Abstract
Objectives and setting
Across sub-Saharan Africa, urogenital schistosomiasis (UGS), in particular female genital schistosomiasis (FGS), is a significant waterborne parasitic disease, with its direct burden on the sexual and reproductive health (SRH) of sufferers infrequently measured. UGS has an established control plan, which in most endemic regions as in Cameroon, still excludes FGS considerations. Highlighting existent associations between UGS and FGS could increase the management of FGS within UGS interventions. This study seeks to identify current associations among FGS and UGS with some reproductive health indicators, to provide formative information for better integrated control.
Participants
304 females aged 5–69 years were all examined for UGS by urine filtration and microscopy. Among these, 193 women and girls were eligible for clinical FGS assessment based on age (>13). After selective questioning for FGS symptoms, a subgroup of 67 women and girls consented for clinical examination for FGS using portable colposcopy, with observed sequelae classified according to the WHO FGS pocket atlas.
Outcome
Overall UGS and FGS prevalence was measured, with FGS-related/UGS-related reproductive health symptoms recorded. Associations between FGS and UGS were investigated by univariate and multivariate logistic regression analyses.
Results
Overall UGS prevalence was 63.8% (194/304), where FGS prevalence (subgroup) was 50.7% (34/67). FGS manifestation increased significantly with increasing age, while a significant decrease with ascending age was observed for UGS. Lower abdominal pain (LAP) vaginal itches (VI) and coital pain (CP) were identified as the main significant shared symptoms of both FGS and UGS, while LAP with menstrual irregularity (MI) appeared a strong symptomatic indicator for FGS.
Conclusion
LAP, MI, CP and VI are the potential SRH indicators that could be exploited in future for targeting of praziquantel provision to FGS sufferers within primary care, complementary with existing praziquantel distribution for UGS sufferers in Schistosoma haematobium endemic areas.
Keywords: Epidemiology, PARASITOLOGY, Colposcopy, Infection control
Strengths and Limitations of this study
This study used clinical colposcopy, which is the recommended diagnostic method for female genital schistosomiasis (FGS), though not very common within primary healthcare settings in sub-Saharan Africa.
Here, questionnaire approach is used to better capture individual experiences of FGS sufferers within endemic areas for urogenital schistosomiasis (UGS).
Clinical diagnosis of girls younger than 14 (about half of the study participants) was not considered, because of the invasive nature of colposcopy examination, especially as non-invasive clinical diagnostic tools are lacking for examination among this age group within low resource schistosomiasis endemic communities.
Clinical diagnosis for FGS was carried out only on a limited sample.
Assessment for Sexually Transmitted Infections (STIs) among participants are not presented here, whereby such results could complement or clarify FGS diagnosis, considering most sexual and reproductive health-related symptoms for UGS present as sexually transmitted infections and can be misdiagnosed.
Introduction
In endemic areas, a definitive diagnosis of urogenital schistosomiasis (UGS) is established by demonstration of viable Schistosoma haematobium eggs (≥1) in urine or haematuria,1–3 while female genital schistosomiasis (FGS) can be diagnosed visually4 for S. haematobium-induced cervical lesions and small fibrotic nodules known as ‘sandy patches’,5 either with the presence or absence of S. haematobium eggs in urine.4 6 7 While both FGS and UGS are caused by infection with S. haematobium,1 4 8 9 a waterborne blood fluke, each appear to have some unclear epidemiological associations, largely due to insufficient disease surveillance.6 7 10–13 In sub-Saharan Africa where UGS is endemic and can be highly prevalent (>50%),14 15 insufficient or infrequent efforts have been undertaken to document FGS specifically,11 16–18 partly as the clinical skills to do so are lacking and uninformed within primary care.10 While active UGS does not readily predict FGS,6 since FGS can occur without the direct presence of schistosome eggs in urine (a cardinal diagnostic for UGS),18 19 rather FGS often presents with a more chronic time frame where schistosome eggs are trapped within the cervicovaginal surfaces.6 20 21 For some, these trapped eggs can accumulate from very early on in life,1 with enduring and typically hidden sequelae.20 22 Based on several biological determinants such as age,1 the mucosal damage and fibrotic scarring of the vaginal and cervical surfaces from FGS proceeds with increasing duration of UGS6; moreover, FGS-specific sequelae maybe slow to resolve on standard antiparasitic treatment of UGS,23 24 that is, single annual administration of praziquantel at 40 mg/g as used in public health campaigns.6 13 20
In many parts of Africa where surveillance of UGS is limited25 26 and that for FGS largely absent,15 27 there is a clear need to better understand the epidemiological associations between UGS and FGS.14 This particularly so, to support earlier diagnosis of cases of FGS, and individualise praziquantel treatment needs (for individual and context-specific case management)15 28 to better avert their disease progression15; as current interventions against UGS do not specifically target adolescent girls or women.23 29 This gap in treatment coverage28 and surveillance13 30 also has considerable bearing on progress towards elimination of schistosomiasis transmission within disease endemic communities.29
In recent years, FGS-focused research and public health education31 has gained traction in certain countries such as Ghana, Tanzania, Madagascar, Nigeria and Mozambique,8 9 although other countries such as Cameroon, currently lag behind.32 33 Schistosomiasis exists in several regions of Cameroon,34 affecting over 10 million people in rural and urban areas.35 The country has a national coordinated control plan for fairly early interventions during childhood years (from 5 to 14 years old),36 which take advantage of school based intervention platforms,37 38 and in certain settings, community based interventions, where their at-risk status (people or communities dependent on schistosomiasis endemic water bodies, for main water source) is high.21 32 36 39 40 Even with improved (>70%) helminth control among children in the last decade,35 some of the adolescent at-risk populations do not always benefit from praziquantel treatment due to existing policy gaps and programme intervention challenges.15 41 42
To address this treatment deficit, capture missed opportunities and ensure the consideration and apprehension of ensuing FGS manifestations within such already identified subgroups (young girls and women); with better knowledge on the precise associations between UGS and FGS; future control policies and intervention campaigns can be revised to better target at-risk populations.
Here, we sought to clarify existing associations between FGS and UGS, highlighting cardinal symptomologies for scalable detection and targeted control of FGS within UGS endemic areas. This supports the need for a future integrated approach for control of schistosomiasis and limits the ‘gap’ concerning FGS surveillance within current primary care.
Materials and methods
Patient and public involvement
We followed inclusive and participative methods to get overall participant and public involvement. Tailored visits for data collection were carried out according to best practices with local engagement of key community members and local health workers.
