Abstract
The recent global outbreak of the monkeypox (mpox) virus in humans was declared a public health emergency by the World Health Organization in July 2022. The smallpox and mpox vaccine (JYNNEOS; Modified Vaccinia Ankara-Bavarian Nordic; MVA-BN), provided as a two-dose regimen, is currently the primary vaccine utilized against mpox. However, the efficacy of MVA-BN against mpox has never been demonstrated in clinical trials to date. Due to the limited supply of vaccines, the World Health Organization has recommended prioritizing the vaccination of high-risk groups. We evaluated the real-world effectiveness of a single, subcutaneous dose of MVA-BN in this observational, retrospective cohort study, which included the analysis of electronic health records of all members of Clalit Health Services eligible for the vaccine on 31 July 2022. We used a Cox proportional hazards regression model with time-dependent covariates to estimate the association between vaccination and mpox while adjusting for sociodemographic and clinical risk factors. In an analysis of 2,054 male individuals who met vaccine eligibility criteria, 1,037 (50%) were vaccinated during the study recruitment period and completed at least 90 d of follow-up. During the study period, 5 and 16 infections were confirmed in vaccinated and unvaccinated individuals, respectively. The adjusted vaccine effectiveness was estimated at 86% (95% confidence interval, 59–95%). Our results suggest that a single dose of subcutaneous MVA-BN in this high-risk cohort is associated with a significantly lower risk of MPXV infection.
Subject terms: Infectious diseases, Epidemiology
Effectiveness of one subcutaneous dose of MVA-BN, the smallpox and mpox vaccine, was estimated to be 86% in a cohort of vaccine-eligible males in Israel, supporting its use to curtail the outbreak of mpox virus.
Main
The human mpox virus (MPXV) is a member of the Orthopoxvirus genus and is closely related to the virus that causes smallpox. The recent global outbreak of MPXV was first recognized in May 2022, when infections were reported in several countries where MPXV cases had not been previously identified1–3. On 23 July 2022, the Director-General of the World Health Organization (WHO) declared mpox a public health emergency of international concern4. By 22 December 2022, over 83,000 laboratory-confirmed cases were reported worldwide5. The smallpox and mpox vaccine (MVA-BN), a live attenuated Orthopoxvirus, is currently the preferred vaccine for mpox2,6. Official US Food and Drug Administration (FDA) prescribing information recommends providing the vaccine as a series of two subcutaneous doses administered 4 weeks apart7. Nevertheless, because of a limited vaccine supply, many countries have implemented a single-dose strategy and later an intradermal 1/6th of a dose to maximize vaccine availability8–10.
MVA-BN was developed initially as a third-generation smallpox vaccine11. The FDA expanded the indication to mpox prophylaxis based on data from an MPXV challenge study conducted in nonhuman primates. However, efficacy data of the vaccine against mpox in humans are lacking. Therefore, evidence for the real-life effectiveness of the vaccine in preventing mpox in humans is still warranted6,12. Our objective was to promptly follow vaccinated individuals to assess the effectiveness of providing one dose of the vaccine in a real-world, at-risk population.
Results
Study population
A total of 2,054 members of Clalit Health Services (CHS) met the study eligibility criteria (Methods). All eligible individuals were male. Of these, 1,037 (50%) were vaccinated with MVA-BN and had at least 90 d of follow-up. The majority of participants belonged to the general Jewish population sector (95.0%), followed by Arabs (2.1%) and ultra-Orthodox Jews (1.9%). The population sector was unknown for 0.9% of the study cohort. All vaccine doses were provided subcutaneously. Follow-up time was 90 to 147 d (average, 141 d).
Vaccine uptake
The characteristics of vaccinated and unvaccinated individuals and the association between these characteristics and vaccine uptake are detailed in Table 1. Vaccine uptake in individuals who attended primary healthcare clinics in the Israeli Tel Aviv District was 2.2-fold higher than in individuals from other regions. Uptake was lower in individuals from the minority population sectors and those with a socioeconomic status score below the median by 55% and 22%, respectively. HIV pre-exposure prophylaxis (HIV-PrEP) utilization, PDE5 inhibitors utilization and recent chlamydia or Neisseria (NE) gonorrhea infections were associated with a 70%, 43% and 34% higher vaccine uptake, respectively.
Table 1.
