Abstract
Primary literature detailing updated management principles of acute ischemic stroke outpaces current guidelines, resulting in heterogenous practices. Recent advancements in neuroimaging have shifted treatment from a time‐based approach to an individualized, image‐guided appraisal directed by the presence or absence of salvageable brain tissue. In addition, tenecteplase appears to be a safe and effective for the treatment of acute ischemic stroke and is becoming an attractive agent due to its practical administration. Several factors must be accounted for when implementing tenecteplase into the health‐system including cost, education, and changes in clinician workflows. Larger studies with broad patient populations are needed to more definitively evaluate whether intravenous thrombolytics should be used in combination with endovascular thrombectomy in patients with anterior large‐vessel occlusions. Although debate regarding the safety and efficacy of various endovascular therapies, delays encountered in the identification, triage, and care of acute ischemic stroke patients increase the likelihood of necrotic core lesion development and loss of salvageable penumbra.
Keywords: acute ischemic stroke, fibrinolytics, neuroimaging, tenecteplase, thrombectomy
1. INTRODUCTION
Acute ischemic stroke remains a leading cause of death and disability within the United States. 1 The pathophysiology of stroke is heterogeneous. Although symptom manifestation can be indistinguishable, 87% of acute stroke is due to ischemia, 10% due to intracranial hemorrhage (ICH), and 3% due to subarachnoid hemorrhage. Ischemic stroke can be further classified into subtypes based on various risk factors and etiologies. 2 , 3 These etiologies are determined through a combination of clinical features, imaging modalities, and clinical assessments. Appropriately determining the etiology of ischemic stroke can influence primary treatment options including reperfusion therapies.
In the early 20th century, it was widely held that damage from acute ischemia was irreversible within minutes of symptom onset, giving rise to the phrase “time is brain.” In the late 1970s, researchers observed that this damage occurs in 2 phases. The central area of infarct with very low perfusion, known as the core, is considered irreversibly damaged at stroke onset. However, the area surrounding the core, known as the penumbra, consists of neurons that are simply idling and potentially salvageable. Dysfunction of these neurons is thought to be due to metabolic and ionic disturbances of ischemia whereas the structural integrity remains intact. Over time, a lack of perfusion to the penumbra results in an enlargement of irreversibly damaged tissue (Figure 1). 4 The goal of reperfusion therapy is to restore blood flow to the ischemic area in an effort to salvage neuronal tissue, thus preserving as much function as possible. Current practice guidelines recommend fibrinolysis in eligible patients using alteplase within 3 to 4.5 hours from symptom onset (Table 1). 5
FIGURE 1.
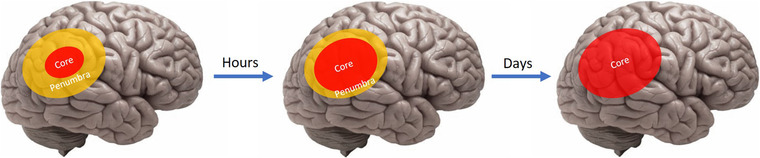
Progression of irreversible brain tissue injury
TABLE 1.
Evidence supporting alteplase within 3–4.5 hours of symptom onset
| Trial | Patient population | Intervention | Outcomes | Takeaways |
|---|---|---|---|---|
| NINDS (1995) |
|
|
|
|
| ECASS III (2008) |
|
|
|
|
Abbreviations: mRS, modified Rankin Scale; sICH, symptomatic intracerebral hemorrhage.
2. ADVANCEMENTS WITH NEUROIMAGING
Neuroimaging has long been employed in patients with suspected stroke to determine stroke etiology, assess the degree of brain injury, and identify vascular lesions responsible for the ischemic deficit (Figure 2). Current guidelines recommend either non‐contrast computed tomography (CT) or magnetic resonance imaging (MRI) with diffusion‐weighted imaging (DWI) for initial assessment. 5 Non‐contrast CT remains the most used initial study due to rapid scan time, relatively inexpensive cost, and high sensitivity for detecting hemorrhages. Although less readily available, MRI with DWI is more sensitive, specific, and accurate for detecting hyperacute ischemia compared to non‐contrast CT. Follow‐up imaging with CT angiography (CTA) or magnetic resonance angiography (MRA) is employed to further identify candidates for endovascular thrombectomy if a large‐vessel‐occlusion (LVO) is present. 6 , 7
FIGURE 2.
