Abstract
Obesity is characterized by metabolic disorder and accompanying an altered and less diverse gut microbiota composition during a fat-enriched diet. Recent studies indicated that sulphated polysaccharide prevents high-fat diet (HFD) induced obesity, reduces metabolic disorder, and restores the gut microbiota. However, there are few studies about Ulva prolifera polysaccharide (UPP) may induce anti-obesogenic effects. Therefore, the present study investigates the enzymatic extracted UPP effects in HFD-fed mice. The results showed that UPP considerably slowed down the HFD-induced weight gain and improved metabolic disorders in HFD-fed mice. Notably, the effects were associated with lower body weight gain, reduced adipose tissue hypertrophy, triglyceride concentration in liver and systemic low-grade inflammation, and improved fasting blood glucose. Moreover, our result reveals that UPP may elevate the expression of AMPK via adiponectin activation. Interestingly, we found that UPP may induce PPARα agonist to enhance β-oxidation since the elevation of CPT-1 and PPARα expression simultaneously. Meanwhile, gut microbiota analysis revealed UPP promoted the growth of Parasutterella, Feacalibaculum, and Bifidobacterium, and reduced the abundance of Acetatifactor, Tyzerella, Ruminococcus_1, and Desulfovibrio. The changes in microbiota may have a positively correlated effect on improving obesity and metabolic abnormalities. UPP may prevent HFD-induced obesity and associated metabolic diseases, as well as modulate the composition of gut microbiota to facilitate the growth of probiotics.
Keywords: Gut microbiota, Metabolic disorder, Obesity, Sulphated polysaccharide, Ulva prolifera
1. Introduction
Obesity and overweight are defined as abnormal or excessive fat accumulation that may lead to harmful effects such as development of type II diabetes mellitus and cardiovascular disease [1]. These conditions are also associated with body weight gain, ectopic fat accumulation, adipocyte hypertrophy [2], metabolic abnormalities [2], chronic inflammation [2], insulin resistance [2], and gut microbiota diversity alteration [3]. Due to the high costs and potentially dangerous adverse effects of drugs, researchers have become interested in identifying compounds derived from natural products, such as algae, that may be therapeutically effective in reducing obesity and alleviating metabolic syndrome.
Marine green macroalgae, species of Chlorophyta in the genus Ulva, are edible seaweeds with numerous health-promoting bioactive components [4]. Polysaccharides of algae are potential candidates to reduce the impact of HFD on the occurrence of metabolic disease. Ulvan is a type of sulphated polysaccharide and is mainly composed of rhamnose, uronic acids (glucuronic acid and iduronic acid), and xylose [4]. Ulva prolifera polysaccharide (UPP) is a soluble sulfated heteropolysaccharide [4] that has recently been exploited for numerous applications. Previous studies have demonstrated that UPP induced a potent improvement in glucose metabolism in diabetic rats and adiponectin in adipose tissue and attenuation of nonalcoholic fatty liver disease [5].
In short, UPP is the component most intensively investigated for medical purposes. However, the anti-obesity pharmacological effects of UPP have been seldom studied. Thus, this study is designed to clarify the molecular mechanisms involved in the effects of enzymatic extracted UPP on lipid metabolism to gain an understanding of how these polysaccharides might prevent obesity and modulate microflora in mice with diet-induced obesity.
2. Materials and methods
2.1. Reagents and chemicals
U. prolifera was obtained from East Green Biotech, Inc. (Taitung, Taiwan). IL-6 and TNF-α) uncoated enzyme-linked immunosorbent assay (ELISA) kits were purchased from Thermo Fisher (Waltham, MA, USA). ACC, anti-AMPK, anti-pAMPK, HSL, anti-pHSL, and anti-β-actin were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-adiponectin, anti-CPT1A, anti-PPARα, and anti-PPARγ were purchased from Abcam (Cambridge, UK). Anti-IL-1β was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Total dietary fiber assay kit was purchased from Sigma-Aldrich (St. Louis, MO, USA). Triglyceride Colorimetric Assay Kit was purchased from Cayman Chemical (Ann Arbor, MI, USA). InnuPREP Stool DNA kit was purchased form Analytic Jena (Germany).
2.2. Ulva prolifera polysaccharide preparation
Polysaccharide extraction was according to the total dietary fiber assay kit was purchased from Merck & Co., Inc (Kenilworth, NJ, USA) instructions. Briefly, 1 g of dried U. prolifera powder was weighed into a beaker. Next, 50 mL of pH 6.0 phosphate buffer was added into the beaker. The sample was gelatinized with 0.10 mL of heat-stable α-amylase and then enzymatically digested with 50 mg/mL of protease and 0.10 mL of amyloglucosidase to remove the protein and starch present in the sample, and incubated for 30 min at 60 °C. Next, four volumes of 95% ethanol was then added to precipitate the soluble dietary fiber. The solution was placed at 4 °C overnight to allow complete precipitation.
2.3. Composition of sugar analysis
Sugar analysis was modified from methods previously described [6]. First, 1 mL of 2 N HCl/MeOH was prepared and reacted with 3 mg of polysaccharide sample in a hydrolytic tube at 70 °C for 8 h. HCl/MeOH was removed by vacuum. Next, the sample was hydrolyzed with 2 mL of 2 N trifluoroacetic acid (TFA) in a sealed hydrolytic tube at 100 °C for 1.5 h. TFA was removed by evaporation under vacuum. The sugar compositions of polysaccharide were determined using HPAEC (Metrohm, Herisau, Switzerland). The column used was a CarboPacPA1 column (Dionex, Sunnyvale, CA, USA). An isocratic elution was performed at 1 mL/min with 10 mM NaOH eluent containing 2 mM Ba(OAc)2.