Association between participant characteristics and vaccine uptake
| Unvaccinated | Vaccinated | All | HRa (95% CI) | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Total N | 1,017 (50%) | 1,037 (50%) | 2,054 (100%) | |
| Sociodemographic variables | ||||
| Age, mean (s.d.) | 33.5 (5.8) | 34.1 (4.9) | 33.8 (5.4) | 1.02 (1.01, 1.03) |
| Sex—male | 1,017 (100) | 1,037 (100) | 2,054 (100) | All male |
| Tel Aviv District | 406 (39.9) | 783 (75.5) | 1,189 (57.9) | 2.23 (1.92, 2.59) |
| Sociodemographic status score below median | 501 (49.3) | 326 (31.4) | 827 (40.3) | 0.78 (0.69, 0.89) |
| Population sector—general Jewish | 935 (91.9) | 1,017 (52) | 1,952 | Reference |
| Population sector—others | 82 (8.1) | 20 (1.9) | 102 (5.0) | 0.45 (0.29, 0.71) |
| Arab | 40 (3.9) | 4 (0.4) | 44 (2.1) | |
| Ultra-Orthodox Jewish | 30 (2.9) | 9 (0.9) | 39 (1.9) | |
| Unknown | 12 (1.2) | 7 (0.7) | 19 (0.9) | |
| Clinical risk factors | ||||
| History of HIV/AIDS | 511 (50.2) | 136 (13.1) | 647 (31.5) | 0.46 (0.34, 0.63) |
| History of syphilis infection | 199 (19.6) | 233 (22.5) | 432 (21.0) | 1.05 (0.89, 1.24) |
| Recentb syphilis infection | 31 (3.0) | 61 (5.9) | 92 (4.5) | 1.05 (0.79, 1.40) |
| Recentb STIc in urinary test | 31 (3.0) | 67 (6.5) | 98 (4.8) | 0.88 (0.65, 1.20) |
| Recentb STIc in pharyngeal test | 46 (4.5) | 159 (15.3) | 205 (10.0) | 1.25 (0.96, 1.64) |
| Recentb STIc in rectal test | 46 (4.5) | 157 (15.1) | 203 (9.9) | 1.02 (0.78, 1.32) |
| Recent chlamydia or NE gonorrhead | 100 (9.8) | 298 (28.7) | 398 (19.4) | 1.34 (0.99, 1.82) |
| Purchase of PDE5 inhibitorsb | 98 (9.6) | 202 (19.5) | 300 (14.6) | 1.43 (1.22, 1.67) |
| Purchase of HIV-PrEPb | 506 (49.8) | 876 (84.5) | 1,382 (67.3) | 1.70 (1.29, 2.24) |
aThe association between all covariates and MVA-BN vaccine uptake was estimated using a multivariate Cox proportional hazards regression model. The higher the HR, the greater the association between the listed characteristic and vaccine uptake.
bRecent: from 01/2022 to 06/2022.
cSexually transmited infection (STI): chlamydia or NE gonorrhea.
dRecent chlamydia or NE gonorrhea: in a urinary, pharyngeal or rectal test.
Assessment of vaccine effectiveness
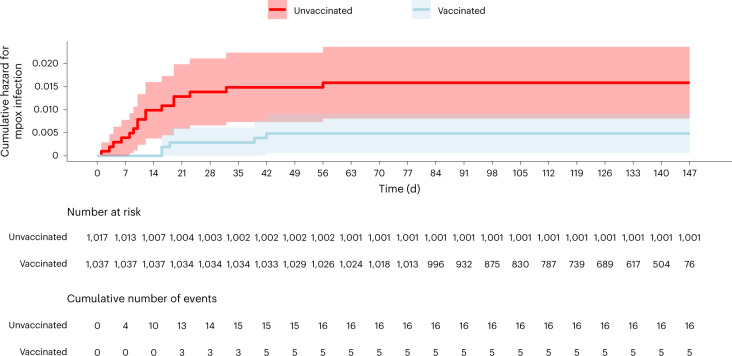
During the study period, MPXV infections occurred in 16 unvaccinated individuals (9.3 per 100,000 person-days) and 5 vaccinated individuals (4.3 per 100,000 person-days). The adjusted hazard ratio (HR) for infection in the vaccinated compared to the unvaccinated population was 0.14 (95% confidence interval (CI) 0.05–0.41). The cumulative HR curves for infection are shown in Fig. 1. Univariable and multivariable Cox proportional hazard analyses assessing the association between participant characteristics and mpox are detailed in Table 2. The Tel Aviv District of the primary healthcare clinic was associated with a fourfold higher risk of mpox.
