Abstract
Diabetic kidney disease (DKD) is a common complication of diabetes and has become the leading cause of end-stage kidney disease. The pathogenesis of DKD is complicated, and oxidative stress is considered as a core of DKD onset. High glucose can lead to increased production of reactive oxygen species (ROS) via the polyol, PKC, AGE/RAGE and hexosamine pathways, resulting in enhanced oxidative stress response. In this way, pathways such as PI3K/Akt, TGF-β1/p38-MAPK and NF-κB are activated, inducing endothelial cell apoptosis, inflammation, autophagy and fibrosis that cause histologic and functional abnormalities of the kidney and finally result in kidney injury. Presently, the treatment for DKD remains an unresolved issue. Traditional Chinese medicine (TCM) has unique advantages for DKD prevention and treatment attributed to its multi-target, multi-component, and multi-pathway characteristics. Numerous studies have proved that Chinese herbs (e.g., Golden Thread, Kudzuvine Root, Tripterygium glycosides, and Ginseng) and patent medicines (e.g., Shenshuaining Tablet, Compound Rhizoma Coptidis Capsule, and Zishen Tongluo Granule) are effective for DKD treatment. The present review described the role of oxidative stress in DKD pathogenesis and the effect of TCM intervention for DKD prevention and treatment, in an attempt to provide evidence for clinical practice.
Keywords: Diabetic kidney disease (DKD), oxidative stress, pathogenesis, traditional Chinese medicine
1. Introduction
According to the 2021 International Diabetes Federation (IDF) Diabetes Atlas (10th edition), approximately 0.537 billion adults (20–79 years old) worldwide were affected by diabetes mellitus (DM), and the number was estimated to increase to 0.643 billion by 2030 while 0.783 billion by 2045 [1]. DM, therefore, has become a major public health issue in the world. Sustained hyperglycemia can lead to severe microvasculopathy which involves the heart, kidney, eyes, nerves, and teeth, etc. Diabetic kidney disease (DKD) is one of the common complications of DM. Clinical research demonstrated that around 20–40% of DM patients developed DKD, and in those who developed end-stage kidney disease, 80% were resulted from an interplay between hyperglycemia and hypertension. In addition, DKD was also reported as closely linked to cardiovascular disease (CVD) [2].
The main pathological changes that lead to DKD include diffuse mesangial expansion and sclerosing, alteration of the glycocalyx of endothelial cells, basement membrane thickening, and podocyte foot process effacement of glomerulus, tubular basement membrane thickening, increased apoptosis, and kidney interstitial fibrosis, which can result in persistent proteinuria and progressive reduction in glomerular filtration rate (GFR) in clinic [3]. The pathogenesis of DKD is highly complicated and remains elusive. Most scholars believe that the DKD onset is involved in multiple factors, such as insulin resistance, glycolipid metabolism disorder, hemodynamics alteration, inflammatory response, cytokine, oxidative stress, and genetic factors. Notably, oxidative stress is core to DKD onset that can be activated by the activation of the polyol pathway, accumulation of advanced glycation end-products (AGEs), and multiple cytokines and then lead to lesions in the kidney small vessels and subsequent exacerbation of kidney injury.
With the advances in purification technique, a large number of experimental studies have suggested that Chinese herbs along with their active ingredients and Chinese patent medicines can play a role in prevention and treatment of DKD with their antioxidant effects. In this review, we summarized the role of oxidative stress in DKD pathogenesis and the efficacy of TCM intervention for DKD treatment, in an attempt to instruct early interventions, help look for new therapeutic strategies and decrease the incidence and mortality of DKD.
2. Oxidative stress in pathogenesis of DKD
2.1. Overview
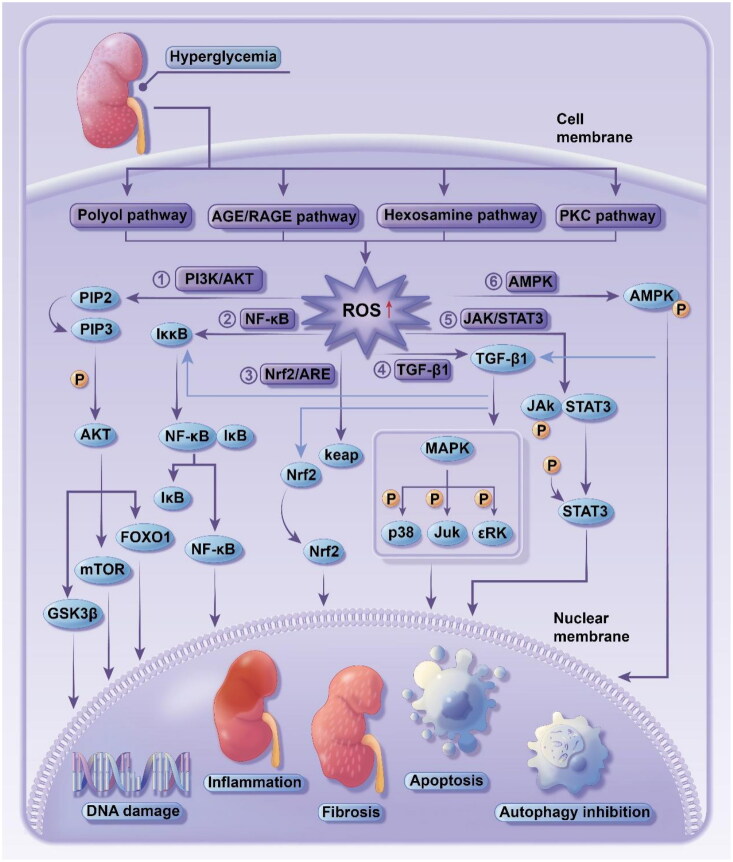
Oxidative stress is defined as a response to oxidant-antioxidant balance disorder [4]. Upon stimulation with adverse factors from the internal and external environment, numerous reactive oxygen and nitrogen radicals are produced in vivo and cannot be completely scavenged with the antioxidant defense system, resulting in a series of physiological and pathological responses in cells and tissues. In addition, the interplay between multiple factors, such as glycolipid metabolism disorder and hemodynamics alteration, activates pathways like polyol and hexosamine pathways to induce substantial ROS production, leading to oxidant-antioxidant balance disorder in the kidney and inducing oxidative stress responses. As a consequence, the downstream cellular signaling pathways are activated, which causes inflammation, autophagy, and fibrosis, etc., accelerating the pathological changes and functional abnormality that led to DKD (Figure 1).
Figure 1.
oxidative stress in pathogenesis of diabetic kidney disease.
2.2. Upstream pathways that activate oxidative stress response
2.2.1. Polyol pathway
Polyol pathway is one of the metabolic pathways [5] from glucose reduced to sorbitol under the actions of aldose reductase (AR) and nicotinamide adenine dinucleotide phosphate (NADPH) and subsequently oxidized to fructose in presence of sorbitol dehydrogenase (SDH) and nicotinamide adenine dinucleotide (NAD). In states of hyperglycemia, AR is activated, leading to increased production of sorbitol. Due to the consistent SDH activity, the produced sorbitols are accumulated in cells, which causes an increase in cell membrane permeability, resulting in exudation of intracellular matters such as inositol and reduced glutathione (GSH) and eventually oxidative stress response [6]. During this metabolic process, the activation of AR is dependent on NADPH, while the metabolism of excessive glucose consumes large amounts of NADPH, leading to reduced GSH production [7] and ROS scavenging capacity, eventually resulting in redox balance disorder in vivo. In the meantime, SDH-induced nicotinamide adenine dinucleotide (NADH) increases and then is oxidized to superoxides and other ROS under respiratory chain functions in mitochondria [8]. The accumulation of end product fructose can also induce oxidative stress and subsequent oxidative damage to tissue. Research revealed that AR inhibitor could reduce oxidative stress in the kidney to protect kidney function, which suggested that polyol induces DKD onset through inducing oxidative stress responses [9].
2.2.2. Ages/RAGE pathway
AGEs are highly active, irreversible end products of non-enzymatic reactions between glucuronyl and free amino groups such as lipid and protein [10]. Sustained hyperglycemia stimulation accelerates AGEs production, while the excessive AGEs directly increase ROS production. Notably, the generated ROS feeds back to stimulate AGEs production, exacerbating oxidative stress and resulting in renal tissue damage.
A large body of research suggested that receptor for AGEs (RAGE) is increasingly expressed by glomerular epithelial cells, mesangial cells, endothelial cells, and podocytes upon hyperglycemia stimulation. The RAGE binding to AGEs activates NADPH oxidase to increase ROS production in endothelial cells, which disturbed molecular conformation and altered enzyme activity, inducing oxidative stress responses. As a consequence, the activated oxidative stress mediates the downstream signaling pathways (e.g., NF-κB, TNF-β, JNK, and p38-MAPK [11]) to increase the release of adhesion molecules, vascular endothelial factors and inflammatory factors [12], leading to multiple mechanisms that contribute to renal injury, such as renal interstitial fibrosis and mesangial expansion.
2.2.3. Hexosamine pathway
In the glycolysis pathway, approximately 2–5% glucose-6-phosphate (G6P) is converted to fructose-6-phosphate (F6P) and then enters the hexosamine pathway [13]. At states of sustained hyperglycemia, excessive F6P is converted to glucosamine-6-phosphate (GlcN6P) under the catalysis of glutamine fructose-6-phosphate aminotransferase (GFAT), followed by generation of uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) with the action of related enzymes. The UDP-GlcNAc is then used as substrate for O-linked N-acetylglucosamine (O-GlcNAc) glycosylation under the catalysis of O-GlcNAc transferase. It was reported that hexosamine can induce endoplasmic reticulum (ER) stress in endothelial cells and macrophages, leading to increased oxidative stress responses. Another study revealed that overexpression of GFAT increases NF-κB promoter activity and TNF-α expression in mesangial cells and stimulates the production of TGF-β1 and PAI-1, inducing inflammatory response, extracellular matrix (ECM) accumulation and diabetic glomerulosclerosis [14].
A recent study showed a close link between O-GlcNAc glycosylation and DKD. The intracellular O-GlcNAc modulates the mitochondrial stress, impairing endothelial nitric oxide synthase (eNOS) activity while increasing uperoxide anion generation to regulate ROS production. In the meantime, O-GlcNAc modifies specific proteins to stimulate the production of large amounts of AGEs, subsequently inducing a series of pathological changes (e.g., apoptosis, mesangial cell proliferation, and fibrosis) that lead to progression of DKD [15].
2.2.4. Pkc pathway
The protein kinase (PKC) pathway is critical in the oxidative stress-induced DKD occurrence and development. Under normal circumstances, the PKC in renal tissue is in an inactivated state. While upon hyperglycemia, it will be activated given the significant increase in intracellular diacylglycerol content [16]. Additionally, PKC can also be activated indirectly via AGE/RAGE and polyol pathways [17]. Activated PKC enhances NADPH oxidase activity and promotes the endothelial and mesangial cells to produce ROS, resulting in damage to renal tissue cells from oxidative stress [18].
Moreover, the activated PKC can also change renal hemodynamics by increasing prostaglandin (PG) and nitric oxide (NO) [19]; elevate the expression of vascular endothelial growth factor (VEGF) and vascular permeability factor (VPF) and decrease glomerular Na+-K+-ATPase activity [20]; increase TGF-β expression to cause renal basement membrane thickening [21]. Besides, the ROS produced following PKC pathway activation can induce apoptosis in cells, exacerbating kidney injury [22].