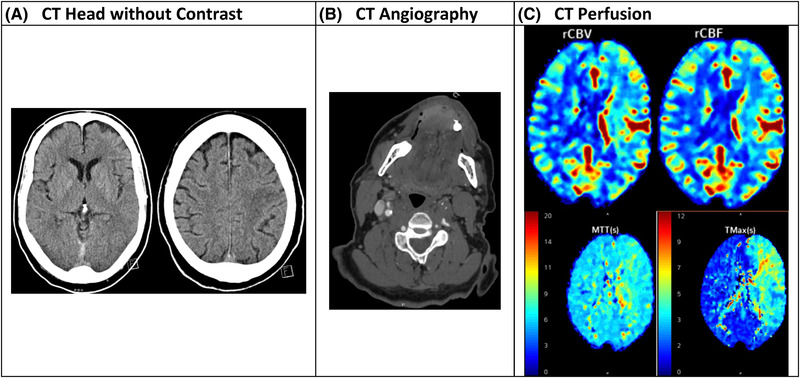
Comparison of various imaging modalities for acute ischemic stroke
Additional imaging modalities have recently been examined in trial design and clinical practice to more accurately assess tissue viability and triage patients for reperfusion intervention. The first of these assays uses MRI to identify a mismatch between DWI and fluid‐attenuated inversion recovery (FLAIR) sequencing modes. Although DWI shows high signal intensity within the first few minutes after an ischemic stroke, FLAIR hyperintensity signaling occurs hours after stroke as a result of gradually developing vasogenic edema. A patient presenting with a stroke occurring within 4.5 hours will likely have hyperintensity on DWI but not on FLAIR, resulting in a DWI/FLAIR mismatch that can help identify patients with salvageable penumbra who may be eligible for reperfusion therapy. 6 , 7
DWI/FLAIR mismatch has subsequently been validated in patients with unknown time from symptom onset. In 2018, WAKE‐UP trial investigators randomly assigned patients with an unknown time of symptom onset and DWI/FLAIR mismatch on MRI to receive alteplase or placebo. They found that intravenous alteplase guided by a DWI/FLAIR mismatch resulted in significantly better functional outcome at 90 days compared to placebo. No difference was seen with symptomatic ICH but there was an increase in parenchymal hemorrhage type 2. Last, although there was a signal toward increased deaths in the alteplase arm, there was no statistically significant difference in mortality. However, the use of a DWI/FLAIR imaging modality prevented 137 patients from receiving therapy due to a negative mismatch. 8
Penumbral imaging has also recently been employed to identify the presence of a mismatch between the core infarction and the salvageable penumbra. CT perfusion (CTP) and magnetic resonance perfusion (MRP) use rapid administration of iodinated media and gadolinium respectively, followed by repeated imaging over at least a 60‐second period to assess collateral blood flow and identify areas of hypoperfusion. 9 Quantitative thresholds are used to estimate a mismatch between the penumbra and core tissue present due to LVO. Patients are more likely to benefit from reperfusion therapy when the penumbra is larger than the core infarction. 6 , 7
Perfusion imaging has also been validated in several prospective studies. In 2006, DEFUSE investigators conducted a prospective, multicenter study assessing whether alteplase was effective within 4 to 6 hours from symptom onset when diffusion‐perfusion mismatch was present on MRI. They found that early reperfusion was associated with increased odds of achieving a favorable clinical outcome in patients found to have a mismatch between perfusion and diffusion. 10 Similarly, the EPITHET trial in 2008 evaluated whether intravenous alteplase was effective in patients with symptom onset within 3 to 6 hours and saw improved functional outcomes in patients with perfusion‐diffusion mismatch on MRI. 11 These studies have further validated the use of advanced neuroimaging to identify patients eligible for reperfusion beyond the established 4.5‐hour timeframe.
3. ADVANCEMENTS WITH FIBRINOLYTICS
Although alteplase has been an industry standard for over 25 years, recent literature surrounding the use of tenecteplase as an alternative thrombolytic has shown favorable outcomes. Tenecteplase is a third‐generation thrombolytic agent bioengineered to retain the full fibrinolytic activity of our endogenous tissue plasminogen activator (Figure 3). Compared to alteplase, it has an 80‐fold increased resistance to plasminogen activator inhibitor type 1 (PAI‐1), which inhibits tissue plasminogen activator from converting plasminogen to plasmin. The resistance to PAI‐1 leads to a clearance that is 4 times longer than alteplase and allows for a bolus intravenous administration over 5 seconds (Table 2). Advantages of bolus administration include the potential to decrease medication errors and a reduction in nursing resources. In addition, tenecteplase may offer a favorable safety profile compared to alteplase due to a 15‐fold higher specificity for clot‐bound fibrin, which may result in a lower risk of systemic fibrinogen depletion and bleeding. 12
FIGURE 3.
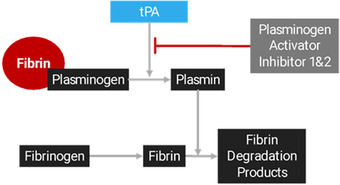
Fibrinolytic mechanism of action
TABLE 2.
Administration of fibrinolytics for acute ischemic stroke
| Tenecteplase | Alteplase |
|---|---|
| IV bolus over 5 seconds | 10% of dose administered as IV bolus over 1 minute, 90% of dose administered as IV infusion of 1 hour |
Endogenous tissue‐type plasminogen activator (tPA) releases from cells within the brain parenchyma exposed to ischemic conditions. Endogenous and recombinant tPA activates fibrin‐bound plasminogen into plasmin. Plasmin is subsequently cleaved from fibrin‐bound plasminogen and breaks up molecules of fibrin into fibrin degradation products. Plasminogen activator inhibitor type 1 and 2 prevent tPA from converting plasminogen into plasmin.
3.1. Dosing
Several small, phase‐2 dose‐finding trials were conducted to evaluate the safety and feasibility of tenecteplase for acute ischemic stroke. In 2005, Haley et al conducted a pilot dose‐escalation study to develop preliminary experience with tenecteplase. 13 They initially designed their study with 6 arms ranging from 0.1 mg/kg to a planned maximum dose of 0.6 mg/kg. However, the study was terminated early after 15% of participants in the 0.5 mg/kg arm experienced symptomatic intracerebral hemorrhage (sICH). Haley et al 13 ultimately demonstrated that tenecteplase dosed in the range of 0.1–0.4 mg/kg appears to be safe in ischemic stroke. In 2015, TEMPO‐1 investigators conducted a prospective, multicenter, 2‐cohort dose‐escalation study in patients with a National Institutes of Health Stroke Scale (NIHSS) <5, intracranial occlusion on CTA, and time from symptom onset within 12 hours. 14 They found that a tenecteplase dose of 0.25 mg/kg resulted in higher rates of recanalization compared to 0.1 mg/kg. 14 Last, TNK EXTEND‐IA Part 2 investigators in 2020 evaluated patients within 4 hours of symptom onset. 15 They found no difference in cerebral perfusion before endovascular thrombectomy and no difference in sICH between 0.25 mg/kg and 0.4 mg/kg dosing strategies. Thus, dosing of 0.25 mg/kg with a maximum dose of 25 mg has largely been established for the indication of AIS.
