Abstract
Background
Gestational diabetes with onset or first recognition during pregnancy is an increasing problem worldwide. Myo‐inositol, an isomer of inositol, is a naturally occurring sugar commonly found in cereals, corn, legumes and meat. Myo‐inositol is one of the intracellular mediators of the insulin signal and correlates with insulin sensitivity in type 2 diabetes. The potential beneficial effect of improving insulin sensitivity suggests that myo‐inositol may be useful for women in preventing gestational diabetes. This is an update of a review first published in 2015.
Objectives
To assess if antenatal dietary supplementation with myo‐inositol is safe and effective, for the mother and fetus, in preventing gestational diabetes.
Search methods
We searched the Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, WHO ICTRP (17 March 2022) and the reference lists of retrieved studies.
Selection criteria
We included published and unpublished randomised controlled trials (RCTs) including cluster‐RCTs and conference abstracts, assessing the effects of myo‐inositol for the prevention of gestational diabetes in pregnant women. We included studies that compared any dose of myo‐inositol, alone or in a combination preparation, with no treatment, placebo or another intervention. Quasi‐randomised and cross‐over trials were not eligible. We excluded women with pre‐existing type 1 or type 2 diabetes.
Data collection and analysis
Two review authors independently assessed studies for inclusion, assessed risk of bias and extracted the data. We checked the data for accuracy. We assessed the certainty of the evidence using the GRADE approach.
Main results
We included seven RCTs (one conducted in Ireland, six conducted in Italy) reporting on 1319 women who were 10 weeks to 24 weeks pregnant at the start of the studies. The studies had relatively small sample sizes and the overall risk of bias was low.
For the primary maternal outcomes, meta‐analysis showed that myo‐inositol may reduce the incidence of gestational diabetes (risk ratio (RR) 0.53, 95% confidence interval (CI) 0.31 to 0.90; 6 studies, 1140 women) and hypertensive disorders of pregnancy (RR 0.34, 95% CI 0.19 to 0.61; 5 studies, 1052 women). However, the certainty of the evidence was low to very low. For the primary neonatal outcomes, only one study measured the risk of a large‐for‐gestational‐age infant and found myo‐inositol was associated with both appreciable benefit and harm (RR 1.40, 95% CI 0.65 to 3.02; 1 study, 234 infants; low‐certainty evidence). None of the included studies reported on the other primary neonatal outcomes (perinatal mortality, mortality or morbidity composite).
For the secondary maternal outcomes, we are unclear about the effect of myo‐inositol on weight gain during pregnancy (mean difference (MD) ‐0.25 kilogram (kg), 95% CI ‐1.26 to 0.75 kg; 4 studies, 831 women) and perineal trauma (RR 4.0, 95% CI 0.45 to 35.25; 1 study, 234 women) because the evidence was assessed as being very low‐certainty. Further, myo‐inositol may result in little to no difference in caesarean section (RR 0.91, 95% CI 0.77 to 1.07; 4 studies, 829 women; low‐certainty evidence). None of the included studies reported on the other secondary maternal outcomes (postnatal depression and the development of subsequent type 2 diabetes mellitus). For the secondary neonatal outcomes, meta‐analysis showed no neonatal hypoglycaemia (RR 3.07, 95% CI 0.90 to 10.52; 4 studies; 671 infants; very low‐certainty evidence). However, myo‐inositol may be associated with a reduction in the incidence of preterm birth (RR 0.35, 95% CI 0.17 to 0.70; 4 studies; 829 infants). There were insufficient data for a number of maternal and neonatal secondary outcomes, and no data were reported for any of the long‐term childhood or adulthood outcomes, or for health service utilisation outcomes.
Authors' conclusions
Evidence from seven studies shows that antenatal dietary supplementation with myo‐inositol during pregnancy may reduce the incidence of gestational diabetes, hypertensive disorders of pregnancy and preterm birth. Limited data suggest that supplementation with myo‐inositol may not reduce the risk of a large‐for‐gestational‐age infant.
The current evidence is based on small studies that were not powered to detect differences in outcomes such as perinatal mortality and serious infant morbidity. Six of the included studies were conducted in Italy and one in Ireland, which raises concerns about the lack of generalisability to other settings. There is evidence of inconsistency among doses of myo‐inositol, the timing of administration and study population. As a result, we downgraded the certainty of the evidence for many outcomes to low or very low certainty.
Further studies for this promising antenatal intervention for preventing gestational diabetes are encouraged and should include pregnant women of different ethnicities and varying risk factors. Myo‐inositol at different doses, frequency and timing of administration, should be compared with placebo, diet and exercise, and pharmacological interventions. Long‐term follow‐up should be considered and outcomes should include potential harms, including adverse effects.
Keywords: Adult; Female; Humans; Pregnancy; Diabetes Mellitus, Type 2; Diabetes Mellitus, Type 2/prevention & control; Diabetes, Gestational; Diabetes, Gestational/prevention & control; Diabetes, Gestational/therapy; Dietary Supplements; Hypertension, Pregnancy-Induced; Inositol; Inositol/therapeutic use; Insulin Resistance; Perinatal Death; Premature Birth
Plain language summary
Taking myo‐inositol as a dietary supplement during pregnancy to prevent the development of gestational diabetes
Key messages
Women who develop gestational diabetes have a higher risk of experiencing complications during pregnancy and birth, as well as developing diabetes later on in life. The babies of mothers who have gestational diabetes can be larger than they should be and might be injured at birth. These babies are at risk of diabetes, even as young children or young adults. The number of women being diagnosed with gestational diabetes is increasing around the world, so finding simple and cost‐effective ways to prevent women from developing this condition is important.
Myo‐inositol is a naturally‐occurring sugar found in cereals, corn, green vegetables, and meat, that has a role in the body's sensitivity to insulin.
What did we want to find out?
We wanted to find out if myo‐inositol is an effective antenatal dietary supplement for preventing gestational diabetes in pregnant women.
What did we do?
We searched for studies that compared myo‐inositol (given alone or in combination with another treatment) with no treatment or another treatment. We compared and summarized the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found seven studies on 1319 women who were 10 weeks to 24 weeks pregnant.
Main results
We are unclear whether supplementation with myo‐inositol is associated with a reduction in the rate of gestational diabetes. However, myo‐inositol may be associated with a reduction of hypertensive disorders of pregnancy. We are unclear whether myo‐inositol supplementation decreases the number of babies who were born large for gestational age.
The studies did not provide any information about the number of babies that died (either before birth or shortly afterwards), depression, or subsequent type 2 diabetes after delivery. There were no maternal adverse effects of therapy in the five studies that reported on this outcome; the other two studies did not mention this.
We are unclear about the effect of supplementation with myo‐inositol on weight gain during pregnancy or on a baby with low blood glucose levels. This review did not find any impact on other outcomes, such as the risk of having a caesarean section or a large baby. This may be due to the studies being too small to detect differences in these outcomes and the outcomes not being reported by all studies. However, myo‐inositol may be associated with a reduction in the rate of preterm birth compared with the control group.
The included studies did not report on many other relevant mother and baby outcomes, nor did they have any data relating to longer‐term outcomes for the mother or infant, or the cost to the health services.
There is not enough evidence to support that giving myo‐inositol as a dietary supplement during pregnancy, prevents gestational diabetes. However, myo‐inositol may prevent hypertensive (high blood pressure) disorders of pregnancy and preterm birth. Further large, well‐designed, randomised controlled trials are required to assess the effectiveness of myo‐inositol in preventing gestational diabetes and improving other health outcomes for mothers and their babies.
What are the limitations of this evidence?
We have little confidence in the evidence because there were not enough studies to be certain about the results and many of our review outcomes were not reported in the studies that we identified. The studies were also limited to populations from high‐income settings and so results may not be applicable to other populations. The studies also had some limitations on how they reported the methods.
How up to date is this evidence?
This evidence is up‐to‐date to December 2022.
Summary of findings
Summary of findings 1. Myo‐inositol for preventing gestational diabetes: maternal outcomes.
| Antenatal supplementation with myo‐inositol for preventing gestational diabetes | ||||||
|
Patient or population: pregnant women (women with pre‐existing type 1 or type 2 diabetes are NOT included)
Intervention: myo‐inositol Setting: hospital Comparison: folic acid or placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with myo‐inositol | |||||
| Gestational diabetes mellitus | Study population | RR 0.53 (0.31 to 0.90) | 1140 (6 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c |
GDM diagnosed using IADPSG 2010 criteria Random‐effects model |
|
| 217 per 1000 | 115 per 1,000 (67 to 196) |
|||||
| Weight gain during pregnancy | Comparator | The mean weight gain during pregnancy in the intervention group was 0.25 kg lower (1.26 kg fewer to 0.76 kg more) | ‐ | 831 (4 RCTs) | ⊕⊝⊝⊝ Very lowb,c,d,e | Random‐effects model |
| Hypertensive disorders of pregnancy | Study population | RR 0.34 (0.19 to 0.61) | 1052 (5 RCTs) | ⊕⊕⊝⊝ Lowc,f | Random‐effects model | |
| 86 per 1,000 | 29 per 1,000 (16 to 53) | |||||
| Caesarean section | Study population | RR 0.91 (0.77 to 1.07) | 829 (4 RCTs) | ⊕⊕⊝⊝ Lowc,g | ||
| 430 per 1,000 | 391 per 1,000 (331 to 460) | |||||
| Perineal trauma | Study population | RR 4.00 (0.45 to 35.25) | 234 (1 RCT) |
⊕⊝⊝⊝ Very lowh,i,j | ||
| 9 per 1,000 | 34 per 1,000 (4 to 301) |
|||||
| Postnatal depression | See comments | Not estimable | (0 studies) | No data reported this outcome in any of the included studies | ||
| Development of subsequent type 2 diabetes mellitus | See comments | Not estimable | (0 studies) | No data reported this outcome in any of the included studies | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
a. Downgraded (‐1) for serious limitations in study design: due to unclear risk of selection bias in two of the six included studies; five of the six included studies were at high risk of performance bias; two of the six included studies were at high risk of detection bias; one study was at high risk of attrition bias.
b. Downgraded (‐1) for serious inconsistency; considerable heterogeneity, possible due to different study populations.
c. Downgraded (‐1) for serious indirectness; only one of the included studies was conducted outside Italy, and the Italian studies only included white women, the generalisability of findings is limited.
d. Downgraded (‐1) for serious limitations in study design: all studies were at high risk of performance bias; one study was at high risk of detection bias.
e. Downgraded (‐1) for serious imprecision; evidence of imprecision with wide confidence intervals crossing the line of no effect.
f. Downgraded (‐1) for serious limitations in study design: all studies were at high risk of performance bias; two studies were at high risk of detection bias.
g. Downgraded (‐1) for serious limitations in study design: all studies were at high risk of performance bias. One study was at high risk of detection bias, and insufficient evidence to judge detection bias and subsequent judgement of unclear risk of bias. Due to insufficient evidence to judge allocation concealment in two studies and subsequent judgement of unclear risk of bias. Due to insufficient evidence to judge attrition bias in two studies and subsequent judgement of unclear risk of bias.
h. Downgraded (‐1) for serious limitations in study design: the study was at high risk of performance bias and detection bias for lack of blinding.
i. Downgraded (‐1) for serious Indirectness: only one study conducted in Ireland reported this outcome.
j. Downgraded (‐1) for serious imprecision: wide confidence intervals with very low event rates.
Summary of findings 2. Myo‐inositol for preventing gestational diabetes: infant, child and adult outcomes.
| Antenatal supplementation with myo‐inositol for preventing gestational diabetes | ||||||
|
Patient or population: infants of pregnant women Setting: hospital Intervention: myo‐inositol Comparison: folic acid or placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with myo‐inositol | |||||
| Large‐for‐gestational age | Study population | RR 1.40 (0.65 to 3.02) | 234 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | ||
| 85 per 1000 | 120 per 1000 (56 to 258) |
|||||
| Perinatal mortality (stillbirth and neonatal mortality) | See comments | Not estimable | (0 studies) | No data reported this outcome in any of the included studies | ||
| Composite of serious neonatal outcomes | See comments | not estimable | (0 studies) | No data reported this outcome in any of the included studies | ||
| Neonatal hypoglycaemia | Study population | RR 3.07 (0.90 to 10.52) | 671 (4 RCTs) | ⊕⊝⊝⊝ Very lowc,d,e | ||
| 9 per 1,000 | 27 per 1000 (8 to 91) | |||||
| Adiposity | See comments | not estimable | (0 studies) | No data reported this outcome in any of the included studies | ||
| Diabetes | See comments | not estimable | (0 studies) | No data reported this outcome in any of the included studies | ||
| Neurosensory disability | See comments | not estimable | (0 studies) | No data reported this outcome in any of the included studies | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
a. Downgraded (‐1) for serious limitations in study design: the study was at high risk of performance bias and detection bias for lack of blinding.
b. Downgraded (‐1) for serious indirectness: only one study conducted in Ireland reported this outcome.
c. Downgraded (‐1) for serious limitations in study design: all studies were at high risk of performance bias; one study was at high risk of detection bias.
d. Downgraded (‐1) for serious indirectness: only one of the included studies was conducted outside Italy, and the Italian studies only included Caucasian women. Thus, the generalisability of findings is limited.
e. Downgraded (‐1) for serious imprecision: evidence of imprecision with wide confidence intervals crossing the line of no effect.
Background
Description of the condition
Gestational diabetes is defined as any degree of glucose intolerance with onset or first recognition during pregnancy (Alberti 1998). Gestational diabetes can lead to complications for affected women and their babies, making it crucial that effective strategies for its prevention are found.
Screening for, and diagnosis of gestational diabetes, is usually undertaken between 24 and 28 weeks' of pregnancy. However, screening regimes vary from country to country, with some countries selectively screening based on risk factors (NICE 2015), and other countries using universal screening of all pregnant women (Nankervis 2013). If thresholds for the oral glucose challenge test (OGCT) are exceeded, a diagnostic oral glucose tolerance test (OGTT) is used to confirm diagnosis, or a diagnostic OGTT can be used without screening by OGCT (MoH 2014).
A number of risk factors are associated with developing gestational diabetes (Nankervis 2013):
previous gestational diabetes;
previously elevated blood glucose level;
ethnicity: south and southeast Asian, Aboriginal, Pacific Islander, Māori, Middle Eastern, African;
age 40 years or over;
family history of diabetes mellitus (first‐degree relative with diabetes mellitus or a sister with gestational diabetes);
obesity, especially body mass index (BMI) greater than 35 kg/m2;
previous macrosomia (baby with birthweight greater than 4500 g or greater than 90th percentile);
polycystic ovarian syndrome;
medications: corticosteroids, antipsychotics;
pregnancy weight gain.
Some studies have reported an increasing prevalence of gestational diabetes (Ferrara 2007; Zhu 2016). As many as 50% of women with gestational diabetes will develop type 2 diabetes within five years of the index pregnancy (Kim 2002; Vounzoulaki 2020). Gestational diabetes increases the risk of serious injury at birth, the likelihood of caesarean delivery, and the incidence of newborn intensive care unit (NICU) admission (Ali 2011). Infants of women with gestational diabetes are at increased risk of developing obesity, impaired glucose tolerance, and diabetes as children or young adults (Boney 2005; Pettitt 1983; Pettitt 1988; Silverman 1998).
Description of the intervention
Both non‐pharmacological and pharmacological interventions have been used to try to prevent gestational diabetes
Metformin, an oral anti‐diabetic drug in the biguanide class, is the first‐line drug of choice for the treatment of type 2 diabetes (Nankervis 2013). Metformin has been used to prevent gestational diabetes in pregnant women with a history of polycystic ovary syndrome (PCOS) with contrasting results (Glueck 2008; Tang 2012). A randomised trial on the effect of metformin on obese pregnant women found that while fasting glucose and insulin were lower at 28 weeks' gestation in the metformin group, there was no difference in the risk of developing gestational diabetes, by either International Association of Diabetes and Pregnancy Study Groups (IADPSG) or World Health Organization (WHO) criteria, between those women who received metformin and those who received placebo (Chiswick 2015).