Study setting
This study was carried out across a group of girls and women residing in remote communities surrounding the Mape Dam, a known transmission focus for S. haematobium35 40 in the Matta Health Area in Cameroon. Most study participants were involved actively in fishing or other household activity that put them in constant contact with the lake water.33 More than 90% of the population lived less than 200 m to infested water source (the Mape Dam), and more than 75% depended fully on the Mape Dam for household water and for an income-generating activity (fishing).13 The Matta Health Area hosts several remote fishing island communities that surround this man-made water body, and for at least 18 years has witnessed high transmission of S. haematobium with prevalence of UGS greater than 50% among children.43
Study design and procedures
This cross-sectional study was conducted between December 2020 and June 2021. The total population estimate of the study site was 5000,44 where females represented approximately 51.0%. About 54.6% of the population of females were aged 5–69 years. The sample size estimation for this study was based on UGS prevalence (considered as key indicator for the study). With no existing records of UGS prevalence among adults within the Matta Health Area, a hypothesis of UGS endemicity among adults was based on recorded school-age schistosomiasis prevalence (>41% in the last decade) within the Matta Health Area.43 45 In this context made up of primarily fishing communities, more than 80% of adults (both men and women) spent long stretches a day in contact with water (for economic—fishing—or household activity purposes).37 Based on this, we assumed that the UGS prevalence in such communities should be higher among women. Hence, we resorted to an estimate of 55% for UGS prevalence in the Matta Health Area, for our sample size calculation. Considering lake proximity and economic activities, 11 main communities were involved within the Matta Health Area: nine secluded water-locked fishing communities (Islands/fishing camps) with habitations mostly less than 200 m from the lake; and two mainland communities (landlocked) with habitation more than 400 m from the lake.33
Following a simple random sampling technique, on the base of attaining a precision rate of 95% with an error margin of 5%, our initial sample size was estimated using the sample size formula for prevalence studies46 given by n=N×X/(X+N–1), where X=Z2×p×(1–p)/MOE2, and Z=Zα/2 is the critical value of the normal distribution at α/2 (for confidence level of 95%, α is 0.05 and critical value is 1.96), MOE is the margin of error (5%), p is an estimate of UGS prevalence in the study area (fixed at 55%) and N is the population of females in the study area (1400). The Finite Population Correction was applied to the sample size formula. Thus, n=1400×3.8/(3.8+1400–1)=387. With an originally determined sample size of 387, due to logistic and cultural constraints, 304 (78.55%) of target recruitment was reached.33
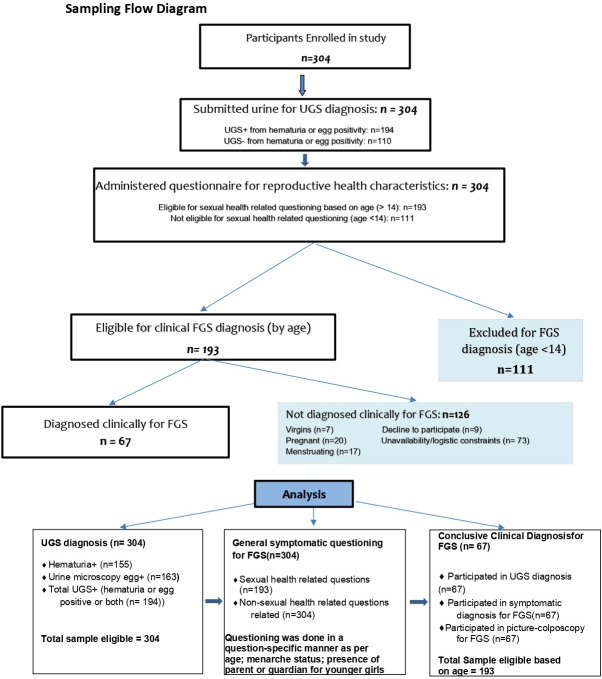
A secondary objective in this study was also to find out reproductive health determinants for FGS. For that, a subsample of 67 females was obtained from the 304 women enrolled in the study (figure 1). Eligibility criteria for this subgroup consisted of the following: being 14 years old (considered as minimum marriage age in this context) and above, not virgin, not menstruating at present, not pregnant and consent/assent from parent or spouse for girls younger than 18. Hence, of the 304 participants enrolled and tested for UGS, 193 were eligible for clinical FGS assessment based on age (figure 1). However, due to participant availability, logistic constraints and consent among others, only 67 among 193 participants were available for clinical FGS diagnosis.
Figure 1.
Study participant selection criteria and numbers with diagnostic methods flow. FGS, female genital schistosomiasis; UGS, urogenital schistosomiasis.
Questionnaires related to sexual and reproductive health (SRH) characteristics were administered to participants based on age and question sensitivity. Hence, varying denominators for different variables.
Due to the secluded nature of study communities (far from healthcare settings) and the preference of a participative nature for recruiting (involvement of formal/informal health workers and some community members), recruitment was contextualised within each community as per the propositions of key community members (including participants themselves).
Within the subgroup for FGS diagnosis, after questioning (questionnaire), consenting participants underwent gynaecological examination by colposcopy with photo documentation by a trained midwife. All participants were recruited and screened within the community, mostly in their homes or a ‘safe’ house prescribed by the women themselves, or the village leader.
Thus, after UGS assessment, a structured questionnaire (see online supplemental file 1) with FGS-related symptoms,33 SRH and sociodemographic questions was administered privately in a one-to-one format to all consenting/assenting participants, mostly based on age and question sensitivity. For girls younger than 14, questions linked to sexual health were avoided and other questioning also depended on parent/guardian availability for aiding/complementing their responses or responding directly for them. For girls/women older than 14, both reproductive and sexual health-related questions were asked where possible. Participants responded to the structured questionnaire and were prompted to discuss further on related symptoms if they wished to share.
bmjopen-2022-063392supp001.pdf (71.5KB, pdf)
SRH-related questions included: sexual activeness, (with age of first encounter or age at marriage), number of children, age of last child, any miscarriage, menstrual irregularities (MIs) or abnormalities (collected as irregular, painful or ceased menstruation), abdominal pain, coital pain (CP) and vaginal itches (VI) with abnormal discharges. Demographic questions asked included: age, level of formal or informal schooling achieved, water contact activities and income-generating activities. Most females encountered during initial sample enrolment were married by age 14, which helped guide the minimum age for the study, in terms of deciphering a general baseline for assessing girls for FGS (through general sexual health-related questions and invasive gynaecological examination). To better explore age-related profiles, three age groups were formed around these context-specific SRH characteristics: adolescence (14–19 years), young adults (20–35 years) and older adults (36+ years). The first age group can be considered to represent active marriage period in this study area, the second, period of high conception probability, and third, post conception period.47
Parasitological and gynaecological examinations
Dipstick diagnosis of microscopic haematuria48 and urine syringe filtration technique with microscopic-based polycarbonate filter examination for urinary eggs were used on a single urine sample for standard UGS detection within this study. At least 10 mL of urine was collected and observed for macrohaematuria, tested for microhaematuria and proteinuria with reagent strips (Siemens Multistix 10 SG), then analysed for S. haematobium eggs, at the local health centre laboratory on the same day of collection. Microscopy for visualisation of schistosome eggs was performed by ×100 magnification using a light compound microscope and stained with Lugol’s iodine. A urine sample was counted positive for UGS on the presence of haematuria3 or at least one terminal-spined ovum seen.49 The number of ova reported where classified as ≥50 (high intensity) or <50 (low intensity).2 Next, consenting eligible girls and women were examined by clinical colposcopy with photo documentation, using a hand-held colposcope (EVA COLPO, Mobile ODT). The obtained images were cross-checked against the WHO FGS pocket atlas5 to record key sequelae. These were then saved in a coded database for the internal validation through blinded evaluation of cervical images from photo colposcopy by external team members, following the cross examination with the WHO Pocket atlas. A minimal clinical indication for FGS was determined on the presence of sandy patches, abnormal blood vessels and/or sandy patches on homogenous yellow areas, in line with the WHO FGS pocket atlas coding5 after cross verification by external specialists.
