion is disadvantageous because of its lesser-defined chemical nature. Also, purity of the HSCs for transplantations after the co-culture was always the cause of concern as it altered the engraftment kinetics in-vivo. Cocktail of cytokines and growth factors was preferred because of their defined chemical nature, however, this method required critical optimization. Genetic manipulation of HSCs was proposed based on the genes that promote self-renewal but risk of transformation affecting their therapeutic potential is suspected. Also, it is difficult to get sufficient number of HSCs from a single cord for any adult patients (47, 48). So, two or more cord bloods were combined for a single recipient, but the failure of engraftment and high rates of mortality was a cause of concern (49). Therefore, ex-vivo expansion of nucleated cells from cord blood is under critical investigation by researchers since years. In-vitro simulation of stem cell niche was performed to better understand the role of these cytokines in expansion and preservation of function. Although yield of HSCs from bone marrow sources are 10 times higher than that of cord blood, but their ex-vivo expansion is significantly lower than cord blood HSCs (50). For ex-vivo expansion purpose, we used freshly isolated cord blood-derived stem cells and incubated on a poly-ether sulfone (PES) nanofiber with serum-free media containing TPO, flt-3, IL3, LDL, SCF and found a significant expansion of stem cells retaining their phenotype (51–53).
4.1.1. Interleukin-3
IL-3 was one of the earliest cytokines implicated in self-renewal, proliferation and differentiation of primitive and mature hematopoietic stem cells (54). IL-3 receptors were found to be present on HSC, B and T cells and myeloid cells (55). The effect of IL-3 on adult HSCs remains controversial. IL-3 was shown to increase (56) and decrease (30) (57) the expansion and/or self-renewal capacity of adult HSCs. Contradictory effects of IL-3 were most likely influenced by its concentration, the presence/absence of other cytokines, serum, culture conditions and tested cell populations. Comparison of the role of IL-3 and IL-11 along with the other cytokines such as Flt3, megakaryocyte developmental factor and c-kit ligand showed that IL-3 and IL-11 both promote efficient self-renewal and proliferation of murine Lin− Sca+ kit+ (LSK) cells with sustained long-term repopulating stability (56). Negative effects of IL-3 and positive effects of IL-11 on self-renewal and the engraftment capacity of adult HSCs were also reported (58).
4.1.2. Interleukin-6
IL6 was used for expansion of the murine HSCs in culture. Since IL6 receptor is not expressed by human HSC, investigators employed a strategy where they supplemented the stem cell media with soluble IL6 receptor (sIL6R) and IL6 along with Flt3L, TPO and stem cell factor (SCF) for the expansion (59). The interaction was mediated through gp130 expressed on the HSCs crucial for ex-vivo HSC expansion. Based on these observations, fusion protein for sIL6R/IL6 was synthesized and supplemented in the stem cell media for expansion of UCB derived HSC (60) and peripheral blood progenitors (61).
4.1.3. Thrombopoietin
Thrombopoietin (TPO), a glycoprotein hormone, plays a crucial role in maintenance of quiescent-HSCs in bone marrow and produced by bone marrow stroma and its receptor, c-mpl is expressed on HSCs (62). However, TPO is not essential for embryogenesis and development. TPO knockout mouse showed a reduced capacity of engraftment of HSCs (63). Report also indicated that combination of TPO with Flt3/Flk2 ligand could support the expansion of HSC derived from UCB (64). Small molecule agonist for the TPO receptor, c-mpl was shown to be more efficient in increasing the numbers of the primitive HSCs and SRC (SCID repopulating cells)(65).
4.1.4. Fetal liver tyrosine kinase 3 ligand
The FLT3 receptor tyrosine kinase (FLT3) was selectively expressed on HSCs and binding of its ligand (FL) induced HSC proliferation (66). Mobilization of HSCs to peripheral blood was reported in the presence of FL in combination of G-CSF and superior engraftment potential was observed after transplantation in murine models of myeloablation (67). FL was shown to be an effective cytokine that increased the number of long-term repopulating cells in cultures of CD34+ human bone marrow cells (68).
4.1.5. Stem cell factor
Stem cell factor (SCF) plays a central role in the regulation of the early hematopoiesis. SCF is extensively studied in ex-vivo expansion experiments as its receptor; c-kit was show to be expressed by hematopoietic cells (69). Also, it was found to exhibit synergistic effects in number of hematopoietic growth factors and was used in treatment of broad spectrum of hemtopoietic diseases (69, 70). None of the above-mentioned cytokines were able to promote expansion of Lin−1Sca +1c-kit+1 HSCs alone, however, combination such as SCF, FL and IL-11 enhanced the production of progenitors and nucleated cells (71).
4.1.6. Signaling molecules
Another possibility of expanding the HSC population is via supplementation and activation of signaling molecules and genes involved in self-renewal of HSCs and enhancing differentiation-inhibitory effects during hematopoiesis (72). Understanding the functions of genes such a HOX-B4, Wnt, Notch signaling molecules and cell cycling genes such as p18, p21 are crucial to achieve successful engraftment in host after ex-vivo expansion of HSC (73–75). Homeobox genes were identified as critical factors for the regulation of early development of hematopoiesis. HOX-B4 gene promoted expansion of HSCs in-vivo and in-vitro and was able to retain HSCs differentiation ability into normal lymphoid and myeloid cells (76, 77). Soluble Sonic-hedgehog showed increased self-renewal of human HSCs, however, expansion was relatively modest (78). Notch signaling preserved the stem cell nature of HSC and signaling was mediated through transmembrane receptors and ligands. Notch signaling was shown to inhibit stem cell differentiation and maintained the self-renewal ability (79) in HSC niche. Notch ligand (Jagged 1) was shown to be presented by osteoblasts and augmented the numbers of the immature HSCs. Soluble Notch ligand (Delta-1) pre-coated-culture plates were used for ex-vivo expansion (80) and showed 4.5 fold expansion of SRC’s (most immature HSCs) (81) and 240-fold increase in nucleated cells over a period of 3–4 weeks. Long-term in vivo myeloid and lymphoid repopulating capacity of the expanded cells was observed (53–67%) (80) by transplantation in the NOG mice (NOD/SCID mice with IL-2 receptor common gamma chain-knockout), known to accept transplanted HSCs and their differentiation into T-cell lineage (82).
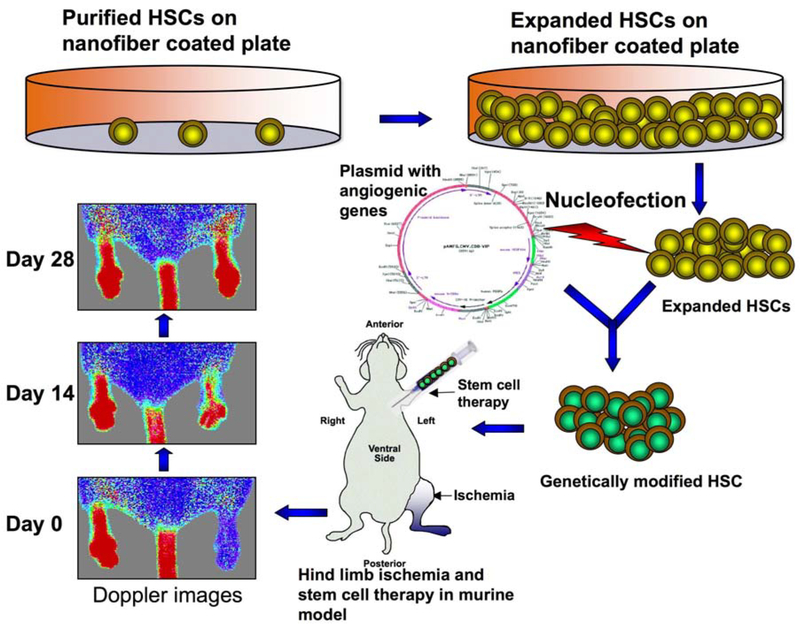
So, the importance of ex vivo expansion of stem cells is critical, as it will provide sufficient number of stem cells for genetic modification, which can be used for therapy not only in hematopoietic disorders but also in degenerative disease such as ischemic diseases, neurological disorders or in autoimmune dysfunctions (Figure 2).
Figure 2.
Stem cell expansion, modification and therapy for ischemia. Purified HSCs were seeded on the nanofiber-coated plate supplemented with serum-free media and growth factors for expansion. Expanded HSCs were genetically modified with pro-angiogenic factors and injected into the circulation via left ventricle of a murine hind limb ischemic model. Doppler images were taken for monitoring revascularization in the limbs.