Myo‐inositol, an isomer of inositol, is commonly found in cereals, legumes and nuts (Croze 2013). It is a nutrient the body requires for cell membrane formation and cellular reactions to environmental messages (Croze 2013). Myo‐inositol is one of the intracellular mediators of the insulin signal and is correlated with insulin sensitivity in type 2 diabetes (Kennington 1990; Suzuki 1994). Due to its role as a second messenger, myo‐inositol has many benefits. When used as a co‐treatment in people with subclinical hypothyroidism and autoimmune thyroiditis, myo‐inositol aided maintenance of euthyroidism (normal production of thyroid hormone; Nordio 2013). Myo‐inositol has been associated with an improvement in a range of conditions. These include: premenstrual dysphoric disorder (PMDD), a mood disorder disrupting the social or occupational life, or both, of affected women (Carlomagno 2011); symptoms of PCOS, a medical condition characterised by insulin resistance (Papaleo 2007); insulin sensitivity and ovulatory function in young women affected by PCOS (Genazzani 2008; Nestler 1999); hyperandrogenism in women with PCOS (Minozzi 2008); and increased number and quality of oocytes in women undergoing in vitro fertilisation (IVF) treatment for a previous history of infertility (Unfer 2011).
Antenatal supplementation with myo‐inositol for the prevention of gestational diabetes is novel, and whether myo‐inositol is viewed as a nutritional supplement or as a medicine requiring prescription, seems to vary in different parts of the world.
How the intervention might work
Given these beneficial effects on improving insulin sensitivity, myo‐inositol may be useful for women with gestational diabetes. A retrospective review of 46 pregnant women treated with myo‐inositol compared with 37 controls described it as safe during the pre‐pregnancy and early pregnancy period when used in insulin‐resistant conditions (D'Anna 2012). No women reported any side effects of treatment.
Why it is important to do this review
Gestational diabetes is an increasing problem worldwide. To date, three Cochrane Reviews on the prevention of gestational diabetes have been conducted. In Dietary advice in pregnancy for preventing gestational diabetes mellitus, Tieu 2017 concluded that while a low glycaemic index (GI) diet was beneficial for some outcomes for the mother (lower maternal fasting glucose concentration) and child (reduction in large‐for‐gestational‐age infants, lower ponderal index), the evidence is limited. Similarly, in Exercise for pregnant women for preventing gestational diabetes mellitus, Han and colleagues concluded that there is limited evidence to support exercise during pregnancy for the prevention of glucose intolerance or gestational diabetes (Han 2012). Bain and colleagues assessed the effects of physical exercise in combination with dietary advice for pregnant women for preventing gestational diabetes, and health consequences for the mother and her infant/child (Bain 2015). They found no clear differences in outcomes between women receiving diet and exercise interventions compared with those receiving no intervention. Thus, identification of effective preventive measures for gestational diabetes remains of great importance. This is an update of a review first published in 2015, that found that myo‐inositol taken during pregnancy may prevent the development of gestational diabetes, but further trials were required (Crawford 2015). Since then further trials have been published that may be eligible for inclusion in this review.
Objectives
To assess if antenatal dietary supplementation with myo‐inositol is safe and effective, for the mother and fetus, in preventing gestational diabetes.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised controlled trials (RCTs) including conference abstracts assessing the effects of myo‐inositol for the prevention of gestational diabetes. We planned to include cluster‐RCTs, but we did not identify any. We excluded quasi‐randomised trials and cross‐over trials.
Types of participants
We included pregnant women but excluded women with pre‐existing type 1 or type 2 diabetes.
Types of interventions
Any dose of myo‐inositol in pregnancy, alone or in a combination preparation, for the purpose of preventing gestational diabetes. We included studies where such intervention was compared with no treatment, placebo or another intervention.
Types of outcome measures
Studies that met the above inclusion criteria were included regardless of whether they reported on the following outcomes for the review.
Primary outcomes
Maternal outcomes
Gestational diabetes (diagnostic criteria as defined in individual studies)
Hypertensive disorders of pregnancy (including pre‐eclampsia, eclampsia, pregnancy‐induced hypertension)
Neonatal outcomes
Large‐for‐gestational age (birthweight greater than the 90th centile; or as defined by individual study)
Perinatal mortality (stillbirth and neonatal mortality)
Mortality or morbidity composite (variously defined by trials, e.g. infant death, shoulder dystocia, bone fracture or nerve palsy)
Secondary outcomes
Maternal outcomes
Caesarean section
Placental abruption
Induction of labour
Perineal trauma
Postpartum hemorrhage
Postpartum infection
Weight gain during pregnancy
Adherence to the intervention (as defined by study authors)
Behaviour changes associated with the intervention (as defined by study authors)
Relevant biomarker changes associated with the intervention (e.g. adiponectin, free fatty acids, triglycerides, high‐density lipoproteins (HDL), low‐density lipoproteins (LDL), insulin)
Sense of well‐being and quality of life
Views of the intervention
Breastfeeding (e.g. at discharge, six weeks postpartum)
Adverse effects of intervention
Long‐term maternal outcomes
Postnatal depression
Postnatal weight retention or return to pre‐pregnancy weight
Body mass index (BMI)
Gestational diabetes in a subsequent pregnancy
Type 1 diabetes mellitus
Type 2 diabetes mellitus
Impaired glucose tolerance
Cardiovascular health (as defined by trialists, including blood pressure (BP), hypertension, cardiovascular disease, metabolic syndrome)
Infant outcomes
Stillbirth
Neonatal mortality
Gestational age at birth
Preterm birth (less than 37 weeks' gestation and less than 32 weeks' gestation)
Apgar score (less than seven at five minutes)
Macrosomia
Small‐for‐gestational age
Birthweight and birthweight z‐score
Head circumference and head circumference z‐score
Length and length z‐score
Ponderal index
Adiposity
Shoulder dystocia
Bone fracture
Nerve palsy
Respiratory distress syndrome
Hypoglycaemia (variously defined)
Hyperbilirubinaemia
Childhood outcomes
Weight and weight z‐score
Height and height z‐score
Head circumference and head circumference z‐score
Adiposity (e.g. as measured by BMI, skinfold thickness)
Blood pressure
Type 1 diabetes mellitus
Type 2 diabetes mellitus
Impaired glucose tolerance
Dyslipidaemia or metabolic syndrome
Neurodisability
Educational achievement
Adulthood outcomes
Weight
Height
Adiposity (e.g. as measured by BMI, skinfold thickness)
Cardiovascular health (as defined by study authors, including BP, hypertension, cardiovascular disease, metabolic syndrome)
Type 1 diabetes mellitus
Type 2 diabetes mellitus
Impaired glucose tolerance
Dyslipidaemia or metabolic syndrome
Employment, education and social status/achievement
Health services cost
Number of hospital or health professional visits (e.g. midwife, obstetrician, physician, dietitian, diabetic nurse)
Number of antenatal visits or admissions
Length of antenatal stay
Neonatal intensive care unit (NICU) admission
Length of postnatal stay (mother)
Length of postnatal stay (baby)
Costs to families associated with the management provided
Costs associated with the intervention
Cost of maternal care
Cost of offspring care
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (17 March 2022).
The Register is a database containing over 34,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register, including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of hand searched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) which includes centralised searches of the WHO International Clinical Trials Registry Platform (ICTRP);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
These search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above are reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics) and is then added to the Register.
The Information Specialist searched the Register for this review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included, Excluded, Awaiting Classification or Ongoing).
In addition, we searched ClinicalTrials.gov and the WHO ICTRP for unpublished, planned and ongoing trial reports (17 March 2022). The search terms used are given in Appendix 1.
Searching other resources
We searched reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
Selection of studies
Three review authors (SM, LL and CC) independently assessed all potential studies identified from the search strategy for inclusion. We resolved any disagreement through discussion. We created a study flow diagram to map out the number of records identified, included and excluded (Figure 1).
1.
Study flow diagram for updated review
Screening eligible studies for scientific integrity or trustworthiness
Two review authors evaluated all studies that initially met our inclusion criteria against predefined criteria to determine which studies, based on available information, were deemed to be sufficiently untrustworthy to be excluded. We used the following criteria.
Research governance
No prospective trial registration for studies published after 2010 without plausible explanation.
When requested, study authors refused to share the protocol and or ethics approval letter.
Study authors refused to engage in communication with the Cochrane Review authors.
Study authors refused to provide individual patient data (IPD) upon request with no justifiable reason.
Baseline characteristics
Characteristics of the study participants being too similar (distribution of mean (SD) excessively narrow or excessively wide, as noted by Carlisle 2017).
Feasibility
Implausible numbers (e.g. 500 women with severe cholestasis of pregnancy recruited in 12 months).
(Close to) zero losses to follow‐up without plausible explanation.
Results
Implausible results (e.g. massive risk reduction for main outcomes with small sample size).
Unexpectedly even numbers of women ‘randomised’ including a mismatch between the numbers and the methods (e.g. if they say no blocking was used but still end up with equal numbers, or they say they used blocks of four, but the final numbers differ by six).
We excluded studies assessed as being potentially high risk. Where a study was classified as high risk for one or more of the above criteria, we attempted to contact the study authors to address any possible lack of information or concerns. If adequate information remained unavailable, we kept the study in Studies awaiting classification and we reported the reasons and communications with the study author (or lack of), in detail.
The process used is described in Figure 2.
2.
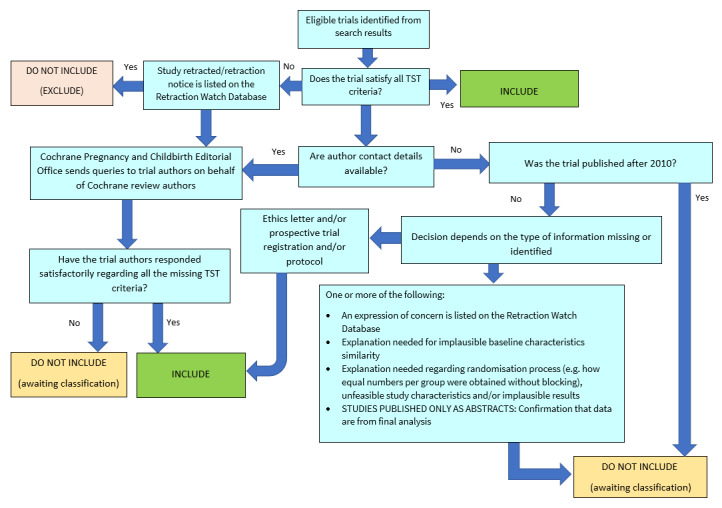
Applying the trustworthiness screening tool criteria. TST: Trustworthiness Screening Tool.
Abstracts
We only included data from abstracts if, in addition to the trustworthiness assessment, the study authors confirmed in writing that the data to be included in the review had come from the final analysis and would not change. If such information was not available or provided, the study remained in Studies awaiting classification (as above).
Data extraction and management
We designed a form to extract data based on the Cochrane Pregnancy and Childbirth Group's data extraction form. For eligible studies, two review authors (SM, LL, JA and CC) independently extracted the data using the agreed form. We resolved discrepancies through discussion. We entered data into Review Manager software (Review Manager 2020) and checked for accuracy. When information regarding any of the above was unclear, we attempted to contact study authors of the original reports to provide further details.
Assessment of risk of bias in included studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). We resolved any disagreement by discussion.
Random sequence generation (checking for possible selection bias)
For each included study we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
Allocation concealment (checking for possible selection bias)
For each included study we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias
Blinding of participants and personnel (checking for possible performance bias)
For each included study we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
Blinding of outcome assessment (checking for possible detection bias)
For each included study we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods used to blind outcome assessment as:
low, high or unclear risk of bias.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each outcome or class of outcomes in each included study, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the study authors, we re‐included missing data in the analyses which we undertook.
We assessed the methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
unclear risk of bias.
Selective reporting (checking for reporting bias)
For each included study we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
Other bias (checking for bias due to problems not covered by the domains above)
For each included study we described any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
Overall risk of bias
We made explicit judgments about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). With reference to the domains above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias by undertaking sensitivity analyses (see Sensitivity analysis).
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratios (RR) with 95% confidence intervals (CIs).
Continuous data
For continuous data where outcomes were measured on the same scale, we presented the mean difference (MD) with 95% CIs. For studies that measured the same outcome on different scales, we planned to report the standardised mean difference (SMD) and 95% CIs.
Unit of analysis issues
Cluster‐RCTs
We did not identify any cluster‐RCTs for inclusion in this review. If we identify cluster‐RCTs for inclusion in future updates of this review, we will include them in the analyses along with individually‐randomised studies. We will make adjustments using the methods described in sections 16.3.4 and 16.3.6 in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021), using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar study or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. We will consider it reasonable to combine the results from both cluster‐RCTs and individually‐randomised studies if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomization unit is considered to be unlikely.
Multiple pregnancy
There may be unit of analysis issues that arise when women randomised have a multiple pregnancy. We present maternal data as per woman randomised and neonatal data as per infant.
Multiple arm studies
In future updates of this review, where a study has multiple intervention arms, we will avoid 'double counting' of participants by combining groups to create a single pair‐wise comparison if possible. Where this is not possible, we will split the 'shared' group into two or more groups with smaller sample size and include two or more (reasonably independent) comparisons.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat (ITT) basis; that is, we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether they received the allocated intervention. The denominator for each outcome in each study was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
We did not undertake investigation of reporting biases because we included only seven studies. In future updates of this review, if 10 or more studies are included in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager (RevMan) software (Review Manager 2020). We used fixed‐effect meta‐analyses for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect (i.e. where studies were examining the same intervention, and the studies’ populations and methods were judged sufficiently similar). If there was sufficient clinical heterogeneity to suggest that the underlying treatment effects differed between studies, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across studies was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects, and we discussed the clinical implications of treatment effects differing between studies. If the average treatment effect was not clinically meaningful, we did not combine studies.
Where we used random‐effects analyses, we present the results as the average treatment effect with 95% CIs, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses where data were available. We considered whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it.
We planned to conduct the following subgroup analyses:
women with polycystic ovary syndrome (PCOS) versus women without PCOS;
obese women versus non‐obese women;
dosage: high versus low dose;
myo‐inositol alone or in combination versus non myo‐inositol combination;
commencement of myo‐inositol supplementation: pre‐pregnancy versus first trimester.
However, we were unable to split the participant data into subgroups, and none of the included studies commenced supplementation with myo‐inositol pre‐pregnancy.
We planned to restrict subgroup analysis to this review's primary outcomes.
In future versions of this review, we will assess subgroup differences by interaction tests available within RevMan (Review Manager 2020). We will report the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
We had insufficient studies to conduct sensitivity analysis for this review. If in future updates there are sufficient studies for analysis, and there is evidence of significant heterogeneity for primary outcomes, we will explore heterogeneity by using the quality of the included studies. We will compare studies that have low risk of bias for allocation concealment with those judged to be of unclear or high risk of bias.
Summary of findings and assessment of the certainty of the evidence
We assessed the certainty of the evidence using the GRADE approach, as outlined in the GRADE handbook, in order to assess the certainty of the body of evidence relating to the following outcomes for the main comparisons. We produced two summary of findings tables for seven maternal outcomes and seven neonatal, child and adult outcomes.
Maternal
Gestational diabetes
Weight gain during pregnancy
Hypertensive disorders of pregnancy (including pre‐eclampsia, eclampsia, and pregnancy‐induced hypertension)
Caesarean section
Perineal trauma
Postnatal depression
Development of subsequent type 2 diabetes mellitus
Neonatal, child, adult outcomes
Large‐for‐gestational age
Perinatal mortality (stillbirth and neonatal mortality)
Composite of serious neonatal outcomes
Neonatal hypoglycaemia (variously defined)
Adiposity (e.g. as measured by BMI, skinfold thickness)
Diabetes
Neurosensory disability
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (Review Manager 2020) in order to create summary of findings tables. We produced a summary of the intervention effect and a measure of certainty for each of the above outcomes using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
See Characteristics of included studies.