3.2. Tenecteplase versus alteplase
Several trials have directly compared the efficacy and safety of tenecteplase against alteplase. In 2015, ATTEST investigators conducted a phase‐2, randomized‐controlled trial comparing tenecteplase 0.25 mg/kg (maximum dose of 25 mg) to standard‐dose alteplase and with similar inclusion criteria of ECASS III, including patients with symptom onset within 4.5 hours and NIHSS ≥1. 16 Using CT perfusion, investigators found no difference in the percentage of penumbral salvage, sICH, or total ICH events between the 2 groups. In the largest randomized controlled trial to date, the NOR‐TEST investigators compared tenecteplase 0.4 mg/kg (maximum dose of 40 mg) to standard‐dose alteplase in patients with symptom onset within 4.5 hours while also including patients with a DWI/FLAIR mismatch in patients who woke up with new‐onset symptoms. 17 NOR‐TEST investigators found no difference in functional outcomes at 3 months and no difference in sICH. However, they did find increased mortality in moderate‐severe stroke at 90 days with tenecteplase. 17
Because of a heavy presence of patients with minor stroke in the original NOR‐TEST trial, the 2022 NOR‐TEST 2 Part A trial sought to establish non‐inferiority of tenecteplase 0.4 mg/kg to alteplase 0.9 mg/kg in patients with moderate to severe ischemic stroke, defined as a NIHSS ≥6. 18 This trial was prematurely terminated due to an imbalance in the rates of symptomatic ICH in the tenecteplase group. In addition, tenecteplase was associated with less frequent favorable functional outcomes and increased mortality at 3 months compared to alteplase. NOR‐TEST Part B is currently ongoing to evaluate a lower dose of tenecteplase 0.25 mg/kg.
Given the practicality of bolus administration without the need for infusion pumps, tenecteplase has recently been evaluated for use in the prehospital setting. The TASTE‐A trial, released in 2022, was a phase 2, randomized, open‐label trial evaluating the use tenecteplase 0.25 mg/kg versus alteplase within mobile stroke units (MSUs) in patients with symptom onset within 4.5 hours. 19 Investigators found that early administration of tenecteplase resulted in a superior rate of early reperfusion, faster clinical recovery, and quicker time to drug initiation compared with alteplase. No safety concerns were noted. These results were further substantiated with the AcT trial, a multicenter, open‐label, phase 3 randomized controlled trial, demonstrating that intravenous tenecteplase dosed at 0.25 mg/kg is comparable to alteplase in terms of safety and efficacy in patients presenting within 4.5 hours of stroke symptom onset. 20
Tenecteplase has also been evaluated in patients with LVO. In 2012, TAAIS investigators enrolled patients with NIHSS >4, symptom onset within 6 hours, and LVO on CTA with >20% mismatch on CTP. They compared both tenecteplase 0.1 mg/kg (maximum dose, 10 mg) and 0.25 mg/kg (maximum dose, 25 mg) to standard‐dose alteplase and found that tenecteplase overall had greater reperfusion and clinical improvement at 24 hours compared to alteplase. The higher dose of tenecteplase was found to be superior for all efficacy outcomes. No significant differences in ICH or serious adverse events were seen between any groups. 21 Finally, in 2018, EXTEND‐IA TNK Part 1 compared TNK 0.25 mg/kg (maximum dose, 25 mg) and standard‐dose alteplase in patients with symptom onset within 4.5 hours and large vessel occlusion on CTA. They found greater rates of reperfusion with tenecteplase with no difference in sICH. 22
Although the current body of literature consists mostly of small, phase‐2, randomized‐controlled trials, supporting evidence for the efficacy and safety of tenecteplase has been shown in meta‐analyses. Burgos and colleagues 23 evaluated 5 randomized‐controlled trials assessing tenecteplase versus alteplase within 6 hours of symptom onset. Collectively, they found no difference in functional outcome at 90 days and no difference in sICH. Katsanos and colleagues 24 analyzed 4 randomized controlled trials in patients with LVO before thrombectomy. They found that patients receiving tenecteplase have 3‐fold higher odds of achieving successful recanalization and 2‐fold higher odds of having favorable clinical outcomes at 3 months compared to those receiving alteplase. 24
Tenecteplase is becoming an attractive fibrinolytic agent for patients with acute ischemic stroke. In addition to its favorable drug characteristics and practical administration, tenecteplase appears to be equally effective in terms of efficacy and safety based on current literature (Table 3). According to the preponderance of evidence, tenecteplase administered as a 0.25 mg/kg push (maximum, 25 mg) appears most appropriate for acute ischemic stroke. In patients with large vessel occlusions, tenecteplase appears to be superior to alteplase in patients undergoing thrombectomy. Several trials are ongoing to continue evaluating the efficacy and safety of tenecteplase (Table 4).
TABLE 3.