Fig. 1. Cumulative hazard for mpox infection (95% CIs).
For unvaccinated participants, time zero corresponds to 31 July 2022, when the vaccination campaign was initiated. For vaccinated participants, time zero corresponds to the date of vaccine uptake. The shaded areas indicate the 95% CIs.
Table 2.
Association of participant characteristics and MPXV infection
| Variables | Results of the univariablea models | Results of the multivariableb model |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Vaccination | 0.30 (0.11, 0.83) | 0.14 (0.05, 0.41) |
| Tel Aviv District | 3.11 (1.05, 9.23) | 3.98 (1.29, 12.33) |
| HIV-PrEP usec | 0.97 (0.39, 2.41) | |
| Purchase of PDE5 inhibitorsc | 1.84 (0.67, 5.02) | 2.14 (0.76, 5.99) |
| History of HIV/AIDS | 0.87 (0.34, 2.24) | |
| Any syphilis infection | 1.89 (0.76, 4.67) | 1.11 (0.39, 3.18) |
| Chlamydia or NE gonorrhea in recentc rectal PCR | 2.15 (0.72, 6.39) | |
| Chlamydia or NE gonorrhea in recentc urine PCR | 3.38 (1.00, 11.48) | |
| Chlamydia or NE gonorrhea in recentc pharyngeal PCR | 0.95 (0.22, 4.09) | |
| Chlamydia or NE gonorrhea in any recentc STI PCR | 2.09 (0.84, 5.19) | 2.53 (0.98, 6.52) |
| Recentc syphilis infection | 3.58 (1.05, 12.15) | 3.20 (0.78, 13.17) |
aThe associations between each of the covariates and mpox were estimated using a series of univariate Cox proportional hazards regression models.
bA multivariate Cox proportional hazards regression model was used to estimate the association between vaccine and mpox while controlling for the covariates found to be associated with mpox in the univariate analyses. The higher the HR, the greater the association between the listed characteristic and vaccine uptake.
cRecent: from 01/2022 to 06/2022. STI: chlamydia or NE gonorrhea.
Discussion
Six months after the initial worldwide spread of mpox, the outbreak seems to be contained4, mainly attributed to vaccination efforts and behavioral changes13. While the vaccines against smallpox were assumed to also protect against mpox, efficacy data are still limited to studies in mice and monkey models14. How well MVA-BN protects against mpox in humans and how much protection against mpox is elicited by providing only a single dose rather than the recommended two doses is still unclear12. Our results demonstrate that vaccination with one subcutaneous dose of MVA-BN was associated with an 86% reduction in the risk for mpox among vaccinated individuals considered at high risk of MPXV infection. Nevertheless, completing the second vaccine dose, per the approved label, may improve this effectiveness and provide longer-lasting protection.
In a recent observational study of 276 individuals at a single hospital setting, who were vaccinated after exposure, 12 participants (4%) had a confirmed MPXV breakthrough infection; of those, 10 participants developed an infection up to 5 d following vaccination15. However, we are unaware of published studies evaluating vaccine effectiveness when provided as PrEP.
The US Centers for Disease Control and Prevention has reported in its 8 December 2022 update that, among 43 US jurisdictions, the mpox rate in individuals who were recommended to receive a vaccine was seven times higher among unvaccinated individuals compared with those vaccinated with one dose, suggesting similar results of vaccine effectiveness as demonstrated in our study. However, these results did not control for possible differences in baseline characteristics such as age, underlying conditions or other differences between the two groups8.
Randomized controlled trials are yet required to provide direct evidence of the efficacy and safety of vaccines against mpox in humans. While such data would have been the gold standard for supporting mpox vaccination guidelines, the time required for such trials to be planned, executed and published would not be sufficient for immediate policy decisions urgently needed to contain the epidemic. Therefore, controlled studies and vaccine effectiveness surveillance are critical to understanding the utility of vaccines against MPXV acquisition16.
Our study has some noteworthy limitations. The primary limitation is the small number of participants, the younger age (18–42 years) and the low number of infections observed during the study period, even in the unvaccinated individuals. Our results should, therefore, be regarded as preliminary, and additional larger studies are still required. However, these promising results may further drive the engagement of individuals and their healthcare providers for vaccination.
