Abstract
Perfluoroalkyl substances (PFASs), as coating materials, possess oil-resistant and waterproof properties. However, their persistency and toxicity have caused concerns. This study developed a method for determining five types of 20 PFASs with ultra-performance liquid chromatography/tandem mass spectrometry, and measured seven categories of 32 commercial samples of oil-resistant food packaging in Taiwan. The assay was validated according to the specification of Taiwan Food and Drug Administration (TFDA). Samples of 100 cm2 were cut into pieces and were ultra-sonicated in 20-mL methanol at 50 °C for 45 minutes. The extracts were concentrated to 1 mL for instrumental analysis. Most matrix effect factors and extraction efficiencies of the analytes were 50%–80% and 52%–99%, respectively. Most limits of detection and limits of quantification were between 0.07–11.3 ng/dm2 and 0.17–18.3 ng/dm2, respectively. Most recoveries ranged from 70% to 117% at three tested levels, and the precisions (%RSD) were lower than 19%. Microwave popcorn paper contained more types and higher levels of PFASs than other packaging, with perfluoroalkyl acids at 8.3–1960 ng/dm2 and fluorotelomer alcohols (FTOHs) at 9.7–7188 ng/dm2. High concentrations of FTOHs were also observed in one oil-proof paper bag at 454–2595 ng/dm2 and in one French fries paper bag at 22.4–167 ng/dm2.
Keywords: Fluorotelomer alcohol, Microwave popcorn paper, Oil-proof paper, Perfluoroalkyl acids, Perfluoroalkyl substances, UPLC-MS/MS
1. Introduction
Perfluoroalkyl substances (PFASs) possess both oil-resistant and waterproof properties because their carbon chains are fully fluorinated and are connected with various polar functional groups. PFASs are widely used in surfactants, lubricants, semi-conductor industries, and surface coating materials [1,2]. PFASs include perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkyl sulfonates, perfluoroalkyl sulfonamides (PFASAs), fluorotelomer alcohols (FTOHs), polyfluoroalkyl phosphoric acid esters (PAPs), and so on [3]. Some relatively volatile PFASs, such as FTOHs and perfluorooctane sulfonamide (PFOSA), can biodegrade or degrade in the atmosphere into ionic PFASs, such as perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) [4].
PFASs, especially those carbon-chain lengths of PFCAs more than eight and sulfonates more than six, are persistent and bioaccumulative, and are ubiquitous in the environment [3,5]. The general population is primarily exposed to PFASs through ingestion of food, especially from fish and seafood; drinking water would contribute about 20% [6,7]; concentrations of PFASs in blood positively associate with their levels in dietary intake [8,9]. PFASs may result in liver, immune, developmental, and reproductive adverse health effects [10,11]. Among the PFASs, PFOA and PFOS have been studied more extensively, have been used for a long period of time, and are listed in the Stockholm Conventions [10]. Some guidelines set advisory levels of PFOA and PFOS at 70 ng/L in drinking water, and 300–7000 ng/L for C4–C7 PFASs [12,13]. The European Food Safety Authority (EFSA) established tolerable weekly intake (TWI) of 6 and 13 ng/kg bw per week for PFOA and PFOS, respectively, in 2018 [14], and further reduced the TWI of total PFASs to 4.4 ng/kg bw per week in 2020 [15].
PFASs may be used in coating, adhesive, and printing inks of food packaging, and could migrate to food [16–18]. The concentrations of PFASs vary in different types of food packaging; for example, paper cups may contain lower PFASs concentrations than fast food packaging [19], but Poothong et al. observed 16.9 ng/dm2 of PFOA in a sample of ice cream cup, which was the highest among seven categories of paper food packaging [16]. Previous studies found PFOA at concentrations of 198–290 ng/g in microwave popcorn packaging [20,21]; a recent report stated decreased concentrations of PFOA and PFOS in microwave packaging from 2005 to 2018 [22]. Despite the decrease of long-chain ionic PFASs in food packaging [1,23], their precursors like PAPs and FTOHs as oil resistant coatings may result in the accumulation of ionic PFASs in humans and the environment [24]; for instance, FTOHs could reach ppm levels in microwave popcorn packaging [25].
Solid-liquid extraction [23,26], ultrasound assisted extraction [17,27], and pressurized liquid extraction (PLE) [1,16,21] are used for extracting PFASs from food packaging with ethanol-water or methanol-water mixtures. These studies focused on PFCAs and perfluoroalkyl sulfonates [16,21,28,29], and only limited researches investigated PAPs and FTOHs [2,17,23,25]. This study developed an analytical method for simultaneously determining five types of 20 PFASs in food packaging with an ultra-performance liquid chromatography/triple-quadrupole tandem mass spectrometer (UPLC-MS/MS). The study optimized the extraction method, evaluated matrix effect and extraction efficiency, and demonstrated good accuracy and precision. The validated method was applied on measuring PFASs in seven categories of commercial oil-resistant food packaging, in the total of 32 samples in Taiwan.
2. Methods
2.1. Materials
Target analytes and their stable isotope-labeled internal standards (ISTDs) were purchased from Wellington Laboratories (Ontario, Canada; purity >98%, 50 ± 2.5 μg/mL in methanol), including perfluoro-n-butanoic acid (PFBA), perfluoropentanoic acid (PFPeA), perfluorohexanoic acid (PFHxA), perfluorooctanoic acid (PFOA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnDA), perfluorododecanoic acid (PFDoDA), perfluorohexadecanoic acid (PFHxDA), perfluorobutane sulfonate (PFBS), perfluorohexane sulfonate (PFHxS), perfluorooctane sulfonate (PFOS), perfluorooctane sulfonamide (PFOSA), N-methyl perfluorooctane sulfonamide (N-MeFOSA), 2-perfluorobutyl ethanol (4:2 FTOH), 2-perfluorohexyl ethanol (6:2 FTOH), 2-perfluorooctyl ethanol (8:2 FTOH), sodium 1H,1H,2H,2H-perfluorooctyl phosphate (sodium 6:2 PAP), sodium 1H,1H,2H,2H-perfluorodecyl phosphate (sodium 8:2 PAP), sodium bis(1H,1H,2H,2H-perfluorooctyl) phosphate (sodium 6:2 diPAP), sodium bis(1H,1H,2H,2H-perfluorodecyl) phosphate (sodium 8:2 diPAP), perfluoro-n-[1,2,3,4-13C4]butanoic acid (13C4-PFBA), perfluoro-n-[3,4,5-13C3]pentanoic acid (13C3-PFPeA), perfluoro-n-[1,2,3,4,6-13C5]hexanoic acid (13C5-PFHxA), perfluoro-n-[13C8]octanoic acid (13C8-PFOA), perfluoro-n-[1,2,3,4,5,6,7-13C7] undecanoic acid (13C7-PFUnDA), sodium perfluoro-1-hexane[18O2]sulfonate (sodium 18O2-PFHxS), sodium perfluoro-1-[1,2,3,4-13C4]octane sulfonate (sodium 13C4-PFOS), N-methyl-D3-perfluoro-1-octane sulfonamide (2D3-N-MeFOSA), 2-perfluorobutyl-[1,1,2,2-2H4]-ethanol (2D4-4:2 FTOH), 2-perfluorohexyl-[ 1,1-2H2]-[1,2-13C2]-ethanol ( ), 2-perfluorooctyl-[1,1-2H2]-[1,2-13C2]-ethanol ( ), sodium 1H,1H,2H,2H-[1,2-13C2] perfluorooctyl phosphate (sodium 13C2-6:2 PAP), sodium 1H,1H,2H,2H-[1,2-13C2]perfluorodecyl phosphate (sodium 13C2-8:2 PAP), sodium bis(1H,1H,2H,2H-[1,2-13C2]perfluorooctyl) phosphate (sodium 13C4-6:2 diPAP), and sodium bis(1H,1H,2H,2H-[1,2-13C2]perfluorodecyl) phosphate (sodium 13C4-8:2 diPAP).
HPLC-grade acetone and methanol, and LC/MS-grade acetonitrile were provided by J.T. Baker (Philipsburg, NJ, USA). Milli-Q water was from a Milli-Q integral water purification system (Merck Millipore, Billerica, MA, USA). LC/MS-grade methanol was purchased from Merck (Darmstadt, Hesse, Germany). HPLC-grade ammonium acetate, acetic acid, and N-methylmorpholine (purity ≥99.5%) were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Nylon Millex filters of 0.20 μm in 33-mm diameter were bought from Merck Millipore (Darmstadt, Hesse, Germany).
2.2. Sample collection
Thirty-two pieces of oil-resistant food packaging in seven categories were bought from markets and restaurants in Taipei, Taiwan in October 2018, including to-go boxes, burger/hot dog paper, fried chicken boxes, French fries paper bags, microwave popcorn paper bags, oil-proof paper, and beverage paper cups (Table S1 and Fig. S1). To avoid the potential influences of food residues on quantitative results, we requested the stores to provide the same materials without contacting with food as analytical samples.
