Abstract
Background
The literature has not distinguished between LF “hypertrophy” and “buckling” when addressing cervical spondylotic myelopathy. The identification of buckling on dynamic magnetic resonance imaging can determine the levels for decompression more accurately and modify the surgical plan accordingly. No studies have been performed in the cervical spine to analyze the factors affecting LF buckling.
Purpose
Our objective was to investigate the factors affecting static ligamentum flavum (LF) “hypertrophy” and dynamic LF “buckling.”
Study Design
Retrospective cohort study.
Methods
We conducted a retrospective study of hospital records and imaging database from January 2014 to January 2020. The relation of age, disc height, and intervertebral instability to LF hypertrophy and buckling were assessed.
Results
Measurements were performed from C2-3 to C7-T1 in 169 patients who satisfied the eligibility criteria, making a total of 1014 levels. The samples were divided into 2 groups: 798 levels with buckling <1 mm (group A) and 216 levels with buckling >1 mm (group B). Of those, 161 levels satisfied the criteria for radiological instability (sagittal translation/rotation). No correlation was observed between age/disc height and buckling. Intervertebral instability showed significant association (P = 0.046) with buckling. No correlation was found between age/intervertebral instability and hypertrophy.
Conclusion
LF buckling but not hypertrophy is related to intervertebral instability in the cervical spine. LF buckling in the cervical spine is not related to age or disc height in the cervical spine.
Clinical Relevance
Intervertebral instability on dynamic x-ray imaging of the cervical spine can be a predictor of ligamentum flavum buckling and can be utilized for surgical planning.
Level of Evidence
3.
Keywords: ligamentum flavum, buckling, hypertrophy, instability, disc height
INTRODUCTION
Cervical spondylotic myelopathy (CSM) is the most common cause of spinal cord dysfunction in adults older than 55 years.1 The diagnosis of CSM is based on clinical symptoms and physical examination and correlated with radiological imaging including x-ray imaging, computed tomography, and magnetic resonance imaging (MRI).2 MRI can demonstrate the status of disc degeneration, the compression on spinal cord, and signal changes within the spinal cord and is considered the gold standard for assessment of CSM. Recent studies have reported that a static MRI may not reveal the cord compression in some cases, and dynamic evaluation of cervical spine is necessary.3–6 A static neutral MRI can reveal factors such as hypertrophy of the ligamentum flavum (LF) and bulging of the posterior margin of the intervertebral disc, which can cause cord compression. Dynamic factors such as LF buckling, in particular, can be revealed using a dynamic MRI. Identifying LF buckling on dynamic MRI can determine the levels for decompression more accurately and modify the surgical plan accordingly.3–5,7
LF thickening on MRI can be due to either LF “hypertrophy” or “buckling.” The literature has not distinguished between LF hypertrophy and buckling when addressing CSM. LF is composed of 80% elastic fibers and 20% collagen fibers. The elastic fibers within the LF prevent it from buckling into the vertebral canal with neck movements.8 LF “hypertrophy” is probably due to inflammation and following hypertrophic scar formation caused by repeated microtears within the ligament.8 Replacement of elastic fibers with collagen can cause the LF to thicken and result in canal stenosis. However, the reasons for LF “buckling” into the spinal canal are unclear. A few studies have reported LF buckling following reduction of the disc space in the lumbar spine.9–11 Studies with contrary findings reported that disc height loss is a gradual process in disc degeneration and the elastic nature of LF prevents it from buckling.12 Mechanical stress has been reported to result in LF thickening in the lumbar spine.13 Dynamic MRI utilizing flexion and extension views can help in distinguishing hypertrophy and buckling by noting the difference in the thickness of LF in flexion and extension.14 To our knowledge, no studies have been performed in the cervical spine to analyze the factors affecting LF buckling.
Objectives of the Study
To investigate the relationship between dynamic LF buckling and intervertebral instability/disc height in the cervical spine.
To investigate the relationship between static LF hypertrophy and intervertebral instability/disc height in the cervical spine.
MATERIALS AND METHODS
A retrospective cohort analysis of hospital records and imaging database of a tertiary care referral center from January 2014 to January 2020 was performed. The place of study accepts retrospective studies without an Institutional Review Board approval. Radiological measurements were performed at all cervical levels from C2-C3 to C7-T1 on the subjects who satisfied the eligibility criteria. The associations between LF buckling and LF hypertrophy with age, disc height, and intervertebral instability were assessed. The inclusion criteria were as follows:
Patients with a documented clinical diagnosis of CSM.
Evidence of radiological findings in support of diagnosis of CSM.
Imaging database has neutral, adequate flexion and extension x-ray images of the cervical spine, neutral static MRI and dynamic (flexion and extension) MRI available.
