Abstract
Background:
Few well-established factors are associated with risk of amyotrophic lateral sclerosis (ALS). We comprehensively evaluate prescription drugs use in administrative health claims from U.S. Medicare beneficiaries in relation to ALS risk to generate hypotheses for further research.
Methods:
This is a population-based case–control study of 10,450 U.S. Medicare participants (ages 66–89 years) diagnosed with ALS, based on Medicare Parts A and B fee-for-service claims, between 1 January 2008, and 31 December 2014, and 104,500 controls (1:10 ratio) frequency-matched on age, sex, and selection year. Odds ratios (ORs) for the ALS association with 685 prescription drugs were estimated using logistic regression models for both a one- and three-year lag period. Covariates included demographic characteristics and key comorbidities, among other factors. Prescription drug use was based on Medicare Part D claims. We adjusted for multiple comparisons using a Bonferroni correction. Additional a priori analyses of sex hormone drugs were also undertaken.
Results:
In the large drug screen, we found 10 drugs significantly associated with lower ALS risk after the multiple-testing correction in a one-year and three-year lag analysis. These included several drugs for hypertension, diabetes, and cardiovascular disease. In a separate a priori inquiry of sex hormone drugs, tamoxifen was related to lower ALS risk, and testosterone to a higher risk in women.
Conclusions:
These associations warrant replication in databases that include information on the severity and duration of medical conditions underlying drug use, and drug use over a longer portion of individuals’ lifespans, to further help evaluate confounding by indication.
Keywords: Amyotrophic lateral sclerosis, medications, Medicare, risk, population based, drug screening
Introduction
Amyotrophic lateral sclerosis (ALS) is a rapidly progressive fatal neurodegenerative disease; about one-half of patients die within two to three years of symptom onset (1,2). ALS is most common among persons over age 60 and 90–95% of cases are considered sporadic, rather than familial, in origin (3). Although much has been learned about genetic mutations linked to ALS risk, the causal pathophysiologic mechanisms are poorly understood (4,5).
Only two drugs, riluzole (anti-glutamatergic, among other functions) and edaravone (free radical scavenger), have been approved by the US Food and Drug Administration for ALS treatment, but both only modestly affect survival (5). Absent effective treatments, identifying new factors, including medications that might influence disease incidence and progression, is important. One cost-effective approach is to investigate drugs approved for other indications. Unintended effects of marketed medications may include an elevated or decreased ALS risk. When risk is lower, this approach may lead to “repurposing” drugs and is especially attractive for rare diseases such as ALS (6). This strategy was also highlighted in a 2011 neurologic meeting as an approach to herald an advanced therapeutic era for ALS (1).
Few studies have investigated the impact of drugs on the risk of ALS incidence. We recently showed that statin use was related to a lower ALS risk in a Medicare population (7). However, many drugs approved for other medical conditions have not been investigated. We therefore conducted this hypothesis-generating investigation to identify approved prescription drugs that may be related to ALS risk. Here, we use claims data from Medicare patients across the U.S. from 2006 to 2014 to assess associations between a wide range of prescription medications and risk of ALS.
Materials and methods
Overview
Medicare is a U.S. federal insurance program for those aged 65 and older; it covers 97% of the age-eligible population. The program offers all participants inpatient care, Part A, and 95% of participants elect to subscribe to Part B (physician and other outpatient services). Since 2006, enrollees could subscribe to Part D, the Medicare Prescription Drug Plan.
ALS case identification
ALS case selection was based on International Classification of Diseases (ICD), 9th Revision Code 335.20. ALS cases were required to have one hospital claim or ≥2 outpatient/health professional claims at least 30 days apart between 1 January 2008, and 31 December 2014 (hospital claims are more thoroughly audited for accuracy) (8). Cases were restricted to those with a first ALS claim (the selection date) between ages 66 through 89. Those aged ≥90 years were excluded because of potential under-diagnosis of ALS.
Additionally, we required cases have 13 months participation in Parts A, B (not in a health maintenance organization (HMO)), and D prior to the selection date. HMO coverage was not counted because Medicare does not receive HMO claims (8).
Control identification
Ten controls per case were randomly selected, frequency-matched on sex, calendar year of case selection (2008, 2009, …, 2014) and age group at diagnosis/selection (66–<70; 70–<75; 75–<80; 80–<85; 85–<90 years). Individuals were ineligible to be selected as a control in or after any year in which they had an ICD 335.20 claim by 30th June of that year. Thirty controls later became cases (0.29% of cases). Controls were assigned 30th June as the selection date for the relevant selection year. Although controls could be sampled multiple times, only 0.25% were selected twice. Like cases, controls were required to have 13 months participation in Parts A/B (non-HMO) and D before selection. Finally, we required controls to have 1+ claims before their selection date to ensure minimum Medicare participation.
Ascertainment of medications
Drug use was based on Medicare Part D prescription claims data. Individuals were considered to have used a medication if they had ≥2 prescriptions on different days, both filled ≥one year before the selection date. Drugs were included in the analysis if they were used by ≥10 individuals (cases and controls) in the selected sample.
Ascertainment of covariates
We controlled for the matching factors, race, indicators of socio-economic status, Medicare use, selected comorbidities, obesity and chronic obstructive pulmonary disease (COPD) as a surrogate for smoking (9). Race/ethnicity was derived from the Research Triangle Institute algorithm (10). We used two indicators of socio-economic status: “LIS”, identifying participants receiving low-income subsidies, and “Medicaid”, identifying participants using a US medical assistance program for poverty. Medical comorbidities identified by Part B claims using ICD 9 codes (restricted to claims at ages ≥65 years, between 1 January 2006, and one year before selection) were dyslipidemia (272); diabetes (250); stroke (434.91); renal disease (585); hypertension (401); acute myocardial infarction (410); and COPD (490, 491, 492, 494, 496). Obesity was derived from the Chronic Conditions Data Warehouse obesity definition, based on both diagnostic and procedural codes (including V85.3 and V85.4). To account for medical surveillance intensity, we calculated the average number of physician visits for six-month intervals between 2006 and selection, omitting the first and last interval. We excluded claims from specialists with limited patient contact (i.e. radiologists, anesthesiologists, and pathologists).
Statistical analyses
We estimated odds ratios (ORs) and 95% confidence intervals (CIs) for the relationship between prescription drugs and ALS risk using adjusted logistic regression models. Because it was so rare, we did not account for multiple control selection. Fully adjusted models included: drug use (ever/ never); sex; age; race; calendar year of selection; duration of Part D coverage prior to selection date (quintiles; ≤30; 31–42; 43–54; 55–78; ≥79 months); Medicare LIS (ever/never); Medicaid (ever/never); comorbidities (dyslipidemia, diabetes, stroke, renal disease, hypertension, acute myocardial infarction, obesity, COPD), and physician visits per six-month period (quintiles, cutpoints: 0.75; 2.06; 3.55; 5.92 visits).