2.4. Animal experiments
Fifty-five 4-week-old wild-type C57BL/6J male mice were purchased from NARlab (Taipei, Taiwan). All experiments were approved by the Institutional Animal Care and Use Committee (NTU-108-EL-00098) of National Taiwan University. Animals were co-housed with three to four mice per cage in a temperature-controlled room (22 °C ± 2 °C) on a 12 h light-dark cycle. The mice were fed with a normal diet for one week to acclimate. After acclimatization, the mice were randomly divided into 5 groups of 11 mice each into the normal diet group (control, 13.5% of energy from fat; LabDiet 5001, Fort Worth, USA) or HFD groups (50% of energy from fat). The mice were supplemented daily with 100 μL normal saline (vehicle) or 100, 300, or 500 mg/kg of body weight polysaccharide (UPPL, UPPM, and UPPH, respectively) by intragastric gavage. After 15 weeks, the mice were fasted 8 h before and then euthanized by carbon dioxide asphyxiation, and blood was withdrawn by cardiac puncture. Liver and adipose tissue were immersed in liquid nitrogen and stored at −80 °C for further analysis. Fasting blood glucose of mice was measured at 8th week and 15th week, respectively.
2.5. Biochemical analysis
The blood sample was withdrawn by cardiac puncture in a tube containing 10 μL of heparin sodium under anesthesia at the end of experiment. The blood centrifuged at 1000 g for 10 min at 4 °C and supernatant (serum) was collected. The serum levels of AST, ALT, TC, TG, LDL, and HDL were analyzed by the National Laboratory Animal Center (Taipei, Taiwan).
2.6. Histopathological examination
Liver and adipose tissues were collected and trimmed to 3–5 μm thickness. Trimmed sections were fixed in 10% paraformaldehyde, followed by dehydration and paraffinization. Formalin-fixed paraffin-embedded tissues were stained with hematoxylin-eosin (H&E) for morphological examination. Adipocyte size and adipocyte number in subcutaneous WAT was estimated using ImageJ software (Rasband, W.S., ImageJ, U.S. National Institutes of Health, Bethesda, MD, USA).
2.7. Oral glucose tolerance test
Oral glucose tolerance test (OGTT) was performed in the 15th week. Animals were fasted 8 h and weighed before measurement. The blood samples were collected from the tail vein by cutting off 2 mm of the tip with a sterile scalpel. The mice were then treated with a glucose solution (2 g glucose per kg mouse) by intragastric gavage. Afterward, the blood glucose level were monitored by using a Onetouch glucometer (LifeScan, Inc., Milpitas, CA, USA) before and 30, 60, and 120 min after the administration of the glucose solution. An area under the curve (AUC) was calculated. AUC between any two time points was calculated as (Time difference in minutes between sequential reads)*(Glucose level 1st time point + Glucose level 2nd time point)/2). The glucose level is measured from the level at time zero to the end time point level.
2.8. Measurement of cytokines
Whole blood was withdrawn by cardiac puncture and centrifuged at 1000 g for 10 min and serum was collected. Retroperitoneal fats were extracted using sterile phosphate buffer and then centrifuged at 1000 g for 30 min at 4 °C. The concentrations of TNFα and IL-6 were measured using ELISA kits according to the manufacturer’s instructions.
2.9. Measurement of triglycerides in liver
Liver was homogenized with sterile phosphate buffer and centrifuged at 1000 g for 30 min at 4 °C. Lipid was obtained from the homogenate. Next, 10 μL of sample was added to each well, followed by 150 μL of diluted Enzyme Mixture solution to initiate the reaction. The plate was incubated for 15 min at room temperature. The absorbance was measured at 530–550 nm using an ELISA reader.
2.10. Western blot analysis
Western blotting was performed according to our earlier report [7]. Adipose tissues were homogenized in an ice-cold lysis buffer (50 mM Tris-HCl, pH 7.4; 1 mM NaF; 150 mM NaCl; 1 mM EGTA; 1 mM phenylmethanesulfonyl fluoride; 1% NP-40; and 10 μg/mL leupeptin) for 1 h. Homogenates were then centrifuged at 12000 g for 30 min at 4 °C. Bio-Rad protein assay (Bio-Rad Laboratories, Inc., Hercules, CA,USA) was used to measure protein concentration.
2.11. Short-chain fatty acids analysis
SCFA quantification was conducted as described in a previous study [8]. SCFAs in fecal samples from mice was extracted with 2-nitrophenylhydrazine (2-NPH) at 60 °C for 20 min. Potassium hydroxide was added, and the samples were then heated for 15 min after. After cooling, phosphoric acid was added to the sample, and the sample was extracted with ethyl ether. The upper organic layer was collected. A SYNAPT G2-S high-definition mass spectrometer (Waters MS Technologies, Manchester, UK) was used for the SCFA quantification in mice feces.
2.12. 16S rDNA gene sequencing and analysis
16s rDNA sequencing was modified from methods previously described [9]. Fecal samples were stored at −80 °C. For gene sequencing, the purified DNA was eluted using the innuSPEED Stool DNA kit (Analytik Jena AG, Jena, Germany) according to the manufacturer’s protocol. The sample DNA quality was assessed by NanoDrop 1000 spectrophotometer and sent to Biotools Co., Ltd., for 16S rRNA gene amplification, sequence library construction, and sequencing. The PCR primer sequences were used to amplify the V3–V4 regions of bacterial 16S rRNA. The library DNA was sequenced using the Illumina HiSeq 2500 platform and paired-end reads (250 bp) were generated. Sequences were chimera-checked and filtered from the data set before the effective tags were grouped by 97% DNA sequence similarity into operational taxonomic units (OTUs).