In addition, the following exclusion criteria were applied:
Patients with a documented clinical diagnosis of cervical myelopathy due to other causes (eg, noncompressive myelopathies, compressive myelopathies due to intradural/ intramedullary compression, compressive myelopathies secondary to trauma/tumor/infection/congenital anomalies).
No evidence of spinal cord compression on radiological imaging.
Imaging database has one or more of the following unavailable: neutral, adequate flexion and extension x-ray images of the cervical spine, neutral static MRI and dynamic (flexion and extension) MRI.
Patients of all ages and both sexes were included in the study. Screening of all medical records was done utilizing the electronic database, and details of patients with documented clinical diagnosis of cervical myelopathy were retrieved. Imaging database was then screened to identify those patients who satisfied the eligibility criteria.
Demographic details such as age, sex, and duration of symptoms were retrieved from the database. Flexion and extension x-ray images were considered adequate if the occiput and superior end plate of T1 were visible, and there was a 30° of movement from the neutral position in either films.15 Intervertebral instability was defined as the change in sagittal translation >3.5 mm or 20% or sagittal plane rotation >12° on flexion extension x-ray images16 (Figures 1 and 2). Neutral static T2-weighted MRI sequences were used to assess the disc height by measuring the distance between the adjacent end plates at the center of each disc (Figure 3). Midsagittal T2-weighted MRI sequences were used to measure the thickness of the LF at each level from C2-C3 to C7-T1 on dynamic MRI (flexion and extension views). The difference in thickness of LF between flexion and extension in the midsagittal cuts was taken as the amount of LF buckling (Figure 4). Two blinded, independent, and experienced spine surgeons (>5 years) performed the measurements, and the mean values were considered.
Figure 1.
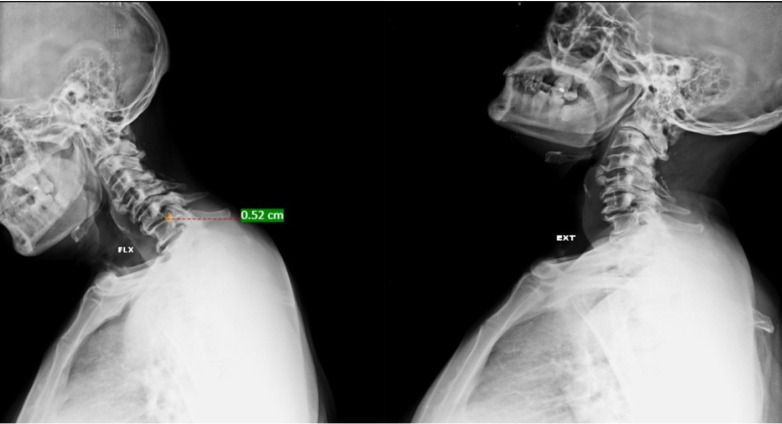
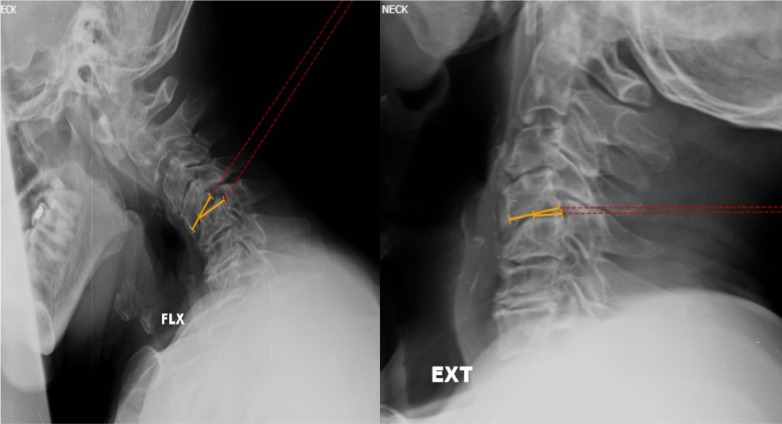
Representative adequate dynamic x-ray images of a patient with cervical myelopathy depicting measurement of sagittal translational instability at C6-C7 level. Lines were drawn along the posterior border of the superior and inferior vertebrae, and the horizontal distance between the lines was measured. A difference of more than 3.5-mm translation or 20% was considered as sagittal translational instability.
Figure 2.
Representative dynamic x-ray images of a patient with cervical myelopathy depicting measurement of sagittal rotational instability at C4-C5 level. Lines were drawn along the inferior end plate of the superior vertebra and the superior end plate of the inferior vertebra, and the angle between these lines was measured. The difference in angles formed between these lines in flexion and extension views was measured. A difference of more than 12° was considered as sagittal rotational instability.