Statistical analysis
All numerical data collected were first imputed into computer system using the Microsoft excel database and later imported into the R (V.4.0.2) software for statistical analyses. In univariate analysis, frequencies and proportions were reported for sociodemographic, syndromic and clinical variables. In bivariate analysis, Pearson’s χ2 tests were used to test the association between the sociodemographic, clinical and syndromic reproductive health-related variables (which serve as independent variables), against the dependent variables FGS and UGS. To further highlight such dependence, univariate logistic regression analyses was used, with the results presented in the form of unadjusted ORs. To identify most relevant variables among the reproductive health-related independent variables associated to each of UGS and FGS, multivariate logistic regressions analyses were used, with the results presented in the form of adjusted ORs (AORs), alongside 95% CI and p values based on the Wald’s test. To fit the models, only factors significantly related to the outcomes at a 25% level of significance in the univariate models were included. Multicollinearity between independent variables in the initial multivariate models was evaluated using the vif function in the car R package and our knowledge on how the variables were measured. The step function in the R package stats was applied to the resulting multivariate models after correcting for multicollinearity to select the ‘best’ fitting model. The global significance of variables in the final models were evaluated based on analysis of deviance tables using the anova function in the stats R package. In all, the level of significance was set at p value<0.05.
Results
General participant characteristics
A total sample population of 304 females were enrolled. All 304 participants were assessed for UGS but not all participants were diagnosed with FGS. Participants were aged from 5 to 69 years (193 of reproductive age, >13 and <70, with mean±SD age of 28±12.7). Also, 88.16% of participants were dependent on and lived within a proximity of ≤200 m to the Mape lake (Island communities), and the remaining 11.82% came from Mainland communities, which were further from the lake (>400 m), having alternative water sources (wells and stand taps) and involved in farming alone without fishing activities. A prevalence of 63.8% (194/304) for UGS was recorded from egg prevalence or haematuria. Furthermore, 27.30% showed proteinuria, and a 51.0% prevalence (155/304) was recorded for haematuria (with 19 showing macrohaematuria). Microhaematuria sensitivity and specificity was calculated against egg positivity, with a specificity and sensitivity of 80.00% (95% CI: 73.58% to 86.42%) and 73.83% (95% CI: 66.91% to 80.75%), respectively. The geometric mean egg count was 33.1 (range: 2–1220) among which 36.2% had heavy (≥50 eggs/10 mL of urine) infection, while 63.8% had light (>50 eggs/10 mL of urine) infection. Macrohaematuria was strongly related to egg density categories (χ2=17.7; p<0.001), where cases of macrohaematuria were directly related to heavy egg load (93.2%). More than half of the study population reporting not having received treatment with praziquantel in more than a year (see table 1). Reported SRH syndromes included miscarriages (58.89%), lower abdominal pain (LAP) (56.95%), lower back pain (44.59%), CP (45.98%), coital bleeding (37.93), VI (68%), abnormal vaginal discharge (42.6%) and MIs (47.74%), all seen to be comparatively higher among participants, compared with stress incontinence (19.47) (see table 1).
Table 1.
General characteristics of all study participants (sociodemographic, syndromic, clinical)
| Variable | Category | Women, n | Percentage |
| Demographic | |||
| Age (groups) (n=304/304*) |
<14 | 111 | 36.51 |
| (14–19) | 62 | 20.39 | |
| (20–35) | 83 | 27.3 | |
| 36+ | 48 | 15.79 | |
| Menarche (n=304/304*) | Pre | 98 | 32.24 |
| Post | 206 | 67.76 | |
| Age at marriage (n=178/193*) |
(13–14) | 71 | 39.89 |
| (15–17) | 88 | 49.44 | |
| 18+ | 19 | 10.67 | |
| Number of children (n=181/193*) |
0 | 44 | 24.31 |
| (1–3) | 72 | 39.78 | |
| (4–6) | 37 | 20.44 | |
| 7+ | 28 | 15.47 | |
| Age of last child (n=130/137*) |
0+ | 10 | 7.69 |
| (1–3) | 67 | 51.54 | |
| (4–8) | 15 | 11.54 | |
| 7+ | 38 | 29.23 | |
| Treatment with praziquantel (n=304/304*) |
<12 months | 1 | 0.3 |
| >12 months | 292 | 96.1 | |
| Never | 11 | 3.6 | |
| Economic activity (n=241/304*) |
Fishing (with/without farming) | 211 | 87.5 |
| Farming (without fishing) | 30 | 12.4 | |
| Proximity to lake (n=304/304*) |
<200 m | 268 | 88.16 |
| >400 m | 36 | 11.84 | |
| Syndromic | |||
| Lower abdominal pain (n=223/304*) | Yes | 127 | 56.95 |
| Coital pain (n=174/193*) | Yes | 80 | 45.98 |
| Coital bleeding (n=174/193*) | Yes | 66 | 37.93 |
| Vaginal itches (n=225/304*) | Yes | 153 | 68.00 |
| Vaginal discharge (n=214/304*) | Yes | 90 | 42.06 |
| External genital itch (n=206/304*) | Yes | 86 | 41.75 |
| Lower back pain (n=223/304*) | Yes | 99 | 44.59 |
| Stress incontinence (n=226/304*) | Yes | 44 | 19.47 |
| Menstrual irregularities (n=199/206*) | Yes | 95 | 47.74 |
| Miscarriages (n=180/193*) | 0 | 74 | 41.11 |
| 1+ | 106 | 58.89 | |
| Clinical | |||
| Parasitemia (n=304/304*) | 0 | 141 | 46.38 |
| (1–50) | 104 | 34.21 | |
| 50+ | 59 | 19.41 | |
| Haematuria (n=304/304*) | + | 155 | 51.0 |
| Proteinuria (n=304/304*) | + | 83 | 27.30 |
*The eligible sample size for each variable. For age of marriage (n=178/193*) for example, 178 out of the 193 eligible women gave information of their marriage age, meaning 15 women had missing information for the variable.
UGS characteristics among sample population
Table 2 presents the relationship between UGS as a dependent factor and each of the sociodemographic and reported reproductive health characteristics based on χ2 tests of independence and univariate logistic regression. The results indicate that the chances of UGS infection among women who lived more than 400 m from the lake were 0.36 (95% CI: 0.17 to 0.72) times than that of women who lived less than 200 m to the lake, implying that significant odds of being infected with UGS was seen with closer lake proximity. A significant decrease in chances of infection with UGS was observed with increasing age. Relative to girls<14 years, girls between 14 and 19 years had a 0.46 (95% CI: 0.22 to 0.95) odds of having UGS, as opposed to 0.29 (95% CI: 0.15 to 0.54) odds for adults ranging from 20 to 35 years, and 0.09 (95% CI: 0.04 to 0.19) odds for women older than 35 years. All reported reproductive health syndromes showed significant relationship with UGS, except for stress incontinence (UOR 1.72 (95% CI: 0.87 to 3.54) p=0.1287). In effect, women with LAP, CP, vaginal itch, MI and coital bleeding showed significantly higher odds (table 2) of UGS.
Table 2.