2.3. Downstream signaling of oxidative stress
2.3.1. Pi3k/Akt pathway
Phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway mainly acts in regulation of cell proliferation, differentiation and apoptosis. Upon an extracellular stimulus, PI3K phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) to generate phosphatidylinositol-3,4,5-triphosphate (PIP3). The generated PIP3 further induces Akt plasma membrane translocation and activates Akt under the catalysis of phosphoinositide-dependent kinase-1 (PDK1) [23]. The activated Akt then plays a role via regulating downstream signaling molecules such as glycogen synthase kinase (GSK) 3β, mammalian target of rapamycin (mTOR) and forkhead box protein O1 (FoxO1) [24].
2.3.1.1. Pi3k/Akt/GSK3β signaling pathway
GSK3β is the downstream target of Akt. Activated Akt phosphorylates GSK3β and blocks its activity to modulate various cellular functions. Previous research found that sustained high glucose levels resulted in suppressed PI3K/Akt signaling, leading to decreased GSK3β activity, reduced Bcl-2 but elevated Bax, which induced apoptotic damage to renal cells [25]. Animal experiment noted that phosphorylated GSK3β augmented NF-κB function to increase inflammation in DKD mice [26]. In addition, another study showed that reduced GSK3β activity increased β-catenin and slowed the degradation of snali, leading to increased trans-differentiation of tubular epithelial cells and promoting renal interstitial fibrosis in diabetic rats [27].
2.3.1.2. Pi3k/Akt/mTOR signaling pathway
mTOR has been the focus of intense research, and it is tightly linked to the Akt-mediated signaling pathways. Activated Akt phosphorylates and activates mTOR and downstream signaling pathways to participate in proliferation, apoptosis and glucose metabolism in cells. Lu Q et al. [28] found that high glucose levels induced tubular epithelial cells NRK-52E to produce ROS, stimulating TGF-β1 generation and Akt activation, while the activated Akt phosphorylated mTOR to play a role in epithelial-mesenchymal transition (EMT) in NRK-52E cells, exacerbating diabetic kidney fibrosis. Another study reported that high glucose levels also activated the mTOR signaling to reduce LC3II/LC3I ratio and Beclin level, thereby decreasing autophagy to make effects on DKD progression [29].
2.3.1.3. Pi3k/Akt/FoxO1 signaling pathway
FoxO1 is a class of highly conserved, ubiquitously expressed transcription factors that has implications for processes such as oxidative stress, inflammation, autophagy and apoptosis upon high glucose stimulation. PI3K/Akt signaling phosphorylates FoxO1 and deactivates it via inducing its nuclear translocation, the process of which is significant for the pathogenesis of diabetic kidney injury [30]. FoxO1 in renal tissue can alleviate the impaired glomerular filtration barrier and apoptosis resulted from the detachment of renal podocytes from the basement membrane because of abnormal glucose metabolism [31]. Early elevation of glucose can stimulate the TGF-β/Smad pathway to prevent the occurrence of kidney fibrosis in DKD [32]. The study of Ma et al. [33] also found that increased phosphorylation of FoxO1 decreased FoxO1 activity and autophagy in DKD animal models, resulting in exacerbation of kidney injury.
2.3.2. Nf-кB signaling pathway
In normal cases, NF-кB is ubiquitously present in various tissue cells in an inactive state. In response to stimulation with activators (e.g., proinflammatory cytokine, Toll-like receptor, p38-MAPK, HO-1, and ROS), NF-κB dimers will be released to nucleus to regulate target gene expression, inducing the body’s immune and inflammatory responses [34]. Multiple studies have proved that DKD development is associated with NF-κB hyper-activation. For example, the produced ROS and inflammatory factors (e.g., TNF-α and IL-6) under high glucose stimulation activated the NF-κB signaling, while the activated NF-κB then promoted the transcription and translation of proinflammatory cytokines, chemokines, adhesion molecules, and TGF-β1, etc., leading to apoptosis and necrosis in cells and tissue fibrosis, which accelerated the development of DKD [35]. Hofmann et al. [36] found that NF-κB expression in DKD patients was significantly increased in comparison to that in non-diabetic patients, and it was positively associated with the degree of proteinuria in this population.
Collectively, suppression of the NF-κB signaling pathway activation may be a direction for research in prevention and treatment of DKD.
2.3.3. Nrf2/ARE signaling pathway
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor important in body’s resistance to oxidative stress. It is involved in regulation of anti-oxidant, anti-inflammatory, anti-apoptosis, and anti-fibrostic processes and is closely associated with the degree of DKD injury. Under normal physiological conditions, Nrf2 exists in cytoplasm in an inactive state as a compound with its inhibitor Kelch-like ECH-associated protein 1 (KEAP1). High glucose-induced oxidative stress dissociates Nrf2 from the compound and activates it to induce nuclear translocation. The activated Nrf2 binds with the antioxidant response elements (AREs) in nucleus to activate the expression of downstream antioxidant factors, playing a protective role in cells and then slowing the development of kidney injury in DKD [37]. An animal experiment demonstrated that Nrf2-/- mice suffered from severer kidney injury than wild mice, which also proved the protective role of Nrf2 in kidney diseases [38]. Combining the findings, Nrf2 could be employed as a key target to prevent DKD and preserve renal function.
Animal experimental data revealed that inhibiting the Nrf2-mediated activities of antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px), exacerbated the kidney injury in mice with spontaneous DKD [39]. Another study noted that inhibition of the Nrf2 signaling while activating the TGF-β1-stimulated fibronectin expression and smad2/3 phosphorylation slowed down the occurrence of renal fibrosis in rats with streptozotocin (STZ) -induced diabetes [40]. Additionally, up-regulation of Nrf2 down-regulated TNF-α, IL-6, Bax and p38 levels, suppressed NF-κB activation and increased Bcl-2 expression, showing anti-inflammatory and anti-apoptotic effects, conducive to preventing the STZ-induced DKD [41].
2.3.4. Tgf-β1 signaling pathway
During the development of DKD, oxidative stress activates the TGF-β1 signaling pathway to induce expression of intracellular signals (protein kinase or cytokine), which promotes ECM accumulation and induces EMT, resulting in renal interstitial fibrosis and glomerulosclerosis [42]. A meta-analysis of randomized controlled trials (RCTs) demonstrated that serum TGF-β1 could be employed as a biomarker for early warning of fibrosis in DKD [43]. In addition, it was proven that TGF-β1 regulators, such as Resveratrol, Salvia Root, Taxol and Calcitriol, could improve the fibrosis in DKD via inhibiting the TGF-β1 signaling pathway [44].
It has been established that the profibrotic effect of TGF-β1 is regulated by multiple signaling pathways, among which the mitogen-activated protein kinase (MAPK) signaling pathway has received increasing attention. The MAPK family contains three important members: p38-MAPK, JNK and ERK, which are intimately associated with the development of DKD. Notably, p38-MAPK shows the strongest relationship with DKD. In response to high glucose, pro-inflammatory factor stimulation and oxidative stress, activated p38-MAPK targets and acts on TGF-β1, promoting the phosphorylation of Smad2/3 and increasing the expression of fibronectin, collagen I and IV (Col-I/IV) [45], thereby playing a role in renal fibrosis. In addition, p38-MAPK with enhanced activity in turn exacerbates the body’s oxidative stress, resulting in increased oxygen radicals [46].
ERK as another member of the MAPK family is also associated with the TGF-β1 signaling. In animal models of kidney fibrosis from many causes, the TGF-β1/ERK pathway was activated, while suppression of the pathway improved the kidney fibrosis [47]. Another study noted that activated ERK signaling induced the transcriptional regulation of hypoxia inducible factor-1α (HIF-1α) to regulate the levels of fibronectin, Col-I and connective tissue growth factors (CTGF) [48], thereby playing a role in multiple pathological processes of DKD such as proliferation, hypertrophy and increased ECM in glomerular mesangial cells.
Therefore, TGF-β1 is a key factor that induces fibrosis in DKD.
2.3.5. Jak2/STAT3 signaling pathway
The Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathway is activated in DKD, while inhibition of this pathway can slow down the progression of this disease [49]. Experiment in DKD rats revealed increased phosphorylation of JAK and STAT3 in renal tissue, leading to up-regulated Bax while down-regulated Bcl-2, which enhanced apoptosis in renal cells [50]. The JAK2/STAT3 pathway can also activate macrophages in the kidney to promote the release of inflammatory factors and the production of ROS, exacerbating kidney injury [51]. It was reported that Berberine alleviated the high glucose-induced EMT and apoptosis in renal podocytes by suppressing the JAK2/STAT3 signaling pathway [52], thereby slowing down the progression of DKD.
Collectively, the JAK2/STAT3 signaling pathway mainly participants in the regulation of immune response, inflammation, oxidative stress and cell apoptosis during the course of DKD.
2.3.6. Ampk signaling pathway
Adenosine monophosphate-activated protein kinase (AMPK) is a sensor of cellular energy, and it has gained intensive attention given its role as a therapeutic target for obesity, diabetes, and metabolic syndrome-related diseases. Clinical research revealed that phosphorylated AMPK (p-AMPK) protein expression significantly decreased in renal tissue of DKD patients, and AMPK activator improved the clinical symptoms and pathological changes and reduced 24-h urinary proteins (UP) in these patients [53]. Additionally, p-AMPK in human glomerular cells upon high glucose reduced the expression of nicotinamide adenine dinucleotide phosphate oxidase (NOX) 4 and TGF-β1 and subsequent ECM accumulation [54]. AMPK also serves as a sensor of intracellular ROS. Activated AMPK up-regulates the expression of SODs to reduce superoxide generation, and it phosphorylates and activates FOXO to exert antioxidant effects. It was proven that the levels of AMPK, p-AMPK and SIRT1 were remarkably decreased in both mouse models of STZ-induced DKD and podocytes exposed to high glucose, activating oxidative stress and then leading to pyroptosis. In addition, inhibiting this process suppressed the injury in DKD [55]. Upon high glucose, AMPK phosphorylation is inhibited, leading to increased PPAR-α expression, lipid accumulation, apoptosis, and expression of pro-inflammatory and profibrotic gene expression [56]. In the in vivo/vitro experiments performed by Han YC et al. [57], AMPK activator promoted mitophagy via activating the p-AMPK-pink1-pinkin pathway, thereby improving the oxidative stress and interstitial fibrosis in the kidney.
3. Advances in Chinese medicine in treatment of DKD
Current treatment for DKD mainly aims at controlling body weight, lowering blood glucose and pressure (antihypertensive drugs such as ACEI/ARB), and inhibiting the renin-angiotensin-aldosterone system (RAAS) system. However, such treatment fails to stop proteinuria progression and the persistent changes of organ functions [58]. Clinical research and in vivo/vitro experiments have revealed that Chinese herbal medicines (e.g., Milkvetch Root, Kudzuvine Root), their bioactive ingredients (e.g., ristocetin, berberine), and Chinese patent medicines (e.g., Yishen Tongluo Granule) can slow down DKD progression via multiple signaling pathways and mechanisms (e.g., anti-oxidant, anti-inflammatory, anti-fibrotic, and podocyte-protective), showing certain advantages with respect to clinical efficacy for DKD treatment [59]. Chinese medicines have great potential for therapeutic applications for DKD treatment. The following part reviewed the recent advances in the representative Chinese herbal medicines, active ingredients and patent medicines with therapeutic efficacy for DKD.
3.1. Chinese herbal medicines and active ingredients
It has been proven that Chinese herbal medicines and their bioactive ingredients, such as polyphenols (e.g., breviscapine, puerarin, resveratrol, safflower yellow, silymarin, curcumin, icariin, and tanshinone), polysaccharides (e.g., lycium barbarum polysaccharides, astragalus polysaccharides, ramulus mori polysaccharides, and Chinese Yam polysaccharides), alkaloids (e.g., berberine, tripterygium glycosides, ligustrazine, and emodin), and saponins (e.g., astragaloside and ginsenoside), can improve glucose metabolism and reduce renal pathological changes via multiple anti-inflammatory and anti-fibrotic pathways involved in oxidative stress, playing a nephroprotective role. See Table 1 for more details.