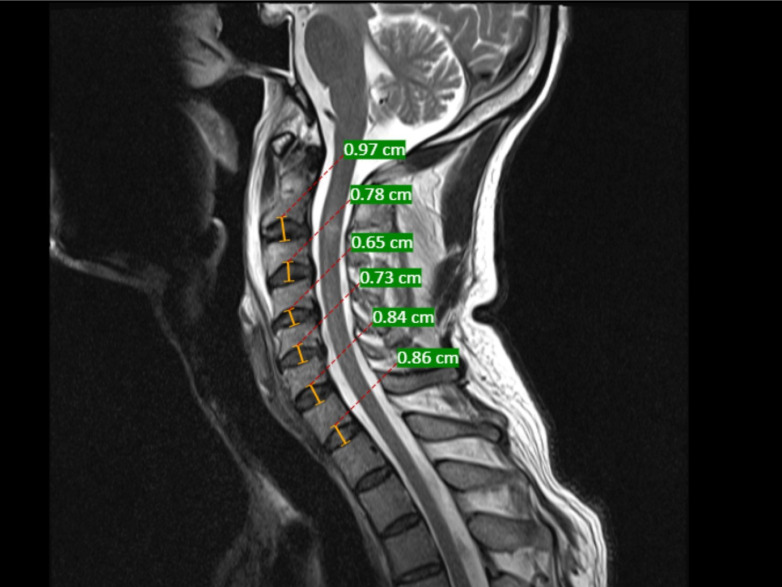
Figure 3.
Representative neutral T2-weighted sagittal magnetic resonance imaging image of the cervical spine depicting the measurement of disc height. Disc height was measured from the center of the inferior end plate of superior vertebra to the center of the superior end plate of the inferior vertebra.
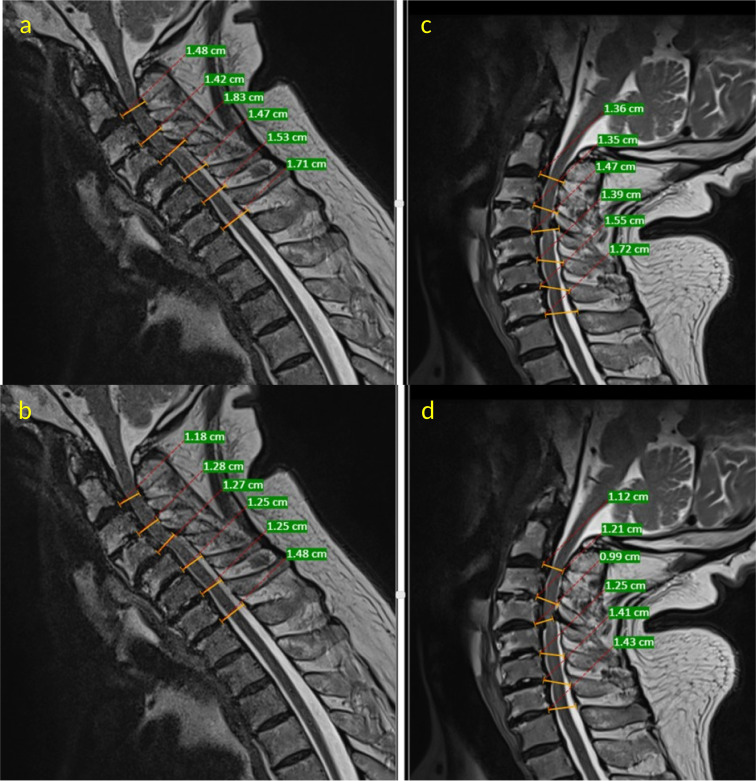
Figure 4.
Representative neutral T2-weighted sagittal magnetic resonance imaging (MRI) images of the cervical spine depicting measurements for ligamentum flavum (LF) thickness and buckling. At the midlevel of the intervertebral disc, 2 linear lines were drawn. One line measured the distance from the posterior margin of the disc to the base of spinous process (A) and another line measured the distance from the posterior margin of the disc to the posterior border of the cerebrospinal fluid (b) in a flexion MRI. The difference in value obtained (A, B) represents the LF thickness in flexion. (C) and (D) represent similar measurements performed on an extension MRI. The LF thickness in extension is represented by value (c-d). The difference in LF thickness in flexion and extension (C, D-[A, B]) is considered as buckling.