Published studies evaluating efficacy and safety of tenecteplase
| Trial | Design | Inclusion criteria | Intervention (n) | Outcomes |
|---|---|---|---|---|
| Haley et al. (2005) 13 |
|
|
|
|
| TAAIS (2012) 21 |
|
|
|
|
| TEMPO‐1 (2005) 14 |
|
|
|
|
| ATTEST (2015) 16 |
|
|
|
|
| NOR‐TEST (2017) 17 |
|
|
|
|
| EXTEND‐IA TNK Part 1 (2018) 22 |
|
|
|
|
| Burgos et al. (2019) 23 |
|
|
|
|
| TNK EXTEND‐IA Part 2 (2020) 15 |
|
|
|
|
| Katsanos et al. (2021) 24 |
|
|
|
|
|
NOR‐TEST 2, Part A (2022) 18 |
|
|
|
|
| TASTE‐A (2022) 19 |
|
|
|
|
| AcT (2022) |
|
|
|
|
Abbreviations: CTA, computer tomography angiography; DWI, diffusion weighted imaging; FLAIR, fluid attenuated inversion recovery; LVO, large‐vessel occlusion; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; RCT, randomized controlled trial; rTPA, alteplase; sICH, symptomatic intracranial hemorrhage; TNK, tenecteplase.
TABLE 4.
Ongoing studies evaluating efficacy and safety of tenecteplase
| Trial | Name | Clinical trial ID |
|---|---|---|
| ATTEST 2 | Alteplase‐tenecteplase trial evaluation for stroke thrombolysis | NCT02814409 |
| BRIDGE‐TNK | Endovascular treatment with versus without intravenous tenecteplase in stroke | NCT04733742 |
| NOR‐TEST 2, Part B | The Norwegian Tenecteplase Stroke Trial 2 | NCT03854500 |
| TEMPO 2 | A randomized controlled trial of TNK‐tPA versus standard of care for minor ischemic stroke with proven occlusion | NCT02398656 |
| TIMELESS | Tenecteplase in stroke patients between 4.5 and 24 hours | NCT03785678 |
| TRACE 2 | Tenecteplase reperfusion therapy in acute ischemic cerebrovascular events‐II | NCT04797013 |
| TWIST | Tenecteplase in wake‐up stroke | NCT03181360 |
3.3. Implementing tenecteplase into a health system
With perceived improvements in patient outcomes due to the transition from alteplase to tenecteplase for acute ischemic stroke (AIS), health systems are likely to begin contemplating how best to integrate this agent into their stroke care protocols. Although a seemingly easy transition, there are several factors that must be accounted for before making the switch: health‐system and patient cost, education, and changes in clinician workflows are among several important to note.
Tenecteplase is supplied commercially as a 50‐mg kit, containing the drug, sterile diluent, abbreviated instructions detailing reconstitution, dosage, and administration information, a 10‐ml syringe with TwinPak dual cannula device, and full prescribing information. 25 Notably, the included information in the kit only pertains to tenecteplase's cardiac indication. Therefore, providers and health care workers unfamiliar with the use of tenecteplase for AIS are likely susceptible to drug errors. To successfully integrate tenecteplase for AIS, a large educational campaign needs to take place, clearly delineating the differences in tenecteplase dose, administration, and monitoring, stratified by indication. Although post‐fibrinolytic monitoring requirements in AIS are the same for both alteplase and tenecteplase, other actions such as the creation of institutional tenecteplase stroke kits with stroke‐specific dosing may offer an added level of error avoidance. Implementation of these kits may be performed by either repackaging the tenecteplase drug contents into stroke‐specific packaging, or by covering the cardiac dosing within the original tenecteplase package with stroke‐specific dosing. Nursing education should focus on administration and dosing differences, and highlight the physical incompatibility of tenecteplase and D5W.
Although ease of administration and dosing are a large stimulus for the transition to tenecteplase therapy, potential benefits in patients undergoing thrombectomy are also important. Moreover, cost savings on both institutional and patient levels are worth noting. The average wholesale price of tenecteplase ($8071.39) is significantly lower than a 100‐mg vial of alteplase ($10,560.43). 26 , 27 In addition, because of tenecteplase's bolus‐dosing administration strategy, other costly items such as administration tubing and infusion pumps are not needed. Although these factors do not consider patient insurance and hospital contract factors, continued uptake by health care systems and subsequent cost‐savings studies will likely confirm these suspicions. Conversely, it is worth mentioning that, unlike alteplase, there is currently no spoilage program to replace unused product for stroke‐related indications because it is not currently Food and Drug Administration‐approved for that disease state.
Last, given the significant nursing shortage ongoing in the United States, bolus dosing with tenecteplase may help ease nursing workload and clinical staff burden. In an era where staff shortages are rampant, and emergency departments are chronically full, this small change has the potential to alleviate some of the strain currently experienced in the emergency department workforce.
