ABSTRACT
During a field survey of cultivated Morchella mushroom diseases, Diploöspora longispora and Paecilomyces penicillatus, causal agents of pileus rot or white mould disease were detected, which resulted in up to 80% of yield losses. Multi-locus phylogenic analysis revealed that the fungi were affiliated in a distinct clade in Hypocreales. We further constructed a phylogenetic tree with broader sampling in Hypocreales and estimated the divergence times. The D. longispora and P. penicillatus clades were estimated to have diverged from Hypocreaceae around 129 MYA and Pseudodiploösporeaceae fam. nov is herein proposed to accommodate species in this clade. Two new genera, i.e. Pseudodiploöspora and Zelopaecilomyceswere, were introduced based on morphological characteristics and phylogenic relationships of Diploöspora longispora and Paecilomyces penicillatus, respectively. Five new combinations – Pseudodiploöspora cubensis, P. longispora, P. fungicola, P. zinniae, and Zelopaecilomyces penicillatus – were proposed.
KEYWORDS: Five new combinations, two new genera, one new family, fungal pathogen, mushroom disease
Introduction
True morels (Morchella, Morchellaceae, Pezizales, Ascomycota) are one of the most popular edible mushrooms with a long history of consumption in Asia, Europe, and North America (Pilz et al. 2017; Liu et al. 2018). Because of their good taste, culinary qualities, and pharmacological performances in antitumor, anti-inflammatory, and antioxidant activities (Tietel and Masaphy 2018; Zhang et al. 2019), demands for morels have significantly increased in the market. In recent years, large-scale field cultivation of Morchella was successfully achieved in China (Liu et al. 2018). The morel cultivation area reached approximately 12,000 ha in the production season of 2021–2020 in China, with an economic value of over RMB 10 billion. However, with the rapid expansion of cultivation, diseases become a bottleneck for morel production, especially for diseases caused by fungi. Several common fungal diseases have been identified in the fruiting bodies of cultivated Morchella: stipe rot disease caused by the Fusarium incarnatum–F. equiseti species complex (Guo et al. 2016) and by Purpureocillium lilacinum (Masaphy, 2022), cobweb disease caused by Hypomyces/Cladobotryum species (Lan et al. 2020), white mould disease caused by Paecilomyces penicillatus (He et al. 2017) and pileus rot disease caused by Diploöspora longispora (He et al. 2018; Liu et al. 2018). Previous investigations showed that white mould diseases and pileus rot resulted in up to 80% of morel yield losses each year, which was attributed to a large number of conidia quickly spreading around the cultivation areas (Wang et al. 2020). However, the fungal pathogens were mainly identified based on sequence similarity of internal transcribed spacer gene region (ITS) but lacked convincing morphological evidence (Hyde et al. 2017, 2018; Liu et al. 2018). When did a blast of the ITS sequence of D. longispora, Tanney et al. (2015) also showed that D. longispora is most closely related to P. penicillatus including its ex-type (CBS 448.69).
The genus Diploöspora was established by Grove (1916) with Diploöspora rosea as the type species. This genus was characterised by producing chains of hyaline, cylindrical to fusiform, aseptate, or 1–3-septate conidia (Tanney et al. 2015). Currently, phylogenetic analysis of the partial sequences of small subunit (SSU) ribosomal RNA gene, internal transcribed spacers (ITS), and large subunit (SSU) ribosomal RNA gene reveals that D. rosea is an onygenalean fungus (Tanney et al. 2015). Diploöspora longispora was firstly isolated from a dead leaf of Colocasia esculenta var. antiquorum in Japan (Matsushima 1976). Two varieties of D. longispora are available, namely Diploöspora longispora var. longispora and Diploöspora longispora var. cubensis, and the latter was originally obtained from the fallen leaves of Leguminosae in Cuba (Castaneda 1987). Tanney et al. (2015) presented that D. longispora and its varieties were most closely related to P. penicillatus belonging to the order Hypocreales and reached affinity with Hypocreaceae. However, apart from conidial chains, there is little morphological similarity between P. penicillatus and D. longispora, namely the penicillate conidiophores of P. penicillatus with their basipetal conidiogenesis versus the branched conidiophores and acropetal conidiogenesis of D. longispora (Tanney et al. 2015).
The genus Paecilomyces was introduced by Bainier (1907) with Paecilomyces variotii as the type species (Samson 1974). This genus was featured by verticillate conidiophores with divergent whorls of phialides, having a cylindrical or inflated base tapering to a long and distinct neck and producing typically hyaline, one-celled conidia. Phylogenetic analysis based on 18S rDNA demonstrates that Paecilomyces is polyphyletic across two classes (Luangsa-Ard et al. 2004). The type species, P. variotii, and its thermophilic relatives were placed in Eurotiales (Eurotiomycetes), while mesophilic species are in Hypocreales (Sordariomycetes) (Luangsa-Ard et al. 2004, 2005). Paecilomyces penicillatus was introduced by Samson in 1974, which was first isolated from rotten mushrooms. Based on the morphological characteristics, it was placed in Eurotiales (Samson 1974). Based on the molecular phylogenetic analysis of 18S rRNA sequences and β-tubulin, P. penicillatus was transferred to the order Hypocreales (Sordariomycetes) and revealed an uncertain affinity with Hypocreaceae (Luangsa-ard et al. 2004, 2005); however, P. penicillatus is still placed in Paecilomyces sensu stricto.
According to Hyde et al. (2020), this order comprises 14 families, including Bionectriaceae, Calcarisporiaceae, Clavicipitaceae, Cocoonihabitaceae, Cordycipitaceae, Flammocladiellaceae, Hypocreaceae, Myrotheciomycetaceae, Nectriaceae, Niessliaceae, Ophiocordycipitaceae, Sarocladiaceae, Stachybotryaceae, and Tilachlidiaceae. Recently, Polycephalomycetaceae was introduced for the accommodation of the fungicolous species from Ophiocordycipitaceae based on a concatenated matrix of six genetic markers (LSU, ITS, SSU, TEF, RPB1, and RPB2, personal communication). These hypocrealean fungi are mostly found as saprobes on decaying wood and in soil, pathogens or endophytes of plants, nematodes, and insects (Zhang et al. 2018, personal communication), as well as parasites on other fungi and lichens (Zhu and Zhuang 2013; Sun et al. 2019a). Generally, hypocrealean fungicolous taxa are the more serious, common pathogens of most cultivated mushrooms (Sun et al. 2019b). Diploöspora longispora and Paecilomyces penicillatus (Hypocreales) are recognised as the serious pathogens of cultivated Morchella, and previous studies indicated that their family ranks are uncertain (Luangsa-Ard et al. 2004; Luangsa-ard et al. 2005; Tanney et al. 2015). Additionally, Tanney et al. (2015) and our analyses presented that D. longispora and its variants were most closely related to several P. penicillatus strains (including CBS 448.69, the ex-type strain) based on an ITS BLAST query. Currently, multi-locus phylogenetic analyses and divergence time estimation have been used to clarify the higher ranking of fungal taxa (Hyde et al. 2017, 2020). This study performed phylogenetic analysis and divergence time estimation using a concatenated matrix of five genetic markers (LSU, ITS, SSU, TEF, and RPB2). Based on the results, a new family, Pseudodiploösporeaceae, and two new genera Pseudodiploöspora and Zelopaecilomyces, are introduced to accommodate the fungal taxa misplaced under Diploöspora and Paecilomyces, respectively. Additionally, we introduce four combinations including the fungal pathogens causing pileus rot disease and white mould disease of cultivated Morchella.