We present a one-year lag (lag1) to maximize generalizability, since longer lag periods select for an older study population, and a three-year lagged (lag3) analysis, to check for reverse causation bias, as ALS has a median survival of 2–3 years from symptom onset (11). In interpreting the results, we focus on those drugs that showed associations at lag1 and lag3.
In the lag1 analysis, we tested ALS associations with 772 drugs, and for lag3, 677 drugs were included. However, logistic models only converged for 685 drugs in lag1 and for 590 drugs in lag3. The lag3 drugs were mostly a subset of lag1 drugs.
In evaluating statistical significance, we adjusted for multiple testing by using a Bonferroni correction that divided the nominal significance level of 0.05 by the number of drugs that were tested in the lag1 analysis (n=685), resulting in a p-value threshold of 7.30(10−5), the “corrected p-value threshold”.
Because the list of drugs tested is so extensive, the main table only presents associations for drugs with a p-value of ≤0.005 in either the lag1 or lag3 analysis. Supplementary e-Table 1 provides the complete list of drugs analyzed for both lagged periods. We also present sex-stratified associations for sex hormonal drugs (e.g. testosterone, estrogen), because some studies suggest that sex hormones may play a role in ALS pathogenesis (12). Because of the a priori hypothesized associations between hormones and ALS, the conventional p<0.05 was applied to these analyses to assess statistical significance.
To further address potential confounding, we also present associations organized by drug categories based on the general medical condition for which the drugs are prescribed (e.g. diabetes, hypertension). This can facilitate hypothesis generation about drugs with disparate relationships to ALS within a drug category, along with the medical conditions underlying the drug prescription.
This study’s use of de-identified Medicare claims was exempt from review by an Institutional Review Board.
Results
We identified 10,450 ALS cases diagnosed between 1 January 2008, and 31 December 2014, and 104,500 controls. The median age at diagnosis was 74 years, and 51% of these cases/controls were female (Table 1). The median number of months of Part D coverage was similar in cases and controls (49 vs 50 months). The distributions of other characteristics by case–control status are presented in Table 1. The three-year lag analysis included 7891 cases and 78,794 controls.
Table 1.
Characteristics of ALS cases and controls in frequency-matched case–control analysis of ALS and medications prior to ALS diagnosis in a U.S. Medicare population, 2006–2014.
| Variable Age at selection, y | ALS Cases | Controls | ||
|---|---|---|---|---|
|
|
|
|||
| n = 10,450 | (Percent) | n = 104,500 | (Percent) | |
| 66–69 | 2079 | (19.9) | 20,790 | (19.9) |
| 70–74 | 3218 | (30.8) | 32,180 | (30.8) |
| 75–79 | 2627 | (25.1) | 26,270 | (25.1) |
| 80–84 | 1727 | (16.5) | 17,270 | (16.5) |
| 85–89 | 799 | (7.7) | 7990 | (7.7) |
| Age at selection, median y | 74.00 | 74.00 | ||
| Sex | ||||
| Men | 5095 | (48.8) | 50,950 | (48.8) |
| Women | 5355 | (51.2) | 53,550 | (51.2) |
| Race/ethnicity | ||||
| White, non-Hispanic | 8969 | (85.8) | 81,990 | (78.5) |
| Black, non-Hispanic | 578 | (5.5) | 8826 | (8.5) |
| Hispanic | 560 | (5.4) | 9011 | (8.6) |
| Other/unknown | 343 | (3.3) | 4673 | (4.5) |
| Selection year | ||||
| 2008 | 1262 | (12.1) | 12,620 | (12.1) |
| 2009 | 1347 | (12.9) | 13,470 | (12.9) |
| 2010 | 1452 | (13.9) | 14,520 | (13.9) |
| 2011 | 1547 | (14.8) | 15,470 | (14.8) |
| 2012 | 1537 | (14.7) | 15,370 | (14.7) |
| 2013 | 1671 | (16.0) | 16,710 | (16.0) |
| 2014 | 1634 | (15.6) | 16,340 | (15.6) |
| Regiona | ||||
| Northeast | 630 | (6.0) | 5074 | (4.9) |
| New York+ | 966 | (9.2) | 10,079 | (9.6) |
| Mid-Atlantic | 1018 | (9.7) | 10,158 | (9.7) |
| Southeast | 2160 | (20.7) | 23,240 | (22.2) |
| Northwest | 412 | (3.9) | 3675 | (3.5) |
| Western Pacific | 1172 | (11.2) | 11 843 | (11.3) |
| Mountain | 325 | (3.1) | 3353 | (3.2) |
| Southwest | 1128 | (10.8) | 12,320 | (11.8) |
| Central | 625 | (6.0) | 6082 | (5.8) |
| Great Lakes | 2000* | (19.1*) | 18,531 | (17.7) |
| State code not in U.S. | – | – | 145 | (0.1) |
| Median mos. of coverage | ||||
| Part A/B/non-HMO coverage | 98.00 | 91.00 | ||
| Part D coverage | 49.00 | 50.00 | ||
| Average physician visits/mo. | 3.71 | 2.75 | ||
| Socioeconomic status | ||||
| Low-income subsidy | 2907 | (27.8) | 32,984 | (31.6) |
| Medicaid eligibility | 2736 | (26.2) | 29,599 | (28.3) |
| Co-morbidities | ||||
| Dyslipidemia | 7026 | (67.2) | 66,705 | (63.8) |
| Diabetes | 2665 | (25.5) | 30,393 | (29.1) |
| Stroke | 341 | (3.3) | 2666 | (2.6) |
| Renal disease | 734 | (7.0) | 8589 | (8.2) |
| Hypertension | 7122 | (68.2) | 70,114 | (67.1) |
| Acute Myocardial Infarction | 284 | (2.7) | 2963 | (2.8) |
| Obesity | 692 | (6.6) | 7866 | (7.5) |
| COPD | 1794 | (17.2) | 17,348 | (16.6) |
ALS: amyotrophic lateral sclerosis; COPD: chronic obstructive pulmonary disease; HMO: health maintenance organization.
New York + refers to New York, New Jersey, Puerto Rico, Virgin Islands; the “*” in the Great Lakes distribution refers to a slight alteration of the frequency to ensure that the numbers for the category “State code not in U.S.”, which are not presented, cannot be calculated through subtraction, as is required by the Centers for Medicare and Medicaid Services for groups of subjects that are ≤10.
