Abstract
Background:
Head and neck squamous cell carcinoma is one of the most important malignancies, worldwide. Oncogenic viruses, such as human papilloma virus (HPV) and Epstein-Barr virus (EBV), are linked to these cancers and studies suggest a possible interaction between HPV and EBV during co-infections to promote oncogenesis. Nonetheless, these reports are controversial and demand more investigations in this regard. The present work to assessed the prevalence of HPV and co-infection with EBV in oral and oropharyngeal squamous cell carcinomas.
Methods:
Formalin-fixed paraffin-embedded tissues were collected from 166 archived oral and oropharyngeal squamous cell carcinoma samples from Ahvaz Imam Khomeini hospital, Ahvaz, Iran, from March 2013 and December 2019. Nested-PCR was used to detect the viruses and type-specific PCR/nested-PCR and sequencing were performed for virus genotyping. Results: Out of the 166 specimens, 84.33% and 16.42% were from oral cavity and oropharynx, respectively; of which, 32 cases (19.3%) were HPV-positive (16.42% of oral cavity and 34.6% of oropharynx). HPV was detected in 36.36%, 25%, and 16.42% of base of tongue, tonsil, and oral tongue tumors, respectively. HPV was more associated with well differentiated tumors (24;18.04%) in compared to moderately and poorly differentiated ones. Regarding HPV-16 genotyping, 7 (21.8%) out of the 32 samples were found to be HPV-16 (4/26 (15.38%) for oropharynx and 3/140 (2.14%) for oral cavity). Moreover, 90 samples were evaluated for EBV infection and co-infection; of which, 4 (4.4%) subjects tested positive for EBV, including two cases with HPV co-infection. All the positive cases were EBV type B, from oral cavity, and histologically well differentiated.
Conclusions:
HPV was more associated with oropharyngeal cancer. This association has been linked to various factors such as repeated oral and oropharyngeal exposure to HPV due to change in patterns of sexual behaviors; a phenomenon that may demand routine HPV vaccination.
Key Words: Oncogenic viruses, human papilloma virus, Epstein-Barr virus, oral squamous cell carcinoma
Introduction
Head and neck squamous cell carcinoma (HNSCC), known as the sixth most frequent malignancy worldwide, is a heterogeneous group of epithelial cancers, developed from the oral cavity, oropharynx, nasopharynx, hypopharynx, and larynx (Vigneswaran et al., 2014). Oral squamous cell carcinoma (OSCC) and oropharyngeal squamous cell carcinoma (OPSCC) are the most prevalent malignancies of the oral cavity and oropharynx, respectively, accounting for more than 90% of oral and oropharyngeal cancers (Mirzaei et al., 2018). The etiology of HNSCCs is a multifactorial process, with various risk factors such as alcohol drinking and tobacco smoking (Kumar et al., 2016). However, despite a decrease in exposure to these factors during the past three decades, the number of new OSCC and OPSCC cases has continued to increase, suggesting other etiological factors, such as infection with human papilloma virus (HPV) and Epstein-Barr virus (EBV), for these cancers (Elrefaey et al., 2014). In fact, HPV and EBV, as two important human oncogenic viruses, have also been implicated in the development of OSCC and OPSCC (Mirzaei et al., 2020); so that, in 2009, the International Agency for Research on Cancer (IARC) classified the sexually transmitted oncogenic viral pathogen HPV-16 as an independent risk factor for OPSCC (Bouvard et al., 2009). Moreover, studies suggest a possible interaction between HR-HPVs (such as HPV-16) and EBV during their co-infections to promote oncogenesis by various mechanisms (Cyprian et al., 2018; Shi et al., 2016). HPVs are DNA viruses with up to 200 genotypes, categorized into two high- and low-risk viruses, according to their tumorigenic potential (Ghedira et al., 2016). While HPV is a well-known etiological factor for OPSCC, its causative role in OSCC has remained controversial (Mirzaei et al., 2018). On the other hand, EBV is also a well-established etiological factor for several cancers with two subtypes (EBV-A and -B), whose causative role in HNSCCs has remained highly controversial (Higa et al., 2002) and needed to be more investigated. In this way, the present work aimed to evaluate the prevalence of HPV and co-infection with EBV among 166 OSCC and OPSCC samples from Ahvaz, Iran, for the first time.
Materials and Methods
Specimens
In this cross-sectional study, 141 OSCC and 25 OPSCC formalin-fixed paraffin-embedded (FFPE) tissues diagnosed from March 2013 and December 2019 were retrieved from the pathology archive of Ahvaz Imam Khomeini hospital, maffiliated to the Ahvaz Jundishapur University of Medical Sciences (AJUMS), Ahvaz, Iran, after obtaining ethical approval (IRAJUMS REC 1398233) from the Ethics Committee of AJUMS. This work used FFPE tissues due to the rarity of HNSCC and the lack of fresh tissues. Tissues were reviewed by an expert pathologist and selected based on their quality for molecular assays. Patient’s information, including gender, age, anatomical site, and histological grades were collected from the patient records. Samples were categorized as OSCC (tumors of the oral tongue, hard palate, floor of mouth, and gingiva) and OPSCC (tumors from the back one-third of the tongue, the soft palate, uvula, and tonsils) (Mirzaei et al., 2018).