5. GENETIC MODIFICATION OF STEM CELLS
Since stem cells can differentiate into various lineages, they offer a great deal of potential for the treatment in regenerative medicine. Genetically modifying stem cells is an attractive approach, where combinations of stem cell and gene therapy act-in-synergy for effectively reverting the disease state. Several methods have been employed to genetically modify stem cells but with variable efficiencies. Procedure of transfection for genetic modification of cells could be of two different types i.e. transient or stable transfection. The transient transfection resulted in high levels of expression of the introduced gene but only for few weeks. However, stable transfection had lower level of expression, than the transient but lasts for longer period of time. Methods employed to introduce foreign DNA into the stem cells include both viral and non-viral-mediated gene delivery system.
5.1. Viral methods
Viruses are infectious agents that cause variety of diseases in human. Viral genome consists of either RNA or DNA enveloped by a protein coat. Viruses replicate in living cell only and utilize and overtake the host cellular machinery. These infectious agents transfer genetic material from one cell to another. This important property makes them clinically important vector for introducing a normal copy of gene into host cells via the mechanism called transduction or transfection. The viral methods of transduction employ variety of viruses as carrier of the candidate genomic sequence as described below.
5.1.1. Retroviruses
Retroviruses are RNA viruses and are the most common mode of stable transfection. Retroviruses carry an enzyme called reverse transcriptase capable of synthesizing cDNA from its RNA genome. The resultant cDNA is single stranded and is replicated as double stranded DNA (dsDNA) genome. Viral genome has an important property of being linear but randomly integrated into the host genome (chromosomal DNA). This feature has been exploited to transduce the hematopoietic stem cells for therapeutic purposes in various experimental procedures and clinical trials, but with limited success (83). Retroviruses are restricted with respect to the size of the transgene as they are able to carry only 9–10 kb (84) compare to non-viral method (electroporation) where 50–100 kb plasmids can be transferred. Low transduction efficiencies of only 10–40% are currently possible with the existing generation of retroviruses (85, 86). However, higher transduction efficiencies of retroviruses could be achieved by concentrating the vector to high titer or by packaging the retrovirus in a heterologous envelope with broad host spectrum. Autologous CD34+ cells were mobilized from the bone marrow and transfected with the multi-drug resistance gene (MDR-1) via retroviral transduction in presence of recombinant fibronectin CH-296 with a median efficiency range of 13 to 24% and engraftment kinetics were not adversely affected. Though promising results were observed in clinical therapies, potential risks were reported. It was reported that in X-SCID gene therapy (83) patients developed leukemia due to insertional mutagenesis caused by retroviral-mediated transfection (87). Other disadvantages would be due to the fusion of the transgene product with the cellular products thus causing some toxic side effects. Loss of homing of HSCs after transfection, variation in copy number of the virus in each cell or the side effects of the regimens used for engraftment procedures in patients was also reported (88). Evidence indicated that the onco-retroviral integration occurs preferably in the genes that are being actively transcribed in HSCs (89), and some preferred transcription start sites of the HSC genes (90), this can potentially affect the functionality of the HSC upon transplantation (91, 92). Also, retroviruses were limited to transduction to the cycling cells compare to the quiescent cells (93), thus posing more restrictions on the choice of cells for transduction along with the above-mentioned limitations.
5.1.2. Lentiviruses
Lentiviruses belong to the group of retroviruses that are known to cause slow and progressive fatal disease in livestock animals. Lentiviruses are being constantly modified due to their ability to transduce non-proliferating cells, thus making gene therapy possible in larger spectrum of tissue and cell targets. HSCs mostly existed in mostly G0/ G1 and were rarely found in G2, S or M phase of the cell cycle (94, 95). Although the possibility to transduce CD34+ HSCs from hUCB is high but transfection efficiency increases if these cells are found in proliferative phase (G2, S or M) (96). It was reported that G0/G1 HSC engraft better than those cycling in G2/S/M2 phase (97). Thus the lentiviral-mediated transfection is advantageous for the HSC (98), however, transduction efficiencies was low (98) compare to the non-viral methods (53, 99).
5.1.3. Adeno-associated viruses
Adeno-associated viruses (AAVs) are DNA viruses have linear single stranded genome (100). It is the smallest virus know, that infects human cells and has been found to be non-malignant and possess some antitumorigenic properties (101, 102). Adeno-associated virus requires helper virus co-infection for efficient replication but in absence of these viruses it has been found to have specific insertion site in human chromosome 19 (102, 103). Variable expression levels of surface receptor limit the transduction of the CD34+ HSC with the AAV2 vectors. It also impaired the ability of the pro-viral genome integration in the host genome due to lack of second strand of DNA and transcriptionally active genome. Varied results regarding the AAV2-mediated transduction have been reported. Transient expression of genes in human CD34+ cells was observed because of inefficient viral genome integration, while others reported successful transduction of primitive human HSCs that presented with successful engraftment in primary and secondary host (104). These studies strongly suggest that there is need for protocol optimization to enhance the transfection efficiencies, and a self-inactivating vector also needs to be introduced without compromising the normal functioning of the HSCs.
Further work needed to be done to generate bio-safe vector design that would ensure higher transfection efficiencies. Thus, there is a strong need to develop safer and more reliable techniques for efficient transfer of genes into stem cells.
5.2. Non-viral methods
Non-viral methods of transfection consist of chemical and physical methods. Many parameters influece the transfection efficiency, including cell type (primary versus transformed cell lines), culture conditions, vector types and choice of the transfection technology. Numbers of different transfection technologies are currently available to the researchers and each of them has their own merits and demerits. Among them, calcium phosphate method, electroporation and liposome mediated transfction and are commonly used and discussed here.
5.2.1. Calcium phosphate
The principle of this method is to mix DNA in a phosphate buffer with calcium chloride to form a precipitate, which adheres to the cell membrane and is endocytosed into the cytosol. This method is suitable for stable transfection; however, variability of DNA transfer and low expression is a common disadvantage. Optimization of protocols is needed and little variations in pH, concentration of DNA, calcium concentrations and phosphate ions, serum and temperature seems to control the precipitate size (i.e. size of DNA-calcium phosphate complexes) and transfection efficiencies (105, 106).
5.2.2. Lipofection
This technique employs lipid based cationic reagents for transfection purposes. Cationic lipids interact electrostatically with negatively charged DNA thus forming a lipid-DNA complex. Since, this complex is lipophillic, it can easily pass through the hydrophobic cell membrane (107). However, their mechanisms of cell endocytosis and cellular trafficking are not clearly understood (108). Lipidbased reagents, such as FuGene, lipofectamine and oligofectamine are being extensively employed for transfection of transformed cell lines as well as primary cells with variable efficiencies. Cationic lipids are easy to prepare and offer negligible toxicity and immunogenicity issues compare to viral vectors (109).
5.2.3. Electroporation
This method involves transient formation of pores on the cell membrane via high-voltage pulses and enables the transfer of the exogenous target DNA to cytoplasm of the recipient cells. This technique proved to be highly useful for efficient transfection; however, mortality rate is high with this procedure. Recently it was shown that increasing the number of cells would decrease the cell mortality (110). Electroporation is best for suspension cells rather than adherent cells. The electroporation technique was used for transient transfection of any cell types (111).
All the above-described methods deliver the DNA into the cytosol, however, their entry into nucleus becomes indispensable for the transfected DNA expression. These methods delay the expression of transfected DNA, as DNA needs to cross the nuclear membrane barrier. Experiments have shown that transfected plasmid DNA is not transported efficiently into the nucleus of quiescent cells (112). During mitosis, the nuclear membrane disintegrates, allowing transfected DNA to enter and access the nuclear transcription machinery. Also, different stages of cell cycle affect the transfection efficiency, confirming that G1 phase cells has poor expression of the cytosolic transfected DNA, compared to actively cycling cell (112). Thus, DNA arrives within the nucleus only when nuclear barrier disintegrates and requires nuclear pore complex to import and is hard to transduce DNA in non-cycling cells (113).
5.2.4. Nucleofection
As earlier discussed, only actively dividing cells
were transduced efficiently, a newer transfection technique, modified version of electroporation called nucleoporation or nucleofection was introduced (Lonza Group Ltd) (114). Nucleofection employs cell-specific electrical parameters and nucleofector solution to deliver genetic material, including DNA, small interfering RNA (siRNA) and oligonucleotides directly to the nucleus. HSCs are well-known quiescent cells and were found to be difficult for gene transfection using above-mentioned techniques.
Our laboratory regularly uses nucleoporation to transduce genes to HSCs with high efficiencies (53, 99). Other cell types such as bone marrow derived stem cells, endothelial cells, keratinocytes are also successfully transduced with this technology (114–116). This experimental procedure offers several advantages such as short sample preparation time; pulsing the cell solution and plating the nucleofected cells take less than 30 minutes to accomplish. Highly significant transduction efficiency overcomes the disadvantage of high mortality rate (117). Direct delivery of nucleic acids to the nucleus, reduces the delay between transfection and expression and cell division is not required. Limited restrictions includes high cost and mortality rate, however, optimization of conditions can control the mortality rate (117).
