ABSTRACT
Background: Treatment guidelines for asthma management are derived almost exclusively from the results of controlled clinical trials undertaken in carefully selected patient populations; meaning that their outcomes may not reflect the true performance of treatments when used in general daily medical practice. The aim of this meta-analysis was to combine the results of observational studies investigating the fluticasone propionate/formoterol (FP/FORM) fixed-dose combination in real-world asthma patients. Methods: A systemic literature review was completed in March 2019 using the PubMed database. We identified 394 studies. Five studies, which included a total of 4756 patients treated with FP/FORM, were judged eligible and included in the meta-analysis. Results: The estimated severe asthma exacerbation rate was 11.47% (95% CI, 5.8 to 18.72%), calculated from the random effect model. A sensitivity analysis excluding 2 studies (one was an outlier, and the exacerbation rate for the studied treatment alone could not be determined in the other) showed a 7.04% rate of severe asthma exacerbations. The estimated relative risk of the incidence of severe asthma exacerbations was 0.323 (95% CI, 0.159 to 0.658). The estimated asthma control rate was 60.6% (95% CI, 55.7% to 65.6%). The odds of achieving asthma control significantly increased by FP/FORM compared with pre-study conditions (estimated odds ratio: 2.214 [95% CI, 1.292 to 3.795]; p < 0.001). Conclusions: The findings of this meta-analysis confirm the effectiveness of FP/FORM for the treatment of asthma patients in a real-world setting beyond the limitations of RCTs.
KEYWORDS: Asthma, real-world setting, fluticasone, formoterol, fixed-dose combination, meta-analysis
Introduction
Guidelines’ recommendations on asthma treatment are largely based on data from controlled randomised clinical trials (RCTs) which are undertaken in carefully selected patient cohorts with minimal comorbidities and risk factors. These restrictive recruitment criteria make trials’ outcomes unlikely to reflect the true performance of treatments when used in real-life settings (where patients may have multiple comorbidities, unreliable compliance, erratic therapy administration techniques, or any multitude of other confounding factors). It is estimated that only 4–5% of asthma patients in the general population would have been eligible to participate in the major RCTs that have defined standard treatment practices [1,2]. Treatment of asthma patients in the real world is complex and real-world observational studies are paramount in determining the applicability of RCTs’ findings to these patients.
Combination therapy with inhaled corticosteroids (ICS) and long-acting β2-agonists (LABA) is the mainstay of asthma therapy in patients with persistent symptoms [3]. Fixed dose combination (FDC) therapies (which employ a single inhaler) offer potential benefits in terms of improved compliance and may also impact outcomes. Currently, numerous FDCs of ICS/LABA inhalers are available on the market [4].
In a recent study [5], the incidence of asthma exacerbations, in two open-label trials of fluticasone/formoterol (FP/FORM) FDC [6,7], was compared to that reported in three Cochrane meta-analyses of other FDCs of ICS/LABA [8–10]. Results showed that the incidence of exacerbations in the two fixed-dose FP/FORM studies was low and less than in the most comparable published studies involving other ICS/LABA combinations. The authors suggested that this finding may be due to the favourable pharmacological profile and inhaler characteristics of the FP/FORM combination compared to other combinations in the same therapeutic class [11].
Given the differences between the meticulous conditions of RCTs compared to the heterogeneous patient populations in real-world clinical practice, the aim of this systematic review and meta-analysis was to compile data from observational real-world studies on the effectiveness of the FP/FORM FDC in real-world asthma patients, and examine whether the outcomes reported in these studies are consistent with the outcomes reported in RCTs for the FP/FORM FDC.
Materials and methods
A systematic review of the literature on FP/FORM was undertaken. We searched PubMed for all articles indexed using the MeSH terms ‘fluticasone’ AND ‘formoterol’. In addition, other observational studies, known to the authors, that had relevant outcomes for the combination were also included for analysis.
Inclusion and exclusion criteria
Observational studies of the impact of fixed-dose FP/FORM pressurised metered dose inhaler (pMDI) on asthma control and severe asthma exacerbation in real-world asthma patients were included. Exclusion criteria were [1]: preclinical studies [2], reviews, perspectives, editorials or case studies [3], studies investigating respiratory diseases other than asthma [4], interventional RCTs [5], studies investigating ICS/LABA combinations apart from FP/FORM, or other classes of asthma treatment [6], studies investigating FP/FORM combination in devices other than a pMDI, and [7] studies that did not have the endpoints of asthma control and/or severe asthma exacerbations.
Relevant citations retrieved from the PubMed search were initially screened for potential articles, and irrelevant articles were excluded. Abstracts of the remaining citations were further evaluated in a similar fashion. Full-text publications were subsequently retrieved for the remaining articles for a more detailed review to determine appropriateness for inclusion. Citations with no abstracts and/or access to the full-text publication in English language were excluded. Studies for inclusion were independently selected by two reviewers and the final studies for inclusion were agreed upon by all authors prior to undertaking the meta-analysis.
Data collation
Relevant data were selected and collated into a predefined table. These data included, but were not limited to, baseline characteristics such as duration of follow-up, use of oral corticosteroids (OCS) and use of short-acting β2-agonist (SABA), and endpoints including asthma control test (ACT®) scores, asthma control questionnaire (ACQ7), overall asthma control (OAC), risk domain asthma control (RDAC), and severe asthma exacerbation.