3.4. Advancements in thrombectomy
Although intravenous thrombolytics have revolutionized the treatment of acute ischemic stroke, ∼80% of patients with cerebral artery occlusions fail to show recanalization with fibrinolysis alone. 28 In addition, the numerous contraindications and narrow treatment window of thrombolytics preclude their use for many patients. Advancements in nonpharmacologic endovascular thrombectomy to mechanically remove clots have significantly improved treatment options in patients with anterior large vessel occlusions. Several landmark trials have shown improved functional outcomes with endovascular thrombectomy compared to thrombolytics alone in patients with anterior large vessel occlusions. In 2015, the landmark MR CLEAN trial conducted a multicenter, randomized clinical trial evaluating functional outcomes of intra‐arterial treatment for emergent revascularization in patients with proximal intracranial arterial occlusion. Patients were eligible if they could be treated within 6 hours of symptom onset. Of the 500 patients enrolled, 89% were treated with intravenous alteplase before randomization and retrievable stents were used in 81.5% of patients assigned to the treatment arm. They found an absolute difference of 13.5% in the rate of functional independence favoring the endovascular intervention group with no significant differences in mortality or sICH. 29 Following MR CLEAN, several other landmark studies including EXTEND‐IA, ESCAPE, SWIFT PRIME, and REVASCAT showed similar outcomes and reinforced the benefit of endovascular thrombectomy in combination with thrombolytics in eligible patients. 30 , 31 , 32 , 33
Similar to intravenous thrombolytic trials, the benefit of reperfusion was found to be dependent on time from symptom onset as a surrogate for salvageable tissue. To determine patient eligibility for endovascular thrombectomy, time from last known well was considered the time of stroke onset, including patients waking up with symptoms. A meta‐analysis of the aforementioned trials found no significant benefit for endovascular thrombectomy beyond 7.3 hours. 34 However, recent advancements in neuroimaging have established a tissue‐based approach over purely relying on time.
In 2018, DAWN investigators conducted a multicenter, randomized controlled trial to investigate the effects of endovascular thrombectomy versus standard of care in patients with occlusion of the intracranial internal carotid artery or proximal middle cerebral artery with a last known well between 6 and 24 hours. In addition, they only included patients who had the presence of a mismatch between the severity of clinical deficit and infarct volume using DWI or CTP. They found that 49% of patients in the endovascular thrombectomy group achieved functional independence at 90 days compared to 13% in the standard of care group with no significant differences in the rate of symptomatic ICH or mortality. 35 Similar results were seen in the DEFUSE‐3 trial which randomized patients with a last known well between 6 and 16 hours with viable tissue identified via perfusion imaging to endovascular thrombectomy or standard of care. They found that endovascular therapy resulted in 45% functional independence compared to 17% in the standard of care group. Mortality and symptomatic ICH remained statistically insignificant. 36
Current guidelines recommend mechanical thrombectomy within 24 hours of last known normal who have LVO in the anterior circulation and meet other DAWN or DEFUSE 3 eligibility criteria. 5 Evidence is limited surrounding thrombectomy for posterior LVOs partially due to the high mortality and poor‐functional outcomes associated with posterior LVO. In a recent meta‐analysis, patients with posterior LVO receiving thrombectomy had a lower likelihood of sICH compared to anterior LVOs receiving thrombectomy but had a higher likelihood for mortality. 37 In addition, patients with posterior LVO had worse functional outcomes. Authors found no difference in the rate of successful recanalization. Given these limitations, thrombectomy in patients with posterior LVO is evaluated on a case‐by‐case basis with considerations for last known well, location of the occlusion, NIHSS, and pre‐morbid mRS score.
3.5. Endovascular thrombectomy alone
Theoretical benefits to intravenous thrombolytics as a bridge to endovascular thrombectomy include potentially faster resolution of ischemia, reduction in clot size, and dissolution of embolic debris downstream of the occlusion. However, potential disadvantages include delaying definitive endovascular procedure, increased risk of symptomatic ICH, and embolization of a large vessel thrombus into a potentially inaccessible vessel. 38 Given these inherent benefits and risks, recent trials have evaluated the efficacy and safety of endovascular thrombectomy alone in eligible patients with large vessel occlusions who present to thrombectomy‐capable centers.
Two randomized controlled trials, DIRECT‐MT and DEVT, found that endovascular thrombectomy alone was non‐inferior to endovascular thrombectomy preceded by intravenous alteplase with regard to functional outcome in Chinese patients experiencing a large‐vessel occlusion. 39 , 40 In contrast, the SKIP randomized clinical trial failed to demonstrate non‐inferiority of endovascular thrombectomy alone in Japanese patients in regards to functional outcome. 41 Notably, these trials have several limitations. The DIRECT‐MT trial was powered for a generous noninferiority margin and had wide confidence intervals around the primary outcome. Both DEVT and SKIP were powered for large noninferior margins selected using the fixed‐margin method rather than the minimal clinically important difference. Most recently, MR CLEAN‐NO IV investigators conducted a multicenter, randomized trial in European patients presenting to a hospital capable of endovascular thrombectomy. When comparing functional outcomes between groups, investigators found that endovascular thrombectomy alone was neither superior nor non‐inferior to intravenous alteplase. 42 Larger studies with broad patient populations are needed to more definitively evaluate whether or not intravenous thrombolytics should be used in combination with endovascular thrombectomy in patients with anterior large‐vessel occlusions. In addition, future studies are needed to address whether alteplase should be considered before transport to an endovascular thrombectomy‐capable center.
3.6. Evolving trends in acute ischemic stroke transitions of care
Although debate regarding the safety and efficacy of various endovascular therapies, thrombolytic agents, and treatment windows is ongoing, one theme continues to permeate acute ischemic stroke care: time is brain. Delays encountered in the identification, triage, and care of acute ischemic stroke patients increase the likelihood of necrotic core lesion development and loss of salvageable penumbra. To expedite stroke care, several aspects of the current process have been examined to improve recognition, optimize transfer to thrombolysis or endovascular therapy, and expedite door‐to‐needle time.
