Abstract
Background:
Papillary breast lesions and neoplasms (PBLs/Ns) are diagnostically challenging lesions in both core needle biopsy (CNB) and radiology.
Aim:
To determine the accuracy and upgrade rate of CNB and BI-RADS diagnosis of PBLs/Ns compared to final excision diagnosis and the factors linked to upgrade.
Methods:
The favored CNB diagnosis and BI-RADS category for 82 PBLs/Ns were assessed based on histopathology, myoepithelial marker immunohistochemistry, mammographic/ultrasonographic findings. The radiological findings were compared to the pathological diagnoses. The accuracies of CNB and BI-RADS were compared to the excision diagnosis of the corresponding PBLs/Ns. The upgrade rates to malignancy were evaluated for both CNB and BI-RADS.
Results:
The presence of solid, irregular masses in breasts with composition A/B with calcification in radiology was significantly associated with the diagnosis of suspicious/malignant CNB, and malignant excision specimens (p<0.05). CNB was more accurate (90%), sensitive and specific with high positive and negative predictive values than BI-RADS. Combined CNB/BI-RADS accuracy was 90.2%. Overall upgrade rate came up to 9.8%. Upgrade rates to carcinoma were 7.3% for CNB and 8.5% for BI-RADS. Factors linked to upgrade were the age, lesion-size, BI-RADS category 4A and C, and histopathological/radiological discordance. All the upgraded PBLs/Ns were diagnosed as benign lesions in CNB with present/focally present myoepithelial diagnosis reflecting a sampling error.
Conclusion:
Up to 9.8% of PBLs/Ns diagnosed on CNB and BI-RADS undergo upgrading upon final excision, despite the high diagnostic accuracy. These evidences should be considered for final decision on whether to excise the lesion or not.
Key Words: Papillary breast, upgrading, accuracy, BI-RADS, core needle biopsy
Introduction
Papillary breast neoplasms (PBNs) are a heterogeneous group of epithelial tumors composed mostly of papillae. These lesions account for less than 3% of breast tumors and their classification rests on the characteristics of the epithelium and the presence and distribution of the myoepithelial cells along the papillae and around the tumor (Nunez et al., 2020; Brogi and Krystel-Whittemore, 2021). Based on the most updated World Health Organization (WHO) classification of breast neoplasms, PBNs include: intraductal papilloma (IDP) with or without atypia, papillary ductal carcinoma in situ (papillary DCIS), encapsulated papillary carcinoma (EPC), solid-papillary carcinoma (SPC; in situ and invasive), and invasive papillary carcinoma (IPC) (WHO Editorial Board, 2019). However, previous studies have suggested that 8.1% of breast biopsies contain lesions with a papillary component (Cuneo et al., 2012). Such lesions range from non-neoplastic lesions as fibrocystic disease with papillomatosis (Batori et al., 2000), and micropapillary usual ductal hyperplasia, to frankly malignant lesions as invasive ductal carcinoma with micropapillary features or with a papillary in situ component (Cuneo et al., 2012; Ni and Tse, 2016; Guan et al., 2020).
Although percutaneous imaging-guided core needle biopsy (CNB) is a reliable method for the diagnosis of breast lesions in general, papillary breast lesions (PBLs) identified at CNB biopsy are diagnostically challenging and can be difficult to interpret histologically (Chen et al., 2019; Brogi and Krystel-Whittemore, 2021). Noteworthy, a limited/fragmented material or a sampling error at CNB may result in missing the presence of atypia or malignancy within or adjacent to the lesion. Therefore, surgical excision following CNB is considered gold standard for treating papillary lesions and ruling out coexisting malignancy when a diagnosis of papilloma with atypia is yielded on CNB (Chen et al., 2019). Upon excision, upgrade of papillary breast lesions to an in situ or invasive carcinoma is reported (Mooney et al., 2016; Zhang et al., 2021). This increases the debate whether all papillary lesions require surgical excision for appropriate management or not. So, studying the upgrading to malignancy in patients with benign papillary lesions in CNB is of utmost importance to determine the management of choice (Park et al., 2020).
Additionally, PBL/Ns not only constitute a pathologically heterogeneous group, but also exhibit diverse clinical and imaging criteria (Chen et al., 2019; Nunez et al., 2020). Recently, the American College of Radiology has proposed the Breast Imaging-Reporting and Data System (BI-RADS) to standardize reporting of breast imaging results and to define clear guidelines for further management. Lesions are classified along 7 main diagnostic categories (0 to 6), with 3 additional subcategories for category 4 (4A, 4B, and 4C). For each BI-RADS category, an increasing positive predictive value (PPV) for malignancy is assigned (American College of Radiology, 2013). For BI-RADS categories 4C and 5, pathological analysis is warranted because of PPV for cancer (Farras et al., 2021).
Aim
The goal of this study was to assess the accuracy of radiology and CNB in the diagnosis of papillary breast lesions and neoplasms (PBL/Ns) compared to the results of surgical excision specimens, and to evaluate the rate of upgrading these lesions, specifically to malignant ones following excision. Furthermore, the clinicopathological characteristics and radiological features of these lesions are designated.
Materials and Methods
Study design and settings
This retrospective-cross sectional study was conducted at the Oncology Centre Mansoura University (OCMU). Based on searching the electronic database system for medical records, cases diagnosed with PBLs/Ns in CNB biopsy during the period from January 2016 to September 2020 and who underwent previous radiological assessment and subsequent surgical excision of the same breast lesion were enrolled. Inclusion criteria were: (1) the accessibility to complete medical records, (2) the availability of ultrasonographic and mammographic radiological data/images, and (3) the presence of archived pathological slides and formalin-fixed, paraffin-embedded tissue blocks (FF-PETB) for both CNB and excision biopsy at the pathology laboratory of the same center.
The procedure of the study was conducted according to the current revision of Helsinki Declaration (The World Medical Association, 2013). The study was approved by the institutional research board (code: R21.08.1405.R1). Informed consent was obtained from the patients as appropriate.