Results of the search
See Figure 1.
In the previous version of the review, we included four studies (seven reports) and excluded two studies. The updated search (March 2022) retrieved 28 new study reports after we removed duplicates. We deemed five of these records not relevant based on title and abstract. We assessed the remaining 23 records, plus one report of an ongoing study (Farren 2017) in the previous review. We classified seven studies (12 reports) as ongoing trials (Amaefule 2018; Asimakopoulos 2020; CTRI/2018/06/014477; Ibrahim 2022; IRCT20120826010664N4; NCT04801485; NL7799). We excluded two studies as the participants did not meet the inclusion criteria of the review (Celentano 2020; Godfrey 2017). We considered four studies (8 records) as eligible for inclusion in the updated review (Farren 2017; Malvasi 2017; Santamaria 2016; Vitale 2019). Facchinetti 2013 was included in the previous version of this review, but we confirmed with the authors that Facchinetti 2013 is an interim report of D'Anna 2015. We classified one study (two reports) (Esmaeilzadeh 2021) as awaiting classification whilst awaiting further details. Therefore, we included seven studies in the updated review.
Screening eligible studies for trustworthiness
From the seven eligible studies identified from the search, we judged that all studies met our criteria for trustworthiness.
Included studies
See Characteristics of included studies.
Study design
We included seven RCTs (D'Anna 2013; D'Anna 2015; Farren 2017; Malvasi 2014; Malvasi 2017; Santamaria 2016; Vitale 2019).
Setting
Six studies were conducted in Italy (D'Anna 2013; D'Anna 2015; Malvasi 2014; Malvasi 2017; Santamaria 2016; Vitale 2019) and one study was conducted in Ireland (Farren 2017). The included studies were conducted between 2010 and 2018.
Participants
All studies were conducted in pregnant women. Gestational age at study entry was 10 to 16 weeks in one study (Farren 2017); 12 to 13 weeks in four studies (D'Anna 2013; D'Anna 2015; Santamaria 2016; Vitale 2019); 13 to 24 weeks in one study (Malvasi 2014); and 24 to 28 weeks in another study (Malvasi 2017). Six studies were on women with a BMI less than 30 kg/m2 (D'Anna 2013; Malvasi 2014; Malvasi 2017; Farren 2017; Santamaria 2016; Vitale 2019) while one study was on obese women with a BMI greater 30 kg/m2 (D'Anna 2015). Three studies included women exclusively of white ethnicity (D'Anna 2013; Santamaria 2016; Vitale 2019). An inclusion criterion in D'Anna 2013 was a first‐degree relative with type 2 diabetes. Women with pre‐existing diabetes mellitus were excluded from all included studies
Five studies (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016; Vitale 2019) used the International Association of Diabetes and Pregnancy Study Groups (IADPSG 2010) to diagnose GDM while the two studies (Malvasi 2014; Malvasi 2017) used the Italian Society of Diabetology criteria.
Groups were comparable at baseline for age, parity and BMI in Malvasi 2014 and Malvasi 2017. In D'Anna 2015; D'Anna 2013; Santamaria 2016; Farren 2017, the participants were comparable between groups at baseline for maternal age, BMI and gestational age at the commencement of treatment. In Vitale 2019, groups were comparable at baseline for age and haematological parameters. D'Anna 2013, Santamaria 2016, and Vitale 2019 included women exclusively of Caucasian ethnicity. An inclusion criterion in D'Anna 2013 was a first‐degree relative with type 2 diabetes. Women with pre‐existing diabetes mellitus were excluded from all included studies.
Intervention
The following doses of myo‐inositol were reported.
4 g myo‐inositol, 400 mcg folic acid daily in divided doses (2 g myo‐inositol plus 200 mcg folic acid twice a day) (D'Anna 2013; D'Anna 2015; Santamaria 2016; Vitale 2019)
1100 mg myo‐inositol, 27.6 mg C‐chiro‐inositol, 400 mcg folic acid per day (Farren 2017)
2 g myo‐inositol, 400 mg D‐chiro‐inositol, 400 mcg folic acid and 10 mg manganese per day in one dose (Malvasi 2014)
200 mg myo‐inositol, 500 mg D‐chiro‐inositol, 80 mg of Revifast (Malvasi 2017)
Comparison
The following comparisons were reported.
200 mcg folic acid (D'Anna 2013; D'Anna 2015; Santamaria 2016; Vitale 2019)
400 mcg folic acid (Farren 2017)
The authors stated women received placebo but did not state what the placebo was (Malvasi 2014; Malvasi 2017).
One study provided nutritional and lifestyle counselling to women in both the treatment and control groups (D'Anna 2015). None of the other included studies reported this.
Diagnostic criteria used to diagnose GDM
International Association of Diabetes and Pregnancy Study Groups (IADPSG 2010): D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016; Vitale 2019.
Italian Society of Diabetology: Malvasi 2014; Malvasi 2017.
Outcomes
Five studies reported on gestational diabetes and provided fasting, one‐ and two‐hour blood glucose results (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016; Vitale 2019). One study reported on gestational diabetes (Malvasi 2017) but did not provide blood glucose results. Five studies reported hypertensive disorders of pregnancy (D'Anna 2013; D'Anna 2015; Santamaria 2016; Vitale 2019; Farren 2017). Five studies reported on adverse effects of intervention (D'Anna 2013; Farren 2017; Malvasi 2014; Malvasi 2017; Santamaria 2016). Four studies reported a number of maternal and infant outcomes such as caesarean section, gestational age at birth, preterm birth, macrosomia, birthweight, neonatal hypoglycaemia, and shoulder dystocia (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016). Four trials reported on weight gain during pregnancy (D'Anna 2013; D'Anna 2015; Santamaria 2016; Vitale 2019). Three studies reported on the use of insulin (D'Anna 2015; Santamaria 2016; Vitale 2019). Two studies reported on respiratory distress syndrome (D'Anna 2013; Farren 2017). Two studies reported on relevant biomarker changes associated with the intervention (Malvasi 2014; Malvasi 2017). Only one study reported on the following maternal and neonatal outcomes: postpartum hemorrhage, adherence to the intervention, perineal trauma, large for gestation age, small for gestational age, nerve palsy, neonatal hyperbilirubinemia and admission to neonatal intensive care unit or special care baby unit (Farren 2017).
Funding sources
Two studies reported no funding source, with participants buying their own supplements (D'Anna 2013; Santamaria 2016). Farren 2017 reported that they did not receive any specific grant. Two studies did not state the source of funding (Malvasi 2014; Vitale 2019). D'Anna 2015 was funded by a grant from Messina University, Italy. Farren 2017 was supported by the Coombe Women & Infants University Hospital, Ireland and the food supplement was provided at no cost from Lo.Li. Pharma. Malvasi 2017 reported the research did not receive a specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors. All seven studies reported that none of the authors had any potential financial conflicts of interest (D'Anna 2013; D'Anna 2015; Farren 2017; Malvasi 2014; Malvasi 2017; Santamaria 2016; Vitale 2019).
Ongoing studies
See Characteristics of ongoing studies.
One record of an ongoing study from the previous version has now been added to the included studies (Farren 2013). In this update, seven ongoing studies (12 records) have been identified for potential inclusion when published (Amaefule 2018; Asimakopoulos 2020; CTRI/2018/06/014477; Ibrahim 2022; IRCT20120826010664N4; NCT04801485; NL7799).
Amaefule 2018 will compare outcomes of participants who take 2 g of myo‐inositol twice daily from 12 + 0 to 15 + 6 weeks’ gestational age until delivery with those who take an identical placebo at the same dose and duration. Their participants will be pregnant women with a singleton pregnancy recruited from 15 + 6 weeks of gestation. Asimakopoulos 2020 aims to compare outcomes between participants who take 4 g of myo‐inositol and 400 mcg of folic acid daily from 11 to 13 + 6 to 26 to 28 weeks of gestation with those who take 400 mcg of folic acid daily for the same duration. Their participants will not have pre‐existing impaired glucose tolerance and will have a singleton pregnancy. CTRI/2018/06/014477 aims to compare outcomes between participants who take myo‐d‐chiro inositol and vitamin D3 sachets twice daily in water and those who take placebo and vitamin D3 sachets twice daily. Their participants will have a BMI ≤ 35. Ibrahim 2022 aims to compare outcomes in pregnant women who will either take myo‐inositol supplementation or a placebo, with all participants completing at least 12 weeks of supplementation prior to undertaking the OGTT at 24 to 28 weeks. IRCT20120826010664N4 will analyse differences in outcomes of their participants who take 2 g of myo‐inositol and 200 mcg of folic acid twice daily from 14 to 28 weeks gestation with those who take only 200 mcg folic acid twice daily. They will recruit participants they define to have a high risk of developing gestational diabetes. NCT04801485 will examine outcomes in women considered at high risk of gestational diabetes who will either take myo‐inositol 1 gram per day as well as health guidance about diet and exercise or a placebo and the same health guidance. NL7799 will examine outcomes in pregnant women with confirmed PCOS who take 4 g myo‐inositol in addition to folic acid supplement, twice daily throughout pregnancy and those with PCOS who take the standard dose of folic acid without the myo‐inositol supplement.
(See Ongoing studies).
Excluded studies
See Characteristics of excluded studies.
In the previous version of this review, two studies were excluded (Corrado 2011; Matarrelli 2013). These studies were ineligible as they used myo‐inositol as a treatment for women already diagnosed with gestational diabetes rather than as a preventative intervention.
In this update, we excluded two studies (Celentano 2020; Godfrey 2017) as their participants did not meet the inclusion criteria for this review. Celentano 2020 recruited pregnant women with elevated fasting glucose levels (> 92 mg/dL and < 126 mg/dL) which may include women with pre‐gestational diabetes. Godfrey 2017 did not recruit pregnant women to their study but recruited women prior to conception.
Risk of bias in included studies
The overall risk of bias for most domains were low. See Figure 3 and Figure 4.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
We judged all seven included studies to be at low risk of selection bias for random allocation. Four studies used a computer‐generated random sequence (D'Anna 2015; D'Anna 2013; Santamaria 2016; Vitale 2019), two used a random number table (Malvasi 2014; Malvasi 2017), and randomisation was carried out by an independent statistician in the other study (Farren 2017).
We judged five studies to be at low risk of selection bias for allocation concealment (D'Anna 2015; Farren 2017; Malvasi 2014; Malvasi 2017; Santamaria 2016). In three studies, allocation was assigned by a centralised contact who was independent of the recruitment process (D'Anna 2015; Malvasi 2014; Malvasi 2017). Two studies used sealed opaque and sequentially numbered envelopes (Farren 2017; Santamaria 2016). We judged two studies to be at unclear risk of bias as allocation concealment was not reported (D'Anna 2013; Vitale 2019).
Blinding
Performance bias
We deemed two studies to be at unclear risk of performance bias as although participants were blinded, the clinicians involved were aware of the treatment allocation (Malvasi 2014; Malvasi 2017). The remaining five studies were open‐label, and we therefore judged them to be at high risk of performance bias (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016; Vitale 2019).
Detection bias
We judged three studies to be at low risk of detection bias as outcome assessors were blinded to allocation group (D'Anna 2015; Malvasi 2014; Malvasi 2017). We judged D'Anna 2013 and Santamaria 2016 as unclear risk due to inadequate reporting of blinding of outcome assessors. In D'Anna 2013, whilst the outcome of incidence of gestational diabetes was diagnosed by a blood test and unlikely to be affected by blinding, other outcomes such as neonatal respiratory distress syndrome are more subjective and may be impacted by knowledge of treatment group. We judged the remaining two studies to be at high risk of detection bias as they were open‐label (Farren 2017; Vitale 2019).
Incomplete outcome data
We judged four studies to be at low risk of attrition bias as losses to follow up were low (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016). In Santamaria 2016, there was a 10.5% overall loss to follow‐up but study authors provided a detailed explanation. We judged two studies to be at unclear risk of attrition bias (Malvasi 2017; Vitale 2019). In Malvasi 2017 randomisation and allocation were conducted after excluding six participants, but the three participants who left the study after the first visit were not treated as lost to follow‐up. Analysis was conducted on the remaining 104 women. Vitale 2019 reported a 10.8% overall loss but did not provide a detailed consort flow diagram. Finally, we judged Malvasi 2014 to be at high risk of attrition bias due to 26% overall attrition (17 women excluded from final analysis). Seven women left the study spontaneously but their group allocation or reasons for withdrawing were not stated.
Selective reporting
We judged five studies to be at low risk of reporting bias as all pre‐specified outcome measures were reported (D'Anna 2015; D'Anna 2013; Malvasi 2014; Malvasi 2017; Santamaria 2016). We judged Farren 2017 to be at low risk of reporting bias as all assessed outcome were reported, one outcome was pre‐specified but was not assessed. We judged Vitale 2019 to be at unclear risk of reporting bias as not all outcomes specified in the methodology section were reported.
Other potential sources of bias
We judged all included studies as being at low risk of other bias. The authors of all included studies declared no potential conflicts of interest.
Effects of interventions
The certainty of the evidence of the included studies is summarised in the Table 1 and Table 2 for the pre‐specified outcomes of this review.
Myo‐inositol versus placebo
All seven included studies compared myo‐inositol and placebo (D'Anna 2015; D'Anna 2013; Farren 2017; Malvasi 2014; Malvasi 2017; Santamaria 2016; Vitale 2019).
Maternal primary outcomes
Gestational diabetes
Six studies reported this outcome (D'Anna 2013: D'Anna 2015; Farren 2017; Malvasi 2017; Santamaria 2016; Vitale 2019). Meta‐analysis showed that supplementation of myo‐inositol may reduce the incidence of gestational diabetes compared with placebo (risk ratio (RR) 0.53, 95% confidence interval (CI) 0.31 to 0.90; 1140 women; very low‐certainty evidence; Analysis 1.1). Caution is required when interpreting the data due to significant heterogeneity (I2 = 71%). The difference is most likely due to differences in the study populations. D'Anna 2015 included only obese pregnant women while Malvasi 2017, Santamaria 2016 and Vitale 2019 recruited overweight women. D'Anna 2013 and Farren 2017 recruited women with a family history of type 1 or type 2 diabetes in a first‐degree relative. Nutritional and lifestyle counselling was provided to both the intervention and control groups in D'Anna 2015, but was not reported as being provided in the other studies.
1.1. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 1: Gestational diabetes mellitus
Five studies reported on blood glucose concentrations at the time of the diagnostic 75 g OGCT for GDM at 24 to 28 weeks' gestation. Myo‐inositol may be associated with a reduction in blood glucose concentrations compared to placebo.
Fasting: mean difference (MD) ‐0.14 mmol/L, 95% CI ‐0.21 to ‐0.07; 1071 women; Analysis 1.2).
One hour: MD ‐0.34 mmol/L, 95% CI ‐0.55 to ‐0.14; 1071 women; Analysis 1.3.
Two hours: MD ‐0.38mmol/L, 95% CI ‐0.77 to 0.01; 1071 women; Analysis 1.4.
1.2. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 2: Fasting OGTT
1.3. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 3: One hour OGTT
1.4. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 4: Two hour OGTT
Hypertensive disorders of pregnancy (including pre‐eclampsia, eclampsia, pregnancy‐induced hypertension)
Five studies reported this outcome (D'Anna 2013; D'Anna 2015; Malvasi 2017; Santamaria 2016; Vitale 2019). Meta‐analysis showed that myo‐inositol may reduce the incidence of gestational hypertension (RR 0.34, 95% CI 0.19 to 0.61; 1052 women; low‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 5: Hypertensive disorders of pregnancy
Infant primary outcomes
Large‐for‐gestational‐age
Farren 2017 reported data on the primary neonatal outcome of large‐for‐gestational‐age and showed no difference between myo‐inositol and placebo (RR 1.40, 95% CI 0.65 to 3.02; 234 infants; low‐certainty evidence; Analysis 1.6).
