In this issue of RPTH, Choi et al analyze the outcomes of 50 cases of COVID-19 vaccination–associated immune thrombocytopenia (ITP) diagnosed in Australia, describing 37 cases of de novo vaccine-associated ITP and 13 cases of relapsed prior ITP [1]. Many of their findings, consistent with prior studies of COVID-19 vaccination in ITP, are highly clinically relevant. They found that most patients with COVID-19 vaccination–associated ITP responded quickly and robustly to ITP therapies, although 1 patient did die because of an early fatal intracerebral hemorrhage. As it has been demonstrated in acute ITP exacerbations long before COVID-19, they found shorter time to treatment responses with dexamethasone-based treatment as compared with prednisone-based treatment. Need for persistent ITP treatment beyond 90 days was more likely in patients presenting with early postvaccination thrombocytopenia (within 10 days) than those presenting later. Ultimately, approximately one-third of patients needed additional therapy beyond corticosteroids and intravenous immunoglobulin and were primarily treated with the nonimmunosuppressive thrombopoietin receptor agonists. No patients had thrombosis or findings concerning for vaccine-induced immune thrombotic thrombocytopenia. Perhaps most intriguing, they found that de novo ITP appeared to be more likely to follow ChAdOx1 nCoV-19 (Vaxzevria, AstraZeneca, adenoviral vector vaccine) administration as compared with BNT162b2 (Comirnaty, Pfizer, mRNA vaccine) administration, with 19-fold higher odds. De novo ITP cases following ChAdOx1 were also observed to be more likely to be persistent, requiring second-line therapy.
Both de novo ITP and acute exacerbation of pre-existing ITP are both well recognized to be triggered by (or become clinically apparent following exposure to) immunologic stimuli. However, prior studies have found no difference in vaccine exposure between patients with newly diagnosed ITP and matched controls [2]. The influenza vaccine, administered to hundreds of millions of people in the world yearly, does not appear to have an association with de novo ITP but may have a possible weak association with acute exacerbation of pre-existing ITP [3,4]. There may be an association with de novo ITP in the 6 weeks following the measles, mumps, and rubella (MMR) vaccine in toddlers [5], but in a study evaluating children who developed ITP after receiving MMR, none had a recurrence after a subsequent dose of MMR vaccine [6].
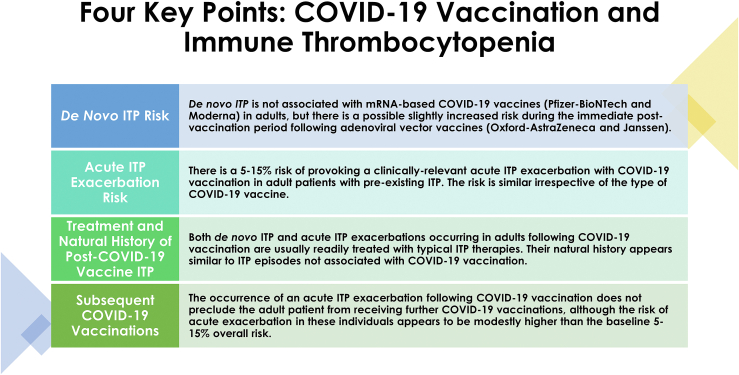
Observational studies investigating vaccine-associated complications are notoriously challenging and almost universally suffer from potential confounding. They are often hampered by voluntary, incomplete, or unverifiable reporting of adverse events of interest. For example, the widely cited US Vaccine Adverse Event Reporting System allows any individual to make a report and does not independently verify reports. Nevertheless, despite these limitations, we can gain a clear picture of the likely potential complications and associations of new vaccinations through an accumulation of independently performed, methodologically sound studies from various groups across the world. Thankfully, this is exactly what the ITP academic community has delivered with regards to COVID-19 vaccination over the past year. The work by Choi et al characterizing COVID-19 vaccination–associated ITP in Australia is quite consistent with the papers that preceded it from other regions of the world, and therefore acts as another source of evidence reinforcing the general conclusions drawn from this collective body of work to date. These general conclusions can be summarized as follows (Figure).