2.13. Statistical analysis
Quantitative data are presented as mean ± standard deviation (SD). Statistical analysis was conducted with one-way analysis of variance (ANOVA) using SPSS 12.0. A p-value less than 0.05 was considered statistically significant, and the Duncan’s multiple range post hoc test was applied if the p-value was less than 0.05. Graphs were created using SigmaPlot 12.5 software.
3. Results
3.1. Monosaccharide composition of Ulva prolifera
Hydrolyzed sample was analyzed and quantified by high-performance anion exchange chromatography (HPAEC) system. UPPconsisted of 70.6± 29.7% rhamnose, 27.4 ± 9.22% glucuronic acid, 12.4 ± 5.28% xylose, 10.6 ± 6.0% glucose, and 2.04 ± 1.51% galactose (Table 1).
Table 1.
The content of monosaccharide composition of Ulva prolifera polysaccharide.
| Sample | Molar ratio of monosaccharide composition (100%) | ||||
|---|---|---|---|---|---|
|
| |||||
| Rha | Gal | Glc | Xyl | Glc A | |
| UP | 70.6 ± 29.7 | 2.04 ± 1.51 | 10.6 ± 6 | 12.4 ± 5.28 | 27.4 ± 9.22 |
Data are expressed as mean ± SD (n = 3).
3.2. UPP reduced body weight in high-fat diet-fed mice
At the end of the experiment, the mean body weight of mice fed a HFD was 22.5% higher than the control group, which demonstrated that HFD successfully induced weight gain in mice. Meanwhile, mice fed a HFD with UPPL, UPPM, or UPPH showed a significantly reduced body weight gain, with a 11.8, 12.0, and 16.2%, respectively, compared with the HFD mice (Fig. S1 & Table 2), suggesting that UPPs slowed down HFD-induced weight gain in HFD-fed mice.
Table 2.
The effect of UPPs on body weight, body weight gain, food intake, and food efficiency ratio.
| Group | Control | HFD | UPPL | UPPM | UPPH |
|---|---|---|---|---|---|
| Initial weight (g) | 18.41 ± 1.3a | 18.43 ± 1.1a | 18.46 ± 0.9a | 18.46 ±1.2a | 18.43 ±0.9a |
| Final weight (g) | 26.74 ± l.7c | 31.35 ± 3.4a | 29.47 ± 1.5ab | 29.41 ±1.5ab | 28.78 ± 2.8b |
| Weight gain (g) | 7.94 ± 1.3c | 12.1 ±1.9a | 9.95 ± 1.5b | 9.9 ± 1.7b | 9.11 ± 2.5bc |
| Food intake (g) | 3.22 ± 0.3a | 2.41 ±0.2b | 2.32 ± 0.2b | 2.39 ± 0.3b | 2.48 ± 0.3b |
| Food efficiency ratio (%) | 2.27 ± 0.3d | 4.69 ± 0.7a | 4.01 ± 0.6b | 3.81 ± 0.6bc | 3.41 ±0.9c |
Food efficiency ratio (%) = (daily weight gain/daily food intake) × 100. Data are expressed as mean ± SD (n = 11 mice/group). The statistical significance of differences among the five groups were analyzed by one-way ANOVA and Duncan’s multiple range tests. The values with different letters (a–d) are significantly different (p < 0.05) between each group.
The food intake of the control group was significantly higher than the HFD-fed group (Table 2) due to the differences in dietary energy density. Furthermore, the FER of the UPPL, UPPM, and UPPH groups was reduced with increasing polysaccharide concentration, while the FER of the HFD group was two times higher than that of the control group (Table 3). These results indicate that the effect of UPP on suppressing body weight gain was not via changing food intake.
Table 3.
Effect of UPPs on serum biochemical parameters in high-fat diet-fed C57BL/6 mice.
| Item | Control | HFD | UPPL | UPPM | UPPH |
|---|---|---|---|---|---|
| AST (U/L) | 95.4 ± 32.6a | 99.3 ± 26.0a | 95.0 ± 38.8a | 83.0 ± 42.2a | 74.2 ± 45.1a |
| ALT (U/L) | 15.3 ± 1.9ab | 14.7 ± 1.5ab | 12.9 ± 2.0b | 17.9 ± 3.2a | 18.0 ± 3.4a |
| TC (mg/dL) | 42.2 ± 5.4d | 101.5 ± 8.8a | 93.4 ± 8.1ab | 94.6 ± 12.3ab | 88.2 ± 11.9c |
| TG (mg/dL) | 27.9 ± 5.5a | 30.9 ± 15.7a | 28.6 ± 6.8a | 27.0 ± 8.2a | 25.0 ± 5.9a |
| HDL-C (mg/dL) | 36.6 ± 4.1b | 79.1 ± 5.4a | 73.8 ± 5.2a | 77.0 ± 9.0a | 75.6 ± 6.8a |
| LDL-C (mg/dL) | 4.7 ± 0.4d | 30.4 ± 4.5a | 25.2 ± 3.0b | 25.6 ± 3.8b | 21.1 ± 3.6c |
| HDL/LDL | 7.6 ± 0.7a | 2.7 ± 0.5c | 2.7 ± 0.3c | 3.0 ± 0.2bc | 3.4 ± 0.3b |
| AI (TC/HDL-C) | 1.2 ± 0.1a | 1.3 ± 0.1b | 1.3 ± 0.1b | 1.3 ± 0.1b | 1.2 ± 0.1a |
Data are expressed as mean ± SD (n = 7–8 mice/group). The statistical significance of differences among the five groups were analyzed by one-way ANOVA and Duncan’s multiple range tests. The values with different letters (a–d) are significantly different (p < 0.05) between each group. AI = atherogenic index.
