ABSTRACT
Introduction: Familial Mediterranean Fever (FMF) is a hereditary autoinflammatory disease that significantly reduces occupational productivity and quality-of-life in affected patients. Italy has an estimated FMF prevalence of 1 in 60,000 people. While colchicine is the primary treatment for FMF, biologics are administered to intolerant and non-responder patients. Anakinra and canakinumab are the only biologics approved and reimbursed for FMF in Italy. Both medicines have demonstrated efficacy in FMF patients yet differ in treatment costs. This study aimed to perform a budget impact analysis (BIA) following anakinra’s reimbursement for FMF treatment, considering pharmaceutical costs from the Italian National Healthcare Service (NHS) perspective. Methods: A ‘Reference scenario’ (all patients treated with canakinumab) was compared to an ‘Alternative scenario’, with increased anakinra market shares. The target population was estimated based on the Italian population, epidemiological and market research data. Drugs costs were estimated based on Summary of Product Characteristics and net ex-factory prices. Sensitivity analyses were implemented to test results’ robustness. Results:The base case analysis showed an overall cumulative expenditure of €30,586,628 for ‘Reference scenario’ and € 16,465,548 for ‘Alternative scenario’. A cumulative savings of €14,121,080 (46.2%) was calculated over 3 years as a result of the reimbursement and increasing uptake of anakinra. The sensitivity analyses, even considering a discount of 50% for canakinumab, confirmed the base case results. Conclusions: Anakinra’s introduction, in FMF treatment, provides a financially sustainable option for Italian patients, with savings increasing according to greater use of anakinra.
KEYWORDS: Anakinra, budget impact analysis, cost savings, familial Mediterranean fever, Italy
Introduction
Familial Mediterranean Fever (FMF) is a hereditary autoinflammatory disease characterised by skin rash, abdominal and joints pain, and periodic febrile episodes associated with serositis and synovitis [1,2]. The disease has an acute onset lasting 12–72 h, and in the interval between attacks, patients are asymptomatic [3].
FMF is an autosomal recessive disease caused by point mutations in the Mediterranean Fever (MEFV) gene located on the short arm of chromosome 16, leading to deficient levels of functional pyrin.).
Pyrin acts in a specific inflammasome or alternatively as an interleukin 1 – beta (IL-1β) inflammation regulator. Mutations in the pyrin gene lead to an overproduction of interleukin-1, the pivotal cytokine in the pathogenesis of FMF [1,3].
Epidemiology
FMF is the most common periodic fever syndrome. It is closely linked to an ethnic distribution among populations in the Mediterranean basin, in particular individuals of Turkish, Armenian, Arabian, and non-Ashkenazi Jewish descent. Although the disease is fairly rare in the rest of the world, cases have been reported in some European countries including Belgium, England, France, Germany, Italy and Spain [4,5].
According to the Eurofever registry for autoinflammatory diseases, there is an estimated overall prevalence in Italy of 1 in 60,000 people [6], with most of the patients from Southern Italy, especially Sicily and Calabria [7].
Clinical symptoms
The initial onset of the disease occurs during childhood. Ninety percent of patients experience their first attack before the age of 20 and in 60% of the cases the onset of FMF occurs under the age of 10, with an average age of disease onset between 6 and 9 years. The onset of the disease is characterised by recurrent episodes of inflammation: fever, serositis, arthritis, pleurisy, erysipelas-like rash, and high levels of inflammatory reagents: C-reactive protein, erythrocyte sedimentation rate, and serum amyloid A associated with neutrophil leukocytosis [2,3,8].
The age of disease onset is significantly correlated with the clinical severity of the disease. More specifically, paediatric patients aged >12 years at disease onset showed a reduced frequency of fever attacks compared to younger patients (<5 years), who showed more severe attacks. The disease is also characterised by a diagnostic delay due to clinical diversity in the frequency, symptoms, and presentation of attacks [9].
Fever is a typical symptom of the disease; it occurs in more than 96% of the inflammatory attacks, with body temperature often reaching 38–40°C, and typically lasts 1–4 days [9]. In addition to fever, other symptoms, such as abdominal pain, may occur as a result of inflammation in the peritoneal area, and are observed in almost 90% of the patients with FMF. Moreover, chest pain associated with pericarditis and/or pleural effusion is observed in 50–60% of the patients. The second most common symptom is arthritis, which typically involves the joints of the lower limbs – hip, knee, and ankle – and is reported in 45% of the patients with FMF. Some patients with FMF experience arthralgia (joint pain) without presenting symptoms of arthritis. Rashes occurring in the form of erysipelas-like erythema are the most typical dermatological symptom experienced by patients with FMF. If FMF patients are not diagnosed or adequately treated, they may develop renal amyloidosis, resulting in kidney failure and death [2,3,8,9].