Relations between UGS and each sociodemographic and syndromic variable in the study sample
| Χ2 test of independence | Univariate logistic regression | ||||||||
| Variables | Category | N |
UGS– n (%) |
UGS+ n (%) |
P value | Unadjusted OR (95% CI) | P value | ||
| Age group (n=304) | <14 | 111 | 20 (18.2) | 91 (46.9) | 1 | ||||
| [14–19] | 62 | 20 (18.2) | 42 (21.6) | 0.46 (0.22 to 0.95) | 0.0352 | ||||
| [20–35] | 83 | 36 (32.7) | 47 (24.2) | 0.29 (0.15 to 0.54) | 0.0002 | ||||
| 36+ | 48 | 34 (30.9) | 14 (7.2) | <0.0001 | 0.09 (0.04 to 0.19) | <0.0001 | |||
| Number of children (n=181) | 0 | 44 | 15 (18.1) | 29 (29.6) | 1 | ||||
| [1–3] | 72 | 33 (39.8) | 39 (39.8) | 0.61 (0.28 to 1.32) | 0.2143 | ||||
| [4–6] | 37 | 16 (19.3) | 21 (21.4) | 0.68 (0.27 to 1.67) | 0.3994 | ||||
| 7+ | 28 | 19 (22.9) | 9 (9.2) | 0.0462 | 0.25 (0.09 to 0.66) | 0.0063 | |||
| Age of last child (n=130) | 0+ | 10 | 4 (6) | 6 (9.5) | 1 | ||||
| [1–3] | 67 | 33 (49.3) | 34 (54) | 0.69 (0.16 to 2.62) | 0.5863 | ||||
| [4–6] | 15 | 4 (6) | 11 (17.5) | 1.83 (0.33 to 10.6) | 0.4862 | ||||
| 7+ | 38 | 26 (38.8) | 12 (19) | 0.0323 | 0.31 (0.07 to 1.27) | 0.1082 | |||
| Miscarriages (n=180) | 0 | 74 | 41 (48.8) | 33 (34.4) | 1 | ||||
| 1 | 52 | 19 (22.6) | 33 (34.4) | 2.16 (1.05 to 4.52) | 0.0382 | ||||
| 2+ | 54 | 24 (28.6) | 30 (31.2) | 0.1055 | 1.55 (0.77 to 3.17) | 0.2216 | |||
| Lower abdominal pain (n=223) | Yes | 127 | 42 (45.2) | 85 (65.4) | 0.0039 | 2.29 (1.33 to 3.98) | 0.0028 | ||
| Coital pain (n=174) | Yes | 80 | 25 (30.5) | 55 (59.8) | 0.0001 | 3.39 (1.82 to 6.43) | 0.0001 | ||
| Coital bleeding (n=174) | Yes | 66 | 19 (23.2) | 47 (51.1) | 0.0002 | 3.46 (1.82 to 6.79) | 0.0002 | ||
| Vaginal itches (n=225) | Yes | 153 | 51 (53.7) | 102 (78.5) | 0.0001 | 3.14 (1.77 to 5.67) | 0.0001 | ||
| Abnormal vaginal discharge (n=214) | Yes | 90 | 27 (29) | 63 (52.1) | 0.0008 | 2.66 (1.51 to 4.76) | 0.0008 | ||
| Lower back pain (n=222) | Yes | 99 | 34 (36.6) | 65 (50.4) | 0.0551 | 1.76 (1.03 to 3.06) | 0.0416 | ||
| Stress incontinence (n=226) | Yes | 44 | 14 (14.7) | 30 (22.9) | 0.1729 | 1.72 (0.87 to 3.54) | 0.1287 | ||
| Genital itches (n=206) | Yes | 86 | 26 (28.6) | 60 (52.2) | 0.0007 | 2.73 (1.53 to 4.94) | 0.0008 | ||
| Menstrual irregularity (n=199) | Yes | 95 | 29 (33) | 66 (59.5) | 0.0002 | 2.98 (1.68 to 5.4) | 0.0002 | ||
| Proximity to lake | <20 m | 268 | 89 (80.9) | 179 (92.3) | 1 | ||||
| >200 m | 36 | 21 (19.1) | 15 (7.7) | 0.0051 | 0.36 (0.17 to 0.72) | 0.0042 | |||
UGS, urogenital schistosomiasis.
Multivariate logistic regression model for reporting SRH risk factors related to UGS
After including all variables significantly related to UGS at a 25% level in a multivariate model, multicollinearity issues were suspected between LAP and lower back pain, and external genital itch and VI. However, considering genital itch responses were most often related to vaginal itch or misreported by respondents due to their literal similarity in Pidgin English or Fulbe used during questioning, we resorted to keeping only VI in the model. Similarly for lower back pain and LAP because of the similarity in responses, but with a more comprehensive responding for LAP, lower back pain was removed. The resulting ‘best’ fitting model included age group, LAP and CP as the most significant SRH risk factors for UGS (table 3). In this result, we also observed a decreasing trend in number of cases of UGS with increasing age. Also, the odds of infection in women with LAP was 6.42 (95% CI: 2.85 to 15.68) times than that for women without the pain. The odds of infection in women with CP was 2.16 (95% CI: 1.05 to 4.46) times than that for women without the pain.
Table 3.
Possible risk factors for UGS among the sociodemographic and SRH included in the study
| SRH indicator | Category | Adjusted OR (95% CI) |
P value |
| Age group | (14–19) | 1.0 | |
| (20–35) | 0.28 (0.10 to 0.70) | 0.0087 | |
| 36+ | 0.11 (0.04 to 0.31) | 0.0001 | |
| Lower abdominal pain | No | 1.0 | |
| Yes | 6.42 (2.85 to 15.68) | 0.0000 | |
| Coital pain | No | 1.0 | |
| Yes | 2.16 (1.05 to 4.46) | 0.0362 |
A multivariate logistic regression model on the effects of sexual and reproductive health factors significantly related to UGS.
FGS, female genital schistosomiasis; UGS, urogenital schistosomiasis.
FGS Characteristics among study participants (subgroup)
Of the total number of participants examined for FGS after UGS (n=67), 40 were confirmed to have ova patent UGS and 34 for FGS, on the presence of homogenous yellow sandy patches, grainy sandy patches and abnormal blood vessels (figure 2). A breakdown of UGS/FGS within the subset of 67 women examined for FGS showed: 26 FGS+/UGS+; 8 FGS+/UGS−; 14 FGS−/UGS+; and 19 FGS−/UGS−. Related reproductive health syndromes (as reported in UGS), similarly, were all found to have some association (p<0.05) with FGS manifestation among females (table 4), except for stress incontinence. Of import among these, MIs or abnormality, also found with UGS, was seen to have 7.9 times higher odds of affecting women with FGS than women without FGS (table 4). Back pain was seen to significantly affect women with FGS manifestations than was the case with UGS. Similarly, odds of having FGS manifestations were seen to ascend with age (table 4), unlike UGS which was significant with descending age. LAP, MI and lower back pain showed the highest odds of manifesting among women with FGS.
Figure 2.
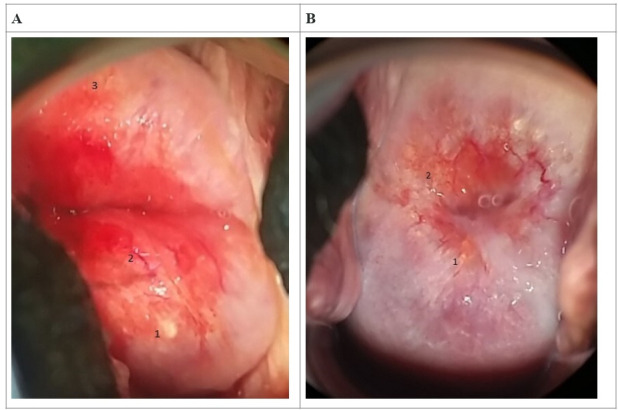
Imagery of female genital schistosomiasis pathology of the cervix with reported lesions (magnification of ×4). (A) 1—rubbery papule, 2—abnormal blood vessel, 3—homogeneous sandy patch (30-year-old woman, +UGS, +lower abdominal pain, +menstrual irregularity). (B) 1,2—grainy sandy patches (45-year-old woman, −UGS, +lower abdominal pain, +menstrual irregularity).