Statistical Analysis
Statistical analysis was performed using IBM Corp (released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp). Results on continuous measurements such as age, LF hypertrophy, LF buckling, disc height, sagittal translation, and sagittal rotation are presented as mean and standard deviation (min-max). Results on categorical measurements such as sex, LF buckling, and instability (translational/rotational) are presented as numbers (n) and percentages. The significance is assessed at 5% level of significance. Repeat measures analysis of variance was used to compare the means of variables such as LF thickness/buckling across different cervical levels. To determine the correlation between 2 variables, Pearson correlation/ Spearman ρ was used as per the distribution and assumption. Χ 2/Fisher exact test was utilized to find the significance of study parameters such as LF buckling and instability on a categorical scale between 2 and more groups. Student t test (2 × one tailed)/Mann-Whitney U test was utilized to find the significance of parameters on a continuous scale between 2 groups as per the distribution of data. To evaluate the cutoff point for LF buckling, receiver operator characteristic (ROC) curve analyses were performed using DeLong criteria. Optimal cutoff values for ROC curves were determined using the Youden index criteria (area under curves, sensitivity, specificity, and 95% CI).
RESULTS
A total of 169 subjects who satisfied the eligibility criteria were included for the study. A flowchart depicting the selection process was provided in Figure 5. Measurements were performed from C2-C3 to C7-T1 in all the subjects, making a total of 1014 levels. Mean age of the study subjects was 59.5 ± 12.0 years (range, 22–82 years). Out of total study subjects 62.7% (106/169) were male patients. The mean distribution of LF thickness, LF buckling, disc height, sagittal translation, and sagittal plane rotation according to the levels, as measured on the dynamic x-ray images was provided in Table 1. Out of the 1014 total levels, 161 levels satisfied the criteria for radiological instability (sagittal translation/rotation). Except for disc height, rest of the parameters showed a significant difference between various cervical levels. The disc height showed a strong negative correlation with age (correlation coefficient = −0.145, P < 0.001). To account for measurement errors, cutoff value of >1 mm of buckling was considered (validated by ROC curve analysis). The entire sample size of 1014 levels are divided into 2 groups based on the cutoff of ≤1 and >1 mm. A total of 798 levels had buckling ≤1 mm (group A) and 216 levels had buckling >1 mm (group B). The distribution of the groups according to the level is provided in Table 2.
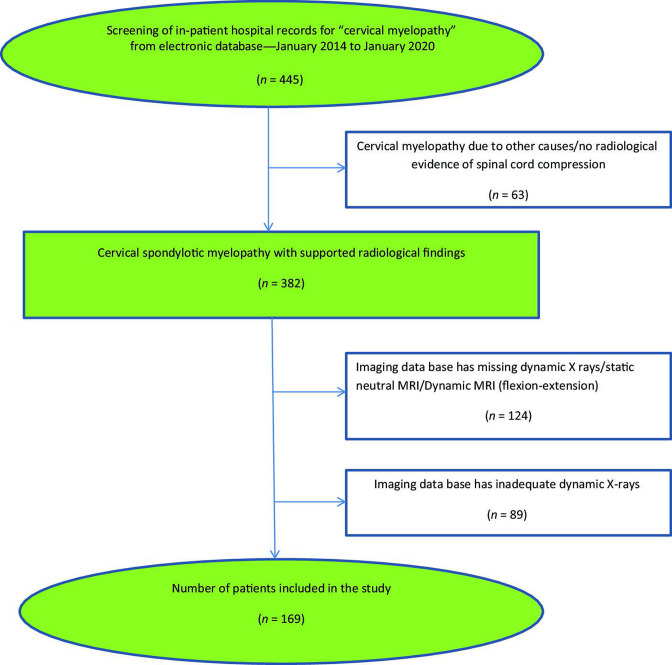
Figure 5.
A flowchart depicting the selection of study samples utilized for the study.
Table 1.
Descriptive statistics (mean ± standard deviation) according to the level. Analysis of variance (ANOVA) was used to compare the means of variables such as ligamentum flavum thickness/buckling across different cervical levels.
| Characteristic | C2-C3 | C3-C4 | C4-C5 | C5-C6 | C6-C7 | C7-T1 | P Valuea |
| Thickness | 0.26 ± 0.06 | 0.28 ± 0.07 | 0.29 ± 0.11 | 0.28 ± 0.09 | 0.27 ± 0.08 | 0.31 ± 0.08 | <0.001 |
| Buckling | 0.03 ± 0.03 | 0.05 ± 0.05 | 0.10 ± 0.08 | 0.08 ± 0.09 | 0.05 ± 0.07 | 0.03 ± 0.04 | <0.001 |
| Disc height | 0.71 ± 0.13 | 0.71 ± 0.21 | 0.72 ± 0.14 | 0.75 ± 0.15 | 0.72 ± 0.16 | 0.72 ± 0.20 | 0.331 |
| Translation | 0.26 ± 0.45 | 0.27 ± 0.35 | 0.27 ± 0.28 | 0.48 ± 0.63 | 0.30 ± 0.41 | 0.26 ± 0.25 | <0.001 |
| Rotation | 3.03 ± 0.87 | 3.32 ± 1.53 | 4.02 ± 3.61 | 3.73 ± 1.77 | 3.27 ± 1.63 | 2.56 ± 1.22 | <0.001 |
aRepeated measures ANOVA (Greenhouse-Geisser).