1.6. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 6: Large‐for‐gestational‐age
Perinatal mortality
None of the included studies reported data on this outcome.
Mortality or morbidity composite (variously defined by trials, e.g. infant death, shoulder dystocia, bone fracture or nerve palsy)
None of the included studies reported data on this outcome.
Maternal secondary outcomes
Caesarean section
Four studies reported this outcome (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016). Meta‐analysis showed that myo‐inositol resulted in little to no effect in caesarean section rate (RR 0.91, 95% CI 0.77 to 1.07; 829 women; low‐certainty evidence; Analysis 1.7).
1.7. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 7: Caesarean section
Weight gain during pregnancy
Four studies reported this outcome (D'Anna 2013; D'Anna 2015; Santamaria 2016; Vitale 2019). Meta‐analysis showed that myo‐inositol resulted in little to no effect on weight gain during pregnancy compared to placebo (MD ‐0.25kg, 95% CI ‐1.26 to 0.76; I2 = 81%, 831 women, very low‐certainty evidence; Analysis 1.8). Caution is required when interpreting the data due to significant heterogeneity (I2 = 81%). The difference is most likely due to differences in the study populations.
1.8. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 8: Weight gain during pregnancy
Relevant biomarker changes associated with the intervention
Three studies on a total of 340 women reported this outcome (Malvasi 2014; Malvasi 2017; Vitale 2019). Meta‐analysis showed that myo‐inositol may reduce total cholesterol (MD ‐29.57 mg/dL, 95% CI ‐32.80 to ‐26.33), low‐density lipoproteins (LDL) (MD ‐22.43 mg/dL, 95% CI ‐25.86 to ‐19.00), high‐density lipoproteins (HDL) (MD ‐1.46 mg/dL, 95% CI ‐2.72 to ‐0.20), and triglycerides (MD ‐24.92 mg/dL, 95% CI ‐27.82 to ‐22.02), compared with the control group (Analysis 1.9).
1.9. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 9: Relevant biomarker changes associated with the intervention
Adverse effects of intervention
Five studies measured this outcome and reported no adverse effects of therapy (D'Anna 2013; Farren 2017; Malvasi 2014; Malvasi 2017; Santamaria 2016). The remaining two studies did not report on this outcome (D'Anna 2015; Vitale 2019).
Perineal trauma
One study reported data on perineal trauma (Farren 2017). The evidence is very uncertain about the effect of myo‐inositol on perineal trauma (RR 4.00, 95% CI 0.45 to 35.25; 234 women; Analysis 1.10).
1.10. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 10: Perineal trauma
Postpartum hemorrhage
One study reported data on postpartum hemorrhage (Farren 2017) and found no difference in the risk of postpartum haemorrhage between myo‐inositol and placebo (RR 0.67, 95% CI 0.31 to 1.42; 234 women; Analysis 1.11).
1.11. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 11: Postpartum haemorrhage
Adherence to the intervention
One study reported data on adherence to the intervention (Farren 2017). There was no difference in the risk of adherence to the intervention between myo‐inositol and placebo (RR 0.99, 95% CI 0.84 to 1.16; 240 women; Analysis 1.12).
1.12. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 12: Adherence to intervention
Other secondary outcomes
No data were reported for any of the other pre‐specified maternal secondary outcomes for this systematic review (placental abruption, induction of labour, postpartum infection, behaviour changes associated with the intervention (as defined by study authors), sense of well‐being and quality of life, views of the intervention, breastfeeding (e.g. at discharge, six weeks postpartum), postnatal depression, postnatal weight retention or return to pre‐pregnancy weight, body mass index (BMI), GDM in a subsequent pregnancy, type I diabetes, type 2 diabetes, impaired glucose tolerance or cardiovascular health (as defined by trialists, including blood pressure (BP), hypertension, cardiovascular disease, metabolic syndrome)).
Other outcomes not pre‐specified
Although the main aim of the included studies was the prevention of GDM, three of the included studies that continued the intervention until the end of pregnancy (D'Anna 2013; D'Anna 2015; Santamaria 2016), reported on the need for additional pharmacological therapy to treat gestational diabetes For interest, we include a summary of these data. There was no difference between myo‐inositol and placebo for the need for use of insulin therapy (RR 0.50, 95% CI 0.17 to 1.52; 595 women; Analysis 1.13).
1.13. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 13: Supplementary insulin
Infant secondary outcomes (infant, child and adult)
There were no differences in secondary infant outcomes between infants of mothers supplemented with myo‐inositol and placebo
Gestational age at birth
Four studies reported this outcome (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016). Meta‐analysis showed no difference in the gestational age at birth between myo‐inositol and placebo (MD 3.69 days, 95%CI ‐1.48 to 8.86; 829 infants; Analysis 1.14). Caution is required when interpreting the data due to significant heterogeneity (I2 = 91%). The difference is most likely due to differences in the populations. D'Anna 2015 included only obese pregnant women while Malvasi 2017, Santamaria 2016 and Vitale 2019 recruited overweight women. D'Anna 2013 and Farren 2017 recruited women with a family history of type 1 or type 2 diabetes in a first‐degree relative. Nutritional and lifestyle counselling was provided to both the intervention and control groups in D'Anna 2015, but was not reported as being provided in the other studies.
1.14. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 14: Gestational age at birth
Preterm birth
Four studies reported this outcome (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016). Meta‐analysis showed that myo‐inositol may be associated with a reduction in the incidence of preterm birth compared with placebo (RR 0.35, 95% CI 0.17 to 0.70; 829 infants; Analysis 1.15).
1.15. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 15: Preterm birth (less than 37 weeks' gestation)
Macrosomia
Four studies reported this outcome (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016). Meta‐analysis showed no difference between myo‐inositol and placebo for the risk of macrosomia (RR 0.55, 95% CI 0.16 to 1.96; 829 infants; Analysis 1.16). Caution is required when interpreting the data due to significant heterogeneity (I2 = 46%). The difference is most likely due to differences in the populations. D'Anna 2015 included only obese pregnant women while Malvasi 2017, Santamaria 2016 and Vitale 2019 recruited overweight women. D'Anna 2013 and Farren 2017 recruited women with a family history of type 1 or type 2 diabetes in a first‐degree relative. Nutritional and lifestyle counselling was provided to both the intervention and control groups in D'Anna 2015, but was not reported as being provided in the other studies.
1.16. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 16: Macrosomia
Birthweight
Four studies reported this outcome (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016). Meta‐analysis showed no difference between myo‐inositol and placebo for birthweight (MD ‐8.65 g, 95% CI ‐140.36 to 123.07; 829 infants; Analysis 1.17) ). No data were reported for birthweight z‐scores. Caution is required when interpreting the data due to significant heterogeneity (I2 = 72%). The difference is most likely due to differences in the populations. D'Anna 2015 included only obese pregnant women while Malvasi 2017, Santamaria 2016 and Vitale 2019 recruited overweight women. D'Anna 2013 and Farren 2017 recruited women with a family history of type 1 or type 2 diabetes in a first‐degree relative. Nutritional and lifestyle counselling was provided to both the intervention and control groups in D'Anna 2015, but was not reported as being provided in the other studies.
1.17. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 17: Birthweight
Shoulder dystocia
Four studies reported this outcome (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016). Meta‐analysis showed no difference between myo‐inositol and placebo on the risk of shoulder dystocia (RR 1.43, 95% CI 0.15 to 13.54; 829 infants; very low‐certainty evidence; Analysis 1.18). Caution is required when interpreting the data due to significant heterogeneity (I2 = 59%). The difference is most likely due to differences in the populations. D'Anna 2015 included only obese pregnant women while Malvasi 2017, Santamaria 2016 and Vitale 2019 recruited overweight women. D'Anna 2013 and Farren 2017 recruited women with a family history of type 1 or type 2 diabetes in a first‐degree relative. Nutritional and lifestyle counselling was provided to both the intervention and control groups in D'Anna 2015, but was not reported as being provided in the other studies.
1.18. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 18: Shoulder dystocia
Respiratory distress syndrome
Two studies reported this outcome (D'Anna 2013; Farren 2017), and showed no benefit of myo‐inositol on the risk of respiratory distress syndrome (RR 1.49, 95% CI 0.25 to 8.85; 2 studies; 431 infants; very low‐certainty evidence; Analysis 1.19)
1.19. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 19: Respiratory distress syndrome
Neonatal hypoglycaemia
Four studies reported this outcome (D'Anna 2013; D'Anna 2015; Farren 2017; Santamaria 2016). Meta‐analysis showed no difference between myo‐inositol and placebo on neonatal hypoglycaemia (RR 3.07, 95% CI 0.90 to 10.52; 671 infants; very low‐certainty evidence; Analysis 1.20) . For infants of women who received myo‐inositol, the risk of neonatal hypoglycaemia ranged from 0.8% to 9.1%; for infants of women given a placebo, the risk of neonatal hypoglycaemia was 0.9%.
1.20. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 20: Neonatal hypoglycaemia
Small‐for‐gestational‐age
Farren 2017 reported data on small‐for‐gestational‐age infants and found no difference between myo‐inositol and placebo (RR 2.33, 95% CI 0.62 to 8.80; 234 infants; Analysis 1.21).
1.21. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 21: Small‐for‐gestational‐age
Nerve palsy
Farren 2017 measured nerve palsy but reported no cases.
Neonatal hyperbilirubinemia
Farren 2017 reported data on neonatal hyperbilirubinemia and found no difference between myo‐inositol and placebo (RR 0.25, 95% CI 0.05 to 1.15; 234 infants; Analysis 1.22).
1.22. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 22: Neonatal hyperbilirubinaemia
Other secondary outcomes
No studies reported the other secondary neonatal (infant, child, adult) outcomes of this systematic review were reported (stillbirth, neonatal mortality, Apgar score < five at seven minutes, head circumference and z score, length and z score, ponderal index, adiposity, bone fracture). For the infant as a child and adult, no data were reported for any of the pre‐specified outcomes (weight, height, adiposity (e.g. as measured by BMI, skinfold thickness), cardiovascular health (as defined by trialists, including blood pressure, hypertension, cardiovascular disease, metabolic syndrome), type I diabetes, type 2 diabetes mellitus, impaired glucose tolerance, dyslipidaemia or metabolic syndrome, employment, education and social status/achievement).
Health service outcomes
D'Anna 2015 and Farren 2017 reported on admission to the neonatal intensive care unit (NICU) and found no difference between myo‐inositol and placebo (RR 0.40, 95% CI 0.14 to 1.18; 435 infants; very low‐certainty evidence; Analysis 1.23).
1.23. Analysis.

Comparison 1: Myo‐inositol versus control, Outcome 23: Admission to neonatal intensive care unit or special care baby unit
None of the included studies reported any of the other health service outcomes (number of hospital or health professional visits (e.g. midwife, obstetrician, physician, dietitian, diabetic nurse), number of antenatal visits or admissions, length of antenatal stay, length of postnatal stay (mother), length of postnatal stay (baby), costs to families associated with the management provided, costs associated with the intervention, cost of maternal care, and cost of offspring care).
Myo‐inositol versus no treatment
None of the included studies assessed myo‐inositol versus no treatment.
Myo‐inositol versus another intervention
None of the included studies assessed myo‐inositol versus another intervention.
Discussion
Summary of main results
In this updated review that included seven RCTs involving 1319 women, we found that the evidence is very uncertain about the effect of supplementation with myo‐inositol on the incidence of gestational diabetes, weight gain during pregnancy or perineal trauma. However, supplementation with myo‐inositol may result in a large reduction in hypertensive disorders of pregnancy but little to no difference in the risk of caesarean section. For infants, the evidence is also very uncertain about the effect of myo‐inositol on the risk of a large‐for‐gestational‐age infant or neonatal hypoglycaemia, but myo‐inositol may be associated with a reduction in the incidence of preterm birth. None of the current trials reported on postnatal depression, development of subsequent type 2 diabetes mellitus, perinatal mortality or serious neonatal outcomes.
Overall completeness and applicability of evidence
The included studies were conducted in healthy women and those considered at high risk of developing gestational diabetes, including obese and non‐obese women, and those with a family history of type 2 diabetes mellitus. However, applicability is limited by six studies being conducted in Italy and only one being conducted elsewhere, in Ireland, and participants were predominantly white women. Further studies in diverse settings, including participants of different ethnicities and varying risk factors, would improve the applicability of the evidence. Not all the outcomes of interest for this review were addressed in the included studies, including pre‐eclampsia, perinatal mortality, and longer‐term maternal and infant health outcomes. Furthermore, we were unable to conduct sensitivity analysis due to the small number of included studies reporting few outcomes. Several factors may influence the outcome effects, including the differences in myo‐inositol doses (that varied from 200 mg to 4 g in the included trials), women at different risks (both obese and non‐obese populations) and different gestational age at study entry.
Quality of the evidence
The current evidence is based on seven RCTs that included a total of 1319 women and their infants. The overall risk of bias was judged to be low. Where studies had a high risk of performance bias and detection bias this was due to their open‐label study design. Where there was an unclear risk of bias this was because of insufficient information provided to enable a judgment of risk to be made.
Using the GRADE method, we assessed the certainty of the body of evidence for the maternal outcomes of gestational diabetes, weight gain during pregnancy, hypertensive disorders of pregnancy, caesarean section, perineal trauma, postnatal depression, and type 2 diabetes, and the neonatal outcomes of large‐for‐gestational age, perinatal mortality, composite of serious neonatal outcomes, neonatal hypoglycaemia, adiposity, diabetes, and neurosensory disability. No data were reported for the maternal outcomes of postnatal depression and type 2 diabetes. No data were reported for the neonatal outcomes of perinatal mortality, composite of serious neonatal outcomes, adiposity, diabetes, and neurosensory disability. The certainty of evidence was downgraded in the Table 1 and Table 2 to low or very low, due to limitation in study design, indirectness and imprecision.
Potential biases in the review process
The Trials Search Co‐ordinator of the Cochrane Pregnancy and Childbirth Group searched multiple databases, without language or date restrictions in an attempt to limit bias by identifying all relevant trials. Where necessary, we contacted trial authors to seek clarification or further information. As per the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021), at least two review authors appraised studies for inclusion and extracted the data in order to minimise bias.
Agreements and disagreements with other studies or reviews
The increasing prevalence of gestational diabetes worldwide has led to greater interest in findings ways to prevent and treat gestational diabetes. The body of evidence for the use of antenatal myo‐inositol supplementation for the prevention of gestational diabetes continues to grow. Other literature (Di Benedetto 2013), a meta‐analysis (Zheng 2015), and systematic reviews (Rogozinska 2015; Guo 2018; Noventa 2016; Vitagliano 2019; Zhang 2019), cited most of the studies included in this updated review, and draw similar conclusions that myo‐inositol shows potential in preventing gestational diabetes. All are unanimous in their call for large, high‐quality RCTs in more diverse populations to further assess this potential treatment. We await the publication of ongoing studies that can be incorporated into future updates of this review.
Authors' conclusions
Implications for practice.
Antenatal supplementation with myo‐inositol for the prevention of gestational diabetes is a comparatively new treatment. Whilst the results of this review show that myo‐inositol has promise in preventing the onset of gestational diabetes, there is currently insufficient evidence to support its routine adoption. The results of future research into the use of antenatal supplementation with myo‐inositol for the prevention of gestational diabetes will provide more robust evidence for informing and guiding practice.
Implications for research.