Data collection, histopathological and radiological evaluation
After collection and tabulation of the clinicopathological data, the radiological parameters were mutually collected from reports and re-assessed from archived radiological images whenever appropriate. These data included: size, shape and number of masses, nature of the mass (solid, cystic, area of parenchymal distortion), ultrasound and mammographic breast composition (A; entirely fatty, B; fibro-glandular, C; heterogeneously dense and D; extremely dense), presence or absence of calcification and its pattern, ultrasonographic mass margin, orientation, echo-pattern, posterior shadowing and the associated features (edema, internal vascularity, architectural distortion and duct prominence-dilation-thick wall), and the presence of an associated lymphadenopathy. The BI-RADS category was evaluated according to the protocol of the American College of Radiology (ACR) (American College of Radiology, 2013), and the favored radiologic diagnosis as benign, or suspicious/malignant was given for each case.
To re-evaluate the CNB, routinely-stained hematoxylin and eosin tissue sections were examined along with the immunohistochemical slides-stained with antibodies against cytokeratin 5/6, smooth muscle action (SMA), and p63 (at least two myoepithelial markers, one cytoplasmic and the other nuclear) in addition to estrogen receptor (ER) or pan-cytokeratin according to our laboratory protocol. Conforming to the recommended criteria, the myoepithelial markers were evaluated both throughout the papillae, and on the periphery of the lesion and classified as: present/focally present or absent (WHO Editorial Board, 2019). The final histopathologic diagnosis of CNB was given upon agreement of the 2 examining histopathologists. The cases were divided into 3 CNB categories: benign, suspicious, and malignant (Uğur Kılınç et al., 2021).
Excision biopsies of the same lesions were re-evaluated independently by 2 pathologists who were blinded of the result of the CNB. After giving the final histopathological diagnosis and its categorization as: excision “benign” or “malignant”, the BIADS category (favored radiological diagnosis) and the CNB histopathologic diagnosis were compared to the final diagnosis obtained from the excision biopsy to assess the accuracy and upgrading rate of these lesions. Upgrading was defined as the identification of atypia, DCIS or carcinoma after surgical excision (Park et al., 2020), which is further classified as “overall upgrade” to atypical papilloma, papilloma with atypical hyperplasia, carcinoma in situ, or invasive carcinoma; and “upgrade to carcinoma” (Zhang et al., 2021).
Basic procedures
CNB was performed using the 14-gauge needle gun method with ultrasonography guidance and infiltration of an anesthetic though the pathway up to the lesion. Optimally, 3 to 5 cores were obtained from different areas in each lesion, usually from the center and close to the borders at the 3, 6, 9 and 12 o’clock positions (Rocha et al., 2013).
Immunohistochemistry (IHC) was accomplished with Autostainer Link 48, using its adjusted reagents with pharmDx kits EnVisionTM FLEX Visualization Systems (Link code K8000) and EnVision FLEX Hematoxylin (Link code K8008) according to the user’s-guide procedure pre-programmed into the autostainer software. Pre-treatment (dewaxing and dehydration) of FF-PE sections with heat-induced epitope retrieval (HIER) using the 3-in-1 specimen preparation procedure was done with these parameters: pre-heat temperature: 65 °C; epitope retrieval: 97 °C for 20 minutes; cool down to 65 °C. The automated protocol is based on an indirect biotin-avidin system and uses a universal biotinylated immunoglobulin secondary antibody, and diaminobenzidine (DAB) colorizing substrate. After the staining procedure has been completed, the sections were dehydrated, cleared and mounted.
Statistical analysis
Analysis was performed using IBM Corp. SPSS (International Business Machines Corporation Statistical Product and Service Solutions), released 2013 for Windows, Version 22.0. Armonk, NY: IBM Corp. Continuous variables were presented as mean± standard deviation or as median and interquartile range (min-max). Categorical variables were expressed as frequencies and percentages. After testing normality using Kolmogorov-Smirnov test, the Pearson’s Chi-Square, Fischer exact and Monte Carlo tests were used to compare categorical variables as appropriate. The diagnostic accuracy was detected using cross-tabulation for calculation of sensitivity, specificity, positive and negative predictive values (PPV, NPV) and accuracy after determination of true-positive and true-negative parameters. Probability (p-value) <0.05 was considered statistically significant.
Results
The study was applied on 82 breast lesions obtained from 81 female and one male patients with a mean age of 58.5 years, the clinical criteria of whom are presented in Table 1. Radiologically, most of the lesions were unilateral (96.3%), single, solid (90.2% each), with a nearly equal distribution of mammographic breast composition as A, B, C and a more frequently homogenous ultrasonographic composition and parallel orientation (62.2% each). Lesions with angular margins (64.6%), irregular shape (57.3%), and hypoechoic pattern (776.8%) were more common. Calcification was evident in 20.7% of lesions and 61% of them were associated with axillary lymphadenopathy. Most of the lesions were classified as BI-RADS category 4 (61 lesions; 74.4%) including: 23 as 4A; 17 as 4B; and 21 as 4C. The given favored radiological diagnosis was suspicious/malignant in 91.5% of cases (Figure 1).
Table 1.