Materials and methods
Specimens, isolates, and morphological observation
Fresh specimens were collected along with the fruiting bodies of cultivated Morchella mushrooms in Kunming Yunnan province, Ankang, Shanxi province, Baoding, Hebei, province, Ningxia Hui Autonomous Region during the 2019 and 2021 cultivation seasons. Fruiting bodies were examined from free-hand sections using a stereomicroscope. The conidia were picked and streaked on potato extract agar (PDA) and incubated at 25°C for 7 days. A Nikon Eclipse 80i light microscope, equipped with differential interference contrast (DIC) optics, was used to capture digital images. Tarosoft (R) v.0.9.7 Image Frame Work was used to measure the morphologic structures, and Adobe Photoshop CS6 Extended version 13.0.1 software (Adobe Systems, USA) to edit the photographic plates.
For observation by SEM, each patch (0.3 × 0.3 cm) of the fresh infected and un-infected M. sextelata was fixed in 2.5% glutaraldehyde in 0.05 M phosphate-buffered saline (BPS, pH 7.2) at 4°C. After 24 hours, the samples were washed with deionised 0.1 M PBS for 7 min three times, then dehydrated in graded ethanol (50%,70%, 80%, 95%) for 15 min, respectively. Subsequently, the samples were dehydrated in 100% ethanol for 15 min three times and dried in a fume hood using critical point dryers (Autosamdri® 931, Tousimis, MD, USA) with CO2. Finally, the samples were sputter-coated with gold by an ion sputter coater (ISC150, SuPro Instruments, Shenzhen, China) with a voltage of 110 V, a frequency of 50/60 Hz, and a current of 10 mA under vacuum of lower than 1–2 Pa for 60 s. The samples were loaded onto the SEM (SU8010, Hitachi, Tokyo, Japan) and observed.
DNA extraction, PCR amplification, and sequencing
Genomic DNA of each strain was extracted from fresh mycelium grown on PDA after 7 days of growth following the rapid “thermolysis” method described by Zhang et al. (2010). For the amplification of SSU, ITS, LSU, RPB2, and TEF1-α gene fragments, the following primer pairs: NS1/NS4 primer pair for partial small subunit ribosomal RNA gene region (SSU), ITS4/ITS5 primer pair for internal transcribed spacer gene region (ITS) (White et al. 1990), LROR/LR5 for partial large subunit rRNA gene region (LSU), 983 F/2218 R for partial translation elongation factor 1-alpha gene region (TEF-1α) (Carbone and Kohn 1999); RPB2-5 F/RPB2-7 R for partial RNA polymerase II largest subunit gene region (RPB2) (Liu et al. 1999) was used. Each PCR reaction consisted of 12.5 μl 2× Taq PCR SuperMix (TianGen Biotech Co., Beijing, China), 1 μl of each forward and reverse primer (10 μM), 0.5 μl DMSO, 3 μl DNA template, and 7 μl double sterilised water. PCR reactions were performed in a fast thermal cycler (LongGene Co., Hangzhou, China), following the protocols described by Gu et al. (2020). The PCR products were sequenced by Beijing Tianyihuiyuan Bioscience and Technology after being evaluated by electrophoresis.
Phylogenetic analysis
SeqMan Pro v. 7.1.0 (DNASTAR Lasergene) was used to trim the low-quality bases at both ends of the raw forward and reverse reads and to assemble them. The newly obtained sequences were queried against the nuclear database of NCBI. For species delimitation, the aligned ITS sequence matrix of 58 taxa including our isolates, Diploöspora, and available species of Paecilomyces and its allied fungi, as well as Alternaria species (outgroup taxa) were used to construct the phylogenetic tree. The SSU, ITS, LSU, RPB2, and TEF sequences of available generic type species and reprehensive of Hypocreales and representative species of all accepted Hypocaceae from recent studies (Sun et al. 2017) were employed for multi-locus phylogenetic analysis. Gelasinospora tetrasperma, Neurospora crassa and Sordaria fimicola were chosen as the outgroup taxa. The alignments were generated by using MAFFT version 7.03 with the Q-INS-I strategy (Katoh and Standley 2013). Conserved blocks were selected from the initial alignments with Gblocks 0.91 b (Castresana 2000). The best nucleotide substitution model for each gene was determined by using jModeltest2.1.1 (Darriba et al. 2012). GTR+G + I was estimated as the best-fit model for ITS; RPB2, TN93 + G was estimated as the best-fit model for SSU; and LSU, TN93 + G + I as the best-fit model for TEF-1α under the output strategy of BIC. The multi-locus phylogenetic analyses included 1403 characters for SSU, 607 characters for ITS, 893 characters for LSU, 1044 characters for RPB2, and 907 characters for TEF. All characters were weighted equally, and gaps were treated as missing characters.
Maximum likelihood (ML) analyses were performed by RAxML2.0 (Edler et al. 2021), using the GTR+GAMMA+I model. The maximum likelihood bootstrap proportions (MLBP) were determined using 1000 replicates. Bayesian inference (BI) analyses were conducted with MrBayes v3.2.7 (Ronquist et al. 2012). Metropolis-coupled Markov Chain Monte Carlo (MCMC) searches were calculated for 10,000,000 generations, sampling every 100th generation with the best-fit model for each gene. Two independent analyses with six chains each (one cold and five heated) were carried out until the average standard deviation of the split frequencies dropped below 0.01. The initial 25% of the generations of MCMC sampling were discarded as burn-in. The refinement of the phylogenetic tree was used for estimating Bayesian inference posterior probability (PP) values. The tree was viewed in FigTree v1.4 (Rambaut 2012), and values of maximum likelihood bootstrap proportion (MLBP) greater than 50% and Bayesian inference posterior probabilities (BIPP), greater than 95% at the nodes, are shown along branches.
Relative divergence time estimation
Molecular dating analysis was performed using BEAST v1.10.4 (Suchard et al. 2018). The aligned data were partitioned for each SSU, ITS, LSU, RPB2, and TEF1 dataset, and these were loaded to BEAUti v1.10.4. to prepare the XML file. The data partitions were set with unlinked substitution and clock models to independently estimate each gene partition. Taxa sets were developed for each calibration of the common ancestor nodes, associated with the most recent common ancestor (TMRCA). The Hypocreales crown with a normal distribution (mean = 216, SD = 27.5, with 97.5% of CI = 269 MYA). Calibration of the core Clavicipitaceae, using a normal distribution (mean = 133.7, SD = 20.8, with 97.5% of CI = 174.5 MYA). Calibration of the Ophiocordyceps crown, using an exponential distribution (offset = 100, mean = 27.5, with 97.5% CI of 200 MYA) (Samarakoon et al. 2016; Hyde et al. 2017). The Yule process tree prior was used to model the speciation of nodes in the topology with a randomly generated starting tree. The analyses were performed for 100 million generations, with sampling parameters every 1000 generations. The effective sample sizes were checked in Tracer v.1.7.2 and the acceptable values are higher than 200. The first 10,000 trees (10%) representing the burn-in phase were discarded based on Tracer v.1.7.2, and 90,000 trees were combined in LogCombiner v1.10.4. The maximum clade credibility (MCC) tree was given by summarised data and estimated in TreeAnnotator v1.10.4. The molecular dating tree was viewed in FigTree v1.4 (Rambaut 2012). In the MCC tree, node bars indicate 90% confidence intervals for the divergence time estimates.