Endpoints
The primary endpoints for the analysis were as follows:
-
Severe asthma exacerbations:
- The rate of severe asthma exacerbations during the observational periods of the studies. Severe exacerbations were defined according to the definition provided by the European Respiratory Society/American Thoracic Society (ERS/ATS) 2015 guidelines (any asthma-related hospitalization or emergency hospital attendance or prescription of an acute course of OCS) [12].
- The relative risk (RR) of severe asthma exacerbations before and during the study. RR was calculated as the ratio of the exacerbation rate during the study and the rate before the study.
(2) Asthma control:- The rate of responders defined by ‘asthma control’ rate.
- The odds-ratio (OR) of asthma control before and during the study. OR was calculated as the proportion of controlled patients before the study and the proportion of controlled patients during the study.
Safety data were summarised as reported in the studies.
Statistical methods
The program used for calculation (MedCalc) uses a Freeman-Tukey transformation (arcsine square root transformation) [13] to calculate the weighted summary proportion under the fixed and random effects model [14]. The assumption of a ‘fixed effects model’ was tested by the ‘Heterogeneity test’. Cochran’s Q and the I2 statistic were used to measure heterogeneity [15].
Risk of bias
We used the ROBINS-I assessment tool (version for cohort-type studies Sterne 2016 [16]) to assess risk of bias for each of the included studies.
Study quality assessment
Study quality was assessed, summarised, and presented according to the modified Quality of Study Rating Form (QSRF) which comprises 18 questions [17] (see Supplementary Table S1). The QSRF incorporates principles of meta-analysis to rate key features in an evaluation study. The maximum original rating score is 100 points. The main purpose of the quality score is to show comparability of the included studies with respect to quality.
Since the included studies used well-defined and accepted endpoints that were also recommended in the respective guidelines, we calculated the total score excluding questions 13 and 14, which check for reliability of endpoints. The maximum rating for this score is 90 points.
Results
The literature search process used to identify the studies included in the current systematic review and meta-analysis is illustrated in Figure 1, and the resulting PRISMA checklist is shown in Supplementary Table S2. The literature search was conducted in March 2019 and identified 394 articles. The articles were initially screened for eligibility based on the title and article information, 331 articles were excluded for the following reasons: preclinical studies (n = 28), review/perspective/case study articles (n = 54), not in the real-world setting (n = 14), out-of-scope patient population (n = 100), out-of-scope treatment (n = 116), out-of-scope device investigated for the FP/FORM combination (n = 3), and out-of-scope outcomes (n = 16) for the objective of the current meta-analysis. The remaining 63 articles underwent a further abstract review for eligibility based on the exclusion criteria described previously. Of these, 50 articles were excluded for the following reasons: review/case study articles (n = 10), not in the real-world setting (n = 5), out-of-scope treatment (n = 29) and out-of-scope outcomes (n = 6). The remaining 13 articles were reviewed, and 9 articles were excluded for the following reasons: editorial article (n = 1), not in the real-world setting (n = 2), and out-of-scope treatment (n = 2). Four articles were further excluded due to the lack of online access to the full-text publication (n = 3) and the lack of access to the publication in English language (n = 1).
Figure 1.
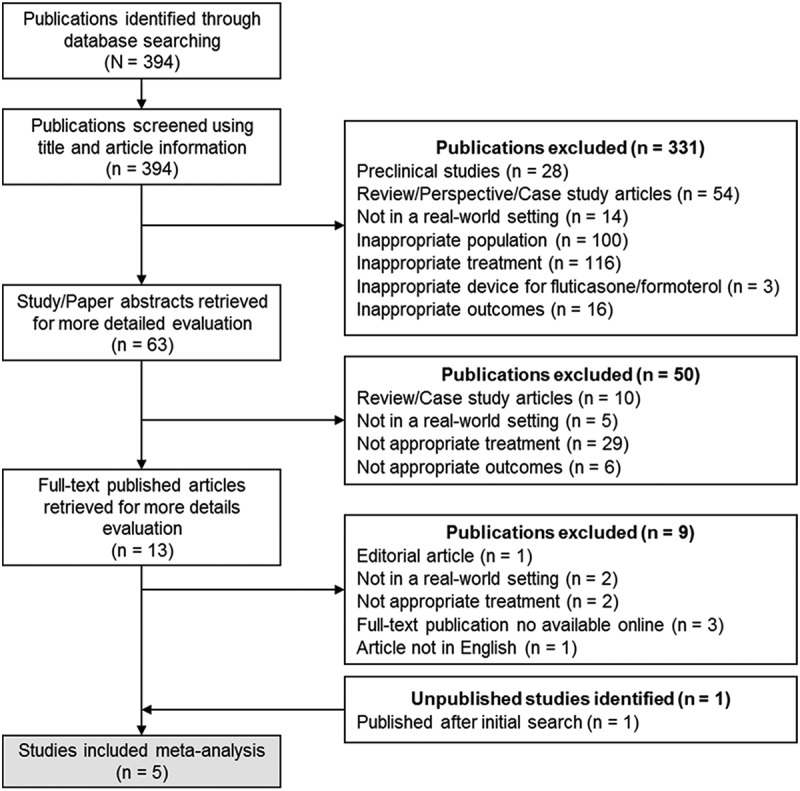
PRISMA diagram showing the study selection process.