Stroke care begins with early recognition by well‐trained emergency medical services personnel, or health care triage staff. Although the acronym F.A.S.T. (facial drooping, arm weakness, speech difficulties, and time) is commonly used by emergency staff due to its incorporation into the advanced cardiac life support stroke algorithm, newer tools have been developed and validated to more accurately identify patients with large vessel occlusions. 43 Of these, the RACE (rapid arterial occlusion evaluation) scale and FAST‐ED (facial palsy, arm weakness, speech changes, time, eye deviation, denial/neglect) scale, have demonstrated improved sensitivity and specificity at detecting LVO. 44 , 45 Recently, the RACE scale was found to more closely mimic the NIHSS area under the curve on a receiver operating characteristic curve for detection of LVO in a pre‐hospital setting, suggesting its superiority for this purpose. 44 Early identification of LVO improves decision‐making, allowing for more rapid transfer to mechanical thrombectomy centers. Additional technologies currently under investigation to expedite rapid transfer of LVO patients to endovascular therapy‐capable centers include the use of portable transcranial Doppler systems, and the volumetric impedance phase shift spectroscopy (VIPS) system. 45 The VIPS system is a portable helmet worn by the patient who can identify large strokes within 30 seconds. 46 Although a pilot study validating use has been performed, large, prospective data are currently lacking.
The decision to transfer patients to a thrombolysis‐capable center or endovascular therapy‐capable center has important ramifications in AIS care. Two models are currently used to describe this early treatment period: the drip‐and‐ship model and the mothership model. 45 In the drip‐and‐ship model, patients are initially treated with a thrombolytic agent within a regional stroke network. However, if a LVO is detected at this facility, the patient is subsequently transferred to the “mothership” hospital for endovascular therapy. In the mothership model, direct EMS transfer to an endovascular therapy‐capable hospital is performed. Although the mothership model has the potential to decrease time‐to‐thrombectomy by 90–120 minutes, it is dependent on the availability of regional thrombectomy centers in proximity to the patient's location and requires early identification of LVO. One tool used to improve this decision‐making capacity is a MSU. 45 Although still not readily adopted by most stroke networks, MSUs are equipped with neuroimaging systems, point‐of‐care laboratories, and telemedicine connections that allow for more thorough patient triage in the pre‐hospital setting. Despite these potential benefits, prospective studies are needed to demonstrate improvements in patient outcomes.
Aside from pre‐hospital assessment and triage, other factors in AIS care may be addressed to more rapidly assess and treat these patients once they arrive to the hospital. Several recent investigations have identified that the presence of an emergency department pharmacist decreases door‐to‐needle times by 5–25 minutes and increases the number of patients receiving thrombolysis within 30 minutes of arrival. 47 , 48 These findings highlight the importance of a pharmacist's presence within stroke care teams, and emergency departments nationwide. Additional ways to decrease door‐to‐needle times include direct transfer of patients from EMS and triage to the CT scanner and initiation of thrombolysis within the CT scanner. 49
Despite these advancements in stroke care, it is important to keep in mind the optimal triage and treatment model is highly dependent on regional network capabilities. Ongoing, prospective studies will hopefully provide further insight and help us continue to improve overall AIS management.
4. CONCLUSION
Ischemic stroke care has undergone significant advancement in recent years. Changes in thrombolytic selection, endovascular therapies, and transitions of care have the potential to reduce door‐to‐needle times, increase salvageable brain tissue, and ultimately provide patients with better clinical outcomes. Although more robust, prospective data are needed to help guide optimization of stroke care, thrombolytic selection, advanced neuroimaging, proper planning, and interdisciplinary teams can potentially improve stroke‐related outcomes.
ACKNOWLEDGMENTS
The authors received no funding from any agency in the public, commercial, or not‐for‐profit sectors.
Robbins BT, Howington GT, Swafford K, Zummer J, Woolum JA. Advancements in the management of acute ischemic stroke: A narrative review. JACEP Open. 2023;4:e12896. 10.1002/emp2.12896
Supervising Editor: Prasanthi Govindarajan, MBBS, MAS.