The burden of disease and quality-of-life
A recent study of Suticen et al. has shown that FMF leads to a considerable reduction in occupational productivity and quality-of-life (QoL) in affected patients [10].
Impairment in work productivity correlated with the number of attacks, disease activity, colchicine resistance, and disease-associated damage, was assessed as both absenteeism (9.3 ± 23.2% vs. 0.7 ± 2.6%, p = 0.013) and presenteeism (35.2 ± 32.6% vs. 9.6 ± 14.7%, p < 0.001), with significantly higher values in patients with FMF than in healthy subjects [10].
In addition, Kosan et al. reported that 38.9% caregivers of children with FMF-perceived moderate (25.6%) or severe (13.3%) care burden [11].
Diagnosis and treatments
Diagnosis of FMF is based on the identification of clinical manifestations, assessment of inflammatory indices during and between attacks, and genetic assays. Colchicine is the main treatment for FMF. Its mechanism of action suppresses pyrin inflammasome activation, thereby inhibiting the production of IL-1β. Colchicine is used as a prophylactic treatment to control the inflammatory attacks, but it is unable to completely prevent the febrile episodes [12,13].
Although it is usually well tolerated, up to 5–10% of the patients are considered resistant or respond inadequately to colchicine, while others (2–5%) are unable to tolerate the side effects of effective doses of colchicine (intolerance) [12–16].
In non-responders, biologics can be given in combination with colchicine. These include anti-IL-1 agents, since high IL-1 levels are associated with an excessive inflammatory response in patients with FMF [12]. In addition, as also reported by Suticen et al., the use of IL-1 antagonists may help to improve occupational productivity and QoL in FMF patients with frequent attacks [10].
Currently, the only biologics approved and reimbursed in Italy for the treatment of FMF patients are anakinra (KINERET) and canakinumab (ILARIS) [17].
Anakinra is a recombinant, non-glycosylated homologue of the human IL-1 receptor antagonist (IL-1Ra) that competes with IL-1α and β [12,13]. Anakinra is indicated and reimbursed in FMF patients (adults, adolescents, children, and infants 8 months of age and older with a body weight of 10 kg or more), in combination with colchicine (if appropriate) [18,19].
Canakinumab is a fully human anti-IL-1β monoclonal antibody [12,20] which is indicated and reimbursed (in combination with colchicine if appropriate) in 2 years and older FMF patients [21,22].
Although studies have demonstrated the efficacy of both anakinra and canakinumab in reducing excessive inflammation in the management of the disease [20,23], the two treatments have different costs [24].
Objectives
Both anakinra and canakinumab have demonstrated efficacy in treating patients with FMF. However, considering the high price difference between the two biologic drugs, this study aimed to estimate a) the costs of the drugs per patient/year; and b) the budget impact following the reimbursement of anakinra, from the perspective of the Italian National Health Service (NHS).
Methods
The budget impact analysis (BIA) was carried out in accordance with the Guidelines issued by a) the Professional Society for Health Economics and Outcomes Research (ISPOR) [25,26] and b) the Italian Medicines Agency (Agenzia Italiana del Farmaco – AIFA) [27].
The methodology consists of a BIA, with a 3-year time horizon, comparing a ‘Reference scenario’ in which canakinumab has a 100% market share with an ‘Alternative Scenario’ in which anakinra has an increasing market share (a) Year 1 = 39.5%; b) Year 2 = 50.6%; c) Year 3 = 59.8%, with canakinumab market shares decreasing accordingly.
The analysis was carried out from the perspective of the Italian NHS, considering a 3-year time horizon (2023–2025) and including pharmaceutical costs related to the FMF management.
The number of eligible patients was estimated by taking into account: a) Italian population data [28]; b) the prevalence and incidence of the disease [6,29]; c) the proportion of colchicine-intolerant and -resistant patients (mean values) [12–16]; and d) market research data [30]. In addition, considering the slightly different indications related to the eligible population for the biological medicinal products considered in the analysis (patients aged 8 months and older for anakinra and patients aged 2 years and older for canakinumab), it was assumed that all FMF patients treated with biologics were older than 2 years of age.
In this model, the prevalence was applied only to the starting population (Year 1, 2023). It was then considered steady across the 3 years of the analysis.
Incidence data were applied to each of the 3 years [6,29]. Subsequently, in order to estimate the target population, incident patients were added to prevalent patients each year. Given the absence of country-specific evidence, data from the NHS England Report was used as a basis for estimating incident patients in Italy [29].
The costs of the medicinal products were calculated by multiplying their consumption (doses and frequencies) by their prices. The doses and frequencies of administration were obtained from the respective Summary of Product Characteristics for each drug [31,32].
For anakinra in patients weighing ≥50 kg the recommended daily dose is 100 mg, and 1–2 mg for patients weighing <50 kg [18].