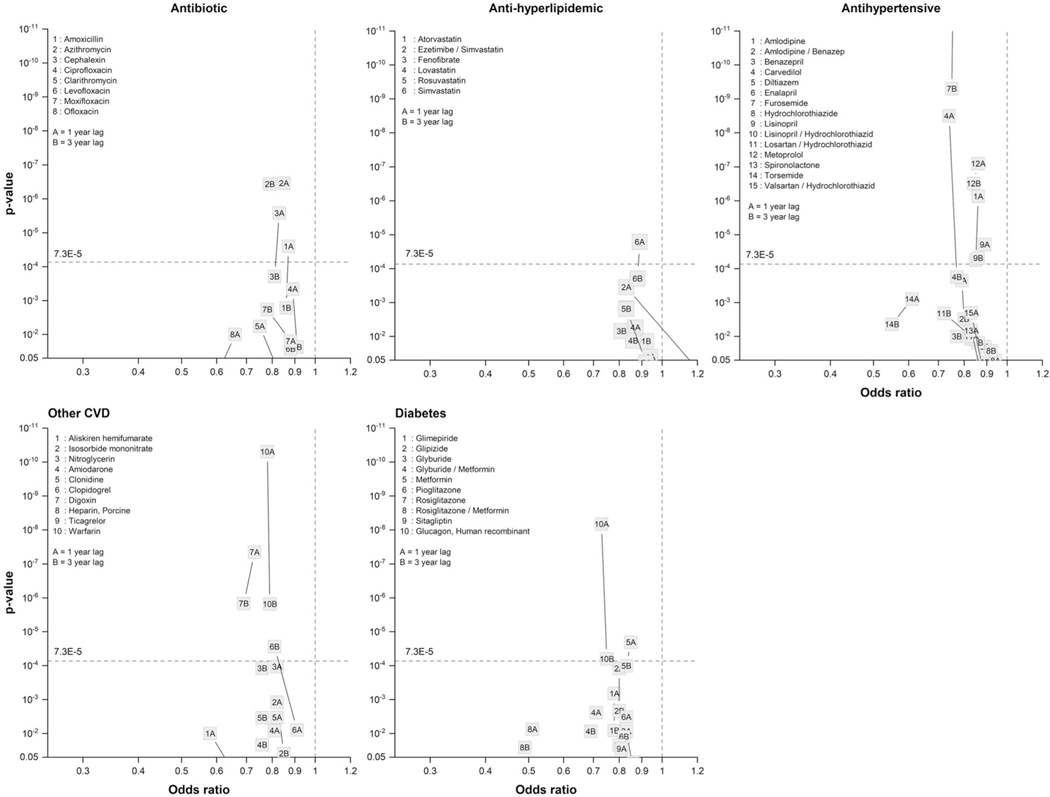
Table 2 presents results for 90 drugs, organized by drug category, that showed an association with ALS risk at a p-value of <0.005 for either the lag1 or lag3 analysis. Figure 1 depicts the ORs for associations and corresponding p-values for five main categories of drugs (antibiotics, antidiabetics, anti-hyperlipidemic, antihypertensive, other cardiovascular (CVD) drugs).
Table 2.
Drugs associated with ALS risk at p < 0.005 in either the one or three-year lagged analysis (percentages based on <11 individuals cannot be disclosed and are marked by ND in the table). P-values that meet the Bonferroni correction are in cells shaded in grey For findings that are significant at both lag times, p-values are presented in bold faced font.
| DrugGroup | GN | Oneyear lag 10,450 cases/104,500 controls | Three-year lag 7891 cases/ 78 794 controls | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| % exposed | % exposed | ||||||||||
|
|
|
|
|
||||||||
| Cases | controls | OR | Std error | p-value | cases | controls | OR | Std error | p-value | ||
| Analgesic | Diclofenac misoprostol | 0.3 | 0.42 | 0.6 | 0.19 | 6.12E-03 | ND | ND | 0.36 | 0.32 | 2.43E-04 |
| Hydrocodone /acetaminophen | 21.8 | 19.83 | 0.94 | 0.03 | 2.00E-02 | 13.98 | 13.35 | 0.87 | 0.04 | 1.36E-04 | |
| Meloxicam | 5.73 | 5.26 | 0.97 | 0.05 | 4.52E-01 | 3.18 | 3.26 | 0.84 | 0.07 | 8.66E-03 | |
| Morphine sulfate | 0.68 | 0.61 | 0.89 | 0.13 | 3.76E-01 | 0.25 | 0.38 | 0.55 | 0.23 | 9.61E-03 | |
| Propoxyphene /acetaminophen | 6.23 | 6.3 | 0.85 | 0.04 | 2.57E-04 | 4.52 | 4.87 | 0.74 | 0.24 | 1.95E-01 | |
| Tramadol /acetaminophen | 1.06 | 1.48 | 0.68 | 0.1 | 8.21E-05 | 0.8 | 1.05 | 0.94 | 0.06 | 1.02E-01 | |
| Antibiotics | Amoxicillin | 11.05 | 10.58 | 0.87 | 0.03 | 2.52E-05 | 7.46 | 7.00 | 0.86 | 0.05 | 1.63E-03 |
| Azithromycin | 14.31 | 13.85 | 0.85 | 0.03 | 3.47E-07 | 8.16 | 8.26 | 0.79 | 0.05 | 3.69E-07 | |
| Cephalexin | 7.95 | 7.78 | 0.83 | 0.04 | 2.63E-06 | 4.85 | 4.82 | 0.81 | 0.06 | 1.95E-04 | |
| Ciprofloxacin | 12.68 | 11.97 | 0.89 | 0.03 | 4.54E-04 | 7.74 | 7.14 | 0.91 | 0.05 | 2.36E-02 | |
| Clarithromycin | 0.97 | 1.05 | 0.75 | 0.11 | 5.73E-03 | 0.72 | 0.69 | 0.84 | 0.14 | 2.03E-01 | |
| Levofloxacin | 8.32 | 7.12 | 0.96 | 0.04 | 3.25E-01 | 5.02 | 4.53 | 0.88 | 0.06 | 2.64E-02 | |
| Moxifloxacin | 4.07 | 4.05 | 0.88 | 0.05 | 1.58E-02 | 2.18 | 2.40 | 0.78 | 0.08 | 1.83E-03 | |
| Ofloxacin | 0.39 | 0.55 | 0.66 | 0.16 | 9.96E-03 | 0.15 | 0.23 | 0.61 | 0.30 | 1.03E-01 | |
| Anti-ulcer | Esomeprazole | 7.72 | 7.18 | 0.94 | 0.04 | 1.17E-01 | 5.45 | 5.54 | 0.85 | 0.05 | 2.20E-03 |
| Omeprazole | 14.69 | 14.63 | 0.91 | 0.03 | 1.50E-03 | 8.73 | 9.27 | 0.83 | 0.04 | 2.54E-05 | |
| Pantoprazole | 5.58 | 5.28 | 0.94 | 0.05 | 1.49E-01 | 3.61 | 3.64 | 0.87 | 0.06 | 3.41E-02 | |
| Rabeprazole | 1.52 | 1.03 | 1.27 | 0.09 | 5.42E-03 | 1.31 | 0.88 | 1.29 | 0.11 | 1.76E-02 | |
| Ranitidine | 3.82 | 4.54 | 0.83 | 0.05 | 6.64E-04 | 2.52 | 3.25 | 0.77 | 0.08 | 6.29E-04 | |
| Anti-allergic | Fexofenadine | 3.61 | 3.67 | 0.85 | 0.06 | 4.72E-03 | 2.84 | 2.94 | 0.83 | 0.07 | 8.01E-03 |
| Anti-asthma treatment | Albuterol | 7.55 | 7.56 | 0.95 | 0.04 | 1.78E-01 | 4.35 | 5.07 | 0.79 | 0.06 | 9.08E-05 |
| Fluticasone/salmeterol | 5.08 | 4.94 | 0.97 | 0.05 | 5.37E-01 | 3.35 | 3.55 | 0.86 | 0.07 | 2.66E-02 | |
| Montelukast | 3.68 | 3.43 | 0.97 | 0.06 | 6.03E-01 | 2.29 | 2.56 | 0.80 | 0.08 | 4.16E-03 | |
| Tiotropium | 3.36 | 3.19 | 0.99 | 0.06 | 8.89E-01 | 1.91 | 1.98 | 0.87 | 0.09 | 1.10E-01 | |
| Corticosteroid | Budesonide | 1.12 | 0.69 | 1.34 | 0.1 | 3.70E-03 | 0.81 | 0.55 | 1.16 | 0.14 | 2.65E-01 |