Table 1.
Clinical efficacy and mechanism of action of Chinese herbs and active ingredients for DKD treatment.
| Categories | Component | Result | Pathway |
|---|---|---|---|
| Tripterygium | Tripterygium glycosides | Reduce 24-h UP, UAER and urinary β2-microglobulin | Up-regulate autophagy through mTOR/Twist1, inhibit TLR4/NF-κB to decrease EMT and apoptosis/PI3K/Akt, immune-inflammatory [60–63] |
| Triptolide | |||
| Polyphenols | Puerarin | Reduce UAER and SCr | RAGE pathway, regulate SIRT1 to induce autophagy and reduce PKC activity [65–68] |
| Breviscapinun | Reduce 24-h UP, SCr, BUN, and elevate TC and TG to decrease dyslipidemia | Regulate the Nrf2 /HO-1, TGF-β1, and PKC pathways [69–71] | |
| Resveratrol | Improve UACR, and increase production of antioxidant enzymes | Inhibit caspase 3, TGF-β/Smad and ERK1/2 to reduce early glomerulosclerosis [73–76] | |
| Icariin | Blood lipid-lowering, anti-aging, and anti-inflammation | TLR4/NF-κB, Keap1/Nrf2, Col-IV/TGF-β1, miR-192-5p/GLR-1R [77–80] | |
| Silymarin | Reduce UACR, urinary TNF-α, and MDA | Protect against kidney fibrosis via the JAK/STAT3/SOCS1 and TGF-β1/Smad pathways [82–84] | |
| Curcumin | Anti-oxidization and anti-inflammation | Regulate Nrf2 to inhibit NF-κB, TGF-β1, CTGF, fibronectin and Col IV [85–88] | |
| Salvianolic acid | Reduce blood glucose | Suppress TNF-α and IL-6 [89] | |
| Safflower amine | Inhibit platelet/neutrophil adhesion, decrease fibrosis [90] | ||
| Polysaccharides | Lycium barbarum polysaccharides | Enhance activity of antioxidant enzymes and scavenge of oxygen radicals | Decrease ERK 1/2 activation via the PKC pathway [91–93] |
| Ramulus mori polysaccharides | Dispel wind and activate collaterals, ease joint movement, dry dampness and alleviate water retention, regulate glycolipid metabolism, and improve the body’s immune function | Up-regulate the expression of SIRT1 and FoxO1 proteins and decrease NF-KB to resist oxidative stress [94] | |
| Chinese yam polysaccharides | Elevate insulin level, decrease glucagon level and FBG | Enhance multiple anti-oxidative stress pathways including the PI3K [95] | |
| Alkaloids | Berberine | Reduce UER, FBG, HbA1c and GPx | Regulate the expression of GRKs in the G protein-AC-cAMP signaling pathway [99–104] |
| Ligustrazine | Reduce 24-h UP, blood glucose, and BUN | Anti-oxidative stress, anti-inflammation, and anti-fibrosis [106–107] | |
| Saponins | Astragalosides | Alleviate albuminuria and glomerulosclerosis, and simultaneously inhibit podocyte apoptosis and recover impaired autophagy | PI3K/Akt/FoxO1, AMPK and SIRT/NF-κB signaling pathways [108–116] |
| Paeoniflorin | Reduce UAER | Alleviate oxidization and inflammation through the TLR2/4 and JAK2/STAT3 pathways, up-regulate p-mTOR level to promote autophagy [117–119] | |
| Ginsenoside | Reduce blood glucose and lipid levels, improve oxidative stress | Akt/GSK-3β, PI3K/Akt, TGF-β1/Smad [121–126] | |
| Notoginsenoside | Reduce FBG, Ccr, UAlb and kidney index | Enhance BMP-7 and Smad7 via inhibiting VEGF and TGF- β1 [127] | |
| Other | Emodin | Reduce 24-h UP | Regulate the AMPK/mTOR-mediated autophagy and activate the PI3K/Akt/GSK-3β pathway to inhibit inflammation [128–131] |
3.1.1. Tripterygium
Tripterygium and its extracts (e.g., triptolide and tripterygium glycosides) have been recognized as important therapeutic agents for clinical treatment of DKD with proved efficacy. A meta-analysis in 2,764 DKD patients from 31 articles revealed that Tripterygium Glycosides Tablet administration for 3 months significantly reduced the levels of 24-h uridine triphosphate (UTP) and serum creatinine (SCr) in DKD patients, and there was a low incidence of adverse events [60]. Another study found that tripterygium glycosides up-regulated the autophagy level and reduced the EMT and apoptosis in DKD rats through the mTOR/Twist1 signaling pathway [61]. Moreover, it was also noted that tripterygium glycosides inhibited tubular fibrosis via inhibiting TLR4/NF-κB to improve EMT or prevented DKD development through suppressing the immune-inflammation system via the PI3K/Akt pathway [62].
Triptolide is another important active ingredient of Tripterygium. Research revealed that triptolide could activate SOD to inhibit renal inflammation, macrophage infiltration and GSK3β phosphorylation, thereby alleviating proteinuria and podocyte injury in DKD rats [63].
3.1.2. Polyphenols
Polyphenols, mainly including flavonoids, tannins and phenolic acids, are natural compounds with multiple phenol groups. They have a wide range of clinical applications due to their strong antioxidant capability and proved efficacy in reducing blood glucose and urinary albumin and increasing GFR.
Puerarin, an extract of Kudzuvine Root, is mainly composed of flavonoid C-glycoside [64]. Clinical research demonstrated that puerarin injection led to remarkable reductions in urinary albumin excretion rate (UAER) and SCr, showing good therapeutic efficacy [65]. While in STZ-induced DKD animal model and high glucose-treated podocytes, puerarin protected the kidney via activating SIRT1 to induce autophagy and through its antioxidant effect [66]. The study of Yuan et al. [67] reported that puerarin inhibited AGEs formation to block AGEs binding to RAGE, thereby improving the pathological changes that lead to DKD. Furthermore, it was also noted that puerarin could decrease PKC activity and down-regulate expression of c-Fos, c-Jun and Col-IV to reduce ECM accumulation [68].
Breviscapinun is the purified flavonoid extracted from Erigeron Breviscapus and mainly comprising breviscapinun-7-glucuronide (90%) and apigenin 7-O-glucoside (4%). In a meta-analysis in 2,320 DKD patients, breviscapinun injection combined with routine treatment contributed to significant reductions in patient 24-h UTP, SCr, blood urea nitrogen (BUN), UAER and albumin (ALB), suggesting advantages of breviscapinun injection in efficacy for DKD [69]. Xu et al. [70] found that breviscapinun was capable of inhibiting the oxidative stress, PKC activity increase and TGF-β1 overexpression in DKD rats, conducive to decreasing the pathological kidney injuries in DKD. While in the study of Jiang et al. [71], breviscapinun was found to exert anti-fibrotic effects in the kidney through reducing the phosphorylation levels of PKCβII, Akt, JNK1/2 and p38.
Resveratrol is a representative polyphenol that exists in Chinese herbal medicines such as Giant Knotweed Rhizome and Cassia Tora as a strong antioxidant. Clinical trial revealed that the combination of resveratrol (500 mg/d) with Losartan (12.5 mg/d) reduced the urinary albumin-creatinine ratio (UACR) (–46.4 mg/g, 95% CI: −64.5 to −28.3 vs 29.9 mg/g, 95% CI: 4.9 to 54.9; p < 0.001) and increased the activity of antioxidant enzymes [72]. A meta-analysis involving 36 animal studies demon strated that resveratrol exerted its antioxidant effects by means of reducing malondialdehyde (MDA) level and restoring activities of SOD, CAT, GSH and GPx [73]. Mechanistic studies showed that resveratrol played its therapeutic role in DKD treatment under multiple mechanisms: (1) ameliorate oxidative stress and reduce apoptosis via up-regulating AMPK expression and activity [74]; (2) regulate autophagy through overexpressing miR-18a-5p to suppress Caspase 3 [75]; (3) alleviate early-stage glomerulosclerosis via inhibiting TGF-β/Smad and ERK1/2 [76].
Icariin is a type of flavonoid derived from the dry cauline leaf of Epimedium Herb, with blood lipid-lowering, anti-aging, and anti-inflammatory pharmacological effects. It is also reported to have a protective role against DKD. Qi MY et al. [77] found that icariin suppressed the TLR4/NF-κB signaling pathway, reduced inflammatory response, and increased the activity of SOD, CAT and GSH-Px. The in vivo/vitro experiments performed by Wang et al. [78] showed that icariin decreased the level of superoxide anion via GPER-mediated Keap1 degradation and Nrf2 activation, enhancing the activity of antioxidant enzymes while reducing apoptosis and fibronectin formation, which subsequently alleviated the kidney injury in DKD. In addition, multiple studies have proved that icariin can delay the progression of DKD with its anti-fibrotic effects. For instance, icariin could down-regulate Col-IV and TGF-β1 expression [79] or enhance autophagy through the miR-192-5p/GLR-1R pathway to reduce tubulointerstitial fibrosis [80].
Silymarin is a natural bioactive substance extracted from the dry fruit of Silybum Marianum. In a RCT with 60 DKD patients, patients administrated with 12-week renin-angiotensin system (RAS) inhibitors plus capsules of silymarin (140 mg/d, tid) had significantly reduced levels of UACR, urinary TNF-α and MDA in comparison to patients administrated with RAS inhibitors alone [81]. Silymarin also has antioxidant effects. Khazim et al. [82] found that silymarin reduced NADPH enzyme, Nox4 activity, and superoxide production and apoptosis in cells, protecting podocyte from damages caused by high glucose. In addition, the study of Chen et al. [83] showed that silymarin was capable of reducing the IL-6 and ICAM-1-induced inflammatory response via inhibiting the JAK/STAT3/SOCS1 pathway, and it also played an anti-fibrotic role through suppressing the TGF-β1/Smad signaling pathway, eventually improving the STZ-induced kidney injury in diabetic rats. Liu et al. [84] also noted that silymarin activated the Akt signaling pathway and reduce the levels of p-GSK-3β and Bax to prevent kidney injury and alleviate oxidative stress in db/db mice. Silymarin, therefore, is promising to be applied as a new agent for DKD treatment.
Curcumin is the active ingredient extracted from the rhizomes of Curcuma longa. Growing studies have proved the protective effect of curcumin against DKD, which is achieved by its anti-oxidant and anti-inflammatory effects. For example, ALTamimi JZ et al. [85] established an animal model of DKD and found that curcumin reduced kidney injury via activating Nrf2 while decreasing NF-κB to inhibit NADPH oxidase and down-regulate the PKCβII/p66Shc axis. Soetikno et al. [86] also identified that curcumin modulated PKC activator-mediated phosphorylate ERK1/2 to reduce oxidative stress, and in the meantime, decreased the expression levels of TGF-β1, CTGF, fibronectin and Col-IV. The study of Tu et al. [87] reported that curcumin administration led to reduced levels of blood glucose, SCr, BUN, and UP, up-regulated expression of E-cadherin and LC3, and suppressed PI3K/Akt/mTOR pathway in rats with STZ-induced DKD, inducing autophagy while decreasing EMT. Moreover, Tikoo et al. [88] noted that the protective effect of curcumin against DKD might be attributed to its actions on post-translational modification of histone H3 and p38-MAPK expression.