Another major limitation is that the characteristics of vaccinated and unvaccinated individuals in our study cohort were significantly different despite all being identified as high risk, according to the Israeli Ministry of Health (MOH) guidelines. The vaccinated and unvaccinated groups differed in most sociodemographic and clinical variables (Table 1). The unvaccinated group had a lower socioeconomic status and included more minority sectors living outside the Tel Aviv District and a higher prevalence of HIV. Although we adjusted for known and measurable confounders, some sources of residual confounding may not have been measured or corrected adequately.
Bias in estimating vaccine effectiveness might be caused by unmeasured differences in lifestyle behaviors, including sexual behaviors, between vaccinated and unvaccinated individuals. Vaccination might also lead to changes in behaviors that could affect the risk of mpox acquisition. Our study was based on a clinical database and, therefore, could not directly capture the sexual behavior patterns of the study participants. Sexual behavior patterns would have been extraordinarily challenging to capture and control, especially since we included all the vaccine-eligible individuals in our study cohort, not only those infected with MPXV. We attempted to overcome behavioral bias by controlling for measurable factors identified by clinicians that may serve as markers for sexual behavior patterns and which include previous STIs detected in rectal, pharyngeal or urine PCR tests, blood tests for syphilis screening (TPHA) and dispense of HIV-PrEP therapy and PDE5 inhibitors (sildenafil, tadalafil or vardenafil)17.
It should be noted that no screening for mpox was carried out in Israel and that the detection of mpox was limited only to individuals who reported symptoms to their physicians. Underreporting could occur if individuals were asymptomatic or because individuals’ symptoms were not attributed to mpox18. However, we assumed that rates of undiagnosed mpox infections are not likely to differ in vaccinated and unvaccinated individuals.
Another possible source of bias is the variation of exposure to mpox during the study period. In our analysis, Schonfeld’s global test confirmed that the proportional hazards assumption was met in the Cox proportional hazards model, suggesting that this variation in the risk for exposure to mpox remained similar in vaccinated and unvaccinated participants throughout the study period.
The initial mpox vaccination policy in Israel focused on PrEP in high-risk individuals, with special per-case approval for post-exposure cases of infection. However, no testing for the existence of mpox was done before vaccine administration. Therefore, some vaccinated individuals may have been infected (but undiagnosed) before vaccine administration, potentially lowering the observed effectiveness. All five cases of infection in the vaccinated individuals were diagnosed at least 21 d after vaccine uptake (21–47 d) and, therefore, probably represent vaccine breakthroughs rather than cases of post-exposure vaccinations.
We could not assess the effectiveness of the recommended two-dose regimen because only 20% of the vaccinated participants in Israel have completed the second dose to date, and due to insufficient follow-up time after the second dose.
In Israel, the vaccine was administered only by a subcutaneous route during the study period, according to FDA prescribing information8. Transition to the intradermal route was implemented in Israel on 26 October 2022, only after the enrollment of participants in our dataset had ended. The change in route of administration was decided, like in many other countries, to advance access, equity and chances of controlling the mpox outbreak, as the subcutaneous dose can be split into up to six intradermal doses10,19,20. Therefore, our results might not be relevant in other healthcare settings where the intradermal, lower-volume dose administration of MVA-BN was adopted earlier.
In conclusion, our results suggest that a single dose of the MVA-BN vaccine administered subcutaneously is associated with a lower risk of mpox in high-risk male individuals in Israel. These findings suggest that providing at least one dose of the vaccine might have contributed to the containment of the current mpox outbreak. Larger randomized and real-world studies are still required to validate the vaccine’s effectiveness over time.
Methods
The study was approved by the CHS Community Institutional Review Board Committee and the CHS Data Utilization Committee. The study was exempt from the requirement to obtain informed consent owing to the retrospective design.
Study participants
This observational, retrospective population-based cohort study was based on data obtained from the electronic medical records of CHS, the largest of four integrated healthcare organizations in Israel, which insures 4.78 million people (52% of the population).