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
REFERENCES
- 1. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141(9):E139‐E596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 2. Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke definitions for use in a multicenter clinical trial. Stroke. 1993; 24(1): 35‐41. doi: 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 3. Mohr JP, Albers GW, Amarenco P, et al. Etiology of stroke. Stroke. 1997; 28(7): 1501‐1506. doi: 10.1161/01.STR.28.7.1501 [DOI] [PubMed] [Google Scholar]
- 4. Jones TH, Morawetz RB, Crowell RM, et al. Thresholds of focal cerebral ischemia in awake monkeys. J Neurosurg. 1981; 54(6): 773‐782. doi: 10.3171/JNS.1981.54.6.0773 [DOI] [PubMed] [Google Scholar]
- 5. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):E344‐E418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 6. Czap AL, Sheth SA. Overview of imaging modalities in stroke. Neurology. 2021; 97(20 Supplement 2): S42‐S51. doi: 10.1212/WNL.0000000000012794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heit JJ, Zaharchuk G, Wintermark M. Advanced neuroimaging of acute ischemic stroke: penumbra and collateral assessment. Neuroimaging Clin N Am. 2018; 28(4): 585‐597. doi: 10.1016/J.NIC.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 8. Thomalla G, Simonsen CZ, Boutitie F, et al. MRI‐guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018; 379(7): 611‐622. doi: 10.1056/NEJMOA1804355/SUPPL_FILE/NEJMOA1804355_DISCLOSURES.PDF [DOI] [PubMed] [Google Scholar]
- 9. Demeestere J, Wouters A, Christensen S, Lemmens R, Lansberg MG. Review of perfusion imaging in acute ischemic stroke: from time to tissue. Stroke. 2020;51(3):1017‐1024. doi: 10.1161/STROKEAHA.119.028337 [DOI] [PubMed] [Google Scholar]
- 10. Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006; 60(5): 508‐517. doi: 10.1002/ANA.20976 [DOI] [PubMed] [Google Scholar]
- 11. Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo‐controlled randomised trial. Lancet Neurol. 2008; 7(4): 299‐309. doi: 10.1016/S1474-4422(08)70044-9 [DOI] [PubMed] [Google Scholar]
- 12. Baruah DB, Dash RN, Chaudhari MR, Kadam SS. Plasminogen activators: a comparison. Vascul Pharmacol. 2006; 44(1): 1‐9. doi: 10.1016/j.vph.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 13. Haley EC, Lyden PD, Johnston KC, Hemmen TM. A pilot dose‐escalation safety study of tenecteplase in acute ischemic stroke. 2005;36(3):607‐612. doi: 10.1161/01.STR.0000154872.73240.e9 [DOI] [PubMed] [Google Scholar]
- 14. Coutts S, Dubuc V, Mandzia J, et al. Tenecteplase‐tissue‐type plasminogen activator evaluation for minor ischemic stroke with proven occlusion. Stroke. 2015; 46(3): 769‐774. doi: 10.1161/STROKEAHA.114.008504 [DOI] [PubMed] [Google Scholar]
- 15.v Campbell BC, Mitchell PJ, Churilov L, et al. Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND‐IA TNK part 2 randomized clinical trial. JAMA. 2020; 323(13): 1257‐1265. doi: 10.1001/jama.2020.1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang X, Cheripelli BK, Lloyd SM, et al. Alteplase versus tenecteplase for thrombolysis after ischaemic stroke (ATTEST): a phase 2, randomised, open‐label, blinded endpoint study. Lancet Neurol. 2015; 14(4):368‐376. doi: 10.1016/S1474-4422(15)70017-7 [DOI] [PubMed] [Google Scholar]
- 17. Logallo N, Novotny V, Assmus J, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR‐TEST): a phase 3, randomised, open‐label, blinded endpoint trial. Lancet Neurol. 2017; 16(10):781‐788. doi: 10.1016/S1474-4422(17)30253-3 [DOI] [PubMed] [Google Scholar]
- 18. Elnan Kvistad C, Naess H, Helleberg BH, et al. Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR‐TEST 2, part A): a phase 3, randomised, open‐label, blinded endpoint, non‐inferiority trial. 2022; 21(6):511‐519. doi: 10.1016/S1474-4422(22)00124-7 [DOI] [PubMed] [Google Scholar]
- 19. Bivard A, Zhao H, Churilov L, et al. Comparison of tenecteplase with alteplase for the early treatment of ischaemic stroke in the Melbourne Mobile Stroke Unit (TASTE‐A): a phase 2, randomised, open‐label trial. 2022;21(6):520‐527. doi: 10.1016/S1474-4422(22)00171-5 [DOI] [PubMed] [Google Scholar]
- 20. Menon BK, Buck BH, Singh N, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open‐label, registry‐linked, randomised, controlled, non‐inferiority trial. Lancet. 2022;400(10347):161‐169. doi: 10.1016/S0140-6736(22)01054-6 [DOI] [PubMed] [Google Scholar]
- 21. Parsons M, Spratt N, Bivard A, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med. 2012; 366(12): 1099‐1107. doi: 10.1056/NEJMOA1109842/SUPPL_FILE/NEJMOA1109842_DISCLOSURES.PDF [DOI] [PubMed] [Google Scholar]
- 22. Campbell BCV, Mitchell PJ, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018; 378(17): 1573‐1582. doi: 10.1056/NEJMOA1716405/SUPPL_FILE/NEJMOA1716405_DISCLOSURES.PDF [DOI] [PubMed] [Google Scholar]
- 23. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta‐analysis of 5 randomized trials. Stroke. 2019; 50(8): 2156‐2162. doi: 10.1161/STROKEAHA.119.025080 [DOI] [PubMed] [Google Scholar]
- 24. Katsanos AH, Safouris A, Sarraj A, et al. Intravenous thrombolysis with tenecteplase in patients with large vessel occlusions: systematic review and meta‐analysis. Stroke. 2021;52(1):308‐312. doi: 10.1161/STROKEAHA.120.030220 [DOI] [PubMed] [Google Scholar]
- 25. TNKase (Tenecteplase) Package Insert. Accessed March 21, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/tenegen060200lb.htm