There are some other polyphenols with therapeutic efficacy against DKD. Salvianolic acids, for example, could inhibit TNF-α and IL-6 to improve the STZ-induced glucose elevation in rats, protecting DKD rats from oxidative stress injury [89]. Safflower yellow is the safflower amine component extracted from Safflower carthamus with characteristics of inhibiting platelet/neutrophil adhesion, vascular endothelial injury and smooth muscle hyperplasia, and improving development of fibrosis in DKD [90].
3.1.3. Polysaccharides
Lycium barbarum polysaccharides (LBP) are main extracts of the fruit of wolfberry, and they have been intensively applied in China for their immunomodulatory, liver protective, anti-oxidant, and anti-tumor effects. Both domestic and abroad studies have proved that LBP have protective effects in DKD. For instance, Du et al. [91] identified that LBP decreased TLR4, Myd88 and NF-κB expression to reduce the release of inflammatory factors such as IL-2, IL-6 and TNF-α and enhance the activity of serum SOD and GSH-Px, thereby exerting anti-inflammatory and anti-oxidant effects. Other studies reported that LBP could reduce mesangial cell proliferation through the PKC and ERK1/2 pathways [92]. In addition, it could also inhibit the binding between endothelin and its receptor, resulting in decrease in UAER level and subsequent improvement of kidney function [93].
Ramulus mori polysaccharides (RMP) are one of the main active ingredients of mulberry branch that have functions of drying dampness and alleviating water retention, lowering blood glucose and lipid, and improving immunity. Research revealed that RMP could improve the oxidative stress-induced alteration of renal function via decreasing the content of MDA and ROS, up-regulating the expression of SIRT1 and FoxO1 proteins, and reducing the levels of inflammatory factor NF-κB [94].
Chinese yam polysaccharides (CYPS) were reported to alleviate the kidney injury in DKD through enhancing the PI3K signaling pathway via actions on the expression of IRS-1 and PI3K [95]. Other polysaccharides, such as the polysaccharides from cyclocarya paliurus, alga and okra also had a protective role in DKD rats via exerting their anti-oxidant effect [96].
3.1.4. Alkaloids
Berberine, the main bioactive extract of Golden Thread and Amur Cork-Tree, is a benzylisoquinoline alkaloid structurally with heteropentacyclic groups [97]. It has broad clinical applications owing to its effects to regulate glycolipid metabolism and protect the kidney via anti-inflammatory and anti-oxidant mechanisms [98]. A previous RCT revealed that berberine led to reductions in 24-h UP, UAER, fasting blood glucose (FBG), glycated hemoglobin (Hb A1c), total cholesterol (TC), C-reactive protein (CRP), and GPx [99]. In addition, an animal experiment found that berberine increased the glucose uptake, lipid oxidization, and insulin sensitivity in diabetic fatty rats [100]. Mechanistic studies showed that berberine regulated the G protein-coupled receptor kinase (GRK) of the G protein-AC-cAMP signaling pathway to increase the cAMP level in STZ-induced diabetic rats [101]; and it reduced interstitial fibrosis in DKD by inhibiting the EMT in tubular epithelial cells via activating Nrf2 while decreasing TGF-β/Smad expression [102]. Moreover, in vitro studies reported that berberine activated PGC-1α to restore mitochondrial function, blocking the excessive production of ROS [103]; and it regulated autophagy via activating the AMPK signaling or inhibited TLR4/NF-κB to improve the high glucose-induced podocyte apoptosis [104].
Ligustrazine is an alkaloid monomer derived from the rhizome of Sichuan Lovage Rhizome. A huge number of clinical and animal studies have demonstrated the preventive effect of ligustrazine on DKD. For instance, a meta-analysis of 25 RCTs involving 1,645 DKD patients (ligustrazine group, n = 858; control group, n = 787) revealed that ligustrazine injection reduced BUN, SCr, 24-h UP, and UAER in these patients, exhibiting certain clinical efficacy [105]. Similarly, in another meta-analysis, the combination of Salvia Root and ligustrazine also decreased the body’s inflammatory response, and it was safe and effective in early DKD treatment as an adjuvant therapy [106]. Furthermore, there was a meta-analysis in animal experiments, which showed that ligustrazine (150 mg/kg) improved the glomerular and tubular pathological changes and the indices of renal function. It was believed that such effects were attributed to the anti-oxidant, anti-apoptotic and anti-inflammatory activities of ligustrazine as well as its effects to reduce renal fibrosis, inhibit mesangial cell proliferation and promote autophagy [107].
3.1.5. Glycosides
Astragaloside is the main bioactive component of Milkvetch Root (astragalus) that has a nephroprotective function. Milkvetch Root is clinically applied for treatment of CVD, kidney and liver diseases, etc., especially common in DKD treatment [108]. Clinical research indicated that astragalus injection reduced the BUN, SCr, UP, and increased the creatinine clearance (Ccr) and se rum albumin in DKD patients [109]. In addition, both astragalus injections with and without ACEI/ARB had significant nephroprotective effect in DKD patients, presenting as remarkably decreasing albuminuria and slowing down progression of glomerulosclerosis. In the meantime, there were no severe adverse events during the treatment as supported by a large number of samples, showing a safety profile [110]. Furthermore, in a meta-analysis that included 424 animals from 24 studies, astragalosides were proven to exert effects in reducing ER stress, inhibiting mitochondrial division and increasing autophagy activity via anti-fibrotic, anti-oxidant, and anti-apoptotic mechanisms [111]. More specifically, astragalosides inhibited the apoptosis in tubular epithelial cells and reduced TGF-β1 expression [112]; regulated Nrf2 and PINK1 to improve the podocyte injury and mitochondrial function in diabetic rats [113]; suppressed the PI3K/Akt/FoxO1 signaling or activated the AMPK to mediate the autophagy activity of renal tissue cells, alleviate albuminuria and glomerulosclerosis, and inhibit podocyte apoptosis [114]; modulated the SIRT1/NF-κB signaling and decreased the excessive ECM production to inhibit high glucose-induced mesangial fibrosis [115]; and inhibited the NLRP3-mediated inflammatory response in turn to improve renal function and podocyte injury [116].
Paeoniflorin is the purified bioactive component of Chinese herbaceous peony. It has been applied for treatment of chronic kidney diseases due to it biological effects such as anti-oxidization and anti-inflammation, and it has also shown a protective role in DKD. Animal experiments demonstrated that paeoniflorin inhibited the TLR2/4 signaling pathway [117], or suppressed the JAK2/STAT3 pathway to block macrophage activation and subsequently reduce kidney injury [118]. In vitro experiments proved that paeoniflorin decreased AGEs generation, increased activity of GPx and catalase, reduced the levels of IL-6 and MCP-1, thereby alleviating oxidative damage and inflammation in cells [119]. In addition, it could also up-regulate p-mTOR level to promote autophagy. Combining the studies, paeoniflorin has the potential as an agent for prevention and treatment of DKD [120].
Ginsenoside, belonging to Araliaceae ginseng species, is mainly composed of Rh2, Rg1, Rg2 and Rb1. It has a high medicinal value because of its anti-oxidant, anti-aging, and anti-tumor effects. There are some studies reporting the role of ginsenoside in prevention and treatment of DKD. For instance, ginsenoside Re played its therapeutic effect against DKD by reducing the levels of blood glucose and lipid and ameliorating oxidative stress [121]. In addition, ginsenoside Rb1 reversed the increase in expression of mitochondrial protein NADPH oxidase 4 (NOX4) to reduce the apoptosis and glomerular injury induced by CytoC and Caspase 9 [122]. Ginsenoside Rg1 was reported to have effects in reducing EMT in podocytes by enhancing the Akt/GSK3β pathway and restoring autophagy [123]. Additionally, it could also reduce inflammation, oxidative stress and apoptosis through promoting FoxO3 nuclear translocation via the PI3K/Akt pathway [124]. Combination of ginsenoside with astragaloside was conducive to improving renal function because of their effects in ameliorating oxidative stress and inhibiting the TGF-β1/Smad signaling [125]. Ginsenoside Rg5 was reported to decrease ROS generation to resist oxidative stress and inhibit the activation of NLRP3 inflammasome to ameliorate the renal tissue inflammation in diabetic mice [126].
Notoginsenoside could inhibit VEGF and TGF-β1 to increase BMP-7 and Smad7, lowering glucose and reducing FBG, CCr, urinary albumin (UAlb) and kidney index [127].
3.1.6. Other
Emodin is a bioactive anthraquinone from Rhubarb. It was reported that emodin reduced the NF-κB-mediated expression of TGF-β1 and FN, thereby decreasing the high glucose-induced EMT [128]. Moreover, the in vivo/vitro experiments performed by Tian NX et al. [129] revealed that emodin suppressed the PERK-elf2α signaling to reduce ER stress, in turn to decrease podocyte apoptosis and improve renal function. Multiple studies also proved that emodin could promote the AMPK/mTOR-mediated autophagy [130] and active the PI3K/Akt/GSK-3β pathway to inhibit inflammation, apoptosis, and prevent DKD [131].
3.2. Chinese patent medicine
Chinese patent medicines are pharmacological agents prepared by single or multiple Chinese herbal medicines on the basis of TCM theory, and have a long history. Because of the long course and complicated mechanism of DKD, multiple Chinese patent medicines, such as Tangshen Formula and Wuling Powder, have achieved good efficacy in clinical practice owing to their multi-component and multi-target properties. See Table 2 for more details.
Table 2.
Clinical efficacy and mechanism of action of Chinese patent medicine for DKD treatment.
| Chinese patent medicine | Result | Pathway |
|---|---|---|
| Tangshen Formula | Reduce blood glucose and urinary albumin to resist inflammation and fibrosis | JAK/STAT/SOCS1, TNF-α and TGF-β/MMP-1 pathways, Col-IV level [133] |
| Huangkui Capsule | Prevent tubulointerstitial fibrosis, reduce SCr and BUN, improve inflammation | NADPH, ERK and TGF-β1/p38-MAPK pathways [135–137] |
| Yishen Tongluo Granule | Improve Ccr, FBG, TG, TC and HbA1c, etc. | Regulate expression of SOD, MDA, ET-1 and VEGF [138] |
| Six-Ingredient Rehmannia Pill | Reduce the UAER and β2-microglobulin in blood and urine | Inhibit ROS production [139–141] |
| Wuling Powder | Reduce blood glucose and improve glycation-mediated kidney injury | Suppress NF-κB and TGF- β /Smads pathways [142–143] |
| Jinshuibao Capsule | Reduce SBP, DBP, and 24-h UP, UACR, SCr, BUN and TG | Regulate glycolipid metabolism, anti-oxidization, and anti-inflammation [144–146] |
| Tongluo Capsule | Improve SCr, BUN, TC, TG, LDL-C, etc. | Decrease serum TGF-β level [147] |
| Shenshuaining Granule | Reduce 24-h UP, SCr and BUN | Inhibit SOD, AOPP, AGEs and IL-8 [148] |
| Compound Rhizoma Coptidis Capsule | Reduce FBG, BUN, SCr, and 24-h UP | Inhibit TGF-β1 and Col-IV [149] |
| Qizhi Jiangtang Capsule | Reduce urinary protein | Anti-oxidative stress [150] |
| Qi-flavor Granule | Anti-inflammation, and anti-oxidative stress | Decrease TGF-β1 expression [151] |
| Chaihuang Yishen Granule | Reduce 24-h UP | Inhibit the TGF-β/Smad3-mediated kidney fibrosis [152] |
| Qidan Dihuang Granule | Improve 24-h urinary proteinuria, total proteinuria, and UACR | Reduce fibrosis in glomerular endothelial and mesangial cells [153] |
Tangshen Formula is prepared by Milkvetch Root, Unprocessed Rehmannia Root, Asiatic Cornelian Cherry Fruit, Sanqi, Winged Euonymus, Cooked Rhubarb and Orange Fruit and has functions of tonifying qi and yin and activating blood. In a clinical trial involving 144 DKD patients, 50 weeks of treatment with the combination of Tangshen Formula and Irbesartan (2-week lead-in period, 24-week intervention period, and 24-week follow-up period) led to a significant reduction in 24-h UP [132]. Scientific research is also conducive for effective clinical treatment. For instance, animal experiment in db/db mice revealed that the Tangshen Formula reduced blood glucose and UAlb via the JAK/STAT/SOCS1 signaling [133]. Moreover, the Tangshen Formula could also protect the kidney via its anti-inflammatory and anti-fibrotic effects by down-regulating TNF-α, inhibiting TGF-β, enhancing MMP-1 and decreasing Col-IV level.
