Abstract
Background and objective:
High LIMCH1 expression in lung and renal cancer is determined as a favorable prognostic factor. However, prognostic value of LIMCH1 expression in breast cancer has not been studied yet. Therefore, this study was performed to determine the prognostic value of LIMCH1 expression in breast cancer patients.
Methods:
This retrospective study included 89 patients with invasive breast carcinoma of no special type. These patients referred to Cancer Research Institute of Tomsk National Research Medical Center from 2007 to 2018. LIMCH1 protein expression in tumor cells was detected by immunohistochemical analysis in this study. Statistical analysis was done to investigate the possible relationship between LIMCH1 protein expression and clinicopathological parameters, risk of metastasis, distant metastasis free survival, and overall survival.
Results:
IHC analysis of breast cancer tissue samples revealed that LIMHC1 protein expression was found in 29.2% (26/89) of the cases. Lymph node and distant metastases were more frequent in patients with LIMCH1 protein expression. LIMCH1 protein expression increased the risk of distant metastasis based on our findings. LIMCH1 protein affected metastatic-free survival regardless of the T, as well as other clinical and pathological parameters (p=0.0146, HR=3.2058 (1.26; 8.17)). Moreover, LIMCH1 protein expression was associated with worse overall survival (p=0.0071, HR=2.73 (1.28; 5.85)) in our breast cancer patients.
Conclusion:
LIMCH1 protein expression was associate with metastases development, providing prognostic stratification. In breast cancer, LIMCH1 protein expression was found as an unfavorable prognostic factor of distant metastasis-free survival based on our findings.
Key Words: LIMCH1, breast cancer, metastasis, invasion, migration
Introduction
Metastasis is a multi-step process. Migration of tumor cells on extracellular matrix is known as an early stage of metastases development. Two modes of tumor cell migration are suggested, namely individual and collective tumor cell migrations (Clark, Vignjevic, 2015). During individual tumor cell migration, tumor cells can have mesenchymal and amoeboid migration. Epithelial-mesenchymal transition (EMT) is a key step in mesenchymal migration, leading to acquisition of invasive phenotype by tumor cells. Elongated morphology, integrin-depended adhesion to the extracellular matrix (ECM), and high proteolytic activity are features of mesenchymal migration (Pearson, 2019). Amoeboid cell migration has spheroid morphology and weak adhesion to the ECM and lacks proteolytic activity (Boekhorst and Friedl, 2016). Collective cell migration is a movement pattern of grouped cells that retain cell-cell connections and migrate coordinately. Leader cells at invasion edge have mesenchymal characteristics during collective cell migration (Yang et al., 2019).
Knowledge about each migration mode is extensive although invasion-targeted therapy does not gain enough success (Wu et al., 2021). It can be associated with plasticity of cancer cell invasion. Tumor cells can switch migration modes depending on microenvironment, mechanical, or biochemical factors (Wu et al., 2021).
However, the involvement of actin cytoskeleton in the formation of traction force is common to all migration modes: formation of blebs in amoeboid migration and lamellipodia in mesenchymal and collective migration (Wu et al., 2021). Studying regulation of the actin cytoskeleton will allow to develop more effective therapeutic approaches to manage tumor cell invasion.
LIMCH1 protein regulates the activity of non-muscle myosin type 2A (NMIIA). It also promotes retrograde actin flow in lamellipodia and increases the strength of focal adhesion, preventing cell migration and increasing cell adhesion (Lin et al., 2017). LIMCH1 protein is described as a favorable prognostic factor in lung (Zhang et al., 2019; Cao et al., 2021) and renal cancers (https://www.proteinatlas.org/ENSG00000064042-LIMCH1/pathology/renal+cancer). However, its role in breast cancer remains unexplored. The aim of this study was to investigate the expression status and the clinical implications of LIMCH1 protein in breast cancer.