6. STEM CELL THERAPY FOR ISCHEMIC DISEASES
Ischemia is a disease state generated by restriction in blood supply due to factors present with in the blood vessel, which results to damage or dysfunction of tissue. Since, oxygen is mainly bound to hemoglobin in red blood cells, insufficient blood supply causes tissue to become hypoxic, or, if no oxygen is supplied at all, anoxic. However, complete cessation of oxygenation in tissues for more than 20 minutes typically results in irreversible damage. Peripheral arterial disease (PAD) is an example of such diseased state, which develops due to occlusion caused by systemic arthrosclerosis (fat and cholesterol deposition) especially, in arteries of iliac femoral system leading to terminal clinical condition known as critical limb ischemia (118). This leads to eventual loss of functional cells, daily life activities with possibility of amputation of affected limbs despite the current advances in endovascular surgery and interventional radiology. Overall, more than 12% of the population is affected by PAD, and men and women were equally affected (119). Formation of new blood vessels or development of collateral vessels from the pre-existing blood vessels is critical for the recovery of the ischemia and is termed as angiogenesis. At cellular levels, the cells of the hypoxic tissue typically undergo oxidative stress and produce hypoxia-inducible factor (120)-1□, which increases production of many genes that regulates and alters the cellular gene expression, angiogenesis, change in metabolic switch and apoptosis (121). The molecules in hypoxic or ischemia-induced response include vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF), and fibroblast growth factors (FGF). At preclinical levels the role of these pro-angiogenic factors are tested in the murine models of hind limb ischemia (53, 122). The treatment for the PAD depends on the restoration of blood flow through therapeutic angiogenesis and vasculogenesis.
6.1. Role of pro-angiogenic factors in ischemia therapy
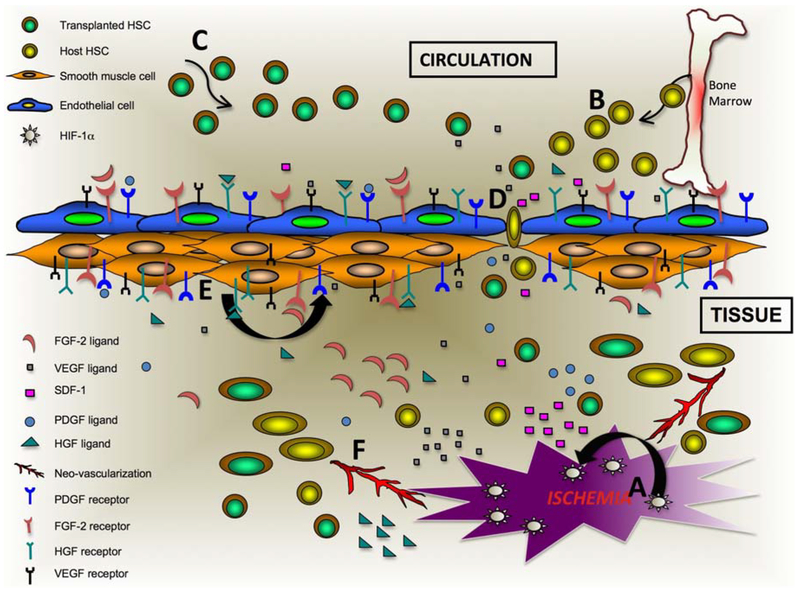
Angiogenesis is induced by a combination of various soluble factors such as growth factors and cytokines, which may be produced by inflammation or tumor. The invasion of endothelial cells into the perivascular tissue and degradation of the basement membrane are important to start the angiogenesis process. Elongation of the new vessel is achieved by the proliferation of endothelial cells and stabilized by synthesis of extra-cellular matrix including the basement membrane and integration of pericytes. All blood vessels in the body are made up of two major cell typesendothelial cells towards the lumen of the vessel and smooth muscle cells regulating contraction and relaxation of the vessel. During embryogenesis, three major events, responsible for the generation of the blood vessels occur (123–125) i.e. vasculogenesis (blood vessel formation from the precursor cells), angiogenesis (blood vessel formation from preexisting vessels and vascular endothelial cells), and vascular remodeling. Vasculogenesis was considered to occur only in pre-natal life; however, the postnatal neovascularization was also demonstrated in murine model of hind limb ischemia. In adult humans, the endothelial progenitor cell (126) plays an important role for the neovascularization. Endothelial progenitor cell express surface epitopes, such as VEGFR-2, CXCR-4, CD34 and CD133 and are derived from bone marrow, peripheral blood or umbilical cord blood (127, 128). In an event of ischemic condition, bone marrow derived EPCs home to the ischemic region and stimulate the resident endothelial cells in the vasculature to mediate vascular repair (129). Critical molecules that regulate function of vascular endothelial cells, and play role in therapeutic angiogenesis are reviewed below (Figure 3).
Figure 3.
Schematic of revascularization process after genetically modified stem cell therapy in ischemic tissue. A. Ischemia generates high level of HIF-1α thus causing upregulation and production of angiogenic factors such as VEGF, PDGF, HGF, FGF-2 and others. B. SDF-1 and VEGF causes migration of EPCs from bone marrow to ischemic region and stabilizes endothelial cells near the ischemic region. C. Migration of injected genetically modified stem cells to the ischemic region via CXCR4-SDF-1 interaction. D. VEGF mediates vascular endothelial permeability and as a result migration of stem cells occur. E. Angiogenic factor causes proliferation of smooth muscle cells. F. Various angiogenic factors in presence of stem cell (both transplanted and host) along with proliferated smooth muscle and endothelial cells and other supporting cells mediate neo-vascularization and angiogenesis to ischemic tissues from pre-existing blood vessels.
6.1.1. Vascular endothelial growth factor
Vascular endothelial growth factor (VEGF) is responsible for the endothelial cell growth and was also identified as vascular permeability factor (130, 131). VEGF in humans have different isoforms, major subtypes comprise of 121, 138, 165 and 189 amino acids. VEGF 165 is the strongest signal transducer among the VEGF family, since it has a very strong affinity to VEGF-R called Flt-1 and binds to other VEGF-receptor called KDR (VEGFR-2). VEGF was shown to be upregulated by physiological conditions such as hypoxia, hormonal induction, tumor promoters, inflammation and by several other growth factors. HIF-1α is the major regulator of the VEGF expression under different oxygen concentrations (132). Knockout mice for VEGF, VEGF-R1 and VEGF-R2 were found embryonic lethal (133). VEGF caused ischemiainduced mobilization of endothelial progenitor cells (126) in mice (122) and humans (134). VEGF was also shown to be the mediator of vasculogenesis in both human and murine models of limb ischemia (134). VEGF acts on the smooth muscle cells (135). It mediates their chemotaxis and migration to the hypoxic area, as smooth muscle cells are required for remodeling of vessels (136). Circulating monocytes and other leukocytes release growth factors essential for vascular growth, and VEGF has chemotactic effects on these cells (137, 138) offering possible cure for ischemia related disorders. VEGF-B is known to cause its effects through VEGFR-1, which causes endothelial cell differentiation by stimulating nitric oxide (NO) release via endothelial NO synthase and Akt pathways (139).
6.1.2. Platelet derived growth factor
Similar to VEGF family of growth factors, platelet derived growth factor (PDGF) family consists of highly conserved growth factor domain, called PDGF/VEGF homology domain. The PDGF family consists of two classical members (PDGF-A and PDGF-B), (140) and two newly discovered novel PGDF’s called PDGF-C and PDGF-D (141, 142). These receptors are expressed by myeloid hematopoietic cells, platelets (143), VSMC, pericytes and endothelial cells (144). PDGF-B exerts angiogenic effects via PDGFR-B receptor signaling [18, 19]. PDGF molecules exist as homodimers or heterodimers and have different receptor affinities (PDGFRα and PDGFRβ). These receptors belong to the tyrosine kinase receptor family and cause cellular alteration in metabolism, proliferation and migration. PDGF in the vascular system is important for recruitment of pericytes to maintain the vessel integrity (145). Deletion of PDGF-B resulted in a phenotype manifested by loss of pericytes in the blood vessel and subsequent hemorrhage and its targeted inactivation caused embryonic lethality (25). Synergism of VEGF and PDGF-B stimulated the revascularization of the ischemic hind limb by promoting vessel growth and maturation (26–29). Effects of PDGF-C were seen in endothelial cells and were shown to cause angiogenesis in ischemic heart (30). PDGF-C stimulated branching in pre-existing blood vessels, SMC growth (15, 30, 31) and caused release of VEGF (32).