The Clinical, Radiological and Pathological Criteria of the Included 82 Patients with Papillary Breast Lesions
| Criteria | Number (%) |
|---|---|
| Clinical criteria | |
| Mean age (range)/years | 58.5 (34-82) |
| Gender | |
| Female | 81 (98.8) |
| Male | 1 (1.2) |
| Family history of breast cancer | |
| No | 68 (82.9) |
| Yes | 14 (17.1) |
| History of oral contraception | |
| No | 70 (85.4) |
| Yes | 12 (14.6) |
| Comorbidity | |
| No | 40 (48.8) |
| Yes | 42 (51.2) |
| Type of comorbidity | |
| Bronchial asthma | 3 (3.7) |
| Cardiac disease | 1 (1.2) |
| Diabetes mellitus | 12 (14.6) |
| Hypertension | 25 (30.5) |
| Rheumatoid arthritis | 1 (1.2) |
| Menopausal status | |
| Pre-menopausal | 55 (67.1) |
| Post-menopausal | 27 (32.9) |
| Body mass index (BMI, mean± standard deviation) | 37.23 ±6.951 |
| ECOG Performance status grade | |
| 0 | 62 (75.6) |
| 1 | 20 (24.4) |
| Skin changes | |
| No | 77 (93.9) |
| Yes (peau d’orange and nipple retraction) | 5 (6.1) |
| Supraclavicular Lymphadenopathy | |
| No | 81 (98.8) |
| Yes | 1 (1.2) |
| Radiological criteria | |
| Tumor size/mm. (median and interquartile range) | 2.7 (2.2-12.9) |
| Laterality | |
| Bilateral | 3 (3.7) |
| Unilateral | 79 (96.3) |
| Number of masses | |
| Single | 74 (90.2) |
| Double | 4 (4.9) |
| Multiple | 4 (4.9) |
| Nature of the mass | |
| Area of parenchymal distortion | 2 (2.4) |
| Cystic | 2 (2.4) |
| Partially cystic partially solid | 4 (4.9) |
| Solid | 74 (90.2) |
| Mammographic breast composition | |
| A | 27 (32.9) |
| B | 28 (34.1) |
| C | 25 (30.5) |
| D | 2 (2.4) |
| Criteria | Number (%) |
| Mammographic calcification | |
| No | 65 (79.3) |
| Yes | 17 (20.7) |
| Ultrasonographic breast composition | |
| Heterogenous | 31 (37.8) |
| Homogenous | 51 (62.2) |
| Ultrasonographic mass shape | |
| Regular (round-oval) | 35 (42.7) |
| Irregular | 47 (57.3) |
| Ultrasonographic mass margin | |
| Circumscribed | 29 (35.4) |
| Angular | 53 (64.6) |
| Ultrasonographic mass orientation | |
| Not parallel | 31 (37.8) |
| Parallel | 51 (62.2) |
| Ultrasonographic echoic pattern | |
| Hypoechoic | 63 (76.8) |
| Complex | 19 (23.2) |
| BI-RADS category | |
| 3 (Probably benign) | 7 (8.5) |
| 4 (Suspicious for malignancy) | 61 (74.4) |
| 5 (Highly suggestive of malignancy) | 4 (4.9) |
| 6 (Known malignancy) | 10 (12.2) |
| Associated malignant lymphadenopathy | |
| No | 32 (39) |
| Yes | 50 (61) |
| Favored radiological diagnosis | |
| Benign | 7 (8.5) |
| Malignant | 75 (91.5) |
| Pathological/surgical criteria | |
| Myoepithelial markers on CNB | |
| Present/ focally present | 37 (45.1) |
| Absent | 41(50) |
| Non-conclusive | 4 (4.9) |
| Favored CNB diagnosis | |
| Benign | 16 (19.5) |
| Malignant | 44 (53.7) |
| Suspicious | 22 (26.8) |
| Surgery type | |
| Conservative breast surgery | 31 (37.8) |
| Modified radical mastectomy | 51(62.2) |
| Final pathological diagnosis | |
| Benign | 10 (12.2) |
| Papillomatosis (fibrocystic) | 3 (3.7) |
| Papilloma (one with atypia)/Benign papillary lesion | 7 (8.5) |
| Malignant | 72 (87.8) |
| Ductal carcinoma in situ | 4 (4.9) |
| Encysted papillary carcinoma | 18 (21.9) |
| Encysted papillary carcinoma with invasion | 3 (3.7) |
| Solid papillary carcinoma with invasion | 7 (8.5) |
| Invasive papillary carcinoma | 29 (35.4) |
| Infiltrating duct carcinoma (NOS) with a papillary component | 11 (13.4) |
ECOG, Eastern Cooperative Oncology Group; BI-RADS; Breast Imaging-Reporting and Data System, CNB; Core needle biopsy
Figure 1.
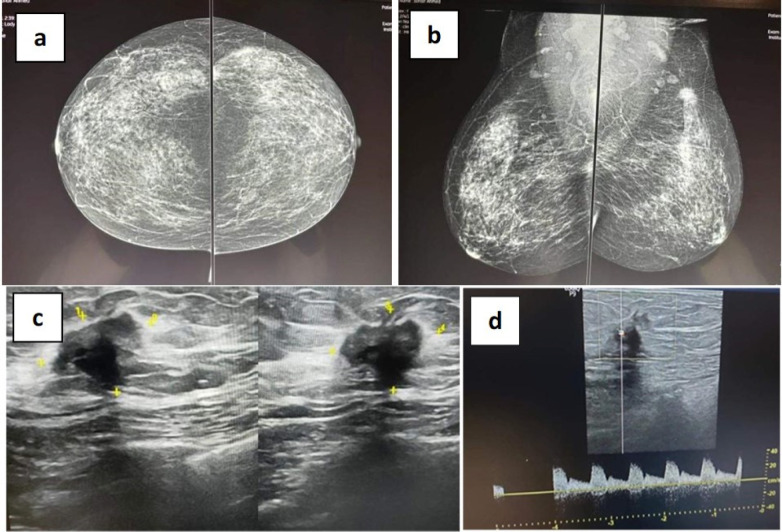
Mammography. Craniocaudal Position Showing Heterogeneously Dense Breast which May Obscure Masses (a). Mediolateral position showing irregular speculated dense mass at upper outer quadrant of left breast associated with nipple retraction and bilateral enlarged axillary lymphadenopathy (b). Ultrasonography. Irregular, non-circumscribed, speculated, hypo-echoic mass showing posterior shadowing, architecture distortion and internal vascularity in the mass on dipper study (c and d). Conclusion: BI-RADS IV C mass with breast density matching category C (American college of radiology)
In CNB, the cases were favorably diagnosed as benign (19.5%; figure 2a-d), suspicious for malignancy or frankly malignant (80.5%; 26.8 and 53.7% respectively; figure 3a-d) based on the combined histopathological interpretation and the expression of myoepithelial markers; that were present/focally present in 45.1%, absent in 50%, and inconclusive in 4.9% of lesions. Most of the cases underwent modified radical mastectomy (MRM) (62.2%; upgrading done during intraoperative frozen section), and the remainder underwent conservative breast surgery (CBS). Upon evaluation of surgical excision specimens (figure 2e-f and 3e-f), 87.8% of lesions were diagnosed as malignant, most of which were invasive papillary carcinomas and encysted papillary carcinomas (35.4 and 21.9% respectively).
Figure 2.