| Methylprednisolone | 4.25 | 4.02 | 0.84 | 0.05 | 1.06E-03 | 2.10 | 2.38 | 0.69 | 0.08 | 5.95E-06 | |
| Triamcinolone | 5.16 | 5.15 | 0.87 | 0.05 | 2.50E-03 | 3.09 | 3.44 | 0.77 | 0.07 | 1.22E-04 | |
| Antihyperlipidemic | Atorvastatin | 17.81 | 17.79 | 0.94 | 0.03 | 4.07E-02 | 14.62 | 14.52 | 0.92 | 0.03 | 1.37E-02 |
| Ezetimibe/simvastatin | 4.3 | 4.74 | 0.83 | 0.05 | 3.51E-04 | 3.70 | 4.22 | 1.40 | 0.06 | 9.50E-01 | |
| Fenofibrate | 3.3 | 3.79 | 0.83 | 0.06 | 1.49E-03 | 2.43 | 2.80 | 0.81 | 0.08 | 6.98E-03 | |
| Lovastatin | 4.57 | 5.45 | 0.87 | 0.05 | 5.40E-03 | 3.88 | 4.55 | 0.86 | 0.06 | 1.33E-02 | |
| Rosuvastatin | 6.26 | 6.36 | 0.92 | 0.04 | 6.00E-02 | 4.08 | 4.51 | 0.83 | 0.06 | 1.53E-03 | |
| Simvastatin | 21.88 | 23.31 | 0.89 | 0.03 | 1.62E-05 | 15.59 | 16.31 | 0.88 | 0.03 | 1.94E-04 | |
| Antihypertensive | Amlodipine | 14.39 | 16.29 | 0.86 | 0.03 | 7.43E-07 | 10.09 | 11.39 | 0.85 | 0.04 | 5.62E-05 |
| Amlodipine/benazepril | 2.73 | 3.52 | 0.79 | 0.06 | 2.24E-04 | 2.41 | 3.02 | 0.80 | 0.08 | 3.05E-03 | |
| Benazepril | 1.92 | 2.36 | 0.82 | 0.07 | 7.68E-03 | 1.41 | 1.78 | 0.77 | 0.10 | 9.93E-03 | |
| Carvedilol | 4.37 | 5.67 | 0.74 | 0.05 | 3.17E-09 | 2.94 | 3.64 | 0.77 | 0.07 | 1.77E-04 | |
| Diltiazem | 4.95 | 5.13 | 0.89 | 0.05 | 2.00E-02 | 3.76 | 4.07 | 0.86 | 0.06 | 1.52E-02 | |
| Enalapril | - | - | - | - | - | 2.72 | 3.32 | 0.83 | 0.07 | 1.21E-02 | |
| Furosemide | 11.41 | 13.68 | 0.76 | 0.03 | 1.97E-15 | 7.77 | 9.62 | 0.75 | 0.05 | 4.99E-10 | |
| Hydrochlorothiazide | 13.39 | 14.22 | 0.94 | 0.03 | 5.25E-02 | 10.45 | 11.15 | 0.92 | 0.04 | 2.66E-02 | |
| Lisinopril | 18.74 | 20.62 | 0.89 | 0.03 | 1.96E-05 | 13.6 | 15.05 | 0.86 | 0.04 | 4.87E-05 | |
| Lisinopril/hydrochlorothiazide | 4.71 | 4.82 | 0.99 | 0.05 | 8.16E-01 | 3.49 | 3.94 | 0.88 | 0.07 | 5.87E-02 | |
| Losartan/hydrochlorothiazide | 2.07 | 2.5 | 0.83 | 0.07 | 9.93E-03 | 1.25 | 1.70 | 0.72 | 0.11 | 2.08E-03 | |
| Metoprolol | 19.24 | 20.46 | 0.86 | 0.03 | 8.01E-08 | 14.65 | 15.67 | 0.84 | 0.04 | 3.08E-07 | |
| Spironolactone | 2.33 | 2.63 | 0.83 | 0.07 | 6.73E-03 | 1.53 | 1.64 | 0.88 | 0.10 | 1.93E-01 | |
| Torsemide | 0.48 | 0.75 | 0.61 | 0.15 | 7.80E-04 | 0.30 | 0.53 | 0.55 | 0.21 | 4.40E-03 | |
| Valsartan/hydrochlorothiazide | 3.11 | 3.73 | 0.83 | 0.06 | 1.98E-03 | 2.71 | 3.01 | 0.88 | 0.07 | 8.90E-02 | |
| CVD/Other | Aliskiren | 0.23 | 0.37 | 0.58 | 0.21 | 9.94E-03 | 0.15 | 0.21 | 0.66 | 0.30 | 1.63E-01 |
| Isosorbide mononitrate | 2.97 | 3.54 | 0.82 | 0.06 | 1.19E-03 | 2.23 | 2.57 | 0.85 | 0.08 | 3.87E-02 | |
| Nitroglycerin | 4.53 | 4.97 | 0.82 | 0.05 | 1.07E-04 | 2.84 | 3.33 | 0.76 | 0.07 | 1.20E-04 | |
| Amiodarone | 1.66 | 1.77 | 0.81 | 0.08 | 8.13E-03 | 1.05 | 1.15 | 0.76 | 0.12 | 2.14E-02 | |
| Clonidine | 2.33 | 2.92 | 0.82 | 0.07 | 3.37E-03 | 1.55 | 2.08 | 0.76 | 0.10 | 3.44E-03 | |
| Clopidogrel | 9.93 | 10.22 | 0.91 | 0.04 | 7.82E-03 | 6.82 | 7.58 | 0.81 | 0.05 | 2.77E-05 | |
| Digoxin | 3.24 | 3.89 | 0.73 | 0.06 | 4.51E-08 | 2.38 | 2.99 | 0.69 | 0.08 | 1.47E-06 | |
| Heparin, porcine | 0.15 | 0.06 | 2.17 | 0.28 | 6.33E-03 | ND | ND | 1.20 | 0.49 | 7.09E-01 | |
| Ticagrelor | ND | ND | 4.92 | 0.6 | 8.33E-03 | - | - | - | - | - | |
| Warfarin | 8.93 | 9.2 | 0.78 | 0.04 | 4.94E-11 | 6.45 | 6.54 | 0.79 | 0.05 | 1.51E-06 | |
| Antidiabetes | Glimepiride | 2.19 | 3.08 | 0.78 | 0.07 | 6.62E-04 | 1.57 | 2.2 | 0.78 | 0.10 | 8.14E-03 |
| Glipizide | 3.51 | 5.12 | 0.8 | 0.06 | 1.21E-04 | 2.78 | 4.07 | 0.80 | 0.07 | 2.16E-03 | |
| Glyburide | 2.12 | 2.97 | 0.83 | 0.07 | 8.58E-03 | 1.79 | 2.42 | 0.86 | 0.09 | 1.06E-01 | |