DNA extraction
After cutting the samples for 15-μm-thick sections, the microtome blade was cleaned repeatedly using 10% chlorox to reduce cross contamination. Moreover, empty paraffin blocks were sectioned after every 10-15 tissue blocks and investigated for HPV DNA intersample contamination. Deparaffinization was done by incubating at 37°C for 1 h with 1 mL xylene, and then, washing with a descending ethanol concentration (100%, 70%, and 50%) for 30 minutes. After drying at 65°C, samples were lysed by adding 300 μL lysis buffer containing 30 µl proteinase K (Bioneer, Korea) and incubation at 37 overnight. The proteins were removed from the suspension by using an equal volume of Phenol;Chloroform;Isoamyl Alcohol (Sigma-Alrdich, USA). The extracted DNA was precipitated via absolute ethanol and checked for concentration and purity by NanoDrop(Thermo Fisher, USA).
PCR assay
Human β-globin gene
To check for DNA quality, a 110 bp human β-globin gene amplicon was amplified by using PCO3/PCO4 primers (Table 1), under the following condition; first denaturation at 95°C for 4 min, 35 cycles with denaturation at 95°C for 30s, annealing at 55°C for 30s, and extension at 72°C for 30s, and finally an extension at 72°C for 4 min. The positive samples were selected for further analysis. All the amplifications from this work used a 25μL PCR reaction containing 12.5μL mastermix (Ampliqon, Denmark), 1μL of forward/revers primers (10pmol), 9.5μL sterile water, and 1μL DNA sample (0.5μL for nested amplification).
Table 1.
Sequence of Primers Used in PCRs
| Primers | Sequences (5’–3’ ) | Amplicon Size (bp) |
|---|---|---|
| PCO3 | ACACAACTGTGTTCACTAGC | 110 |
| PCO4 | CAACTTCATCCACGTTCACC | |
| SB01 | CAAWTRTTYAATAARCCWTATTGG | 495 |
| SB02 | AAAAAYTTYCGWCCMARRGG | |
| MY09 | CGTCCMARRGGAWACTGATC | 450 |
| MY011 | GCMCAGGGWCATAAYAATGG | |
| GP5 | TTTGTTACTGTGGTAGATACTAC | 150 |
| GP6 | GAAAAATAAACTGTAAATCATATTC | |
| E7F1 | TAGCTGTAAACATGCATGGAA | 307 |
| E7R1 | TTATGGTTTCTGAGAACAGAB | |
| E7F2 | TGCAACCAGAGACAACTGAT | 183 |
| E7R2 | TGTCTACGTGTGTGCTTTGT | |
| EBNA1F1 | GTAGAAGGCCATTTTTCCAC | 609 |
| EBNA1R1 | CTCCATCGTCAAAGCTGC | |
| EBNA1F2 | AGATGACCCAGGAGAAGGCCCAAGG | 309 |
| EBNA1R2 | CAAAGGGGAGACGACTCAATGGTG | |
| EBNA3CF | CGG AAG AGG TGG AAA ACA AA | 246, 153 |
| EBNA3CR | GTG GGG GTC GTC ATC ATC TC |
HPV Detection and HPV-16 Genotyping
A nested PCR was done to detect HPV L1 gene by using two primer pairs (outer primers; MY09/11 (450 bp) and nested primers; GP5/6 (150 bp)) (Table 1). The amplifications were done under the following conditions: for outer PCR; 35 cycles of 95°C for 30s, 50°C for 1min, and 72°C for 1min, with a pre-denaturation at 95°C for 5min and an additional extension at 72°C for 8min, and for nested-PCR; 5min at 95°C followed by 30 cycles of 95°C for 30s, 50°C for 30s, 72°C for 30s, and finally 8min at 72°C. For HPV-16 genotyping, the HPV-positive samples were evaluated by a HPV-16 E7 gene type-specific nested-PCR to amplify a 183 bp fragment of HPV-16 E7 via two primer pairs (Table 1). To perform this, after a pre-denaturation at 94°C for 3min, the first and second amplifications were done through 35 cycles at 94°C for 30s, 45°C for 30s (for nested-PCR, 30s at 52°C), and 72°C for 30s, and also 5min at 72°C as final extension. For HPV-16 sequencing, the L1 gene was first amplified by SB01/02 primers for a 495 bp product (under the following condition; 5min at 95°C as an initial denaturation, 10 cycles of denaturation at 95°C for 1min, annealing at 42°C for 1 min, extension at 72°C for 1min, followed by 28 cycles of 95°C for 20s, 42°C for 40s, 72°C for 1min, and a final extension at 72°C for 8min), and then by MY09/11 primers. Moreover, a PUC57 plasmid containing full-length HPV-16 E7 gene, supplied by Karbalaie Niya et al.(Niya et al., 2018) was used as positive controls. The presence of PCR products was checked using agarose gel electrophoresis and visualized by UV transilluminator(GENE FLASH , UK).