Materials and Methods
This retrospective study included 89 patients with invasive breast carcinoma of no special type T1-4N0-3M0. They were admitted to Cancer Research Institute of the Tomsk National Research Medical Center. About 41,6% (37/89) of patients were treated by neoadjuvant chemotherapy. The patients’ mean age was 52.2 ± 13.7 years. The study was approved by the Local Committee for Medical Ethics of Tomsk National Research Medical Center (24 May 2021, the approval No. 7). Before initiating the study, informed consent was obtained from all the patients. All patient was divided into two cohorts by LIMCH1 protein expression. We then compared the differences between clinicopathological characteristics in two cohorts of breast cancer patients (Table 2). Clinicopathological characteristics of the patients are shown in Table 1.
Table 2.
Clinicopathological Parameters in the Group of Breast Cancer Patients with Respect to LIMCH1 Protein Expression
| Parameters | LIMCH1-, Abs. f., (%) | LIMCH1+, Abs. f., (%) | P level | |
|---|---|---|---|---|
| Age | < 35 | 5/63 (7.94) | 2/26 (7.69) | 1 |
| 35-50 | 20/63 (31.75) | 11/26 (42.3) | 0.4634 | |
| > 50 | 38/63 (60.32) | 13/26 (50) | 0.4804 | |
| Menopause | No | 21/63 (33.33) | 14/26 (53.85) | 0.0956 |
| Yes | 42/63 (66.67) | 12/26 (46.15) | 0.0956 | |
| Tumor size | < 20 mm | 19/63 (30.16) | 3/26 (11.54) | 0.1032 |
| 20-50 mm | 40/63 (63.49) | 22/26 (84.6) | 0.0746 | |
| > 50 mm | 4/63 (6.35) | 1/26 (3.85) | 1 | |
| Molecular type | Luminal A | 15/63 (23.81) | 5/26 (19.23) | 0.7829 |
| Luminal B | 23/63 (36.51) | 12/26 (46.15) | 0.4763 | |
| Triple-negative | 15/63 (23.81) | 6/26 (23.08) | 1 | |
| HER2-positive | 10/63 (15.87) | 3/26 (11.54) | 0.7483 | |
| T | T1 | 20/63 (31.75) | 5/26 (19.23) | 0.3035 |
| T2 | 33/63 (52.38) | 13/26 (50) | 1 | |
| T3 | 4/63 (6.35) | 4/26 (15.38) | 0.2249 | |
| T4 | 6/63 (9.52) | 4/26 (15.38) | 0.4696 | |
| N | N0 | 42/63 (66.67) | 3/26 (11.54) | 0 |
| N1 | 16/63 (25.40) | 14/26 (53.85) | 0.0139 | |
| N2 | 3/63 (4.76) | 7/26 (26.92) | 0.0058 | |
| N3 | 2/63 (3.17) | 2/26 (7.69) | 0.5771 | |
| NACT | 28/63 (44.44) | 9/26 (34.62) | 0.4809 | |
| Lymph node metastases | 18/63 (28.57) | 21/26 (80.77) | 0 | |
| ACT | 35/63 (55.56) | 14/26 (53.85) | 1 | |
| Chemotherapy regimen | Conventional | 20/63 (31.75) | 9/26 (34.62) | 0.8077 |
| Conventional + Targeted | 4/63 (6.35) | 1/26 (3.85) | 1 | |
| Targeted | 7/63 (11.11) | 1/26 (3.85) | 0.4286 | |
| Hormonal | 4/63 (6.35) | 3/26 (11.54) | 0.6743 | |
| Grade | 2 | 54/63 (85.71) | 23/26 (88.46) | 1 |
| 3 | 9/63 (14.29) | 3/26 (11.54) | 1 | |
| Distant metastases | 13/63 (20.63) | 22/26 (84.62) | 0 |
Table 1.