6.1.3. Fibroblast growth factor-2
Fibroblast growth factor (FGF)-2 molecule exists in different isoforms with molecular weight ranging from 18–25 kd (146, 147). FGF-2 interacts with specific cell surface receptor, which share a common feature including a conserved cytoplasmic tyrosine kinase domain, transmembrane domain and an extra-cellular ligandbinding domain (148). Fibroblast growth factor has pleiotropic effects on various cells and organs. It mediates differentiation and regulates the normal functioning and tissue repair of various organs (149, 150). FGF-2 induced differentiation of endothelial cells and hematopoietic cells in vitro (151) and was found to be a potent angiogenic molecule both in vitro and in vivo (152). FGF-2 was found to stimulate the growth of the small blood vessels in embryonic tissue. Marked synergistic effects of the FGF-2 and PDGF-B was observed on neo-vascularization when transplanted in mouse corneas (153), however, the mechanism of the synergism is not known. Studies of the synergistic effects of the FGF-2 with VEGF-B also showed increased development of collateral blood vessels and improved the recovery of calf-blood pressure in the hind limb ischemia model (154).
6.1.4. Placental growth factor
Placenta growth factor (PIGF), VEGF homologue has been shown to play an important role in angiogenesis via its receptor VEGFR-1 present on endothelial cells (155). Impaired collateral growth or areriogenesis was observed in mice lacking either PIGF or VEGFR-1 and restoration of angiogenesis was observed via exogenous gene delivery in ischemic limb (155, 156). PIGF was shown to play an important role in angiogenesis through inflammatory macrophage or monocytes mediated mechanism or by enhancement in EPC recruitment (157). Induction of PIGF expression was stimulated by FGF-2 gene in murine hind-limb ischemia model and was regulated via VEGF/PKC-MEK and NF-κB signaling pathway (158).
6.1.5. Hepatocyte growth factor
Hepatocyte growth factor (HGF) or scatter factor (SF) is a large multi-domain protein, initially identified as the mitogen for the hepatocytes (159) but recently found to be potent mitogen for the endothelial cells (160). HGF induced VEGF production via endothelial cells and surrounding vascular smooh muscle cells (161). VEGF and HGF were shown to act in synergy, caused regulation of endothelial cell cycle (162) and angiogenesis in a paracrine manner (163). However, HGF could also act independent of the VEGF, via direct activation of Akt ad Erks (164). It was also shown that the vascular levels of the HGF decreased in the ischemic conditions, however, with the delivery of the rHGF via gene therapy induced therapeutic angiogenesis in the hind limb ischemia model (165). Moreover, intravenous delivery of the HGF caused collateral formation of the blood vessels in the hind limb ischemic model (165).
The contribution of each of the above angiogenic factors was studied in various hind-limb ischemic models and was translated into clinical trials with limited success. Our recent work showed that ex-vivo expanded HSCs transduced with bicistronic vector expressing VEGF and PDGF genes, administered via intra-ventricular injection into the hind limb ischemia model, augmented vasculogenesis resulting in restoration of blood flow (Figure 2).
7. CONCLUSIONS AND FUTURE DIRECTIONS
Stem cell therapies are currently being developed for clinical applications in the field of regenerative medicine and HSCs are already the gold standard for the hematological disorders. Ex-vivo expansion is challenging, as delicate balance exists between self-renewal versus commitment, requires the use of specific concentration and combination of cytokines for expansion of biologically functional HSC population. Techniques employing viral proteins and viral vectors need more critical evaluation as viruses present potential risk of transformation. Newer bio-safe strategies for successful ex-vivo expansion such as tissue engineering, use of 3D nanofiber with the chemical modifications, cocktail of cytokines of defined biological effects need to be established before offering stem cell transplantation as a curative alternative to other available treatments. Paracrine effects of the transplanted stem cells influence the microenvironment of the host tissue towards functional regeneration of damaged organ. Ex-vivo expansion and genetic modification of stem cells provides promising results in the preclinical studies. However, before its induction to the routine clinical application, more validation is necessary, specially evaluating long-term effects.
8. ACKNOWLEDGEMENTS
This work was supported in part by National Institutes of Health grants, K01 AR054114 (NIAMS), SBIR R44 HL092706–01 (NHLBI), Third Frontier Projects, Ohio Technology, BRCP Grant and The Ohio State University start-up fund for stem cell research.
9. REFERENCES
- 1.Price J and Williams BP: Neural stem cells. Curr Opin Neurobiol, 11(5), 564–7 (2001) [DOI] [PubMed] [Google Scholar]
- 2.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS and Jones JM: Embryonic stem cell lines derived from human blastocysts. Science, 282(5391), 1145–7 (1998) [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131(5), 861–72 (2007) [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K and Yamanaka S: Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663–76 (2006) [DOI] [PubMed] [Google Scholar]
- 5.Wernig M, Meissner A, Cassady JP and Jaenisch R: c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell, 2(1), 10–2 (2008) [DOI] [PubMed] [Google Scholar]
- 6.Okita K, Nakagawa M, Hyenjong H, Ichisaka T and Yamanaka S: Generation of mouse induced pluripotent stem cells without viral vectors. Science, 322(5903), 949–53 (2008) [DOI] [PubMed] [Google Scholar]
- 7.Schofield R: The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells, 4(1–2), 7–25 (1978) [PubMed] [Google Scholar]
- 8.Spradling A, Drummond-Barbosa D and Kai T: Stem cells find their niche. Nature, 414(6859), 98–104 (2001) [DOI] [PubMed] [Google Scholar]
- 9.Scadden DT: The stem-cell niche as an entity of action. Nature, 441(7097), 1075–9 (2006) [DOI] [PubMed] [Google Scholar]
- 10.Taichman RS: Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood, 105(7), 2631–9 (2005) [DOI] [PubMed] [Google Scholar]
- 11.Adams GB and Scadden DT: The hematopoietic stem cell in its place. Nat Immunol, 7(4), 333–7 (2006) [DOI] [PubMed] [Google Scholar]
- 12.Can A: Haematopoietic stem cells niches: interrelations between structure and function. Transfus Apher Sci, 38(3), 261–8 (2008) [DOI] [PubMed] [Google Scholar]
- 13.Rafii S, Shapiro F, Pettengell R, Ferris B, Nachman RL, Moore MA and Asch AS: Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood, 86(9), 3353–63 (1995) [PubMed] [Google Scholar]
- 14.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM and Scadden DT: Osteoblastic cells regulate the haematopoietic stem cell niche. Nature, 425(6960), 841–6 (2003) [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG,Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y and Li L: Identification of the haematopoietic stem cell niche and control of the niche size. Nature, 425(6960), 836–41 (2003) [DOI] [PubMed] [Google Scholar]
- 16.Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J and Aguila HL: Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood, 103(9), 3258–64 (2004) [DOI] [PubMed] [Google Scholar]
- 17.Taichman RS and Emerson SG: The role of osteoblasts in the hematopoietic microenvironment. Stem Cells, 16(1), 7–15 (1998) [DOI] [PubMed] [Google Scholar]
- 18.Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, Arenzana-Seisdedos F, Magerus A, Caruz A, Fujii N, Nagler A, Lahav M, Szyper-Kravitz M, Zipori D and Lapidot T: Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest, 106(11), 1331–9 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ and Riggs BL: The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res, 15(1), 2–12 (2000) [DOI] [PubMed] [Google Scholar]
- 20.Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grunewald E, Cheng T, Dombkowski D, Calvi LM, Rittling SR and Scadden DT: Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med, 201(11), 1781–91 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taichman RS, Reilly MJ and Emerson SG: Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. Blood, 87(2), 518–24 (1996) [PubMed] [Google Scholar]