The recommended starting dose of canakinumab in FMF patients is as follows: 150 mg for patients with body weight >40 kg; 2 mg/kg for patients with body weight ≥7.5 kg and ≤40 kg [21].
Drug wastage was considered in the analysis. For every anakinra and canakinumab administration, a standard drug’ consumption was assumed for both adult and paediatric patients, independent of body weight. Therefore, it was considered a) consumption of one 100-mg syringe once a day for anakinra and b) consumption of one 150-mg vial every 4 weeks (i.e., 13 administrations per year) for canakinumab.
Net ex-factory prices were considered when estimating the cost of the treatments: a) for anakinra, the official net ex-factory prices (including mandatory discounts: −5%, −5%) [24]; b) for canakinumab, which at the time of the analysis was subject to a payback agreement, there was a −5% discount on the ex-factory price, thus an additional −5% discount was considered, in line with previous Italian studies [33,34]. Therefore, the base case analysis was carried out considering a price of € 28.47 for a 100-mg syringe of anakinra and € 9,927.50 for a 150-mg vial of canakinumab.
Two sensitivity analyses were also performed to test the robustness of the results.
The first sensitivity analysis considered a price of € 4,963.75 for the 150 mg vial of canakinumab (with a 50% discount on the actual price).
The second sensitivity analysis assumed that 10% of the adult patients did not achieve a satisfactory response and were therefore considered non-responders. In this analysis, non-responders to canakinumab were treated with 300 mg of canakinumab every 4 weeks, whilst no difference was considered for the anakinra group, according to the drugs’ SmPC [18,21]. The proportion of adult patients was estimated on the basis of up-to-date Italian population data [28].
All the assumptions used in the analysis were supported by an Italian clinician expert in the FMF management (Key Opinion Leader – KOL).
Results
Base case
The budget impact was estimated from the number of patients being treated with each drug, multiplied by the drug’s annual costs.
The target population, i.e., the number of eligible FMF patients treated with anakinra and canakinumab, ranged from 76 to 82 per year (Table 1).
Table 1.
Target population*† .
| Year 1 | Year 2 | Year 3 | Reference | |
|---|---|---|---|---|
| Italian population | ||||
| General population | 58,928,122 | [28] | ||
| Prevalent FMF PTS | ||||
| Prevalence of FMF | 1/60,000 | [6] | ||
| FMF Prevalent PTS (n) | 982 | |||
| FMF PTS intolerant to colchicine | 3.5% | [12–16] | ||
| FMF PTS resistant/non-responder to colchicine | 7.5% | [12–16] | ||
| FMF prevalent PTS intolerant OR resistant/non-responder to colchicine (n) | 105 | |||
| Incident FMF PTS | ||||
| Incident FMF PTS (n) | 40 | 40 | 40 | [29] |
| FMF Pts intolerant to the colchicine | 3.5% | 3.5% | 3.5% | [12–16] |
| FMF PTS resistant/non-responder to colchicine | 7.5% | 7.5% | 7.5% | [12–16] |
| FMF prevalent PTS intolerant OR resistant/non-responder to colchicine (n) | 4 | 4 | 4 | |
| Target population | ||||
| Total FMF PTS treated with biologics (n) | 109 | 113 | 117 | |
| Percent of PTS treated with anakinra and canakinumab (on-label biologicals) | 70% | 70% | 70% | [30] |
| Of whom PTS treated with anakinra and canakinumab (on-label biologicals) (n) | 76 | 79 | 82 | |
Legend: FMF: Familial Mediterranean Fever; PTS: Patients; n: number.
*Number of patients rounded at the first integer. †Percentages rounded to the first decimal number.
The ‘Reference scenario’ was analysed assuming that the whole target population was managed with canakinumab, whereas in the ‘Alternative Scenario’, the market shares of anakinra were assumed to increase from about 40% (Year 1) to 60% (Year 3), resulting in a range of 30 to 49 treated patients, respectively (Table 2).
Table 2.
Estimated patients and market shares broken down by year and therapy: base case and sensitivity analysis 1*†.
| Year 1 | Year 2 | Year 3 | |
|---|---|---|---|
| Reference Scenario | |||
| Anakinra | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Canakinumab | 76 (100.0%) | 79 (100.0%) | 82 (100.0%) |
| Total | 76 (100.0%) | 79 (100.0%) | 82 (100.0%) |
| Alternative Scenario | |||
| Anakinra | 30 (39.5%) | 40 (50.6%) | 49 (59.8%) |
| Canakinumab | 46 (60.5%) | 39 (49.4%) | 33 (40.2%) |
| Total | 76 (100.0%) | 79 (100.0%) | 82 (100.0%) |
*Number of patients rounded at the first integer. †Percentages rounded to the first decimal number.
Overall, in the base case, the mean annual cost per patient was € 129,058 with canakinumab and € 10393 with anakinra (Table 3).
Table 3.