Clinicopathological Parameters of Breast Cancer Patients
| Parameters | Abs. f., (%) | |
|---|---|---|
| Age | < 35 | 7/89 (7.87) |
| 35-50 | 31/89 (34.83) | |
| > 50 | 51/89 (57.3) | |
| Menopause | No | 35/89 (39.33) |
| Yes | 54/89 (60.67) | |
| Tumor size | < 20 mm | 22/89 (24.72) |
| 20-50 mm | 62/89 (69.66) | |
| > 50 mm | 5/89 (5.62) | |
| Molecular type | Luminal A | 20/89 (22.47) |
| Luminal B | 35/89 (39.33) | |
| Triple-negative | 21/89 (23.6) | |
| HER2-positive | 13/89 (14.6) | |
| T | T1 | 25/89 (28.9) |
| T2 | 46/89 (51.69) | |
| T3 | 8/89 (8.99) | |
| T4 | 10/89 (11.24) | |
| N | N0 | 45/89 (50.56) |
| N1 | 30/89 (33.71) | |
| N2 | 10/89 (11.24) | |
| N3 | 4/89 (4.49) | |
| NACT | 37/89 (41.57) | |
| Lymph node metastases | 39/89 (43.82) | |
| ACT | 49/89 (55.06) | |
| Chemotherapy regimen | Conventional | 29/89 (32.58) |
| Conventional + Targeted | 5/89 (5.62) | |
| Targeted | 8/89 (8.99) | |
| Hormonal | 7/89 (7.87) | |
| Grade | 2 | 77/89 (86.52) |
| 3 | 12/89 (13.48) | |
| Frequency of distant metastases | 35/89 (39.33) | |
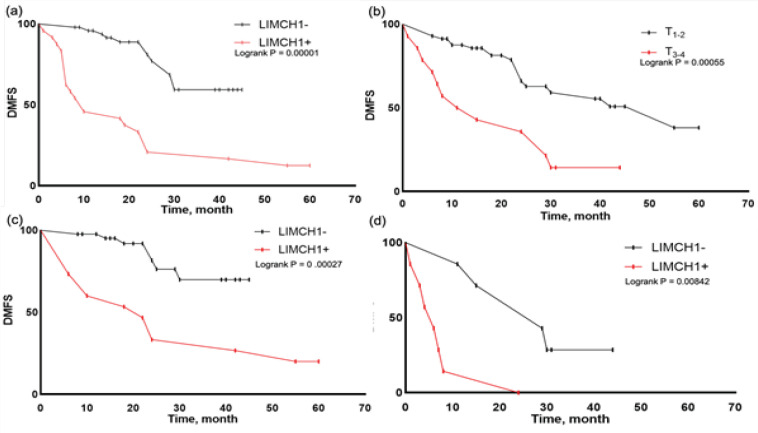
Formalin-fixed and paraffin-embedded tissue were collected for IHC analysis. We were also keen to evaluate whether the LIMCH1 expression would be a prognostic factor predicting lymph node and distant metastases in breast cancer patients. Prognostic parameters used in clinical routine today also were included as variables in univariate and multivariate regression analysis (Table 3). In addition, the relationship between LIMCH1 protein expression, T3-4, and DMFS was analyzed using Kaplan–Meier method (Figure 3).
Table 3.
Univariate and Multivariate Regression Analysis of the Risk Lymph Node and Distant Metastases
| Parameters | Univariate analyse | Multivariate analyse | ||
|---|---|---|---|---|
| OR (95% Cl) | P value | OR (95% Cl) | P value | |
| Menstrual status (Menopause vs pre-menopause) | 0.48 (0.20; 1.19) | 0.1132 | 0.62 (0.16; 2.35) | 0.4803 |
| Ki67 (>20% vs <20%) | 1.00 (0.97; 1.02) | 0.705 | 0.99 (0.95; 1.03) | 0.5314 |
| Tumor size (20– 50 mm vs <20 mm) | 0.90 (0.38; 2.10) | 0.8032 | 0.45 (0.13; 1.57) | 0.2122 |
| NACT (presence vs absence) | 1.19 (0.50; 2.83) | 0.6893 | 2.06 (0.50; 8.38) | 0.3151 |
| Grade (3 vs 2) | 0.39 (0.10; 1.58) | 0.1876 | 0.32 (0.04; 2.34) | 0.2613 |
| LIMCH1 (presence vs absence) | 12.83 (385; 42.67) | 0.00003 | 3471 (5.21; 231.50) | 0.0002 |
Figure 3.