The remaining 4 studies met all the inclusion criteria for the meta-analysis. Of these, the study by Usmani and colleagues was included but only the first observational phase of the study was analysed, because this was the phase which included a fixed dose regimen (given that the aim of our study was to evaluate the real-world effectiveness of fixed-dose FP/FORM). Phase 2 of the study was not included because it was a ‘stepping-down’ treatment phase. In addition, only ‘controlled’ patients were included in phase 2, so including phase 2 from that study would have introduced bias.
One additional unpublished observational study, known to the authors to fulfil the inclusion criteria, was also included for analysis; this study was subsequently published at the time of the meta-analysis [18]. The current meta-analysis therefore included the following 5 studies: AFFIRM [19], EFFECTIVENESS [20], FFAIRNESS [21], TRANSFFORM2 [18] and FFLUX [22]. The key baseline characteristics of the patients included in these 5 studies are summarised in Supplementary Table s3.
Data were extracted from the five published studies (AFFIRM, EFFECTIVENESS, FFAIRNESS, TRANSFFORM2 and FFLUX) with respect to the incidence of severe exacerbations and asthma control. Table 1 summarizes the data used for the meta-analysis. One of the limitations we encountered was that asthma control was measured using different instruments. The AFFIRM and FFAIRNESS studies used the 5-item ACT® score, which defines the extent of asthma control as: controlled (score≥20), somewhat controlled (score 16–19), or poorly controlled (score≤15). Asthma control was defined as an ACT total score≥20 [19,21]. The TRANSFFORM2 and EFFECTIVENESS studies used the risk domain asthma control (RDAC) defined as the absence of asthma-related hospital admissions AND asthma-related A&E attendance AND an acute course of OCS AND asthma-related antibiotics without upper respiratory diagnosis [18,20]. The FFLUX study used the GINA definition, which defined asthma control as having no asthma symptoms in the past four weeks as determined by four ‘level of asthma symptom control’ questions [22]. The limitations of the individual studies are listed in Supplementary Table 3.
Table 1.
Summary of data used for the meta-analysis.
| Study ID | Number of patients | Observation time [months] |
Severe exacerbation (N) |
Asthma control (N) |
||||
|---|---|---|---|---|---|---|---|---|
| Before study | During study | Available (N) | Before study | During study | Available (N) | |||
| AFFIRM (Backer 2018) [19] |
N = 2539 | 12 | 909 | 248 | 2539 | 723 | 1496a | 2220 |
| TRANSFFFORM2 (Park 2019) [18] |
N = 85; (FP/FORM n = 38) |
12 | 24 | 13 | 85 | 49 | 64b | 85 |
| FFAIRNESS (Schmidt 2017) [21] |
N = 1410 | 12 | 575 | 83 | 1410 | 421 | 850a | 1363 |
| EFFECTIVENESS (Wan 2017) [20] |
N = 2472; (FP/FORM n = 618) |
12 | 188 | 160 | 618 | 354 | 383b | 618 |
| FFLUX (Usmani 2017) [22] |
N = 225; (FP/FORM N = 151) | 3 | 38 | 7 | 151 | 68 | 71c | 126 |
Definition of asthma control: aACT total score≥20; bRDAC; cGINA.
FP/FORM: fluticasone propionate and formoterol fumarate; n: number of patients.
Risk of bias & study quality
When it comes to the risk of bias according to the ROBINS-I assessment, we found that a low or moderate risk of bias could be evaluated for 4 studies. Only one study – TRANSFFFORM2 – was classified as having a serious risk of bias because the number of severe exacerbations was reported overall not by treatment. The number of exacerbations in the sub-group of patients treated with beclomethasone dipropionate/FORM did not change after initiation of the study treatment and it cannot be determined whether this is in favor of the treatment under investigation. A summary of the risk-of-bias assessments and results of the QSRF assessment are provided in Table 2. There was no major discrepancy in quality among the 5 studies.
Table 2.
Risk-of-bias (ROBINS-I assessment tool) and total quality scores for individual studies.
| Study ID | Bias domains |
Overall risk of bias |
Total score (% of max) | Total reduced score* (% of max) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| i | ii | iii | iv | v | vi | vii | ||||
| FFAIRNESS | M | L | M | L | M | M | L | Moderate | 56% | 66% |
| AFFIRM | M | L | M | L | M | M | L | Moderate | 66% | 78% |
| TRANSFFFORM2 | S | M | L | L | M | M | L | Serious | 61% | 72% |
| EFFECTIVENESS | L | L | L | L | M | L | L | Low | 65% | 76% |
| FFLUX | M | L | L | L | M | L | L | Moderate | 75% | 88% |
Bias domains are as follows: I. Bias due to confounding; II. Bias in selection of participants into the study; III. Bias in classification of interventions; IV. Bias due to deviations from intended interventions; V. Bias due to missing data; VI. Bias in measurement of outcomes; VII. Bias in selection of the reported results. L: low; M: moderate; S: serious.
*Excluding questions 13 and 14.
Rate of severe asthma exacerbations
The estimated exacerbation rate from the meta-analysis was 11.47% (95%-confidence interval [CI], 5.8% to 18.72%), calculated from the random effect model (Table 3). This result was driven by the high rate (25.89%) in the EFFECTIVENESS study [20]. Heterogeneity among studies was significant (I2: 96.9%) (Figure 2).
Table 3.