Serum biochemical parameters were analyzed to evaluate the impact of UPP administered to the HFD-fed mice. Furthermore, an additional of cholesterol (0.5%) was added into the HFD to promote the development of lipid disorder. As the results in Table 3 demonstrate, TC and LDL-C levels were significantly higher in the HFD group than control group after animal were fed a HFD for three months. Subsequently, TC and LDL-C levels presented a decreasing trend in the UPPL, UPPM, and UPPH groups. TC levels in the UPPL, UPPM, and UPPH groups were reduced by 8, 6.6, and 13.1%, respectively. In parallel, LDL-C levels were statistically significant reduced by 17.4, 15.8, and 30.6%, respectively. However, TC and LDL-C levels in the UPPH group was significantly lower than the UPPL and UPPM groups after 15-week administration. Finally, the HDL-C levels in the intervention groups had no statistical significant difference compared with that in the HFD group.
3.3. UPP reduced visceral fat pad mass and prevented adipocyte hypertrophy in high-fat dietfed mice
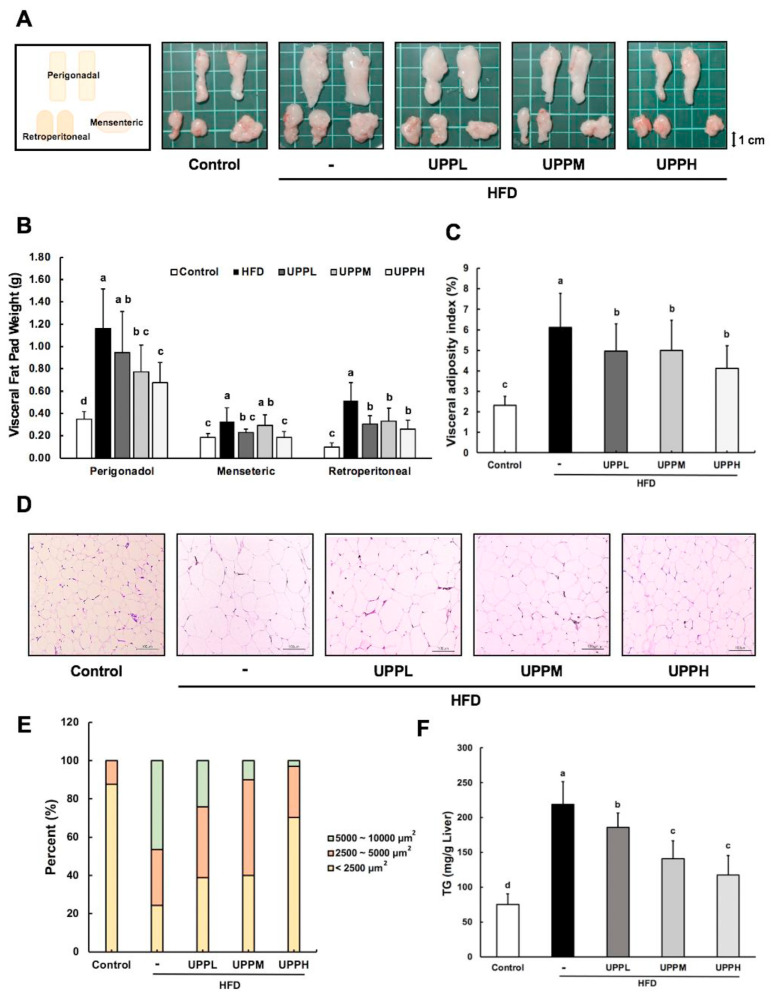
The average weights of the perigonadal, mesenteric, and retroperitoneal WAT of the HFD group was remarkably heavier than those of the control group, while the visceral fat pad weights of mice in the UPPL, UPPM, and UPPH groups tended to decrease after 15 weeks of administration of UPP (Fig. 1A, B). In addition, the inhibitory effects of UPPH on HFD-induced elevation of fat pad weight and visceral adiposity index were slightly higher than those of UPPL and UPPM (Fig. 1B, C). Furthermore, to evaluate the effect of UPP on adipocyte morphology in HFD-fed mice, biopsy of perigonadal fat pads was observed in a microscopic view with H&E staining. It is clear from Fig. 1D that larger and more hypertrophic perigonadal fat pads in HFD-fed mice compared to mice in the control group. The quantification of adipocyte composition is presented in Fig. 1E. In the HFD group, the percentages of adipocytes 5000–10,000 μm2 was 46.34%. It was higher than those found in the UPPL, UPPM, and UPPH group, which were 24.07, 10.00, and 2.97%, respectively. Moreover, UPP treatment at high dose in the HFD group had a lower but similar of <2500 μm2 adipocyte distribution (70.29%) compared to control group (87.00%). Collectively, the results showed that UPP limits the accumulation of WAT, as smaller adipocytes were found in UPP-treated mice compared to HFD-fed mice.
Fig. 1.
Effect of Ulva prolifera polysaccharide on epididymal white adipose tissue and liver in high-fat diet-fed mice.