Kaplan-Meier DMFS Analysis in Groups of Patients: a, by LIMCH1 protein expression (p = 0.00001); b, by the T parameter (p = 0.00055); c, in the T1-2 group by LIMCH1 protein expression (p = 0 .00027); d, in the T3-4 group according to LIMCH1 protein expression (p = 0.00842). The curves were statistically matched using the log-rank test
Immunohistochemistry analysis
Immunohistochemistry was used to evaluate the expression of LIMCH1 protein in tumor cells. The anti-LIMCH1 antibody (HPA004184) was used at a dilution of 1:1,000. Visualization was performed using the EnVision detection system (Dako, Agilent, USA). Then, tissue samples were stained with hematoxylin and embedded in Vitrogel medium (Biovitrum, Russia). The expression of LIMCH1 protein in cells was assessed using AxioScope A1 (Zeiss, Germany).
Bioinformatics analysis
The Kaplan–Meier plotter public database (https://kmplot.com/analysis) was used to perform overall survival analysis.
Statistical analysis
Statistical analysis was carried out using Prism 9 (GraphPad, USA) and Statistica 10.0 (StatSoft, USA) software package. The variables were compared the two-tailed Fisher’s exact test. Differences were considered significant at p < 0.05. The univariate and multivariate COX analysis was applied to explore whether LIMCH1 expression could be an independent predictor of distant metastatic-free survival (DMFS) in the breast cancer patients. Factors with statistical significance, after performing univariable COX regression analysis (p value: entry 0.05, removal 0.10), were put into the multivariable COX regression model. Metastatic-free survival was calculated using the Kaplan–Meier method, and differences among patient groups were evaluated by running a log-rank test.
Results
LIMCH1 expression by tumor cells in breast cancer patients
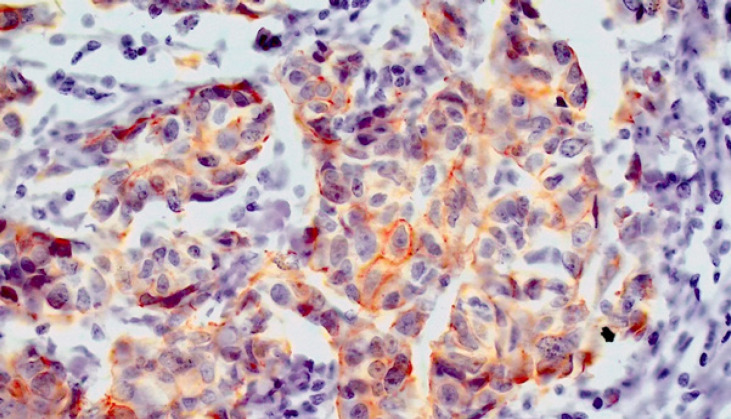
The IHC results revealed that 29.2% (26/89) of patients had tumor cells with LIMCH1 protein expression (Figure 1). According to the Human-protein atlas data, 50% of patients had LIMCH1 protein expression (https://www.proteinatlas.org/ENSG00000064042-LIMCH1/pathology/breast+cancer). There were no significant differences between two groups in terms of age, menstrual status, tumor size, molecular type, and T grade. Lymph node (p = 0.0000) and distant (p = 0.0000) metastases were more often in LIMCH1-positive cohort.
Figure 1.