Mean cost per patient/year: all analyses.
| Cost per ADM | Number of ADM per year | Total cost* | |
|---|---|---|---|
| Anakinra, adult and paediatric PTS: all analyses | € 28.47 (100 mg) |
365 | € 10,393 |
| Canakinumab, paediatric and adult PTS: base case analysis | € 9,927.50 (150 mg) |
13 | € 129,058 |
| Canakinumab, paediatric and adult PTS: sensitivity analysis 1 | € 4,963.75 (150 mg) |
13 | € 64,529 |
| Canakinumab, paediatric and responder adult PTS: sensitivity analysis 2 | € 9,927.50 (150 mg) |
13 | € 129,058 |
| Canakinumab, non-responder adult PTS: sensitivity analysis 2 | € 19,855.00 (300 mg) |
13 | € 258,115 |
| Canakinumab, mean adult PTS (90% responders and 10% non-responders): sensitivity analysis 2 | € 10,920.25 | 13 | € 141,963 |
Legend: ADM: Administration; PTS: Patients.
*Total costs rounded at the first integer.
As reported in the methods, in the analysis it was assumed wastage for both medicinal products and therefore considered one whole syringe/vial per administration for both paediatric and adult patients. Detailed results are provided in Table 4, Figures 1–2.
Table 4.
Budget Impact analysis: base case analysis.
| Year 1 | Year 2 | Year 3 | |
|---|---|---|---|
| Reference Scenario | |||
| Anakinra | € 0 | € 0 | € 0 |
| Canakinumab | € 9,808,370 | € 10,195,543 | € 10,582,715 |
| Total | € 9,808,370 | € 10,195,543 | € 10,582,715 |
| Alternative Scenario | |||
| Anakinra | € 311,789 | € 415,719 | € 509,255 |
| Canakinumab | € 5,936,645 | € 5,033,243 | € 4,258,898 |
| Total | € 6,248,434 | € 5,448,961 | € 4,768,153 |
| Δ Alternative – Reference scenario | |||
| Budget Impact: per year | -€ 3,559,936 (−36.3%) |
-€ 4,746,581 (−46.6%) |
-€ 5,814,562 (−54.9%) |
| Budget Impact: cumulative at 3 years | -€ 14,121,080 (−46.2%) |
||
Legend: ADM: Administration; PTS: Patients.
*Costs rounded at the first integer. †Percentages rounded to the first decimal number.
Figure 1.
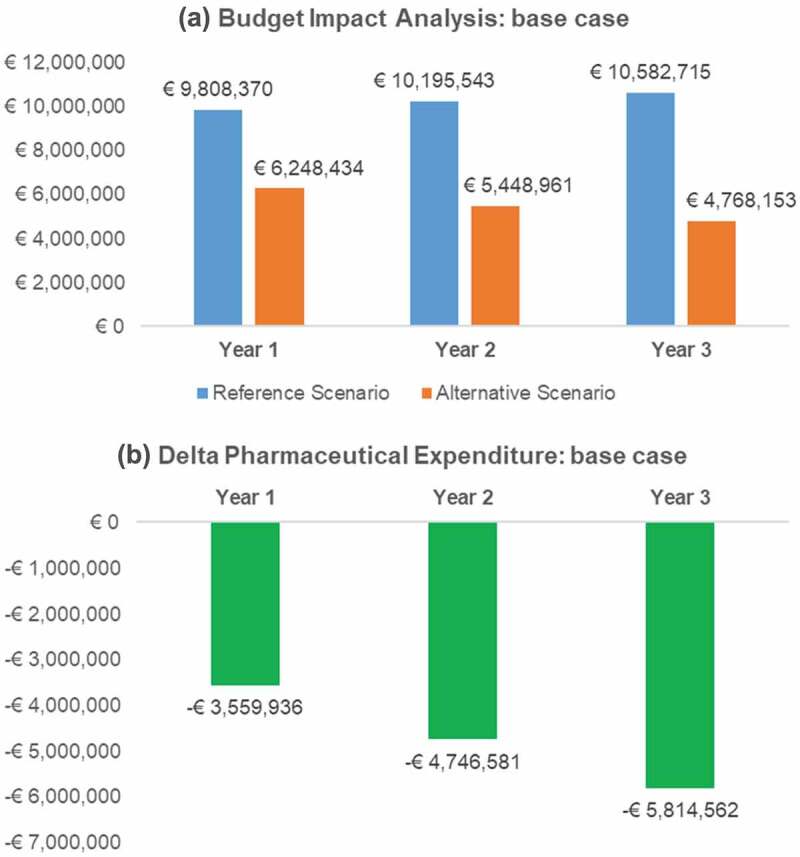
Budget Impact and pharmaceutical expenditure variation (Alternative – Reference scenario) per year: base case analysis.
Figure 2.