In response to the current mpox outbreak, the Israeli MOH initiated a vaccination campaign on 31 July 2022, for individuals considered at high risk for infection. The eligibility criteria were males aged 18–42 years who were (a) dispensed HIV-PrEP at least for 1 month since 1 January 2022, or (b) diagnosed with HIV and also were diagnosed with one or more STIs since 1 January 2022. However, due to the limited vaccine supply, the policy in Israel when the vaccination campaign was initiated was to administer only a single subcutaneous dose of the vaccine. The cohort included all CHS members eligible for the vaccine according to the Israeli MOH guidelines when the study commenced who had completed at least 90 d of follow-up after vaccination. Individuals who were infected with mpox before the study period were excluded.
Study design and timeline
The follow-up of participant data started on 31 July 2022, when the vaccination campaign was initiated in CHS. The data were collected until 25 December 2022, and participants vaccinated after 26 September 2022 were excluded to allow sufficient follow-up time. Vaccination with a second dose of MVA-BN was introduced in Israel on 13 September 2022. Therefore, follow-up of individuals who received a second vaccine dose was censored at the date of the second vaccine.
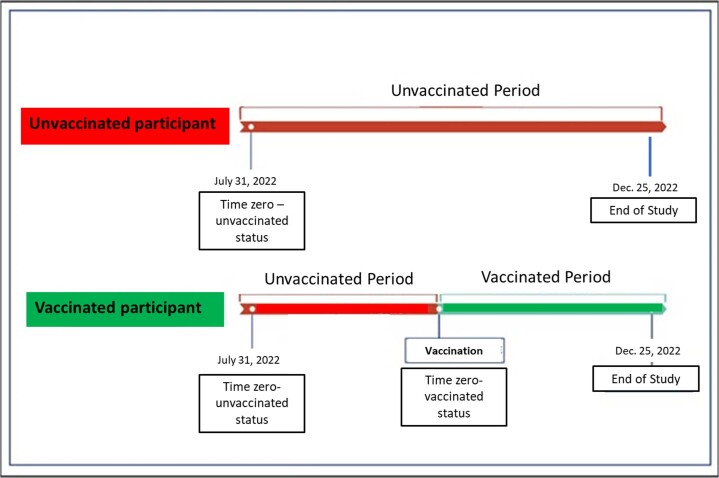
Participants were evaluated as part of the unvaccinated group until the vaccination date. For unvaccinated participants, time zero corresponds to 31 July 2022, when the vaccination campaign was initiated. For vaccinated participants, time zero corresponds to the date of vaccine uptake. Participants moved from the unvaccinated group to the vaccinated group on the day they were vaccinated. In the vaccinated participants, the first time period accounts for the days from the follow-up start date (31 July 2022, when the vaccination campaign was initiated) until the vaccination date. During this period, the follow-up days of the participants are a part of the ‘unvaccinated status.’ The second follow-up period of the vaccinated participants accounts for the days from the vaccination day until the end of follow-up (25 December 2022) and is counted as part of the ‘vaccinated status’. The study timeline and the transition between the unvaccinated and vaccinated status are depicted in the Extended Data Fig. 1.
Extended Data Fig. 1.
Study timeline.For unvaccinated participants, time zero corresponds to 31 July 2022, when the vaccination campaign was initiated. For vaccinated participants, time zero corresponds to the date of vaccine uptake. The figure depicts the transition from unvaccinated to vaccinated status, as applied in the time-dependent Cox model.
The study’s primary endpoint was mpox diagnosis, determined by a laboratory-confirmed RT–PCR test. Because the minimum time between infection and symptom onset was initially reported to be 5 d21, the estimated date of infection was defined as the earlier 5 d before the positive PCR test result or of a physician-documented suspected diagnosis of mpox. Any infection according to the above definition occurring later than the date of vaccination was considered as a breakthrough infection.
Data extraction
The following data were extracted for each participant: MVA-BN vaccination, mpox diagnosis, RT–PCR laboratory results, age, geographical district of primary healthcare clinic, population sector, the score for socioeconomic status, history of HIV/AIDS; STIs detected in rectal, pharyngeal or urine PCR tests; blood test for syphilis screening (TPHA) and dispense of HIV-PrEP therapy and PDE5 inhibitors (sildenafil, tadalafil or vardenafil).
The CHS data repositories and the definition of the sociodemographic variables were previously described in published coronavirus disease 2019 studies22. Data were extracted on 29 December 2022.