Table 2.
Distribution of the 2 groups of buckling (groups A and B) by cervical level.
| Level | No. of Levels (%) | |
| Group A, Buckling≤0.1 mm (n = 798) | Group B, Buckling>0.1 mm (n = 216) | |
| C2-C3 | 156 (19.5) | 13 (6.0) |
| C3-C4 | 136(17.0) | 33 (15.3) |
| C4-C5 | 97 (12.2) | 72 (33.3) |
| C5-C6 | 112 (14.0) | 57 (26.4) |
| C6-C7 | 148 (18.6) | 21 (9.7) |
| C7-T1 | 149 (18.7) | 20 (9.3) |
LF Buckling
1. Correlation With Age/Gender
Using Spearman ρ, no correlation was observed between buckling and age (P = 0.869). On correlation analysis of LF buckling with age at different levels using Pearson correlation coefficient, there was no significant correlation except at C7-T1 level (Table 3). Using Χ2 test, no correlation was observed between gender and LF buckling.
Table 3.
Correlation analysis of buckling with age at different levels. Only C7-T1 level had correlation of ligamentum flavum buckling with age.
| Age | C2-C3 | C3-C4 | C4-C5 | C5-C6 | C6-C7 | C7-T1 |
| Pearson correlation | 0.001 | 0.054 | 0.041 | −0.129 | −0.079 | 0.187a |
| P value | 0.986 | 0.487 | 0.594 | 0.095 | 0.310 | 0.015 |
aCorrelation is significant at the 0.05 level (2 tailed).
2. Correlation With Instability
Out of 798 levels in group A, 117 levels satisfied whereas 681 levels did not satisfy the criteria for instability. In group B, 44 out of 216 levels satisfied whereas 172 levels did not satisfy the criteria for instability. Fisher exact test showed significant association (exact significance [2 sided] = 0.042) between the instability and LF buckling.
3. Correlation With Disc Height
The disc height is significantly different in the 2 groups (groups A and B) for C3-C4 level (P < 0.001) and C6-C7 level (P < 0.001) (Table 4). Using Spearman ρ, no correlation was found between the disc height and amount of LF buckling (correlation coefficient = 0.055, P = 0.078). On analysis at each cervical level, only C4-C5 level was found to have a significant negative correlation between the disc height and LF buckling (correlation coefficient = −0.26, P = 0.001).
Table 4.
Disc height at different levels between the 2 groups of buckling.
| Level | Group A, Buckling≤0.1 mm (n = 798) | Group B, Buckling>0.1 mm (n = 216) | P Value | ||
| n | Mean (SD) | n | Mean (SD) | ||
| C2-C3 | 156 | 0.71 (0.14) | 13 | 0.74 (0.09) | 0.435 |
| C3-C4 | 136 | 0.68 (0.13) | 33 | 0.68 (0.13) | <0.001 |
| C4-C5 | 97 | 0.72 (0.71) | 72 | 0.71 (0.11) | 0.545 |
| C5-C6 | 112 | 0.76 (0.17) | 57 | 0.72 (0.10) | 0.068 |
| C6-C7 | 148 | 0.70 (0.17) | 21 | 0.83 (0.02) | <0.001 |
| C7-T1 | 149 | 0.72 (0.19) | 20 | 0.71 (0.28) | 0.901 |
LF Hypertrophy
1. Correlation With Age
No correlation was found between age and LF hypertrophy (correlation coefficient = 0.045, P = 0.157).
2. Correlation With Instability
The LF hypertrophy was not found to be significantly associated with instability (Pearson correlation, P = 0.199). Independent sample t test also does not show significant difference between the 2 categories of instability for LF hypertrophy (mean difference = 0.00935; P = 0.199).
3. Correlation With Disc Height
No correlation was found between LF thickness and disc height (correlation coefficient = 0.131, P < 0.001).
DISCUSSION
A significant association of intervertebral instability in the cervical spine with LF buckling but not with LF hypertrophy was observed in our study. Majority of the studies on LF hypertrophy and buckling have addressed the lumbar region. LF in the cervical region is thinner, broader, and longer compared to the lumbar spine. However, the relative canal occupancy is significant in the cervical spine and LF buckling may cause significant clinical symptoms.
LF Thickening: Buckling vs Hypertrophy
A normal LF constricts naturally in extension due to the presence of yellow elastin and prevents buckling of LF. Hypertrophied component of LF would maintain its thickness irrespective of the position of spine whereas the buckling would be revealed during extension of spine when the laminae get closer. Dynamic MRI would therefore, be able to differentiate the 2 components.