IHC Analysis of LIMCH1 Expression in Breast Cancer Tissue (magnification ×200)
LIMCH1 expression, lymph node, and distant metastases in the patients
LIMCH1 protein expression was found as an independent prognostic factor predicting lymph node (p=0.0004, OR=34.71 (5.21; 231.50)) and distant metastases (p=0.0021, OR= 25.44 (3.12; 207.17)).
Association of LIMCH1 protein expression with DMFS and OS
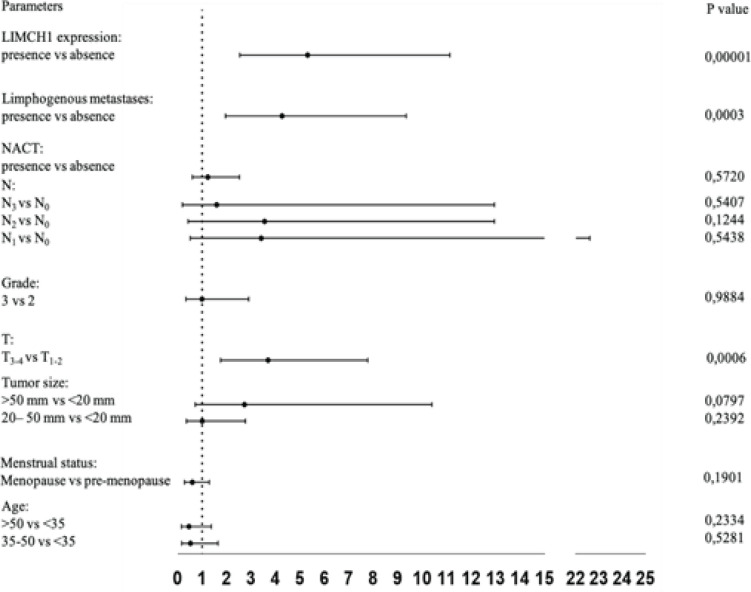
Cox proportional hazards regression analysis were used to identify independent prognostic factors predicting distant metastatic-free survival (Figure 2, Table 4).
Figure 2.
COX Analysis of DMFS
Table 4.
COX Analysis of DMFS
| Parameters | HR (95% Cl) | P value |
|---|---|---|
| LIMCH1 expression | 5.3116 (2.5356; 11.1267) | 0.00001 |
| presence vs absence | ||
| Limphogenous metastases: | 712 (1.9516; 9.3480) | 0.0003 |
| presence vs absence | ||
| NACT: | 1.2302 (0.5997; 2.5237) | 0.5720 |
| presence vs absence | ||
| N: | 1.5921 (0.1957; 12.9537) | 0.5407 |
| N3 vs N0 | 3.5506 (0.4241; 12.9537) | 0.1244 |
| N2 vs N0 | 3.4130 (0.5154; 22.6008) | 0.5438 |
| N1 vs N0 | ||
| Grade: | 0.9921 (0.3391; 2.9025) | 0.9884 |
| 3 vs 2 | ||
| T: | 3.6937 (1.7557; 7.7707) | 0.0006 |
| T3-4 vs T1-2 | ||
| Tumor size: | 2.7302 (0.7170; 10.3957) | 0.0797 |
| >50 mm vs <20 mm | 1.0017 (0.3623; 2.7690) | 0.2392 |
| 20– 50 mm vs <20 mm | ||
| Menstrual status: | 0.5976 (0.2767; 1.2908) | 0.1901 |
| Menopause vs pre-menopause | ||
| Age: | 0.4583 (0.1531; 1.3716) | 0.2334 |
| >50 vs <35 | 0.5219 (0.1651; 1.6498) | 0.5281 |
| 35-50 vs <35 | ||
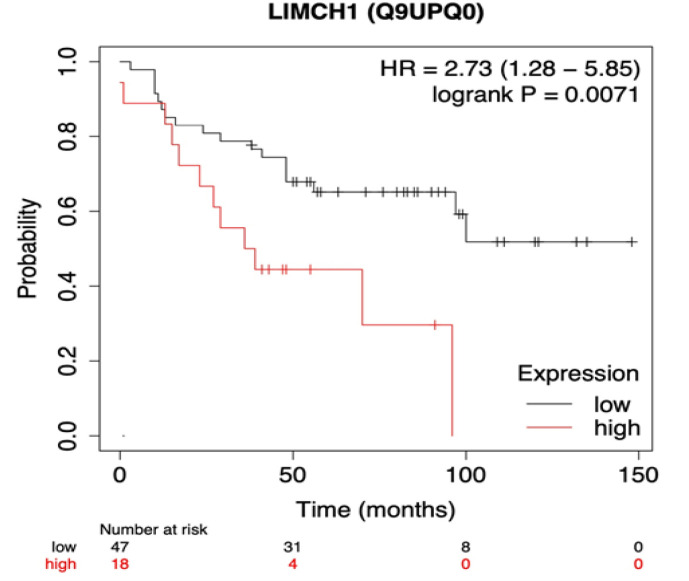
LIMCH1 protein expression (p=0.0146, HR=3.21 (1.26; 8.17) and T3-4 (p=0.0002, HR=4.27 (1.99; 9.18)) were found as unfavorable predictors of DMFS. In the T1-2 and T3-4 groups, patients without LIMCH1 protein expression had improved DMFS compared to patients with LIMCH1 protein expression. The LIMCH1 protein expression and overall survival were investigated in patients with breast cancer using the Kaplan-Meier plotter database (Figure 4). The findings revealed that high mRNA expression levels of LIMCH1 were associated with worse overall survival in breast cancer patients (p=0.0071, HR=2.73 (1.28; 5.85)).
Figure 4.