A) Representative images of visceral fat pads (epididymal white adipose tissue) from each group of mice are shown at the end of week 15. Perigonadal, retroperitoneal, and mesenteric fat pad, respectively, are depicted. B) Visceral fat pad weights were measured after 15 weeks of treatment. C) Visceral adiposity index (%) was calculated as {[perigonadal + mesenteric + retroperitoneal fat pad weight (g)]/BW (g) * 100}. D) Representative images of hematoxylin and eosin (H&E)-stained perigonadal fat pads of mice in each group. Scale bar, 100 μm. Data are expressed as mean ± SD (B–C, n = 11 mice/group). E) Adipocytes size in visceral adipose tissue was determined by Image J. F) Hepatic triglyceride levels. Scale bar, 100 μm. Data are expressed as mean ± SD (C, n = 8 mice/group). The statistical significance of differences among the five groups were analyzed by one-way ANOVA and Duncan’s multiple range tests. The values with different letters (a–d) are significantly different ( p < 0.05) between each group.
3.4. UPP possessed hepatoprotective effects in highfat diet-fed mice
Livers morphological analysis demonstrated that the livers of mice in the control group were bright red in color, while yellowish and dull fatty livers were observed in HFD-fed mice (Fig. S2A). This effect might be due to lipid accumulation. Next, liver tissue was confirmed in histopathological observations by H&E staining. Liver H&E demonstrated normal histology in control group with no lipid accumulation. In contrast, HFD-fed mice exhibited numerous fat vacuoles in the HFD group, indicating that the mice developed a high degree of macrovascular steatosis induced by HFD. Marked effects were not observed for suppression of steatosis by 15-week ministration of low dose polysaccharide. However, hepatocyte hypertrophy and hepatic steatosis in mice fed an HFD, which was alleviated after the UPPM and UPPH intervention. These findings suggest UPP mitigated steatosis in an HFD-induced obesity mouse model (Fig. S2B). In addaction, TGs are strongly associated with non-alcoholic fatty liver disease; therefore, hepatic TG levels were compared among the groups. As shown in Fig. 1F, the HFD group had a significant increase in TG levels (218.81 ± 32.7 mg/g) compared to the control group. Meanwhile, the TG levels in the UPPL, UPPM, and UPPH groups were reduced by 15.1, 35.6, and 46.4%, respectively. UPP therefore ameliorated HFD-induced fatty liver.
3.5. UPP maintained blood glucose homeostasis in high-fat diet-fed mice
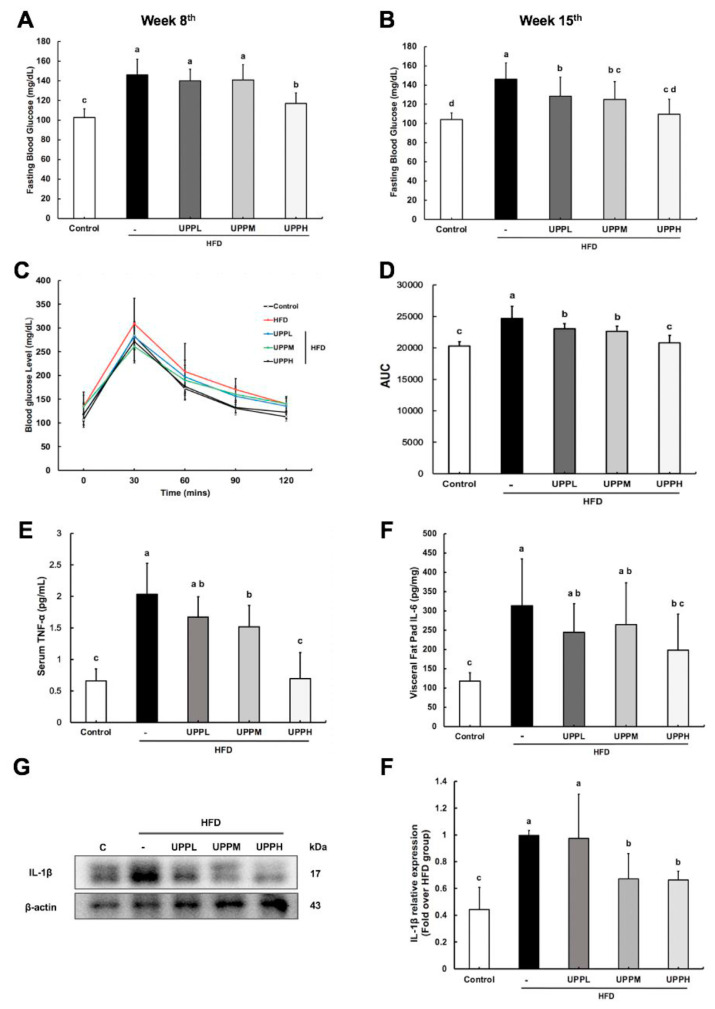
There was a significant increase in fasting blood glucose (FBG) in the HFD group (Fig. 2A,B), which meant that hyperglycemia was established successfully by obesity. The FBG was lower in the UPPH group after eight weeks of treatment, but FBG levels in the UPPL and UPPM groups were not significantly different compared to the HFD group. Notably, the hyperglycemic state of HFD-fed mice in the UPPL, UPPM, and UPPH groups was ameliorated after 15 weeks of polysaccharide administration. The effect of UPP on glucose tolerance in HFD-fed mice is shown in Fig. 2C, D. Moreover, there was an apparent low glucose tolerance in HFD-fed mice. In contrast, the AUC of mice administered UPPL and UPPM was significantly improved compared with HFD-fed mice, and UPPH reversed the effect back to the level observed in the controlled. In short, UPP exhibited a potential ability to modulate blood glucose levels.