Table 4.
Relations between FGS and sociodemographic and syndromic variables in the sub-group of girls and women diagnosed for FGS
| Χ2 test of independence | Univariate logistic regression | ||||||
| Variables (n=67) | Category | N | FGS–, n (%) | FGS+, n (%) | P value | Unadjusted OR (95% CI) | P value |
| Age group | [14–19] | 19 | 15 (45.5) | 4 (11.8) | 1 | ||
| [20–35] | 29 | 11 (33.3) | 18 (52.9) | 6.14 (1.73 to 26.1) | 0.0077 | ||
| 36+ | 19 | 7 (21.2) | 12 (35.3) | 0.0091 | 6.43 (1.62 to 30.35) | 0.0116 | |
| Age at marriage | [13–14] | 23 | 10 (30.3) | 13 (38.2) | 1 | ||
| [15–17] | 38 | 22 (66.7) | 16 (47.1) | 0.56 (0.19 to 1.58) | 0.2765 | ||
| 18+ | 6 | 1 (3) | 5 (14.7) | 0.1286 | 3.85 (0.51 to 79.99) | 0.2510 | |
| Number of children | 0 | 16 | 12 (36.4) | 4 (11.8) | 1 | ||
| [1–3] | 22 | 12 (36.4) | 10 (29.4) | 2.5 (0.64 to 11.23) | 0.2024 | ||
| [4–6] | 15 | 4 (12.1) | 11 (32.4) | 8.25 (1.79 to 46.95) | 0.0102 | ||
| 7+ | 14 | 5 (15.2) | 9 (26.5) | 0.0363 | 5.4 (1.19 to 28.98) | 0.0357 | |
| Age of last child | 0+ | 1 | 1 (4.8) | 0 (0) | 1 | ||
| [1–3] | 27 | 14 (66.7) | 13 (46.4) | Inf (0 to Inf) | 0.9965 | ||
| [4–6] | 4 | 0 (0) | 4 (14.3) | Inf (0 to Inf) | 0.9937 | ||
| 7+ | 17 | 6 (28.6) | 11 (39.3) | 0.1199 | Inf (0 to Inf) | 0.9963 | |
| Miscarriages | 0 | 26 | 18 (54.5) | 8 (23.5) | 1 | ||
| 1 | 22 | 8 (24.2) | 14 (41.2) | 3.94 (1.22 to 13.78) | 0.0256 | ||
| 2+ | 19 | 7 (21.2) | 12 (35.3) | 0.0362 | 3.86 (1.14 to 14.19) | 0.0343 | |
| Lower abdominal pain | Yes | 47 | 15 (45.5) | 32 (94.1) | 0 | 19.2 (4.74 to 131.08) | 0.0003 |
| Coital pain | Yes | 32 | 10 (30.3) | 22 (64.7) | 0.0071 | 4.22 (1.55 to 12.16) | 0.0058 |
| Coital bleeding | Yes | 29 | 8 (24.2) | 21 (61.8) | 0.0029 | 5.05 (1.82 to 15.19) | 0.0026 |
| Vaginal itches | Yes | 49 | 18 (54.5) | 31 (91.2) | 0.0009 | 8.61 (2.44 to 40.95) | 0.0021 |
| Vaginal discharge | Yes | 32 | 9 (27.3) | 23 (67.6) | 0.0014 | 5.58 (2.01 to 16.67) | 0.0013 |
| Back pain | Yes | 41 | 12 (36.4) | 29 (85.3) | 0 | 10.15 (3.31 to 36.45) | 0.0001 |
| Stress incontinence | Yes | 10 | 0 (0) | 10 (29.4) | 0.0009 | Inf (0 to Inf) | 0.9927 |
| Genital itch | Yes | 32 | 9 (27.3) | 23 (67.6) | 0.0014 | 5.58 (2.01 to 16.67) | 0.0013 |
| Menstrual irregularities | Yes | 34 | 9 (27.3) | 25 (73.5) | 0.0002 | 7.41 (2.61 to 23) | 0.0003 |
| Proximity | <20 m | 60 | 30 (90.9) | 30 (88.2) | 1 | 1 | |
| >200 m | 7 | 3 (9.1) | 4 (11.8) | 1 | 1.33 (0.27 to 7.25) | 0.7212 | |
FGS, female genital schistosomiasis.
Multivariate logistic regression model for reporting SRH risk factors related to FGS
Similar to UGS, a multivariate model was constructed with all variables significantly related to FGS at a 25% level. As well, multicollinearity checks revealed lower back pain and genital itch (for same reasons) with the variables age group, CP, VI and LAP (table 5) retained in the ‘best’ fitting model.
Table 5.
Possible risk factors among SRH for FGS
| SRH indicator | Category | Adjusted OR (95% CI) |
P value |
| Age group | (14–19) | ||
| (20–35) | 20.15 (2.92 to 240.94) | 0.0061 | |
| 36+ | 41.29 (4.16 to 946.69) | 0.0054 | |
| Coital pain | No | 1.0 | |
| Yes | 10.44 (2.12 to 90.91) | 0.0105 | |
| Vaginal itches | No | 1.0 | |
| Yes | 12.50 (1.92 to 128.77) | 0.0151 | |
| Lower abdominal pain | No | 1.0 | |
| Yes | 28.80 (3.36 to 578.24) | 0.0081 |
A multivariate logistic regression model on the effects of FGS on sexual and reproductive health factors.
FGS, female genital schistosomiasis; SRH, sexual and reproductive health.
UGS, FGS and associations with reproductive health characteristics
Age, number of children, age of last child, menstrual abnormality, miscarriages and LAP were identified as possible reproductive health factors associated with S. haematobium infection and were used within this study. Generally, both FGS and UGS were not significantly (p value>0.05) related to number of children, age of last child and miscarriages. In multivariate logistic regression models, after selection of the best fitting models, the results show that the most significant risk factors for UGS are age group, LAP and CP (table 4), whereas age group and LAP, CP and VI were identified as the most significant risk factors for FGS (table 5). Chances of FGS manifestations among women with LAP was AOR 9.5 (95% CI: 1.7 to 81.8) times than that of women without the pain (table 5). Analysis of deviance tables for both best fitting models (FGS and UGS), with p values based on likelihood ratio tests are reported in online supplemental file 2.
bmjopen-2022-063392supp002.pdf (15.6KB, pdf)
Discussion
Understanding the risks and associations of UGS and FGS, especially within different contexts of women’s health,41 50 sheds greater light on the disease epidemiology, which could foster improved and coordinated control measures both locally and nationally.41 Furthermore, precisely documenting existing associations between both UGS and FGS could clarify further the need for precision mapping of schistosomiasis in endemic regions, for formulating a better targeted integrated response.48 Though a non-significant association was observed between egg intensity in urine and FGS from the onset of this study (table 2), parasitemia association has been shown to be misleading in UGS7 12 13 from several other studies13 and reports on FGS, particularly when only a single urine sample is examined which is usually the case for population-based surveillance.6 Considering this questionnaire (for symptoms),10 21 as well as visual examination of cervix and vaginal walls by colposcopy,12 18 51 offers an added strength to single sample urinalysis for detection of FGS, as carried out in this study, and several others.10 18 The possibility of the presence of FGS in UGS populations has been often raised,8 21with projections of about 360 million girls and women possibly having UGS,15 but today it is thought that at least 56 million adolescent girls and women are suffering from FGS.15 17 Our results seem to show an even higher rate of FGS among the UGS-infected population with an approximate FGS/UGS ratio of 34/40. Our study, given our application of portable colposcopy, is the first formal attempt to document the pathology of FGS in a primary care setting in Cameroon.