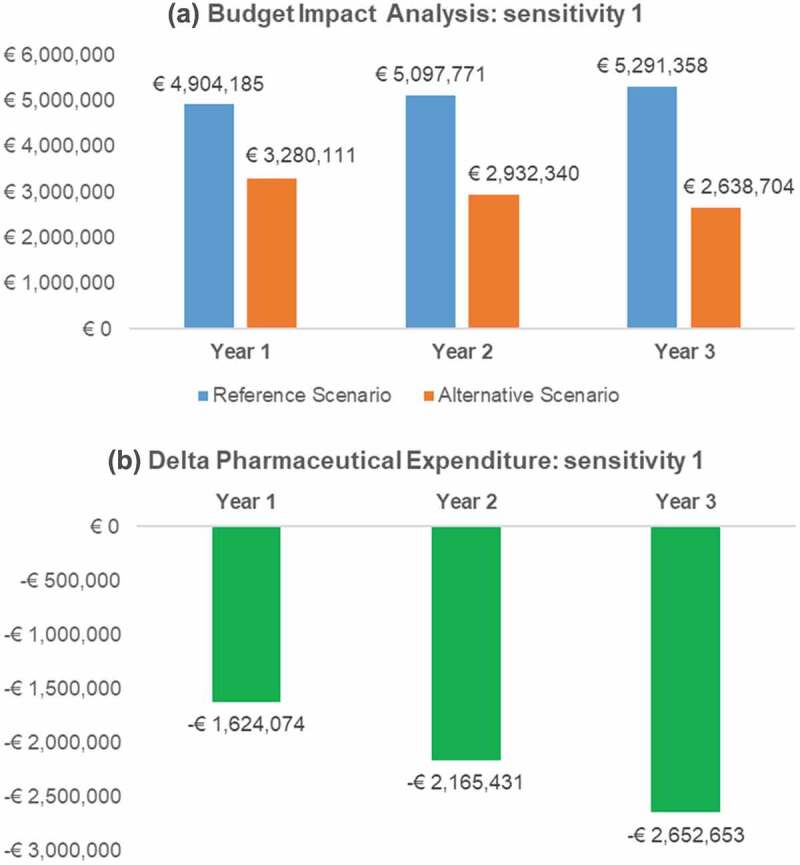
Budget Impact and pharmaceutical expenditure variation (Alternative – Reference scenario) per year: sensitivity analysis 1.
The base case analysis showed a total cumulative cost over 3 years of € 30586,628 in the ‘Reference scenario’ and € 16465,548 in the ‘Alternative scenario’.
In the ‘Alternative scenario’ as a consequence of the reimbursement of anakinra and the estimated increased number of patients treated with it, the annual expenditure for anakinra ranged from € 311,789 to € 509,255. The total savings ranged from €3,559,936 to €5,814,562 compared to the ‘Reference scenario’. Detailed results are provided in Table 4 and Figure 1.
Sensitivity Analyses
In the first sensitivity analysis, a 50% price discount was assumed for canakinumab, with a mean annual patient cost of € 64,529 (Table 3). Nevertheless, the BIA results were still favourable for the introduction of anakinra to the Italian market, with annual savings in the ‘Alternative scenario’, ranging from € 1,624,074 to € 2,652,653 compared to the ‘Reference scenario’ (Figure 2).
In the second sensitivity analysis, it was assumed that the 10% of the adult patients who would not respond to administration of 150 mg of canakinumab would subsequently be treated with 300 mg, resulting in an average cost of € 141,963 for adult patients and € 129,057 for paediatric patients (Table 3). According to the Italian population data, it was estimated that approximately 85% of the patients in the target population are adults; therefore, canakinumab would be administered to between 64 (Year 1) and 70 (Year 3) adult patients in the ‘Reference scenario’ and to between 39 (Year 1) and 28 (Year 3) in the ‘Alternative scenario’ (Table 5).
Table 5.
Estimated patients and market shares broken down by year and therapy: sensitivity analysis 2.
| Year 1 | Year 2 | Year 3 | |
|---|---|---|---|
| Reference Scenario | |||
| Anakinra, adult and paediatric PTS | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Canakinumab, paediatric PTS | 12 (15.8%) | 12 (15.2%) | 12 (14.6%) |
| Canakinumab, adult PTS | 64 (84.2%) | 67 (84.8%) | 70 (85.4%) |
| Total | 76 (100.0%) | 79 (100.0%) | 82 (100.0%) |
| Alternative Scenario | |||
| Anakinra, adult and paediatric PTS | 30 (39.5%) | 40 (50.6%) | 49 (59.8%) |
| Canakinumab, paediatric PTS | 7 (9.2%) | 6 (7.6%) | 5 (6.1%) |
| Canakinumab, adult PTS | 39 (51.3%) | 33 (41.8%) | 28 (34.1%) |
| Total | 76 (100.0%) | 79 (100.0%) | 82 (100.0%) |
Legend: PTS: Patients.