The current evidence indicates that the effect of antenatal supplementation with myo‐inositol in reducing the incidence of gestational diabetes is unclear but may result in a reduction of the incidence of hypertensive disorders of pregnancy. However, higher‐certainty evidence is needed to confirm or refute this finding. The effect on other important infant outcomes is unclear. Further well‐designed randomised controlled trials are required and should be sufficiently powered to detect differences in relevant maternal and infant outcomes. They should include participants of varying ethnicities, with various risk factors for gestational diabetes such as obesity, polycystic ovarian syndrome, family history, and previous gestational diabetes; explore the optimal dose, frequency, and timing of supplementation; and report long‐term maternal, infant, and childhood outcomes. It is important that trials report on potential harms, including any adverse effects. In view of the availability of myo‐inositol as a dietary supplement and its relatively low cost compared with traditional interventions for preventing gestational diabetes, future RCTs should include economic analysis, or at least report on health service use and costs. If the efficacy of antenatal supplementation with myo‐inositol compared with placebo is established, then it will also be useful to conduct trials that compare the use of myo‐inositol with other preventative interventions, such as lifestyle (diet and exercise) or pharmacological interventions, such as metformin.
What's new
| Date | Event | Description |
|---|---|---|
| 14 February 2023 | New search has been performed | Search updated and four new studies identified, so total of seven studies included in this review update. Conclusions similar to previous version of the review. |
| 14 February 2023 | New citation required but conclusions have not changed | Conclusions remain unchanged. |
History
Protocol first published: Issue 2, 2015 Review first published: Issue 12, 2015
Acknowledgements
Portions of the methods section of this protocol are based on a standard template used by the Cochrane Pregnancy and Childbirth Review Group. Outcomes may be similar to other Cochrane reviews for preventing gestational diabetes to provide consistent core outcomes for reviews on this condition.
We acknowledge the support from the Cochrane Pregnancy and Childbirth Review Group editorial team in Liverpool, the Australian and New Zealand Satellite of the Cochrane Pregnancy and Childbirth Review Group, and the support from the Liggins Institute, University of Auckland, Auckland, New Zealand.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), and the Group's Statistical Adviser. The authors are grateful to the following peer reviewers for their time and comments: Diane Farrar, Bradford Institute for Health Research, Bradford, UK; Shanshan Han PhD, Master of Nutrition and Dietetics, Bachelor of Medicine, Adelaide, Australia.
We thank Julie Brown and Susan Kruse for their contribution as authors in previous versions of this review and Dahineswari Rajamanickam who assisted the initial review authors in preparing the first draft of the protocol.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
We thank Lindsay Robertson for her help in copy editing the final draft of this review.
Appendices
Appendix 1. Search terms for ICTRP and ClinicalTrials.gov
ClinicalTrials.gov (searched via Cochrane Register of Studies (CRS))
Study type: interventional studies, intervention: myoinositol, condition: gestational diabetes
Study type: interventional studies, intervention: inositol, condition: gestational diabetes
ICTRP
Each line searched separately and all synonyms searched
myoinositol AND pregnancy
myoinsitol AND pregnant
myoinositol AND gestational
Data and analyses
Comparison 1. Myo‐inositol versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Gestational diabetes mellitus | 6 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.31, 0.90] |
| 1.2 Fasting OGTT | 5 | 1071 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.21, ‐0.07] |
| 1.3 One hour OGTT | 5 | 1071 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.55, ‐0.14] |
| 1.4 Two hour OGTT | 5 | 1071 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.77, 0.01] |
| 1.5 Hypertensive disorders of pregnancy | 5 | 1052 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.19, 0.61] |
| 1.6 Large‐for‐gestational‐age | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.65, 3.02] |
| 1.7 Caesarean section | 4 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.77, 1.07] |
| 1.8 Weight gain during pregnancy | 4 | 831 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐1.26, 0.76] |
| 1.9 Relevant biomarker changes associated with the intervention | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.9.1 Total cholesterol | 3 | 340 | Mean Difference (IV, Fixed, 95% CI) | ‐29.57 [‐32.80, ‐26.33] |
| 1.9.2 Low density lipoprotein | 3 | 340 | Mean Difference (IV, Fixed, 95% CI) | ‐22.43 [‐25.86, ‐19.00] |
| 1.9.3 High density lipoprotein | 3 | 340 | Mean Difference (IV, Fixed, 95% CI) | ‐1.46 [‐2.72, ‐0.20] |
| 1.9.4 Triglycerides | 3 | 340 | Mean Difference (IV, Fixed, 95% CI) | ‐24.92 [‐27.82, ‐22.02] |
| 1.10 Perineal trauma | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.00 [0.45, 35.25] |
| 1.11 Postpartum haemorrhage | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.31, 1.42] |
| 1.12 Adherence to intervention | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.84, 1.16] |
| 1.13 Supplementary insulin | 3 | 595 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.17, 1.52] |
| 1.14 Gestational age at birth | 4 | 829 | Mean Difference (IV, Random, 95% CI) | 3.69 [‐1.48, 8.86] |
| 1.15 Preterm birth (less than 37 weeks' gestation) | 4 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.17, 0.70] |
| 1.16 Macrosomia | 4 | 829 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.16, 1.96] |
| 1.17 Birthweight | 4 | 829 | Mean Difference (IV, Random, 95% CI) | ‐8.65 [‐140.36, 123.07] |
| 1.18 Shoulder dystocia | 4 | 829 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.15, 13.54] |
| 1.19 Respiratory distress syndrome | 2 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.25, 8.85] |
| 1.20 Neonatal hypoglycaemia | 4 | 671 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.07 [0.90, 10.52] |
| 1.21 Small‐for‐gestational‐age | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.62, 8.80] |
| 1.22 Neonatal hyperbilirubinaemia | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.05, 1.15] |
| 1.23 Admission to neonatal intensive care unit or special care baby unit | 2 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.14, 1.18] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
D'Anna 2013.
| Study characteristics | ||
| Methods | Study type: parallel RCT | |
| Participants | 220 women from Italy Inclusion criteria: first‐degree relative (mother, father or both) affected by type 2 diabetes, pre‐pregnancy BMI < 30 kg/m2, fasting plasma glucose < 126 mg/dL and random glycaemia < 200 mg/dL, singleton pregnancy, Caucasian. Women were 12 to 13 weeks' gestation at study entry. Exclusion criteria: pre‐pregnancy BMI ≥ 30 kg/m2, previous gestational diabetes, pre‐gestational diabetes, first trimester glycosuria, first‐degree relative (mother or father) not affected by type 2 diabetes, fasting plasma glucose ≥ 126 mg/dL or random glycaemia ≥ 200 mg/dL, twin pregnancy, associated therapy with corticosteroids, PCOS. Location: Department of Gynecology and Obstetrics, University of Messina, Messina, Italy Timeframe: 2010 to 2012 |
|
| Interventions | Intervention: 4 g myo‐inositol plus 400 mcg folic acid daily (2 g myo‐inositol plus 200 mcg folic acid twice a day) (N = 110) Duration of myo‐inositol supplementation: from trial entry until the end of pregnancy Comparison: 400 mcg folic acid daily (200 mcg folic acid twice a day) as placebo (N = 110) |
|
| Outcomes |
Maternal: incidence of gestational diabetes, gestational hypertension, caesarean section Criteria used to diagnose gestational diabetes: IADPSG Infant: fetal macrosomia (> 4000 g), preterm birth, shoulder dystocia, neonatal hypoglycaemia, respiratory distress syndrome |
|
| Notes |
Sample size calculation: not stated Intention‐to‐treat analysis: yes (carried out but not reported) Losses to follow‐up: 11 women in the intervention group and 12 in the comparison group Funding source: none, the women bought the supplement on their own Conflict of interest: none reported Further information was received following email contact with the authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer randomization was used" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. Blinding not carried out |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Primary outcome of incidence of gestational diabetes diagnosed by blood test so blinding unlikely to impact assessment of this outcome. However, other secondary outcomes are more subjective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall 10% loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcome measures were reported. |
| Other bias | Low risk | Intention‐to‐treat analysis was carried out on the available data. |
D'Anna 2015.
| Study characteristics | ||
| Methods | Study type: parallel RCT | |
| Participants | 220 obese pregnant women from Italy Inclusion criteria: pre‐pregnancy BMI ≥ 30 kg/m2, singleton gestation Women were 12 to 13 weeks' gestation at study entry. Exclusion criteria: previous gestational diabetes, pre‐gestational diabetes, first trimester glycosuria (urine glucose value 10 mg/dL or greater), first trimester fasting plasma glucose 126 mg/dL or greater, or random plasma glucose 200 mg/dL or greater, concomitant treatment with corticosteroids, hypertension or renal or hepatic disease. Location: obstetric departments of 2 university hospitals located in Messina and Modena, Italy Timeframe: January 2011 to April 2014 |
|
| Interventions |
Intervention: 4 g myo‐inositol plus 400 mg folic acid daily (2 g myo‐inositol + 200 mg folic acid orally twice a day), and nutritional and lifestyle counselling (N = 110) Duration of myo‐inositol supplementation: from trial entry until the end of pregnancy Comparison: 400 mg folic acid daily (200 mg folic acid orally twice a day), and nutritional and lifestyle counselling (N = 110) |
|
| Outcomes |
Maternal: occurrence of gestational diabetes, changes of insulin resistance from the first trimester to the performance of the OGTT performed at 24‐28 weeks as measured by the homeostasis model assessment of insulin resistance, caesarean section, gestational hypertensive disorders Criteria used to diagnose gestational diabetes: IADPSG Infant: preterm delivery, shoulder dystocia, macrosomia (birthweight > 4000 g), neonatal hypoglycaemia, neonatal transfer to intensive care unit |
|
| Notes |
Sample size calculation: conducted Intention‐to‐treat analysis: yes Funding source: grant from Messina University Conflict of interest: none reported ClinicalTrials.gov trial registration NCT01047982 Further information was received following email contact with the authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated random number list prepared by an investigator with no clinical involvement with the trial." |
| Allocation concealment (selection bias) | Low risk | "Allocation concealment was ensured by central randomisation." "After the research investigator had obtained the patients consent, he telephoned a contact who was independent of the recruitment process for allocation assignment." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial was open label so blinding of participants and clinicians was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Data collectors were blinded to treatment allocation and the data came from the patients record." "objective measurements of primary laboratory outcomes." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9% loss to follow‐up overall. More participants chose to drop out of the myo‐inositol group (n = 8) than the 'placebo' group (n = 0). |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes are reported on. |
| Other bias | Low risk | Appears free of other bias. The authors do not report any potential conflicts of interest. |
Farren 2017.
| Study characteristics | ||
| Methods | Study type: single‐centre, parallel RCT | |
| Participants | 240 pregnant women from Ireland Inclusion criteria: women were 10 to 16 weeks' gestation at trial entry. Pregnant women with a family history in a first‐degree relative of diabetes, either type 1 or type 2, were eligible for inclusion. Exclusion criteria: age younger than 18 years, multiple pregnancies, limited comprehension of English, and any pre‐existing liver or kidney disease or diabetes. Timeframe: January 2014 to January 2016 |
|
| Interventions |
Intervention: combination of myo‐inositol 1,100mg, C‐chiro‐inositol 27.6mg, and 400 microgram folic acid daily (N = 120) Duration of myo‐inositol supplementation; not stated Comparison: 400 microgram folic acid daily (N = 120) |
|
| Outcomes |
Maternal: occurrence of gestational diabetes, gestational hypertension, induction of labour, the mode of delivery, perineal trauma Criteria used to diagnose GDM: IADPSG Infant: birth weight, shoulder dystocia, brachial plexus palsy, neonatal intensive care unit (NICU) admission, neonatal hypoglycaemia, respiratory distress syndrome Further information was received following email contact with the authors. |
|
| Notes |
Sample size calculation: yes Intention‐to‐treat analysis: yes Induction of labour was not reported in the study. Funding source: supported by the Coombe Women & Infants University Hospital. The food supplement was provided at no cost from Lo.Li. Pharma. Conflicts of interest: none reported Ethical approval was granted by the Hospital Research Ethics Committee in June 2013. The trial was registered with the ISRCTN registry and assigned the trial number ISRCTN92466608. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out by an independent statistician. |
| Allocation concealment (selection bias) | Low risk | The author confirmed that they used sealed opaque and sequentially numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The Investigators knew which supplement was dispensed and the women knew which supplement they were taking, but the clinicians managing the pregnancy and delivery were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Trial was open label, investigators were aware of which supplement was dispensed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors provide a list of the flow of participants through their trial. |
| Selective reporting (reporting bias) | Low risk | All collected outcomes have been reported. |
| Other bias | Low risk | Authors had no potential conflicts of interest related to the article. |
Malvasi 2014.
| Study characteristics | ||
| Methods | Study type: parallel RCT | |
| Participants | 65 pregnant women from Italy Inclusion criteria: healthy pregnant women, aged between 30 to 40, between 13 and 24 weeks' gestation, BMI between 25 to 30 kg/m2. Exclusion criteria: diabetes mellitus, cardiovascular disease, chronic hypertension, autoimmune disease, dysthyroidism. Location: Bari, Italy Timeframe: January to December 2012 |
|
| Interventions |
Intervention: a combination of 2000 mg myo‐inositol, 400 mg d‐chiro‐inositol, 400 mcg folic acid, 10 mg manganese Duration of myo‐inositol supplementation: 60 days Comparison: placebo (but not stated what placebo was) |
|
| Outcomes |
Maternal: total cholesterol, LDL, HDL, blood glucose Criteria used to diagnose gestational diabetes: not stated Infant: not stated |
|
| Notes |
Sample size calculation: not stated Funding source: not stated Conflicts of interest: none reported Further information was received following email contact with the authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was generated by a random number table. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by an independent statistician who assigned numbered patients to groups using sealed numbered containers. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were blinded. Clinicians were aware of treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 65 women were initially enrolled, 17 of which were excluded; 6 did not meet inclusion criteria, 4 refused to participate, 7 left the study spontaneously. Analysis was conducted on the remaining 48 women. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes are reported on (total cholesterol, LDL, HDL, blood glucose). No other maternal, pregnancy or neonatal outcomes are specified or reported. |
| Other bias | Low risk | Appears free of other bias. The authors do not report any potential conflicts of interest. |
Malvasi 2017.
| Study characteristics | ||
| Methods | Study type: prospective, double‐blinded, placebo‐controlled, single‐centre RCT | |
| Participants | 104 pregnant women from Italy Inclusion criteria: BMI between 25 to 30 kg/m2 in the first trimester, aged between 25 to 40 years, singleton pregnancy. Women were 10 to 16 weeks’ gestation at study entry. Exclusion criteria: diabetes mellitus, cardiovascular disease, chronic hypertension, autoimmune disease, thyroid disease, ART. Location: Bari, Italy Timeframe: January to December 2016 |
|
| Interventions |
Group I: 80 mg of Revifast®, 200 mg of myo‐inositol, 500 mg D‐chiro‐inositol Group II: 138 mg of myo‐inositol, 550 mg D‐chiro‐inositol. Duration of myo‐inositol supplementation: 60 days Group III: placebo (but not stated what placebo was) |
|
| Outcomes |
Maternal: lipid profile (total cholesterol, LDL, HDL, triglycerides), glucose levels, blood pressure (systolic pressure and diastolic pressure) at baseline and after 30 and 60 days of therapy Criteria used to diagnose gestational diabetes: not stated Infant: not stated |
|
| Notes |
Sample size calculation: not stated Funding source: no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors Conflict of interests: none reported Data collected in the form compares group 2 with group 3 (no revifast group (1) comparison) Further information was received following email contact with the authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation sequence was generated by a random number table. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by an independent statistician who assigned numbered patients to groups using sealed numbered containers. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | During the enrolment of 110 pregnant women, six patients were excluded: three left after the first visit, three did not meet inclusion criteria. The remaining 104 women have been allocated in the three groups as follows: 35 in group I, 34 in group II and 35 in group III. It seems that the randomization and allocation were conducted after excluding the 6 people, but the three participants who left after the first visit should be treated as lost to follow‐up. Analysis was conducted on the remaining 104 women. |
| Selective reporting (reporting bias) | Low risk | All outcome specified in the methods section have been reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. The authors do not report any potential conflicts of interest. |
Santamaria 2016.