Elsewhere, the clinical pathology of FGS has been described resulting from the complex inflammatory responses to antigens released by adult worms and viable eggs,6 14 which persists until sometime after adult worms are stopped egg-laying or are destroyed by praziquantel.24 Thereafter, various signs and symptoms (or effects) may present months or even years after treatment.23 42
From present results, and within the general literature,15 17 one of such effects noted is an effect on menstrual health. More than half of women within the study who reported poor menstrual health (FGS=73.5%; UGS=54.6%), either as irregular, painful or seized menstruation, were found to either shed eggs in urine (positive for UGS) or were positive for FGS, but without eggs in urine; showing more women positive for FGS reporting abnormal menstruation, than for UGS. This confirms recent analysis17 33 and suggests strong linkages between menstrual health management and FGS,15 17 an under-researched area. This can be credited to the fact that symptoms perhaps diminished after a while with UGS (maybe as a result of treatment in mass drug administration campaigns), but later resurface with more chronic sequelae of FGS,17 and with more dire symptoms and negative impact on menstrual health.17 33 In our study context, postmenarche females already faced a substantial challenge with limited access to hygienic material and information on menstrual health management, typically relying on self-made clothes and absorbent plant leaves during menstruation, due to lack of finances or general knowledge.
Still related to FGS and menstrual health, narratives from a previous study33 which used qualitative probing showed women having manifestations of FGS and not shedding eggs in urine, gave a history of having lived in their earlier years in heavily infested S. haematobium foci. This explained their later manifestation of FGS symptoms, even after having moved away to a less infested area, with more than 90% limit in fresh water contact.33 This is similar to our findings, where lake proximity was seen to be not very significant to FGS manifestation (table 4), same as egg shedding, still pointing to early-in-life infection and later chronicity. Significant difference in menstrual abnormalities among UGS positive women (n=22 (56.4%)) and UGS negative women (n=12 (44.4%)), alerts to future chronicity of FGS after UGS, especially if not managed with more readily available praziquantel treatment(s).42 52
On its own, LAP observed significant association (in adjusted and unadjusted regression models) in both UGS and FGS. The chances of having LAP were significantly higher in females with either FGS (94.1%) or UGS (84.2%). Similarly with CP and VI, these reported as key indicators for UGS and FGS, directing early diagnosis of UGS and future FGS in endemic communities, thereby promoting the verticalisation of control strategies for both diseases.
Another trend observed in this study is that intensity of infection or egg patent prevalence (for UGS) reduces while chronic disease or morbidity for FGS increases as women age. The chances of FGS after UGS increased significantly (AOR 6.43, 95% CI 1.62 to 30.35, p=0.0091) as women aged (36+). Similar findings have been reported in other studies in different geographical locations6 14 53 54 and recently in this area33; emphasising on the level of present intensity for UGS, and possible future occurrence of FGS.14 54 UGS at a younger age will in some cases manifest into FGS when the female is older,14 causing more intense gynaecological symptoms and effects. This offers a possible guiding tool for better control policies, related to early diagnosis and treatment.41 55 This surpasses need alone for school-based MDAs,13 28 56 but considers and encourages individual therapy in different contexts for FGS (and MGS).6
Although only a few among the extensive list of reproductive health determinants54 were identified in this study to be statistically significant, where mostly reported symptoms were collected, clinical and biological examinations carried out, enabled confirmation of how future self-reported symptoms with UGS and FGS might be best used.33 These results support the advocated need for further attention on post-transmission schistosomiasis,57 and also, the availability of praziquantel in lowest level (health areas and community) healthcare for individual therapy,54 as well as treatment from a younger age,15 28 41 52 buoyed with the recent development of paediatric praziquantel.58
Study limitations
Though described as gold standard,49 active UGS was only detected through observation of eggs in urine samples by microscopic-based polycarbonate filter examination, as well as recommended dipstick assays for urinary haematuria detection. Alternative molecular assays such as PCR for schistosome detection in human serum and urine samples12 were not considered for added sensitivity for UGS and FGS (in vaginal lavage analysis).3 59 Though recommended,12 21 only visual examination through inspection for lesions on the cervix, the fornices and the vaginal walls with a colposcope5 51 and screening with questionnaires10 was considered in the detection of FGS in this study. Lastly, clinical diagnosis of FGS was carried out only on a limited subgroup of females, where more precise conclusions may be obtained by using an appropriate sample size.
Conclusion
While millions of women in Cameroon have UGS, its epidemiological connection with FGS is not fully appreciated, which creates an unfortunate knowledge holdup for effective control at the public health level. In our chosen study location, which is broadly typical for endemic areas of UGS in Cameroon,21 34 36 40 strong epidemiological associations between UGS and FGS were found against certain key sexual and reproductive determinants: age, LAP and menstrual health. This formative knowledge could be used to tackle and ultimately prevent FGS, with a more targeted integrated control for UGS in Cameroon and elsewhere in endemics areas for UGS globally. This study further adds detailed insight into the connection of FGS and UGS within primary care in endemic communities, denoting those with cardinal symptomologies more explicitly for scalable detection and targeted control of FGS within UGS endemic areas.
Supplementary Material
Acknowledgments
The authors most specifically acknowledge and appreciate the immense contribution of the reviewers for this revised and very much updated version of this manuscript. The authors recognise here the National Programme for the fight against Schistosomiasis and Soil-Transmitted Helminths in Cameroon (PNLSHi) for their effort in the control of schistosomiasis in Cameroon. Thanks to the contribution of Dr Wepnje Godlove and Marlene Nstinda Tchoffo in this research, and to all health workers and study participants at the Matta Health Area for their support and sacrifice. The authors express appreciation to Dr Savadogo Bonaventure and Dr Patty Ngassa of the MTN-OCEAC Project for their technical advice and coordination.
Footnotes
Twitter: @masongbye
Contributors: CMM and RS conceptualised the study and planned the methodology. ENA, VAG and CMM carried out field investigation. NBF, CMM, VAG, RS, ASO and ENA analysed and interpreted the data for this manuscript. CMM acquired funding for study and wrote the original draft of the manuscript. RS supervised the study and was a major contributor in the conceptualisation and writing of the manuscript. ENA coordinated field activities within the Malanteoun Health District. ENA, NBF, VAG and ASO reviewed and edited the manuscript. CMM is responsible for the overall content and guarantor for this manuscript. All authors approved the final version of the paper before submission.