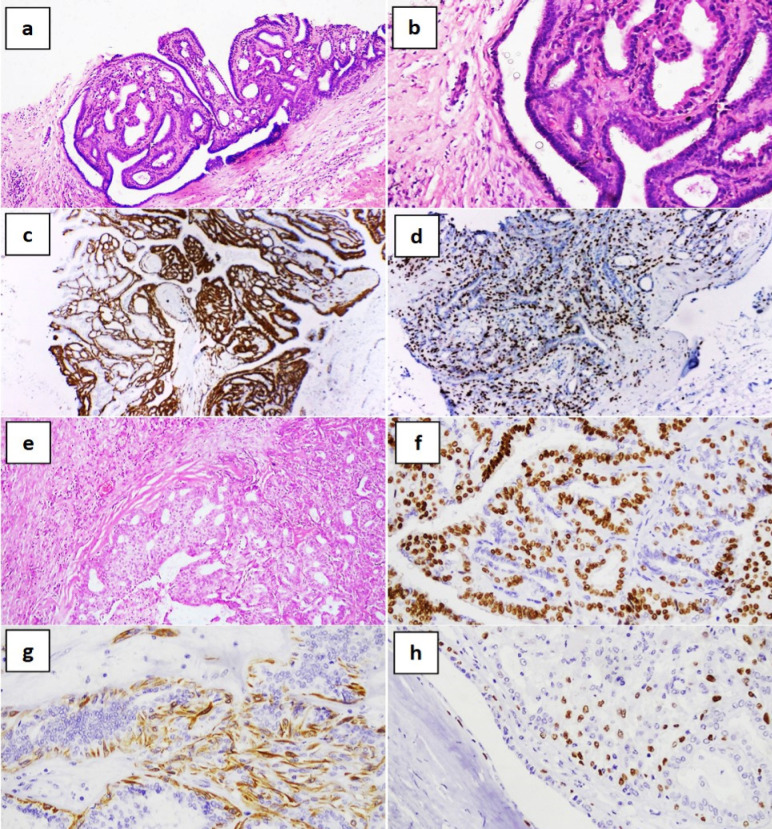
Benign Papilloma of the Breast. Core needle biopsy showing a papillary breast lesion with connective tissue core (a; hematoxylin and eosin [H&E] x100 and b; H&E x200) with dual positivity for ER and p63 (c and d respectively; immunohistochemistry, diaminobenzidine x200). Excision specimen of the same mass showing circumscribed papillary breast lesion (e; H&E x200) with positive staining for ER, CK5/6 and p63 (f-h respectively; immunohistochemistry, diaminobenzidine x200)
Figure 3.
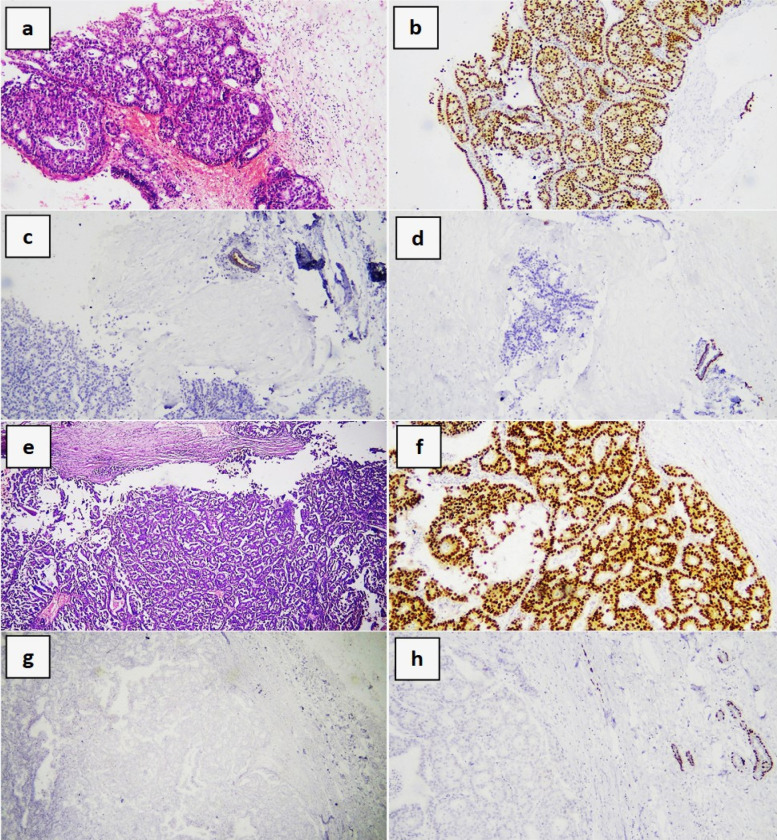
Encysted Papillary Carcinoma of the Breast. Core needle biopsy showing a complex papillary breast lesion (a; hematoxylin and eosin [H&E] x100) showing positivity for ER and negativity for both CK5/6 and p63 (b, c and d respectively; immunohistochemistry, diaminobenzidine x100). Excision specimen of the same mass (e; H&E x40) with positive staining for ER and negative staining for both CK5/6 and p63 (f-h respectively; immunohistochemistry, diaminobenzidine x200). Note the positive internal controls for CK5/6 and p63
When the radiological findings of the 82 breast lesions were compared to the final excision pathological diagnosis, significant statistical associations were observed with the following criteria: (1) Nature of the mass (p<0.001), as most of the malignant masses were entirely solid, while 60% of benign ones were solid and 30% contained partially a cystic component; (2) Mammographic breast composition (p=0.038), as most of the malignant lesions were diagnosed in breasts with compositions A and B (36.1% each), while most of the benign lesions were diagnosed in breasts with composition C (60%); (3) Ultrasonographic mass shape (p=0.02), as most of the malignant masses were irregular in comparison to the benign lesions (62.5 vs 20%) that were more frequently round to oval. The reminder of the radiological findings was not significantly associated with the results of the final pathological diagnosis (all p≥0.05). Of particular concern, the BI-RADS category was not associated with the final excision biopsy diagnosis (p=0.75), as most of either the malignant or the benign masses were categorized as class 4 BI-RADS (75 and 70% respectively); and the presence of calcification (p=0.65) that was seen in around 20% of either the malignant or the benign masses (Table 2).
Table 3.