-
1)
De novo ITP does not appear to be associated with mRNA-based COVID-19 vaccines, but adenoviral vector vaccines may have an association with de novo ITP, with a slightly increased risk during the immediate postvaccination period in adult patients.
Figure.
The 4 key points regarding COVID-19 vaccination and immune thrombocytopenia based on data published in 2021 and 2022
Although the work by Choi et al found a substantially higher odds of development of de novo ITP following ChAdOx1 vaccination compared with BNT162b2 vaccination, it was not the first study to find such an association. A large, nested case-control study from investigators in Scotland evaluating 2.53 million vaccinated persons found an approximately 6-fold greater risk of development of de novo ITP following ChAdOx1 vaccination compared with BNT162b2 in the 4 weeks following vaccination [7]. This equated to an incidence of 1.13 cases per 100,000 doses of vaccine administered (for context, the background incidence of ITP in western populations is approximately 3 cases per 100,000 persons per year [8]). There was no association with increased de novo ITP risk with BNT162b2 administration. These findings were echoed in a large French registry study [9] and an analysis of the EudraVigilance, UK MHRA, and Health-Infobase databases [10] and are consistent with the findings regarding de novo ITP incidence in the United States, which did not employ ChAdOx1 and administered comparatively few doses of any adenoviral vector vaccine. In a study analyzing the US Vaccine Adverse Event Reporting System, 77 possible de novo ITP cases (nearly all reported in the 4 weeks following vaccination) had been reported after 35 million individuals had been vaccinated with exclusively mRNA-based vaccines, an overall incidence consistent with the background rate of de novo ITP [11,12]. Although there is a paucity of data regarding potential risk with Ad26.COV2.S (Jcovden, Janssen, adenoviral vector vaccine), in January 2022 Janssen requested that the US FDA update the existing emergency use authorization for this vaccine with a warning advising providers and the public of an increased risk of de novo ITP during the 6 weeks following vaccination and of the potential need for platelet monitoring in patients with known ITP receiving it. Of note, all of the aforementioned studies were performed in adult patients, and the risk of de novo ITP with COVID vaccination in pediatric patients remains unknown.
-
2)
There is an approximately 5%–15% risk of provoking a clinically relevant acute ITP exacerbation with COVID-19 vaccination in adult patients with pre-existing ITP. This risk appears to be similar irrespective of the type of COVID-19 vaccine.
Numerous cohorts have been published assessing the risk of acute ITP exacerbation in adults with ITP receiving COVID-19 vaccination [11,[13], [14], [15], [16], [17]]. Rates of exacerbation ranged between approximately 5% and 15%, although each study defined an acute exacerbation differently. Definitions ranged from relatively liberal (i.e., a relative decrease in platelet count of ≥50% without any requirement for severe thrombocytopenia below a certain threshold or bleeding symptoms) to much more stringent (i.e., a requirement for new or escalated ITP-directed treatment or new bleeding requiring medical attention). Unsurprisingly, the studies with more stringent definitions reported lower acute exacerbation rates, and those with more liberal definitions reported higher acute exacerbation rates. Serious bleeding was rare in patients developing acute exacerbations. Multiple studies compared rates of acute exacerbation between the different vaccines, and none found a relevant difference regarding risk. Again, these data are in adult patients; no comparable cohorts have been published to date in the pediatric ITP population.
-
3)
When de novo ITP or acute exacerbations of pre-existing ITP occur following COVID-19 vaccination, they are usually readily treated with corticosteroids, IVIG, or standard second-line ITP therapies (such as thrombopoietin receptor agonists). The natural history of these ITP episodes appears similar to those in patients experiencing them not in association with COVID-19 vaccination.
Essentially every study published describing the outcomes of treatment of large cohorts of patients with COVID-19 vaccination–associated ITP describes similar findings to that of Choi et al: when it occurs, de novo ITP and acute exacerbations of pre-existing ITP are readily treated with typical ITP therapies in the vast majority of patients [11,[13], [14], [15], [16]]. Some series and a number of case reports describe refractory ITP cases following vaccination, but this is clearly a significant minority. Therefore, COVID-19 vaccination–associated ITP should be managed similarly to typical exacerbations of primary ITP and the prognosis appears similar.