Results of the meta-analysis of severe exacerbation rates.
| Study | Sample size | Proportion (%) |
95% CI | Weight (%) |
|
|---|---|---|---|---|---|
| Fixed | Random | ||||
| Backer 2018 | 2539 | 9.8 | 8.6–10.9 | 52.8 | 21.4 |
| Park 2019 | 85 | 15.3 | 8.4–24.7 | 1.8 | 17.5 |
| Schmidt 2017 | 1410 | 5.9 | 4.7–7.3 | 29.4 | 21.2 |
| Wan 2017 | 618 | 25.9 | 22.5–29.5 | 12.9 | 20.9 |
| Usmani 2017 | 151 | 4.6 | 1.9–9.3 | 3.2 | 19.0 |
| Total (fixed effects) | 4803 | 10.1 | 9.3–10.9 | 100.0 | 100.0 |
| Total (random effects) | 4803 | 11.5 | 5.8–18.7 | 100.0 | 100.0 |
Figure 2.
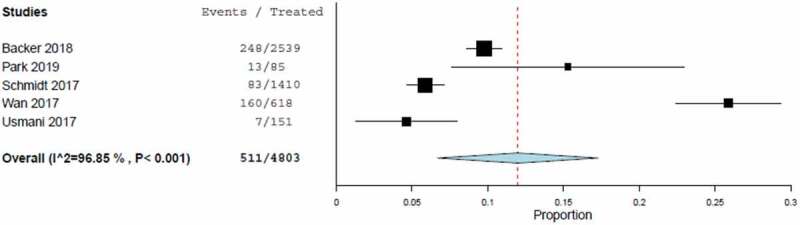
Forest plot of exacerbation rates including estimated combined effect (random effect model).
A funnel plot, displaying possible bias which might be introduced by outliers, showed no trend towards extreme proportions with larger or smaller studies. The only values which showed a tendency for outliers were the exacerbation rates from the EFFECTIVENESS study [20]. A sensitivity analysis, excluding the EFFECTIVENESS study [20] (because of its detection as an outlier in the funnel plot) and the TRANSFFORM2 study [18] (because the exacerbation rate could only be determined for the complete cohort rather than the FP/FORM pMDI treatment alone) showed slightly lower point estimates for the random effect model compared to the results of all studies (7.04% versus 11.47%).
Relative risk of severe asthma exacerbations before and during the study
The estimated RR from the meta-analysis was 0.323 (95% CI, 0.159 to 0.658), calculated from the random effect model (Table 4 and Figure 3). The rate of severe asthma exacerbations was significantly lower in patients treated with FP/FORM compared with pre-study conditions (p < 0.001). This result was driven by the values in the EFFECTIVENESS and TRANSFFORM2 studies [18,20]. Heterogeneity among studies was significant (I2: 97.7%).
Table 4.
Results of the meta-analysis of relative risk of severe asthma exacerbation.
| Study | Sample size | Relative-Risk | 95% CI | Weight (%) |
|
|---|---|---|---|---|---|
| Fixed | Random | ||||
| Backer 2018 | 2539 | 0.27 | 0.24–0.31 | 51.4 | 21.5 |
| Park 2019 | 85 | 0.54 | 0.3–0.99 | 2.4 | 18.7 |
| Schmidt 2017 | 1410 | 0.14 | 0.12–0.18 | 18.1 | 21.2 |
| Wan 2017 | 618 | 0.85 | 0.71–1.02 | 26.8 | 21.4 |
| Usmani 2017 | 151 | 0.18 | 0.09–0.40 | 1.4 | 17.2 |
| Total (fixed effects) | 4803 | 0.33 | 0.30–0.37 | 100.0 | 100.0 |
| Total (random effects) | 4803 | 0.32 | 0.16–0.66 | 100.0 | 100.0 |
Figure 3.
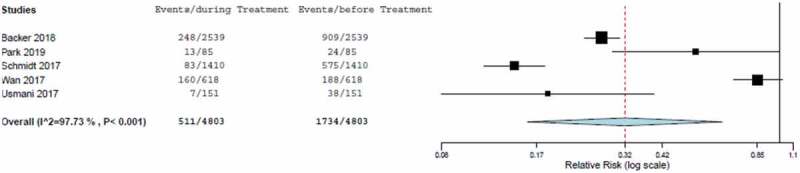
Forest plot of relative risk of severe asthma exacerbation including estimated combined effect (random effect model).
Asthma control rate
The estimated asthma control rate from the meta-analysis was 60.6% (95% CI, 55.7% to 65.6%), calculated from the random effect model (Table 5 and Figure 4). This result was driven by the low rate (45.2%) in the FFLUX study [22]. Heterogeneity among studies was significant (I2: 87.8%).
Table 5.
Results of the meta-analysis of asthma control.
| Study | Sample size |
Proportion (%) |
95% CI | Weight (%) |
|
|---|---|---|---|---|---|
| Fixed | Random | ||||
| Backer 2018 | 2220 | 67.4 | 65.4–69.3 | 52.1 | 25.5 |
| Park 2019 | 85 | 58.8 | 48.4–69.3 | 1.8 | 12.1 |
| Schmidt 2017 | 1363 | 62.4 | 59.8–64.9 | 29.9 | 24.8 |
| Wan 2017 | 618 | 60.0 | 58.1–65.8 | 13.5 | 22.9 |
| Usmani 2017 | 126 | 45.2 | 36.5–53.9 | 2.6 | 14.6 |
| Total (fixed effects) | 4412 | 64.4 | 63.0–65.8 | 100.0 | 100.0 |
| Total (random effects) | 4412 | 60.6 | 55.7–65.6 | 100.0 | 100.0 |
Figure 4.