| Study characteristics | ||
| Methods | Study type: parallel, open‐label RCT | |
| Participants | 220 overweight non‐obese pregnant women from Italy. Inclusion criteria: pre‐pregnancy BMI > 25 and < 30 kg/m2, first trimester fasting plasma glucose ≤ 126 mg/dL and/or random glycaemia < 200 mg/dL, single gestation, Caucasian ethnicity. Women were 12 to13 weeks' gestation at study entry. Exclusion criteria: pre‐pregnancy BMI < 25 and ≥ 30 kg/m2, previous GDM, pre‐gestational diabetes, first trimester glycosuria (urine glucose value 10 mg/dL or greater), treatment with corticosteroids. Location: obstetric departments of 2 university hospitals located in Messina and Modena, Italy Timeframe: January 2012 to December 2014 |
|
| Interventions |
Intervention: 4 g myo‐inositol plus 400 mg folic acid daily (2 g myo‐inositol + 200 mg folic acid orally twice a day), (N = 110) Duration of myo‐inositol supplementation: from trial entry until the end of pregnancy Comparison: 400 mg folic acid daily (200 mg folic acid orally twice a day), (N = 110) |
|
| Outcomes |
Maternal: occurrence of gestational diabetes, rate of caesarean section, pregnancy induced hypertension, occurrence of side effects, Homeostasis Model Assessment‐Insulin Resistance index (HOMA‐IR) Criteria used to diagnose gestational diabetes: IADPSG Neonatal: fetal macrosomia (birthweight > 4000 g), preterm delivery (< 37 weeks), shoulder dystocia, neonatal hypoglycaemia, transfer to NICU |
|
| Notes |
Sample size calculation: yes Funding source: none, the women bought the supplement on their own Conflicts of interest: none reported ClinicalTrials.gov trial registration NCT01047982 Further information was received following email contact with the authors. This trial was conducted at the same time as the D'Anna 2015 trial, with women being recruited to the appropriate trial depending on eligibility criteria. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list was obtained by using a computer‐generated random allocation |
| Allocation concealment (selection bias) | Low risk | The allocations were sealed in numbered white envelopes, which were kept in the midwifery facility. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Abstract states "open‐label" Personnel were not blinded: "because of the design of the study, the gynaecologist knew the group allocation of the patient". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention is made of blinding of outcome assessors, although primary outcome of occurrence of GDM is objective and based on laboratory results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 110 women were initially recruited to each group. 15 women were excluded from the myo‐inositol group (one mid‐trimester miscarriage, 2 abandoned the trial not attending the OGTT, 5 delivered elsewhere, and 7 dropped out). Analysis was performed on the remaining 95 women. 8 women were excluded from the placebo group (one mid‐trimester miscarriage, 2 abandoned the trial not attending the OGTT, and 5 delivered elsewhere). Analysis was performed on the remaining 102 women. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes are reported on. |
| Other bias | Low risk | Appears free of other bias. The authors reported no potential conflicts of interest. |
Vitale 2019.
| Study characteristics | ||
| Methods | Study type: prospective, open‐label, placebo‐controlled RCT | |
| Participants | 250 pregnant women from Italy Inclusion criteria: pre‐pregnancy BMI > 25 and < 30 kg/m2, first‐trimester fasting plasma glucose ≤ 126 mg/dl and/or random glycaemia <200 mg/dl, single pregnancy, and Caucasian ethnicity. Exclusion criteria: women who had a pre‐pregnancy BMI < 25 and ≥ 30 kg/m2, previous gestational diabetes, pre‐gestational diabetes, first‐trimester glycosuria, and in treatment with corticosteroids. Location: Gynaecology and Obstetrics of the Department of Human Pathology in Adulthood and Childhood, University of Messina, Italy Timeframe: started at the beginning of 2016 and lasted 2 years |
|
| Interventions |
Intervention: 4 g myo‐inositol plus 400 mg folic acid (2 g myo‐inositol plus 200 mg folic acid twice/day—InofolicVR ; Loli Pharma, Rome, Italy), and followed the same diet according to the ADA recommendations (N = 125) Duration: the treatment lasted until three weeks after delivery Comparison: 400 mg folic acid only (200 mg twice/day), and followed the same diet according to the ADA recommendations (N = 125) |
|
| Outcomes |
Maternal: occurrence of gestational diabetes and body water distribution, changes in lipid metabolism (total cholesterol, HDL, LDL and triglycerides serum levels), rate of caesarean section in emergency, pregnancy induced hypertension (PIH) and pre‐eclampsia Criteria used to diagnose gestational diabetes: IADPSG Infant: prevalence of fetal macrosomia (fetal birth weight >4500 g at delivery), preterm delivery (< 37 weeks), , the occurrence of shoulder dystocia, neonatal hypoglycaemia, the need for transfer to the Neonatal Intensive Care Unit (NICU) |
|
| Notes |
Sample size calculation: yes Funding source: not reported Conflict of interest: none reported The trial is registered with the number NCT01047982, the same as D’Anna 2015 The ethical was approval by the Ethical Committee of Messina University Hospital(E347/2008) No response was received following email contact with the authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated random sampling method with a 1:1 ratio was used. |
| Allocation concealment (selection bias) | Unclear risk | A nurse sealed and randomly numbered the allocations in white envelopes according to the computer‐generated scheme. Sealed envelopes should be opaque and sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label trial. Blinding not carried out |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label trial. Blinding not carried out |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10.8% lost to follow up overall |
| Selective reporting (reporting bias) | Unclear risk | Not all outcomes specified in the methodology section have been reported. |
| Other bias | Low risk | Appears free of other bias. The authors do not report any potential conflicts of interest. |
ADA: American Diabetes Assocation BMI: body mass index GDM: gestational diabetes HbA1c: glycated haemoglobin HDL: high density lipoprotein IADPSG: International Association of Diabetes and Pregnancy Study Groups LDL: low density lipoprotein OGTT: oral glucose tolerance test PCOS: polycystic ovary syndrome RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Celentano 2020 | The study may include women with pre‐gestational diabetes as all women recruited had an elevated fasting glucose level (> 92 mg/dL and < 126 mg/dL) as part of the inclusion criteria. |
| Corrado 2011 | Used myo‐inositol as a treatment intervention in women diagnosed with gestational diabetes, not as a preventative measure |
| Godfrey 2017 | Participants were women recruited before pregnancy and therefore do not meet the inclusion criteria for this review. |
| Matarrelli 2013 | Used myo‐inositol as a treatment intervention in women diagnosed with gestational diabetes, not as a preventative measure |
Characteristics of studies awaiting classification [ordered by study ID]
Esmaeilzadeh 2021.
| Methods | Study type: double‐blind randomised controlled trial |
| Participants | Inclusion criteria: 76 singleton, overweight, pregnant women (pre‐pregnancy body mass index ≥ 25 and < 30 kg/m2), aged 18 to 40, were enrolled at their first visit. |
| Interventions |
Intervention: daily intake of 2000 mg myo‐inositol plus 200 micrograms folic acid from 14 to 24 gestational weeks Control: daily intake of 400 µg of folic acid from 14 to 24 gestational weeks |
| Outcomes |
Primary outcome: gestational diabetes at 24 to 28 gestational weeks Secondary outcomes: evaluation of insulin resistance and lipid profile, insulin therapy, inappropriate gestational weight gain, caesarean section, pregnancy‐induced hypertension, pre‐eclampsia, preterm delivery, fetal macrosomia, shoulder dystocia, neonatal respiratory distress syndrome, and neonatal intensive care unit (NICU) admissions. The occurrence of adverse drug effects caused by intervention such as the presence of uterine contractions, headache, nausea, vomiting, diarrhoea, tiredness, and atulence were all assessed during follow‐up visits. |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Amaefule 2018.
| Study name | Effectiveness and acceptability of myoinositol nutritional supplement in the prevention of gestational diabetes (EMmY): a protocol for a randomised, placebo‐controlled, double‐blind pilot trial |
| Methods | Study type: multi‐centre randomised placebo‐controlled, double‐blind, pilot trial with a nested qualitative evaluation |
| Participants |
Inclusion criteria: pregnant women with singleton from 15+6 weeks’ gestation and one of the following risk factors: first degree family history of diabetes, previous gestational diabetes, obesity, minority ethnic family origin with a high prevalence of diabetes, PCOS or previous macrosomic baby. Target sample size: 200 women randomised to either intervention or placebo. An attrition rate of 20% is expected. |
| Interventions |
Intervention: myo‐inositol powder supplement to be taken in a dose of 2 g twice daily from 12+0 to 15+6 weeks’ gestational age until delivery Control: identical placebo in colour, flavour and texture to myo‐inositol powder taken at same dose and for the same duration |
| Outcomes |
Primary outcomes: proportion of eligible, consented and randomised participants Secondary outcomes: acceptability of the study and the intervention as well as the proportion of outcome measures obtained in the trial. Laboratory outcomes include diagnosis of gestational diabetes through fasting and 2‐hour postprandial 75 g OGTT. |
| Starting date | 1 April 2017 |
| Contact information | Dr Zoe Drymoussi Yvonne Carter Building 58 Turner Street London E1 2AB United Kingdom |
| Notes | ISRCTN48872100; Intention to publish date on protocol; 1 April 2020 |
Asimakopoulos 2020.
| Study name | Effect of dietary myo‐inositol supplementation on the insulin resistance and the prevention of gestational diabetes mellitus: study protocol for a randomised controlled trial |
| Methods | Study type: single‐centre, open‐label, randomised controlled trial |
| Participants |
Inclusion criteria: patients over 18 years old without pre‐existing impaired glucose tolerance and have a singleton pregnancy. Target sample size: 160 women to be enrolled. An estimated 10% rate of withdrawal and loss to follow‐up among participants. |
| Interventions |
Intervention: 4000 mg of myo‐inositol + 400 mcg of folic acid per day from 11 to 13+6 weeks of gestation until 26 to 28 weeks of gestation Control: 400 mcg of folic acid orally per day for the same time duration |
| Outcomes | Primary outcome: gestational diabetes incidence rate at 26 to 28 weeks of gestation |
| Starting date | 1 December 2017 |
| Contact information | Mr Georgios Asimakopoulos 24 Agias Elenis Street Athens 15772 Greece |
| Notes | ISRCTN16142533 Additional contact: Prof Georgios Daskalakis 80 Vasilissis Sofias Avenue Athens 11528 Greece Intention to publish date on protocol: 1 November 2022 |
CTRI/2018/06/014477.
| Study name | A clinical study to assess the potential of myo‐d‐chiro‐inositol in prevention of the development of gestational diabetes in pregnant women |
| Methods | Study type: randomised, parallel group, placebo‐controlled trial |
| Participants |
Inclusion criteria: 20 to 40 year‐old pregnant women between 11 to 14 weeks of gestation with no known history of diabetes and a pre‐gestational BMI ≤ 35. Target sample size: 1500 women |
| Interventions |
Intervention: Myo‐d‐chiro inositol + vitamin D3 sachets twice a day in water Control: placebo + vitamin D3 sachets twice a day in water |
| Outcomes | Primary outcome: preventing the development of gestational diabetes in pregnant women at 11 to 14 weeks and 24 to 28 weeks of gestation |
| Starting date | Date of first enrollment (India): 11 June 2018 |
| Contact information | Dr Hema Divakar ‐ Principal investigator and Clinical Director (Scientific Query) drhemadivakar@gmail.com |
| Notes | Alternative contact: Jestin V Thomas ‐ Director (Public Query) jestin.leadsclinbio@gmail.com |
Ibrahim 2022.
| Study name | Effect of antenatal dietary myo‐inositol supplementation on the incidence of gestational diabetes mellitus and fetal outcome |
| Methods | Study type: prospective, randomised, double‐blind, placebo‐controlled clinical trial |
| Participants | Inclusion criteria: 640 pregnant women attending antenatal care at Sidra Medicine |
| Interventions |
Intervention: myo‐inositol Control: placebo |
| Outcomes |
Primary outcome: gestational diabetes Secondary outcomes: gestational weight gain; need for metformin or insulin therapy; mode of delivery; hypertensive disorders of pregnancy; large for gestational age at delivery; small for gestational age at delivery; macrosomia; shoulder dystocia and birth injury; polyhydramnios; neonatal Intensive Care Unit (NICU) admission for > 24 hours; neonatal hypoglycaemia requiring intravenous glucose; preterm delivery (< 37 weeks gestation); transient tachypnoea of the newborn; respiratory distress syndrome (RDS). |
| Starting date | 15 October 2021 |
| Contact information | Ibrahim Ibrahim Ibrahim2002@doctors.org.uk |
| Notes |
IRCT20120826010664N4.
| Study name | The effect of myoinositol supplementation on the prevention of gestational diabetes mellitus in high‐risk women |
| Methods | Study type: randomised, multi‐center, clinical trial with parallel groups |
| Participants |
Inclusion criteria: BMI > 30, family history of type 2 diabetes, previous history of gestational diabetes, glucosoria, glucose metabolism disorder, and history of infant macrosomia. Exclusion criteria: history of diabetes mellitus, multiple pregnancies, acute infection during pregnancy, impaired OGTT in first‐trimester routine tests. Target sample size: 276 women |
| Interventions |
Intervention: 2 g of myo‐inositol + 200 mcg of folic acid twice per day from 14 to 28 weeks gestation. Control: 200 mcg folic acid twice per day |
| Outcomes | Primary outcome: rate of gestational diabetes and other side effects of gestational diabetes |
| Starting date | Expected recruitment start date: 20 February 2019 |
| Contact information | Dr Reihaneh Pirjani (Associate Professor) Tehran University of Medical Sciences pirjani@razi.tums.ac.ir |
| Notes | The study is not blinded and it states that placebo is not used. Registration date: 08 May 2019 Last update: 08 May 2019 |
NCT04801485.
| Study name | Myo‐inositol in prevention of gestational diabetes mellitus in China |
| Methods | Study type: randomised, double‐centred, placebo‐controlled study |
| Participants | Inclusion criteria: 360 pregnant women who is in high risk for gestational diabetes |
| Interventions |
Intervention: myo‐inositol 1 gram per day as well as health guidance about diet and exercise. From recruitment until OGTT Control: placebo (similar appearance but not containing myo‐inositol) 1 gram per day before meals. Similar health guidance about diet and exercise. From recruitment until OGTT |
| Outcomes |
Primary outcome: gestational diabetes Secondary outcome: macrosomia, weight gain during pregnancy, caesarean‐section incidence |
| Starting date | 1 January 2021 |
| Contact information | Danqing Chen Chendq@zju.edu.cn |
| Notes |
NL7799.
| Study name | MYPP‐trial: Myo‐inositol Supplementation to Prevent Pregnancy Complications in Women with Polycystic Ovary Syndrome: a multi‐centre double‐blind randomised controlled trial |
| Methods | Study type: multi‐centre double‐blind randomised controlled trial |
| Participants |
Inclusion criteria: women ≥ 18 years old with a singleton viable pregnancy, confirmed PCOS according to the Rotterdam consensus criteria, and ability to start supplements between 8+0 and 16+0 weeks gestational age. Target sample size: 464 women |
| Interventions |
Intervention: 4 g myo‐inositol in addition to folic acid supplement, divided over two daily sachets of sugary powder throughout pregnancy. Control: similar‐looking supplement sachets which contain the standard dose of folic acid without the added myo‐inositol supplement. |
| Outcomes | Primary outcome: composite outcome of either gestational diabetes mellitus, and or preeclampsia and or preterm birth |
| Starting date | 17 June 2019 |
| Contact information | Chryselle Frank Erasmus MC, University Medical Center Rotterdam c.frank@erasmusmc.nl |
| Notes | Study stop date: 17 June 2019 |
BMI: body mass index GDM: gestational diabetes mellitus OGTT: oral glucose tolerance test PCOS: polycystic ovarian syndrome
Differences between protocol and review
This updated review published in 2021 followed the criteria and outcomes used in the review published in 2015.