- 26. Alteplase . Lexi‐Drugs. Lexi‐Comp, Inc. Updated December 21, 2022. Lexicomp. https://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6310?cesid=3cvSgw1RHO9 [Google Scholar]
- 27. Tenecteplase . Lexi‐Drugs. Lexi‐Comp, Inc. Updated December 8, 2022. Lexicomp. https://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7779?cesid=afC5roPJYC6 [Google Scholar]
- 28. Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke real‐world experience and a call for action. 2010;41(10):2254‐2258. doi: 10.1161/STROKEAHA.110.592535 [DOI] [PubMed] [Google Scholar]
- 29. Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015; 372(1): 11‐20. doi: 10.1056/NEJMOA1411587/SUPPL_FILE/NEJMOA1411587_DISCLOSURES.PDF [DOI] [PubMed] [Google Scholar]
- 30. Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015; 372(24): 2296‐2306. doi: 10.1056/NEJMOA1503780/SUPPL_FILE/NEJMOA1503780_DISCLOSURES.PDF [DOI] [PubMed] [Google Scholar]
- 31. Saver JL, Goyal M, Bonafe A, et al. Stent‐retriever thrombectomy after intravenous t‐PA vs. t‐PA alone in stroke. N Engl J Med. 2015; 372(24):2285‐2295. doi: 10.1056/NEJMOA1415061/SUPPL_FILE/NEJMOA1415061_DISCLOSURES.PDF [DOI] [PubMed] [Google Scholar]
- 32. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015; 372(11): 1019‐1030. doi: 10.1056/NEJMOA1414905/SUPPL_FILE/NEJMOA1414905_DISCLOSURES.PDF [DOI] [PubMed] [Google Scholar]
- 33. Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion‐imaging selection. N Engl J Med. 2015; 372(11): 1009‐1018. doi: 10.1056/NEJMOA1414792/SUPPL_FILE/NEJMOA1414792_DISCLOSURES.PDF [DOI] [PubMed] [Google Scholar]
- 34. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large‐vessel ischaemic stroke: a meta‐analysis of individual patient data from five randomised trials. Lancet. 2016; 387(10029): 1723‐1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 35. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018; 378(1): 11‐21. doi: 10.1056/NEJMOA1706442/SUPPL_FILE/NEJMOA1706442_DISCLOSURES.PDF [DOI] [PubMed] [Google Scholar]
- 36. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018; 378(8): 708‐718. doi: 10.1056/NEJMOA1713973/SUPPL_FILE/NEJMOA1713973_DISCLOSURES.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao W, Ma P, Zhao W, et al. The safety and efficacy of mechanical thrombectomy in posterior vs. anterior emergent large vessel occlusion: a systematic review and meta‐analysis. J Stroke Cerebrovasc Dis. 2020; 29(3). doi: 10.1016/J.JSTROKECEREBROVASDIS.2019.104545 [DOI] [PubMed] [Google Scholar]
- 38. Saver JL, Adeoye O. Intravenous thrombolysis before endovascular thrombectomy for acute ischemic stroke. JAMA. 2021; 325(3): 229‐231. doi: 10.1001/JAMA.2020.22388 [DOI] [PubMed] [Google Scholar]
- 39. Zi W, Qiu Z, Li F, et al. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT randomized clinical trial. JAMA. 2021;325(3): 234‐243. doi: 10.1001/JAMA.2020.23523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang P, Zhang Y, Zhang L, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020; 382(21): 1981‐1993. doi: 10.1056/NEJMOA2001123/SUPPL_FILE/NEJMOA2001123_DATA-SHARING.PDF [DOI] [PubMed] [Google Scholar]
- 41. Suzuki K, Matsumaru Y, Takeuchi M, et al. Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: the SKIP randomized clinical trial. JAMA. 2021; 325(3): 244‐253. doi: 10.1001/JAMA.2020.23522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. LeCouffe NE, Kappelhof M, Treurniet KM, et al. A randomized trial of intravenous alteplase before endovascular treatment for stroke. N Engl J Med. 2021; 385(20): 1833‐1844. doi: 10.1056/NEJMOA2107727/SUPPL_FILE/NEJMOA2107727_DATA-SHARING.PDF [DOI] [PubMed] [Google Scholar]
- 43. Merchant RM, Topjian AA, Panchal AR, et al. Part 1: executive summary: 2020 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020; 142(16_suppl_2): S337‐S357. doi: 10.1161/CIR.0000000000000918 [DOI] [PubMed] [Google Scholar]
- 44. Duvekot MHC, Venema E, Rozeman AD, et al. Comparison of eight prehospital stroke scales to detect intracranial large‐vessel occlusion in suspected stroke (PRESTO): a prospective observational study. Lancet Neurol. 2021; 20(3): 213‐221. doi: 10.1016/S1474-4422(20)30439-7 [DOI] [PubMed] [Google Scholar]
- 45. Ramos A, Guerrero WR, Pérez de la Ossa N. Prehospital stroke triage. Neurology. 2021; 97(20 Suppl 2): S25‐S33. doi: 10.1212/WNL.0000000000012792 [DOI] [PubMed] [Google Scholar]
- 46. Kellner CP, Sauvageau E, Snyder Kv, et al. The VITAL study and overall pooled analysis with the VIPS non‐invasive stroke detection device. J Neurointerv Surg. 2018; 10(11): 1079‐1084. doi: 10.1136/NEURINTSURG-2017-013690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barbour J, Hushen P, Newman GC, Vidal J. Impact of an emergency medicine pharmacist on door to needle alteplase time and patient outcomes in acute ischemic stroke. Am J Emerg Med. 2022; 51: 358‐362. doi: 10.1016/J.AJEM.2021.11.015 [DOI] [PubMed] [Google Scholar]
- 48. Rech MA, Bennett S, Donahey E. Pharmacist participation in acute ischemic stroke decreases door‐to‐needle time to recombinant tissue plasminogen activator. Ann Pharmacother. 2017; 51(12): 1084‐1089. doi: 10.1177/1060028017724804 [DOI] [PubMed] [Google Scholar]
- 49. Urdaneta AE, Bhalla P. Cutting edge acute ischemic stroke management. Emerg Med Clin North Am. 2019; 37(3): 365‐379. doi: 10.1016/J.EMC.2019.03.001 [DOI] [PubMed] [Google Scholar]