Capogna et al reported that there is no significant difference in the thickness of LF between flexed and supine positions in the lumbar region.17 Sayit et al noted significant difference in thickness of LF between neutral and flexion positions at C5-C6 and C6-C7 levels but not at other levels.14 However, when flexion and extension positions were compared, significant differences were found at C3-C4, C4-C5, C5-C6, and C6-C7 levels.14 These studies have not introspected into the biomechanical reasons for difference in LF thickening with neck position.
Factors Affecting LF Thickness
Factors such as age, spinal level, mechanical stress, and growth factors have been described as causes for LF thickening. Some authors claim that narrowing of the spinal canal is due to hypertrophy of LF whereas others claim that structural abnormalities and deformities of LF inside the spinal canal cause neural compression.18,19 Postacchini et al reported that an LF of normal thickness in the lumbar spine may bulge into the spinal canal in standing position.10 He proposed that disc space collapse with age-related degeneration may be a reason for LF buckling. Altinkaya et al in his study reported a significant correlation between LF thickening and disc degeneration.9 On the contrary, Sakamaki et al and Mattar et al did not find a correlation between LF thickening and disc degeneration.20,21 Safak et al also found no association in the thickness of the LF with respect to gender or age.19 This was similar to the findings in our study. Yoshiiwa et al conducted a study on 57 patients with normal disc height to eliminate buckling.13 He found that LF thickening was associated with age.
Disc Degeneration and LF Thickening
Disc degeneration, leading to facetal degenerative changes and a resulting instability, has been proposed to cause LF hypertrophy.13 Chokshi et al suggested that there may be an independent relationship between disc degeneration and LF hypertrophy.22 We could not find similar results in our study of the cervical spine.
Instability and LF Thickening
Based on observations of increased LF thickening at the L3-L4 and L4-L5 levels compared to L5-S1 level, Abbas et al reported hypermobility as the cause of LF thickening.23 Fukuyama et al in his observation of increased LF thickening at the levels with degenerative radiological signs such as spondylolisthesis and vaccum phenomenon suggested that mechanical instability may be a reason for LF thickening.24 The contributory role of instability to LF thickening has not been established in the cervical spine. We found that instability is related to buckling of LF rather than hypertrophy.
Pathogenesis of Buckling
LF may undergo degeneration due to age or trauma. The degenerative changes may cause the ligament to lose its elastic nature and can result in LF buckling. However, we could not find an association of LF buckling with age in our study. The LF maintains its elastic nature within the normal limits of motion and prevents buckling. In cases of intervertebral instability, the LF stretches beyond its physiological limits and could not keep its elastic behavior (Figure 6).25 As individual fibrils within the ligament fail, damage accumulates, stiffness reduces, and the ligament begins to fail. We hypothesize that this LF stretch beyond the physiological limits in case of intervertebral instability could lead to a failure of the elastic nature of the ligament and thereby LF buckling.
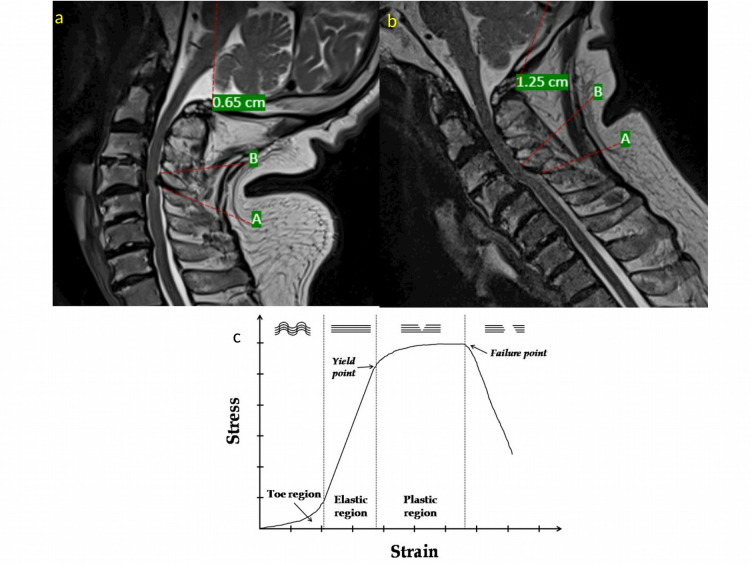
Figure 6.