Huangkui Capsule is prepared by the extracts of the flower of Abelmoschus manihot(L.), and it is the agent with various functions such as anti-oxidization, anti-inflammation and immunomodulation. It has been recommended for clinical treatment of DKD and chronic nephritis, etc. Evidence-based data proved that Huangkui Capsule had significant therapeutic efficacy for proteinuria and could effectively delay the deterioration of renal function [134]. Pharmacological studies demonstrated that Huangkui Capsule administration could inhibit NADPH oxidase activity, decrease ROS production, and block the ERK signaling [135] or suppress the TGF-β1/p38MAPK pathway [136] to prevent tubulointerstitial fibrosis. In addition, it could reduce SCr, BUN and activate PPAR to ameliorate ER stress, improving lipid metabolic disorders and alleviating inflammation [137]. Given the good therapeutic efficacy, Huangkui Capsule deserves clinical promotion.
Yishen Tongluo Granule, prepared by Salvia Root, Milkvetch Root, Asiatic Cornelian Cherry Fruit, Rhubarb, Prepared Rehmannia Root, Dragon’s Blood and Euonymus alatus, is one of the classical Chinese compound formulae used for DKD treatment. It mainly acts to tonify the kidney and dredge collaterals. Clinical research reported that Yishen Tongluo Granule was superior to Benazepril in improving renal function and decreasing the levels of endogenous CCr, FBG, HbA1c, TC and triglyceride (TG). It was believed that Yishen Tongluo Granule exhibited these effects via inhibiting SOD and MDA and regulating ET-1 and VEGF expression [138].
Six-Ingredient Rehmannia Pill, prepared by Prepared Rehmannia Root, Common Yam Rhizome, Asiatic Cornelian Cherry Fruit, Tree Peony Root Bark, Poria and Oriental Waterplantain Rhizome, has good clinical therapeutic efficacy for DKD. Hsu PC et al. [139] established a regression model for predicting the risk of developing renal failure in DKD patients with and without Six-Ingredient Rehmannia Pill, and they found that administration of Six-Ingredient Rehmannia Pill during diabetic care slowed down the occurrence of DKD. Another Cox proportional hazard regression model based on 70,036 diabetic patients revealed that Six-Ingredient Rehmannia Pill administration significantly delayed the use of hypoglycemic agents and insulin [140]. Additionally, experiments demonstrated that Six-Ingredient Rehmannia Pill exhibited significant nephroprotective effects via reducing glomerular mesangial matrix expansion and basement membrane thickening. In the meantime, it also suppressed the activity of AR in hemocytes and reduced the levels of UAER and β2-microglobulin in the blood and urine without affecting the blood glucose and lipid and mean arterial pressure (MAP), which might be associated with the inhibition of ROS production [141].
Wuling Powder is also known as Poria Five-herb Formula due to its five components including Poria, Oriental Waterplantain Rhizome, Chuling, White Atractylodes Rhizome and Cassia Twig. It is a representative formula for treatment of greater yang water retention syndrome and can ameliorate the edema in late-stage DKD. Clinical trial suggested that Wuling Powder used as an adjuvant of Rosiglitazone alleviated edema and improved lipid metabolism while increasing urinary volume and decreasing proteinuria, reaching a response rate of up to 94.1% (76.5% in control group) [142]. In addition, animal experiment also showed that Wuling Powder decreased the NF-κB and TGF-β1 ex pression in STZ-induced diabetic rats, which lowered blood glucose and improved the glycation-mediated kidney injury, showing a nephroprotective role [143].
Jinshuibao Capsule, mainly prepared by the artificially fermented cordyceps sinensis Cs-4 strain, has extensive applications in clinical prevention and treatment of diabetes complicated by kidney injury and other chronic kidney diseases. It has nephroprotective and hepatoprotective roles as well as functions of immunomodulation and anti-inflammation, which have been proven in clinical practice [144]. Fermented cordyceps sinens is contains multiple amino acids, vitamins and trace elements, which allow for improvement of the amino acid metabolism inside and outside cells and the extracellular mitochondrial respiratory function, conducive to accelerating cell damage repair [145]. A meta-analysis investigated 4,562 patients from 48 studies and found that the combination of Jinshuibao Capsule with ACEI/ARB had superior efficacy and safety to ACEI/ARB alone in reducing 24-h UP, UACR, SCr, BUN and TG, HbA1c, TNF-α, and CRP [146].
Beside the common Chinese patent medicines mentioned above, Tongluo Capsule was reported to be more effective in improving the levels of SCr, BUN, TC, TG, low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein (HDL) [147]. Shenshuaining Tablet could significantly decrease the 24-h urinary microalbumin, SCr, and BUN [148]. Compound Rhizoma Coptidis Capsule, prepared by Golden Thread, Kudzuvine Root, Sarrette and Loquat Leaf, could inhibit the expression of TGF-β1 and Col-IV to protect kidney function and slow down DKD progression [149]. Qizhi Jiangtang Capsule, composed of Milkvetch Root, Leech, Rehmannia Root and Crystalline Lens, could effectively reduce UP to delay the progression of renal disorder [150]. Qi-flavor Granule, comprising Milkvetch Root, Prepared Rehmannia Root, Winged Euonymus and Rhubarb, could ameliorate the kidney lesions and decrease TGF-β1 expression in type 2 DM KK-Ay mice [151]. Chaihuang Yishen Electuary is composed of Milkvetch Root, Chinese Yam, Chinese Thorowax Root, Chinese Angelica, Baical Skullcap Root, Chuling and Leech and could be employed as an agent for DKD treatment because of its effect to block the TGF-β/Smad3-mediated kidney fibrosis [152]. Qidan Dihuang Granule could profoundly improve the levels of 24-h urinary proteinuria, total proteinuria and 4-d UACR [153]. Danggui Shaoyao Powder, a combination of Debark Peony Root, Chinese Angelica, Sichuan Lovage Rhizome, Poria, White Atractylodes Rhizome and Oriental Waterplantain Rhizome, could protect kidney function in STZ-induced diabetic rats via regulating blood glucose and decreasing the expression of AGEs in diabetic glomerulus [154]. Xueshuangtong Compound Granule, composed of Sanqi, Salvia Root, Figwort Root and Milkvetch Root, could prevent glomerular hypertrophy and mesangial matrix expansion, and decrease the activity of SOD and MDA [155].
4. Summary and prospect
DKD is one of the important microvascular complications of diabetes, and its onset is closely associated with multiple mechanisms such as inflammation, blood lipid metabolic disorder, and hemodynamics abnormality. Notably, oxidative stress is the core of various mechanisms. In the present review, we summarized the upstream pathways that induce oxidative stress and the downstream pathways that are activated by oxidative stress, in an attempt to explore the role of oxidative stress in pathogenesis of DKD.
Upon sustained high glucose, multiple pathways such as AGEs, PKC and hexosamine pathways are activated, stimulating ROS production and triggering oxidative stress. As a result, the body’s oxidant-antioxidant balance is impaired, resulting in cascade reactions involved in multiple downstream pathways (e.g., P13K/AKT, TGF-β1/p38-MAPK, and NF-κB) that lead to inflammation, fibrosis and apoptosis, eventually exacerbating the progression of DKD. The body’s oxidant and antioxidant systems are complicated and interacted. ROS production induces oxidative stress, while the oxidative stress response in turn stimulates ROS accumulation via multiple pathways. These processes interact to form a vicious cycle, which can cause damage to podocytes and kidney tissue. In this context, enhancing the ability of the antioxidant defense system (such as increasing the activity of antioxidant enzymes) and blocking oxidative stress-related pathways might be a new direction for research and development of therapeutic agents for DKD.
Chinese medicine is a treasure of the Chinese nation. Either herbal medicines or their active ingredients or patent medicines take an active role in DKD prevention because of their multi-component and multi-target properties. The present review described the current advances in TCM intervention for DKD treatment, so as to provide evidence for further research and development of Chinese medicines applicable for clinical DKD treatment. It has been recognized that the protective role of Chinese medicines in DKD is largely attributed to their anti-oxidant, anti-inflammatory, anti-fibrotic, and podocyte-protective effects. Nonetheless, there are still some issues that need to be addressed. For example, the specific mechanism by which Chinese medicine prevents DKD via interfering with oxidative stress is still an open issue and lacks comprehensive studies. Presently, the research into some active ingredients of Chinese medicines or patent medicines is limited to cellular and animal experiments, without support from clinical trials. Besides, in spite of the certain clinical efficacy, further validation toward safety is in demand due to the small sample size. Therefore, it is necessary to carry out multi-center, multi-level, large-scale clinical trials to ensure the effectiveness, tolerance, and safety of Chinese medicines. Moreover, relevant signaling pathways that are involved in their mechanisms of action are not explored yet, requiring further comprehensive studies to help improve the clinical service. Research directions in the future may include, but are not limited to DNA methylation, histone modification, RNA editing, miRNA, siRNA, lncRNA, and their crosstalks with traditional pathways.
To conclude, oxidative stress is critical for DKD pathogenesis, while Chinese medicines can inhibit the occurrence of oxidative stress to enhance the body’s anti-oxidant ability thereby to delay disease progression.
Funding Statement
This work was supported by the Science and Technology department of Sichuan Province [No. 2018SZ007].