Kaplan-Meier Overall Survival Analysis in All Breast Cancer Subtypes. The threshold level of LIMCH1 protein expression was automatically selected by the website (https://kmplot.com/analysis). The curves were statistically matched using the log-rank test
Discussion
Metastasis is the most common cause of death in breast cancer patients. Therefore, it is important to discover new biomarkers that can predict metastasis. LIMCH1 protein is a kind of actin-binding proteins. LIMCH1 regulates cell motility by binding and activating NMIIA. Depletion of LIMCH1 in HeLa cells leads to a decrease in actin stress fibers and an increase in the cell ability to migrate (Lin et al., 2017). It is also shown that LIMCH1 protein is a transcription factor that promotes the expression of p53 (Zhang et al., 2019).
LIMCH1 protein is regarded as a typical tumor suppressor due to its functions such as suppressing cells migration and promoting expression of p53. Actually, in lung (Zhang et al., 2019; Cao et al., 2021) and renal (https://www.proteinatlas.org/ENSG00000064042-LIMCH1/pathology/renal+cancer) carcinoma, it has been shown that LIMCH1 protein expression can be a favorable prognostic factor. However, the clinical and prognostic value of LIMCH1 protein expression in breast cancer is not enough explored. LIMCH1 protein expression was not found in some of our cases, suggesting that it is not constitutive in breast cancer.
The results of our study showed that the LIMCH1 protein is associated with lymph node and distant metastasis. In the cohort of patients with LIMCH1 protein expression lymph node and distant metastases developed more often. The mechanisms of lymph node and distant metastasis are different. It has been shown that the development of lymph node metastases is possible both through collective tumor cell migration and amoeboid migration (Wong and Hynes, 2006; Giampieri et al., 2009; Graziani et al., 2021). Distant metastasis is carried out mainly by a single tumor cell, which are characterized by an individual migration (Giampieri et al., 2009). Single breast cancer tumor cells can saved epithelial morphology (Tashireva et al., 2020). Single tumor cells have potencies for both mesenchymal and amoeboid migration modes.