EBV Amplifications
For EBV detection, 90 samples (including 58 and 32 HPV-negative and -positive samples, respectively) were tested by two EBV-EBNA-1 gene primer pairs(Table 1). After 4min at 94°C, the first and second amplifications were performed through 35 cycles at 94°C for 45s, 54°C for 45s (for nested-PCR, 45s at 58°C), and 72°C for 45s, and also 5 min at 72°C as final extension. For EBV typing, a strain-specific region of EBNA-3C gene(246 and 153 bp for EBV type B and type A, respectively) was amplified by a primer pair (Table 1) and evaluated by agarose gel electrophoresis. The amplification condition was the same as the EBNA-1 gene protocol (the EBNA-3C annealing temperature was 53°C).
Sequencing
The HPV-16-PCR products were sequenced in two directions using MY09/11 primers, by a 3130xl Applied Biosystems ABI sequencer(Applied Biosystems,
Foster City, CA). SnapGene(GSL Biotech) software was employed to analyze the results. The sequences were submitted in GenBank database with the following accession numbers; MW078991- MW078993 and MW079001.
Phylogenetic Analysis
Four HPV-16 L1 sequences were blasted by online tools (www.ncbi.nlm.nih.gov/BLAST). The phylogenetic tree was constructed by Neighbor joining method by using Mega 5.0 software.
Statistical analysis
This was done by IBM SPSS Software(Version 22), using the Independent Samples T Test and Fisher’s Exact Test. A P value<0.05 was considered statistically significant.
Results
HPV results
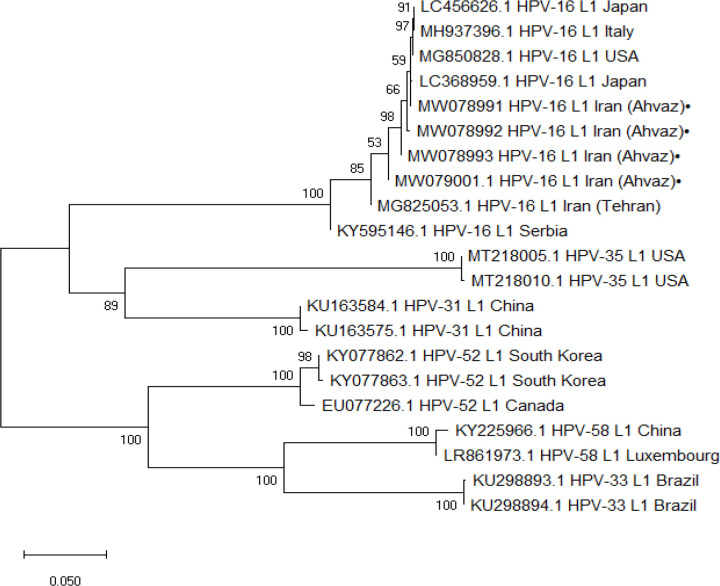
Of the 166 specimens, 140(84.33%) and 26(16.42%) cases were OSCCs and OPSCCs, respectively; of which, 87(52.4%) subjects were male and 79(47.6%) subjects were female, with a total mean age of 53.23 ± 15.19 years (Table 2). The most common anatomical sub-sites were the oral tongue (140(84.33%)) and the base of tongue (22(13.25%)), followed by the tonsils (4 (2.4%)). Moreover, 133 (80.1%), 23 (13.9%), and 10 (6%) out of the cases were histologically well, moderately, and poorly differentiated, respectively. Regarding PCR results, 32 (19.3%) out of the 166 cases tested positive for HPV, including 18 (18/87; 20.7%) males and 14 (14/79; 17.7%) females(P>0.05). For the anatomical sites, 16.42%(23/140) of the OSCCs and 34.6%(9/26) of the OPSCCs were HPV-positive(P<0.05); the virus was detected in 36.36% (8 out of 22), 25%(1 out of 4), and 16.42% (23 out of 140) of the base of tongue, the tonsil, and the oral tongue tumors, respectively(P=0.085). Additionally, 24 (18.04%) out of the HPV-positive cases were well differentiated, five (21.73%) moderately differentiated and three (30%) poorly differentiated(P>0.05). For HPV-16 genotyping, all the HPV-positive samples were tested by type-specific primers and 7 cases (7/32 (21.87%); 7/166 (4.2%)) were found to be HPV-16, including 4 cases (4/26; 15.38%) from OPSCC and 3 cases (3/140; 2.14%) from OSCC (P>0.05)(Figure 1). Six and one HPV-16-positive cases were histologicaly well and moderately differentiated, respectively (P>0.05). Four of the HPV-16 cases were sequenced and clustered with the virus from different regions of the world, such as Serbia, Japan, USA, Italy, and Iran (Tehran) (Figure 2).