*Number of patients rounded at the first integer. †Percentages rounded to the first decimal number.
The results of this analysis proved to be more favourable following the reimbursement of anakinra for the treatment of FMF, with annual cost savings of between € 3,882,580 and € 6,343,698 (Figure 3).
Figure 3.
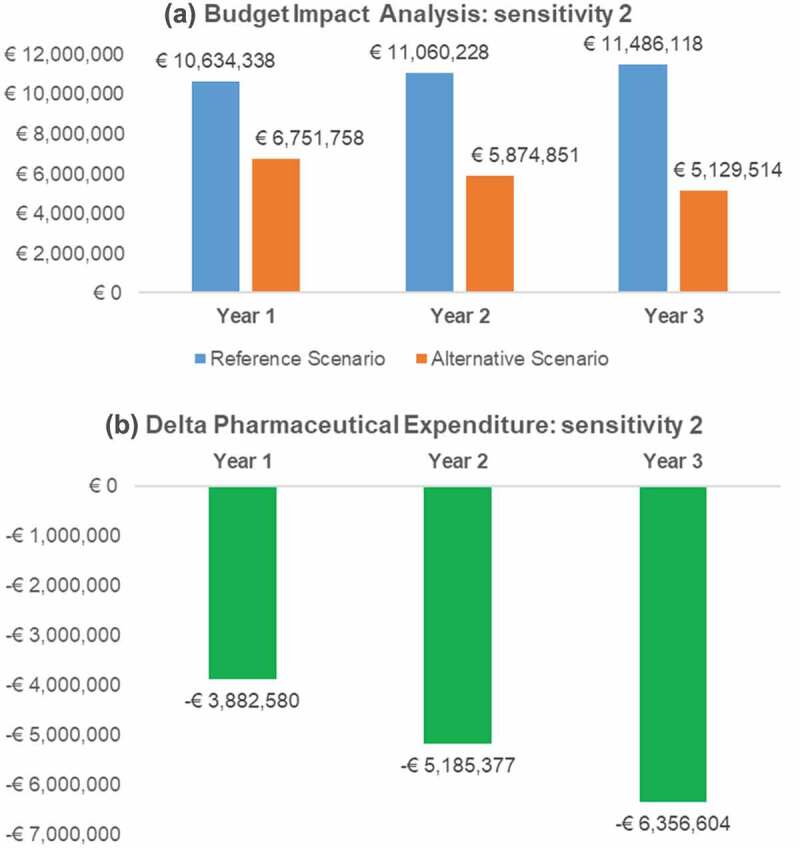
Budget Impact and pharmaceutical expenditure variation (Alternative – Reference scenario) per year: sensitivity analysis 2.
Discussion
This BIA estimated the impact of reimbursement and uptake of anakinra for the treatment of FMF in Italian patients from the NHS perspective. As the purpose of the model was to estimate pharmaceutical expenditure, its structure was based on population target estimation, market share assumption, and currently reimbursed treatment costs. In the base case scenario, the uptake assumptions and lower average annual cost per patient treated with anakinra result in a significant reduction in NHS pharmaceutical expenditure of € 14,121,080 (46.2%), over 3 years.
In the sensitivity analyses, the cost savings at 3 years, ranged from € 6,442,158 to € 15,424,560. The results show that even considering a significant discount of 50% on the price of canakinumab, the cost savings are more than 42%.
If 100% of the patients in the ‘Alternative scenario’ were to be treated with anakinra, the model estimates a 91.9% reduction in expenditure, from € 30.5 million in the ‘Reference scenario’ to € 2.5 million in the ‘Alternative scenario’.
Comparable BIAs with these biologics in FMF have not been reported, therefore limiting extrapolation of our results outside of Italy. Nevertheless, two economic studies from Turkey concluded that the main costs of the disease are associated with the pharmaceutical therapies and that expenditure for FMF has an upward trend, with a higher cost for patients treated with biologics and those who develop complications [35,36].
The first limitation of this study is that it was not possible to develop a more complete economic analysis taking into account non-pharmaceutical health-care costs, due to a lack of evidence. Nevertheless, it is important to emphasise that given the high cost of biological therapy, especially for canakinumab, the pharmaceutical costs are likely to be the main driver for the analysis presented. This was also confirmed by the Italian KOL. Furthermore, both treatments have proved to be effective and well tolerated [23], and therefore considering medicinal product costs alone seemed to be the most appropriate and reasonable approach.
The second limit of the analysis is the lack of incidence data for Italy; however, the assumption of 40 new FMF patients per year, based on the UK data [29]. In any case, the incidence data have a very limited impact on the final target population.
The third and final limit is that the annual treatment costs were calculated assuming 100% patient compliance, which is unlikely to be the case in clinical practice.