- 22.Taichman RS, Reilly MJ and Matthews LS: Human osteoblast-like cells and osteosarcoma cell lines synthesize macrophage inhibitory protein 1alpha in response to interleukin 1beta and tumour necrosis factor alpha stimulation in vitro. Br J Haematol, 108(2), 275–83 (2000) [DOI] [PubMed] [Google Scholar]
- 23.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C and Morrison SJ: SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell, 121(7), 1109–21 (2005) [DOI] [PubMed] [Google Scholar]
- 24.Sugiyama T, Kohara H, Noda M and Nagasawa T: Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity, 25(6), 977–88 (2006) [DOI] [PubMed] [Google Scholar]
- 25.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D and Lapidot T: Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science, 283(5403), 845–8 (1999) [DOI] [PubMed] [Google Scholar]
- 26.Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, Elson A and Lapidot T: Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med, 12(6), 657–64 (2006) [DOI] [PubMed] [Google Scholar]
- 27.Nilsson SK, Johnston HM and Coverdale JA: Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood, 97(8), 2293–9 (2001) [DOI] [PubMed] [Google Scholar]
- 28.Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N and Reya T: Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol, 6(3), 314–22 (2005) [DOI] [PubMed] [Google Scholar]
- 29.Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD and Sklar J: TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell, 66(4), 649–61 (1991) [DOI] [PubMed] [Google Scholar]
- 30.Pereira RM, Delany AM, Durant D and Canalis E: Cortisol regulates the expression of Notch in osteoblasts. J Cell Biochem, 85(2), 252–8 (2002) [DOI] [PubMed] [Google Scholar]
- 31.Weber JM, Forsythe SR, Christianson CA, Frisch BJ, Gigliotti BJ, Jordan CT, Milner LA, Guzman ML and Calvi LM: Parathyroid hormone stimulates expression of the Notch ligand Jagged1 in osteoblastic cells. Bone, 39(3), 485–93 (2006) [DOI] [PubMed] [Google Scholar]
- 32.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R and Weissman IL: A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature, 423(6938), 409–14 (2003) [DOI] [PubMed] [Google Scholar]
- 33.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR 3rd and Nusse R: Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature, 423(6938), 448–52 (2003) [DOI] [PubMed] [Google Scholar]
- 34.Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M and Scadden DT: Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science, 287(5459), 1804–8 (2000) [DOI] [PubMed] [Google Scholar]
- 35.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY and Suda T: Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell, 118(2), 149–61 (2004) [DOI] [PubMed] [Google Scholar]
- 36.House MG, Kohlmeier L, Chattopadhyay N, Kifor O, Yamaguchi T, Leboff MS, Glowacki J and Brown EM: Expression of an extracellular calcium-sensing receptor in human and mouse bone marrow cells. J Bone Miner Res, 12(12), 1959–70 (1997) [DOI] [PubMed] [Google Scholar]
- 37.Antoniou ES, Sund S, Homsi EN, Challenger LF and Rameshwar P: A theoretical simulation of hematopoietic stem cells during oxygen fluctuations: prediction of bone marrow responses during hemorrhagic shock. Shock, 22(5), 415–22 (2004) [DOI] [PubMed] [Google Scholar]
- 38.Danet GH, Pan Y, Luongo JL, Bonnet DA and Simon MC: Expansion of human SCID-repopulating cells under hypoxic conditions. J Clin Invest, 112(1), 126–35 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP and Gurtner GC: Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med, 10(8), 858–64 (2004) [DOI] [PubMed] [Google Scholar]
- 40.Dao MA, Creer MH, Nolta JA and Verfaillie CM: Biology of umbilical cord blood progenitors in bone marrow niches. Blood, 110(1), 74–81 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL and Chen TH: Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood, 103(5), 1669–75 (2004) [DOI] [PubMed] [Google Scholar]
- 42.Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S and Bischoff J: In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood, 109(11), 4761–8 (2007) [DOI] [PubMed] [Google Scholar]
- 43.Rubinstein P, Rosenfield RE, Adamson JW and Stevens CE: Stored placental blood for unrelated bone marrow reconstitution. Blood, 81(7), 1679–90 (1993) [PubMed] [Google Scholar]
- 44.Broxmeyer HE and Vadhan-Raj S: Preclinical and clinical studies with the hematopoietic colony-stimulating factors and related interleukins. Immunol Res, 8(3), 185–201 (1989) [DOI] [PubMed] [Google Scholar]
- 45.Apperley JF: Umbilical cord blood progenitor cell transplantation. The International Conference Workshop on Cord Blood Transplantation, Indianapolis, November 1993. Bone Marrow Transplant, 14(2), 187–96 (1994) [PubMed] [Google Scholar]
- 46.Locatelli F, Rocha V, Chastang C, Arcese W, Michel G, Abecasis M, Messina C, Ortega J, BadellSerra I, Plouvier E, Souillet G, Jouet JP, Pasquini R, Ferreira E, Garnier F and Gluckman E: Factors associated with outcome after cord blood transplantation in children with acute leukemia. Eurocord-Cord Blood Transplant Group. Blood, 93(11), 3662–71 (1999) [PubMed] [Google Scholar]
- 47.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS and Wagner JE: Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood, 102(5), 1915–9 (2003) [DOI] [PubMed] [Google Scholar]
- 48.De Lima M, St John LS, Wieder ED, Lee MS, McMannis J, Karandish S, Giralt S, Beran M, Couriel D, Korbling M, Bibawi S, Champlin R and Komanduri KV: Double-chimaerism after transplantation of two human leucocyte antigen mismatched, unrelated cord blood units. Br J Haematol, 119(3), 773–6 (2002) [DOI] [PubMed] [Google Scholar]
- 49.Lekakis L, Giralt S, Couriel D, Shpall EJ, Hosing C, Khouri IF, Anderlini P, Korbling M, Martin T, Champlin RE and de Lima M: Phase II study of unrelated cord blood transplantation for adults with high-risk hematologic malignancies. Bone Marrow Transplant, 38(6), 421–6 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mazurier F, Doedens M, Gan OI and Dick JE: Characterization of cord blood hematopoietic stem cells. Ann N Y Acad Sci, 996, 67–71 (2003) [DOI] [PubMed] [Google Scholar]
- 51.Li Y, Ma T, Kniss DA, Yang ST and Lasky LC: Human cord cell hematopoiesis in three-dimensional nonwoven fibrous matrices: in vitro simulation of the marrow microenvironment. J Hematother Stem Cell Res, 10(3), 355–68 (2001) [DOI] [PubMed] [Google Scholar]
- 52.Okamoto T, Takagi M, Soma T, Ogawa H, Kawakami M, Mukubo M, Kubo K, Sato R, Toma K and Yoshida T: Effect of heparin addition on expansion of cord blood hematopoietic progenitor cells in three-dimensional coculture with stromal cells in nonwoven fabrics. J Artif Organs, 7(4), 194–202 (2004) [DOI] [PubMed] [Google Scholar]
- 53.Das H, Abdulhameed N, Joseph M, Sakthivel R, Mao HQ and Pompili VJ: Ex vivo nanofiber expansion and genetic modification of human cord blood-derived progenitor/stem cells enhances vasculogenesis. Cell Transplant, 18(3), 305–18 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ihle JN, Keller J, Oroszlan S, Henderson LE, Copeland TD, Fitch F, Prystowsky MB, Goldwasser E, Schrader JW, Palaszynski E, Dy M and Lebel B: Biologic properties of homogeneous interleukin 3. I. Demonstration of WEHI-3 growth factor activity, mast cell growth factor activity, p cell-stimulating factor activity, colony-stimulating factor activity, and histamine-producing cell-stimulating factor activity. J Immunol, 131(1), 282–7 (1983) [PubMed] [Google Scholar]
- 55.Morikawa Y, Tohya K, Hara T, Kitamura T and Miyajima A: Expression of IL-3 receptor in testis. Biochem Biophys Res Commun, 226(1), 107–12 (1996) [DOI] [PubMed] [Google Scholar]
- 56.Bryder D and Jacobsen SE: Interleukin-3 supports expansion of long-term multilineage repopulating activity after multiple stem cell divisions in vitro. Blood, 96(5), 1748–55 (2000) [PubMed] [Google Scholar]