2.3. Sample preparation
Samples were cut into a size of 10 cm × 10 cm; the places with ink were avoided. After weighing, the samples were further cut into pieces of smaller than 1 cm2 with scissors, and were put into 50-mL polypropylene (PP) centrifuge tubes. The sample was soaked with 20 mL of methanol, which was spiked with 100 μL of isotope-labeled internal standards in methanol at the concentration of 1.0 μg/mL; after 30-sec vortex, the mixture was extracted for 45 minutes at 50 °C with an LEO IACF-1502S ultrasonic cleaner (New Taipei City, Taiwan) at 150 W. The PP tube was centrifuged by a KUBOTA 2010 centrifuge (Tokyo, Japan) for 15 minutes at 3000 rpm (1910×g); the supernatant was transferred into a 40-mL deactivated (silanized) amber glass tube, concentrated to 1.8 mL at 50 °C by a SpeedVac (Savant SPD 1010, Thermo Fisher Scientific, Waltham, MA, USA), and was filtered through a methanol-prewashed 0.20-μm nylon syringe filter using a Samplicity filtration system (Merck Millipore, Darmstadt, Hesse, Germany) into a 2-mL deactivated vials (Waters, Milford, MA, USA). The filtrate was further concentrated to 1.0 mL at 50 °C by the SpeedVac and was mixed with 500 μL of Milli-Q water; the solution was concentrated again to a final volume of 1.0 mL. Four microliters of the residue were injected for instrumental analysis.
2.4. Instrumental analysis
The analysis was performed on a Waters ACQUITY UPLC coupled with a Waters Quattro Premier XE triple-quadrupole mass spectrometer using negative electrospray ionization (ESI−) at multiple-reaction monitoring mode (MRM). The two most abundant ion transitions of each analyte were acquired as quantifiers and qualifiers, respectively; the exceptions were PFBA and PFPeA, which can only form one stable ion transition. Cone voltages and collision energies were optimized on each analyte (Table 1). The capillary voltage and extractor voltage were 1.5 kV and 4 V, respectively. Both the desolvation gas (flow rate 900 L/h) and the cone gas (flow rate 50 L/h) were nitrogen. The source temperature and desolvation temperature were 120 °C and 450 °C, respectively. The collision gas was argon at the pressure of 3.03 × 10−3 mbar, with a flow rate of 0.17 mL/min.
Table 1.
Parameters of tandem mass spectrometer of the 20 analytes and the 15 isotope-labeled internal standards, and analyte limits of detection, limits of quantification, linear ranges, and r2 of calibration curves.
| Compounds | ISTDsa | Retention time (min) | LODs (n = 3)b | LOQs (n = 3)b | Linear rangec |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
| Cone voltage (V); | ng/dm2 (ng/g) | ng/dm2 (ng/g) | (ng/mL), r2 | ||
| Precursor ion > product ion I, II (collision energy, V) | |||||
| PFBA | 13C4-PFBA | 0.93 | 0.41 ± 0.06 | 1.35 ± 0.19 | 0.1–1000 |
| 12; 213.0 > 169.0 (10) | 12; 217.0 > 172.0 (10) | (0.50 ± 0.07) | (1.67 ± 0.24) | 0.997 | |
| PFPeA | 13C3-PFPeA | 2.65 | 0.35 ± 0.18 | 1.16 ± 0.62 | 0.1–1000 |
| 15; 263.0 > 218.9 (10) | 12; 266.0 > 222.0 (9) | (0.43 ± 0.23) | (1.43 ± 0.76) | 0.999 | |
| PFHxA | 13C5-PFHxA | 3.90 | 0.51 ± 0.09 | 1.50 ± 0.61 | 1.0–1000 |
| 12; 313.0 > 268.9 (12), 118.8 (18) | 12; 317.9 > 272.1 (10) | (0.63 ± 0.11) | (1.85 ± 0.75) | 0.998 | |
| PFOA | 13C8-PFOA | 5.34 | 0.28 ± 0.06 | 0.65 ± 0.06 | 0.5–1000 |
| 13; 412.9 > 368.9 (10), 168.8 (18) | 13; 421.1 > 376.1 (10) | (0.34 ± 0.07) | (0.80 ± 0.07) | 0.997 | |
| PFDA | 13C7-PFUnDA | 6.19 | 0.11 ± 0.01 | 0.19 ± 0.03 | 1.0–1000 |
| 20; 512.9 > 469.1 (11), 218.9 (16) | (0.13 ± 0.01) | (0.23 ± 0.04) | 0.995 | ||
| PFUnDA | 13C7-PFUnDA | 6.51 | 0.07 ± 0.01 | 0.19 ± 0.02 | 0.5–1000 |
| 17; 562.9 > 519.0 (13), 269.1 (17) | 17; 570.1 > 525.1 (10) | (0.09 ± 0.01) | (0.23 ± 0.02) | 0.995 | |
| PFDoDA | 13C7-PFUnDA | 6.76 | 0.13 ± 0.03 | 0.29 ± 0.08 | 0.5–1000 |
| 20; 612.9 > 568.9 (14), 269.1 (17) | (0.16 ± 0.03) | (0.36 ± 0.10) | 0.996 | ||
| PFHxDA | 13C7-PFUnDA | 7.57 | 0.94 ± 0.26 | 3.19 ± 0.44 | 0.5–1000 |
| 20; 812.9 > 769.0 (15), 419.1 (30) | (1.16 ± 0.32) | (3.93 ± 0.55) | 0.997 | ||
| PFBS | 18O2-PFHxS | 3.17 | 0.74 ± 0.09 | 1.15 ± 0.26 | 0.1–1000 |
| 20; 299.0 > 79.7 (35), 98.7 (30) | (0.92 ± 0.11) | (1.42 ± 0.32) | 0.999 | ||
| PFHxS | 18O2-PFHxS | 4.87 | 0.17 ± 0.01 | 0.35 ± 0.12 | 0.5–1000 |
| 22; 398.8 > 79.7 (31), 98.7 (31) | 25; 403.1 > 83.7 (35) | (0.21 ± 0.02) | (0.43 ± 0.15) | 0.995 | |
| PFOS | 13C4-PFOS | 5.84 | 0.18 ± 0.03 | 0.25 ± 0.03 | 0.5–1000 |
| 18; 499.1 > 79.5 (40), 98.6 (42) | 22; 503.1 > 79.7 (42) | (0.22 ± 0.04) | (0.31 ± 0.04) | 0.996 | |
| PFOSA | 2D3-N-MeFOSA | 6.20 | 0.11 ± 0.01 | 0.17 ± 0.03 | 0.5–1000 |
| 23; 498.1 > 77.6 (30), 168.7(27) | (0.13 ± 0.01) | (0.20 ± 0.04) | 0.991 | ||
| N-MeFOSA | 2D3-N-MeFOSA | 7.85 | 2.05 ± 0.77 | 12.5 ± 1.1 | 0.5–1000 |
| 32; 512.1 > 168.8 (28), 128.9 (24) | 38; 515.1 > 168.9 (25) | (2.53 ± 0.95) | (15.4 ± 1.33) | 0.998 | |
| 4:2 FTOH | 2D4-4:2 FTOH | 6.23 | 35.9 ± 21.1 | 124 ± 13.7 | 50–1000 |
| 10; 262.9 > 203.0 (10), 154.7 (18) | 10; 266.2 > 204.1 (10) | (44.3 ± 26.0) | (152 ± 16.9) | 0.973 | |
| 6:2 FTOH | 7.34 | 6.08 ± 1.71 | 18.3 ± 8.0 | 5.0–1000 | |
| 13; 363.0 > 303.0 (9), 255.0 (20) | 10; 367.2 > 306.1 (11) | (7.50 ± 2.11) | (22.6 ± 9.8) | 0.995 | |
| 8:2 FTOH | 8.04 | 11.3 ± 6.60 | 41.7 ± 27.3 | 1.0–1000 | |
| 14; 463.0 > 403.0 (10), 355.0 (20) | 12; 467.2 > 406.1 (12) | (13.9 ± 8.15) | (51.4 ± 33.7) | 0.995 | |
| 6:2 PAP | 13C2-6:2 PAP | 3.64 | 2.04 ± 0.40 | 3.86 ± 1.27 | 1.0–1000 |
| 20; 443.0 > 96.6 (15), 423.1 (12) | 20; 445.2 > 96.7 (15) | (2.52 ± 0.49) | (4.77 ± 1.56) | 0.999 | |
| 8:2 PAP | 13C2-8:2 PAP | 5.48 | 1.54 ± 0.40 | 4.00 ± 0.50 | 1.0–1000 |
| 23; 543.0 > 96.6 (18), 523.0 (15) | 23; 545.1 > 96.7 (20) | (1.91 ± 0.50) | (4.94 ± 0.62) | 0.995 | |
| 6:2 diPAP | 13C4-6:2 diPAP | 7.25 | 0.08 ± 0.00 | 0.32 ± 0.05 | 0.5–1000 |
| 35; 788.9 > 96.6 (25), 443.1 (18) | 30; 793.2 > 96.7 (28) | (0.10 ± 0.00) | (0.40 ± 0.07) | 0.997 | |
| 8:2 diPAP | 13C4-8:2 diPAP | 7.81 | 0.21 ± 0.05 | 0.74 ± 0.20 | 0.1–1000 |
| 40; 988.8 > 96.6 (35), 543.1 (21) | 40; 993.2 > 96.7 (33) | (0.26 ± 0.06) | (0.92 ± 0.25) | 0.997 | |
ISTDs: isotope-labeled internal standards.