Given these limits and to reduce the uncertainty of the data, sensitivity analyses were carried out to test the robustness of the results. The sensitivity analyses showed that, even considering a very large discount for canakinumab, the results are still favourable for an increased use of anakinra, due to the high difference in treatment costs per patient (annualised € 10,393 with anakinra vs € 129,058 with canakinumab). From a pharmacoeconomic perspective, it is unlikely this difference can be offset.
Conclusion
This BIA estimated the economic impact of biologic treatments for FMF in Italy. The cumulative results over a 3-year time horizon showed the potential savings resulting from the reimbursement and increased use of anakinra as a replacement for canakinumab with total nationwide savings of € 14,121,080 (46.2%). Moreover, anakinra represents the only biological medicinal product for the treatment of FMF children between 8 months and 2 years of age. The robustness of the results was confirmed by the sensitivity analyses.
Disclosure statement
Conflict of interest: a) AA, EEM, and MP are employees of Intexo Società Benefit S.r.l.; b) LC and CT are employees of Sobi S.r.l., Milan, Italy; c) RM reports personal fees from Swedish Orphan Biovitrum (Sobi), Sanofi – Genzyme, Novartis
Financial support: Sobi S.r.l., Milan, Italy, has provided financial support to cover the cost of this project and the editorial assistance, but has not influenced the manuscript content.
References
- [1].Alghamdi M. Familial Mediterranean fever, review of the literature. Clin Rheumatol. 2017;36(8):1707–9. [DOI] [PubMed] [Google Scholar]
- [2].Ozdogan H, Ugurlu S. Familial Mediterranean fever. InternetAvailable from Press Med. 2019;481:e61–76. 10.1016/j.lpm.2018.08.014 [DOI] [PubMed] [Google Scholar]
- [3].Sari I, Birlik M, Kasifoglu T. Familial Mediterranean fever: an updated review. Eur J Rheumatol. 2014;1(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Özen S. Update on the epidemiology and disease outcome of familial Mediterranean fever. Best Pract Res Clin Rheumatol. 2018;32(2):254–260. [DOI] [PubMed] [Google Scholar]
- [5].La Regina M, Nucera G, Diaco M, et al. Familial Mediterranean fever is no longer a rare disease in Italy. Eur J Hum Genet. 2003;11(1):50–56. DOI: 10.1038/sj.ejhg.5200916 [DOI] [PubMed] [Google Scholar]
- [6].Cerrito L, Sicignano LL, Verrecchia E, et al. Epidemiology of FMF Worldwide. In. 2015:81–90 Available from. doi: 10.1007/978-3-319-14615-7_5 [DOI] [Google Scholar]
- [7].Bustaffa M, Koné-Paut I, Ozen S, et al. The impact of the Eurofever criteria and the new InFevers MEFV classification in real life: results from a large international FMF cohort. Semin Arthritis Rheum. 2022;52:1–6. [DOI] [PubMed] [Google Scholar]
- [8].Portincasa PC. Biologic agents and more for the treatment of familial Mediterranean fever. the old, the new, and the rare. Curr Med Chem. 2015;23(1):60–86. [DOI] [PubMed] [Google Scholar]
- [9].Maggio MC, Corsello G. FMF is not always “fever”: from clinical presentation to “treat to target. Ital J Pediatr. 2020;46(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Suticen E, Atas N, Guler AA, et al. Work productivity impairment in patients with familial Mediterranean fever and effects of interleukin-1 antagonists. Clin Rheumatol. 2021. Jul 1;40(7):2865–2871. InternetAvailable from. DOI: 10.1007/s10067-021-05617-7 [DOI] [PubMed] [Google Scholar]
- [11].Koşan Z, Yılmaz S, Bilge Yerli E, et al. Evaluation of the burden of care and the quality of life in the parents of Turkish children with familial Mediterranean fever. J Pediatr Nurs. 2019;48(xxxx):e21–6. [DOI] [PubMed] [Google Scholar]
- [12].El Hasbani G, Jawad A, Uthman I. Update on the management of colchicine resistant familial Mediterranean fever (FMF). Orphanet J Rare Dis. 2019;14(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hentgen V, Grateau G, Kone-Paut I, et al. Evidence-based recommendations for the practical management of familial Mediterranean fever. Semin Arthritis Rheum. 2013;43(3):387–391. DOI: 10.1016/j.semarthrit.2013.04.011 InternetAvailable from. [DOI] [PubMed] [Google Scholar]
- [14].Ozen S, Bilginer Y. A clinical guide to autoinflammatory diseases: familial Mediterranean fever and next-of-kin. InternetAvailable from Nat Rev Rheumatol. 2014;103:135–147. 10.1038/nrrheum.2013.174 [DOI] [PubMed] [Google Scholar]
- [15].Ozen S, Kone-Paut I, Gül A. Colchicine resistance and intolerance in familial Mediterranean fever: definition, causes, and alternative treatments. Semin Arthritis Rheum. 2017;47(1):115–120. [DOI] [PubMed] [Google Scholar]
- [16].Ozturk MA, Kanbay M, Kasapoglu B, et al. Therapeutic approach to familial Mediterranean fever: a review update. Clin Exp Rheumatol. 2011;29(4 SUPPL. 67):S77–86. [PubMed] [Google Scholar]
- [17].Agenzia Italana del Farmaco . Lists of class a and class H medicinal products [Internet]. [cited 2022 Apr 14]. Available from: https://www.aifa.gov.it/en/liste-farmaci-a-h
- [18].European Medicine Agency . Kineret - European public assessment report (EPAR) [Internet]. 2022. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/kineret
- [19].Gazzetta Ufficiale . GU n.177 del 26-7-2021 Kineret. 2011. p. 1–4.