Fig. 2.
Effect of Ulva prolifera polysaccharide on fasting blood glucose, glucose tolerance, and cytokine in high-fat diet-fed C57BL/6 mice.
A) Fasting blood glucose on week 8th and B) week 15th. C) Oral glucose tolerance test and D) the corresponding area under the curve. E) Serum tumor necrosis factor α (TNF-α) and F) visceral adipose tissue interleukin 6 (IL-6) levels were analyzed by enzyme-linked immunosorbent assay. G) Protein levels of interleukin-1β (IL-1β) in visceral adipose tissues were analyzed by western blot; H) IL-1β protein was quantified using β-actin as the loading control. Data are expressed as mean ± SD (A–D, n = 7–8 mice/group). The statistical significance of differences among the five groups were analyzed by one-way ANOVA and Duncan’s multiple range tests. The values with different letters (a–d) are significantly different ( p < 0.05) between each group.
3.6. UPP suppressed inflammation in high-fat dietfed mice
Atremendous augmentation in serum TNF-α levels was observed in the HFD group (2.03 ± 0.49 pg/mL) (Fig. 2E): levels were increased 3-fold compared to the control group (0.67 ± 0.19 pg/mL). Strikingly, high-dose polysaccharide administration significantly decrease the serum concentration of pro-inflammatory cytokine TNF-α to 0.69 ± 0.41 pg/mL. The evidence strongly demonstrated that high dose of UPP inhibited the basal release of TNF-α in HFD-fed mice. However, there was no statistically significant difference was detected between UPPL group (1.67 ± 0.32 pg/mL) and HFD group (2.03 ± 0.49 pg/mL). Furthermore, the levels of visceral fat pad IL-6 and IL-1β were tested (Fig. 2F–H). The results showed that HFD-fed mice produced higher levels of IL-6 and IL-1β without UPP intervention. Low-dose UPP did not have any apparent effect on levels of these cytokines, but medium- and high-dose UPP altered the IL-6 and IL-1β expression, resulting in expression levels closer to the control group. These results indicated that UPP could reduce low-grade inflammation in HFD-fed mice in serum and adipose tissue.
3.7. UPP might activate AMPK in high-fat diet-fed mice by increasing adiponectin expression
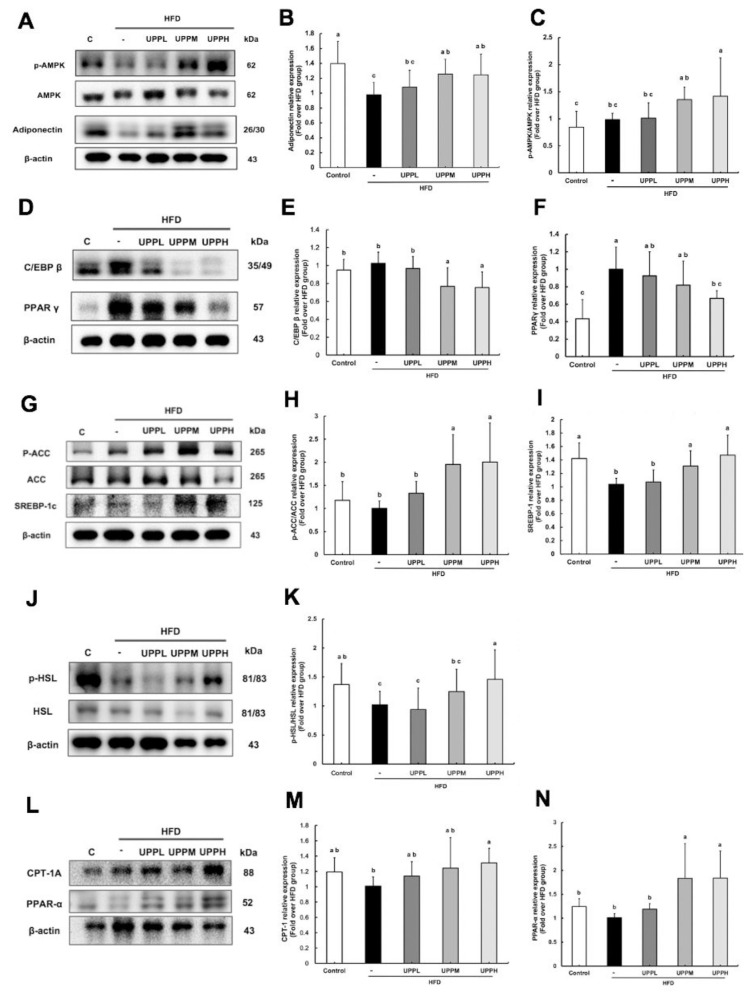
Previous studies have suggested that adiponectin could decrease hyperglycemia, as ectopic lipid accumulation was rescued and sustained weight loss without affecting food intake was observed after replenishment with recombinant adiponectin in mice [10]. As stated above, the body weight gain and visceral fat pad weight of the treatment groups were lower than those of the HFD group (Fig. 1 & Table 2). To test the hypothesis that adiponectin played a role in these effects, adiponectin expression was tested. We found that treatment with UPP led to a dose-dependent increase in adiponectin expression in adipose tissue (Fig. 3A, B). This analysis also indicated that the HFD group had the lowest adiponectin level and the adverse effect was reversed significantly by treatment with UPPM and UPPH. Next, based on a previous study by Yamauchi and colleagues [11] reporting that the effects of adiponectin require AMPK activation. As shown in Fig. 3C, compared with the control group, UPPM and UPPH treatment effectively enhanced phosphorylation of AMPK, with a 1.5-fold increase in phosphorylated AMPK in these groups compared to the control, HFD, and UPPL groups.