Table 2.
Characteristics of the Evaluated Population and HPV-Status
| Variable | Study Population (%) (n= 166) | HPV+ (n=32) | HPV- (n=134) | P-value |
|---|---|---|---|---|
| Mean age (SD) | 53.3 ± 15.48 | 51.09±16.60 | 53.68±15.31 | 0.471 |
| Gender | ||||
| Male (%) | 87 (52.4) | 18 (20.68) | 69 (79.3) | 0.628 |
| Female (%) | 79 (47.6) | 14 (17.72) | 65 (82.27) | |
| Age | ||||
| <51 years (%) | 72 (43.37) | 15 (20.83) | 57 (79.16) | 0.851 |
| ≥51 years (%) | 94 (56.62) | 17 (18.8) | 77 (81.91) | |
| Anatomical sites | ||||
| Oral cavity (%) | 140 (84.33) | 23 (16.42) | 117 (83.57) | 0.031 |
| Oropharynx (%) | 26 (15.66) | 9 (34.6) | 17 (65.38) | |
| Anatomical sub-sites | ||||
| Oral tongue (%) | 140 (84.33) | 23 (16.42) | 117 (83.57) | 0.085 |
| Base of tongue (%) | 22 (13.25) | 8 (36.36) | 14 (63.63) | |
| Tonsil (%) | 4 (2.4) | 1 (25) | 3 (75) | |
| Histological grade | ||||
| Well (%) | 133 (80.12) | 24 (18.04) | 109 (81.95) | |
| Moderately (%) | 23 (13.85) | 5 (21.73) | 18 (78.26) | 0.619 |
| Poorly (%) | 10 (6.02) | 3 (30) | 7 (70) |
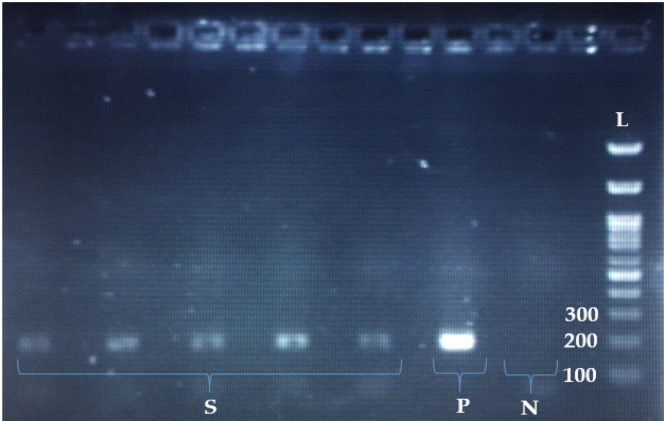
Figure 1.
The Results of E7 PCR for HPV-16 Genotyping. S; 5 HPV-16 (positive for 183 bp E7), N; negative control, P; positive control, L; DNA Molecular Marker (100bp size)
Figure 2.
The Phylogenetic Tree was Constructed by Neighbor Joining for L1 Region of HPV Genome Isolated from Ahvaz City. The detected L1 region of isolated HPV from Ahvaz with accession numbers MW078991- MW078993 and MW079001 were compared with different L1 genotypes retrieved from GenBank. The detected L1 HPV genotype 16 with black circle were clustered with L1 HPV genotype 16 isolated from different regions of world including KY595146.1 Serbia, LC644188.1 Japan, MG850828.1 USA,LC368959.1 Japan MH937396.1 Italy MG825053.1 and Iran(Tehran). The accuracy of the tree was assessed by 1000 bootstrap replicates. The scale bars is equal to 0.05
EBV results
Ninety samples (including 58 and 32 HPV-negative and -positive samples, respectively) were evaluated for EBV infection and co-infection with HPV. All the characteristics of 90 subjects are summarized in Table 3. In short, among the cases (79 (87.7%) and 11 (12.2%) cases with OSCC and OPSCC, respectively), 4 (4.4%) subjects were positive for EBV, including one male (1/52; 1.92%) and three females (3/38; 7.89%); of which two samples (2/90; 2.2%) had co-infection with HPV. Indeed, among the 166 cases, two subjects (1.2%) resulted positive for HPV-16/EBV co-infection. All the EBV-positive cases were from OSCC; three subjects histologically well differentiated, and one moderately differentiated (P>0.05). However, there was no significant association between EBV and other parameters (P>0.05). Moreover, all the EBV-positive cases were EBV-type B (Figure 3).