LODs: limits of detection; LOQs: limits of quantification. The values were shown in mean ± standard deviation.
The concentrations of the chemical standards.
Analytes were separated on an ACQUITY UPLC BEH C18 column (50 × 2.1 mm, 1.7 μm; Waters) with a gradient elution at 55 °C at a flow rate of 0.4 mL/ min. The mobile phases were composed of (A) 10-mM N-methylmorpholine(aq) (pH 9.6) and (B) methanol. The chromatographic gradient began from 95% of A and 5% of B for 0.5 min; the portion of methanol was increased to 100% in 8.5 min and was held for 0.2 min. The gradient returned to the initial composition in 0.3 min and the column was re-equilibrated for 2.0 min. The total chromatographic time took 11.5 min. An isolator column (ACQUITY UPLC BEH C18, 50 × 2.1 mm, 2.6 μm) was installed between the liquid chromatograph mixer and the autosampler injection port to prevent potential interferences of PFASs from the LC system and the mobile phases (Fig. S2).
2.5. Method validation, quality assurance, quality control, and data analysis
This study used microwave popcorn packaging for evaluating matrix effect and extraction efficiency. Linear regression curves were established based on analyte peak areas versus analyte concentrations for pre- and post-spiking samples at five spiked concentrations with three duplicates per concentration. Spiked levels of analytes in the solutions for instrumental analysis were 5, 20, 50, 125, and 250 ng/ mL except for those of 4:2 FTOH and 6:2 FTOH, which were 75, 100, 200, 250, and 500 ng/mL. For pre-spiking, chemical standards in acetone were added to sample surfaces and the samples were shaken slowly for 10 min to ensure homogenous contact; thereafter, the samples were covered with aluminum foil and were left for three days before extraction. Matrix effect factors were calculated as the slope ratios of the regression curves of the post-spiked samples to those of the chemical standards in methanol. Extraction efficiency was assessed by comparing the slopes of the regression curves from the pre-spiked samples to those of post-spiked samples.
Method validation followed the validation guidelines for food chemistry testing of Taiwan Food and Drug Administration (TFDA) [30]. The accuracy and precision were evaluated within the same day (intra-day) and five different days (inter-day) at three spiked concentrations (n = 5 at each concentration) using a brand of microwave popcorn paper bag; the packaging contained some PFASs, and the spiked amount of chemical standards must be at least 2–3 times higher than the endogenous levels. Therefore, the spiked levels of the analytes were divided into three groups: 5.0, 10, and 50 ng/dm2 for PFCAs, perfluoroalkyl sulfonates, PFOSA, and PAPs, in the total of 16 analytes as Group I; 50, 100, 500 ng/dm2 for N-MeFOSA, 6:2 FTOH, and 8:2 FTOH (Group II); 200, 400, 1000 ng/dm2 for 4:2 FTOH (Group III).
Calibration curves were established by the least-squares linear regression with the weighting factor of 1/χ using at least six points of concentrations (most ranged from 0.1 to 1000 ng/mL, Table 1). The peak areas of native analyte standards were normalized to those of the isotope-labeled internal standards for quantifying the analyte concentrations. The coefficients of determination (r2) of calibration curves were greater than 0.99 excluding 4:2 FTOH (0.973) (Table 1). The Waters Mass Lynx 4.1 software was used for data acquisition and processing; further data analysis was conducted on Microsoft Excel 2013. The limits of detection (LODs) and limits of quantification (LOQs) were determined when the qualitative ions and the quantitative ions provided the signal-to-noise (S/N) ratios at three and 10, respectively.
Deactivated (silanized) glass centrifuge tubes and vials were used to prevent analytes from adsorption on the glass surface. After using, glassware was rinsed twice with Milli-Q water, acetone, and methanol sequentially; thereafter, the glassware was covered with aluminum foil and was dried in a chemical fume hood. Each batch of analysis included four QC samples, which were reagent blank, lab spike, QC spike, and QC duplicate. The spiked concentrations were 10 ng/dm2 for lab spike and QC spike excluding 4:2 FTOH and 8:2 FTOH at 100 ng/dm2. The chemical standards of the middle point of the calibration curves were injected at the start, middle, and the end of the run for checking the instrumental performance.
3. Results and discussion
3.1. LC conditions
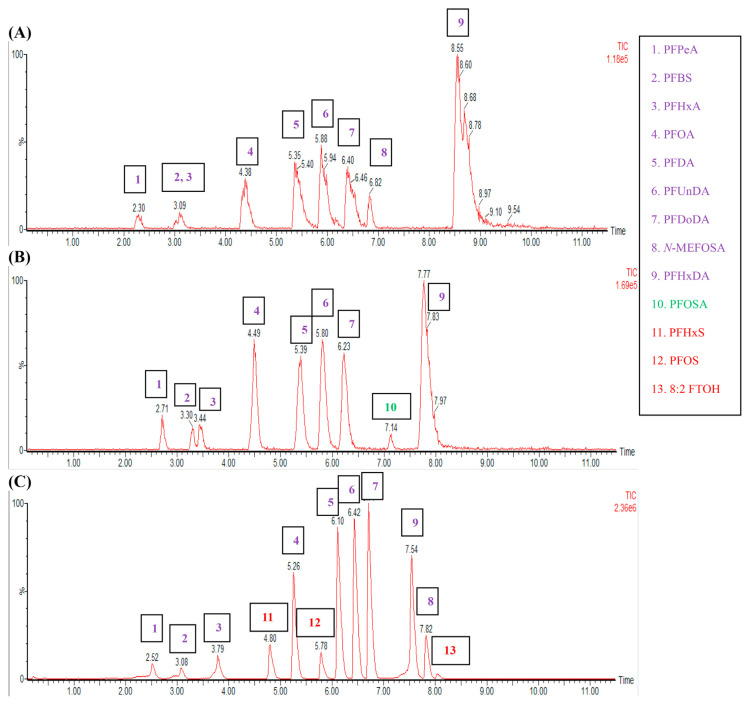
An ACQUITY UPLC BEH C18 column (50 × 2.1 mm, 1.7 μm) provided a better separation on the analytes than a CORTECS UPLC C18 column (30 × 2.1 mm, 1.6 μm). This study tested the two columns using the mobile phase composed of (A) 0.04% acetic acid in acetonitrile/water (5 : 95 (v/v), pH 3.45) and (B) 0.04% acetic acid in acetonitrile/ water (90 : 10 (v/v), pH 3.91) with a simple gradient elution from 5% (B) to 100% (B) within 9 min. The analyte peaks were narrower (mostly at 0.2 min in peak width), sharper, and more symmetric at the BEH C18 column than those at the CORTECS C18 column (0.3 min or wider in peak width) (Fig. 1 (A) and (B)). When the mobile phase above was replaced with the composition of (A) 10-mM N-methylmorpholine(aq) (pH 9.6) and (B) methanol, the analyte signal intensities were about 10 times higher on the BEH C18 column (Fig. 1 (C)).
Fig. 1.
Optimization of columns and mobile phases (500 ng/mL of chemical standards in methanol, injection 4 μL). (A) CORTECS UPLC C18 column (30 × 2.1 mm, 1.6 μm) with 0.04% acetic acid in acetonitrile/water (5:95, v/v) and 0.04% acetic acid in acetonitrile/water (90:10, v/v); (B) ACQUITY UPLC BEH C18 column (50 × 2.1 mm, 1.7 μm) with the same mobile phase compositions as the above; (C) ACQUITY UPLC BEH C18 column (50 × 2.1 mm, 1.7 μm) with 10-mM N-methylmorpholine(aq) and methanol.