- 57.Yonemura Y, Ku H, Hirayama F, Souza LM and Ogawa M: Interleukin 3 or interleukin 1 abrogates the reconstituting ability of hematopoietic stem cells. Proc Natl Acad Sci U S A, 93(9), 4040–4 (1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peters SO, Kittler EL, Ramshaw HS and Quesenberry PJ: Ex vivo expansion of murine marrow cells with interleukin-3 (IL-3), IL-6, IL-11, and stem cell factor leads to impaired engraftment in irradiated hosts. Blood, 87(1), 30–7 (1996) [PubMed] [Google Scholar]
- 59.Ueda T, Tsuji K, Yoshino H, Ebihara Y, Yagasaki H, Hisakawa H, Mitsui T, Manabe A, Tanaka R, Kobayashi K, Ito M, Yasukawa K and Nakahata T: Expansion of human NOD/SCID-repopulating cells by stem cell factor, Flk2/Flt3 ligand, thrombopoietin, IL-6, and soluble IL-6 receptor. J Clin Invest, 105(7), 1013–21 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kollet O, Aviram R, Chebath J, ben-Hur H, Nagler A, Shultz L, Revel M and Lapidot T: The soluble interleukin-6 (IL-6) receptor/IL-6 fusion protein enhances in vitro maintenance and proliferation of human CD34(+)CD38(-/low) cells capable of repopulating severe combined immunodeficiency mice. Blood, 94(3), 923–31 (1999) [PubMed] [Google Scholar]
- 61.Kimura T, Wang J, Minamiguchi H, Fujiki H, Harada S, Okuda K, Kaneko H, Yokota S, Yasukawa K, Abe T and Sonoda Y: Signal through gp130 activated by soluble interleukin (IL)-6 receptor (R) and IL-6 or IL-6R/IL-6 fusion protein enhances ex vivo expansion of human peripheral blood-derived hematopoietic progenitors. Stem Cells, 18(6), 444–52 (2000) [DOI] [PubMed] [Google Scholar]
- 62.Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, Gomei Y, Iwasaki H, Matsuoka S, Miyamoto K, Miyazaki H, Takahashi T and Suda T: Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell, 1(6), 685–97 (2007) [DOI] [PubMed] [Google Scholar]
- 63.Fox N, Priestley G, Papayannopoulou T and Kaushansky K: Thrombopoietin expands hematopoietic stem cells after transplantation. J Clin Invest, 110(3), 389–94 (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Piacibello W, Sanavio F, Garetto L, Severino A, Bergandi D, Ferrario J, Fagioli F, Berger M and Aglietta M: Extensive amplification and self-renewal of human primitive hematopoietic stem cells from cord blood. Blood, 89(8), 2644–53 (1997) [PubMed] [Google Scholar]
- 65.Nishino T, Miyaji K, Ishiwata N, Arai K, Yui M, Asai Y, Nakauchi H and Iwama A: Ex vivo expansion of human hematopoietic stem cells by a small-molecule agonist of c-MPL. Exp Hematol, 37(11), 1364–1377 e4 (2009) [DOI] [PubMed] [Google Scholar]
- 66.Matthews W, Jordan CT, Wiegand GW, Pardoll D and Lemischka IR: A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell, 65(7), 1143–52 (1991) [DOI] [PubMed] [Google Scholar]
- 67.Molineux G, McCrea C, Yan XQ, Kerzic P and McNiece I: Flt-3 ligand synergizes with granulocyte colony-stimulating factor to increase neutrophil numbers and to mobilize peripheral blood stem cells with long-term repopulating potential. Blood, 89(11), 3998–4004 (1997) [PubMed] [Google Scholar]
- 68.Petzer AL, Zandstra PW, Piret JM and Eaves CJ: Differential cytokine effects on primitive (CD34+CD38-) human hematopoietic cells: novel responses to Flt3-ligand and thrombopoietin. J Exp Med, 183(6), 2551–8 (1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galli SJ, Zsebo KM and Geissler EN: The kit ligand, stem cell factor. Adv Immunol, 55, 1–96 (1994) [DOI] [PubMed] [Google Scholar]
- 70.Williams DE, de Vries P, Namen AE, Widmer MB and Lyman SD: The Steel factor. Dev Biol, 151(2), 368–76 (1992) [DOI] [PubMed] [Google Scholar]
- 71.Yonemura Y, Ku H, Lyman SD and Ogawa M: In vitro expansion of hematopoietic progenitors and maintenance of stem cells: comparison between FLT3/FLK-2 ligand and KIT ligand. Blood, 89(6), 1915–21 (1997) [PubMed] [Google Scholar]
- 72.Molofsky AV, Pardal R and Morrison SJ: Diverse mechanisms regulate stem cell self-renewal. Curr Opin Cell Biol, 16(6), 700–7 (2004) [DOI] [PubMed] [Google Scholar]
- 73.Suzuki T and Chiba S: Notch signaling in hematopoietic stem cells. Int J Hematol, 82(4), 285–94 (2005) [DOI] [PubMed] [Google Scholar]
- 74.Buske C and Humphries RK: Homeobox genes in leukemogenesis. Int J Hematol, 71(4), 301–8 (2000) [PubMed] [Google Scholar]
- 75.Kleber M and Sommer L: Wnt signaling and the regulation of stem cell function. Curr Opin Cell Biol, 16(6), 681–7 (2004) [DOI] [PubMed] [Google Scholar]
- 76.Sauvageau G, Thorsteinsdottir U, Eaves CJ, Lawrence HJ, Largman C, Lansdorp PM and Humphries RK: Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev, 9(14), 1753–65 (1995) [DOI] [PubMed] [Google Scholar]
- 77.Thorsteinsdottir U, Sauvageau G and Humphries RK: Enhanced in vivo regenerative potential of HOXB4-transduced hematopoietic stem cells with regulation of their pool size. Blood, 94(8), 2605–12 (1999) [PubMed] [Google Scholar]
- 78.Bhardwaj G, Murdoch B, Wu D, Baker DP, Williams KP, Chadwick K, Ling LE, Karanu FN and Bhatia M: Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol, 2(2), 172–80 (2001) [DOI] [PubMed] [Google Scholar]
- 79.Henrique D, Hirsinger E, Adam J, Le Roux I, Pourquie O, Ish-Horowicz D and Lewis J: Maintenance of neuroepithelial progenitor cells by Delta-Notch signalling in the embryonic chick retina. Curr Biol, 7(9), 661–70 (1997) [DOI] [PubMed] [Google Scholar]
- 80.Suzuki T, Yokoyama Y, Kumano K, Takanashi M, Kozuma S, Takato T, Nakahata T, Nishikawa M, Sakano S, Kurokawa M, Ogawa S and Chiba S: Highly efficient ex vivo expansion of human hematopoietic stem cells using Delta1-Fc chimeric protein. Stem Cells, 24(11), 2456–65 (2006) [DOI] [PubMed] [Google Scholar]
- 81.Heike T and Nakahata T: Ex vivo expansion of hematopoietic stem cells by cytokines. Biochim Biophys Acta, 1592(3), 313–21 (2002) [DOI] [PubMed] [Google Scholar]
- 82.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T and Nakahata T: NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood, 100(9), 3175–82 (2002) [DOI] [PubMed] [Google Scholar]
- 83.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL, Bousso P, Deist FL and Fischer A: Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science, 288(5466), 669–72 (2000) [DOI] [PubMed] [Google Scholar]
- 84.Hu WS and Pathak VK: Design of retroviral vectors and helper cells for gene therapy. Pharmacol Rev, 52(4), 493–511 (2000) [PubMed] [Google Scholar]
- 85.Fraser CC, Eaves CJ, Szilvassy SJ and Humphries RK: Expansion in vitro of retrovirally marked totipotent hematopoietic stem cells. Blood, 76(6), 1071–6 (1990) [PubMed] [Google Scholar]
- 86.Cassel A, Cottler-Fox M, Doren S and Dunbar CE: Retroviral-mediated gene transfer into CD34-enriched human peripheral blood stem cells. Exp Hematol, 21(4), 585–91 (1993) [PubMed] [Google Scholar]
- 87.Check E: A tragic setback. Nature, 420(6912), 116–8 (2002) [DOI] [PubMed] [Google Scholar]
- 88.Baum C, Dullmann J, Li Z, Fehse B, Meyer J, Williams DA and von Kalle C: Side effects of retroviral gene transfer into hematopoietic stem cells. Blood, 101(6), 2099–114 (2003) [DOI] [PubMed] [Google Scholar]
- 89.Wagner W, Laufs S, Blake J, Schwager C, Wu X, Zeller JW, Ho AD and Fruehauf S: Retroviral integration sites correlate with expressed genes in hematopoietic stem cells. Stem Cells, 23(8), 1050–8 (2005) [DOI] [PubMed] [Google Scholar]
- 90.Wu X, Li Y, Crise B and Burgess SM: Transcription start regions in the human genome are favored targets for MLV integration. Science, 300(5626), 1749–51 (2003) [DOI] [PubMed] [Google Scholar]
- 91.Laufs S, Gentner B, Nagy KZ, Jauch A, Benner A, Naundorf S, Kuehlcke K, Schiedlmeier B, Ho AD, Zeller WJ and Fruehauf S: Retroviral vector integration occurs in preferred genomic targets of human bone marrow-repopulating cells. Blood, 101(6), 2191–8 (2003) [DOI] [PubMed] [Google Scholar]
- 92.Mitchell RS, Beitzel BF, Schroder AR, Shinn P, Chen H, Berry CC, Ecker JR and Bushman FD: Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol, 2(8), E234 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dorrell C, Gan OI, Pereira DS, Hawley RG and Dick JE: Expansion of human cord blood CD34(+)CD38(-) cells in ex vivo culture during retroviral transduction without a corresponding increase in SCID repopulating cell (SRC) frequency: dissociation of SRC phenotype and function. Blood, 95(1), 102–10 (2000) [PubMed] [Google Scholar]