Table 3.
Characteristics of the Evaluated Population and EBV-Status
| Variable | Study Population (%) (n= 90) | EBV+ (n=4) | EBV- (n=86) | P-value |
|---|---|---|---|---|
| Mean age (SD) | 52.12 ± 16.63 | 49.75±16.58 | 52.23±16.73 | 0.615 |
| Gender | ||||
| Male (%) | 52 (57.77) | 1 (1.92) | 51 (98.7) | 0.175 |
| Female (%) | 38 (42.22) | 3 (7.89) | 35 (92.1) | |
| Age | ||||
| <51 years (%) | 42 (46.6) | 2 (4.76) | 40 (95.2) | 0.891 |
| ≥51 years (%) | 48 (53.3) | 2 (4.16) | 46 (95.8) | |
| Anatomical sites | ||||
| Oral cavity (%) | 79 (87.7) | 4 (5.06) | 75 (94.93) | 0.445 |
| Oropharynx (%) | 11 (12.2) | 0 | 11 (100) | |
| Anatomical sub-sites | ||||
| Oral tongue (%) | 79 (87.77) | 4 (16.42) | 75 (94.9) | 0.747 |
| Base of tongue (%) | 7 (7.77) | 0 | 7 (100) | |
| Tonsil (%) | 4 (4.44) | 0 | 4 (100) | |
| Histological grade | ||||
| Well (%) | 73 (81.11) | 3 (4.1) | 70 (95.89) | |
| Moderately (%) | 12 (13.33) | 1 (8.33) | 11 (91.66) | 0.712 |
| Poorly (%) | 5 (5.55) | 0 | 5 (100) |
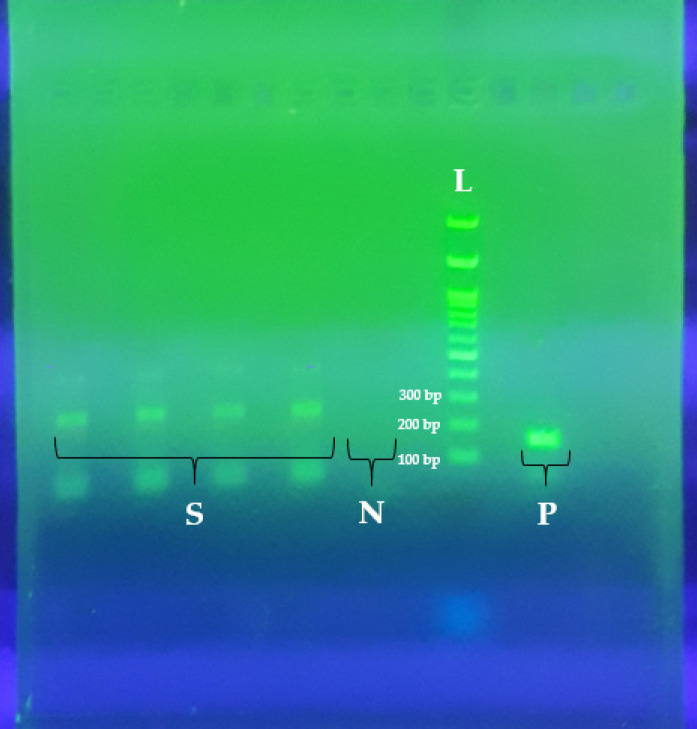
Figure 3.
The Results of EBNA3C PCR for EBV Genotyping. S; 4 EBV type B (positive for 246 bp EBNA3C), N; negative control, P; positive control of EBV type A (153 bp), L; DNA Molecular Marker (100bp size).