Results
Phylogenetic analyses
The phylogenetic trees showed that generic type species of Diploöspora rosea (DAOM 250100) and Paecilomyces variotii (CBS 101075) were positioned in the class Eurotiomycetes, while isolates of Diploöspora longispora and Paecilomyces penicillatus were placed in the class Sordariomycetes (Figure 1). Within Sordariomycetes, D. longispora (UAMH 340, UAMH 6404, UAMH 6367, strain 60319, and strain 60320), D. longispora var. cubensis (CBS 727.87), and P. penicillatus (CBS 448.69, IMI 186962) clustered together with maximum support (MLBP/BIBP = 100%/1.00, Figure 1). In the phylogenetic tree, those fungi also showed affinities with hypocrealean fungi, especially close to Hypomyces corticiicola (MLBP/BIBP = 100%/1.00, Figure 1).
Figure 1.

Phylogenetic analysis of Diploöspora longispora and Paecilomyces penicillatus based on ITS data set. The tree is rooted with three Alternaria species (Dothideomycetes). Bootstrap values higher than 50% from RAxML (BSML) (left) are given above the nodes. Bayesian posterior probabilities greater than 0.95 are indicated (BYPP) (right). Hyphens indicate bootstrap values less than 50% or Bayesian posterior probability values lower than 0.90. T indicates the type. The type species of Diploöspora and Paecilomyces are in blue.
To determine the family placement of Diploöspora longispora and Paecilomyces variotii, a phylogenetic tree was constructed with a sequence matrix of five alignment files including SSU (1043 bp), ITS (607), LSU (884 bp), EF1-α (907 bp), and RPB2 (1044 bp) sequence data (a total of 4853 characters) from 111 taxa of Hypocreales and three outgroup taxa (Gelasinospora tetrasperma, Neurospora crassa, and Sordaria fimicola; Figure 2). The phylogenetic tree well explained the phylogenetic relationship within Hypocreales. There were 15 clades formed in Hypocreales corresponding to the families Bionectriaceae, Calcarisporiaceae, Clavicipitaceae, Cordycipitaceae, Flammocladiellaceae, Cocoonihabitaceae, Hypocreaceae, Nectriaceae, Niessliaceae, Ophiocordycipitaceae, Polycephalomycetaceae, Sarocladiaceae, Stachybotryaceae, and the clade comprising Diploöspora longispora and Paecilomyces penicillatus. Diploöspora longispora and P. penicillatus are phylogenetically distinct from the type species of their respective genera and are better accommodated in as-yet undescribed genera. They are hereafter referred to as Pseudodiploöspora longispora and Zelopaecilomyces penicillatus, respectively, and formally described below. Pseudodiploöspora longispora and Z. penicillatus form a strongly supported (MLBP/BIBP = 100%/1.00; Figure 2) distinct clade sister to Hypocreaceae with robust support (MLBP/BIBP = 94%/1.00; Figure 2). Based on its phylogenetic distinction from Hypocreaceae, this clade is described below as Pseudodiploösporeaceae fam. nov.
Figure 2.

Multi-locus phylogenetic analysis of Hypocreales based on a combined SSU, ITS, LSU, TEF, and RPB2 data set. The tree is rooted with Gelasinospora tetrasperma, Neurospora crassa, and Sordaria fimicola. Bootstrap values higher than 50% from RAxML (BSML) (left) are given above the nodes. Bayesian posterior probabilities greater than 0.90 are indicated (BYPP) (right). Hyphens indicate bootstrap values less than 50% or Bayesian posterior probability values lower than 0.90. The generic type species are in blue.
Relative divergence time estimation
According to the divergence time estimates, the crown age of Hypocreales is around 206 (165–246) MYA (Figure 3). Based on our analysis, Sarocladiaceae was the earlier diverged family in Hypocreales, which diverged from other hypocrealean fungi at approximately 159 MYA. Flammocladiellaceae and Tilachlidiaceae were the youngest families within Hypocreales, which diverged from each other about 116 MYA. In general, the divergence time for the currently accepted 15 families is within the range of 116–159 MYA, suggesting that a family can at best be as young as 116 MYA. In the MCC tree, our newly generated Pseudodiploösporeaceae diverged from Hypocreaceae at about 129 MYA, falling within the temporal band of families.
Figure 3.

The MCC tree of Hypocreales, including some representative strains of Sordariales, was obtained from a Bayesian approach (BEAST). Bars correspond to the 95% highest posterior density (HPD) intervals. The fossil minimum age constraints and second calibrations used in this study are marked with green dots. The divergence time of orders is marked in purple dots and families with blue dots. The generic type species are in blue.
Taxonomy
Pseudodiploösporeaceae Jing Z. Sun, X.Z. Liu & H.W. Liu, fam. nov.
Fungal name: FN 571280
Etymology: Pseudodiploöspor-, from the genus name Pseudodiploöspora, and -aceae is the family suffix
Type genus: Pseudodiploöspora Jing Z. Sun, X.Z. Liu & H.W. Liu, gen. nov.
Description: Saprobic or fungicolous; Sexual morph: Undetermined. Asexual morph: Colonies on natural substrate effuse, whitish. Mycelia, superficial or immersed; Hyphae branched, septate, hyaline. Conidiophores micronematous to macronematous, mononematous, penicillate. Conidiogenous cells sympodial, acropetal, basipetal, hyaline. Conidia cylindrical, ellipsoidal, limoniform, solitary, or catenate in simple or branched chains. Ramoconidia cylindrical or fusiform, aseptate or septate, truncate at the base, with terminal scars.
Note: Both phylogenetic analysis and molecular clock evidence based on SSU, ITS, LSU, TEF, and RPB2 sequence data support Pseudodiploösporeaceae as a sister group of Hypocreaceae. The MCC tree estimates that Pseudodiploösporeaceae split from Hypocreaceae around 129 MYA, falling within the temporal band of families (50–150 MYA) (Hyde et al. 2017). Therefore, Pseudodiploösporeaceae is introduced as a new family within Hypocreales, to accommodate Pseudodiploöspora and Zelopaecilomyces.
Pseudodiploöspora Jing Z. Sun, X.Z. Liu & H.W. Liu, gen. nov.
Fungal name: FN 571281
Etymology: pseudo, in Latin, meaning “false or spurious thing”, referring to members of this genus being morphologically similar to Diploöspora but phylogenetically distinct to the Diploöspora species
Type species: Pseudodiploöspora longispora (Matsush.) Jing Z. Sun, X.Z. Liu & H.W. Liu, comb. nov.