Funding: This study is funded through a fellowship offered by “l'Organisation de Coordination pour la lutte contre les Endémies en Afrique Centrale”, financed by the Ministry for Economic Cooperation and Development (BMZ) of the Federal Republic of Germany, through the KfW (German Development Bank). “Projet Lutte Contre Les Maladies Tropicales Négligées en Afrique Centrale” (MTN) BMZ-Nr 2015.69.227 BMZ 2016.68.797. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Materials and methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data generated or analysed during this study are included in this published article (and its online supplemental files).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants. Ethical clearance for this study was provided by the Cameroon National Ethics committee on Human Health Research (Ref N° 2020/07/1266/CE/CNERSH/SP), with administrative authorisations from both the Regional Delegation of Public Health for the West region of Cameroon (Ref N° 679/L/MINSANTE/SG/DRSPO/CBF) and the district Health Office of the Malantouen Health District (Ref N° 078/L/MINSANTE/DRSPO/DSMLT). Informed written and oral consent was obtained from all participants for parasitological and gynaecological examinations. For participants<18 years, parents, husbands, or guardian gave informed permission and assent was obtained from the participants. Privacy and confidentiality of medical information were protected during and after the study.
References
- 1.Freer JB, Bourke CD, Durhuus GH, et al. Schistosomiasis in the first 1000 days. Lancet Infect Dis 2018;18:e193–203. 10.1016/S1473-3099(17)30490-5 [DOI] [PubMed] [Google Scholar]
- 2.WHO . Prevention and control of schistosomiasis and soil transmitted helminths. Geneva: WHO; 2002. Available: http://apps.who.int/iris/bitstream/handle/10665/42588/WHO_TRS_912.pdf;jsessionid=297C20DDFCBFFF9F9AAE9DF120C0F834?sequence=1 [Google Scholar]
- 3.Utzinger J, Becker SL, van Lieshout L, et al. New diagnostic tools in schistosomiasis. Clin Microbiol Infect 2015;21:529–42. 10.1016/j.cmi.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 4.Søfteland S, Sebitloane MH, Taylor M, et al. A systematic review of handheld tools in lieu of colposcopy for cervical neoplasia and female genital schistosomiasis. Int J Gynaecol Obstet 2021;153:190–9. 10.1002/ijgo.13538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO . Female genital schistosomiasis: A pocket atlas for clinical health-care professionals. Geneva: WHO Press, 2015. Available: https://www.who.int/publications/i/item/9789241509299 [Google Scholar]
- 6.Kjetland EF, Leutscher PDC, Ndhlovu PD. A review of female genital schistosomiasis. Trends Parasitol 2012;28:58–65. 10.1016/j.pt.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 7.Poggensee G, Kiwelu I, Saria M, et al. Schistosomiasis of the lower reproductive tract without egg excretion in urine. Am J Trop Med Hyg 1998;59:782–3. 10.4269/ajtmh.1998.59.782 [DOI] [PubMed] [Google Scholar]
- 8.Christinet V, Lazdins-Helds JK, Stothard JR, et al. Female genital schistosomiasis (FGS): from case reports to a call for concerted action against this neglected gynaecological disease. Int J Parasitol 2016;46:395–404. 10.1016/j.ijpara.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 9.Shams M, Khazaei S, Ghasemi E, et al. Prevalence of urinary schistosomiasis in women: a systematic review and meta-analysis of recently published literature (2016-2020). Trop Med Health 2022;50:12. 10.1186/s41182-022-00402-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekpo UF, Odeyemi OM, Sam-wobo SO, et al. Female genital schistosomiasis (FGS) in ogun state, Nigeria: a pilot survey on genital symptoms and clinical findings. Parasitology Open 2017;3. 10.1017/pao.2017.11 [DOI] [Google Scholar]
- 11.Hotez PJ, Engels D, Gyapong M, et al. Female genital schistosomiasis. N Engl J Med 2019;381:2493–5. 10.1056/NEJMp1914709 [DOI] [PubMed] [Google Scholar]
- 12.Galappaththi-Arachchige HN, Holmen S, Koukounari A, et al. Evaluating diagnostic indicators of urogenital Schistosoma haematobium infection in young women: a cross sectional study in rural South Africa. PLoS One 2018;13:e0191459. 10.1371/journal.pone.0191459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiegand RE, Fleming FM, de Vlas SJ, et al. Defining elimination as a public health problem for schistosomiasis control programmes: beyond prevalence of heavy-intensity infections. Lancet Glob Health 2022;10:e1355–9. 10.1016/S2214-109X(22)00287-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hegertun IEA, Sulheim Gundersen KM, Kleppa E, et al. S. haematobium as a common cause of genital morbidity in girls: a cross-sectional study of children in South Africa. PLoS Negl Trop Dis 2013;7:e2104. 10.1371/journal.pntd.0002104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UNAIDS . No more neglect:female genital schistosomiasis and HIV. 2019. Available: www.unaids.org/en/resources/documents/2019/
- 16.Hotez P. Speaking of medicine and health. In: PLOS BLOGS. 2013. Available: https://speakingofmedicine.plos.org/2013/05/06/female-genital-schistosomiasis-fgs-sub-saharan-africas-secret-scourge-of-girls-and-women/ [Google Scholar]
- 17.Stothard JR, Odiere MR, Phillips-Howard PA. Connecting female genital schistosomiasis and menstrual hygiene initiatives. Trends Parasitol 2020;36:410–2. 10.1016/j.pt.2020.02.007 [DOI] [PubMed] [Google Scholar]
- 18.Bustinduy AL, Randriansolo B, Sturt AS, et al. An update on female and male genital schistosomiasis and a call to integrate efforts to escalate diagnosis, treatment and awareness in endemic and non-endemic settings: the time is now. Adv Parasitol 2022;115:1–44. 10.1016/bs.apar.2021.12.003 [DOI] [PubMed] [Google Scholar]
- 19.Majangara Karaga R. Concealed urogenital schistosomiasis causing chronic pelvic pain: a case report. Clin Case Rep 2021;9:1860–4. 10.1002/ccr3.3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stothard JR. Female genital schistosomiasis-icebergs of morbidity ahead? Trends Parasitol 2012;28:174–5. 10.1016/j.pt.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 21.Campbell SJ, Stothard JR, O’Halloran F, et al. Urogenital schistosomiasis and soil-transmitted helminthiasis (STh) in Cameroon: an epidemiological update at barombi mbo and barombi kotto crater lakes assessing prospects for intensified control interventions. Infect Dis Poverty 2017;6:49. 10.1186/s40249-017-0264-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King CH. Parasites and poverty: the case of schistosomiasis. Acta Trop 2010;113:95–104. 10.1016/j.actatropica.2009.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kjetland EF, Mduluza T, Ndhlovu PD, et al. Genital schistosomiasis in women: a clinical 12-month in vivo study following treatment with praziquantel. Trans R Soc Trop Med Hyg 2006;100:740–52. 10.1016/j.trstmh.2005.09.010 [DOI] [PubMed] [Google Scholar]
- 24.Treatment of female genital schistosomiasis (FGS) with praziquantel: A proof-of-concept study (NCT04115072). NIH: ClinicalTrials.gov archive, 2020. [Google Scholar]