Discussion
HPV-associated HNSCC has emerged as a distinct entity in compared to HPV-unrelated ones. In fact, HPV is currently known as a well-established etiological factor for a subset of HNSCCs, in particular for those originated from the oropharynx (Vokes et al., 2015), so that it is suggested about 30% of OPSCCs are mediated by HPV (Tumban, 2019), where HR-HPV-16 is the most prevalent type, accounting for more than 90% of all HPV-positive OPSCCs (Kobayashi et al., 2018). While HPV is a well-known etiological factor for OPSCC, its causative role in OSCC is still poorly understood (Mirzaei et al., 2018). This work aimed to evaluate the HPV prevalence among 166 OSCCs and OPSCCs from Ahvaz, Iran. The current work found that 19.3% of the cases were positive for HPV, a prevalence rate that is in line with previously reported HPV frequencies among HNSCCs, worldwide (0%-70%) (Ni et al., 2019; Shaikh et al., 2017). HPV-16 type distribution from this work also showed that about 21.87% of the 32 positive samples were positive for this high-risk type, which agreed with previous reports, where HPV-16 accounted for a vast majority of all HPV-positive HNSCCs; an observation that may be a consequence of lower HPV-16 clearance rate (in compared to other HPV types) in the head and neck region (Faust et al., 2016; Gillison et al., 2015; Tumban, 2019). Similarly, Shaikh et al., and Quintero et al., reported that 21% and 18.9% of HNSCCs in their studies were HPV-positive, respectively, where 91.6% and 82% of the cases were HPV-16 (Quintero et al., 2013; Shaikh et al., 2017). In the same way, Faust et al., found a 74% prevalence rate for HPV-16 in HNSCCs (Faust et al., 2016), and Gillison et al., (2015) reported HPV-16 as the cause of about 82% of HPV infections in HNSCCs. The results of phylogenetic tree revealed that the detected HPV-16 genotypes (MW078991- MW078993 and MW079001) were cluster with HPV-16 genotypes isolated from different regions of the world including KY595146.1 Serbia, MG850828.1 USA, LC368959.1 Japan MH937396.1 Italy, MG825053.1 Iran (Tehran), and LC456626.1 Japan (Figure 2). Regarding the anatomical sites, a higher rate of HPV positivity was found in OPSCCs (34.6%), compared to OSCCs (16.42%)(P<0.05), suggesting a trend towards OPSCCs to have more HPV infection. These observations are in accordance with other studies reporting a higher site-specific HPV prevalence in OPSCCs (de Martel et al., 2017; Tumban, 2019). Additionally, our OPSCC finding agreed with previous reportes that about 30% of OPSCCs are associated with HPV, particularly with HPV-16 (Tumban, 2019). In OSCC, HPV prevalence demonstrates much variation, worldwide; and while some studies proposed an etiological role for HPV (Kaminagakura et al., 2012; Mirzaei et al., 2018), other studies failed to establish a causal relationship(Mirzaei et al., 2018; Scapoli et al., 2009). However, our OSCC result is also comparable with another study from Iran by Seraj et al., who reported that 26.6% of OSCCs were HPV-positive, of which 38.5% and 61.5% were HPV-16 and -18, respectively (Seraj et al., 2011). Similarly, Saghravanian et al., from Iran reported a 13.16% prevalence rate for HPV in OSCCs (Saghravanian et al., 2016); nevertheless, HR-HPVs were not found in that study. In the anatomical sub-sites, HPV is commonly associated with cancers of the tonsil(94%), the base of tongue(62%) and the oral tongue (50%)(Yete et al., 2018); accordingly, in this work, the most frequent HPV-positive sites were the base of tongue (36%) and the tonsil (25%) (Table 2).
The present study also evaluated EBV infection/co-infection among 90 HNSCCs. EBV is an oncogenic virus from the Herpesviridae family, associated with several malignancies, whose causative role in HNSCC has remained highly controversial (Mirzaei et al., 2018). In our study, 4.4%(4/90) of the cases tested positive for EBV, where all the cases were EBV-type B and from OSCCs. Indeed, EBV infection among the HNSCCs was low for OSCC (4.4%)(and negative for OPSCC) compared to Shahrabi-Farahani et al. and Sarvani et al. studies from Iran, who reported EBV in 72.3% and 16.7% of OSCCs, respectively(Shahrabi-Farahani et al., 2018). The prevalence of EBV in OSCC shows a high variation all over the world, ranging from 0 to 100% (Sand et al., 2002). However, our results are comparable with those studies with low or without EBV detection (Delavarian et al., 2010; Talacko et al., 1991). In accordance with previous reports, our findings exhibited a higher EBV positivity in well-differentiated OSCCs (3/4); nonetheless, this association was not statistically significant (Table 3)(Sand et al., 2002). Studies suggest a possible interaction between HR-HPVs and EBV during co-infections to promote oral and oropharyngeal oncogenesis (Cyprian et al., 2018; Heawchaiyaphum et al., 2022; Moore-Medlin et al., 2018; Mostafaei et al., 2020; Shi et al., 2016). Accordingly, in this study, two cases (2/166;1.2%) had HPV/EBV co-infection, as other studies reported it in HNSCCs (Moore-Medlin et al., 2018; Shi et al., 2016). For instance, Medlin et al. reported that 13% of 134 patients with OPSCC had HPV/EBV co-infection in their study (Moore-Medlin et al., 2018). This phenomenon that has also been addressed for other malingnancies (Abudoukadeer et al., 2015; Khenchouche et al., 2013; Shi et al., 2016), may be mediated by various mechanisms such as increasing cell invasiveness (Moore-Medlin et al., 2018), which is needed to be more investigated in future studies.