Given that LIMCH1 protein expression can prevent the formation of lamellipodia, the mesenchymal migration mode can switch to the amoeboid. It is possible that this even leads to a more pronounced ability of breast cancer cells to initiate the metastases. LIMCH1 protein expression is associated with lymph node and distant metastases in breast cancer. Probably, the LIMCH1 protein is associated with formation of phenotype of the metastatic tumor cells.
In addition, we found that LIMCH1 protein expression influenced the DMFS and OS of breast cancer patients. LIMCH1 protein expression was associated with worse DMFS and OS in breast cancer. COX regression analysis revealed that LIMCH1 protein expression could serve as an independent prognostic factor predicting lymph node and distant metastases in breast cancer patients.
In conclusion, we found that LIMCH1 protein expression could be an independent prognostic factor predicting lymph node and distant metastases in breast cancer patients. The prognostic role of LIMCH1 protein expression was different in breast cancer compared to lung and renal cancers. This finding can be due to the switch type of migration from mesenchymal to amoeboid, indicating the exclusive role of the amoeboid migration in the development of metastasis in breast cancer.
Author Contribution Statement
All authors contributed equally in this study.
Acknowledgements
Funding Statement
This work was supported by the Russian Science Foundation (grant No. 19-75-30016).
Ethical Declaration
The study was approved by the Local Committee for Medical Ethics of Tomsk National Research Medical Center (24 May 2021, the approval No. 7).
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Cao H, Zhao J, Chen Z, et al. Loss of LIMCH1 predicts poor prognosis in patients with surgically resected lung adenocarcinoma: a study based on Immunohistochemical analysis and bioinformatics. J Cancer. 2021;12:181. doi: 10.7150/jca.47883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AG, Vignjevic DM. Modes of cancer cell invasion and the role of the microenvironment. Curr Opinion Cell Biol. 2015;36:13–22. doi: 10.1016/j.ceb.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Giampieri S, Manning C, Hooper S, et al. Localized and reversible TGFβ signaling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–96. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani V, Rodriguez-Hernandez I, Maiques O, Sanz-Moreno V. The amoeboid state as part of the epithelial-to-mesenchymal transition program. Trends Cell Biol. 2021:2021. doi: 10.1016/j.tcb.2021.10.004. [DOI] [PubMed] [Google Scholar]
- Lin YH, Zhen YY, Chien KY, et al. LIMCH1 regulates nonmuscle myosin-II activity and suppresses cell migration. Mol Biol Cell. 2017;28:1054–106. doi: 10.1091/mbc.E15-04-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson GW. Control of invasion by epithelial-to-mesenchymal transition programs during metastases. J Clin Med. 2019;8:646. doi: 10.3390/jcm8050646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashireva LA, Zavyalova MV, Savelieva OE, et al. Single tumor cells with epithelial-like morphology are associated with breast cancer metastases. Front Oncol. 2020:50. doi: 10.3389/fonc.2020.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Te Boekhorst V, Friedl P. Plasticity of cancer cell invasion—Mechanisms and implications for therapy. Adv Cancer Res. 2016;132:209–26. doi: 10.1016/bs.acr.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Wong SY, Hynes RO. Lymphatic or distant dissemination: how does a metastatic tumor cell decide? Cell Cycle. 2006;5:812–7. doi: 10.4161/cc.5.8.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Jiang J, Chen BJ, et al. Plasticity of cancer cell invasion: Patterns and mechanisms. Translational Oncol. 2021;14:100899. doi: 10.1016/j.tranon.2020.100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zheng H, Zhan Y, Fan S. An emerging tumor invasion mechanism about the collective cell migration. Am J Translational Rre. 2019;11:5301. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang Y, Xu H. LIMCH1 suppress the growth of lung cancer by interacting with HUWE1 to sustain p53 stability. Gene. 2019;712:143963. doi: 10.1016/j.gene.2019.143963. [DOI] [PubMed] [Google Scholar]