(A, B) T2-weighted magnetic resonance images showing the changes in ligamentum flavum (LF) with neck motion. As the neck goes into flexion (B) from extension (A), the distance between the origin (marked A) and insertion (marked B) increases and puts strain on the LF. (C) Representation of a typical stress-strain curve of a ligament. The x-axis represents the strain on the ligament, and y-axis represents the stress applied to the ligament. Within the elastic region, repeated increases or decreases in tension load will not change the ligament length. The elastic region represents the tension loads that occur within the physiological range of motion of the neck. Beyond a point, the curve tends to flatten, indicating that the ligament deforms more per given increase in tension load (ie, the ligament undergoes permanent deformation [plastic deformation]). This is called the yield point. After the plastic region, the sudden failure of the ligament occurs and stress disappears. This is called the failure point. With permanent deformation or lengthening of the ligament, the LF tends to buckle into the spinal canal in extension because of the closer contact between the laminae (points A and B in Figure 6A and B).
Limitations
There are limitations to the study. First, there is a lack of control group. No data exist on dynamic MRI measurements in normal individuals. However, previous histological and clinical studies have reported that normal LF does not buckle into the spinal canal during extension due to presence of elastin. Second, it is a radiological study. Hence, errors such as exaggerated spinal components on MRI and issues with image resolution are possible. Also, we utilized sagittal T2-weighted MRI sequences to measure LF thickness due to lack of dynamic axial sequences. We utilized standard 1.5 Tesla MRI sequencing in all cases, and measurements were performed by 2 blinded and independent assessors to reduce errors. Third, we used dynamic x-ray images to identify segmental instability. Dynamic x-ray images are not highly sensitive, and there may be false negatives. However, dynamic x-ray images are being used at many centers to identify instability, and there is no other highly sensitive investigation to establish instability.
CONCLUSIONS
LF buckling can be predicted using dynamic x-ray images of the cervical spine. LF buckling and not hypertrophy is related to intervertebral instability in the cervical spine. LF buckling and hypertrophy in the cervical spine are not related to age or disc height in the cervical spine.
References
- 1.Montgomery DM, Brower RS. Cervical myelopathy: clinical syndrome and natural history. OrthopClin North Am. 1992;23:487–493. 10.1016/S0030-5898(20)31760-0 [DOI] [PubMed] [Google Scholar]
- 2.Lebl DR, Bono CM. Update on the diagnosis and management of cervical spondylotic myelopathy. J Am Acad Orthop Surg. 2015;23(11):648–660. 10.5435/JAAOS-D-14-00250 [DOI] [PubMed] [Google Scholar]
- 3.Dalbayrak S, Yaman O, Firidin MN, Yilmaz T, Yilmaz M. The contribution of cervical dynamic magnetic resonance imaging to the surgical treatment of cervical spondylotic myelopathy. Turk Neurosurg. 2015;25(1):36–42. 10.5137/1019-5149.JTN.9082-13.1 [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Zeitoun D, Rangel A, Lazennec JY, Catonné Y, Pascal-Moussellard H. Preoperative evaluation of the cervical spondylotic myelopathy with flexion-extension magnetic resonance imaging: about a prospective study of fifty patients. Spine (Phila Pa 1976). 2011;36(17):E1134-9. 10.1097/BRS.0b013e3181f822c7 [DOI] [PubMed] [Google Scholar]
- 5.Kim CH, Chung CK, Kim K-J, et al. Cervical extension magnetic resonance imaging in evaluating cervical spondylotic myelopathy. Acta Neurochir (Wien). 2014;156(2):259–266. 10.1007/s00701-013-1951-2 [DOI] [PubMed] [Google Scholar]
- 6.Xu N, Wang S, Yuan H, Liu X, Liu Z. Does dynamic supine magnetic resonance imaging improve the diagnostic accuracy of cervical spondylotic myelopathy? A review of the current evidence. World Neurosurg. 2017;100:474–479. 10.1016/j.wneu.2017.01.047 [DOI] [PubMed] [Google Scholar]
- 7.Harada T, Tsuji Y, Mikami Y, et al. The clinical usefulness of preoperative dynamic MRI to select decompression levels for cervical spondylotic myelopathy. Magn Reson Imaging. 2010;28(6):820–825. 10.1016/j.mri.2010.03.038 [DOI] [PubMed] [Google Scholar]