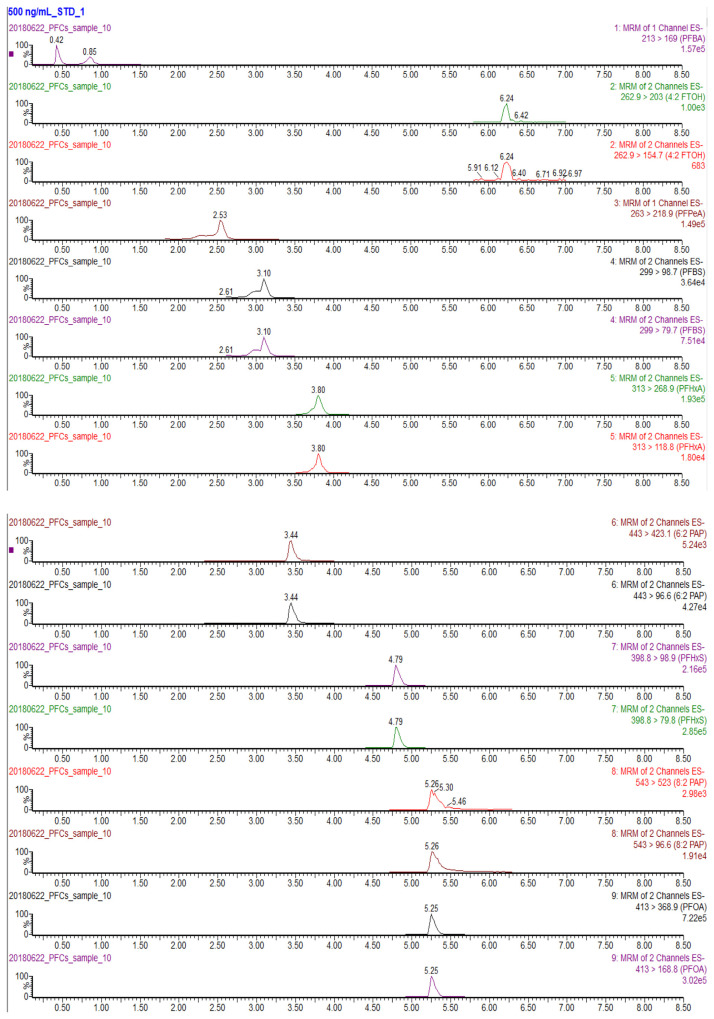
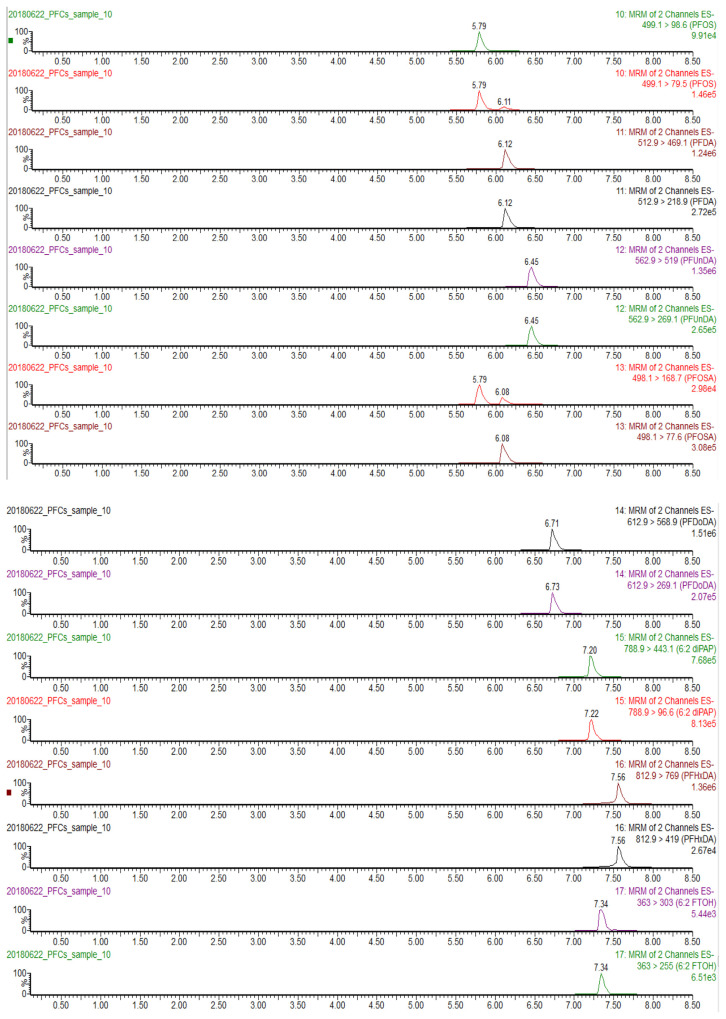
A gradient elution at 11.2%/min increase of methanol (5%–100% in 8.5 min) provided a better chromatographic separation on the BEH C18 column than those at 21.1%/min (5%–100% in 4.5 min) and 8.26%/min (5%–100% in 11.5 min). At the increase of 21.1%/min, most analytes were eluted between 4.0 and 5.0 min, and a few analytes were eluted during the period of column re-equilibrium (5.3–7.5 min). At the increase of 11.2%/min (the total chromatographic time 11.5 min including column re-equilibrium) (Fig. 2), the peak shapes were sharp and the separation of analytes was much better than that at 21.1%/min; the gradient also accommodated more segments and elongated dwelling time for the MRM acquisition, which obtained better signal intensities (about two-fold higher). When the gradient was further reduced to 8.26%/min, each run spent additional 4 min but gained no better separation and signal intensities.
Fig. 2.
Chromatograms of 20 PFASs at the MRM mode (500 ng/mL of chemical standards in methanol, injection 4 μL).
3.2. Sample preparation
Sonication at 50 °C for 45 min obtained the optimal extraction efficiency using methanol, which was the most used extraction solvent in former studies [1,2,16,23,25,26]. This study investigated the combinations of two extraction temperature (30 °C and 50 °C) and five lengths of extracting time (15, 30, 45, 60, 90 min) with three duplicates at each condition; PFBA, PFPeA, and 6:2 FTOH were detected in the tested microwave popcorn packaging. At 30 °C, PFBA became detectable after the extraction of 30 min at 0.57–0.87 ng/dm2; PFPeA were at 0.10–0.13 ng/dm2 after extracting 15–30 min, and then increased to 0.50–0.63 ng/dm2 between 45–90 min; the concentrations of 6:2 FTOH were 24.7–39.3 ng/dm2 between 15–60 min extraction, and increased to 53.9 ng/dm2 at 90-min extraction. At 50 °C, PFBA was detectable at 0.47 ng/dm2 after 15-min extraction, and increased to 0.70–1.03 ng/ dm2 between 30–90 min extractions; the concentrations of PFPeA were 1.13–1.33 ng/dm2 between 15–45 min extractions, but decreased to 0.97 and 0.93 ng/dm2 at 60- and 90-min extractions, respectively; 6:2 FTOH was at 31.1 and 32.8 ng/dm2, respectively, after 15- and 30-min extractions, then increased to 63.8 ng/dm2 at 45-min extraction, and slightly decreased to 42.2 ng/dm2 and 61.6 ng/dm2, respectively, after 60- and 90-min extractions. Consequently, the extraction at 50 °C obtained higher concentrations of PFBA, PFPeA, and 6:2 FTOH faster than that at 30 °C, and the concentrations reached the highest at 45 min. In addition, to investigate if potential thermodegradation of the analytes deteriorated the extraction efficiency, the samples also underwent static soaking for 18 hours at room temperature, but the extracted concentrations were lower those using ultrasonic extraction.
The LEO ultrasonicator offered stable temperature of water bath between 51.1 °C and 52.0 °C at either the middle or the edge places within the ultrasonicator during 15–60 min. Because the boiling point of methanol is 64.7 °C, this study did not test a higher extraction temperature for safety reasons.
3.3. Method validation
LODs and LOQs on the microwave popcorn packaging were 0.07–2.05 ng/dm2 (0.09–2.53 ng/g) and 0.17–12.5 ng/dm2 (0.20–15.4 ng/g), respectively, except for FTOHs (6.08–35.9 ng/dm2 (7.50–44.3 ng/ g) and 18.3–124 ng/dm2 (22.6–152 ng/g), respectively) (Table 1). The LODs and LOQs on some PFCAs and sulfonates in this study are up to 10-fold lower comparing with previous reports [1,21,28]. In contrast, Yuan et al. reached LOQs of 0.2–0.6 ng/g on FTOHs [25], which are much lower than ours; this would result from an additional cleanup of WAX cartridges after the sonication with methanol. The ion suppression of most analytes was lower than 50%, though high matrix effect was observed on PFHxDA, N-MeFOSA, 8:2 FTOH, and 8:2 diPAP. The matrix effect factors of PFCAs and the three perfluorosulfonic acids were 52.5%–78.0% and 61.1%–80.3%, respectively, except for PFHxDA (18.8%). The matrix effector factors of PFOSA and N-MeFOSA were 40.0% and 13.2%, respectively; those of 4:2 FTOH, 6:2 FTOH, and 8:2 FTOH were 69.9%, 65.5%, and 30.2%, individually. Those of substituted polyfluorinated phosphate esters ranged from 50.2% to 61.1% excluding 8:2 diPAP (17.3%). The extraction efficiencies of 15 PFASs were 51.5%–98.7%; however, those of the three FTOHs and two perfluorosulfonamides (PFOSA, N-MeFOSA) were less than 5%, which would result from their volatilization during the three-day aging period of the spiking process rather than not being able to extract the five analytes.