| Glyburide/metformin | 0.84 | 1.44 | 0.71 | 0.11 | 2.40E-03 | 0.71 | 1.25 | 0.69 | 0.14 | 8.67E-03 | |
| Metformin | 10.35 | 13.32 | 0.85 | 0.04 | 2.03E-05 | 7.86 | 10.21 | 0.83 | 0.05 | 1.01E-04 | |
| Pioglitazone | 3.03 | 4.14 | 0.83 | 0.06 | 3.24E-03 | 2.45 | 3.34 | 0.82 | 0.08 | 1.23E-02 | |
| Rosiglitazone | 1.68 | 2.25 | 0.86 | 0.08 | 6.92E-02 | 1.51 | 2.18 | 0.80 | 0.10 | 2.22E-02 | |
| Rosiglitazone/metformin | 0.16 | 0.37 | 0.51 | 0.25 | 7.48E-03 | - | - | 0.49 | 0.32 | 2.58E-02 | |
| Sitagliptin | 1.24 | 1.68 | 0.81 | 0.09 | 2.81E-02 | 0.75 | 0.94 | 0.82 | 0.28 | 4.76E-01 | |
| Glucagon, human recombinant | 4.1 | 6.09 | 0.73 | 0.06 | 6.67E-09 | 2.93 | 4.37 | 0.75 | 0.07 | 6.33E-05 | |
| Central Nervous system (CNS) | Baclofen | 1.98 | 0.63 | 2.73 | 0.08 | 3.21E-34 | 1.03 | 0.39 | 2.21 | 0.13 | 5.57E-10 |
| Tizanidine | 1.08 | 0.59 | 1.5 | 0.1 | 9.16E-05 | 0.51 | 0.35 | 1.18 | 0.17 | 3.39E-01 | |
| Gabapentin | 8.79 | 6.98 | 1.16 | 0.04 | 1.51E-04 | 5.08 | 4.41 | 1.33 | 0.06 | 5.56E-01 | |
| Oxcarbazepine | 0.34 | 0.17 | 1.64 | 0.19 | 7.48E-03 | 0.24 | 0.13 | 1.59 | 0.25 | 6.77E-02 | |
| Carbidopa/levodopa | 2.74 | 1.34 | 1.73 | 0.07 | 3.06E-16 | 1.39 | 0.89 | 1.30 | 0.10 | 1.26E-02 | |
| Ropinirole | 1.74 | 1.17 | 1.26 | 0.08 | 4.50E-03 | 1.17 | 0.78 | 1.26 | 0.11 | 4.25E-02 | |
| Peripheral nervous system | Pyridostigmine | 0.53 | 0.1 | 4.45 | 0.17 | 1.28E-18 | 0.16 | 0.07 | 2.12 | 0.31 | 1.70E-02 |
| Antimuscarinic | Oxybutynin | 3.13 | 2.36 | 1.18 | 0.06 | 5.77E-03 | 1.71 | 1.52 | 0.99 | 0.09 | 9.49E-01 |
| Solifenacin | 1.73 | 1.19 | 1.24 | 0.08 | 7.59E-03 | 1 | 0.71 | 1.19 | 0.12 | 1.65E-01 | |
| Psychiatric / Antidepressant SSRI | Fluoxetine | 3.17 | 2.35 | 1.22 | 0.06 | 7.90E-04 | 2.34 | 1.73 | 1.21 | 0.08 | 1.73E-02 |
| Sertraline | 5.48 | 4.3 | 1.14 | 0.05 | 4.80E-03 | 3.23 | 2.99 | 0.94 | 0.07 | 3.58E-01 | |
| Psychiatric / Anti-anxiety | Meprobamate | 0.12 | 0.05 | 2.32 | 0.32 | 7.68E-03 | - | - | - | - | - |
| Psychiatric / Antipsychotic | Quetiapine | 1.4 | 1.54 | 0.77 | 0.09 | 2.75E-03 | 0.79 | 0.92 | 0.74 | 0.13 | 2.62E-02 |
| Misc. / anti-emetic | Promethazine | 2.3 | 2.31 | 0.83 | 0.07 | 7.32E-03 | 1.51 | 1.49 | 0.86 | 0.10 | 1.20E-01 |
| Misc. / Antibody | Immune globulin γ-caprylate | ND | ND | 9.7 | 0.47 | 1.19E-06 | - | - | - | - | - |
| Misc. / Dietary supplement | Omega-3 | 1.07 | 1.12 | 0.87 | 0.10 | 1.58E-01 | 0.49 | 0.7 | 0.62 | 0.17 | 4.00E-03 |
| Misc./Nutritive Agent | Potassium chloride | 9.98 | 11.03 | 0.82 | 0.04 | 5.63E-08 | 6.92 | 7.92 | 0.80 | 0.05 | 2.12E-06 |
| Misc./Antigout | Allopurinol | 3.67 | 4.2 | 0.85 | 0.06 | 4.31E-03 | 2.67 | 3.01 | 0.85 | 0.07 | 3.26E-02 |
Figure 1.
p-values of association and corresponding odds ratios (ORs) for various drug categories. The horizontal line corresponds to the multiple testing corrected p-value threshold.
Three of the eight antibiotic medications—amoxicillin, cephalexin, and azithromycin—were significantly associated (multiple-testing corrected) with lower ALS risk at lag1; only azithromycin showed associations at both lag periods. All eight drugs showed a 10–20% lower ALS risk in both lag periods (Table 2; Figure 1).
None of the four anti-ulcer medications were associated at both lags. Only omeprazole was statistically significant (multiple-testing corrected) at lag3 with a reduced risk (~20–25%) similar to other antiulcer drugs (Table 2).
One of the three corticosteroids, methyprednisolone was statistically significant (multiple-testing corrected) at lag3. It was related to lower ALS risk, and showed declining risk from lag1 to lag3.