- 25.Brindley PJ, Hotez PJ. Break out: urogenital schistosomiasis and Schistosoma haematobium infection in the post-genomic era. PLoS Negl Trop Dis 2013;7:e1961. 10.1371/journal.pntd.0001961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Secor WE, Colley DG. When should the emphasis on schistosomiasis control move to elimination? TropicalMed 2018;3:85. 10.3390/tropicalmed3030085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kukula VA, MacPherson EE, Tsey IH, et al. A major hurdle in the elimination of urogenital schistosomiasis revealed: identifying key gaps in knowledge and understanding of female genital schistosomiasis within communities and local health workers. PLoS Negl Trop Dis 2019;13:e0007207. 10.1371/journal.pntd.0007207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faust CL, Osakunor DNM, Downs JA, et al. Schistosomiasis control: leave no age group behind. Trends in Parasitology 2020;36:582–91. 10.1016/j.pt.2020.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organisation . WHO guideline on control and elimination of human schistosomiasis. WHO; 2022. Available: https://www.who.int/publications/i/item/9789240041608 [PubMed] [Google Scholar]
- 30.Bergquist R, Yang G-J, Knopp S, et al. Surveillance and response: tools and approaches for the elimination stage of neglected tropical diseases. Acta Trop 2015;141(Pt B):229–34. 10.1016/j.actatropica.2014.09.017 [DOI] [PubMed] [Google Scholar]
- 31.SCI Foundation . FAST PACKAGE PROJECT. 2021. Available: https://schistosomiasiscontrolinitiative.org/projects/fast-research-project
- 32.Ndassi VD, Anchang-Kimbi JK, Sumbele IUN, et al. The epidemiological status of urogenital schistosomiasis among reproductive aged individuals in the tiko health area- a semi-urban setting in the Mount Cameroon area. PLOS Negl Trop Dis 2021;15:e0008978. 10.1371/journal.pntd.0008978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masong MC, Wepnje GB, Marlene NT, et al. Female genital schistosomiasis (FGS) in Cameroon: a formative epidemiological and socioeconomic investigation in eleven rural fishing communities. PLOS Glob Public Health 2021;1:e0000007. 10.1371/journal.pgph.0000007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tchuenté LAT, N’goran EK. Schistosomiasis and soil-transmitted helminthiasis control in Cameroon and Côte d’Ivoire: implementing control on a limited budget. Parasitology 2009;136:1739–45. 10.1017/S0031182009005988 [DOI] [PubMed] [Google Scholar]
- 35.National Program for the control of Schistosomiaisis and intestinal helminthiases MoPHC . Towards elimination of schistosomiasis and soil-transmitted helminthiasis and in cameroon: A road map for paradigm shift 2021-2030. Yaoundé: MINSANTE, 2022. [Google Scholar]
- 36.National Program for the control of Schistosomiaisis and intestinal helminthiases . Progress report cameroon 2013-2019. Yaoundé: MINSANTE, 2019. [Google Scholar]
- 37.World health organization. global update on implementation of preventive chemotherapy against neglected tropical diseases in 2018. Weekly Epidemiological Record 2019;94:425–38. [Google Scholar]
- 38.Crompton DWT, WHO . World health organization. preventive chemotherapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. Geneva: World Health Organization, 2006. [Google Scholar]
- 39.Christine Masong M, Ozano K, Tagne MS, et al. Achieving equity in UHC interventions: who is left behind by neglected tropical disease programmes in cameroon? Glob Health Action 2021;14:1886457. 10.1080/16549716.2021.1886457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Njunda AL, Ndzi EN, Assob JCN, et al. Prevalence and factors associated with urogenital schistosomiasis among primary school children in barrage, magba sub-division of Cameroon. BMC Public Health 2017;17:618. 10.1186/s12889-017-4539-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deworming adolescent girls and women of reproductive age . Policy brief [press release]. Geneva: WHO2022, [Google Scholar]
- 42.Nour NM. Schistosomiasis: health effects on women. Rev Obstet Gynecol 2010;3:28–32. [PMC free article] [PubMed] [Google Scholar]
- 43.MINSANTE . Schistosomiasis distribution in malanteoun health district ntdmaps.org. 2018. Available: https://ntdmaps.org/
- 44.National Institute of Statistics Cameroon . Socio-economic statistics of cameroon, 2015 cameroon2004-2014. 2015. Available: https://cameroon.opendataforafrica.org/SESC2015/socio-economic-statistics-of-cameroon-2015
- 45.Leonard KTD. n.d. Some haematological parameters among urinary schistosomiasis-malaria coinfected children in suburb of malentouen health district, west region cameroon. IJTDH;2020:34–44. 10.9734/ijtdh/2020/v41i730295 [DOI] [Google Scholar]
- 46.Daniels W. Biostastistics: A foundation for analysis in the health sciences. New York: Wiley & sons, 1999. [Google Scholar]
- 47.Gnoth C, Godehardt E, Frank-Herrmann P, et al. Definition and prevalence of subfertility and infertility. Hum Reprod 2005;20:1144–7. 10.1093/humrep/deh870 [DOI] [PubMed] [Google Scholar]
- 48.Colley DG, Bustinduy AL, Secor WE, et al. Human schistosomiasis. Lancet 2014;383:2253–64. 10.1016/S0140-6736(13)61949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organisation . Schistosomiasis WHO: WHO. 2022. Available: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis
- 50.World Health Oganization . Regional office for the western pacific. People Centered Health care: A policy framework, 2007. [Google Scholar]
- 51.Kjetland EF, Norseth HM, Taylor M, et al. Classification of the lesions observed in female genital schistosomiasis. Int J Gynaecol Obstet 2014;127:227–8. 10.1016/j.ijgo.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 52.Bustinduy AL, Friedman JF, Kjetland EF, et al. Expanding praziquantel (PZQ) access beyond mass drug administration programs: paving a way forward for a pediatric PZQ formulation for schistosomiasis. PLOS Negl Trop Dis 2016;10:e0004946. 10.1371/journal.pntd.0004946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chetty A, Omondi MA, Butters C, et al. Impact of helminth infections on female reproductive health and associated diseases. Front Immunol 2020;11:577516. 10.3389/fimmu.2020.577516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stothard JR, Stanton MC, Bustinduy AL, et al. Diagnostics for schistosomiasis in Africa and Arabia: a review of present options in control and future needs for elimination. Parasitology 2014;141:1947–61. 10.1017/S0031182014001152 [DOI] [PubMed] [Google Scholar]
- 55.Richardson ST, Franklin AL, Rome ES, et al. Global health: urogenital schistosomiasis in the adolescent girl. J Pediatr Adolesc Gynecol 2016;29:326–32. 10.1016/j.jpag.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 56.Oluwole AS, Ekpo UF, Nebe OJ, et al. The new who guideline for control and elimination of human schistosomiasis: implications for the schistosomiasis elimination programme in Nigeria. Infect Dis Poverty 2022;11:111. 10.1186/s40249-022-01034-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giboda M, Bergquist R, Utzinger J. Schistosomiasis at the crossroad to elimination: review of eclipsed research with emphasis on the post-transmission agenda. Trop Med Infect Dis 2022;7:55. 10.3390/tropicalmed7040055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Consortuim . Contract manufacturing agreement signed for production of new pediatric treatment for schistosomiasis. 2021. Available: http//:https://www.pediatricpraziquantelconsortium.org/
- 59.Sturt AS, Webb EL, Phiri CR, et al. Genital self-sampling compared with cervicovaginal lavage for the diagnosis of female genital schistosomiasis in Zambian women: the BILHIV study. PLOS Negl Trop Dis 2020;14:e0008337. 10.1371/journal.pntd.0008337 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-063392supp001.pdf (71.5KB, pdf)
bmjopen-2022-063392supp002.pdf (15.6KB, pdf)
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its online supplemental files).