Author contributions
TShen designed the research. XJM wrote this manuscript. RJM, TL, ZZY and YQL were contributors in collecting the datas. TTH reviewed the manuscript.TL, ZZY, QYL, TTH and JRM participated in data analysis and drew the figures. XJM and RJM were co-first authors of the article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Federation ID . IDF Diabetes Atlas. 2021. [Google Scholar]
- 2.Filippatos G, Anker SD, Agarwal R, et al. Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation. 2021;143(6):540–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shim K, Begum R, Yang C, et al. Complement activation in obesity, insulin resistance, and type 2 diabetes mellitus. World J Diabetes. 2020;11(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kao MPC, Ang DSC, Pall A, et al. Oxidative stress in renal dysfunction: mechanisms, clinical sequelae and therapeutic options. J Hum Hypertens. 2010;24(1):1–8. [DOI] [PubMed] [Google Scholar]
- 5.Bonnefont-Rousselot D. Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care. 2002;5(5):561–568. [DOI] [PubMed] [Google Scholar]
- 6.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. [DOI] [PubMed] [Google Scholar]
- 7.Tilton RG, Chang K, Nyengaard JR, et al. Inhibition of sorbitol dehydrogenase. Effects on vascular and neural dysfunction in streptozocin-induced diabetic rats. Diabetes. 1995;44(2):234–242. [DOI] [PubMed] [Google Scholar]
- 8.Garg SS, Gupta J.. Polyol pathway and redox balance in diabetes. Pharmacol Res. 2022;182:106326. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X, Liu Z, Ying K, et al. WJ-39, an aldose reductase inhibitor, ameliorates renal lesions in diabetic nephropathy by activating Nrf2 signaling. Oxid Med Cell Longev. 2020;2020:7950457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawarada Y, Inoue Y, Kawasaki F, et al. TGF-β induces p53/smads complex formation in the PAI-1 promoter to activate transcription. Sci Rep. 2016;6:35483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yonekura H, Yamamoto Y, Sakurai S, et al. Roles of the receptor for advanced glycation endproducts in diabetes-induced vascular injury. J Pharmacol Sci. 2005;97(3):305–311. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt AM, Hori O, Chen JX, et al. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice: a potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96(3):1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh LP, Cheng DW, Kowluru R, et al. Hexosamine induction of oxidative stress, hypertrophy and laminin expression in renal mesangial cells: effect of the anti-oxidant alpha-lipoic acid. Cell Biochem Funct. 2007;25(5):537–550. [DOI] [PubMed] [Google Scholar]
- 14.Yi H, Juhong Y.. Relationship between OGlcNAcylation and diabetic nephropathy. Chin J Diabetes. 2021;13(03):282–286. [Google Scholar]
- 15.Thallas-Bonke V, Thorpe SR, Coughlan MT, et al. Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes. 2008;57(2):460–469. [DOI] [PubMed] [Google Scholar]
- 16.Mochly-Rosen D, Das K, Grimes KV.. Protein kinase C, an elusive therapeutic target? Nat Rev Drug Discov. 2012;11(12):937–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang Q, Yang C.. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37:101799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, et al. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018;9(2):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma K, Danoff TM, DePiero A, et al. Enhanced expression of inducible nitric oxide synthase in murine macrophages and glomerular mesangial cells by elevated glucose levels: possible mediation via protein kinase C. Biochem Biophys Res Commun. 1995;207(1):80–88. [DOI] [PubMed] [Google Scholar]
- 20.Satoh T, Cohen HT, Katz AI.. Intracellular signaling in the regulation of renal Na-K-ATPase. I. Role of cyclic AMP and phospholipase A2. J Clin Invest. 1992;89(5):1496–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geraldes P, King GL.. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106(8):1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jha JC, Dai A, Garzarella J, et al. Independent of renox, NOX5 promotes renal inflammation and fibrosis in diabetes by activating ROS-Sensitive pathways. Diabetes. 2022;71(6):1282–1298. [DOI] [PubMed] [Google Scholar]
- 23.Wang R, Song F, Li S, et al. Salvianolic acid a attenuates CCl(4)-induced liver fibrosis by regulating the PI3K/AKT/mTOR, Bcl-2/bax and caspase-3/cleaved caspase-3 signaling pathways. Drug Des Devel Ther. 2019;13:1889–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hay N. Interplay between FOXO, TOR, and Akt. Biochim Biophys Acta. 2011;1813(11):1965–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ying C, Mao Y, Chen L, et al. Bamboo leaf extract ameliorates diabetic nephropathy through activating the AKT signaling pathway in rats. Int J Biol Macromol. 2017;105(Pt 3):1587–1594. [DOI] [PubMed] [Google Scholar]
- 26.Guo J, Liu Z, Gong R.. Long noncoding RNA: an emerging player in diabetes and diabetic kidney disease. Clin Sci. 2019;133(12):1321–1339. [DOI] [PubMed] [Google Scholar]
- 27.Bolós V, Peinado H, Pérez-Moreno MA, et al. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116(Pt 3):499–511. [DOI] [PubMed] [Google Scholar]
- 28.Lu Q, Wang W-W, Zhang M-Z, et al. ROS induces epithelial-mesenchymal transition via the TGF-β1/PI3K/Akt/mTOR pathway in diabetic nephropathy. Exp Ther Med. 2019;17(1):835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qing Y, Dong X, Hongli L, et al. Berberine promoted myocardial protection of postoperative patients through regulating myocardial autophagy. Biomed Pharmacother. 2018;105:1050–1053. [DOI] [PubMed] [Google Scholar]
- 30.Barthel A, Schmoll D, Unterman TG.. FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab. 2005;16(4):183–189. [DOI] [PubMed] [Google Scholar]
- 31.Li W, Wang Q, Du M, et al. Effects of overexpressing FoxO1 on apoptosis in glomeruli of diabetic mice and in podocytes cultured in high glucose medium. Biochem Biophys Res Commun. 2016;478(2):612–617. [DOI] [PubMed] [Google Scholar]
- 32.Qin G, Zhou Y, Guo F, et al. Overexpression of the FoxO1 ameliorates mesangial cell dysfunction in male diabetic rats. Mol Endocrinol. 2015;29(7):1080–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ke-Ke M, et al. Effect of astragaloside IV on regulation of PI3K/akt/FoxO1 signal in kidney of type 2 diabetic nephropathy rats. Chin J Exp Trad Med Formulae. 2019;25(02):74–81. [Google Scholar]
- 34.Wen C, Ying Y, Zhao H, et al. Resistance exercise affects catheter-related thrombosis in rats through miR-92a-3p, oxidative stress and the MAPK/NF-κB pathway. BMC Cardiovasc Disord. 2021;21(1):440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zoccali C, Mallamaci F.. Nonproteinuric progressive diabetic kidney disease. Curr Opin Nephrol Hypertens. 2019;28(3):227–232. [DOI] [PubMed] [Google Scholar]
- 36.Hofmann MA, Schiekofer S, Isermann B, et al. Peripheral blood mononuclear cells isolated from patients with diabetic nephropathy show increased activation of the oxidative-stress sensitive transcription factor NF-kappaB. Diabetologia. 1999;42(2):222–232. [DOI] [PubMed] [Google Scholar]
- 37.Zheng H, Whitman SA, Wu W, et al. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes. 2011;60(11):3055–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang T, Huang Z, Lin Y, et al. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes. 2010;59(4):850–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma L, Wu F, Shao Q, et al. Baicalin alleviates oxidative stress and inflammation in diabetic nephropathy via Nrf2 and MAPK signaling pathway. Drug Des Devel Ther. 2021;15:3207–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sen Z, Weida W, Jie M, et al. Coumarin glycosides from Hydrangea paniculata slow down the progression of diabetic nephropathy by targeting Nrf2 anti-oxidation and smad2/3-mediated profibrosis. Phytomedicine. 2019;57:385–395. [DOI] [PubMed] [Google Scholar]
- 41.Alshehri AS. Kaempferol attenuates diabetic nephropathy in streptozotocin-induced diabetic rats by a hypoglycaemic effect and concomitant activation of the Nrf-2/Ho-1/antioxidants axis. Arch Physiol Biochem. 2021;2021:1–14. [DOI] [PubMed] [Google Scholar]
- 42.Huang W, Liang Y, Dong J, et al. SUMO E3 ligase PIASy mediates high glucose-induced activation of NF-κB inflammatory signaling in rat mesangial cells. Mediators Inflamm. 2017;2017:1685194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iyengar PV. Regulation of ubiquitin enzymes in the TGF-β pathway. Int J Mol Sci. 2017;18(4):877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iyengar PV, Jaynes P, Rodon L, et al. USP15 Regulates SMURF2 kinetics through C-lobe mediated deubiquitination. Sci Rep. 2015;5:14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ono K, Han J.. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12(1):1–13. [DOI] [PubMed] [Google Scholar]
- 46.Qiao Y, Gao K, Wang Y, et al. Resveratrol ameliorates diabetic nephropathy in rats through negative regulation of the p38 MAPK/TGF-β1 pathway. Exp Ther Med. 2017;13(6):3223–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Y, Wang L, Deng D, et al. Renalase protects against renal fibrosis by inhibiting the activation of the ERK signaling pathways. Int J Mol Sci. 2017;18(5):855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X, Lin L, Li Q, et al. ERK1/2 communicates GPCR and EGFR signaling pathways to promote CTGF-mediated hypertrophic cardiomyopathy upon Ang-II stimulation. BMC Mol Cell Biol. 2019;20(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu M, Wang H, Chen J, et al. Sinomenine improve diabetic nephropathy by inhibiting fibrosis and regulating the JAK2/STAT3/SOCS1 pathway in streptozotocin-induced diabetic rats. Life Sci. 2021;265:118855. [DOI] [PubMed] [Google Scholar]
- 50.Yu J, Wu H, Liu Z-Y, et al. Advanced glycation end products induce the apoptosis of and inflammation in mouse podocytes through CXCL9-mediated JAK2/STAT3 pathway activation. Int J Mol Med. 2017;40(4):1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chow F, Ozols E, Nikolic-Paterson DJ, et al. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65(1):116–128. [DOI] [PubMed] [Google Scholar]
- 52.Hao W, Lin Y, Liqin T, et al. Berberine inhibits JAK2/STAT3 signaling pathway to alleviate high glucose induced podocyte EMT and apoptosis. Acta Universitatis Medicinalis Anhui. 2022;57(08):1189–1194. [Google Scholar]
- 53.Dugan LL, You Y-H, Ali SS, et al. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest. 2013;123(11):4888–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang B, Guo X, Li Y, et al. d-Chiro inositol ameliorates endothelial dysfunction via inhibition of oxidative stress and mitochondrial fission. Mol Nutr Food Res. 2017;61(8):mnfr.201600710. [DOI] [PubMed] [Google Scholar]
- 55.Li F, Chen Y, Li Y, et al. Geniposide alleviates diabetic nephropathy of mice through AMPK/SIRT1/NF-κB pathway. Eur J Pharmacol. 2020;886:173449. [DOI] [PubMed] [Google Scholar]
- 56.Wu L, Liu C, Chang D-Y, et al. The attenuation of diabetic nephropathy by annexin A1 via regulation of lipid metabolism through the AMPK/PPARα/CPT1b pathway. Diabetes. 2021;70(10):2192–2203. [DOI] [PubMed] [Google Scholar]