- 8.Cramer GD, Bakkum BW. Microscopic anatomy of the zygapophysial joints, intervertebral discs, and other major tissues of the back. In: Cramer CD, Darby SA, eds. Clinical Anatomy of the Spine, Spinal Cord, and Ans. 3rd ed. Amsterdam: Elsevier; 2014:586–637. [Google Scholar]
- 9.Altinkaya N, Yildirim T, Demir S, Alkan O, Sarica FB. Factors associated with the thickness of the ligamentum flavum: is ligamentum flavum thickening due to hypertrophy or buckling? Spine (Phila Pa 1976). 2011;36(16):E1093-7. 10.1097/BRS.0b013e318203e2b5 [DOI] [PubMed] [Google Scholar]
- 10.Postacchini F, Gumina S, Cinotti G, Perugia D, DeMartino C. Ligamenta flava in Lumbar disc herniation and spinal stenosis. Spine. 1994;19(8):917–922. 10.1097/00007632-199404150-00009 [DOI] [PubMed] [Google Scholar]
- 11.Okuda T, Baba I, Fujimoto Y, et al. The pathology of ligamentum flavum in degenerative lumbar disease. Spine (Phila Pa 1976). 2004;29(15):1689–1697. 10.1097/01.brs.0000132510.25378.8c [DOI] [PubMed] [Google Scholar]
- 12.Munns JJ, Lee JYB, Espinoza Orías AA, et al. Ligamentum flavum hypertrophy in asymptomatic and chronic low back pain subjects. PLoS One. 2015;10(5):e0128321. 10.1371/journal.pone.0128321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshiiwa T, Miyazaki M, Notani N, Ishihara T, Kawano M, Tsumura H. Analysis of the relationship between ligamentum flavum thickening and Lumbar segmental instability, disc degeneration, and facet joint osteoarthritis in Lumbar spinal stenosis. Asian Spine J. 2016;10(6):1132–1140. 10.4184/asj.2016.10.6.1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sayit E, Daubs MD, Aghdasi B, et al. Dynamic changes of the ligamentum flavum in the cervical spine assessed with kinetic magnetic resonance imaging. Global Spine J. 2013;3(2):69–74. 10.1055/s-0033-1337121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan SN, Erickson G, Sena MJ, Gupta MC. Use of flexion and extension radiographs of the cervical spine to rule out acute instability in patients with negative computed tomography scans. J Orthop Trauma. 2011;25(1):51–56. 10.1097/BOT.0b013e3181dc54bf [DOI] [PubMed] [Google Scholar]
- 16.White AA, Johnson RM, Panjabi MM, Southwick WO. Biomechanical analysis of clinical stability in the cervical spine. Clin Orthop Relat Res. 1975;(109):85–96. 10.1097/00003086-197506000-00011 [DOI] [PubMed] [Google Scholar]
- 17.Capogna G, Celleno D, Simonetti C, Lupoi D. Anatomy of the lumbar epidural region using magnetic resonance imaging: a study of dimensions and a comparison of two postures. Int J Obstet Anesth. 1997;6(2):97–100. 10.1016/s0959-289x(97)80005-2 [DOI] [PubMed] [Google Scholar]
- 18.Ahmadi SA, Suzuki A, Terai H, et al. Anatomical analysis of the human ligamentum flavum in the thoracic spine: clinical implications for posterior thoracic spinal surgery. J Orthop Sci. 2019;24(1):62–67. 10.1016/j.jos.2018.08.023 [DOI] [PubMed] [Google Scholar]
- 19.Safak AA, Is M, Sevinc O, et al. The thickness of the ligamentum flavum in relation to age and gender. Clin Anat. 2010;23(1):79–83. 10.1002/ca.20883 [DOI] [PubMed] [Google Scholar]
- 20.Sakamaki T, Sairyo K, Sakai T, Tamura T, Okada Y, Mikami H. Measurements of ligamentum flavum thickening at lumbar spine using MRI. Arch Orthop Trauma Surg. 2009;129(10):1415–1419. 10.1007/s00402-009-0849-1 [DOI] [PubMed] [Google Scholar]
- 21.Mattar T, Costa AB, Appolonio PR, Cesar AEM, Rodrigues LMR. Thickness of the ligamentum flavum of the spine and its relationship with disc degeneration. Coluna/Columna. 2014;13(2):112–115. 10.1590/S1808-18512014130200321 [DOI] [Google Scholar]
- 22.Chokshi FH, Quencer RM, Smoker WRK. The “thickened” ligamentum flavum: is it buckling or enlargement? AJNR Am J Neuroradiol. 2010;31(10):1813–1816. 10.3174/ajnr.A2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbas J, Hamoud K, Masharawi YM, et al. Ligamentum flavum thickness in normal and stenotic lumbar spines. Spine (Phila Pa 1976). 2010;35(12):1225–1230. 10.1097/BRS.0b013e3181bfca15 [DOI] [PubMed] [Google Scholar]
- 24.Fukuyama S, Nakamura T, Ikeda T, Takagi K. The effect of mechanical stress on hypertrophy of the lumbar ligamentum flavum. J Spinal Disord. 1995;8(2):126–130. 10.1097/00002517-199504000-00006 [DOI] [PubMed] [Google Scholar]
- 25.Korhonen RK, Saarakkala S. Biomechanics and Modeling of Skeletal Soft Tissues, Theoretical Biomechanics, Vaclav Klika [IntechOpen]. 2011. https://www.intechopen.com/books/theoretical-biomechanics/biomechanics-and-modeling-of-skeletal-soft-tissues. 10.5772/19975 [DOI]