The microwave popcorn paper bag for evaluating the accuracy and precision contained PFBA, PFHxA, and PFOA between 0.10 and 0.93 ng/dm2 in average (n = 3), and PFPeA at 4.73 ng/dm2; the concentrations of PFBS and PFHxS in the bag were 1.60 and 0.17 ng/dm2, respectively. 6:2 FTOH (at 57.8 ng/dm2) was the only detectable FTOH, and the concentrations of PAPs ranged from 0.40 to 2.57 ng/dm2.
Regarding the 16 analytes of Group I at the three spiked concentrations, the intra-day and inter-day recoveries ranged from 84.6% to 112% and from 83.0% to 112%, respectively, except for the inter-day recoveries of PFBA (117%) and PFBS (69.4%) at 5 ng/ dm2 (Table 2). All the above recoveries complied with the food chemical test method validation guide issued by the TFDA [30]. Zabaleta et al. also reported a higher recovery of PFBA (117%) at 25 ng/g [2]. The intra-day recoveries of the Group II analytes (N-MeFOSA, 6:2 FTOH, 8:2 FTOH) at the three spiked levels were between 89.1% and 116% other than 228% of 6:2 FTOH at 50 ng/dm2; the inter-day recoveries ranged from 93.8% to 106% aside from 6:2 FTOH at 50 ng/dm2 (201%) and 100 ng/dm2 (121%) (Table 2). These recoveries fulfilled with the TFDA validation guide [30] excluding those of 6:2 FTOH at 50 ng/dm2, which the high recoveries would result from the endogenous 6:2 FTOH at 57.8 ng/dm2 in average in the microwave popcorn paper bag. The intra-day recoveries of 4:2 FTOH were 90.2%–100% at the three spiked levels, and its inter-day recoveries were 77.0%–116% (Table 2).
Table 2.
Method intra-day and inter-day accuracy and precision.
| Compounds | Spiked levels (ng/dm2) | Intra-day (n = 5) | Inter-day (n = 5) | ||
|---|---|---|---|---|---|
|
|
|
||||
| Recovery | %RSDa | Recovery | %RSD | ||
| PFBA | 5 | 112% | 4.8% | 117% | 4.0% |
| 10 | 106% | 3.6% | 108% | 3.0% | |
| 50 | 112% | 3.4% | 112% | 2.7% | |
| PFPeA | 5 | 112% | 11.5% | 83.0% | 14.0% |
| 10 | 98.7% | 19.7% | 108% | 8.7% | |
| 50 | 109% | 6.1% | 98.2% | 3.4% | |
| PFHxA | 5 | 101% | 5.0% | 96.2% | 4.6% |
| 10 | 98.3% | 2.3% | 97.1% | 2.1% | |
| 50 | 96.7% | 1.8% | 92.4% | 2.7% | |
| PFOA | 5 | 94.0% | 4.0% | 93.2% | 5.1% |
| 10 | 91.8% | 1.4% | 92.8% | 3.1% | |
| 50 | 93.7% | 2.2% | 94.4% | 2.4% | |
| PFDA | 5 | 106% | 4.9% | 99.2% | 8.4% |
| 10 | 103% | 2.8% | 97.6% | 8.8% | |
| 50 | 101% | 5.8% | 92.8% | 6.3% | |
| PFUnDA | 5 | 95.6% | 4.8% | 87.2% | 4.5% |
| 10 | 91.2% | 2.1% | 89.2% | 2.4% | |
| 50 | 90.4% | 1.9% | 91.8% | 3.3% | |
| PFDoDA | 5 | 108% | 10.2% | 98.7% | 5.3% |
| 10 | 97.6% | 7.8% | 107% | 10.9% | |
| 50 | 106% | 3.6% | 106% | 6.7% | |
| PFHxDA | 5 | 94.3% | 5.9% | 95.9% | 17.8% |
| 10 | 99.8% | 9.9% | 88.8% | 6.7% | |
| 50 | 93.4% | 5.4% | 86.3% | 8.7% | |
| PFBS | 5 | 100% | 9.2% | 69.4% | 14.4% |
| 10 | 97.5% | 3.4% | 105% | 7.8% | |
| 50 | 101% | 14.6% | 108% | 4.2% | |
| PFHxS | 5 | 95.8% | 2.7% | 99.8% | 4.7% |
| 10 | 92.3% | 5.4% | 95.9% | 5.1% | |
| 50 | 92.9% | 3.7% | 98.8% | 2.8% | |
| PFOS | 5 | 96.8% | 6.1% | 97.6% | 5.3% |
| 10 | 95.2% | 2.9% | 97.8% | 5.3% | |
| 50 | 92.0% | 2.0% | 97.2% | 5.8% | |
| PFOSA | 5 | 99.7% | 3.5% | 101% | 8.4% |
| 10 | 108% | 7.4% | 105% | 7.4% | |
| 50 | 108% | 2.4% | 103% | 3.3% | |
| N-MeFOSA | 50 | 93.3% | 3.7% | 95.0% | 2.1% |
| 100 | 93.1% | 2.6% | 97.8% | 1.3% | |
| 500 | 96.0% | 2.8% | 97.2% | 1.7% | |
| 4:2 FTOH | 200 | 100% | 6.6% | 116% | 20.9% |
| 400 | 98.3% | 4.3% | 79.7% | 42.5% | |
| 1000 | 90.2% | 12.9% | 77.0% | 35.5% | |
| 6:2 FTOH | 50 | 228% | 25.4% | 201% | 47.8% |
| 100 | 91.7% | 17.4% | 121% | 16.3% | |
| 500 | 89.1% | 11.9% | 106% | 12.3% | |
| 8:2 FTOH | 50 | 116% | 8.4% | 98.8% | 7.4% |
| 100 | 90.7% | 5.3% | 93.8% | 7.8% | |
| 500 | 98.9% | 5.5% | 98.7% | 2.1% | |
| 6:2 PAP | 5 | 97.8% | 6.4% | 89.4% | 7.3% |
| 10 | 102% | 4.0% | 96.9% | 5.8% | |
| 50 | 98.3% | 2.5% | 98.2% | 1.2% | |
| 8:2 PAP | 5 | 98.6% | 4.7% | 88.2% | 5.8% |
| 10 | 98.5% | 4.5% | 98.1% | 4.7% | |
| 50 | 94.5% | 3.5% | 92.3% | 2.8% | |
| 6:2 diPAP | 5 | 84.6% | 4.4% | 92.6% | 9.6% |
| 10 | 88.3% | 4.5% | 90.7% | 6.9% | |
| 50 | 94.1% | 3.2% | 89.4% | 1.8% | |
| 8:2 diPAP | 5 | 95.8% | 2.8% | 93.0% | 6.0% |
| 10 | 94.1% | 4.0% | 93.1% | 3.7% | |
| 50 | 99.0% | 3.9% | 95.6% | 1.9% | |
%RSD: Relative standard deviation.
The repeatability (intra-day precision) in relative standard deviation (%RSD) of the 16 Group I analytes was 1.4%–14.6% at the three spiked levels except for 19.7% of PFPeA at 10 ng/dm2 (Table 2); the intermediate precision (inter-day precision) in % RSD was 1.2%–14.4% except for 17.8% of PFHxDA at 5 ng/dm2 (Table 2). All the precision above complied with the TFDA validation guide [30]. The results are similar to the repeatability (%RSD 2–20%) and the intermediate precision (%RSD 3–24%) in previous studies [2,21]. The repeatability and the intermediate precision of the three analytes in Group II were with %RSD of 2.6%–17.4% and 1.3%–16.3%, respectively, except for 25.4% and 47.8% of 6:2 FTOH at 50 ng/dm2 (Table 2). The TFDA validation guide required %RSD lower than 20% and 22%, respectively [30]; the higher %RSD on 6:2 FTOH would result from the endogenous amount in the tested matrix. The repeatability of 4:2 FTOH in %RSD ranged from 4.3% to 12.9% at the three spiked levels; nevertheless, the intermediate precision in %RSD ranged from 20.9% to 42.5% (Table 2), which would arise from varied volatile loss of 4:2 FTOH during the different aging lengths of the spiking process on the samples of five different days.