Description: Saprobic or fungicolous; Sexual morph: Undetermined. Asexual morph: Colonies on natural substrate effuse, whitish. Mycelia, superficial or immersed; Hyphae branched, septate, hyaline. Conidiophores micronematous to macronematous, aseptate or septate. Conidiogenous cells sympodial, acropetal, hyaline. Conidia cylindrical, ellipsoidal, fusiform, catenate in simple or branched chains, hyaline. Ramoconidia cylindrical or fusiform, truncate at the base, with terminal scars, hyaline.
Note: The genus Diploöspora was established by Grove (1916) with Diploöspora rosea as the type species. Phylogenetic evidence supported that D. rosea is an onygenalean fungus within Eurotiomycetes (Tanney et al. 2015). Diploöspora longispora and its two variants, D. longispora var. longispora and D. longispora var. cubensis, were isolated originally from the fallen leaves (Matsushima 1976; Castañeda 1987). Based on an ITS BLAST query, Tanney et al. (2015) proposed that D. longispora and its varieties belong to the order Hypocreales, and reached affinity with Hypocreaceae. While, our phylogenetic analysis based on the ITS sequence data also showed strains of D. longispora (UAMH 340, UAMH 6404, UAMH 6367, strain 60,319, and strain 60,320), and D. longispora var. cubensis (CBS 727.87, IMI 186962) grouped with strong support (MLBP/BIBP = 100%/1.00, Figure 1) in Sordariomyetes rather than in Eurotiomyetes. In our multi-locus phylogenetic tree, those taxa clustered in a distinct clade within Hypocreales but do not belong to Hypocreaceae (Figure 2), representing a new genus rank. Morphologically, despite those taxa and Diploöspora producing conidial chains, they are distinct from Diploöspora in acropetal conidiogenesis and the shape and size of conidia. Pseudodiploöspora is therefore introduced herein to accommodate those species misplaced in Diploöspora.
Pseudodiploöspora fungicola (R.F. Castañeda) Jing Z. Sun, X.Z. Liu & H.W. Liu, comb. nov.
Fungal name: FN 571282
Basionym: Diploöspora fungicola R.F. Castañeda, Fungi Cubenses II: 4 (1987)
Type: INIFAT C86/132 (Holotype)
Description: See the original description in Castañeda Ruiz, R.F. (1987), Fungi Cubenses II, p. 22
Substrate/Host: On dead basidioma of Auricularia
Distribution: Cuba
Note: There is no available sequence of Diploöspora fungicola, and its morphological characters are highly similar to Pseudodiploöspora longispora. Additionally, this species colonised the basidioma of Auricularia, which suggests a similar fungicolous ecology relating it to Pseudodiploöspora longispora (Castañeda Ruiz, R.F. 1987).
Pseudodiploöspora longispora (Matsush.) Jing Z. Sun, X.Z. Liu & H.W. Liu, comb. nov. Figures 4 and 5
Figure 4.
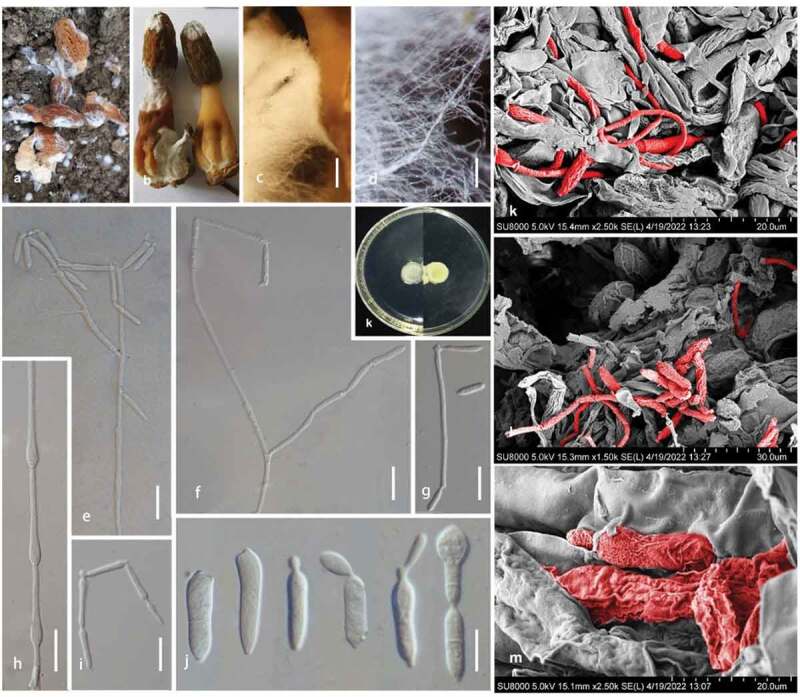
Pseudodiploöspora longispora (CGMCC 3.23768). (a, b) Pseudodiploöspora longispora and its host fungus (Morchella sextelata). (c, d) Mycelia on a fruiting body of M. sextelata. (e–h) Conidiophores with conidia. (i) Conidiogenous cell. (j) Conidiogenous scars and conidia. (k–m) Hypha and conidia of P. longispora (in red) across the fruiting body of M. sextelata. Scale bars: c = 200 μm; d = 100 μm; e-g, i = 25 μm; h, j = 10 μm.
Figure 5.
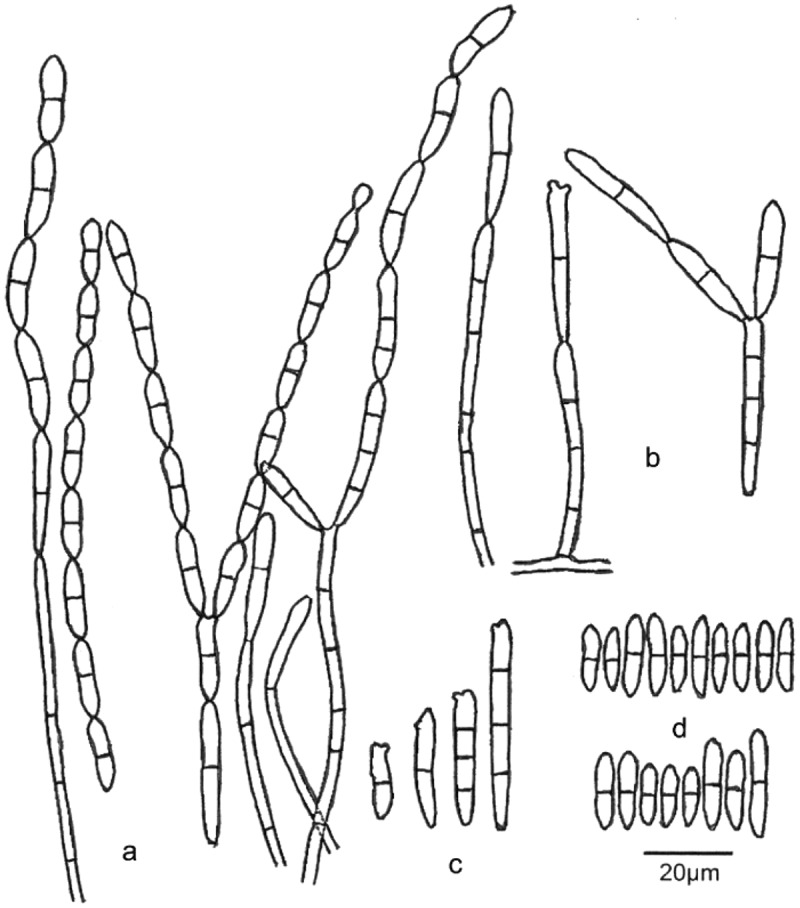
Pseudodiploöspora longispora (Diploöspora longispora, INIFAT C87/58, Holotype!). (a, b) Conidiophores with conidia. (). Conidiogenous cell. (d) Conidiogenous scars and conidia. Scale bars = 20 μm. Redrawn from Matsushima (1975).