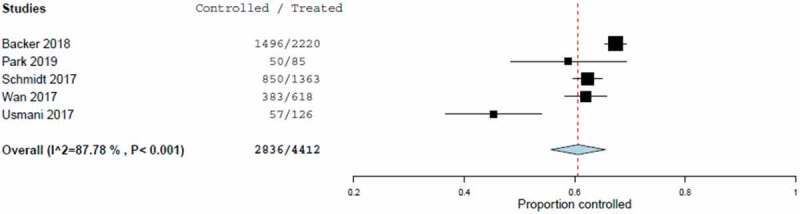
Forest plot of asthma control rates including estimated combined effect (random effect model).
Odds-ratio of asthma control rate before and during the study
The estimated OR from the meta-analysis was 2.214 (95% CI, 1.292 to 3.795) calculated from the random effect model (Table 6 and Figure 5). The odds of achieving asthma control during the study was significantly increased by FP/FORM compared with pre-study conditions (p < 0.001). This result was driven by the values in the EFFECTIVENESS and FFLUX studies [20,22]. Heterogeneity among the studies was significant (I2: 96.4%).
Table 6.
Results of meta-analysis of odds ratio of asthma control rate.
| Study | Sample size |
Odds-ratio | 95% CI | Weight (%) |
|
|---|---|---|---|---|---|
| Fixed | Random | ||||
| Backer 2018 | 2220 | 4.28 | 3.77–4.85 | 49.3 | 21.8 |
| Park 2019 | 85 | 2.24 | 1.16–4.31 | 1.8 | 16.7 |
| Schmidt 2017 | 1363 | 3.71 | 3.16–4.35 | 30.8 | 21.7 |
| Wan 2017 | 618 | 1.22 | 0.97–1.53 | 15.0 | 21.3 |
| Usmani 2017 | 126 | 1.10 | 0.67–1.81 | 3.2 | 18.6 |
| Total (fixed effects) | 4412 | 3.21 | 2.94–3.51 | 100.0 | 100.0 |
| Total (random effects) | 4412 | 2.21 | 1.29–3.80 | 100.0 | 100.0 |
Figure 5.
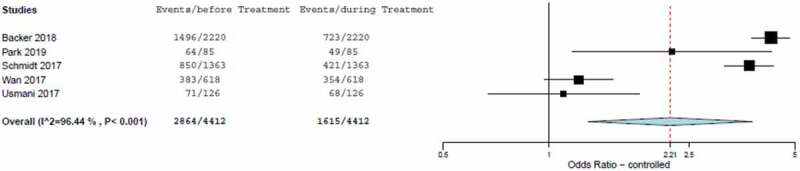
Forest-plot of odds-ratio of asthma control rate including estimated combined effect (random effect model).
Summary of reported safety data
Only three of the five studies included in this meta-analysis reported safety as part of their evaluation. Table 7 summarises the treatment-related adverse events (TRAEs) reported across these three studies (AFFIRM [19], FFAIRNESS [21] and FFLUX [22]). The incidence of oropharyngeal adverse events was 0.6% for oral candidiasis, 0.2% for oral fungal infections, 0.5% for oropharyngeal pain, 0.2% for dry mouth, 2.2% for dysphonia and 0.9% for cough. Bronchopneumonia was reported in 0.1% of patients who received FP/FORM in the AFFIRM study. The incidence of heart palpitations was 0.6% (only reported in the AFFIRM study) [19].
Table 7.
Summary of treatment related adverse events.
| First author and year | Backer 2018 | Schmidt 2017 | Usmani 2017 |
|---|---|---|---|
| Study ID | AFFIRM* | FFAIRNESS ** | FFLUX *** |
| Safety population | n = 2539 | n = 1563 | n = 151 (phase 1) |
| Treatment related adverse events by system organ class n (%) | |||
| Cardiac Disorders | NR | NR | 3 (2.0%) |
| Gastrointestinal disorders | NR | NR | 5 (3.3%) |
| Infections and infestations | NR | NR | 4 (2.6%) |
| Respiratory, thoracic and mediastinal disorders | NR | NR | 8 (5.3%) |
| Treatment related adverse events by preferred term n (%) | |||
| Asthma | 50 (2.0%) | 7 (0.4%) | NR |
| Bronchopneumonia | 3 (0.1%) | NR | NR |
| Cough | 28 (1.1%) | 10 (0.6%) | NR |
| Dizziness | 3 (0.1%) | 7 (0.4%) | NR |
| Dry mouth | 4 (0.2%) | 6 (0.4%) | NR |
| Dysphonia | 46 (1.8%) | 44 (2.8%) | NR |
| Dyspepsia | 3 (0.1%) | NR | NR |
| Dyspnoea | 5 (0.2%) | NR | NR |
| Headache | 10 (0.4%) | 6 (0.4%) | NR |
| Lower RTI | 7 (0.3%) | NR | NR |
| Nasopharyngitis | 3 (0.1%) | NR | NR |
| Nausea | 6 (0.2%) | NR | NR |
| Oral candidiasis | 17 (0.7%) | 8 (0.5%) | NR |
| Oral fungal infection | 4 (0.2%) | NR | NR |
| Oropharyngeal candidiasis | 3 (0.1%) | NR | NR |
| Oropharyngeal pain | 13 (0.5%) | 6 (0.4%) | NR |
| Palpitations | 14 (0.6%) | NR | NR |
| RTI | 3 (0.1%) | NR | NR |
| Sleep disorder | NR | 6 (0.4%) | NR |
| Tachycardia | 7 (0.3%) | 5 (0.3%) | NR |
| Tremor | 16 (0.6%) | 11 (0.7%) | NR |
| Upper airway cough syndrome | 3 (0.1%) | NR | NR |
| Upper RTI | 4 (0.2%) | NR | NR |
* TRAEs reported in more than 2 patients.