3.4. Analysis of real samples
Microwave popcorn packaging contained higher levels of PFCAs and FTOHs than other materials in this study, and the concentrations varied a lot (Table 3). For example, samples of E-1 and E-2 were from a web store; all PFCAs (except for PFHxDA) were between 8.3–217 ng/dm2 (8.5–223 ng/g) in the E-2 sample, such as long-chain PFOA at 217 ng/dm2 (223 ng/g) and PFDA at 210 ng/dm2 (216 ng/g), but none of PFCAs was detectable in the E-1 sample. The E-2 sample also had 8:2 FTOH reaching 7188 ng/dm2 (7373 ng/g), which was much higher than that (9.7 ng/dm2; 11.5 ng/g) in the E-1 sample. The other two microwave popcorn paper bags, E-3 and E-4, were from convenient stores. Sample E-3 contained 188–1960 ng/dm2 (186–1934 ng/g) of short-chain PFCAs and 13.4 ng/dm2 (13.2 ng/g) of PFOA, but E-4 only had 6.9 ng/dm2 (6.4 ng/g) of PFBA (Table 3). 6:2 FTOH was detected in E-1 and E-4 samples at 57.0 (67.5) and 121 (112) ng/dm2 (ng/ g), respectively (Table 3). In terms of microwave popcorn packaging, Sinclair et al. found C5–C12 PFCAs at 40–600 ng/dm2, and 6:2 FTOH and 8:2 FTOH at 160–340 ng/dm2 [31]; Zafeiraki et al. reported 276 ng/g of PFBA and 341 ng/g of PFHxA [1]; Zabaleta et al. observed 291 ng/g of PFBA, 20.5 ng/g of PFPeA, 255 ng/g of PFHxA, and 12.1 ng/g of 8:2 diPAP [2]. The concentrations and profiles of PFCAs and FTOHs in this study were comparable to those in these previous reports except that over 1000 ng/ dm2 of PFBA and PFHxA were found in one sample and higher than 7000 ng/dm2 of 8:2 FTOH was observed in the other. Zabaleta et al. quantified 24 PFASs in microwave popcorn bags from European, American, and Asian countries; PAPs and diPAPs were detectable in a few samples [24]. In contrast, PAPs and diPAPs were undetectable in our study.
Table 3.
PFASs concentrations in seven types of oil-resistant food packaging (ng/dm2 (ng/g))a.
| Category | Sample | PFBA | PFPeA | PFHxA | PFOA | PFDA | PFUnDA | PFDoDA | PFHxDA | PFBS | N-MeFOSA | 6:2 FTOH | 8:2 FTOH | 6:2 PAP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| To-go boxes | A-2 | N.D.b | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 7.7 (2.7) | N.D. | N.D. | N.D. |
| Others (n = 5) | N.D. | N.D. | N.D | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | |
| Burger/hot dog paper | Burger paper (n = 4) | N.D. | N.D. | N.D. | N.D | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Hot dog paper (n = 1) | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | |
| Fried chicken boxes | C-3 | 12.5 (5.8) | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 21.6 (10.0) | N.D. | N.D. | N.D. | N.D. | N.D. |
| Others (n = 4) | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | |
| French fries paper bags | D-2 (small) | N.D. | N.D. | 3.6 (9.0) | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 167 (417) | 22.4 (55.9) | N.D. |
| D-3 (large) | 40.3 (18.0) | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 5.0 (2.2) | 10.5 (4.7) | N.D. | N.D. | N.D. | |
| Others (n = 2) | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | |
| Microwave popcorn paper bags | E-1 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 57.0 (67.5) | 9.7 (11.5) | N.D. |
| E-2 | 10.0 (10.3) | 8.3 (8.5) | 35.4 (36.3) | 217 (223) | 210 (216) | 19.6 (20.1) | 31.8 (32.6) | N.D. | N.D. | N.D. | N.D. | 7188 (7373) | N.D. | |
| E-3 | 1960 (1934) | 188 (186) | 1300 (1283) | 13.4 (13.2) | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | |
| E-4 | 6.9 (6.4) | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 121 (112) | N.D. | N.D. | |
| Oil-proof paper | F-1 | 14.8 (31.5) | 4.0 (8.5) | 17.5 (37.2) | 48.6 (103) | 30.6 (65.1) | 5.7 (12.1) | 17.5 (37.3) | 7.5 (16.1) | 2.9 (6.1) | N.D. | 454 (966) | 2595 (5521) | N.D. |
| F-2 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 66.6 (137) | N.D. | |
| F-3 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | |
| Beverage paper cups | G-4 tea cup (large) | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 8.6 (2.6) |
| Others (n = 4) | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
PFHxS, PFOS, PFOSA, 4:2 FTOH, 8:2 PAP, 6:2 diPAP, and 8:2 diPAP were undetectable in all samples.
N.D.: non-detectable.
Among the three all-purpose oil-proof paper bag samples, only sample F-1 contained all PFCAs and PFBS (a sulfonate) from 2.9 (6.1) to 48.6 (103) ng/dm2 (ng/g) (Table 3). The F-1 sample also had high levels of 6:2 FTOH (454 ng/dm2; 966 ng/g) and 8:2 FTOH (2595 ng/dm2; 5521 ng/g), while only 66.6 ng/dm2 (137 ng/g) of 8:2 FTOH was detected in another sample (F-2) (Table 3). Surma et al. found PFOA at 1.0–15.7 ng/dm2 and perfluoroalkyl sulfonates at 13.8–51.7 ng/dm2 in roasting bag samples [29]; the concentrations of sulfonates in our samples (2.9 ng/dm2 of PFBS in F-1; ND in F-2 and F-3) were much lower comparing with those of Surma et al., but the levels of PFOA (48.6 ng/dm2) and PFDA (30.6 ng/ dm2) in the F-1 sample were higher.
Most beverage paper cups are coated with polyethylene rather than perfluoroalkyl substances. Consequently, only 8.6 ng/dm2 (2.6 ng/g) of 6:2 PAP among the analytes was found in one (G-4) of the five beverage cup samples (Table 3). Poothong et al. detected PFOA (3.01–16.9 ng/dm2) and PFOS (6.41–11.5 ng/dm2) in beverage cups (n = 5) [16], which the two analytes were undetectable in our samples. The coating on to-go boxes and fried chicken boxes would be similar to that of paper cups; this study only observed 7.7 ng/dm2 (2.7 ng/g) of N-MeFOSA in one (A-2) of six to-go box samples, plus 12.5 ng/dm2 (5.8 ng/g) of PFBA and 21.6 ng/dm2 (10.0 ng/g) of PFHxDA in one (C-3) of five fried chicken box samples (Table 3). In contrast, Surma et al. indicated 25.4–66.0 ng/dm2 of PFCAs in breakfast bag samples [29].
A few analytes were detectable in two samples of French fries paper bags from the same fast food restaurant (Table 3). There were 3.6 ng/dm2 (9.0 ng/ g) of PFHxA, 167 ng/dm2 (417 ng/g) of 6:2 FTOH, and 22.4 ng/dm2 (55.9 ng/g) of 8:2 FTOH in the small paper bag (D-2); there were 40.3 ng/dm2 (18.0 ng/g) of PFBA, 5.0 ng/dm2 (2.2 ng/g) of PFBS, and 10.5 ng/ dm2 (4.7 ng/g) of N-MeFOSA in the large paper bag (D-3). Instead, all analytes were undetectable in the other two French fries paper bags collected from two other fast food restaurants. Regarding burger/ hot dog paper samples, no analytes were detectable (Table 3).
4. Conclusions
This study developed and validated a method for determining five types of 20 PFASs in food packaging with an UPLC-MS/MS. This study optimized the parameters of ultrasonic extraction and UPLC-MS/MS. Matrix effect factors and extraction efficiencies of most analytes were 50.2%–80% and 51.5%–98.7%, respectively. The sample preparation was simple and the method was sensitive; most LODs were 0.07–11.3 ng/dm2 (0.09–13.9 ng/g), and most LOQs were 0.17–18.3 ng/dm2 (0.20–22.6 ng/g). The assay was validated according to the TFDA specification to demonstrate good accuracy and precision; the recoveries ranged from 70% to 117% on most analytes at the three tested levels, and the %RSD of precision were lower than 19% (n = 5). The optimized method was applied on measuring 32 samples of commercial oil-resistant food packaging in seven categories. Microwave popcorn paper bags could contain short-chain PFCAs higher than 1000 ng/dm2 and FTOHs exceeding 7000 ng/dm2. One oil-proof paper bag had several PFCAs at 6–49 ng/dm2 and FTOHs at 454–2595 ng/dm2. Although most observed concentrations of the analytes were at ppb levels, the adverse health effects warrant further attention for long-term and simultaneous exposure to some high levels of short-chain PFCAs and FTOHs.