The Association between the Radiological Findings and the Pathological Diagnosis from Core Needle Biopsy (CNB)
| Radiological criteria | CNB diagnosis | Chi square | |||
|---|---|---|---|---|---|
| Benign (16;19.5%) | Suspicious | Malignant | P-value | ||
| (22; 53.7%) | (44; 26.8%) | ||||
| Nature of the mass | |||||
| Area of parenchymal distortion | 2 (2.4) | 0 | 0 | 2 (4.5) | 11.3 |
| Cystic | 2 (2.4) | 1 (6.2) | 1 (4.5) | 0 | 0.186 |
| Partially cystic partially solid | 4 (4.9) | 2 (12.5) | 1 (4.5) | 1 (2.3) | |
| Solid | 74 (90.2) | 13 (81.3) | 20 (90.9) | 41 (93.2) | |
| Mammographic breast composition | |||||
| A | 27 (32.9) | 2 (12.5) | 10 (45.5) | 15 (34.1) | 13.9 |
| B | 28 (34.1) | 3 (18.8) | 6 (27.3) | 19 (43.2) | 0.03* |
| C | 25 (30.5) | 10 (62.5) | 6 (27.3) | 9 (20.5) | |
| D | 2 (2.4) | 1 (6.2) | 0 | 1 (2.3) | |
| Mammographic calcification | |||||
| No | 65 (79.3) | 15 (93.8) | 12 (54.5) | 38 (86.4) | 11.5 |
| Yes | 17 (20.7) | 1 (6.2) | 10 (45.5) | 6 (13.6) | 0.003* |
| Ultrasonographic breast composition | |||||
| Heterogenous | 31 (37.8) | 5 (31.2) | 10 (45.5) | 16 (36.4) | 0.87 |
| Homogenous | 51 (62.2) | 11 (68.8) | 12 (54.5) | 28 (63.6) | 0.64 |
| Ultrasonographic mass shape | |||||
| Regular (round-oval) | 35 (42.7) | 12 (75) | 6 (27.3) | 17 (38.6) | 9.5 |
| Irregular | 47 (57.3) | 4 (25) | 16 (72.7) | 27 (61.4) | 0.04* |
| Ultrasonographic mass margin | |||||
| Circumscribed | 29 (35.4) | 10 (62.5) | 6 (27.3) | 13 (29.5) | 6.4 |
| Angular | 53 (64.6) | 6 (37.5) | 16 (72.7) | 31 (70.5) | 0.04 |
| Ultrasonographic mass orientation | |||||
| Not parallel | 31 (37.8) | 4 (25.0) | 10 (45.5) | 17 (38.6) | 1.4 |
| Parallel | 51 (62.2) | 12 (75.0) | 12 (54.5) | 27(61.4) | 0.43 |
| Ultrasonographic echoic pattern | |||||
| Hypoechoic | 63 (76.8) | 14 (87.5) | 17 (77.3) | 32 (72.7) | 1.4 |
| Complex | 19 (23.2) | 2 (12.5) | 5 (22.7) | 12 (27.3) | 0.48 |
| BI-RADS category | |||||
| 3 | 7 (8.5) | 2 (12.5) | 2 (9.1) | 3 (6.8) | 6.2 |
| 4 | 61 (74.4) | 12 (75.0) | 19 (86.4) | 30 (68.2) | 0.4 |
| 5 | 4 (4.9) | 0 | 1 (4.5) | 3 (6.8) | |
| 6 | 10 (12.2) | 2 (12.5) | 0 | 8 (18.2) | |
BI-RADS; Breast Imaging-Reporting and Data System, CNB; Core needle biopsy, *p-value is significant if ≤0.05%.
Moreover, the radiological findings of the 82 breast lesions were compared to CNB diagnosis. Significant statistical associations were observed with the following criteria: (1) Mammographic breast composition (p=0.03), as most of the CNB suspicious and malignant lesions were diagnosed in breasts with compositions A (45.5 and 34.1%) and B (27.3 and 43.2), while most of the benign lesions were diagnosed in breasts with composition C (62.5%); (2) Mammographic calcification (p=0.003), as a high percentage of the CNB suspicious lesions showed calcification on radiology when compared to the CNB benign and the malignant lesions (45.5 vs 6.2 and 13.6% respectively) (3) Ultrasonographic mass shape (p=0.04), as most of the CNB suspicious and malignant masses were irregular in comparison to the benign lesions (72.7 and 61.4 vs 25% respectively) that were more frequently round to oval. The reminder of the radiological findings was not significantly associated with the results of the final pathological diagnosis (all p≥0.05), notably, the BI-RADS category was not associated with the final excision biopsy diagnosis (p=0.4), as most of the malignant, suspicious or the benign masses were categorized as class 4 BI-RADS (86.4, 68.2 and 75% respectively) (Table 3).
Table 4.
The Accuracy of Radiological and the Core Needle Biopsy (CNB) Pathological Diagnoses as Compared to the Final Pathological Diagnosis of Surgical Excision Specimens
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |
|---|---|---|---|---|---|
| Radiology | 88.20 | 16.70 | 93.10 | 10 | 82.90 |
| CNB | 90.30 | 90.00 | 98.50 | 56.20 | 90 |
| Combined | 90.30 | 90.00 | 97.90 | 53.30 | 90.20 |
PPV; Positive predictive value, NPV; Negative predictive value
As compared to the final excision pathological diagnosis, the accuracy of radiological and CNB in diagnosing papillary breast lesions were shown to be 82.9 and 90% when calculated separately and 90.2% when combined together. The CNB was more sensitive and by far more specific in this regard compared to radiology (90.3 and 90.0% vs and 88.2 and 16.7% respectively). The CNB alone had a higher positive- and negative- predictive values (PPV; 98.5 and NPV; 56.2%) for the final excisional diagnosis when compared to radiology alone or combined CNB and radiology (Table 4).
Table 5.