** TRAEs which occurred at a frequency of≥0.3%.
*** TRAEs were reported by system organ class only, preferred term was not reported.
NR: not reported, RTI: respiratory tract infection, TRAEs: treatment-related adverse event.
Discussion
Due to the difference in the conditions in which RCTs are conducted and real-world clinical practice, findings from RCTs may have limited applicability in everyday medicine [1,2,23]. Accordingly, information about the effectiveness of asthma treatments in real-world asthma patients is necessary. This meta-analysis examines the effect of the FDC FP/FORM on asthma exacerbation and control in patients treated in a real-world setting, addressing the issues highlighted by Papi and colleagues [5] to affirm the low incidence of asthma exacerbations with FP/FORM observed in RCTs.
In the current analysis, the estimated rate of severe asthma exacerbation (11.47% [or 7.04% with the exclusion of two studies based on the sensitivity analysis]) was higher than that reported for FP/FORM in RCTs (2.9% reported as the pooled respiratory exacerbation rate [95% CI, 1.7 to 4.1]) [5] – likely due to the more diverse study population (as expected for studies conducted in real-world setting, some patients in the included studies had concurrent allergic rhinitis, obesity, sleep apnoea syndrome, respiratory tract infection or chronic obstructive pulmonary disease. Further, the study cohorts comprised both treatment-naïve patients and those who had received prior treatment for asthma, and there were varying levels of SABA, ICS and oral corticosteroid use at baseline. In addition, the included studies were conducted in a wide range of countries [including the United Kingdom, Czech Republic, Denmark, France, Ireland, Norway, Slovak Republic, Sweden, Germany and Korea]).
Given all these factors, the heterogeneity among the studies included in this meta-analysis was a significant finding for every outcome assessed, which is why we conducted a sensitivity analysis and resorted to reporting results provided by the random effects model.
Importantly, the meta-analysis shows a significant reduction in the estimated RR of severe exacerbation versus pre-study conditions despite the higher estimated rate versus RCT. This was supported by a significant increase of more than two times the odds of achieving asthma control with FP/FORM from baseline in the current analysis. Furthermore, the results show an estimated asthma control rate of 60.6% with FP/FORM, which is higher than that reported in some of the recent real-world epidemiological studies where between 17.8% and 20.1% of patients had controlled disease in accordance with GINA-defined criteria [24,25]. Along with consideration of the diverse patient population included in the analysis, the significant results for RR of severe exacerbation and odds of achieving asthma control are aligned and further substantiate the fact that the favourable pharmacological and inhaler characteristics may potentially account for the low exacerbation rates reported with FP/FORM in RCTs [5].
Poor asthma control could be attributed to different causes, including poor adherence and device usability. An additional contributing factor could be ascribed to the lack of drug reaching and targeting inflammation in the whole respiratory tree and especially the small airways [26]. Optimal delivery of drugs to the small airways (<2 mm) favours effective asthma management [27]. The prevalence of small airways dysfunction was shown to be present in approximating 50% of asthma patients, irrespective of severity [28]. Factors impacting drug deposition in the lungs include but are not limited to inefficient device, sub-optimal particle size of the inhaled medication and a patient’s poor inhalation capacity [29].
The plume characteristics of an inhaler device – plume force, velocity, duration, angle of dispersion and temperature – as well as particle size, affect lung deposition [30,31]. A gentler, warmer plume results in less drug impacting the oropharyngeal region, a reduction in the ‘cold-Freon’ effect and an increased opportunity for inhaled medications to reach the peripheral lungs [30,32]. Concurrently, a smaller particle size (<5 μm) and greater distribution of fine particles, the higher the resultant fine-particle fraction (FPF) and the increased likelihood of drug deposition in the peripheral lungs [33].
The potential synergistic activity unique to the FP/FORM combination was well-discussed by Papi et al [5]. Of further interest, an in vitro study found that FORM was more effective than salmeterol in suppressing neutrophilic activity [34]. Increased levels of neutrophils have been associated with severe asthma and asthma exacerbation [35–37]. FORM may thus have additional anti-inflammatory activity which could work synergistically with FP. However, further studies are warranted to fully understand the synergism of FP and FORM in asthma management.
It would be of interest to compare the outcomes of this meta-analysis with those reported for other ICS/LABAs. However, the varying outcomes explored in real-world studies makes comparisons challenging. The study by Price et al is perhaps one of the few that reported outcomes close to those examined in the current analysis [38]. Notably, the authors examined asthma control and severe exacerbation rates in patients who switched from FP/salmeterol to beclomethasone/FORM versus those who remained on FP/salmeterol [38]. The rate of severe exacerbation remained relatively unchanged at 12% following the switch to beclomethasone/FORM – the current study showed a severe asthma exacerbation rate of 11.47%, or 7.04% following sensitivity analysis, with FP/FORM.
Of the five studies included in the meta-analysis, only three reported adverse events as part of the study design. There was notably a low incidence of oropharyngeal adverse events which aligns with the gentler plume reported with the FP/FORM pMDI device [32] and lung deposition study [39]. The high dose of fluticasone was previously associated with concerns of pneumonia [40,41]. However, the AFFIRM study reported bronchopneumonia in only 0.1% of patients who received FP/FORM, and pneumonia was not reported as an undesirable effect in the summary of product characteristics [42].