For the review published in 2015 there were some differences between published protocol (Brown 2015) as detailed below.
The title was listed as Myo‐inositol for preventing gestational diabetes in our published protocol but we have edited this to Antenatal dietary supplementation with myo‐inositol in women during pregnancy for preventing gestational diabetes in order to allow more clarity around the intervention, population and outcome.
Methods/criteria for considering studies for this review
Types of interventions: we have expanded this section to include myo‐inositol in a combination preparation; this is also reflected in our list of planned subgroup analyses.
Types of participants: we have clarified that participants will be pregnant women rather than pregnant women at risk of gestational diabetes.
We have incorporated the use of GRADE to assess the quality of the body of evidence and have included summary of findings tables; this was not pre‐specified in our published protocol.
We have reported on the outcome need for supplementary insulin therapy; whilst this is not listed in our methods and outcomes section (and was not pre‐specified in our published protocol), we report on this outcome for interest.
Following a consultative process with Professor Caroline Crowther, Dr Julie Brown, Dr Philippa Middleton, Emily Bain, and Tineke Crawford, a core set of primary and secondary outcomes for GDM systematic reviews and core outcomes for GRADE assessment for GDM systematic reviews were drawn up. This has resulted in a number of changes detailed below. These core outcomes were agreed upon after this review had been submitted for peer review.
Additionally, as this is a review on the use of a dietary supplement as an intervention, adverse effects of the intervention has been added as an outcome.
Previous maternal primary outcomes listed in protocol
Incidence of gestational diabetes (diagnostic criteria as defined in individual studies)
Pre‐eclampsia
Caesarean section
Updated maternal primary outcomes used in review
Gestational diabetes
Hypertensive disorders of pregnancy (including pre‐eclampsia, eclampsia, pregnancy‐induced hypertension)
Previous neonatal primary outcomes listed in protocol
Large‐for‐gestational age (birthweight greater than the 90th centile; or as defined by individual study)
Perinatal mortality
Death or morbidity composite (variously defined by studies, e.g. infant death, shoulder dystocia, bone fracture or nerve palsy)
Updated neonatal primary outcomes used in review
Large‐for‐gestational age (birthweight greater than the 90th centile; or as defined by individual study)
Perinatal mortality (stillbirth and neonatal mortality)
Mortality or morbidity composite (variously defined by studies, e.g. infant death, shoulder dystocia, bone fracture or nerve palsy)
Previous maternal secondary outcomes listed in protocol
Postnatal weight retention
Body mass index (BMI)
Development of type 1 diabetes mellitus
Development of type 2 diabetes mellitus
Impaired glucose tolerance (as defined in individual studies)
Insulin sensitivity (as defined in individual studies)
Incidence of pregnancy hyperglycaemia not meeting gestational diabetes diagnostic criteria (diagnostic criteria as defined in individual studies)
Induction of labour
Perineal trauma
Weight gain during pregnancy
Adiponectin levels
Gestational age at screening for gestational diabetes
Postpartum hemorrhage
Postpartum infection
Placental abruption
Polyhydramnios
Compliance with treatment
Breastfeeding at discharge, six weeks' postpartum
Women’s sense of well‐being and quality of life (as defined in individual studies)
Women’s view of intervention
Updated maternal secondary outcomes used in review
Caesarean section
Placental abruption
Induction of labour
Perineal trauma
Postpartum hemorrhage
Postpartum infection
Weight gain during pregnancy
Adherence to the intervention (as defined by trialists)
Behaviour changes associated with the intervention (as defined by trialists)
Relevant biomarker changes associated with the intervention (e.g. adiponectin, free fatty acids, triglycerides, high density lipoproteins, low density lipoproteins, insulin)
Sense of well‐being and quality of life
Views of the intervention
Breastfeeding (e.g. at discharge, six weeks postpartum)
Adverse effects of intervention
Long‐term maternal outcomes
Postnatal depression
Postnatal weight retention or return to pre‐pregnancy weight
Body mass index (BMI)
Gestational diabetes in a subsequent pregnancy
Type 1 diabetes mellitus
Type 2 diabetes mellitus
Impaired glucose tolerance
Cardiovascular health (as defined by trialists, including blood pressure (BP), hypertension, cardiovascular disease, metabolic syndrome)
Previous neonatal secondary outcomes listed in protocol
Macrosomia (as defined in individual studies)
Birthweight and z‐score
Head circumference and z‐score
Length and z‐score
Small‐for‐gestational age (as defined in individual studies)
Neonatal hypoglycaemia requiring treatment (as defined in individual studies)
Gestational age at birth
Preterm birth (less than 37 weeks’ gestational age)
Shoulder dystocia
Bone fracture
Nerve palsy
Respiratory distress syndrome
Hyperbilirubinaemia requiring treatment (as defined in individual studies)
Apgar scores (less than seven at five minutes)
Ponderal index
Fetal adiposity (as defined in individual studies)
Neonatal glucose concentration
Infant mortality (fetal, neonatal, perinatal)
Updated secondary outcomes used in review
Stillbirth
Neonatal mortality
Gestational age at birth
Preterm birth (less than 37 weeks' gestation and less than 32 weeks' gestation)
Apgar score (less than seven at five minutes)
Macrosomia
Small‐for‐gestational age
Birthweight and z‐score
Head circumference and z‐score
Length and z‐score
Ponderal index
Adiposity
Shoulder dystocia
Bone fracture
Nerve palsy
Respiratory distress syndrome
Hypoglycaemia (variously defined)
Hyperbilirubinaemia
Previous childhood outcomes listed in protocol
Weight
Height
Head circumference
Body mass index
Adiposity (fat mass/fat free mass (variously measured))
Blood pressure
Impaired glucose tolerance (as defined in individual studies)
Development of type 1 diabetes mellitus
Development of type 2 diabetes mellitus
Insulin sensitivity
Dyslipidaemia or metabolic syndrome
Neurodisability
Educational achievement
Updated childhood outcomes used in review
Weight and z scores
Height and z scores
Head circumference and z scores
Adiposity (e.g. as measured by BMI, skinfold thickness)
Blood pressure
Type 1 diabetes mellitus
Type 2 diabetes mellitus
Impaired glucose tolerance
Dyslipidaemia or metabolic syndrome
Neurodisability
Educational achievement
Previous adulthood outcomes listed in protocol
Weight
Height
BMI
Adiposity (fat mass/fat‐free mass (variously measured))
Blood pressure
Impaired glucose tolerance (as defined in individual studies)
Development of type 1 diabetes
Development of type 2 diabetes
Insulin sensitivity (as defined in individual studies)
Dyslipidaemia or metabolic syndrome
Educational achievement
Updated adulthood outcomes used in review
Weight
Height
Adiposity (e.g. as measured by BMI, skinfold thickness)
Cardiovascular health (as defined by trialists, including BP, hypertension, cardiovascular disease, metabolic syndrome)
Type 1 diabetes mellitus
Type 2 diabetes mellitus
Impaired glucose tolerance
Dyslipidaemia or metabolic syndrome
Employment, education and social status/achievement
Previous health services cost outcomes listed in protocol
Number of hospital visits or health professional visits (e.g. midwife, obstetrician, physician, dietitian)
Antenatal visits for mother
Direct costs to families in relation to the management provided
Length of postnatal stay (mother)
Admission to neonatal ward/ neonatal intensive care unit
Length of postnatal stay (baby)
Cost of maternal care
Cost of offspring care
Updated health services cost outcomes used in review
Number of hospital or health professional visits (e.g. midwife, obstetrician, physician, dietitian, diabetic nurse)
Number of antenatal visits or admissions
Length of antenatal stay
Neonatal intensive care unit admission
Length of postnatal stay (mother)
Length of postnatal stay (baby)
Costs to families associated with the management provided
Costs associated with the intervention
Cost of maternal care
Cost of offspring care
Previous GRADE outcomes listed in protocol
Incidence of gestational diabetes (diagnostic criteria as defined in individual studies)
Pre‐eclampsia
Mode of birth
Large‐for‐gestational age (birthweight greater than the 90th centile; or as defined by individual study)
Perinatal mortality
Fetal adiposity
Impaired glucose tolerance as child/adult
Updated GRADE outcomes used in review
Maternal
Diagnosis of gestational diabetes
Gestational weight gain
Hypertensive disorders of pregnancy (including pre‐eclampsia, eclampsia, pregnancy‐induced hypertension)
Caesarean Section
Perineal trauma
Postnatal depression
Development of subsequent type 2 diabetes mellitus
Offspring (infant, child, adult)
Large‐for‐gestational age
Perinatal mortality (stillbirth and neonatal mortality)
Composite of serious neonatal outcomes
Neonatal hypoglycaemia (variously defined)
Offspring adiposity (e.g. as measured by BMI, skinfold thickness)
Offspring diabetes
Neurosensory disability
Contributions of authors
Luling Lin is guarantor for this review.
Soana Motuhifonua and Luling Lin, with support from Jane Alsweiler and Caroline Crowther, screened the search results, retrieved relevant papers, screened retrieved papers against eligibility criteria, appraised quality of papers, extracted data from papers, wrote to authors of papers for additional information, entered data into RevMan, analysed and interpreted data, wrote the first draft of this updated review, and incorporated feedback into subsequent versions of the review.
Jane Alsweiler provided a neonatal clinical perspective, and contributed to all versions of the review.
Tineke Crawford provided feedback on versions of the review.
Caroline Crowther provided a maternal fetal medicine clinical and methodological perspective and contributed to all versions of the review.
Sources of support
Internal sources
-
The Liggins Institute, The University of Auckland, New Zealand
Provision of support for authors preparing reviews in pregnancy, childbirth and neonatal care.
External sources
-
The Australian and New Zealand Satellite of the Cochrane Pregnancy and Childbirth Review Group, Auckland, New Zealand
Provision of support for authors preparing reviews in pregnancy, childbirth and neonatal care within Australia and New Zealand.
-
The Cochrane Pregnancy and Childbirth Review Group editorial team, Liverpool, UK
Providing full editorial support.
Declarations of interest
Soana K Motuhifonua: reports no conflicts of interest.
Jane Alsweiler: reports working as a health professional as a Neonatal Paediatrician, Auckland District Health Board, but no other conflicts of interest.
Tineke J Crawford: reports no conflicts of interest.
Luling Lin: reports no conflicts of interest.
Caroline A Crowther: reports no conflicts of interest.
These authors contributed equally to this work
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
D'Anna 2013 {published data only}
- D'Anna R, Scilipoti A, Giordano D, Caruso C, Cannata ML, Interdonato ML, et al. Myo-inositol supplementation and onset of gestational diabetes mellitus in pregnant women with a family history of type 2 diabetes: a prospective, randomized, placebo-controlled study. Diabetes Care 2013;36(4):854-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01511835. Efficacy of Myo-inositol in preventing gestational diabetes in high-risk pregnant women. clinicaltrials.gov/ct2/show/NCT01511835 (first received 2011).
- Santamaria A, Alibrandi A, Di Benedetto A, Pintaudi B, Corrado F, Facchinetti F, et al. Clinical and metabolic outcomes in pregnant women at risk for gestational diabetes mellitus supplemented with myo-inositol: a secondary analysis from 3 RCTs. American Journal of Obstetrics and Gynecology 2018;219(3):300.e1-300.e6. [DOI] [PubMed] [Google Scholar]
D'Anna 2015 {published data only}
- D'Anna R, Di Benedetto A, Scilipoti A, Santamaria A, Interdonato ML, Petrella E, et al. Myo-Inositol supplementation for prevention of gestational diabetes in obese pregnant women. a randomized controlled trial. Obstetrics and Gynecology 2015;126(2):310-5. [DOI] [PubMed] [Google Scholar]
- D'Anna R, Santamaria A, Corrado F, Di Benedetto A, Petrella E, Facchinetti F. Myo-inositol in the prevention of gestational diabetes and its complications. Pregnancy Hypertension 2015;5(1):6. [DOI: 10.1016/j.preghy.2014.10.015] [DOI] [Google Scholar]
- Facchinetti F, Pignatti L, Interdonato ML, Neri I, Bellei G, D'Anna R. Myoinositol supplementation in pregnancies at risk for gestational diabetes. Interim analysis of a randomized controlled trial. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl):S36. [Google Scholar]
- NCT01047982. Myo-inositol for preventing gestational diabetes in overweight and obese women. clinicaltrials.gov/show/NCT01047982 (first received 2010).
- Santamaria A, Alibrandi A, Di Benedetto A, Pintaudi B, Corrado F, Facchinetti F, et al. Clinical and metabolic outcomes in pregnant women at risk for gestational diabetes mellitus supplemented with myo-inositol: a secondary analysis from 3 RCTs. American Journal of Obstetrics and Gynecology 2018;219(3):300.e1-300.e6. [DOI] [PubMed] [Google Scholar]
Farren 2017 {published data only}
- Farren M, Daly N, McKeating A, Kinsley B, Turner MJ, Daly S. The prevention of gestational diabetes mellitus with antenatal oral inositol supplementation: a randomized controlled trial. Diabetes Care 2017;40(6):759-763. [DOI] [PubMed] [Google Scholar]
- Farren M, Daly N, McKeating A, Turner MJ, Daly S. The role of myo-inositol/D-chiro-inositol in a physiological ratio of 40: 1 in preventing the onset of gestational diabetes mellitus. British Journal of Obstetrics and Gynaecology 2016;123(13):e3. [Google Scholar]
- ISRCTN92466608. A trial to investigate the role of the food supplement inositol in the general health of those at risk of developing gestational diabetes mellitus. www.isrctn.com/ISRCTN92466608 (first received 2013).