- 94.Cheshier SH, Morrison SJ, Liao X and Weissman IL: In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci U S A, 96(6), 3120–5 (1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gothot A, van der Loo JC, Clapp DW and Srour EF: Cell cycle-related changes in repopulating capacity of human mobilized peripheral blood CD34(+) cells in non-obese diabetic/severe combined immune-deficient mice. Blood, 92(8), 2641–9 (1998) [PubMed] [Google Scholar]
- 96.Sutton RE, Reitsma MJ, Uchida N and Brown PO: Transduction of human progenitor hematopoietic stem cells by human immunodeficiency virus type 1-based vectors is cell cycle dependent. J Virol, 73(5), 3649–60 (1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Glimm H, Oh IH and Eaves CJ: Human hematopoietic stem cells stimulated to proliferate in vitro lose engraftment potential during their S/G(2)/M transit and do not reenter G(0). Blood, 96(13), 4185–93 (2000) [PubMed] [Google Scholar]
- 98.Woods NB, Fahlman C, Mikkola H, Hamaguchi I, Olsson K, Zufferey R, Jacobsen SE, Trono D and Karlsson S: Lentiviral gene transfer into primary and secondary NOD/SCID repopulating cells. Blood, 96(12), 3725–33 (2000) [PubMed] [Google Scholar]
- 99.Das H, George JC, Joseph M, Das M, Abdulhameed N, Blitz A, Khan M, Sakthivel R, Mao HQ, Hoit BD, Kuppusamy P and Pompili VJ: Stem cell therapy with overexpressed VEGF and PDGF genes improves cardiac function in a rat infarct model. PLoS One, 4(10), e7325 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Srivastava A, Lusby EW and Berns KI: Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol, 45(2), 555–64 (1983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li C, Bowles DE, van Dyke T and Samulski RJ: Adeno-associated virus vectors: potential applications for cancer gene therapy. Cancer Gene Ther, 12(12), 913–25 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Samulski RJ, Zhu X, Xiao X, Brook JD, Housman DE, Epstein N and Hunter LA: Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J, 10(12), 3941–50 (1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kotin RM, Siniscalco M, Samulski RJ, Zhu XD, Hunter L, Laughlin CA, McLaughlin S, Muzyczka N, Rocchi M and Berns KI: Site-specific integration by adenoassociated virus. Proc Natl Acad Sci U S A, 87(6), 2211–5 (1990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Santat L, Paz H, Wong C, Li L, Macer J, Forman S, Wong KK and Chatterjee S: Recombinant AAV2 transduction of primitive human hematopoietic stem cells capable of serial engraftment in immune-deficient mice. Proc Natl Acad Sci U S A, 102(31), 11053–8 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen C and Okayama H: High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol, 7(8), 2745–52 (1987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kjer KM and Fallon AM: Efficient transfection of mosquito cells is influenced by the temperature at which DNA-calcium phosphate coprecipitates are prepared. Arch Insect Biochem Physiol, 16(3), 189–200 (1991) [DOI] [PubMed] [Google Scholar]
- 107.Schenborn ET and Oler J: Liposome-mediated transfection of mammalian cells. Methods Mol Biol, 130, 155–64 (2000) [DOI] [PubMed] [Google Scholar]
- 108.Ewert K, Evans HM, Ahmad A, Slack NL, Lin AJ, Martin-Herranz A and Safinya CR: Lipoplex structures and their distinct cellular pathways. Adv Genet, 53, 119–55 (2005) [PubMed] [Google Scholar]
- 109.Gao X and Huang L: Cationic liposome-mediated gene transfer. Gene Ther, 2(10), 710–22 (1995) [PubMed] [Google Scholar]
- 110.Chen C, Smye SW, Robinson MP and Evans JA: Membrane electroporation theories: a review. Med Biol Eng Comput, 44(1–2), 5–14 (2006) [DOI] [PubMed] [Google Scholar]
- 111.Lakshmipathy U, Pelacho B, Sudo K, Linehan JL, Coucouvanis E, Kaufman DS and Verfaillie CM: Efficient transfection of embryonic and adult stem cells. Stem Cells, 22(4), 531–43 (2004) [DOI] [PubMed] [Google Scholar]
- 112.Wilke M, Fortunati E, van den Broek M, Hoogeveen AT and Scholte BJ: Efficacy of a peptide-based gene delivery system depends on mitotic activity. Gene Ther, 3(12), 1133–42 (1996) [PubMed] [Google Scholar]
- 113.Brisson M, Tseng WC, Almonte C, Watkins S and Huang L: Subcellular trafficking of the cytoplasmic expression system. Hum Gene Ther, 10(16), 2601–13 (1999) [DOI] [PubMed] [Google Scholar]
- 114.Distler JH, Jungel A, Kurowska-Stolarska M, Michel BA, Gay RE, Gay S and Distler O: Nucleofection: a new, highly efficient transfection method for primary human keratinocytes*. Exp Dermatol, 14(4), 315–20 (2005) [DOI] [PubMed] [Google Scholar]
- 115.Aluigi M, Fogli M, Curti A, Isidori A, Gruppioni E, Chiodoni C, Colombo MP, Versura P, D’Errico-Grigioni A, Ferri E, Baccarani M and Lemoli RM: Nucleofection is an efficient nonviral transfection technique for human bone marrow-derived mesenchymal stem cells. Stem Cells, 24(2), 454–61 (2006) [DOI] [PubMed] [Google Scholar]
- 116.Thiel C and Nix M: Efficient transfection of primary cells relevant for cardiovascular research by nucleofection. Methods Mol Med, 129, 255–66 (2006) [DOI] [PubMed] [Google Scholar]
- 117.Han SY, Gai W, Yancovitz M, Osman I, Di Como CJ and Polsky D: Nucleofection is a highly effective gene transfer technique for human melanoma cell lines. Exp Dermatol, 17(5), 405–11 (2008) [DOI] [PubMed] [Google Scholar]
- 118.Stewart KJ, Hiatt WR, Regensteiner JG and Hirsch AT: Exercise training for claudication. N Engl J Med, 347(24), 1941–51 (2002) [DOI] [PubMed] [Google Scholar]
- 119.Newman AB, Shemanski L, Manolio TA, Cushman M, Mittelmark M, Polak JF, Powe NR and Siscovick D: Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol, 19(3), 538–45 (1999) [DOI] [PubMed] [Google Scholar]
- 120.Rosnet O, Schiff C, Pebusque MJ, Marchetto S, Tonnelle C, Toiron Y, Birg F and Birnbaum D: Human FLT3/FLK2 gene: cDNA cloning and expression in hematopoietic cells. Blood, 82(4), 1110–9 (1993) [PubMed] [Google Scholar]
- 121.Bae SK, Baek JH, Lee YM, Lee OH and Kim KW: Hypoxia-induced apoptosis in human hepatocellular carcinoma cells: a possible involvement of the 6-TG-sensitive protein kinase(s)-dependent signaling pathway. Cancer Lett, 126(1), 97–104 (1998) [DOI] [PubMed] [Google Scholar]
- 122.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M and Isner JM: VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J, 18(14), 3964–72 (1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Isner JM, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T, Rosenfield K, Razvi S, Walsh K and Symes JF: Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet, 348(9024), 370–4 (1996) [DOI] [PubMed] [Google Scholar]
- 124.Isner JM, Walsh K, Symes J, Pieczek A, Takeshita S, Lowry J, Rossow S, Rosenfield K, Weir L, Brogi E and et al. : Arterial gene therapy for therapeutic angiogenesis in patients with peripheral artery disease. Circulation, 91(11), 2687–92 (1995) [DOI] [PubMed] [Google Scholar]
- 125.Risau W, Drexler H, Mironov V, Smits A, Siegbahn A, Funa K and Heldin CH: Platelet-derived growth factor is angiogenic in vivo. Growth Factors, 7(4), 261–6 (1992) [DOI] [PubMed] [Google Scholar]
- 126.Kaufman DB, Iii GW, Bruce DS, Johnson CP, Gaber AO, Sutherland DE, Merion RM, Gruber SA, Schweitzer E, Leone JP, Marsh CL, Alfrey E, Concepcion W, Stegall MD, Schulak JA, Gores PF, Benedetti E, Smith C, Henning AK, Kuehnel F, King S and Fitzsimmons WE: Prospective, randomized, multicenter trial of antibody induction therapy in simultaneous pancreas-kidney transplantation. Am J Transplant, 3(7), 855–64 (2003) [DOI] [PubMed] [Google Scholar]
- 127.Rustemeyer P, Wittkowski W, Greve B and Stehling M: Flow-cytometric identification, enumeration, purification, and expansion of CD133+ and VEGF-R2+ endothelial progenitor cells from peripheral blood. J Immunoassay Immunochem, 28(1), 13–23 (2007) [DOI] [PubMed] [Google Scholar]
- 128.Khan SS, Solomon MA and McCoy JP Jr.: Detection of circulating endothelial cells and endothelial progenitor cells by flow cytometry. Cytometry B Clin Cytom, 64(1), 1–8 (2005) [DOI] [PubMed] [Google Scholar]
- 129.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM: Isolation of putative progenitor endothelial cells for angiogenesis. Science, 275(5302), 964–7 (1997) [DOI] [PubMed] [Google Scholar]