The BI-RADS and CNB Upgraded Cases after Final Pathological Diagnosis of Surgical Excision Specimens
| Case | CNB | BI-RAD | Age | Size | Lymphadenopathy | Myoepithelial markers |
Final Pathological Diagnosis Upgrade |
|---|---|---|---|---|---|---|---|
| (7 cases; 8.5%) | (8 cases; 9.8%) | (Mean; 60.5y) | (Mean; 3.7cm) | ||||
| 1 | Papillomatosis | 4A | 81 | 5 | None | Positive | Invasive ductal carcinoma |
| 2 | Benign papillary lesion |
4A | 57 | 2 | None | Positive | Papilloma with atypical ductal hyperplasia |
| 3 | Duct papilloma | 4C | 52 | 4.5 | None | Focal | Invasive papillary carcinoma |
| 4 | Duct papilloma | 4C | 34 | 6 | None | Focal | Encysted papillary carcinoma |
| 5 | Benign papillary lesion |
4A | 73 | 3.5 | Yes/ Inflammatory | Positive | Papillary ductal carcinoma in situ |
| 6 | Benign papillary lesion |
3 | 73 | 3.5 | None | Focal | Encysted papillary carcinoma |
| 7 | Duct papilloma | 4C | 70 | 4 | Yes/ Inflammatory | Positive | Encysted papillary carcinoma |
| 8 | Invasive papillary carcinoma |
4A | 44 | 1.3 | None | Negative | Invasive papillary carcinoma |
BI-RADS; Breast Imaging-Reporting and Data System, CNB; core needle biopsy
Table 5 shows the CNB and BI-RADS-diagnosed lesions that were upgraded on final excision diagnosis; including 7 and 8 cases respectively with “overall upgrade” rates of 8.5% (7/82 cases) for CNB and 9.8% (8/82 cases) for BI-RADS; and “upgrade to carcinoma” rates of 7.3% (6/82 cases) for CNB; all of which were diagnosed as papilloma/benign papillary lesion, and 8.5% (7/82 cases) for BI-RADS, of which 42.9% were originally classified as BI-RADS class 4C (high suspicion for malignancy), and 42.9% as 4A (low suspicion for malignancy). The mean age of upgraded cases was 60.5 years, the mean lesion size in radiology was 3.7cm. and only 2 cases (25%) had enlarged axillary lymph nodes that showed inflammatory criteria, while the remainder has no lymph nodes. None of the cases were presented with nipple discharge, skin changes or showed mammographic calcification. The myoepithelial markers were present or focally present in all the CNB biopsy lesions upgraded to carcinoma (3/6 cases; 50% each).
Table 2.
The Association between the Radiological Findings and the Final Pathological Diagnosis from Surgical Excision Specimens
| Radiological criteria | Final Pathological Diagnosis | Chi square | ||
|---|---|---|---|---|
| Benign (10; 12.2%) | Malignant (72; 87.8%) | P value | ||
| Nature of the mass | ||||
| Area of parenchymal distortion | 2 (2.4) | 0 | 2 (2.8) | 23.5 |
| Cystic | 2 (2.4) | 1(10) | 1 (1.4) | <0.001* |
| Partially cystic partially solid | 4 (4.9) | 3 (30) | 1 (1.4) | |
| Solid | 74 (90.2) | 6 (60) | 68 (94.4) | |
| Mammographic breast composition | ||||
| A | 27 (32.9) | 1 (10) | 26 (36.1) | 8.409 |
| B | 28 (34.1) | 2 (20) | 26 (36.1) | 0.038* |
| C | 25 (30.5) | 6 (60) | 19 (26.4) | |
| D | 2 (2.4) | 1 (10) | 1 (1.4) | |
| Mammographic calcification | ||||
| No | 65 (79.3) | 8 (80) | 57 (79.2) | 0.004 |
| Yes | 17 (20.7) | 2 (20) | 15 (20.8) | 0.65 |
| Ultrasonographic breast composition | ||||
| Heterogenous | 31 (37.8) | 3(30) | 28 (38.9) | 2.4 |
| Homogenous | 51 (62.2) | 0 (0) | 2 (2.8) | 0.64 |
| Ultrasonographic mass shape | ||||
| Regular (round-oval) | 35 (42.7) | 8 (80) | 27 (37.5) | 7.08 |
| Irregular | 47 (57.3) | 2 (20) | 45 (62.5) | 0.02* |
| Ultrasonographic mass margin | ||||
| Circumscribed | 29 (35.4) | 6 (60) | 23 (31.9) | 3.02 |
| Angular | 53 (64.6) | 4 (40) | 49 (68.1) | 0.08 |
| Ultrasonographic mass orientation | ||||
| Not parallel | 31 (37.8) | 3 (30) | 28 (38.9) | 0.29 |
| Parallel | 51 (62.2) | 7 (70) | 44 (61.1) | 0.43 |
| Ultrasonographic echoic pattern | ||||
| Hypoechoic | 63 (76.8) | 9 | 54(75) | 1.1 |
| Complex | 19 (23.2) | 1 | 18(25) | 0.27 |
| BI-RADS category | ||||
| 3 | 7 (8.5) | 1 (10) | 6 (8.3) | 1.1 |
| 4 | 61 (74.4) | 7 (70) | 54 (75) | 0.75 |
| 5 | 4 (4.9) | 0 | 4 (5.6) | |
| 6 | 10 (12.2) | 2 (20) | 8 (11.1) | |
BI-RADS; Breast Imaging-Reporting and Data System, *p-value is significant if ≤0.05%.
Discussion
Up till now, there is no consensus regarding the management of benign papillary breast lesions and neoplasms (PBLs/Ns) diagnosed on CNB (Kuehner et al., 2019). In recent years, routine excision of these lesions has been questioned and controversy exists over when excision is necessary (Liu et al., 2019). Therefore, it is of utmost importance to evaluate the accuracy of radiology and CNB diagnoses compared to final pathological diagnosis from excision specimens, and to determine the upgrade rate of benign lesions to carcinomas following the final excision.
Despite the advance in radiological diagnostic approaches including the mammographic and ultrasonographic beast imaging modalities and the introduction of the BI-RAD system into clinical practice (Kim et al., 2012), the radiological findings that were significantly associated with either the CNB or final excision pathological diagnoses were limited in this study. Lesions diagnosed as malignant on final excision, were more frequently solid, irregular and diagnosed in entirely fatty breasts (composition A) or breasts with scattered areas of fibro-glandular density (composition B). Concordantly, lesions diagnosed as malignant or suspicious of malignancy in CNB were more frequently irregular, present in breasts with composition A or B and were more commonly associated with mammographic calcification on radiologic examinations. Such features were found to vary considerably from a study to another. A detailed ultrasonographic study verified that; malignant breast lesions are more frequently irregular with a non-parallel orientation, however, lesion size and location, calcifications, breast composition, margin, echogenicity, and posterior features were not significantly different between benign and malignant lesions (Park et al., 2016). In contrast, shape, echogenicity and posterior attenuation showed a statistical power in determining the nature of breast lesions in the study by Farras Roca et al., (2021).