All anti-hyperlipidemic drugs (five statin or statin combination drugs and one fibrate) were related to lower ALS risk at both lag times. However, only simvastatin was statistically significant (multiple-testing corrected) at lag1, with similar lag1 and lag3 risk magnitudes (OR = 0.89 and OR = 0.88, respectively) (Table 2; Figure 1). Risks generally declined from lag1 to lag3 in all.
The group of 15 antihypertensive drugs included beta blockers (BBs), calcium channel blockers (CCBs), angiotensin-converting enzyme (ACE) inhibitors, diuretics, and angiotensin2 receptor blockers (A2RBs). Four drugs were significantly (multiple-testing corrected) associated with lower ALS risk at both lag times: amlodipine (CCB), furosemide (diuretic), lisinopril (ACE), and metoprolol (BB). Risks at lag3 ranged from OR = 0.75 for furosemide to OR= 0.88 for lisinopril and OR = 0.85 for amlodipine (Figure 1). Carvedilol showed a significantly reduced risk (OR = 0.74) only in the lag1 analysis, but with a similar lag3 OR magnitude (OR = 0.77). Risks largely declined from lag1 to lag3.
In a “miscellaneous” category of 10 CVD drugs, three (clopidogrel, digoxin, warfarin) were significantly associated (multiple-testing corrected) at lag3, and digoxin and warfarin at lag1 as well, both with 20–30% lower risks at the two lags (Table 2; Figure 1). Most drugs in that category followed a similar pattern, though the risks were not quite as low.
All 11 diabetes drugs in Table 2 were related to lower ALS risks (Figure 1), but only human recombinant glucagon was statistically significant at both lags (multiple-testing corrected), with ~25% reduced ALS risks.
All six drugs in the central nervous system group showed elevated risks for ALS at lag1, with statistically associations for baclofen and carbidopa/levodopa. For all drugs besides gabapentin, the magnitude of the adverse association declined between lag1 and lag3, and remained statistically significant only for baclofen, an anti-spasticity drug.
Pyridostigmine was associated with a strongly increased ALS risk at lag1 (OR= 4.45), but the association was weaker and no longer significant at lag3 (Table 2).
In a miscellaneous drug category, potassium chloride was significantly associated with reduced ALS risk (multiple-testing corrected) at both lag-times. Immune globulin, γ-caprylate had a highly elevated risk in lag1, but the model did not converge at lag3 (Table 2).
No drugs were statistically significant in the groupings of analgesics, anti-asthma medications, anti-muscarinic medications, and psychiatric drugs (Table 2).
Table 3 presents sex-stratified associations at lag1 and lag3 for male and female hormones. As noted in Methods, statistical significance for this group with a priori hypotheses refers to p<0.05. Among the estrogen associations we analyzed, tamoxifen, a selective estrogen receptor modulator (SERM), was significant at both lags, with OR = 0.62 (p=0.011) for lag1 and OR = 0.61(p=0.04) for lag3. In contrast, another SERM, raloxifene, was unrelated to ALS risk. In women, testosterone had an OR = 3.93 (p=0.002) at lag1, and OR = 4.92 (p=0.003) at lag3. In men, testosterone was linked to a modestly increased risk only at lag1.
Table 3.
Odds ratios (ORs) and standard errors (Std Errors) from fully adjusted logistic regression models for hormone-related drugs in women and men. Here p-values <0.05 were considered statistically significant and are in shaded cells.
| Drug | 1-year lag analysis | 3-year lag analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| % exposed | % exposed | |||||||||
|
|
|
|
|
|||||||
| Cases | Controls | OR | Std Error | p-value | Cases | Controls | OR | Std Error | p-value | |
| Women | ||||||||||
| Estrogens, conjugated | 8.09 | 5.94 | 1.16 | 0.05 | 0.007 | 6.17 | 5.10 | 0.99 | 0.07 | 0.90 |
| Estradiol | 5.53 | 4.32 | 1.01 | 0.065 | 0.851 | 4.06 | 3.24 | 0.94 | 0.09 | 0.49 |
| Estradiol/levonorgestrel | – | – | 0.42 | 1.03 | 0.407 | 0.69 | 0.14 | 0.58 | 1.05 | 0.60 |
| Estradiol/norethindrone | – | – | 0.68 | 0.37 | 0.293 | – | – | 0.83 | 0.43 | 0.66 |
| Estrogen,con/m-progest | 0.67 | 0.67 | 0.85 | 0.18 | 0.355 | 0.52 | 0.55 | 0.77 | 0.26 | 0.23 |
| Raloxifene | 3.60 | 3.42 | 0.94 | 0.08 | 0.447 | 3.16 | 2.96 | 0.91 | 0.10 | 0.33 |
| Tamoxifen | 0.56 | 0.75 | 0.62 | 0.19 | 0.011 | 0.45 | 0.59 | 0.61 | 0.25 | 0.04 |
| Testosterone | – | – | 3.93 | 0.45 | 0.002 | – | – | 4.92 | 0.54 | 0.003 |
| Men | – | – | – | – | – | – | – | – | – | – |
| Testosterone | 2.22 | 1.41 | 1.27 | 0.10 | 0.027 | 1.29 | 0.90 | 1.12 | 0.15 | 0.47 |
| Tamoxifen | – | – | 1.65 | 0.65 | 0.442 | – | – | – | – | – |
| Estrogens, conjugated | – | – | 1.48 | 0.78 | 0.616 | – | – | – | – | – |
Discussion
In this Medicare study of nearly 700 drugs approved for various medical conditions, we found 10 drugs significantly associated with risk of ALS incidence after correcting for multiple comparisons at both, a one and a three-year lagged analysis. Nine drugs from five different categories were significantly associated with decreased ALS risk. These associations were generally more inverse in the longer lag period, evidence against a reverse causation bias. The drug associated with significantly elevated ALS risk, baclofen, is prescribed for spasticity and showed a lessening risk with longer lag time, consistent with a reverse causation bias. Thus, rather than the drug elevating risk, it may have been prescribed in response to early symptoms of yet undiagnosed ALS. There were also some significant associations with sex hormone drugs tested as a priori hypotheses.
Confounding by indication could, however, influence these findings. Within each drug category defined by underlying medical conditions, more than one drug frequently met the corrected statistical threshold, and there were other medications inversely associated with ALS to the same degree, even though not statistically significant. Thus, one cannot easily discern whether the associations are due to the drugs or the medical conditions underlying the prescriptions. Antidiabetes medications illustrate this problem. In Table 2, all 11 diabetes drugs were inversely related to ALS risk, one significantly. It is not possible to disentangle if the reduced risks relate to the physiological conditions associated with diabetes or the medications prescribed for it. Several studies suggest that diabetes or metabolic syndrome is associated with lower ALS risk among older populations (13–15) or overall (16), but did not adjust for drug use in their models, so these associations are ambiguous as well. That we could control for medical conditions on a yes/no basis is insufficient to distinguish clearly between drug use and the prescribing condition, when virtually all drug users have the underlying condition.