Fig. 3.
Effect of Ulva prolifera polysaccharide on lipogenic protein expression in high-fat diet-fed C57BL/6 mice.
Western blot analysis of proteins involved in lipid metabolism in visceral adipose tissues. The proteins analyzed are involved in adiponectin, phosphorylated 5′AMP-activated protein kinase (p-AMPK)/AMPK), adipogenesis (peroxisome proliferator activated receptor γ [PPARγ], CCAAT), lipogenesis (acetyl-CoA carboxylase [ACC], Sterol regulatory element-binding protein 1c [SREBP1c]), lipolysis (hormone-sensitive lipase [HSL]), and β-oxidation (peroxisome proliferator activated receptor α (PPARα); carnitine palmitoyltransferase-1 [CPT-1]). For quantification of protein expression by western blotting, β-actin was used as the loading control. Data are expressed as mean ± SD (n = 7–8 mice/group). The statistical significance of differences among the five groups were analyzed by one-way ANOVA and Duncan’s multiple range tests. The values with different letters (a–c) are significantly different (p < 0.05) between each group.
3.8. UPP exerted anti-obesogenic effects by regulating lipogenic protein expression in high-fat diet-fed mice
Activation of AMPK associated with suppression of adipogenic differentiation was validated by Chen et al. [12]. Therefore, we aimed to determine the effect of UPP on adipogenesis. PPARγ expression showed the greatest increase in HFD-fed mice (Fig. 3D, F). Notably, the expression of PPARγ was suppressed effectively in the presence of high-dose UPP. C/EBPβ is expressed early during adipogenesis and is also required along with PPARγ for robust adipocyte-specific gene expression [13]. As shown in Fig. 3E, no significant differences in the C/EBPβ expression level were observed in the control, HFD, and UPPL groups, but treatment with UPPM and UPPH attenuated the expression of C/EBPβ.
As shown in Fig. 3H, compared with the control group, phosphorylation of ACC was effectively enhanced with UPP treatment. This result indicated that AMPK phosphorylates ACC to inhibit enzyme activity, leading to downregulation of lipogenesis. Moreover, HFD significantly reduced SREBP-1c gene expression, and UPPL had no apparent effect on this (Fig. 3I). In contrast, SREBP-1c gene expression was higher in the UPPM and UPPH groups than in the HFD group, indicating that UPP suppressed SREBP-1c processing and thereby prevented the feed-forward transcription of their own genes.
Daval et al. observed that activation of AMPK could increase HSL phosphorylation in adipocytes and translocation to the lipid droplet was inhibited [14]. As shown in Fig. 3J, K, p-HSL was downregulated in the HFD group, while its levels were increased in the HFD-fed mice treated with UPPM and UPPH.
Shuttling esters of fatty acids into the mitochondria is accomplished through an acyl-carnitine intermediate, which itself is generated by the action of the CPT-1 enzyme for β-oxidation [15]. The expression of CPT-1A was significantly lower in HFD-treated mice compared with those in the control group (Fig. 3M). Surprisingly, UPP administration increased CPT-1A expression, rebalancing the dysregulated lipid metabolism in adipocytes. PPARα is a master regulator of genes involved in triglyceride metabolism, particularly β-oxidation [16]. As shown in Fig. 3N, the protein level of PPARα was increased up to 2-fold in the UPPM and UPPH groups compared to the HFD group. In contrast, PPARα expression was not significantly different in the control and UPPL groups compared to the HFD group. In short, upregulation of PPARα protein level may further stimulate CPT-1A expression to enhance β-oxidation.
3.9. UPP modulated the high-fat diet-induced gut dysbiosis
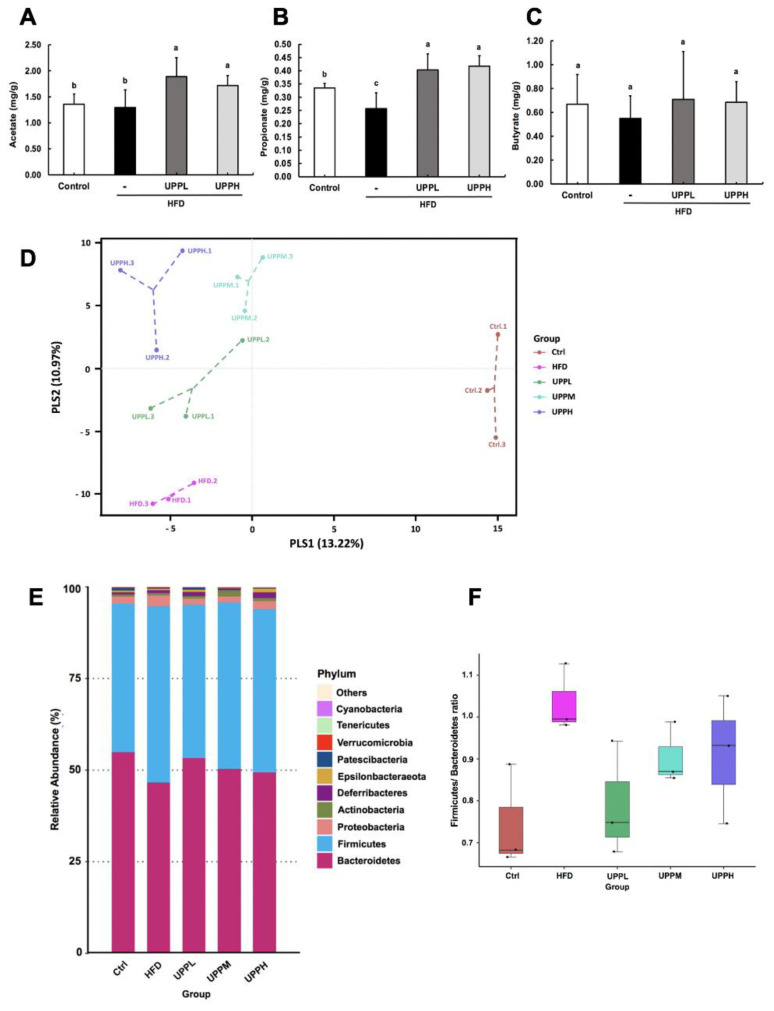
To investigate whether the concentration of SCFAs was affected by polysaccharide concentration, we chose to compare SCFA concentration in the UPPL and UPPH groups. UPP administration significantly increased the levels of acetate (Fig. 4A) and propionate (Fig. 4B) compared to the HFD-fed group. In contrast, the difference in levels of butyrate was not significant between the groups (Fig. 4C). However, the acetate, propionate, and butyrate concentration were not significantly different between the UPPL and UPPH groups.