Fungal name: FN 571283
Basionym: Diploöspora longispora Matsush., Icones Microfungorum a Matsushima lectorum: 61 (1975) Figure 5
Synonym: Diploöspora longispora var. longispora Matsush., Icones Microfungorum a Matsushima lectorum: 61 (1975)
Type: INIFAT C87/58 (Holotype)
Description: See the original description in Matsushima, T. (Matsushima and Matsushima 1976), Icones Microfungorum a Matsushima Lectorum, p. 61.
Substrate/Host: On dead leaf of Colocasia esculenta var. antiquorum, Japan (Matsushima and Matsushima 1976). On the fruiting body of cultivated Morchella spp., China (CGMCC 3.23768, CGMCC 3.23769, CGMCC 3.23770, CGMCC 3.23771). Skin and foot, Canada (UAMH 340) Canada (https://www.uamh.ca/index.html)
Distribution: Japan, China
Note: Diploöspora longispora was first isolated from a dead leaf of Colocasia esculenta var. antiquorum in Japan (Matsushima and Matsushima 1976). We introduce a new combination of Pseudodiploöspora longispora to accommodate D. longispora. Both analyses in Tanney et al. (2015) and this study suggested that Pseudodiploöspora longispora is most closely related to Paecilomyces penicillatus. However, the latter differs from Pseudodiploöspora longispora in the penicillate conidiophore, and basipetal conidiogenesis, as well as the shape and size of conidia (Figures 5 and 6). We did not treat Paecilomyces penicillatus as a synonym of Pseudodiploöspora longispora herein because of the great morphological differences.
Figure 6.
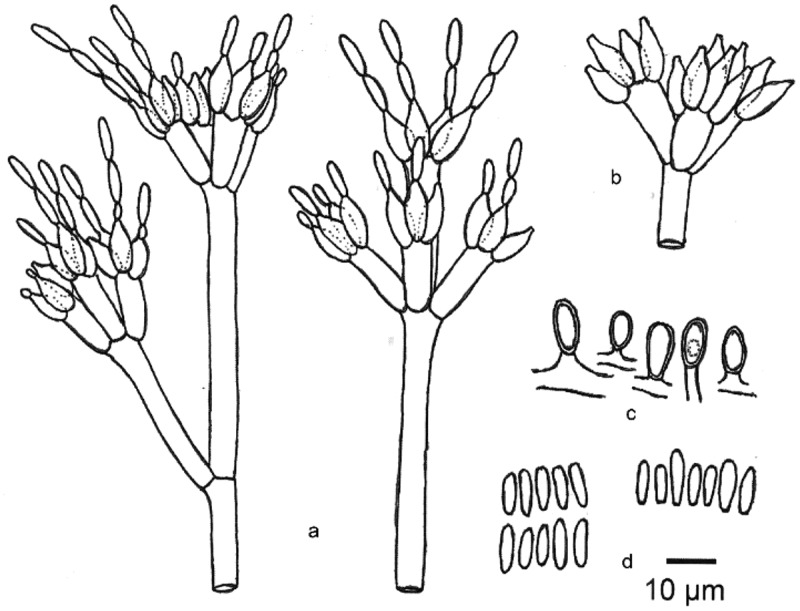
Zelopaecilomyces penicillatus (CBS 448.69, ex-type strain). (a) Conidiophores with conidia (CBS 448.69). (b) Phialides (type Spicaria penicillate). (c) Chlamydospores. (d) Conidia. Scale bars = 10 μm. Redrawn from Samson (1974).
Pseudodiploöspora cubensis (R.F. Castañeda) Jing Z. Sun, X.Z. Liu & H.W. Liu, comb. nov. Figures 4 and 5
Fungal name: FN 5712997
Synonym: Diploöspora longispora var. cubensis R.F. Castañeda, Fungi Cubenses II: 5 (1987)
Type: CBS 727.87 (ex-type strain)
Description: See the original description in Castañeda (1987), Fungi Cubenses II.:1-22
Substrate/Host: On fallen leaves of Leguminosae: Cuba (Castaneda 1987). On porcupine dung in a cave (Including UAMH 6367, UAMH 6404) (https://www.uamh.ca/index.html)
Distribution: Cuba
Note: Pseudodiploöspora cubensis was originally obtained from the fallen leaves of Leguminosae in Cuba (Castaneda 1987). The ITS sequence of CBS 727.87 is 96% similar to Pseudodiploöspora longispora (identities, 514/537, gaps, 5/537). Additionally, regarding the ellipsoidal conidia of Pseudodiploöspora cubensis against cylindrical or ramoconidia of P. longispora, as well as the original isolation resource and location, we introduce a new combination of Pseudodiploöspora cubensis to accommodate D. longispora var. cubensis.
Pseudodiploöspora zinniae (Matsush.) Jing Z. Sun, X.Z. Liu & H.W. Liu, comb. nov.
Fungal name: FN 571284
Basionym: Diploöspora zinniae Matsush., Matsushima Mycological Memoirs 2: 8 (Matsushima 1981)
Type: MBT 70959
Description: See the original description in Matsush. (1981), Matsushima Mycological Memoirs 2, p. 8
Substrate/Host: Seed of Zinnia elegans
Distribution: Japan
Note: There is no available sequence of Diploöspora zinnia, and we transfer this fungus to Pseudodiploöspora based on its sympodial, acropetal conidiogenesis, and the cylindrical-fusiform conidia (Matsushima 1981).
Zelopaecilomyces Jing Z. Sun, X.Z. Liu & H.W. Liu, gen. nov.
Fungal name: FN 571285
Etymology: Zelo, meaning “emulation”, refers to members of this genus being morphologically similar to Paecilomyces but phylogenetically distinct from the true Paecilomyces species
Type species: Zelopaecilomyces penicillatus (Samson) Jing Z. Sun, X.Z. Liu & H.W. Liu, comb. nov.
Description: Saprobic or fungicolous; Sexual morph: Undetermined. Asexual morph: Colonies on natural substrate effuse, whitish. Mycelia, superficial or immersed; Hyphae branched, septate, hyaline. Conidiophores mononematous, penicillate, with whorls of phialides. Phialides cylindrical, basal portion with distinct neck. Conidiogenous cells basipetal, hyaline. Conidia cylindrical, ellipsoidal, solitary, or catenate in simple or in chains, aseptate, truncate at the base, with terminal scars. Chlamydospores produced submerged in the agar, single, ellipsoidal to pyriform, aseptate.