This study was inherently limited by its meta-analysis approach which pooled together results from various studies with different study designs, patient populations, outcomes, and baseline characteristics. It should be noted that real-world, observational studies come with their own set of limitations as well; they lack internal validity, there is often little or no control over the quality of collected data, and unmeasured confounding cannot be eliminated [43]. Therefore, assessment of the efficacy and safety of a certain intervention is best done by considering the totality of real-world evidence and evidence from RCTs.
In conclusion, findings of this meta-analysis indicate that the rate of severe asthma exacerbations is higher in the real world than that reported in RCTs. It also confirms that FP/FORM reduces the risk of severe asthma exacerbations and increases the odds of achieving asthma control in real-world asthmatic patients. With more than 50% of the cohort previously receiving other ICS/LABA combinations, these results may provide support for the use of FP/FORM in suitable patients with uncontrolled asthma on previous ICS/LABA therapy.
Acknowledgments
The authors would like to acknowledge the contribution of Alison Coletta, Principal Medical Writer, CROS NT Italy in the preparation of this manuscript.
Funding Statement
Funding for this meta-analysis and medical writing support was provided by Mundipharma Singapore Holdings Pte. Limited.
Disclosure statement
Mundipharma was not involved in the study design, data collection, analysis or interpretation. Mundipharma reviewed the written manuscript and was involved in the decision to submit it for publication.
References
- [1].Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007. Mar 1;62(3):219–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Herland K, Akselsen J-P, Skjønsberg OH, et al. How representative are clinical study patients with asthma or COPD for a larger “real life” population of patients with obstructive lung disease? Respir med. 2005. Jan;99(1):11–19. [DOI] [PubMed] [Google Scholar]
- [3].Global Initiative for Asthma . Global strategy for asthma management and prevention. USA: GINA; 2022. https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf. [Google Scholar]
- [4].Cazzola M, Matera MG.. Fixed-dose combination inhalers. In: Page, Clive, Barnes, Peter,, editors. Pharmacology and Therapeutics of Asthma and COPD. Handbook of experimental pharmacology, vol. 237. Switzerland: Springer, Cham; 2016. pp. 117–129. doi: 10.1007/164_2016_66. [DOI] [PubMed] [Google Scholar]
- [5].Papi A, Mansur AH, Pertseva T, et al. Long-term fluticasone propionate/formoterol fumarate combination therapy is associated with a low incidence of severe asthma exacerbations. J Aerosol Med Pulm Drug Deliv. 2016;29(4):346–361. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kaiser K, Pertseva T. Long-term safety and efficacy of fluticasone propionate/formoterol fumarate combination therapy in patients with asthma. Prim Care Respir J. 2013;22:A1–18. [Google Scholar]
- [7].Mansur AH, Kaiser K. Long-term safety and efficacy of fluticasone/formoterol combination therapy in asthma. J Aerosol Med Pulm Drug Deliv. 2013. Aug;26(4):190–199. [DOI] [PubMed] [Google Scholar]
- [8].Ducharme FM, Ni Chroinin M, Greenstone I, et al. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev. 2010. Apr 14; DOI: 10.1002/14651858.CD005533.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lasserson TJ, Ferrara G, Casali L. Combination fluticasone and salmeterol versus fixed dose combination budesonide and formoterol for chronic asthma in adults and children. Cochrane Database Syst Rev. 2011. Dec 7; DOI: 10.1002/14651858.CD004106.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chauhan BF, Ducharme FM. Addition to inhaled corticosteroids of long-acting beta 2 -agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2014. Jan 24; DOI: 10.1002/14651858.CD003137.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Adcock IM, Maneechotesuwan K, Usmani O. Molecular interactions between glucocorticoids and long-acting β2-agonists. J Allergy Clin Immunol. 2002. Dec;110(6):S261–8. [DOI] [PubMed] [Google Scholar]
- [12].Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014. Feb 1;43(2):343–373. [DOI] [PubMed] [Google Scholar]
- [13].Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950. Dec;21(4):607–611. [Google Scholar]
- [14].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986. Sep;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- [15].Higgins JPT. Measuring inconsistency in meta-analyses. BMJ. 2003. Sep 6;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016. Oct 12;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gibbs LE. Quality of study rating form: an instrument for synthesizing evaluation studies. J Soc Work Educ. 1989. Jan 25;25(1):55–67. [Google Scholar]
- [18].Park H, Yoon D, Lee HY, et al. Real‐life effectiveness of inhaler device switch from dry powder inhalers to pressurized metred‐dose inhalers in patients with asthma treated with ICS/LABA. Respirology. 2019. Oct 30;24(10):972–979. [DOI] [PubMed] [Google Scholar]