Statistical analysis
Descriptive statistics were used to characterize the study participants, and the study population was divided into two groups—those who had received the vaccine and those who had not. The geographical district of the primary clinic where each participant is registered was based on the administrative classification of CHS, dividing the entire state of Israel into nine districts. This covariate was categorized as Tel Aviv versus the other eight geographical districts, as most of the study population (58%) resided in this area. The population sector was based on CHS’s administrative classification of the primary clinic where each participant is registered: general Jewish, Arab and Jewish ultra-Orthodox. This covariate was dichotomously divided into the majority sector (general Jewish, 95.0% of the study population) and minority sectors (including Arabs, Jewish ultra-Orthodox and those with unknown sector classification). The sociodemographic status score was categorized as below the median versus median score or higher. A multivariate Cox proportional hazards regression model was used to estimate the association of all covariates and uptake of the MVA-BN vaccine.
To avoid immortal time bias23, we performed a time-dependent analysis in which a time-varying covariate was used to indicate the initiation of vaccination for each vaccinated participant. Participants were transferred from the 'unvaccinated' group to the 'vaccinated' group when vaccinated, modifying their vaccination status from unvaccinated to vaccinated. Consequently, the follow-up of vaccinated participants started at the end of the immortal period.
The association between MVA-BN vaccination and mpox was estimated as follows: first, a univariate Kaplan–Meier analysis with a log-rank test was applied to test the associations of each independent candidate variable with the primary outcome. The threshold for the first testing criterion was set at P < 0.25 (ref. 24). Then, the proportional hazard assumption was validated for those variables using Schoenfeld’s global test. Variables that met these two testing criteria served as inputs for multivariable Cox proportional hazards analysis. Vaccine effectiveness was defined as 1 minus the HR. All reported P values are two-tailed.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41591-023-02229-3.
Supplementary information
STROBE checklist
Acknowledgements
No financial or in-kind support was provided for the conduct of this study.
Extended data
Author contributions
All authors contributed to the study design and execution. E.B. extracted the data under the supervision of Y.W. W.A. and N.R. cleaned and analyzed the data with the guidance of Y.W. and R.A. Y.W., R.Z., A.H. and R.A. drafted the initial manuscript and made the primary revisions. R.Z., H.M., N.G.A., G.C., A.M.-A., G.W.-K., H.D.-B. and A.P. planned, revised and approved all the clinical aspects of the study. S.Y. performed internal quality-control checks. G.L. and D.N. contributed to the policy aspects of the study. R.A., G.L. and D.N. oversaw the study design and conduct. All authors revised the manuscript for critical content and clarity and approved it.
Peer review
Peer review information
Nature Medicine thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Alison Farrell, in collaboration with the Nature Medicine team.
Data availability
Due to CHS data privacy regulations and according to the Declaration of Helsinki and institutional data use committee approvals for this study, the data used for this study cannot be shared.
Code availability
R statistical software version 4.0.1 (R Project for Statistical Computing) was used for the univariate and multivariate survival analysis with time-dependent covariates. The following R packages were used: survival (3.2–13), ggplot2 (3.3.5), ggpubr (0.4.0), survminer (0.4.9) and table1 (1.4.2). All R packages are freely available.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yael Wolff Sagy, Roy Zucker, Ariel Hammerman.
These authors jointly supervised this work: Ronen Arbel, Gil Lavie, Doron Netzer.
Extended data
is available for this paper at 10.1038/s41591-023-02229-3.
Supplementary information
The online version contains supplementary material available at 10.1038/s41591-023-02229-3.
References
- 1.Thornhill JP, et al. SHARE-net clinical group. Monkeypox virus infection in humans across 16 countries—April–June 2022. N. Engl. J. Med. 2022;387:679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 2.US Food & Drug Administration. Key facts about monkeypox vaccine. Available from: https://www.fda.gov/vaccines-blood-biologics/vaccines/key-facts-about-monkeypox-vaccine. Extracted on 1 October 2022.
- 3.World Health Organization. Vaccines and immunization for monkeypox. Interim guidance—14 June 2022. https://apps.who.int/iris/bitstream/handle/10665/356120/WHO-MPX-Immunization-2022.1-eng.pdf
- 4.Nuzzo, J. B., Borio, L. L. & Gostin, L. O. The WHO declaration of monkeypox as a global public health emergency. JAMA10.1001/jama.2022.12513 (2022). [DOI] [PubMed]
- 5.Mathieu, E., et al. Monkeypox. Our world in data 2022 https://ourworldindata.org/monkeypox. Extracted on 14 November 2022.