Note: The genus Paecilomyces was introduced by Bainier (1907) with Paecilomyces variotii as the type species. The type species, P. variotii, and its thermophilic relatives were placed in Eurotiales (Eurotiomycetes), while entomopathogenic mesophilic species were placed in Hypocreales (Sordariomycetes) under the genus Isaria but did not include Paecilomyces penicillatus (Luangsa et al. 2004; Luangsa-Ard et al. 2005). Those taxa placed in Isaria were accepted in Samsoniella (Hypocreales, Cordycipitaceae) (Mongkolsamrit et al. 2018). Our phylogenetic analyses showed that Z. penicillatus (CBS 448.69, ex-type strain) was positioned in Pseudodiploösporeaceae (Figure 2). Despite a more than 99% similarity of the SSU (identities, 1589/1590, gap, 1/1590) and ITS sequence (identities, 502/505; gaps, 3/505 gaps) between Z. penicillatus (CBS 448.69) and Pseudodiploöspora loogispora, respectively. The ITS of Z. penicillatus is 4% different from Pseudodiploöspora cubensis (identities, 471/477; gaps, 6/477). Additionally, Z. penicillatus differs from both P. cubensis and P. loogispora in having penicillate conidiophores and basipetal conidiogenesis. Herein, we introduce Zelopaecilomyces for the accommodation of P. penicillatus based on its morphological distinctions.
Zelopaecilomyces penicillatus (Höhn.) Jing Z. Sun, X.Z. Liu & H.W. Liu, comb. nov. Figure 6
Fungal name: FN 571286
Basionym: Paecilomyces penicillatus (Höhn.) Samson, Studies in Mycology 6: 72 (1974)
Spicaria penicillata Höhn., Annales Mycologici 2 (1): 56 (1904)
Type: ex-type strain CBS 448.69
Description: See the original description in Samson (1974), Paecilomyces and some allied Hyphomycetes, Studies in Mycology, 6, p.72
Substrate/Host: Peridia of Arcyria cinerea, rotting Agaricus bisporus mushroom
Distribution: Austria, Belgium
Note: Zelopaecilomyces penicillatus (Spicaria penicillata) was introduced by Höhnel (1904) based on its morphological characteristics. It was first isolated from the peridia of the myxomycete Arcyria cinerea and later isolated from a rotten Agaricus bisporus mushroom, with the resulting strain (CBS 448.69) treated as the ex-type strain (Samson 1974). Herein, we introduce a new combination, Zelopaecilomyces penicillatus, in consideration of the distinct phylogenetic position and morphological features of P. penicillatus.
Discussion
A combination of phylogenetic analyses and divergence time estimation has been widely used in solving the classification schemes and higher ranking of taxa (Hyde et al. 2017). According to this polyphasic approach, a large number of taxonomic positions of fungi have been refined (Hyde et al. 2020; He et al. 2022). Hyde et al. (2020) gave an update of Sordariomycetes based on phylogenetic analyses and divergence time estimation. According to their results, Hypocreales contained 14 families: Bionectriaceae, Calcarisporiaceae, Clavicipitaceae, Cocoonihabitaceae, Cordycipitaceae, Flammocladiellaceae, Hypocreaceae, Myrotheciomycetaceae, Nectriaceae, Niessliaceae, Ophiocordycipitaceae, Sarocladiaceae, Stachybotryaceae, and Tilachlidiaceae. Both our multi-locus phylogeny and divergence time evidence reveal the proposed natural classification of Hypocreales. Multi-locus phylogeny reals a family rank for Pseudodiploösporeaceae because its taxa formed a strongly supported and distinct clade sister to Hypocreaceae. Hyde et al. (2017) introduced a temporal banding for Ascomycota, and time ranges of 150–250 MYA and 50–150 MYA were recommended as the boundary for orders and families, respectively. Our MCC results presented that the crown age of Hypocreales is around 206 (165–246) MYA (Figure 3), which concurs with the previous results (Hyde et al. 2017, 2020). Within Hypocreales, the divergence time for currently accepted families is within the range of 116–159 MYA suggesting that a family can at best be as young as 116 MYA in Hypocreales. Divergence time showed that the family Pseudodiploösporeaceae divorced from Hypocreaceae about 129 MYA, falling within the temporal band of families. Additionally, Polycephalomycetaceae was recently introduced as a new family based on a concatenated matrix of six genetic markers (SSU, ITS, LSU, RPB1, RPB2, and TEF) (personal communication), both our phylogenetic tree and MCC tree also support its family rank in Hypocreales herein. Vu et al. (Vu et al. 2019) proposed a taxonomic threshold predicted for filamentous fungal identification, and 88.5% similarity of ITS barcodes was suggested for family rank. A BLAST querying the ITS sequence of species from Pseudodiploösporeaceae presented less than 89% similarity against that species from Hypocreales, which also supported distinct family rank for Pseudodiploösporeaceae.
The taxonomic position of Diploöspora Grove was confirmed as a member of Eurotiomycetes by re-examination of its generic type species D. rosea (Tanney et al. 2015). Several species including Pseudodiploöspora longispora (previously known as Diploöspora longispora) and Pseudodiploöspora cubensis (previously known as Diploöspora longispora var. cubensis) placed previously in Diploöspora were shown an affinity for Hypocrealean fungi (Sordariomycetes) based on the phylogenetic analysis (Luangsa-Ard et al. 2004, 2005; Tanney et al. 2015). Our phylogenetic analysis also supported that P. longispora and P. cubensis were more closely related to Hypocreaceae (Figure 1–2). In our multi-locus phylogenetic tree, those taxa clustered in a distinct clade within Hypocreales but were outside of the core Hypocreaceae (Figure 2), representing a new family and subsequent genera. Pseudodiploöspora is therefore introduced herein to accommodate those species misplaced in Diploöspora concerning the original nomenclature. Pseudodiploöspora is distinct from Diploöspora in having head-to-tail (acropetal) arrays of conidiogenesis against the latter of tail-to-head (basipetal) arrays of conidiogenesis. Additionally, the conidia of Pseudodiploöspora are longer but more slender than that of Diploöspora (Tanney et al. 2015). We introduce P. longispora and P. cubensis for accepting D. longispora and D. longispora var. cubensis regarding the 95.66% similarity of ITS sequence between P. cubensis (CSB 727.877) and other P. longispora isolates. Despite lacking molecular data on Diploöspora fungicola and Diploöspora zinnia, we enrolled them in Pseudodiploöspora according to the morphological features in the original description. Diploöspora coprophilia with phialides and producing subglobose conidia is unlikely to be related to Diploöspora rosea (Tanney et al. 2015) and Pseudodiploöspora longispora. Its taxonomic position needs to be further demonstrated. It was suggested that Diploöspora indica producing brown conidiophores may be better placed in Parapleurotheciopsis but not in Diploöspora (Tanney et al. 2015). We also excluded D. indica from Pseudodiploöspora in consideration of the brown conidiophore of the fungus.
Both analyses by Tanney et al. (2015) and this study presented that P. longispora is most closely related to Z. penicillatus (CBS 448.86) (Figure1–2). Vu et al. (2019) proposed a 99.6% similarity of ITS barcode for a species taxonomic threshold. When comparing the similarity of the ITS sequence, Z. penicillatus presented less than 98.63% similarity to that of P. longispora, and showed less than 94.3% similarity to that of P. cubensis (KT279809, CBS 727.87, ex-living type, previously known as Diploöspora longispora var. cubensis), respectively. There were no available EF1-α and RPB2 sequences in GenBank. We did not compare the similarity of EF1-α and RPB2 sequences. However, P. penicillatus differs from the latter in having penicillate conidiophores and basipetal conidiogenesis. Herein, we introduce a new genus, Zelopaecilomyces, for the accommodation of P. penicillatus.