Acknowledgements
This work was supported by the Food and Drug Administration, Ministry of Health and Welfare, Taiwan (TFDA) (107TFDA-A-111).
Appendix
Table S1.
Details of the 32 pieces of oil-resistant food packaging
| Categories | # of samples | NO. | Package name | Sampling location | Weight (g) of 100 cm2 |
|---|---|---|---|---|---|
| To-go box | 6 | A-1 | X Buffet disposable box - large | The food court of a hospital in Taipei City | 3.9880 g |
| A-2 | X Buffet disposal box - small | The food court of a hospital in Taipei City | 2.8580 g | ||
| A-3 | Fried dumpling box | A dumpling chain store in Taipei City | 3.0930 g | ||
| A-4 | Noodle bowl | A dumpling chain store in Taipei City | 2.9807 g | ||
| A-5 | Vegetarian diet disposable box | Q Square in Taipei City | 3.6350 g | ||
| A-6 | A bowl of instant noodles | A convenient store in Taipei City | 3.0880 g | ||
| Burger/hot dog paper | 5 | B-1 | The wrapping for double cheese burger | A fast food chain restaurant in Taipei City | 0.3050 g |
| B-2 | The wrapping for fried chicken legs | A fast food chain restaurant in Taipei City | 0.4430 g | ||
| B-3 | Hamburg wrapping paper | The food court of a hospital in Taipei City | 0.3900 g | ||
| B-4 | Sandwich wrapping paper | A fast food chain restaurant in Taipei City | 0.4058 g | ||
| B-5 | Hot dog wrapping paper | A convenient store in Taipei City | 0.4530 g | ||
| Fried chicken box | 5 | C-1 | Fried chicken barrel | A fast food chain restaurant in Taipei City | 3.6350 g |
| C-2 | Fried chicken barrel | A fast food chain restaurant in Taipei railway station | 3.4500 g | ||
| C-3 | Paper box for chicken nuggets | A fast food chain restaurant in Taipei City | 2.1687 g | ||
| C-4 | Fried chicken paper bag | A fast food chain restaurant in Taipei City | 0.4871 g | ||
| C-5 | Crispy chicken paper bag | A fast food chain restaurant in Taipei City | 0.4845 g | ||
| French fries paper bag | 4 | D-1 | French fries paper bag | A fast food chain restaurant in Taipei City | 0.4905 g |
| D-2 | French fries paper bag - small | A fast food chain restaurant in Taipei City | 0.4004 g | ||
| D-3 | French fries paper bag - large | A fast food chain restaurant in Taipei City | 2.2425 g | ||
| D-4 | French fries paper bag | A fast food chain store in the food court of a hospital in Taipei City | 0.4855 g | ||
| Microwave popcorn paper bag | 4 | E-1 | Microwave popcorn paper bag (Brand A) | Ordered from the Internet | 0.8450 g |
| E-2 | Microwave popcorn paper bag (Brand B) | Ordered from the Internet | 0.9750 g | ||
| E-3 | Microwave popcorn paper bag S | A convenient store in Taipei City | 1.0130 g | ||
| E-4 | Microwave popcorn paper bag F | A convenient store in Taipei City | 1.0770 g | ||
| Oil-proof paper | 3 | F-1 | Oil-proof paper bag - large | A retail store in Taipei City | 0.4700 g |
| F-2 | Oil-proof paper bag | A retail store in New Taipei City | 0.4850 g | ||
| F-3 | Baking paper | A retail store in New Taipei City | 0.4035 g | ||
| Beverage paper cup | 5 | G-1 | Milk tea cup - large | A chain tea shop in Taipei City | 2.5438 g |
| G-2 | Coffee cup - large | A chain coffee shop in Taipei City | 3.5136 g | ||
| G-3 | Latte coffee cup - large | A convenient store in Taipei City | 3.6100 g | ||
| G-4 | Milk tea cup - large | A chain coffee shop in Taipei City | 3.3270 g | ||
| G-5 | Coke cup - medium | A fast food chain restaurant in Taipei City | 3.3270 g |
32 pieces of oil-resistant food packaging from markets in Taiwan.
An isolator column (ACQUITY UPLC BEH C18, 50 × 2.1 mm, 2.6 μm) was installed between the mobile phase mixer and the autosampler.
Funding Statement
This work was supported by the Food and Drug Administration, Ministry of Health and Welfare, Taiwan (TFDA) (107TFDA-A-111).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Zafeiraki E, Costopoulou D, Vassiliadou I, Bakeas E, Leondiadis L. Determination of perfluorinated compounds (PFCs) in various foodstuff packaging materials used in the Greek market. Chemosphere. 2014;94:169–76. doi: 10.1016/j.chemosphere.2013.09.092. [DOI] [PubMed] [Google Scholar]
- 2.Zabaleta I, Bizkarguenaga E, Bilbao D, Etxebarria N, Prieto A, Zuloaga O. Fast and simple determination of perfluorinated compounds and their potential precursors in different packaging materials. Talanta. 2016;152:353–63. doi: 10.1016/j.talanta.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Ahrens L, Bundschuh M. Fate and effects of poly- and perfluoroalkyl substances in the aquatic environment: a review. Environ Toxicol Chem. 2014;33:1921–9. doi: 10.1002/etc.2663. [DOI] [PubMed] [Google Scholar]
- 4.Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol. 2006;40:32–44. doi: 10.1021/es0512475. [DOI] [PubMed] [Google Scholar]
- 5.Sharifan H, Bagheri M, Wang D, Burken JG, Higgins CP, Liang Y, et al. Fate and transport of per- and polyfluoroalkyl substances (PFASs) in the vadose zone. Sci Total Environ. 2021;771:145427. doi: 10.1016/j.scitotenv.2021.145427. [DOI] [PubMed] [Google Scholar]
- 6.Fromme H, Tittlemier SA, Volkel W, Wilhelm M, Twardella D. Perfluorinated compounds–exposure assessment for the general population in Western countries. Int J Hyg Environ Health. 2009;212:239–70. doi: 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Kedikoglou K, Costopoulou D, Vassiliadou I, Leondiadis L. Preliminary assessment of general population exposure to perfluoroalkyl substances through diet in Greece. Environ Res. 2019;177:108617. doi: 10.1016/j.envres.2019.108617. [DOI] [PubMed] [Google Scholar]
- 8.Lin PID, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert MF, et al. Dietary characteristics associated with plasma concentrations of per- and polyfluoroalkyl substances among adults with pre-diabetes: cross-sectional results from the Diabetes Prevention Program Trial. Environ Int. 2020;137:105217. doi: 10.1016/j.envint.2019.105217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Susmann HP, Schaider LA, Rodgers KM, Rudel RA. Dietary habits related to food packaging and population exposure to PFASs. Environ Health Perspect. 2019;127:107003. doi: 10.1289/EHP4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buekers J, Colles A, Cornelis C, Morrens B, Govarts E, Schoeters G. Socio-economic status and health: evaluation of human biomonitored chemical exposure to per- and polyfluorinated substances across status. Int J Environ Res Publ Health. 2018;15:2818. doi: 10.3390/ijerph15122818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grandjean P, Budtz-Jorgensen E. Immunotoxicity of perfluorinated alkylates: calculation of benchmark doses based on serum concentrations in children. Environ Health. 2013;12:35. doi: 10.1186/1476-069X-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilhelm M, Bergmann S, Dieter HH. Occurrence of perfluorinated compounds (PFCs) in drinking water of North Rhine-Westphalia, Germany and new approach to assess drinking water contamination by shorter-chained C4-C7 PFCs. Int J Hyg Environ Health. 2010;213:224–32. doi: 10.1016/j.ijheh.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Environmental Protection Agency (USEPA. Fact sheet: PFOA & PFOS drinking water health advisories. [Accessed 20 July 2021]. Available at: https://www.epa.gov/sites/default/files/2016-06/documents/drinkingwaterhealthadvisories_pfoa_pfos_updated_5.31.16.pdf.