Taken togather and in line with previous studies, our findings show that EBV is more frequent in OSCC, while HPV seems to be more associated with OPSCC (Broccolo et al., 2018). A vast majority of our samples were HPV-positive, particularly HPV-16; a phenomenon that is linked to various factors such as repeated oral and oropharyngeal exposure to HPV due to change in patterns of sexual behaviors (Chaturvedi et al., 2016). Hence, it seems, as the CDC recommends, HPV vaccination should be performed routinely for both genders (Pan et al., 2018).
Author Contribution Statement
All the listed authors have contributed substantially to the production of this research work.
Acknowledgments
This paper issued a part of the thesis from Ph.D by Habibollah Mirzaei with registration number OG-9808. It was financially supported by Infectious and Tropical Diseases Research Center, Health Research Institute, Ahvaz Jundishapur University of Medical Sciences (AJUMS), Ahvaz, Iran. Ethical Approval: This work was approved by the Ethics Committee of AJUMS (Ethical code; IRAJUMS REC 1398233).
Conflict of Interest
The authors have no relevant conflicts of interest to declare.
References
- Abudoukadeer A, Niyazi M, Aikula A, et al. Association of EBV and HPV co-infection with the development of cervical cancer in ethnic Uyghur women. Eur J Gynaecol Oncol. 2015;36:546–50. [PubMed] [Google Scholar]
- Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- Broccolo F, Ciccarese G, Rossi A, et al. Human papillomavirus (HPV) and Epstein-Barr virus (EBV) in keratinizing versus non-keratinizing squamous cell carcinoma of the oropharynx. Infect Agent Cancer. 2018;13:1–5. doi: 10.1186/s13027-018-0205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi AK, Song H, Rosenberg PS, et al. Tonsillectomy and incidence of oropharyngeal cancers. Cancer Epidemiol Biomarkers Prev. 2016;25:944–50. doi: 10.1158/1055-9965.EPI-15-0907. [DOI] [PubMed] [Google Scholar]
- Cyprian FS, Al-Farsi HF, Vranic S, et al. Epstein–Barr virus and human papillomaviruses interactions and their roles in the initiation of epithelial–mesenchymal transition and cancer progression. Front Oncol. 2018;8:111. doi: 10.3389/fonc.2018.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141:664–70. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delavarian Z, Pakfetrat A, Falaki F, et al. The role of viruses in oral squamous cell carcinoma in young patients in Khorasan (Northeast of Iran) 2010:981–5. [Google Scholar]
- Elrefaey S, Massaro M, Chiocca S, et al. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital. 2014;34:299. [PMC free article] [PubMed] [Google Scholar]
- Faust H, Alwan EE, Roslin A, et al. Prevalence of human papillomavirus types, viral load and physical status of HPV16 in head and neck squamous cell carcinoma from the South Swedish Health Care Region. J Gen Virol. 2016;97:2949–56. doi: 10.1099/jgv.0.000611. [DOI] [PubMed] [Google Scholar]
- Ghedira R, Mahfoudh W, Hadhri S, et al. Human papillomavirus genotypes and HPV-16 variants distribution among Tunisian women with normal cytology and squamous intraepithelial lesions. Infect Agent Cancer. 2016;11:1–10. doi: 10.1186/s13027-016-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillison ML, Chaturvedi AK, Anderson WF, et al. Epidemiology of human papillomavirus–positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33:3235. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heawchaiyaphum C, Ekalaksananan T, Patarapadungkit N, et al. Association of human papillomavirus and Epstein-Barr virus infection with Tonsil cancer in Northeastern Thailand. Asian Pac J Cancer Prev. 2022;23:781–87. doi: 10.31557/APJCP.2022.23.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa M, Kinjo T, Kamiyama K, et al. Epstein-Barr virus (EBV) subtype in EBV related oral squamous cell carcinoma in Okinawa, a subtropical island in southern Japan, compared with Kitakyushu and Kumamoto in mainland Japan. J Clin Pathol. 2002;55:414–23. doi: 10.1136/jcp.55.6.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminagakura E, Villa LL, Andreoli MA, et al. High-risk human papillomavirus in oral squamous cell carcinoma of young patients. Int J Cancer. 2012;130:1726–32. doi: 10.1002/ijc.26185. [DOI] [PubMed] [Google Scholar]
- Khenchouche A, Sadouki N, Boudriche A, et al. Human papillomavirus and Epstein-Barr virus co-infection in cervical carcinoma in Algerian women. Virol J. 2013;10:1–8. doi: 10.1186/1743-422X-10-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Hisamatsu K, Suzui N, et al. A review of HPV-related head and neck cancer. J Clin Med. 2018;7:241. doi: 10.3390/jcm7090241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Nanavati R, Modi TG, et al. Oral cancer: Etiology and risk factors: A review. J Cancer Res Ther. 2016;12:458. doi: 10.4103/0973-1482.186696. [DOI] [PubMed] [Google Scholar]