- [19].Backer V, Ellery A, Borzova S, et al. Non-interventional study of the safety and effectiveness of fluticasone propionate/formoterol fumarate in real-world asthma management. Ther Adv Respir Dis. 2018. Jan 20;12:175346661879698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yau Ming S W, Haughney J, Small I, et al. Initiating or changing to a fixed-dose combination of Fluticasone propionate/Formoterol over Fluticasone propionate/Salmeterol: a real-life effectiveness and cost impact evaluation. Respir med. 2017. Aug;129:199–206. [DOI] [PubMed] [Google Scholar]
- [21].Schmidt O, Petro W, Hoheisel G, et al. Real-life effectiveness of asthma treatment with a fixed-dose fluticasone/formoterol pressurised metered-dose inhaler – Results from a non-interventional study. Respir med. 2017. Oct;131:166–174. [DOI] [PubMed] [Google Scholar]
- [22].Usmani OS, Kemppinen A, Gardener E, et al. A randomized pragmatic trial of changing to and stepping down fluticasone/formoterol in asthma. J Allergy Clin Immunol Pract. 2017;5(5):1378–1387.e5. Sep. [DOI] [PubMed] [Google Scholar]
- [23].Woodcock A, Vestbo J, Bakerly ND, et al. Effectiveness of fluticasone furoate plus vilanterol on asthma control in clinical practice: an open-label, parallel group, randomised controlled trial. Lancet. 2017;390(10109):2247–2255. Nov. [DOI] [PubMed] [Google Scholar]
- [24].Price D, Fletcher M, van der Molen T. Asthma control and management in 8,000 European patients: the REcognise asthma and link to symptoms and experience (REALISE) survey. NPJ Prim Care Respir Med. 2014. Nov 12;24(1):14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Price D, David-Wang A, Cho S-H, et al. Time for a new language for asthma control: results from REALISE Asia. J Asthma Allergy. 2015. Sep;93:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bonini M, Usmani OS. The role of the small airways in the pathophysiology of asthma and chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2015. Dec 2;9(6):281–293. [DOI] [PubMed] [Google Scholar]
- [27].Usmani OS. Small airways dysfunction in asthma: evaluation and management to improve asthma control. Allergy Asthma Immunol Res. 2014;6(5):376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Usmani OS, Singh D, Spinola M, et al. The prevalence of small airways disease in adult asthma: a systematic literature review. Respir med. 2016. Jul;116:19–27. [DOI] [PubMed] [Google Scholar]
- [29].Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003. Dec;56(6):588–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bell J, Newman S. The rejuvenated pressurised metered dose inhaler. Expert Opin Drug Deliv. 2007. May 9;4(3):215–234. [DOI] [PubMed] [Google Scholar]
- [31].Haughney J, Price D, Barnes NC, et al. Choosing inhaler devices for people with asthma: current knowledge and outstanding research needs. Respir med. 2010. Sep;104(9):1237–1245. [DOI] [PubMed] [Google Scholar]
- [32].Johal B, Murphy S, Tuohy J, et al. Plume characteristics of two hfa-driven inhaled corticosteroid/long-acting beta2-agonist combination pressurized metered-dose inhalers. Adv Ther. 2015. Jun 23;32(6):567–579. [DOI] [PubMed] [Google Scholar]
- [33].Usmani OS, Biddiscombe MF, Barnes PJ. Regional lung deposition and bronchodilator response as a function of β 2 -agonist particle size. Am J Respir Crit Care Med. 2005. Dec 15;172(12):1497–1504. [DOI] [PubMed] [Google Scholar]
- [34].Tintinger GR, Theron AJ, Steel HC, et al. Formoterol is more effective than salmeterol in suppressing neutrophil reactivity. ERJ Open Res. 2015. May;1(1):00014–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wenzel SE, Szefler SJ, Leung DYM, et al. Bronchoscopic evaluation of severe asthma. Am J Respir Crit Care Med. 1997. Sep;156(3):737–743. [DOI] [PubMed] [Google Scholar]
- [36].Jatakanon A, Uasuf C, Maziak W, et al. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med. 1999. Nov;160(5):1532–1539. [DOI] [PubMed] [Google Scholar]
- [37].Norzila MZ, Fakes K, Henry RL, et al. Interleukin-8 secretion and neutrophil recruitment accompanies induced sputum eosinophil activation in children with acute asthma. Am J Respir Crit Care Med. 2000. Mar;161(3):769–774. [DOI] [PubMed] [Google Scholar]
- [38].Price D, Small I, Haughney J, et al. Clinical and cost effectiveness of switching asthma patients from fluticasone-salmeterol to extra-fine particle beclometasone-formoterol: a retrospective matched observational study of real-world patients. Prim Care Respir J. 2013. Nov 2;22(4):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Iwanaga T, Kozuka T, Nakanishi J, et al. Aerosol deposition of inhaled corticosteroids/long-acting β2-agonists in the peripheral airways of patients with asthma using functional respiratory imaging, a novel imaging technology. Pulm Ther. 2017. Jun 3;3(1):219–231. [Google Scholar]
- [40].Qian CJ, Coulombe J, Suissa S, et al. Pneumonia risk in asthma patients using inhaled corticosteroids: a quasi-cohort study. Br J Clin Pharmacol. 2017. Sep;83(9):2077–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].O’byrne PM, Pedersen S, Carlsson L-G, et al. Risks of pneumonia in patients with asthma taking inhaled corticosteroids. Am J Respir Crit Care Med. 2011;183(5):589–595. Mar. [DOI] [PubMed] [Google Scholar]
- [42].Napp Pharmaceuticals Limited . 2018. Summary of product characteristics for flutiform 125/5, inhalation suspension. Available from: https://www.medicines.org.uk/emc/product/7649/smpc#DOCREVISION
- [43].Camm AJ, Fox KAA. Strengths and weaknesses of ‘ real- world ’ studies involving non-vitamin K antagonist oral anticoagulants. Open Heart. 2018;5(e000788):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