The taxonomic position of Paecilomyces was revised and refined by phylogenetic analyses, habitats, host range, etc. (Luangsa-Ard et al. 2004, 2005). The entomopathogenic mesophilic species were placed in the class Sordariomycetes belonging to Hypocreales (Luangsa et al. 2004, 2005). Generally, those taxa were placed in Isaria, which were accepted by a new genus Samsoniella (Hypocreales, Cordycipitaceae) currently (Mongkolsamrit et al. 2018). Our phylogenetic analyses presented that Z. penicillatus (CBS 448.69, ex-type strain) was positioned in Pseudodiploösporeaceae (Figure 2), which was owing to a higher similarity of the SSU and ITS sequence between P. penicillatus and D. longispora. However, previous phylogenetic studies have evidenced that the SSU and ITS sequence data alone are insufficient to provide good resolution in most of the groups in Sordariomycetes(Hyde et al. 2020). Morphologically, Z. penicillatus differs from P. longispora by penicillate conidiophore, basipetal conidiogenesis, and the shape and size of conidia (Figure 5 and 6). Additionally, the divergence time revealed that Z. penicillatus diverged from P. longispora about 14 MYA (Figure 3). Tanney et al. (2015) thought that P. longispora and Z. penicillatus may be two extremes of a continuum, However, we treat P. penicillatus as a distinct species other than a synonym of D. longispora not only relying on morphological differences but also following the divergence time.
Both P. longispora and Z. penicillatus were originally isolated from decaying leaves and found on the rotten mushroom successively (Samson 1974; Matsushima 1976; Castaneda 1987). He et al. (2017) identified Z. penicillatus (as Paecilomyces penicillatus) as the causing agent of the white mould disease of cultivated Morchella only relying on ITS phylogenetic analysis but lacking morphological evidence. Liu et al. (2019) reported P. longispora (as D. longispora) infecting cultivated Morchella, resulting in pileus rot but not offered the typical morphological feature of P. longispora. The phylogenetic analyses in both Tanney et al. (2015) and this study revealed that ITS and SSU are unable to adequately distinguish D. longispora and Z. penicillatus, but our study offered robust morphological evidence on the taxonomy of P. longispora and Z. penicillatus. Since P. longispora has been reported as a serious fungal pathogen (Hyde et al. 2017; Liu et al. 2018), reliably taxonomic information will facilitate tracing the origin and understanding of pathogenesis.
Acknowledgements
This research was supported by the Natural Science Foundation of China (no. 32072645). The authors would like to thank Chun-Li Li from the Institute of Microbiology, Chinese Academy of Sciences, for their instructions on the sample process and observations by SEM. The authors would like to thank Prof. Liwei Zhou for his suggestions on phylogenetic analysis and divergence time estimation. We also thank Prof. Rob Samson for the permission to redraw the plates of Paecilomyces penicillatus.
Funding Statement
This work was supported by the The Natural Science Foundation of China [no. 32072645].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Carbone I, Kohn LM.. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 91(3):553–556. doi: 10.1080/00275514.1999.12061051 [DOI] [Google Scholar]
- Castaneda R. 1987. Fungi cubenses II: instituto de investigaciones fundamentales en agricultura tropical “Alejandro Humboldt”. Cuba (Academia de Ciencas de Cuba): la Habana. [Google Scholar]
- Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evo. 17(4):540–552. doi: 10.1093/oxfordjournals.molbev.a026334 [DOI] [PubMed] [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Meth. 9(8):772. doi: 10.1038/nmeth.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edler D, Klein J, Antonelli A, Silvestro D. 2021. raxmlGUI 2.0: a graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol Evol. 12(2):373–377. doi: 10.1111/2041-210X.13512 [DOI] [Google Scholar]
- Grove WB. 1916. New or noteworthy fungi. Part V. The London J Bot. 54:220. [Google Scholar]
- Guo MP, Chen K, Wang G, Bian YB. 2016. First report of stipe rot disease on Morchella importuna caused by Fusarium incarnatum-F. equiseti species complex in China. Plant Dis. 100:2530. doi: 10.1094/PDIS-05-16-0633-PDN [DOI] [Google Scholar]
- Gu X, Wang R, Sun Q, Wu B, Sun JZ. 2020. Four new species of Trichoderma in the Harzianum clade from northern China. MycoKeys. 73:109–132. doi: 10.3897/mycokeys.73.51424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Li CC, Cai YL, Zhang Y, Bian YB, Liu W. 2018. First report of pileus rot disease on cultivated Morchella importuna caused by Diploöspora longispora in China. J Gen Plant Pathol. 84(1):65–69. doi: 10.1007/s10327-017-0754-3 [DOI] [Google Scholar]
- He MQ, Zhao RL, Liu DM, Denchev TT, Begerow D, Yurkov A, Kemler M, Millanes AM, Wedin M, McTaggart AR, et al. 2022. Species diversity of Basidiomycota. Fungal Divers. 114(1):281–325. doi: 10.1007/s13225-021-00497-3 [DOI] [Google Scholar]
- Hyde KD, Maharachchikumbura SSN, Hongsanan S, Samarakoon MC, Lücking R, Pem D, Harishchandra D, Jeewon R, Zhao RL, Xu JC, et al. 2017. The ranking of fungi: a tribute to David L. Hawksworth on his 70th birthday. Fungal Divers. 84(1):1–23. doi: 10.1007/s13225-017-0383-3 [DOI] [Google Scholar]
- Hyde KD, Norphanphoun C, Maharachchikumbura SSN, Bhat, DJ, Jones, EBG, Bundhun, D, Chen, YJ, Bao, DF, Boonmee, S, Calabon, MS, Chaiwan, N, Chethana, KWT, Dai, DQ, Dayarathne, MC, Devadatha, B, Dissanayake, AJ, Dissanayake, LS, Doilom, M, Dong, W, Fan, XL, Goonasekara, AJ, Dissanayake LS, Doilom M, Dong W, Fan XL, Goonasekara ID, Hongsanan S, Huang SK, Jayawardena RS, Jeewon R, Karunarathna A, Konta S, Kumar V, Lin CG, Liu JK Liu NG, Luangsa-ard J, Lumyong S, Luo ZL, Marasinghe DS, McKenzie EHC, Niego AGT, Niranjan M, Perera RH, Phukhamsakda C, Rathnayaka AR, Samarakoon MC, Samarakoon SMBC, Sarma VV, Senanayake IC, Shang QJ, Stadler M, Tibpromma S, Wanasinghe DN, Wei DP, Wijayawardene NN, Xiao YP, Yang J Zeng XY, Zhang SN, Xiang MM. 2020. Refined families of Sordariomycetes. Mycosphere. 11(1):305–1059. doi: 10.5943/mycosphere/11/1/7 [DOI] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780. doi: 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan YF, Cong QQ, Wang QW, Tang LN, Li XM, Yu QW, Cui X, An XR, Kong FH, Li XD. 2020. First Report of Cladobotryum protrusum causing cobweb disease on cultivated Morchella importuna. Plant Dis. 104(3):977–978. doi: 10.1094/PDIS-07-14-0757-PDN [DOI] [Google Scholar]