The same issue, confounding by indication, may arise for the antihyperlipidemic drugs in Table 2. Although all antihyperlipidemic drugs were associated with lower ALS risk, Simvastatin use was the only statistically significantly associated drug in that class, likely due to larger numbers than all other antihyperlipidemic drug. Two recent studies found that hyperlipidemia was associated with increased ALS risk using Mendelian randomization techniques (17,18), which was confirmed in our case–control population by a higher prevalence of dyslipidemia in cases compared to controls. The fact that the antihyperlipidemic drugs were associated with lower ALS risk, whereas hyperlipidemia appears to be positively related to ALS makes it unlikely that hyperlipidemia accounts for the inverse associations with antihyperlipidemic drugs. Additional evidence is provided by data presented using the same population from a previous publication (Table 4(e); 7), that indicate that the overall association of statins is protective both in those with and those without hyperlipidemia.
Future studies that account for duration and severity of the underlying medical conditions, as well as intensity of medication use over the treatment period may help elucidate the contribution of drugs and conditions to observed associations.
Despite these difficulties, we note several findings that are supported by experimental or other studies. The antibiotic azithromycin in conjunction with another compound, extended survival (beyond that expected from the other compound alone) in an animal model for spinal muscular atrophy, another motor neuron disease (19). Prior evidence supports the role of ACE inhibitors as modulators of neurodegenerative disorders (20). In our study, an ACE inhibitor, lisinopril, was significantly associated with lower ALS risk. A population-based case–control study in Taiwan also found that ACE inhibitors taken over several years were related to lower ALS risk (21).
Peroxisome proliferator-activated receptor (PPAR) γ-agonists have also shown promise as neuroprotective agents for Alzheimer’s disease, Parkinson’s disease, and ALS (22–24). Here, PPAR γ-agonists, including pioglitazone, rosiglitazone, and the PPAR α-agonist, fenofibrate, were suggestively inversely related to ALS.
In addition to exploring nearly 700 drugs, we also hypothesized a priori relationships between sex hormones and ALS risk. Prior evidence suggests that estrogens may protect against ALS. Experimental research supports a role for estrogens in promoting neuronal survival and recovery (25). A recent epidemiologic study showed some exogenous estrogens and progestogens were related to reduced risk (26). In an animal ALS model ovariectomized female mice had earlier disease onset (27). Also, males generally have higher sporadic ALS incidence (2). While we did not observe inverse associations with female hormones or estrogenic drugs generally, one SERM, tamoxifen, was related to reduced risk. Although a clinical trial found that higher tamoxifen doses extended survival after ALS onset (28), to our knowledge, no study examined tamoxifen in relation to incident risk. However, there was no relationship between ALS and raloxifene, another SERM hypothesized to have therapeutic neuroprotective effects (29).
While some evidence points to a neuroprotective role of testosterone (25), the higher ALS incidence in men (3), and a study showing higher testosterone levels in women with ALS compared to controls (30), suggest that male hormones may increase risk. We found testosterone use related to elevated risk, particularly in women, with a stronger association in lag3, supporting a causal relationship.
Strengths of this study include the large number of ALS cases (more than 10-fold larger than any other study), using incident cases identified by physician and hospital visits; the population-based, nationwide design; multiple races/ethnicities; and adjusting for surveillance intensity and underlying conditions. Due to the large numbers of cases, we could investigate drugs that are rarely used, such as testosterone in women. We applied a strict multiple-testing adjustment, and of note, an even stricter adjustment for the number of both lag1 and lag3 tests combined, would still lead to the same conclusions, as only two drugs, lisinopril and glucagon, human recombinant, would not be significant using the stricter threshold.
One limitation is the restriction of the study population to those aged 66+ years, when the median age of onset is somewhat younger (31). Other limitations include reliance on administrative data and thus an inability to apply clinical criteria such as the Airlie House diagnostic criteria, to identify cases. However, these criteria have been found to be too restrictive (32), and when we previously defined ALS cases more conservatively, associations of ALS risk with statins use did not change (7).
Using administrative data also leads to missing information on covariates such as fitness, and inadequately defined factors, including obesity and smoking. BMI has been negatively related to ALS while smoking probably increases ALS risk (33). Because both smoking and BMI are important risk factors for cardiovascular disease, and BMI is a risk factor for diabetes, smoking and BMI are likely positively related to drug prescriptions intended to treat these medical conditions. Our limited ability to adjust for BMI could thus potentially have contributed to the negative association we observed with several drugs. In contrast, because smoking increases ALS risk (assuming it is related to risk), inadequately controlling for smoking would not have contributed to the inverse relationships that we observed for many drugs.
Another potential limitation is that individuals signing up for part D coverage could do so because of preexisting conditions requiring medications, and thus, our controls could be enriched by those with diabetes or cardiovascular diseases. However, both ALS cases and controls had similar durations of Medicare Part D coverage before selection, and ALS cases would have had to enroll due to ALS-related clinical symptoms, which seem unlikely to have been present 3+ years before diagnosis.
In summary, in this exploratory study of nearly 700 drugs using nationwide Medicare data, nine drugs were significantly associated with lower ALS risk at both, a one-year and a three-year lag, after a strict multiple-testing correction. In a focused inquiry of sex hormone drugs, tamoxifen was related to lower and testosterone, to higher ALS risk in women. Future studies able to adjust more fully for BMI and other possible confounders should replicate our findings on the associations of the identified drugs with ALS risk and possibly examine their associations also in relation to duration and intensity of medication use and medical conditions.
Supplementary Material
Acknowledgments
The authors thank Matthew Chaloux of Information Management Services, Inc. for biomedical computer assistance, and Adrienne Rolls for help with the references.
Funding
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the U.S. Public Health Service.