- 57.Han Y-C, Tang S-Q, Liu Y-T, et al. AMPK agonist alleviate renal tubulointerstitial fibrosis via activating mitophagy in high fat and streptozotocin induced diabetic mice. Cell Death Dis. 2021;12(10):925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiang L, Jiang P, Zhou L, et al. Additive effect of qidan dihuang grain, a traditional chinese medicine, and angiotensin receptor blockers on albuminuria levels in patients with diabetic nephropathy: a randomized, Parallel-Controlled trial. Evid Based Complement Alternat Med. 2016;2016:1064924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun G-D, Li C-Y, Cui W-P, et al. Review of herbal traditional chinese medicine for the treatment of diabetic nephropathy. J Diabetes Res. 2016;2016:5749857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi H, Deng P, Dong C, et al. Quality of evidence supporting the role of tripterygium glycosides for the treatment of diabetic kidney disease: an overview of systematic reviews and meta-analyses. Drug Des Devel Ther. 2022;16:1647–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tao M, Zheng D, Liang X, et al. Tripterygium glycoside suppresses epithelial‑to‑mesenchymal transition of diabetic kidney disease podocytes by targeting autophagy through the mTOR/Twist1 pathway. Mol Med Rep. 2021;24(2):592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma Z-J, Zhang X-N, Li L, et al. Tripterygium glycosides tablet ameliorates renal tubulointerstitial fibrosis via the Toll-Like receptor 4/nuclear factor kappa B signaling pathway in High-Fat diet fed and Streptozotocin-Induced diabetic rats. J Diabetes Res. 2015;2015:390428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma R, Liu L, Liu X, et al. Triptolide markedly attenuates albuminuria and podocyte injury in an animal model of diabetic nephropathy. Exp Ther Med. 2013;6(3):649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou YX, Zhang H, Peng C.. Puerarin: a review of pharmacological effects. Phytother Res. 2014;28(7):961–975. [DOI] [PubMed] [Google Scholar]
- 65.Wang B, Chen S, Yan X, et al. The therapeutic effect and possible harm of puerarin for treatment of stage III diabetic nephropathy: a meta-analysis. Altern Ther Health Med. 2015;21(1):36–44. [PubMed] [Google Scholar]
- 66.Li X, Zhu Q, Zheng R, et al. Puerarin attenuates diabetic nephropathy by promoting autophagy in podocytes. Front Physiol. 2020;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yuan Y, et al. Inhibition of puerarin on formation of advanced glycation end products in vivo and in vitro. Chinese Traditional and Herbal Drugs. 2017;48(07):1386–1390. [Google Scholar]
- 68.Mao CP, Gu ZL.. Puerarin reduces increased c-fos, c-jun, and type IV collagen expression caused by high glucose in glomerular mesangial cells. Acta Pharmacol Sin. 2005;26(8):982–986. [DOI] [PubMed] [Google Scholar]
- 69.Liu X, Yao L, Sun D, et al. Effect of breviscapine injection on clinical parameters in diabetic nephropathy: a meta-analysis of randomized controlled trials. Exp Ther Med. 2016;12(3):1383–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu X-X, Zhang W, Zhang P, et al. Superior renoprotective effects of the combination of breviscapine with enalapril and its mechanism in diabetic rats. Phytomedicine. 2013;20(10):820–827. [DOI] [PubMed] [Google Scholar]
- 71.Jiang W, Li Z, Zhao W, et al. Breviscapine attenuatted contrast medium-induced nephropathy via PKC/Akt/MAPK signalling in diabetic mice. Am J Transl Res. 2016;8(2):329–341. [PMC free article] [PubMed] [Google Scholar]
- 72.Sattarinezhad A, Roozbeh J, Shirazi Yeganeh B, et al. Resveratrol reduces albuminuria in diabetic nephropathy: a randomized double-blind placebo-controlled clinical trial. Diabetes Metab. 2019;45(1):53–59. [DOI] [PubMed] [Google Scholar]
- 73.Hu H-C, Lei Y-H, Zhang W-H, et al. Antioxidant and anti-inflammatory properties of resveratrol in diabetic nephropathy: a systematic review and meta-analysis of animal studies. Front Pharmacol. 2022;13:841818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang C-C, Chang C-Y, Wu Y-T, et al. Resveratrol retards progression of diabetic nephropathy through modulations of oxidative stress, proinflammatory cytokines, and AMP-activated protein kinase. J Biomed Sci. 2011;18(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu X-H, Ding D-F, Yong H-J, et al. Resveratrol transcriptionally regulates miRNA-18a-5p expression ameliorating diabetic nephropathy via increasing autophagy. Eur Rev Med Pharmacol Sci. 2017;21(21):4952–4965. [PubMed] [Google Scholar]
- 76.Chen K-H, Hung C-C, Hsu H-H, et al. Resveratrol ameliorates early diabetic nephropathy associated with suppression of augmented TGF-β/smad and ERK1/2 signaling in streptozotocin-induced diabetic rats. Chem Biol Interact. 2011;190(1):45–53. [DOI] [PubMed] [Google Scholar]
- 77.Qi M-Y, He Y-H, Cheng Y, et al. Icariin ameliorates streptozocin-induced diabetic nephropathy through suppressing the TLR4/NF-κB signal pathway. Food Funct. 2021;12(3):1241–1251. [DOI] [PubMed] [Google Scholar]
- 78.Wang K, Zheng X, Pan Z, et al. Icariin prevents extracellular matrix accumulation and ameliorates experimental diabetic kidney disease by inhibiting oxidative stress via GPER mediated p62-Dependent Keap1 degradation and Nrf2 activation. Front Cell Dev Biol. 2020;8:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qi M-Y, Liu H-R, Su Y-h, et al. Protective effect of Icariin on the early stage of experimental diabetic nephropathy induced by streptozotocin via modulating transforming growth factor β1 and type IV collagen expression in rats. J Ethnopharmacol. 2011;138(3):731–736. [DOI] [PubMed] [Google Scholar]
- 80.Jia Z, Wang K, Zhang Y, et al. Icariin ameliorates diabetic renal tubulointerstitial fibrosis by restoring autophagy via regulation of the miR-192-5p/GLP-1R pathway. Front Pharmacol. 2021;12:720387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fallahzadeh MK, Dormanesh B, Sagheb MM, et al. Effect of addition of silymarin to renin-angiotensin system inhibitors on proteinuria in type 2 diabetic patients with overt nephropathy: a randomized, double-blind, placebo-controlled trial. Am J Kidney Dis. 2012;60(6):896–903. [DOI] [PubMed] [Google Scholar]
- 82.Khazim K, Gorin Y, Cavaglieri RC, et al. The antioxidant silybin prevents high glucose-induced oxidative stress and podocyte injury in vitro and in vivo. Am J Physiol Renal Physiol. 2013;305(5):F691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Y, Chen L, Yang T.. Silymarin nanoliposomes attenuate renal injury on diabetic nephropathy rats via co-suppressing TGF-β/Smad and JAK2/STAT3/SOCS1 pathway. Life Sci. 2021;271:119197. [DOI] [PubMed] [Google Scholar]
- 84.Liu Y, Ye J, Cao Y, et al. Silibinin ameliorates diabetic nephropathy via improving diabetic condition in the mice. Eur J Pharmacol. 2019;845:24–31. [DOI] [PubMed] [Google Scholar]
- 85.Jz AL, et al. Curcumin reverses diabetic nephropathy in streptozotocin-induced diabetes in rats by inhibition of PKCβ/p(66)Shc axis and activation of FOXO-3a. J Nutr Biochem. 2021;87:108515. [DOI] [PubMed] [Google Scholar]
- 86.Soetikno V, Watanabe K, Sari FR, et al. Curcumin attenuates diabetic nephropathy by inhibiting PKC-α and PKC-β1 activity in streptozotocin-induced type I diabetic rats. Mol Nutr Food Res. 2011;55(11):1655–1665. [DOI] [PubMed] [Google Scholar]
- 87.Tu Q, Li Y, Jin J, et al. Curcumin alleviates diabetic nephropathy via inhibiting podocyte mesenchymal transdifferentiation and inducing autophagy in rats and MPC5 cells. Pharm Biol. 2019;57(1):778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tikoo K, Meena RL, Kabra DG, et al. Change in post-translational modifications of histone H3, heat-shock protein-27 and MAP kinase p38 expression by curcumin in streptozotocin-induced type I diabetic nephropathy. Br J Pharmacol. 2008;153(6):1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Voroneanu L, Siriopol D, Dumea R, et al. Addition of silymarin to renin-angiotensin system blockers in normotensive patients with type 2 diabetes mellitus and proteinuria: a prospective randomized trial. Int Urol Nephrol. 2017;49(12):2195–2204. [DOI] [PubMed] [Google Scholar]
- 90.Wang X, Xu Y, Chu C, et al. Effect of safflower yellow on early type II diabetic nephropathy: a systematic review and meta-analysis of randomized controlled trials. J Pediatr Endocrinol Metab. 2019;32(7):653–665. [DOI] [PubMed] [Google Scholar]
- 91.Du M, Hu X, Kou L, et al. Lycium barbarum polysaccharide mediated the antidiabetic and antinephritic effects in Diet-Streptozotocin-induced diabetic Sprague Dawley rats via regulation of NF-κB. Biomed Res Int. 2016;2016:3140290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wan F, Ma F, Wu J, et al. Effect of Lycium Barbarum polysaccharide on decreasing serum amyloid A3 expression through inhibiting NF-κB activation in a mouse model of diabetic nephropathy. Anal Cell Pathol. 2022;2022:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao R, Li Q-W, Li J, et al. Protective effect of Lycium barbarum polysaccharide 4 on kidneys in streptozotocin-induced diabetic rats. Can J Physiol Pharmacol. 2009;87(9):711–719. [DOI] [PubMed] [Google Scholar]
- 94.Fu-Tuan G, et al. The anti-oxidative effect of ramulus mori polysaccharides on diabetic nephropathy mice. Chin Pharmacol Bull. 2016;32(08):1148–1152. [Google Scholar]
- 95.Xiao-Ling G, et al. Effect of astragalus polysaccharides on apoptosis, transdifferentiation and ROS content in renal tubular epithelial cells of diabetic nephropathy. Chin J Immunol. 2018;34(03):388–392. [Google Scholar]
- 96.Cheng-de L, et al. Effects of astragalus polysaccharide on renal TGF-β1/smads signaling pathway in rats with diabetes mellitus. Chin Pharmacol Bull. 2018;34(04):512–516. [Google Scholar]
- 97.Qin X, Zhao Y, Gong J, et al. Berberine protects glomerular podocytes via inhibiting Drp1-mediated mitochondrial fission and dysfunction. Theranostics. 2019;9(6):1698–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pang B, Zhao L-H, Zhou Q, et al. Application of berberine on treating type 2 diabetes mellitus. Int J Endocrinol. 2015;2015:905749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ni WJ, Ding HH, Tang LQ.. Berberine as a promising anti-diabetic nephropathy drug: an analysis of its effects and mechanisms. Eur J Pharmacol. 2015;760:103–112. [DOI] [PubMed] [Google Scholar]
- 100.Zhao H-L, Sui Y, Qiao C-F, et al. Sustained antidiabetic effects of a berberine-containing chinese herbal medicine through regulation of hepatic gene expression. Diabetes. 2012;61(4):933–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang FL, Tang LQ, Yang F, et al. Renoprotective effects of berberine and its possible molecular mechanisms in combination of high-fat diet and low-dose streptozotocin-induced diabetic rats. Mol Biol Rep. 2013;40(3):2405–2418. [DOI] [PubMed] [Google Scholar]
- 102.Zhang X, He H, Liang D, et al. Protective effects of berberine on renal injury in streptozotocin (STZ)-induced diabetic mice. Int J Mol Sci. 2016;17(8):1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qin X, Jiang M, Zhao Y, et al. Berberine protects against diabetic kidney disease via promoting PGC-1α-regulated mitochondrial energy homeostasis. Br J Pharmacol. 2020;177(16):3646–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu L, Han J, Yuan R, et al. Berberine ameliorates diabetic nephropathy by inhibiting TLR4/NF-κB pathway. Biol Res. 2018;51(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang B, Ni Q, Wang X, et al. Meta-analysis of the clinical effect of ligustrazine on diabetic nephropathy. Am J Chin Med. 2012;40(1):25–37. [DOI] [PubMed] [Google Scholar]
- 106.Xie F, Zhang B, Dai S, et al. Efficacy and safety of Salvia miltiorrhiza (Salvia miltiorrhiza Bunge) and ligustrazine injection in the adjuvant treatment of early-stage diabetic kidney disease: a systematic review and meta-analysis. J Ethnopharmacol. 2021;281:114346. [DOI] [PubMed] [Google Scholar]
- 107.Zhuang Z, Wang Z-H, Huang Y-Y, et al. Protective effect and possible mechanisms of ligustrazine isolated from Ligusticum wallichii on nephropathy in rats with diabetes: a preclinical systematic review and meta-analysis. J Ethnopharmacol. 2020;252:112568. [DOI] [PubMed] [Google Scholar]