- 14.Knutsen HK, Alexander J, Barregård L, Bignami M, Brüschweiler B, Ceccatelli S, et al. Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. [Accessed 20 July 2021];EFSA J. 2018 16:5194. doi: 10.2903/j.efsa.2018.5194. Available at: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2018.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrenk D, Bignami M, Bodin L, Chipman JK, Mazo Jd, Grasl-Kraupp B, et al. Risk to human health related to the presence of perfluoroalkyl substances in food. [Accessed 20 July 2021];EFSA J. 2020 18:6223. doi: 10.2903/j.efsa.2020.6223. Available at: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2020.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poothong S, Boontanon SK, Boontanon N. Determination of perfluorooctane sulfonate and perfluorooctanoic acid in food packaging using liquid chromatography coupled with tandem mass spectrometry. J Hazard Mater. 2012;205–206:139–43. doi: 10.1016/j.jhazmat.2011.12.050. [DOI] [PubMed] [Google Scholar]
- 17.Trier X, Granby K, Christensen JH. Polyfluorinated surfactants (PFS) in paper and board coatings for food packaging. Environ Sci Pollut Res Int. 2011;18:1108–20. doi: 10.1007/s11356-010-0439-3. [DOI] [PubMed] [Google Scholar]
- 18.Vavrouš A, Vápenka L, Sosnovcová J, Kejlová K, Vrbík K, Jírová D. Method for analysis of 68 organic contaminants in food contact paper using gas and liquid chromatography coupled with tandem mass spectrometry. Food Control. 2016;60:221–9. [Google Scholar]
- 19.Schaider LA, Balan SA, Blum A, Andrews DQ, Strynar MJ, Dickinson ME, et al. Fluorinated compounds in U.S. fast food packaging. Environ Sci Technol Lett. 2017;4:105–11. doi: 10.1021/acs.estlett.6b00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Begley TH, White K, Honigfort P, Twaroski ML, Neches R, Walker RA. Perfluorochemicals: potential sources of and migration from food packaging. Food Addit Contam. 2005;22:1023–31. doi: 10.1080/02652030500183474. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Moral MP, Tena MT. Determination of perfluorocompounds in popcorn packaging by pressurised liquid extraction and ultra-performance liquid chromatography-tandem mass spectrometry. Talanta. 2012;101:104–9. doi: 10.1016/j.talanta.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Monge Brenes AL, Curtzwiler G, Dixon P, Harrata K, Talbert J, Vorst K. PFOA and PFOS levels in microwave paper packaging between 2005 and 2018. Food Addit Contam Part B Surveill. 2019;12:191–8. doi: 10.1080/19393210.2019.1592238. [DOI] [PubMed] [Google Scholar]
- 23.Gebbink WA, Ullah S, Sandblom O, Berger U. Polyfluoroalkyl phosphate esters and perfluoroalkyl carboxylic acids in target food samples and packaging–method development and screening. Environ Sci Pollut Res Int. 2013;20:7949–58. doi: 10.1007/s11356-013-1596-y. [DOI] [PubMed] [Google Scholar]
- 24.Zabaleta I, Negreira N, Bizkarguenaga E, Prieto A, Covaci A, Zuloaga O. Screening and identification of per- and polyfluoroalkyl substances in microwave popcorn bags. Food Chem. 2017;230:497–506. doi: 10.1016/j.foodchem.2017.03.074. [DOI] [PubMed] [Google Scholar]
- 25.Yuan G, Peng H, Huang C, Hu J. Ubiquitous occurrence of fluorotelomer alcohols in eco-friendly paper-made food-contact materials and their implication for human exposure. Environ Sci Technol. 2016;50:942–50. doi: 10.1021/acs.est.5b03806. [DOI] [PubMed] [Google Scholar]
- 26.Chinthakindi S, Zhu H, Kannan K. An exploratory analysis of poly- and per-fluoroalkyl substances in pet food packaging from the United States. Environ Technol Innovat. 2021;21:101247. [Google Scholar]
- 27.Dolman S, Pelzing M. An optimized method for the determination of perfluorooctanoic acid, perfluorooctane sulfonate and other perfluorochemicals in different matrices using liquid chromatography/ion-trap mass spectrometry. J Chromatogr B. 2011;879:2043–50. doi: 10.1016/j.jchromb.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 28.Moreta C, Tena MT. Fast determination of perfluorocompounds in packaging by focused ultrasound solid-liquid extraction and liquid chromatography coupled to quadrupole-time of flight mass spectrometry. J Chromatogr A. 2013;1302:88–94. doi: 10.1016/j.chroma.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 29.Surma M, Wiczkowski W, Zieliński H, Cieślik E. Determination of selected perfluorinated acids (PFCAs) and perfluorinated sulfonates (PFASs) in food contact materials using LC-MS/MS. Packag Technol Sci. 2015;28:789–99. [Google Scholar]
- 30.Taiwan Food and Drug Administration. Version 2. Food chemical test method validation guide. Chinese. [Accessed 20 July 2021]. Available at: https://www.fda.gov.tw/tc/includes/GetFile.ashx?id=f636921436165222394.
- 31.Sinclair E, Kim S-K, Akinleye H, Kannan K. Quantitation of gas-phase perfluoroalkyl surfactants and fluorotelomer alcohols released from nonstick cookware and microwave popcorn bags. Environ Sci Technol. 2007;41:1180–5. doi: 10.1021/es062377w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Details of the 32 pieces of oil-resistant food packaging
| Categories | # of samples | NO. | Package name | Sampling location | Weight (g) of 100 cm2 |
|---|---|---|---|---|---|
| To-go box | 6 | A-1 | X Buffet disposable box - large | The food court of a hospital in Taipei City | 3.9880 g |
| A-2 | X Buffet disposal box - small | The food court of a hospital in Taipei City | 2.8580 g | ||
| A-3 | Fried dumpling box | A dumpling chain store in Taipei City | 3.0930 g | ||
| A-4 | Noodle bowl | A dumpling chain store in Taipei City | 2.9807 g | ||
| A-5 | Vegetarian diet disposable box | Q Square in Taipei City | 3.6350 g | ||
| A-6 | A bowl of instant noodles | A convenient store in Taipei City | 3.0880 g | ||
| Burger/hot dog paper | 5 | B-1 | The wrapping for double cheese burger | A fast food chain restaurant in Taipei City | 0.3050 g |
| B-2 | The wrapping for fried chicken legs | A fast food chain restaurant in Taipei City | 0.4430 g | ||
| B-3 | Hamburg wrapping paper | The food court of a hospital in Taipei City | 0.3900 g | ||
| B-4 | Sandwich wrapping paper | A fast food chain restaurant in Taipei City | 0.4058 g | ||
| B-5 | Hot dog wrapping paper | A convenient store in Taipei City | 0.4530 g | ||
| Fried chicken box | 5 | C-1 | Fried chicken barrel | A fast food chain restaurant in Taipei City | 3.6350 g |
| C-2 | Fried chicken barrel | A fast food chain restaurant in Taipei railway station | 3.4500 g | ||
| C-3 | Paper box for chicken nuggets | A fast food chain restaurant in Taipei City | 2.1687 g | ||
| C-4 | Fried chicken paper bag | A fast food chain restaurant in Taipei City | 0.4871 g | ||
| C-5 | Crispy chicken paper bag | A fast food chain restaurant in Taipei City | 0.4845 g | ||
| French fries paper bag | 4 | D-1 | French fries paper bag | A fast food chain restaurant in Taipei City | 0.4905 g |
| D-2 | French fries paper bag - small | A fast food chain restaurant in Taipei City | 0.4004 g | ||
| D-3 | French fries paper bag - large | A fast food chain restaurant in Taipei City | 2.2425 g | ||
| D-4 | French fries paper bag | A fast food chain store in the food court of a hospital in Taipei City | 0.4855 g | ||
| Microwave popcorn paper bag | 4 | E-1 | Microwave popcorn paper bag (Brand A) | Ordered from the Internet | 0.8450 g |
| E-2 | Microwave popcorn paper bag (Brand B) | Ordered from the Internet | 0.9750 g | ||
| E-3 | Microwave popcorn paper bag S | A convenient store in Taipei City | 1.0130 g | ||
| E-4 | Microwave popcorn paper bag F | A convenient store in Taipei City | 1.0770 g | ||
| Oil-proof paper | 3 | F-1 | Oil-proof paper bag - large | A retail store in Taipei City | 0.4700 g |
| F-2 | Oil-proof paper bag | A retail store in New Taipei City | 0.4850 g | ||
| F-3 | Baking paper | A retail store in New Taipei City | 0.4035 g | ||
| Beverage paper cup | 5 | G-1 | Milk tea cup - large | A chain tea shop in Taipei City | 2.5438 g |
| G-2 | Coffee cup - large | A chain coffee shop in Taipei City | 3.5136 g | ||
| G-3 | Latte coffee cup - large | A convenient store in Taipei City | 3.6100 g | ||
| G-4 | Milk tea cup - large | A chain coffee shop in Taipei City | 3.3270 g | ||
| G-5 | Coke cup - medium | A fast food chain restaurant in Taipei City | 3.3270 g |
32 pieces of oil-resistant food packaging from markets in Taiwan.
An isolator column (ACQUITY UPLC BEH C18, 50 × 2.1 mm, 2.6 μm) was installed between the mobile phase mixer and the autosampler.