- 6.World Health Organization. 2022 monkeypox outbreak: global trends. https://worldhealthorg.shinyapps.io/mpx_global/. Extracted on 12 November 2022.
- 7.US Food & Drug Administation. JYNEOS prescribing information. https://www.fda.gov/media/131078/download. Extracted on 1 October 2022.
- 8.Centers for Disease Control and Prevention. Rates of monkeypox cases by vaccination status. Updated 8 December 2022. https://www.cdc.gov/poxvirus/monkeypox/cases-data/mpx-vaccine-effectiveness.html. Extracted on 29 December 2022.
- 9.Del Rio C, Malani PN. Update on the monkeypox outbreak. JAMA. 2022;328:921–922. doi: 10.1001/jama.2022.14857. [DOI] [PubMed] [Google Scholar]
- 10.Hazra, A. et al. Human monkeypox virus infection in the immediate period after receiving modified Vaccinia Ankara vaccine. JAMA10.1001/jama.2022.18320 (2022). [DOI] [PMC free article] [PubMed]
- 11.Pittman PR, et al. Phase 3 efficacy trial of modified Vaccinia Ankara as a vaccine against smallpox. N. Engl. J. Med. 2019;381:1897–1908. doi: 10.1056/NEJMoa1817307. [DOI] [PubMed] [Google Scholar]
- 12.Kupferschmidt K. Scientists scramble to set up monkeypox vaccine trials. Science. 2022;377:696–697. doi: 10.1126/science.ade3371. [DOI] [PubMed] [Google Scholar]
- 13.Reardon S. What does the future look like for monkeypox? Nature. 2022;610:250–252. doi: 10.1038/d41586-022-03204-7. [DOI] [PubMed] [Google Scholar]
- 14.Earl PL, et al. Immunogenicity of a highly attenuated MVA-BN smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 15.Thy M, et al. Breakthrough infections after post-exposure vaccination against mpox. N. Engl. J. Med. 2022;387:2477–2479. doi: 10.1056/NEJMc2211944. [DOI] [PubMed] [Google Scholar]
- 16.Rubin EJ, et al. Audio interview: responding to monkeypox. N. Engl. J. Med. 2022;387:e21. doi: 10.1056/NEJMe2210967. [DOI] [PubMed] [Google Scholar]
- 17.Zucker, R. et al. Risk assessment of human monkeypox infections for vaccine prioritization. 10.21203/rs.3.rs-1904714/v2 (2022).
- 18.Baetselier, I. et al. Retrospective detection of asymptomatic monkeypox virus infections among male sexual health clinic attendees in Belgium. Nat. Med.10.1038/s41591-022-02004-w (2022).. [DOI] [PMC free article] [PubMed]
- 19.US Food & Drug Administration. Fact sheet for healthcare providers administering vaccine: emergency use authorization of JYNNEOS. https://www.fda.gov/media/160774/download. Extracted on 1 October 2022.
- 20.Brooks JT, Marks P, Goldstein RH, Walensky RP. Intradermal vaccination for monkeypox—benefits for individual and public health. N. Engl. J. Med. 2022;387:1151–1153. doi: 10.1056/NEJMp2211311. [DOI] [PubMed] [Google Scholar]
- 21.WHO. Monkeypox. Key facts. May 19 2022. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- 22.Arbel R, et al. Nirmatrelvir use and severe COVID-19 outcomes during the omicron surge. N. Engl. J. Med. 2022;387:790–798. doi: 10.1056/NEJMoa2204919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lévesque LE, et al. Problem of immortal time bias in cohort tudies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087. doi: 10.1136/bmj.b5087. [DOI] [PubMed] [Google Scholar]
- 24.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol. Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
STROBE checklist
Data Availability Statement
Due to CHS data privacy regulations and according to the Declaration of Helsinki and institutional data use committee approvals for this study, the data used for this study cannot be shared.
R statistical software version 4.0.1 (R Project for Statistical Computing) was used for the univariate and multivariate survival analysis with time-dependent covariates. The following R packages were used: survival (3.2–13), ggplot2 (3.3.5), ggpubr (0.4.0), survminer (0.4.9) and table1 (1.4.2). All R packages are freely available.