- 130.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS and Dvorak HF: Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science, 219(4587), 983–5 (1983) [DOI] [PubMed] [Google Scholar]
- 131.Leung DW, Cachianes G, Kuang WJ, Goeddel DV and Ferrara N: Vascular endothelial growth factor is a secreted angiogenic mitogen. Science, 246(4935), 1306–9 (1989) [DOI] [PubMed] [Google Scholar]
- 132.Shih SC and Claffey KP: Regulation of human vascular endothelial growth factor mRNA stability in hypoxia by heterogeneous nuclear ribonucleoprotein L. J Biol Chem, 274(3), 1359–65 (1999) [DOI] [PubMed] [Google Scholar]
- 133.Neufeld G, Cohen T, Gengrinovitch S and Poltorak Z: Vascular endothelial growth factor (VEGF) and its receptors. FASEB J, 13(1), 9–22 (1999) [PubMed] [Google Scholar]
- 134.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM and Asahara T: Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A, 97(7), 3422–7 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Grosskreutz CL, Anand-Apte B, Duplaa C, Quinn TP, Terman BI, Zetter B and D’Amore PA: Vascular endothelial growth factor-induced migration of vascular smooth muscle cells in vitro. Microvasc Res, 58(2), 128–36 (1999) [DOI] [PubMed] [Google Scholar]
- 136.Schaper W and Ito WD: Molecular mechanisms of coronary collateral vessel growth. Circ Res, 79(5), 911–9 (1996) [DOI] [PubMed] [Google Scholar]
- 137.Arras M, Ito WD, Scholz D, Winkler B, Schaper J and Schaper W: Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest, 101(1), 40–50 (1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Couffinhal T, Silver M, Kearney M, Sullivan A, Witzenbichler B, Magner M, Annex B, Peters K and Isner JM: Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in ApoE−/− mice. Circulation, 99(24), 3188–98 (1999) [DOI] [PubMed] [Google Scholar]
- 139.Silvestre JS, Tamarat R, Ebrahimian TG, Le-Roux A, Clergue M, Emmanuel F, Duriez M, Schwartz B, Branellec D and Levy BI: Vascular endothelial growth factor-B promotes in vivo angiogenesis. Circ Res, 93(2), 114–23 (2003) [DOI] [PubMed] [Google Scholar]
- 140.Deuel TF, Huang JS, Proffitt RT, Baenziger JU, Chang D and Kennedy BB: Human platelet-derived growth factor. Purification and resolution into two active protein fractions. J Biol Chem, 256(17), 8896–9 (1981) [PubMed] [Google Scholar]
- 141.Li X, Ponten A, Aase K, Karlsson L, Abramsson A, Uutela M, Backstrom G, Hellstrom M, Bostrom H, Li H, Soriano P, Betsholtz C, Heldin CH, Alitalo K, Ostman A and Eriksson U: PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol, 2(5), 302–9 (2000) [DOI] [PubMed] [Google Scholar]
- 142.LaRochelle WJ, Jeffers M, McDonald WF, Chillakuru RA, Giese NA, Lokker NA, Sullivan C, Boldog FL, Yang M, Vernet C, Burgess CE, Fernandes E, Deegler LL, Rittman B, Shimkets J, Shimkets RA, Rothberg JM and Lichenstein HS: PDGF-D, a new protease-activated growth factor. Nat Cell Biol, 3(5), 517–21 (2001) [DOI] [PubMed] [Google Scholar]
- 143.Vassbotn FS, Havnen OK, Heldin CH and Holmsen H: Negative feedback regulation of human platelets via autocrine activation of the platelet-derived growth factor alphareceptor. J Biol Chem, 269(19), 13874–9 (1994) [PubMed] [Google Scholar]
- 144.Lindahl P, Johansson BR, Leveen P and Betsholtz C: Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science, 277(5323), 242–5 (1997) [DOI] [PubMed] [Google Scholar]
- 145.Crosby JR, Seifert RA, Soriano P and Bowen-Pope DF: Chimaeric analysis reveals role of Pdgf receptors in all muscle lineages. Nat Genet, 18(4), 385–8 (1998) [DOI] [PubMed] [Google Scholar]
- 146.Basilico C and Moscatelli D: The FGF family of growth factors and oncogenes. Adv Cancer Res, 59, 115–65 (1992) [DOI] [PubMed] [Google Scholar]
- 147.Miyamoto M, Naruo K, Seko C, Matsumoto S, Kondo T and Kurokawa T: Molecular cloning of a novel cytokine cDNA encoding the ninth member of the fibroblast growth factor family, which has a unique secretion property. Mol Cell Biol, 13(7), 4251–9 (1993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jaye M, Schlessinger J and Dionne CA: Fibroblast growth factor receptor tyrosine kinases: molecular analysis and signal transduction. Biochim Biophys Acta, 1135(2), 185–99 (1992) [DOI] [PubMed] [Google Scholar]
- 149.Logan A, Frautschy SA and Baird A: Basic fibroblast growth factor and central nervous system injury. Ann N Y Acad Sci, 638, 474–6 (1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.McAvoy JW, Chamberlain CG, de Iongh RU, Richardson NA and Lovicu FJ: The role of fibroblast growth factor in eye lens development. Ann N Y Acad Sci, 638, 256–74 (1991) [DOI] [PubMed] [Google Scholar]
- 151.Flamme I and Risau W: Induction of vasculogenesis and hematopoiesis in vitro. Development, 116(2), 435–9 (1992) [DOI] [PubMed] [Google Scholar]
- 152.Schwartz SM and Liaw L: Growth control and morphogenesis in the development and pathology of arteries. J Cardiovasc Pharmacol, 21 Suppl 1, S31–49 (1993) [DOI] [PubMed] [Google Scholar]
- 153.Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P and Cao Y: Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med, 9(5), 604–13 (2003) [DOI] [PubMed] [Google Scholar]
- 154.Wafai R, Tudor EM, Angus JA and Wright CE: Vascular effects of FGF-2 and VEGF-B in rabbits with bilateral hind limb ischemia. J Vasc Res, 46(1), 45–54 (2009) [DOI] [PubMed] [Google Scholar]
- 155.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, Vandendriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D and Persico MG: Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med, 7(5), 575–83 (2001) [DOI] [PubMed] [Google Scholar]
- 156.Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, Kroll J, Plaisance S, DeMol M, Bono F, Kliche S, Fellbrich G, Ballmer-Hofer K, Maglione D, Mayr-Beyrle U, Dewerchin M, Dombrowski S, Stanimirovic D, Van Hummelen P, Dehio C, Hicklin DJ, Persico G, Herbert JM, Shibuya M, Collen D, Conway EM and Carmeliet P: Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med, 9(7), 936–43 (2003) [DOI] [PubMed] [Google Scholar]
- 157.Li B, Sharpe EE, Maupin AB, Teleron AA, Pyle AL, Carmeliet P and Young PP: VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovascularization. FASEB J, 20(9), 1495–7 (2006) [DOI] [PubMed] [Google Scholar]
- 158.Fujii T, Yonemitsu Y, Onimaru M, Inoue M, Hasegawa M, Kuwano H and Sueishi K: VEGF function for upregulation of endogenous PlGF expression during FGF-2-mediated therapeutic angiogenesis. Atherosclerosis, 200(1), 51–7 (2008) [DOI] [PubMed] [Google Scholar]
- 159.Nakamura T, Teramoto H and Ichihara A: Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc Natl Acad Sci U S A, 83(17), 6489–93 (1986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A and Comoglio PM: Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol, 119(3), 629–41 (1992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Van Belle E, Witzenbichler B, Chen D, Silver M, Chang L, Schwall R and Isner JM: Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor: the case for paracrine amplification of angiogenesis. Circulation, 97(4), 381–90 (1998) [DOI] [PubMed] [Google Scholar]
- 162.Gerritsen ME, Tomlinson JE, Zlot C, Ziman M and Hwang S: Using gene expression profiling to identify the molecular basis of the synergistic actions of hepatocyte growth factor and vascular endothelial growth factor in human endothelial cells. Br J Pharmacol, 140(4), 595–610 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xin X, Yang S, Ingle G, Zlot C, Rangell L, Kowalski J, Schwall R, Ferrara N and Gerritsen ME: Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am J Pathol, 158(3), 1111–20 (2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sengupta S, Gherardi E, Sellers LA, Wood JM, Sasisekharan R and Fan TP: Hepatocyte growth factor/scatter factor can induce angiogenesis independently of vascular endothelial growth factor. Arterioscler Thromb Vasc Biol, 23(1), 69–75 (2003) [DOI] [PubMed] [Google Scholar]
- 165.Morishita R, Nakamura S, Hayashi S, Taniyama Y, Moriguchi A, Nagano T, Taiji M, Noguchi H, Takeshita S, Matsumoto K, Nakamura T, Higaki J and Ogihara T: Therapeutic angiogenesis induced by human recombinant hepatocyte growth factor in rabbit hind limb ischemia model as cytokine supplement therapy. Hypertension, 33(6), 1379–84 (1999) [DOI] [PubMed] [Google Scholar]