- Liu W, Cai YL, He PX, Ma XL, Bian YB. 2019. Occurrence and control of pests and diseases in field cultivation of Morchella mushrooms. Acta Edulis Fungi. 26(2):128–134. doi: 10.16488/j.cnki.1005-9873.2019.02.018 [DOI] [Google Scholar]
- Liu QZ, Ma HS, Zhang Y, Dong CH. 2018. Artificial cultivation of true morels: current state, issues, and perspectives. Crit Rev Biotechnol. 38(2):259–271. doi: 10.1080/07388551.2017.1333082 [DOI] [PubMed] [Google Scholar]
- Liu YJ, Whelen S, Hall BD. (1999). Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Mol Biol Evol. 16(12), 1799–1808. 10.1093/oxfordjournals.molbev.a026092 [DOI] [PubMed] [Google Scholar]
- Luangsa-ard JJ, Hywel-Jones NL, Manoch L, Samson RA. 2005. On the relationships of Paecilomyces sect Isarioidea Species. Mycol Res. 109(Pt 5):581–589. doi: 10.1017/S0953756205002741 [DOI] [PubMed] [Google Scholar]
- Luangsa-ard JJ, Hywel-Jones NL, Samson RA. 2004. The polyphyletic nature of Paecilomyces sensu lato based on 18S-generated rDNA phylogeny. Mycologia. 96(4):773–780. doi: 10.1080/15572536.2005.11832925 [DOI] [PubMed] [Google Scholar]
- Masaphy S. 2022. First report on Purpureocillium lilacinum infection of indoor-cultivated morel primordia. Agriculture. 12(5):695. doi: 10.3390/agriculture12050695 [DOI] [Google Scholar]
- Matsushima T. 1976. Icones Microfungorum a Matsushima Lectorum. Mycologia. 68(4):955. doi: 10.2307/3758819 [DOI] [Google Scholar]
- Mongkolsamrit S, Noisripoom W, Thanakitpipattana D, Wutikhun T, Spatafora JW, Luangsa A. 2018. Disentangling cryptic species with Isaria-like morphs in Cordycipitaceae. Mycologia. 110(1):230–257. doi: 10.1080/00275514.2018.1446651 [DOI] [PubMed] [Google Scholar]
- Pilz D, McLain R, Alexander S, Villarreal-Ruiz L, Shannon B, Wurtz TL, Parks CG, McFarlane E, Baker B, Molina R, et al. 2017. Ecology and management of morels harvested from the forests of western North America. Portland (OR): U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station; p. 166. [Google Scholar]
- Rambaut A. 2012. FigTree v1. 4. Molecular evolution, phylogenetics and epidemiology. Edinburgh: University of Edinburgh, Institute of Evolutionary Biology. [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542. doi: 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarakoon MC, Hyde KD, Promputtha I, Ariyawansa HA, Hongsanan S. 2016. Divergence and ranking of taxa across the kingdoms Animalia, Fungi and Plantae. Mycosphere. 7(11):1678–1689. doi: 10.5943/mycosphere/7/11/5 [DOI] [Google Scholar]
- Samson RA. 1974. Paecilomyces and some allied Hyphomycetes. Stud Mycol. 6:1–119. [Google Scholar]
- Suchard MA, Lemey P, Baele G, Ayres DL, Drummond AJ, Rambaut A. 2018. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 4(1):vey016. doi: 10.1093/ve/vey016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JZ, Li YL, Lin CG, Tian Q, Zhao Q, Xiao YP, Hyde KD, Nilthong S. 2019. Fifteen fungicolous Ascomycetes on edible and medicinal mushrooms in China and Thailand. Asian J Mycol. 2(1):129–169. doi: 10.5943/ajom/2/1/7 [DOI] [Google Scholar]
- Sun JZ, Liu XZ, Hyde KD, Zhao Q, Maharachchikumbura SS, Camporesi E, Nilthong S, Lumyong S, Lumyong S. 2017. Calcarisporium xylariicola sp. nov. and introduction of Calcarisporiaceae fam. nov. in Hypocreales. Mycol Prog. 16(4):433–445. doi: 10.1007/s11557-017-1290-4 [DOI] [Google Scholar]
- Sun JZ, Liu XZ, McKenzie EHC, Jeewon R, Liu JK, Zhang XL, Zhao Q, Hyde KD. 2019a. Fungicolous fungi: terminology, diversity, distribution, evolution, and species checklist. Fungal Divers. 95(1):337–430. doi: 10.1007/s13225-019-00422-9 [DOI] [Google Scholar]
- Tanney JB, Nguyen HDT, Pinzari F, Seifert KA. 2015. A century later: rediscovery, culturing and phylogenetic analysis of Diploöspora rosea, a rare onygenalean hyphomycete. Anton Leeuw Int J G. 108(5):1023–1035. doi: 10.1007/s10482-015-0555-7 [DOI] [PubMed] [Google Scholar]
- Tietel Z, Masaphy S. 2018. True morels (Morchella)-nutritional and phytochemical composition, health benefits and flavor: a review. Crit Rev Food Sci Nutr. 58(11):1888–1901. doi: 10.1080/10408398.2017.1285269 [DOI] [PubMed] [Google Scholar]
- von HFXR. 1904. Mycologische fragmente. (Fortsetzung) [XLII-LXIX]. Ann Mycol. 2(1):38–60. [Google Scholar]
- Vu D, Groenewald M, de Vries M, Gehrmann T, Stielow B, Eberhardt U, Al-Hatmi A, Groenewald JZ, Cardinali G, Houbraken J, et al. 2019. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud Mycol. 92(1):135–154. doi: 10.1016/j.simyco.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XX, Peng JY, Sun L, Bonito G, Guo YX, Li Y, Fu YP. 2020. Genome Sequencing of Paecilomyces Penicillatus Provides Insights into Its Phylogenetic Placement and Mycoparasitism Mechanisms on Morel Mushrooms. Pathogens. 9(10):834. doi: 10.3390/pathogens9100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. London (UK): Academic Press; p. 315–322. [Google Scholar]
- Zhang Q, Cai W, Wang T, Sun YJ, Li TT, Fan GJ. 2019. Improvement of biological activity of morchella esculenta protein hydrolysate by microwave-assisted selenization. J Food Sci. 84(1):73–79. doi: 10.1111/1750-3841.14411 [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Zhang S, Liu XZ, Wen HA, Wang M. 2010. A simple method of genomic DNA extraction suitable for analysis of bulk fungal strains. Lett Appl Microbiol. 51(1):114–118. doi: 10.1111/j.1472-765X.2010.02867.x [DOI] [PubMed] [Google Scholar]
- Zhang WW, Zhang XL, Li K, Wang CS, Cai L, Zhuang WY, Xiang MC, Liu XZ. 2018. Introgression and gene family contraction drive the evolution of lifestyle and host shifts of hypocrealean fungi. Mycology. 9(3):176–188. doi: 10.1080/21501203.2018.1478333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ZX, Zhuang WY. 2013. Resources of nonlichenized fungicolous Ascomycota from China. Mycosystema. 32(suppl):79–88. in Chinese. [Google Scholar]