Malvasi 2014 {published data only}
- Malvasi A, Casciaro F, Minervini MM, Kosmas I, Mynbaev OA, Pacella E, et al. Myo-inositol, D-chiro-inositol, folic acid and manganese in second trimester of pregnancy: a preliminary investigation. European Review for Medical and Pharmacological Sciences 2014;18(2):270-4. [PubMed] [Google Scholar]
Malvasi 2017 {published data only}
- Malvasi A, Kosmas I, Mynbaev OA, Sparic R, Gustapane S, Guido M, Tinelli A. Can transresveratrol plus d-chiro-inositol and myo-inositol improve maternal metabolic profile in overweight pregnant patients? Clinica Terapeutica 2017;168(4):e240-7. [DOI] [PubMed] [Google Scholar]
Santamaria 2016 {published data only}
- Santamaria A, Alibrandi A, Di Benedetto A, Pintaudi B, Corrado F, Facchinetti F, et al. Clinical and metabolic outcomes in pregnant women at risk for gestational diabetes mellitus supplemented with myo-inositol: a secondary analysis from 3 RCTs. American Journal of Obstetrics and Gynecology 2018;219(3):300.e1-300.e6. [DOI] [PubMed] [Google Scholar]
- Santamaria A, Di Benedetto A, Petrella E, Pintaudi B, Corrado F, D'Anna R, et al. Myo-inositol may prevent gestational diabetes onset in overweight women: a randomized, controlled trial. Journal of Maternal-Fetal and Neonatal Medicine 2016;29(19):3234-37. [DOI] [PubMed] [Google Scholar]
Vitale 2019 {published data only}
- Vitale SG, Corrado F, Caruso S, Di Benedetto A, Giunta L, Cianci A, et al. Myo-inositol supplementation to prevent gestational diabetes in overweight non-obese women: bioelectrical impedance analysis, metabolic aspects, obstetric and neonatal outcomes; a randomized and open-label, placebo-controlled clinical trial. International Journal of Food Sciences and Nutrition 2021;72(5):670-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Vitale SG, Corrado F. Myo-inositol supplementation to prevent gestational diabetes and effect on bioelectrical impendence analysis in overweight non-obese women: a randomized placebo-controlled trial. European Journal of Obstetrics Gynecology and Reproductive Biology 2019;234:e150. [Google Scholar]
References to studies excluded from this review
Celentano 2020 {published data only}
- Celentano C, Matarrelli B, Pavone G, Vitacolonna E, Mattei PA, Berghella V, et al. The influence of different inositol stereoisomers supplementation in pregnancy on maternal gestational diabetes mellitus and fetal outcomes in high-risk patients: a randomized controlled trial. Journal of Maternal Fetal and Neonatal Medicine 2020;33(5):743-51. [DOI] [PubMed] [Google Scholar]
Corrado 2011 {published data only}
- Corrado F, D'Anna R, Di Vieste G, Giordano D, Pintaudi B, Santamaria A, et al. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabetic Medicine 2011;28(8):972-5. [DOI] [PubMed] [Google Scholar]
Godfrey 2017 {published data only}
- Godfrey KM, Cutfield W, Chan S-Y, Baker PN, Chong Y-S, Aris IB, et al. Nutritional Intervention Preconception and During Pregnancy to Maintain Healthy Glucose Metabolism and Offspring Health ("NiPPeR"): study protocol for a randomised controlled trial. Trials 2017;18(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matarrelli 2013 {published data only}
- Matarrelli B, Vitacolonna E, D'angelo M, Pavone G, Mattei PA, Liberati M, et al. Effect of dietary myo-inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: a randomized controlled trial. Journal of Maternal-Fetal and Neonatal Medicine 2013;26(10):967-72. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Esmaeilzadeh 2021 {published data only}
- Esmaeilzadeh S, Ghadimi R, Mashayekhamiri S, Delavar MA, Basirat Z. The effect of myo-inositol supplementation on the prevention of gestational diabetes in overweight pregnant women: a randomized, double-blind, controlled trial; 16 April 2021, PREPRINT (Version 1). www.researchsquare.com/article/rs-260476/v1 (accessed April 2021). [DOI: 10.21203/rs.3.rs-260476/v1] [DOI]
- IRCT20160208026446N2. The impact of myo-inositol supplementation to gestational diabetes in overweight pregnant women. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20160208026446N2 (first received 2017). [CENTRAL: CN-01903667]
References to ongoing studies
Amaefule 2018 {published data only}
- Amaefule CE, Drymoussi Z, Dodds J, Sweeney L, Pizzo E, Daru J, et al. Effectiveness and acceptability of myo-inositol nutritional supplement in the prevention of gestational diabetes (EMmY): a protocol for a randomised, placebo-controlled, double-blind pilot trial. BMJ Open 2018;8(9):e022831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaefule CE, Sweeney L, Thangaratinam S. Effectiveness and acceptability of myo-inositol nutritional supplement in the prevention of gestational diabetes (EMmY): a randomised, placebo-controlled, double-blind multi-centred pilot trial with nested qualitative evaluation. British Journal of Obstetrics and Gynaecology 2019;126(S1):26. [CENTRAL: CN-01936587] [EMBASE: 627142813] [Google Scholar]
- Heighway JM, Drymoussi Z, Lanz D, Thangaratinam S. Approaches to data cleaning in a pilot study: (EMmY) Effectiveness and acceptability of myo-inositol nutritional supplement in the prevention of gestational diabetes: a pilot placebo controlled double blind randomised trial. Trials 2019;20:10. [CENTRAL: CN-02006813] [EMBASE: 629760350] [Google Scholar]
- ISRCTN48872100. Preventing gestational diabetes with myo-inositol supplement. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN48872100 (first received 2018). [CENTRAL: CN-01902369]
Asimakopoulos 2020 {published data only}
- Asimakopoulos G, Pergialiotis V, Anastasiou E, Antsaklis P, Theodora M, Vogiatzi E, et al. Effect of dietary myo-inositol supplementation on the insulin resistance and the prevention of gestational diabetes mellitus: study protocol for a randomized controlled trial. Trials 2020;21(1):633. [CENTRAL: CN-02130113] [EMBASE: 144473532] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN16142533. Myo-inositol supplementation for the prevention of gestational diabetes. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN16142533 (first received 2017). [CENTRAL: CN-01856278]
CTRI/2018/06/014477 {published data only}
- CTRI/2018/06/014477. A clinical study to assess the potential of myo-d-chiro-inositol in prevention of the development of GDM in pregnant women. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/06/014477 (first received 2018). [CENTRAL: CN-01905638]
Ibrahim 2022 {published data only}
- Ibrahim I, Abdullahi H, Fagier Y, Ortashi O, Terrangera A, Okunoye G. Effect of antenatal dietary myo-inositol supplementation on the incidence of gestational diabetes mellitus and fetal outcome: protocol for a double-blind randomised controlled trial. BMJ Open 2022;12(1):e055314. [CENTRAL: CN-02361232] [EMBASE: 636884973] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN16448440. The study of myo-inositol supplements for reducing the risk of gestational diabetes mellitus [Effect of antenatal dietary Myo-inositol supplementation in women during pregnancy on the incidence of gestational diabetes mellitus (GDM) and fetal outcome: a randomized controlled trial]. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN16448440 (first received 2021). [CENTRAL: CN-02279815]
IRCT20120826010664N4 {published data only}
- IRCT20120826010664N4. The effect of myoinositol supplementation on the prevention of gestational diabetes mellitus. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20120826010664N4 (first received 2019). [CENTRAL: CN-01971330]
NCT04801485 {published data only}
- NCT04801485. Myo-inositol in prevention of gestational diabetes mellitus in China [Myo-inositol in prevention of gestational diabetes mellitus in high risk pregnant women in China]. clinicaltrials.gov/show/NCT04801485 (first received 17 March 2021). [CENTRAL: CN-02242297]
NL7799 {published data only}
- NL7799. Mypp-trial: myo-inositol supplementation to prevent pregnancy complications in women with polycystic ovary syndrome: a multicentre double-blind randomised controlled trial. www.who.int/trialsearch/Trial2.aspx?TrialID=NL7799 (first received 2019). [CENTRAL: CN-01971105]
Additional references
Alberti 1998
- Alberti K, Zimmet P. Definition, diagnosis and classification of diabetes mellitus. Part 1: Diagnosis and classification of diabetes mellitus: World Health Organization Report. Diabetic Medicine 1998;15(7):539-53. [DOI] [PubMed] [Google Scholar]
Ali 2011
- Ali S, Dornhorst A. Diabetes in pregnancy: health risks and management. Postgraduate Medical Journal 2011;87(1028):417-27. [DOI] [PubMed] [Google Scholar]
Bain 2015
- Bain E, Crane M, Tieu J, Han S, Crowther CA, Middleton P. Diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2015, Issue 4. Art. No: CD010443. [DOI: 10.1002/14651858.CD010443.pub2] [DOI] [PubMed] [Google Scholar]
Boney 2005
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;115:e290–6. [DOI] [PubMed] [Google Scholar]
Carlisle 2017
- Carlisle JB. Data fabrication and other reasons for non-random sampling in 5087 randomised, controlled trial in anaesthetic and general medical journals. Anaesthesia 2017;72:944-95. [DOI] [PubMed] [Google Scholar]
Carlomagno 2011
- Carlomagno G, Unfer V, Buffo S, D'Ambrosio F. Myo-inositol in the treatment of premenstrual dysphoric disorder. Human Psychopharmacology: Clinical and Experimental 2011;26(7):526-30. [DOI] [PubMed] [Google Scholar]
Chiswick 2015
- Chiswick C, Reynolds R, Denison F, Drake A, Forbes S, Newby D, et al. Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinology 2015;3(10):778-86. [DOI: 10.1016/S2213-8587(15)00219-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Croze 2013
- Croze ML, Soulage CO. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013;95(10):1811-27. [DOI] [PubMed] [Google Scholar]
D'Anna 2012
- D'Anna R, Di Bendetto V, Rizzo P, Raffone E, Interdanto ML, Corado F, et al. Myo-inositol may prevent gestational diabetes in PCOS women. Gynecological Endocrinology 2012;28(6):440-2. [DOI] [PubMed] [Google Scholar]
Di Benedetto 2013
- Di Benedetto A, Giunta A, Ruffo MC, Cannizzaro D. New evidence on inositol supplementation in gestational diabetes [Nuove evidenze sul ruolo della supplementazione con inositolo nel diabete gestazionale]. Giornale Italiano di Diabetologia e Metabolismo 2013;33(4):199-203. [Google Scholar]
Ferrara 2007
- Ferrara A. Increasing prevalence of gestational diabetes mellitus. Diabetes Care 2007;30 (Suppl 2):S141-6. [DOI] [PubMed] [Google Scholar]
Genazzani 2008
- Genazzani AD, Lanzoni C, Ricchieri F, Jasonni VM. Myo-inositol administration positively affects hyperinsulinemia and hormonal parameters in overweight patients with polycystic ovarian syndrome. Gynecological Endocrinology 2008;24:139-44. [DOI] [PubMed] [Google Scholar]
Glueck 2008
- Glueck CJ, Pranikoff J, Aregawi D, Wang P. Prevention of gestational diabetes by metformin plus diet in patients with polycystic ovarian syndrome. Fertility and Sterility 2008;89:625-34. [DOI] [PubMed] [Google Scholar]
Guo 2018
- Guo X, Guo S, Miao Z, Li Z, Zhang H. Myo-inositol lowers the risk of developing gestational diabetic mellitus in pregnancies: a systematic review and meta-analysis of randomized controlled trials with trial sequential analysis. Journal of Diabetes and Its Complications 2018;32:342-8. [DOI] [PubMed] [Google Scholar]
Han 2012
- Han S, Crowther CA, Middleton P. Exercise for pregnant women for preventing gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2012, Issue 7. Art. No: CD009021. [DOI: 10.1002/14651858.CD009021.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.cochrane-handbook.org.
IADPSG 2010
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33(3):676-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kennington 1990
- Kennington AS, Hill CR, Craig J, Bogardus C, Raz I, Ortmeyer HK, et al. Low urinary chiro-inositol excretion in non-insulin-dependent diabetes mellitus. New England Journal of Medicine 1990;323:373-8. [DOI] [PubMed] [Google Scholar]
Kim 2002
- Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25:1862-8. [DOI] [PubMed] [Google Scholar]
Minozzi 2008
- Minozzi M, D'Andrea G, Unfer V. Treatment of hirsutism with myo-inositol: a prospective clinical study. Reproductive Biomedicine Online 2008;17(4):579-82. [DOI] [PubMed] [Google Scholar]
MoH 2014
- MInistry of Health. Screening, diagnosis and management of gestational diabetes in New Zealand: a clinical practice guideline. Wellington: Ministry of Health, 2014. [Google Scholar]
Nankervis 2013
- Nankervis A, Conn J. Gestational diabetes mellitus-negotiating the confusion. Australian Family Physician 2013;42(8):528-31. [PubMed] [Google Scholar]
Nestler 1999
- Nestler JE, Jakubowicz DJ, Reamer P, Gunn RD, Allan G. Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome. New England Journal of Medicine 1999;340:1314-20. [DOI] [PubMed] [Google Scholar]
NICE 2015
- National Institute for Health and Clinical Excellence. Diabetes in Pregnancy: Management of Diabetes and its Complications from Pre-conception to the Postnatal Period. NICE guidelines [NG3]. London (UK): NICE, 2015. [Google Scholar]
Nordio 2013
- Nordio M, Pajalich R. Combined treatment with myo-inositol and selenium ensures euthyroidism in subclinical hypothyroidism patients with autoimmune thyroiditis. Journal of Thyroid Research 2013;2013:424163. [DOI: 10.1155/2013/424163] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noventa 2016
- Noventa M, Vitagliano A, Quaranta M, Borgato S, Abdulrahim B, Gizzo S. Preventive and therapeutic role of dietary inositol supplementation in periconceptional period and during pregnancy: a summary of evidences and future applications. Reproductive Sciences 2016;23(3):278-88. [DOI] [PubMed] [Google Scholar]
Papaleo 2007
- Papaleo E, Unfer V, Baillargeon JP, De Santis L, Fusi F, Brigante C, et al. Myoinositol in patients with polycystic ovarian syndrome: a novel method for ovulation induction. Gynecological Endocrinology 2007;23:700-3. [DOI] [PubMed] [Google Scholar]
Pettitt 1983
- Pettitt DJ, Baird HR, Aleck KA, Bennett PH, Knowler WC. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. New England Journal of Medicine 1983;308:242-5. [DOI] [PubMed] [Google Scholar]
Pettitt 1988
- Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC. Congenital susceptibility to NIDDM: role of intrauterine environment. Diabetes 1988;37:622-8. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Rogozinska 2015
- Rogozinska E, Chamillard M, Hitman G, Khan K, Thangaratinam S. Nutritional manipulation for the primary prevention of gestational diabetes mellitus: a meta-analysis of randomised studies. PLoS One 2015;10(2):e0115526. [DOI: 10.1371/journal.pone.0115526] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silverman 1998
- Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment: The Northwestern University Diabetes in Pregnancy Center. Diabetes Care 1998;21 (Suppl 2):B142-B149. [PubMed] [Google Scholar]
Suzuki 1994
- Suzuki S, Kawasaki H, Satoh Y. Urinary chiro-inositol excretion is an index marker of insulin sensitivity in Japanese type 2 diabetes. Diabetes Care 1994;17:1465-8. [DOI] [PubMed] [Google Scholar]
Tang 2012
- Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database of Systematic Reviews 2012, Issue 5. Art. No: CD003053. [DOI: 10.1002/14651858.CD003053.pub5] [DOI] [PubMed] [Google Scholar]
Tieu 2017
- Tieu J, Shepherd E, Middleton P, Crowther CA. Dietary advice in pregnancy for preventing gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No: CD006674. [DOI: 10.1002/14651858.CD006674] [DOI] [Google Scholar]
Unfer 2011
- Unfer V, Raffone E, Rizzo P, Buffo S. Effect of a supplementation with myo-inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: a prospective, longitudinal, cohort study. Gynecological Endocrinology 2011;27:857-61. [DOI] [PubMed] [Google Scholar]
Vitagliano 2019
- Vitagliano A, Saccone G, Cosmi E, Visentin S, Dessole F, Ambrosini G, et al. Inositol for the prevention of gestational diabetes: a systematic review and meta‑analysis of randomized controlled trials. Archives of Gynecology and Obstetrics 2019;299:55-68. [DOI] [PubMed] [Google Scholar]
Vounzoulaki 2020
- Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ 2020;369:m1361. [DOI: 10.1136/bmj.m1361.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2019
- Zhang H, Lv Y, Li Z, Sun L, Guo W. The efficacy of myo-inositol supplementation to prevent gestational diabetes onset: a meta-analysis of randomized controlled trials. Journal of Maternal-fetal & Neonatal Medicine 2019;32(13):2249-55. [DOI] [PubMed] [Google Scholar]
Zheng 2015
- Zheng X, Liu Z, Zhang Y, Lin Y, Song J, Zheng L, Lin S. Relationship between myo-inositol supplementary and gestational diabetes mellitus. Medicine 2015;94(42):e1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2016
- Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Current Diabetes Reports 2016;16(1):7. [DOI: 10.1007/s11892-015-0699-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Brown 2015
- Brown J, Crawford TJ, Alsweiler J, Crowther CA. Myo-inositol for preventing gestational diabetes. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD011507. [DOI: 10.1002/14651858.CD011507] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crawford 2015
- Crawford TJ, Crowther CA, Alsweiler J, Brown J. Antenatal dietary supplementation with myo-inositol in women during pregnancy for preventing gestational diabetes. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD011507. [DOI: 10.1002/14651858.CD011507.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