- 108.Zhang L, Shergis JL, Yang L, et al. Astragalus membranaceus (Huang Qi) as adjunctive therapy for diabetic kidney disease: an updated systematic review and meta-analysis. J Ethnopharmacol. 2019;239:111921. [DOI] [PubMed] [Google Scholar]
- 109.Xuqin D, et al. Astragalus injection for early diabetic nephropathoes:a meta-analysis. Chin Arch Trad Chin Med. 2019;37(04):869–872. [Google Scholar]
- 110.Li M, Wang W, Xue J, et al. Meta-analysis of the clinical value of Astragalus membranaceus in diabetic nephropathy. J Ethnopharmacol. 2011;133(2):412–419. [DOI] [PubMed] [Google Scholar]
- 111.Wang H, Zhuang Z, Huang Y-Y, et al. Protective effect and possible mechanisms of astragaloside IV in animal models of diabetic nephropathy: a preclinical systematic review and meta-Analysis. Front Pharmacol. 2020;11:988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Q, Shao X, Xu W, et al. Astragalosides IV inhibits high glucose-induced cell apoptosis through HGF activation in cultured human tubular epithelial cells. Ren Fail. 2014;36(3):400–406. [DOI] [PubMed] [Google Scholar]
- 113.Su J, Gao C, Xie L, et al. Astragaloside II ameliorated podocyte injury and mitochondrial dysfunction in streptozotocin-induced diabetic rats. Front Pharmacol. 2021;12:638422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guo H, Wang Y, Zhang X, et al. Astragaloside IV protects against podocyte injury via SERCA2-dependent ER stress reduction and AMPKα-regulated autophagy induction in streptozotocin-induced diabetic nephropathy. Sci Rep. 2017;7(1):6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang X, Gao Y, Tian N, et al. Astragaloside IV represses high glucose-induced mesangial cells activation by enhancing autophagy via SIRT1 deacetylation of NF-κB p65 subunit. Drug Des Devel Ther. 2018;12:2971–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Feng H, Zhu X, Tang Y, et al. Astragaloside IV ameliorates diabetic nephropathy in db/db mice by inhibiting NLRP3 inflammasome‑mediated inflammation. Int J Mol Med. 2021;48(2):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang T, Zhu Q, Shao Y, et al. Paeoniflorin prevents TLR2/4-mediated inflammation in type 2 diabetic nephropathy. Biosci Trends. 2017;11(3):308–318. [DOI] [PubMed] [Google Scholar]
- 118.Li X, Wang Y, Wang K, et al. Renal protective effect of Paeoniflorin by inhibition of JAK2/STAT3 signaling pathway in diabetic mice. Biosci Trends. 2018;12(2):168–176. [DOI] [PubMed] [Google Scholar]
- 119.Zhang M-h, Feng L, Zhu M-M, et al. Antioxidative and anti-inflammatory activities of paeoniflorin and oxypaeoniflora on AGEs-induced mesangial cell damage. Planta Med. 2013;79(14):1319–1323. [DOI] [PubMed] [Google Scholar]
- 120.Chen J, Zhao D, Zhu M, et al. Paeoniflorin ameliorates AGEs-induced mesangial cell injury through inhibiting RAGE/mTOR/autophagy pathway. Biomed Pharmacother. 2017;89:1362–1369. [DOI] [PubMed] [Google Scholar]
- 121.Wang H, Teng Y, Li S, et al. UHPLC-MS-Based serum and urine metabolomics reveals the anti-diabetic mechanism of ginsenoside re in type 2 diabetic rats. Molecules. 2021;26(21):6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.He J-Y, Hong Q, Chen B-X, et al. Ginsenoside Rb1 alleviates diabetic kidney podocyte injury by inhibiting aldose reductase activity. Acta Pharmacol Sin. 2022;43(2):342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shi Y, Gao Y, Wang T, et al. Ginsenoside Rg1 alleviates podocyte EMT passage by regulating AKT/GSK3 β/β-Catenin pathway by restoring autophagic activity. Evid Based Complement Alternat Med. 2020;2020:1903627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu H, Chen W, Lu P, et al. Ginsenoside Rg1 attenuates the inflammation and oxidative stress induced by diabetic nephropathy through regulating the PI3K/AKT/FOXO3 pathway. Ann Transl Med. 2021;9(24):1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Du N, Xu Z, Gao M, et al. Combination of Ginsenoside Rg1 and Astragaloside IV reduces oxidative stress and inhibits TGF-β1/Smads signaling Cascade on renal fibrosis in rats with diabetic nephropathy. Drug Des Devel Ther. 2018;12:3517–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhu Y, Zhu C, Yang H, et al. Protective effect of ginsenoside Rg5 against kidney injury via inhibition of NLRP3 inflammasome activation and the MAPK signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. Pharmacol Res. 2020;155:104746. [DOI] [PubMed] [Google Scholar]
- 127.Tu QN, Dong H, Lu FE.. Effects of Panax notoginoside on the nephropathy in rats with type 1 diabetes mellitus. Chin J Integr Med. 2011;17(8):612–615. [DOI] [PubMed] [Google Scholar]
- 128.Yang J, Zeng Z, Wu T, et al. Emodin attenuates high glucose-induced TGF-β1 and fibronectin expression in mesangial cells through inhibition of NF-κB pathway. Exp Cell Res. 2013;319(20):3182–3189. [DOI] [PubMed] [Google Scholar]
- 129.Tian N, Gao Y, Wang X, et al. Emodin mitigates podocytes apoptosis induced by endoplasmic reticulum stress through the inhibition of the PERK pathway in diabetic nephropathy. Drug Des Devel Ther. 2018;12:2195–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu H, Wang Q, Shi G, et al. Emodin ameliorates renal damage and podocyte injury in a rat model of diabetic nephropathy via regulating AMPK/mTOR-mediated autophagy signaling pathway. Diabetes Metab Syndr Obes. 2021;14:1253–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jing D, Bai H, Yin S.. Renoprotective effects of emodin against diabetic nephropathy in rat models are mediated via PI3K/Akt/GSK-3β and Bax/caspase-3 signaling pathways. Exp Ther Med. 2017;14(5):5163–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yan M, Wen Y, Yang L, et al. Chinese herbal medicine Tangshen Formula treatment of patients with type 2 diabetic kidney disease with macroalbuminuria: study protocol for a randomized controlled trial. Trials. 2016;17(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hu J, Fan X, Meng X, et al. Evidence for the involvement of JAK/STAT/SOCS pathway in the mechanism of Tangshen formula-treated diabetic nephropathy. Planta Med. 2014;80(8–9):614–621. [DOI] [PubMed] [Google Scholar]
- 134.An W, Huang Y, Chen S, et al. Efficacy and safety of Huangkui capsule for diabetic nephropathy: a protocol for systematic review and meta-analysis. Medicine. 2021;100(42):e27569. ), [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cai H-D, Su S-L, Qian D-W, et al. Renal protective effect and action mechanism of Huangkui capsule and its main five flavonoids. J Ethnopharmacol. 2017;206:152–159. [DOI] [PubMed] [Google Scholar]
- 136.Gu L-Y, Tang H-T, Xu Z-X, et al. Huangkui capsule in combination with metformin ameliorates diabetic nephropathy via the Klotho/TGF-β1/p38MAPK signaling pathway. J Ethnopharmacol. 2021;281:113548. [DOI] [PubMed] [Google Scholar]
- 137.Ge J, Miao J-J, Sun X-Y, et al. Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, improves diabetic nephropathy via activating peroxisome proliferator-activated receptor (PPAR)-α/γ and attenuating endoplasmic reticulum stress in rats. J Ethnopharmacol. 2016;189:238–249. [DOI] [PubMed] [Google Scholar]
- 138.Ma J, Xu L, Dong J, et al. Effects of zishentongluo in patients with early-stage diabetic nephropathy. Am J Chin Med. 2013;41(2):333–340. [DOI] [PubMed] [Google Scholar]
- 139.Hsu P-C, Tsai Y-T, Lai J-N, et al. Integrating traditional Chinese medicine healthcare into diabetes care by reducing the risk of developing kidney failure among type 2 diabetic patients: a population-based case control study. J Ethnopharmacol. 2014;156:358–364. [DOI] [PubMed] [Google Scholar]
- 140.Chen HH, Wu C-T, Tsai Y-T, et al. Liu Wei Di Huang Wan and the Delay of insulin use in patients with type 2 diabetes in Taiwan: a nationwide study. Evid Based Complement Alternat Med. 2021;2021:1298487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Song XY, Chen Q, Qi XY.. Effect of Liuwei Dihuang Pill on erythrocyte aldose reductase activity in early diabetic nephropathy patients . Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24(12):1087–1090. [PubMed] [Google Scholar]
- 142.Renzhi J, et al. Clinical efficacy of wuling powder combined with rosiglitazone on diabetic nephropathy. Pharma Clin Chin Materia Medica. 2017;33(04):176–178. [Google Scholar]
- 143.Liu I-M, Tzeng T-F, Liou S-S, et al. The amelioration of streptozotocin diabetes-induced renal damage by Wu-Ling-San (Hoelen Five Herb Formula), a traditional Chinese prescription. J Ethnopharmacol. 2009;124(2):211–218. [DOI] [PubMed] [Google Scholar]
- 144.Xi-Mou Z, et al. Fermented cordyceps powder combined with ACEI/ARB in treatment of diabetic kidney disease:a systematic review. Chin J Exp Trad Med Form. 2021;27(18):169–175. [Google Scholar]
- 145.Haotao M. Effects of Jinshuibao capsule combined with compound α-keto acid tablets on endothelial function and renal artery blood flow in patients with diabetic nephropathy. Pract Clin J Integr Trad Chin Western Med. 2022;22(07):27–30. [Google Scholar]
- 146.Lu Q, Li C, Chen W, et al. Clinical efficacy of Jinshuibao capsules combined with angiotensin receptor blockers in patients with early diabetic nephropathy: a meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2018;2018:6806943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sun WS, Wu XL, Qiao CL. [Clinical study on effect of tongluo capsule in treating diabetic nephropathy caused chronic renal failure. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24(8):704–706. [PubMed] [Google Scholar]
- 148.Li B-y, Peng H, Xiong D-L, et al. Efficacy observation of treating diabetic nephropathy by shenshuaining granule combined telmisartan tablet. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015;35(2):142–146. [PubMed] [Google Scholar]
- 149.Liu S, Tang L-Q, Chen L-M, et al. Effect of compound Rhizoma Coptidis capsule on expression of transforming growth factor-beta1 and type IV collagen proteins in renal tissue of diabetic rats with nephropathy. Zhongguo Zhong Yao Za Zhi. 2008;33(1):68–72. [PubMed] [Google Scholar]
- 150.Guo Z-A, Yu C-J, Liu G, et al. Treatment of stage 3b diabetic kidney disease patients with macroalbuminuria by qizhi jiangtang capsule: a multicenter randomized control clinical study. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34(9):1047–1052. [PubMed] [Google Scholar]
- 151.Li MZ, Gao YB, Ma MF.. Effects of qiwei granule on the protein and mRNA expressions of renal tissue transforming growth factor-beta1 in KK-Ay mice with spontaneous type 2 diabetes mellitus. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32(12):1675–1678. [PubMed] [Google Scholar]
- 152.Zhao TT, Zhang HJ, Lu XG, et al. Chaihuang-Yishen granule inhibits diabetic kidney disease in rats through blocking TGF-β/Smad3 signaling. PLoS One. 2014;9(3):e90807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ma F, Li L, Wang Q, et al. Qi-dan-di-huang decoction alleviates diabetic nephropathy by inhibiting the NF-kappaB pathway. Front Biosci. 2019;24(8):1477–1486. [DOI] [PubMed] [Google Scholar]
- 154.Liu I-M, Tzeng T-F, Liou S-S, et al. Beneficial effect of traditional chinese medicinal formula danggui-shaoyao-san on advanced glycation end-product-mediated renal injury in streptozotocin-diabetic rats. Evid Based Complement Alternat Med. 2012;2012:140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fang D, Wan X, Deng W, et al. Fufang Xue Shuan Tong capsules inhibit renal oxidative stress markers and indices of nephropathy in diabetic rats. Exp Ther Med. 2012;4(5):871–876. [DOI] [PMC free article] [PubMed] [Google Scholar]