In this study, the BI-RADS category was not significantly associated with either the final excision or the CNB pathological diagnoses. The most acceptable explanation of this unexpected finding is that most of the breast lesions in this study were classified as BI-RADS category 4 (74.4%), with a nearly close distribution of the sub-categories 4A (low), B (moderate), and C (high suspicion of malignancy) amidst the different pathological benign, suspicious and malignant groups. Likely, a recent study disclosed that a diverse array of benign breast lesions can be classified as BI-RADS category 4C or even 5, although the PPV for cancer is high in these categories (Farras Roca et al., 2021). Notable that category 4 BI-RADS could be considered as an equivocal class that can be espoused to misleading radiological lesions, thus rendering insignificant statistical data. Therefore, lesions of category 4 BI-RADS are generally recommended for tissue biopsy (Kim et al., 2012).
Previous studies have demonstrated that CNB have a very high diagnostic accuracy, with a sensitivity, specificity, positive predictive value and negative predictive value of 90% or higher (Nunez et al., 2020). In the present study, the accuracy, sensitivity and specificity of CNB in the diagnosis of PBLs/Ns were about 90% with a PPV of 98.5%. Interestingly, combining CNB to radiological diagnosis showed a trivial rise of the diagnostic accuracy to 90.2% and a decline in the PPV to 97.9% with no change in either the sensitivity or specificity, as the radiological diagnosis alone exhibited a relatively lower diagnostic accuracy of 82.9% and a lower PPV of 93.1%. Thus, our data indicate that CNB alone can be more informative in the diagnosis of PBLs/Ns compared to CNB combined and radiology.
Upgrading rate of PBLs/Ns is currently one of the weightiest determinants of the diagnostic accuracy of these lesions in CNB and radiology; and henceforth the management of patient’s as well. In the current study, the “overall upgrade” rates were 8.5% for CNB and 9.8% for BI-RADS; and “upgrade to carcinoma” rates were 7.3% for CNB and 8.5% for BI-RADS. All of the CNB lesions that underwent upgrading to carcinoma were diagnosed as papilloma/benign papillary lesion. Based on studies with upgrade rates ranging between 5 and 10%, some investigators have recommended surgical excision for all intraduct papillomas diagnosed at CNB, regardless of the presence or absence of atypia (Brogi and Krystel-Whittemore, 2021). In agreement with our finding, Park et al., (2020) reported an upgrade rate to malignancy of 7.6% in papillary lesions without atypia in CNB. Current literature reports variable upgrade rates following surgical excision of CNB diagnosed PBLs/Ns, ranging from 2.4 to 26.2% (Kuehner et al., 2019; Liu et al., 2019; Nunez et al., 2020, Zhang et al., 2021). According to Choi et al. (2019) and Kuehner et al. (2019), the low upgrade rate is attributed to using a vacuum-assisted breast biopsy (VAB) in some studies, thus improving markedly the accuracy of CNB diagnosis. However, many of these studies are single institutional, which limits the generalizability of results (Liu et al., 2019).
Factors that were linked to the upgraded lesions in this study included the older age of patients (60.5 vs 58.5 years), larger mean lesion size in radiology (3.7 vs 2.7 cm.) and category 4A and 4C BI-RADS, however, the statistical analysis for significance was unplausible to be applied for these factors due to the limited number of upgraded cases. None of the lesions showed calcification or were associated with nipple discharge, skin changes or significant lymphadenopathy. The myoepithelial markers were present or focally present in all the CNB biopsy lesions upgraded to carcinoma (50% each), indicating that the original lesion was missed during CNB sampling and an area adjacent to the lesion was sampled instead, rendering a false-negative result. As specified in a study that included 988 CNBs, the main causes of false-negative results are sampling from an inappropriate site and histopathological non-homogeneity of cancer infiltration (Boba et al., 2011).
An additional predictive factor for upgrading is the histopathological/radiological discordance that was demonstrated in this study; all the lesions that underwent upgrading to carcinoma were interpreted as “benign” in CNB, while most of them were interpreted as “lesions with suspicion of malignancy” on the BI-RADS (class 4 A and C). Noteworthy that, 7 lesions were diagnosed as suspicious of malignancy/malignant in radiology then were downgraded to benign ones after final excision diagnosis, thus posing more discordance between histopathology and radiology among our cases. Similarly, Kim et al., (2012) noted that some benign breast lesions are initially wrongly interpreted as highly suspicious for malignancy. Yet, the description of a papillary breast lesion as histopathologically/radiologically discordant, by itself mandates excision (Brogi and Krystel-Whittemore, 2021).
In large cohort studies and metanalyses, the predictive factors for upgrade rate were the patient’s ag, presence of bloody nipple discharge, palpability of the mass, lesions above 1 cm, peripheral lesions, radiologic microcalcification, BI-RADS category 4C or 5, histopathological /radiological discordance, the presence of atypia in CNB and contralateral breast carcinoma suggesting that patients with one or more of these predictive factors are more likely to benefit from surgical excision (Kuehner et al., 2019; Liu et al., 2019; Brogi and Krystel-Whittemore, 2021, Park et al., 2020; Zhang et al., 2021).
In conclusion, this study demonstrated that up to 9.8% of PBLs/Ns diagnosed on CNB and BI-RADS undergo upgrading upon final excision, despite the high diagnostic accuracy of CNB alone or combined CNB and BI-RADS that came up to 90.2%. Factors linked to upgrade include the age, size, category 4 BI-RADS and the presence of histopathological/radiological discordance, regardless the state of myoepithelial markers that can be influenced by sampling errors. These data are interesting to note as it can then affect the surgeon’s final decision on whether to excise the lesion or not.
Author Contribution Statement
• M.F. and H.Sh. Conceptualization, collection of data, histopathological and immunohistochemical interpretation, diagnosis, and photography.
• A.A. Conceptualization, literature review, interpretation of results, writing the original draft and the final submitted version of the manuscript.
• D.H. Conceptualization, radiological procedures, collection of data and reporting.
• M.Z. Conceptualization, surgical procedures, collection of data and reporting.
• R.A and D.H.S Conceptualization, clinical data collection reporting.
• All authors have reviewed and agreed upon the final manuscript version.