- [20].Ozen S, Demirkaya E, Erer B, et al. EULAR recommendations for the management of familial Mediterranean fever. Ann Rheum Dis. 2016;75(4):644–651. DOI: 10.1136/annrheumdis-2015-208690 [DOI] [PubMed] [Google Scholar]
- [21].European Medicine Agency . Ilaris - European public assessment report (EPAR) [Internet]. 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/ilaris
- [22].Gazzetta Ufficiale . GU n.122 del 26-5-2022 - Rinegoziazione Ilaris. 2011. p. 1–4.
- [23].Sąhin A, Derin ME, Albayrak F, et al. Assessment of effectiveness of anakinra and canakinumab in patients with colchicine-resistant/unresponsive familial Mediterranean fever. Adv Rheumatol. 2020;60(1):1–7. [DOI] [PubMed] [Google Scholar]
- [24].Patient access monitor . Prezzi Farmaci [Internet] [Internet]. [cited 2022 Apr 14]. Available from: http://pamonitor.it/
- [25].Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR task force on good research practices - budget impact analysis. Value Heal. 2007;10(5):336–347. DOI: 10.1111/j.1524-4733.2007.00187.x [DOI] [PubMed] [Google Scholar]
- [26].Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis - Principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Heal. 2014;17(1):5–14. DOI: 10.1016/j.jval.2013.08.2291 [DOI] [PubMed] [Google Scholar]
- [27].AIFA . Linee guida per la compilazione del dossier a supporto della domanda di rimborsabilità e prezzo di un medicinale [Internet]. 2020. [cited 2022 Mar 22]. Available from: https://www.aifa.gov.it/documents/20142/1283800/Linee_guida_dossier_domanda_rimborsabilita.pdf.
- [28].Istituto Nazionale di Statistica . Previsioni della popolazione Anni 2020-2070 [Internet]. [cited 2022 May 12]. Available from: https://demo.istat.it/previsioni2017/index.php?lingua=ita
- [29].National Health Service England . Clinical commissioning policy: anakinra to treat periodic fevers and autoinflammatory diseases (all ages) [Internet]. 2018. Available from: https://www.england.nhs.uk/wp-content/uploads/2018/07/1713-anakinra-for-periodic-fever.pdf
- [30].Swedish Orphan Biovitrum . Familial Mediterranean fever mapping research. Data on file. 2021.
- [31].European Medicine Agency . Kineret: ePAR – product information. Last updated 22 December 2021 [Internet]. [cited 2022 May 12]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/kineret#product-information-section
- [32].Medicine Agency E. Ilaris: ePAR - product information. Last updated 15 October 2021 [Internet]. [cited 2022 May 12]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/ilaris#product-information-section
- [33].Perna GP, Ravasio R, Ricciardelli A. Analisi di Budget Impact di Ticagrelor nel Trattamento di Prevenzione in Pazienti con Sindrome Coronarica Acuta. Glob Reg Heal Technol Assess Ital North Eur Spanish. 2017;4(1):grhta.5000255. [Google Scholar]
- [34].Aiello A, Ritrovato D, Pitotti C. Budget impact model of indacaterol/glycopyrronium in the treatment of chronic obstructive pulmonary disease in Italy based on the FLAME study. Glob Reg Heal Technol Assess Ital North Eur Spanish. 2018;2018:228424031880480. 2018. DOI: 10.1177/2284240318804808. [DOI] [Google Scholar]
- [35].Yucel IK, Seyahi E, Kasapcopur O, et al. Economic impact of juvenile idiopathic arthritis and familial Mediterranean fever. Rheumatol Int. 2012;32(7):1955–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Aksu K, Dokuyucu O, Ertenli AI, et al. Cost of familial Mediterranean fever (Fmf) disease in Turkey. Value Heal. 2015 Nov 1 18(7):A666. DOI: 10.1016/j.jval.2015.09.2427InternetAvailable from [DOI] [Google Scholar]