- Mirzaei H, Ghorbani S, Khanizadeh S, et al. Histone deacetylases in virus-associated cancers. Rev Med Virol. 2020;30:e2085. doi: 10.1002/rmv.2085. [DOI] [PubMed] [Google Scholar]
- Mirzaei H, Goudarzi H, Eslami G, et al. Role of viruses in gastrointestinal cancer. J Cell Physiol. 2018;233:4000–14. doi: 10.1002/jcp.26194. [DOI] [PubMed] [Google Scholar]
- Moore-Medlin T, Asarkar A, Ma X, et al. The role of human papillomavirus and Epstein-Barr virus co-infection in oropharyngeal squamous cell tumor differentiation. Int J Dent. 2018;100:1352–53. [Google Scholar]
- Mostafaei S, Kazemnejad A, Norooznezhad AH, et al. Simultaneous effects of viral factors of human papilloma virus and Epstein-Barr virus on progression of breast and thyroid cancers: application of structural equation modeling. Asian Pac J Cancer Prev. 2020;21:1431. doi: 10.31557/APJCP.2020.21.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni G, Huang K, Luan Y, et al. Human papillomavirus infection among head and neck squamous cell carcinomas in southern China. PLoS One. 2019;14:e0221045. doi: 10.1371/journal.pone.0221045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niya MHK, Keyvani H, Tameshkel FS, et al. Human papillomavirus type 16 integration analysis by real-time PCR assay in associated cancers. Transl Oncol. 2018;11:593–98. doi: 10.1016/j.tranon.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C, Issaeva N, Yarbrough WGJCoth, et al. HPV-driven oropharyngeal cancer: current knowledge of molecular biology and mechanisms of carcinogenesis. Cancers Head Neck. 2018;3:1–11. doi: 10.1186/s41199-018-0039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero K, Giraldo GA, Uribe ML, et al. Human papillomavirus types in cases of squamous cell carcinoma of head and neck in Colombia. Braz J Otorhinolaryngol. 2013;79:375–81. doi: 10.5935/1808-8694.20130065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghravanian N, Zamanzadeh M, Meshkat Z, et al. Evaluation of the prevalence rate and the prognostic effect of human papilloma virus infection in a group of patients with oral cavity squamous cell carcinoma. Iran J Cancer Prev. 2016:9. doi: 10.17795/ijcp-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand LP, Jalouli J, Larsson P-A, et al. Prevalence of Epstein-Barr virus in oral squamous cell carcinoma, oral lichen planus, and normal oral mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:586–92. doi: 10.1067/moe.2002.124462. [DOI] [PubMed] [Google Scholar]
- Scapoli L, Palmieri A, Rubini C, et al. Low prevalence of human papillomavirus in squamous-cell carcinoma limited to oral cavity proper. Mod Pathol. 2009;22:366–72. doi: 10.1038/modpathol.2008.180. [DOI] [PubMed] [Google Scholar]
- Seraj JM, Yazdani N, Ashtiani ZO, et al. TP53 gene expression in HPV-positive oral tongue SCC and its correlation with nodal metastasis. Pathol Res Pract. 2011;207:758–61. doi: 10.1016/j.prp.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Shahrabi-Farahani M, Karimi E, Mostaan LV, et al. Association between epstein barr virus and tongue squamous cell carcinoma in iranian patients. Pathol Res Pract. 2018;214:130–33. doi: 10.1016/j.prp.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Shaikh MH, Khan AI, Sadat A, et al. Prevalence and types of high-risk human papillomaviruses in head and neck cancers from Bangladesh. BMC Cancer. 2017;17:792. doi: 10.1186/s12885-017-3789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Peng SL, Yang LF, et al. Co-infection of Epstein-Barr virus and human papillomavirus in human tumorigenesis. Chin J Cancer. 2016;35:1–9. doi: 10.1186/s40880-016-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talacko A, Teo C, Griffin B, et al. Epstein-Barr virus receptors but not viral DMA are present in normal and malignant oral epithelium. J Oral Pathol Med. 1991;20:20–5. doi: 10.1111/j.1600-0714.1991.tb00882.x. [DOI] [PubMed] [Google Scholar]
- Tumban EJV. A current update on human papillomavirus-associated head and neck cancers. Viruses. 2019;11:922. doi: 10.3390/v11100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneswaran N, Williams MDJO, Clinics MS. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26:123–41. doi: 10.1016/j.coms.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vokes EE, Agrawal N, Seiwert TYJJJotNCI. HPV-associated head and neck cancer. Cancers (Basel) 2015:107. doi: 10.1093/jnci/djv344. [DOI] [PubMed] [Google Scholar]
- Yete S, D’Souza W, Saranath DJO. High-risk human papillomavirus in oral cancer: clinical implications. Oncology. 2018;94:133–41. doi: 10.1159/000485322. [DOI] [PubMed] [Google Scholar]