Acknowledgements
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Approval
The work is approved by the Institutional Research Board (IRB) at Faculty of Medicine, Mansoura University, Egypt/ it is not a part of an approved student thesis.
Ethical issues
Ethical approval was obtained from the Institutional Research Board (IRB, code: R21.08.1405.R1). Informed consent was obtained from the patients as appropriate. Patient identification is not possible through the presented data and images.
Data availability statement
Data will be available upon reasonable request.
Conflict of interest
The authors declare no relevant financial affiliations or conflicts of interest.
References
- American College of Radiology. ACR BI- RADS Atlas, Breast Imaging Reporting and Data System. 5th ed. American College of Radiology ; 2013. [Accessed at BIRADS-Reference-Card.pdf (acr.org) on 20th September 2021]. [Google Scholar]
- Batori M, Gallinaro LS, D’Urso A, et al. Papillomatosis and breast cancer: a case report and a review of the literature. Eur Rev Med Pharmacol Sci. 2000;4:99–103. [PubMed] [Google Scholar]
- Boba M, Kołtun U, Bobek-Billewicz B, et al. False-negative results of breast core needle biopsies - retrospective analysis of 988 biopsies. Pol J Radiol. 2011;76:25–9. [PMC free article] [PubMed] [Google Scholar]
- Brogi E, Krystel-Whittemore M. Papillary neoplasms of the breast including upgrade rates and management of intraductal papilloma without atypia diagnosed at core needle biopsy. Mod Pathol. 2021;34:78–93. doi: 10.1038/s41379-020-00706-5. [DOI] [PubMed] [Google Scholar]
- Chen P, Zhou D, Wang C, et al. Treatment and Outcome of 341 Papillary breast lesions. World J Surg. 2019;43:2477–82. doi: 10.1007/s00268-019-05047-2. [DOI] [PubMed] [Google Scholar]
- Choi HY, Kim SM, Jang M, et al. Benign breast Papilloma without Atypia: Outcomes of Surgical Excision versus US-guided Directional Vacuum-assisted Removal or US Follow-up. Radiology. 2019;293:72–80. doi: 10.1148/radiol.2019190096. [DOI] [PubMed] [Google Scholar]
- Cuneo KC, Dash RC, Wilke LG et al. Risk of invasive breast cancer and ductal carcinoma in situ in women with atypical papillary lesions of the breast. Breast J. 2012;18:475–8. doi: 10.1111/j.1524-4741.2012.01276.x. [DOI] [PubMed] [Google Scholar]
- Farras Roca JA, Tardivon A, Thibault F ET AL. Correlation of ultrasound, cytological, and histological features of 110 benign BI-RADS categories 4C and 5 nonpalpable breast lesions. The Institut Curie’s experience. Cancer Cytopathol. 2021;129:479–88. doi: 10.1002/cncy.22402. [DOI] [PubMed] [Google Scholar]
- Guan X, Xu G, Shi A et al. Comparison of clinicopathological characteristics and prognosis among patients with pure invasive ductal carcinoma, invasive ductal carcinoma coexisted with invasive micropapillary carcinoma, and invasive ductal carcinoma coexisted with ductal carcinoma in situ: A retrospective cohort study. Medicine (Baltimore) 2020;99:e23487. doi: 10.1097/MD.0000000000023487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Kim D, Jung W, et al. Histological analysis of benign breast imaging reporting and data system categories 4C and 5 breast lesions in imaging study. Yonsei Med J. 2012;3:1203–10. doi: 10.3349/ymj.2012.53.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehner G, Darbinian J, Habel L, et al. Benign Papillary Breast Mass Lesions: Favorable Outcomes with Surgical Excision or Imaging Surveillance. Ann Surg Oncol. 2019;26:1695–703. doi: 10.1245/s10434-019-07180-7. [DOI] [PubMed] [Google Scholar]
- Liu C, Sidhu R, Ostry A, et al. Risk of malignancy in papillary neoplasms of the breast. Breast Cancer Res Treat. 2019;178:87–94. doi: 10.1007/s10549-019-05367-w. [DOI] [PubMed] [Google Scholar]
- Mooney KL, Bassett LW, Apple SK. Upgrade rates of high-risk breast lesions diagnosed on core needle biopsy: a single-institution experience and literature review. Mod Pathol. 2016;29:1471–84. doi: 10.1038/modpathol.2016.127. [DOI] [PubMed] [Google Scholar]
- Ni YB, Tse GM. Pathological criteria and practical issues in papillary lesions of the breast-a review. Histopathol. 2016;68:22–32. doi: 10.1111/his.12866. [DOI] [PubMed] [Google Scholar]
- Nunez DL, Gonzaez FC, Ibarguengoitia MC, et al. Papillary lesions of the breast: a review. Breast Cancer Manag. 2020;9:BMT52. [Google Scholar]
- Park CJ, Kim EK, Moon HJ et al. Reliability of breast ultrasound BI-RADS final assessment in mammographically negative patients with nipple discharge and radiologic predictors of malignancy. J Breast Cancer. 2016;19:308–15. doi: 10.4048/jbc.2016.19.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Ko S, Yoon CS et al. Factors associated with disease upgrading in patients with papillary breast lesion in core-needle biopsy. Gland Surg. 2020;9:919–24. doi: 10.21037/gs-20-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha RD, Pinto RR, Aquino D, et al. Step-by-step of ultrasound-guided core-needle biopsy of the breast: review and technique. Radiol Bras. 2013;46:234–41. [Google Scholar]
- The World Medical Association. Declaration of Helsinki. Ethical principles for medical research involving human subject. JAMA. 2013;310:2191–4. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- Uğur Kılınç AN, Bayramoğlu Z, Ünlü Y et al. Results of excision of unknown papillary neoplasms detected on core biopsy. Eur J Breast Health. 2021;17:258–64. doi: 10.4274/ejbh.galenos.2021.6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Editorial Board. Classification of Breast Tumours. Vol 2. 5th ed. Lyon. 2019;France: IARC:pp 49–67. [Google Scholar]
- Zhang X, Liu W, Hai T, et al. Upgrade rate and predictive factors for breast benign intraductal papilloma diagnosed at biopsy: A Meta-Analysis. Ann Surg Oncol. 2021;28:8643–50. doi: 10.1245/s10434-021-10188-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available upon reasonable request.