-
4)
The occurrence of an acute ITP exacerbation following COVID-19 vaccination does not preclude that patient from receiving further COVID-19 vaccination. Most such patients are able to receive subsequent doses without a recurrent exacerbation, although their risk with these doses may be higher than the baseline ∼5%–15% overall risk.
In a multicenter US/UK study evaluating 117 adults with ITP receiving COVID-19 vaccination, those patients with drops in their platelet counts of >20% following their first COVID-19 vaccination had a 44% risk of having a drop of >20% after their second dose, suggesting a potential patient-specific platelet count sensitivity to vaccination [12]. However, of the patients who had a significant enough exacerbation to require rescue treatment after the first dose, 80% did not require rescue treatment again after the second dose. Therefore, patients developing an acute ITP exacerbation after a COVID-19 vaccination should have close monitoring of platelet counts around the time of subsequent vaccinations but excepting an extremely complicated and protracted exacerbation after a prior dose, subsequent COVID-19 vaccination and boosters should not be withheld from these patients.
Ultimately, the most important conclusions from the accumulated data are that adults with ITP and without other vaccine contraindications should receive COVID-19 vaccination and that the risk of de novo ITP following COVID-19 vaccination is either negligible (mRNA-based vaccines) or very small (adenoviral vector vaccines). There are no consensus guidelines for monitoring of patients with ITP around the time of receipt of COVID-19 vaccination, and consideration of the patient-specific disease history (severity, chronicity, refractoriness to treatment, prior exacerbation following infection or vaccination, and comorbidities) should be considered when formulating a plan for peri-vaccine monitoring. Given the state of our knowledge of this topic and the recognition that approximately 5%–15% of patients will have a clinically significant drop in platelet count, develop bleeding manifestations, and/or require rescue therapy, it is reasonable to obtain a baseline platelet count just before vaccination and repeat platelet count once or twice during the 4-week postvaccination period. Patients should also be counseled to remain vigilant for bleeding and other symptoms they may associate with ITP exacerbation. On the other hand, there is no role for laboratory monitoring persons without ITP for the development of ITP after COVID-19 vaccination in the absence of symptoms compatible with ITP, including those patients receiving adenoviral vector vaccines. Again, all of these statements apply to adult patients; they may hold true in pediatric patients, but we currently lack any significant data in children in this area.
As newer COVID-19 vaccine formulations have been released and continue to be developed, additional studies will be needed to confirm the aforementioned principles continue to hold true. Until then, the work by Choi et al and others like it will continue to form the basis for our understanding of this important potential complication of COVID-19 vaccination.
Author contributions
As the sole author, H.Al-S. was responsible for all aspects of the manuscript.
Relationship Disclosures
H.Al-S. has no disclosures relevant to the manuscript. Universal disclosures include Consultancy (Agios, Sobi, Argenx, Rigel, Novartis, Moderna, Forma) and Research Funding (Agios, Sobi, Amgen).
Acknowledgments
H.Al-S. is the recipient of the American Society of Hematology Scholar Award. H.Al-S. is funded by the National Heart, Lung, and Blood Institute (1K23HL159313).