Footnotes
Ethics approval
The study was exempt from institutional research board review.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
This work was authored as part of the Contributor’s official duties as an Employee of the United States Government and is therefore a work of the United States Government. In accordance with 17 USC. 105, no copyright protection is available for such works under US Law
References
- 1.Turner MR, Hardiman O, Benatar M, Brooks BR, Chio A, de Carvalho M. Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol. 2013;12: 310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol. 2013;9:617–28. [DOI] [PubMed] [Google Scholar]
- 3.Mehta P, Kaye W, Raymond J, Punjani R, Larson T, Cohen J, et al. Prevalence of amyotrophic lateral sclerosis — United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67:1285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen PM, Al-Chalabi A. Clinical genetics of amyotrophic lateral sclerosis: what do we really know? Nat Rev Neurol. 2011;7:603–15. [DOI] [PubMed] [Google Scholar]
- 5.Oskarsson B, Gendron TF, Staff NP. Amyotrophic lateral sclerosis: an update for 2018. Mayo Clin Proc. 2018; 93: 1617–28. [DOI] [PubMed] [Google Scholar]
- 6.Martinez A, Palomo Ruiz MD, Perez DI, Gil C. Drugs in clinical development for the treatment of amyotrophic lateral sclerosis. Expert Opin Investig Drugs. 2017;26: 403–14. [DOI] [PubMed] [Google Scholar]
- 7.Freedman DM, Kuncl RW, Cahoon EK, Rivera DR, Pfeiffer RM. Relationship of statins and other cholesterol-lowering medications and risk of amyotrophic lateral sclerosis in the US elderly. Amyotroph Lat Scler Fr. 2018; 19:538–46. [DOI] [PubMed] [Google Scholar]
- 8.Engels EA, Pfeiffer RM, Ricker W, Wheeler W, Parsons R, Warren JL. Use of surveillance, epidemiology, and end results-medicare data to conduct case-control studies of cancer among the US elderly. Am J Epidemiol. 2011;174: 860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oskarsson B, Horton DK, Mitsumoto H. Potential environmental factors in amyotrophic lateral sclerosis. Neurol Clin. 2015;33:877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonito AJ, Bann C, Eicheldinger C, Carpenter L. Creation of new race-ethnicity codes and socioeconomic status (SES) indicators for Medicare beneficiaries. Rockville, MD: Agency for Healthcare Research and Quality and Center for Medicare and Medicaid Services; 2008. [Google Scholar]
- 11.Traxinger K, Kelly C, Johnson BA, Lyles RH, Glass JD. Prognosis and epidemiology of amyotrophic lateral sclerosis: analysis of a clinic population, 1997–2011. Neurol Clin Pract. 2013;3:313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blasco H, Guennoc AM, Veyrat-Durebex C, Gordon PH, Andres CR, Camu W, et al. Amyotrophic lateral sclerosis: a hormonal condition?. Amyotroph Lateral Scler. 2012; 13:585–8. [DOI] [PubMed] [Google Scholar]
- 13.Kioumourtzoglou MA, Rotem RS, Seals RM, Gredal O, Hansen J, Weisskopf MG. Diabetes mellitus, obesity, and diagnosis of amyotrophic lateral sclerosis: a population-based study. JAMA Neurol. 2015;72:905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariosa D, Kamel F, Bellocco R, Ye W, Fang F. Association between diabetes and amyotrophic lateral sclerosis in Sweden. Eur J Neurol. 2015;22:1436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai CP, Lee JKW, Lee C. Type II diabetes mellitus and the incidence of amyotrophic lateral sclerosis. J Neurol. 2019; 266:2233. [DOI] [PubMed] [Google Scholar]
- 16.D’Ovidio F, d’Errico A, Carna P, Calvo A, Costa G, Chio A. The role of pre-morbid diabetes on developing amyotrophic lateral sclerosis. Eur J Neurol. 2018; 25: 164–70. [DOI] [PubMed] [Google Scholar]
- 17.Zeng P, Zhou X. Causal effects of blood lipids on amyotrophic lateral sclerosis: a mendelian randomization study [Internet]. Hum Mol Genet. 2019; 28:688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bandres-Ciga S, Noyce AJ, Hemani G, Nicolas A, Calvo A, Mora G, et al. Shared polygenic risk and causal inferences in amyotrophic lateral sclerosis. Ann Neurol. 2019;85:470–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osman EY, Washington CW, Simon ME, Megiddo D, Greif H, Lorson CL. Analysis of azithromycin monohydrate as a single or a combinatorial therapy in a mouse model of severe spinal muscular atrophy. J Neuromuscul Dis. 2017;4:237–49. [DOI] [PubMed] [Google Scholar]
- 20.Kaur P, Muthuraman A, Kaur M. The implications of angiotensin-converting enzymes and their modulators in neurodegenerative disorders: current and future perspectives. ACS Chem Neurosci. 2015;6:508–21. [DOI] [PubMed] [Google Scholar]
- 21.Lin FC, Tsai CP, Kuang-Wu Lee J, Wu MT, Tzu-Chi Lee C. Angiotensin-converting enzyme inhibitors and amyotrophic lateral sclerosis risk. JAMA Neurol. 2015;72: 40–8. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal S, Yadav A, Chaturvedi RK. Peroxisome proliferator-activated receptors (PPARs) as therapeutic target in neurodegenerative disorders. Biochem Biophys Res Commun. 2017;483:116–1177. [DOI] [PubMed] [Google Scholar]
- 23.Mosley RL, Gendelman HE. Control of neuroinflammation as a therapeutic strategy for amyotrophic lateral sclerosis and other neurodegenerative disorders. Exp Neurol. 2010;222:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zufirí M, Gil-Bea FJ, Fernandez-Torrón R, Poza JJ, Muñoz-Blanco JL, Rojas-García R, et al. ALS: A bucket of genes, environment, metabolism and unknown ingredients. Prog Neurobiol. 2016;142:104–29. [DOI] [PubMed] [Google Scholar]
- 25.Saldanha C, Duncan K, Walters B. Neuroprotective actions of brain aromatase. Front Neuroendocrinol. 2009;30:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rooney JPK, Visser AE, D’Ovidio F, Vermeulen R, Beghi E, Chio A, et al. A case-control study of hormonal exposures as etiologic factors for ALS in women. Neurology. 2017;89:1283–90. [DOI] [PubMed] [Google Scholar]
- 27.Yan L, Liu Y, Sun C, Zheng Q, Hao P, Zhai J, et al. Effects of ovariectomy in an hSOD1-G93A transgenic mouse model of amyotrophic lateral sclerosis (ALS). Med Sci Monit. 2018;24:678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orsini M, Oliveira AB, Nascimento OJM, Reis CHM, Leite MAA, De Souza JA, et al. Amyotrophic lateral sclerosis: new perpectives and update. Neurol Int. 2015;7: 5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou F, Dong H, Liu Y, Yan L, Sun C, Hao P, et al. Raloxifene, a promising estrogen replacement, limits TDP-25 cell death by enhancing autophagy and suppressing apoptosis. Brain Res Bull. 2018; 140:281–90. [DOI] [PubMed] [Google Scholar]
- 30.Gargiulo-Monachelli GM, Sivori M, Meyer M, Sica R, De Nicola A, Gonzalez-Deniselle MC. Circulating gonadal and adrenal steroids in amyotrophic lateral sclerosis: possible markers of susceptibility and outcome. Horm Metab Res. 2014;46:433–9. [DOI] [PubMed] [Google Scholar]
- 31.Van Es MA, Hardiman O, Chio A, Al-Chalabi A, Pasterkamp RJ, Veldink JH, et al. Amyotrophic lateral sclerosis. Lancet. 2017;390:2084–98. [DOI] [PubMed] [Google Scholar]
- 32.Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman OM. Clinical features of amyotrophic lateral sclerosis according to the El Escorial and Airlie House diagnostic criteria - a population-based study. Arch Neurol. 2000;57:1171–6. [DOI] [PubMed] [Google Scholar]
- 33.Ingre C, Roos RM, Piehl F, Kamel F, Fang FC. Risk factors for amyotrophic lateral sclerosis. Clin Epidemiol. 2015;7:181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.