Footnotes
Handling Editor: Pantep Angchaisuksiri
References
- 1.Young-Ill Choi P., Hsu D., Tran H.A., Tan C.W., Enjeti A., Yee Chen V.M., et al. Immune thrombocytopenia and COVID-19 vaccination: outcomes and comparisons to pre-pandemic patients. Res Pract Thromb Haemost. 2023;7:100009. doi: 10.1016/j.rpth.2022.100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimaldi-Bensouda L., Michel M., Aubrun E., Leighton P., Viallard J.F., Adoue D., et al. A case-control study to assess the risk of immune thrombocytopenia associated with vaccines. Blood. 2012;120:4938–4944. doi: 10.1182/blood-2012-05-431098. [DOI] [PubMed] [Google Scholar]
- 3.Garbe E., Andersohn F., Bronder E., Salama A., Klimpel A., Thomae M., et al. Drug-induced immune thrombocytopaenia: results from the Berlin Case-Control Surveillance Study. Eur J Clin Pharmacol. 2012;68:821–832. doi: 10.1007/s00228-011-1184-3. [DOI] [PubMed] [Google Scholar]
- 4.Lafaurie M., Lapeyre-Mestre M., Sailler L., Sommet A., Moulis G. Risk of immune thrombocytopenia after influenza vaccine. JAMA Intern Med. 2022;182:444–445. doi: 10.1001/jamainternmed.2021.8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black C., Kaye J.A., Jick H. MMR vaccine and idiopathic thrombocytopaenic purpura. Br J Clin Pharmacol. 2003;55:107–111. doi: 10.1046/j.1365-2125.2003.01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stowe J., Kafatos G., Andrews N., Miller E. Idiopathic thrombocytopenic purpura and the second dose of MMR. Arch Dis Child. 2008;93:182–183. doi: 10.1136/adc.2007.126003. [DOI] [PubMed] [Google Scholar]
- 7.Simpson C.R., Shi T., Vasileiou E., Katikireddi S.V., Kerr S., Moore E., et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021;27:1290–1297. doi: 10.1038/s41591-021-01408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michel M. Immune thrombocytopenic purpura: epidemiology and implications for patients. Eur J Haematol Suppl. 2009;82:3–7. doi: 10.1111/j.1600-0609.2008.01206.x. [DOI] [PubMed] [Google Scholar]
- 9.Moulis G., Crickx E., Thomas L., Massy N., Mahevas M., Valnet-Rabier M.B., et al. De novo and relapsed immune thrombocytopenia after COVID-19 vaccines: results of French safety monitoring. Blood. 2022;139:2561–2565. doi: 10.1182/blood.2022015470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mingot-Castellano M.E., Butta N., Canaro M., Gomez Del Castillo Solano M.D.C., Sanchez-Gonzalez B., Jimenez-Barcenas R., et al. (Basel); Vaccines: 2022. COVID-19 vaccines and autoimmune hematologic disorders; p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee E.J., Beltrami-Moreira M., Al-Samkari H., Cuker A., DiRaimo J., Gernsheimer T., et al. SARS-CoV-2 vaccination and ITP in patients with de novo or preexisting ITP. Blood. 2022;139:1564–1574. doi: 10.1182/blood.2021013411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee E.J., Cines D.B., Gernsheimer T., Kessler C., Michel M., Tarantino M.D., et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96:534–537. doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuter D.J. Exacerbation of immune thrombocytopenia following COVID-19 vaccination. Br J Haematol. 2021;195:365–370. doi: 10.1111/bjh.17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visser C., Swinkels M., van Werkhoven E.D., Croles F.N., Noordzij-Nooteboom H.S., Eefting M., et al. COVID-19 vaccination in patients with immune thrombocytopenia. Blood Adv. 2022;6:1637–1644. doi: 10.1182/bloodadvances.2021006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woolley P., Tailor A., Shah R., Westwood J.P., Scully M. Real-world, single-center experience of SARS-CoV-2 vaccination in immune thrombocytopenia. J Thromb Haemost. 2022;20:1476–1484. doi: 10.1111/jth.15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aharoni M., Leader A., Shochat T., Raanani P., Spectre G. Exacerbation of immune thrombocytopenia following initial and booster vaccination with Pfizer-BioNTech COVID-19 vaccine. Platelets. 2022;33:781–786. doi: 10.1080/09537104.2022.2071856. [DOI] [PubMed] [Google Scholar]
- 17.Crickx E., Moulis G., Ebbo M., Terriou L., Briantais A., Languille L., et al. Safety of anti-SARS-CoV-2 vaccination for patients with immune thrombocytopenia. Br J Haematol. 2021;195:703–705. doi: 10.1111/bjh.17813. [DOI] [PMC free article] [PubMed] [Google Scholar]