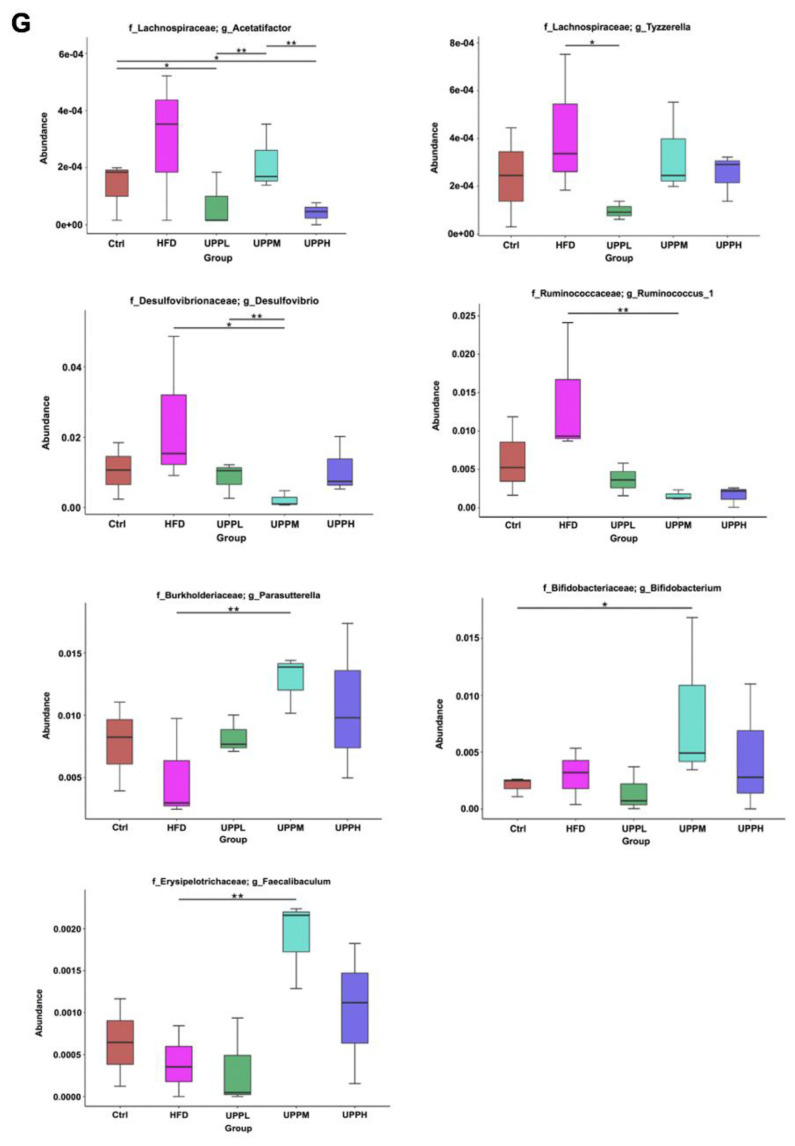
Fig. 4.
Effect of Ulva prolifera polysaccharide on short-chain fatty acid concentration and gut microbiota composition analysis.
A) Acetate, B) propionate, and C) butyrate were quantified in the colons of chow-fed and high-fat diet (HFD)-fed mice after 15 weeks of treatment. D) Partial least squares discriminant analysis (PLS-DA) demonstrates distinct colon microbial composition in the different groups. Each data point represents one sample and each color represents each group (percent variation explained by each PLS is shown in parameters). E) 16s rRNA gene sequencing analysis of colon contents at the phylum level. F) Variability in the Firmicutes/Bacteroidetes ratio in the gut microbiota. G) Box plot of 16s rRNA gene sequencing analysis of colon contents at the genus level. Data are expressed as mean ± SD. The statistical significance of differences among the five groups were analyzed by one-way ANOVA and Duncan’s multiple range tests. Symbols indicates significant differences compared to the HFD group, *p < 0.05; **p < 0.01.
Next, we analyzed the effects of UPP intervention on the gut microbiota composition in the colon. PLS-DA revealed distinct clustering of microbiota composition for each treatment group (Fig. 4D). At the phylum level, HFD induced an increase in the abundance of Firmicutes and Proteobacteria, but a decrease in Bacteroidetes (Fig. 4E). Obese mice demonstrated an increase in F/B ratios; however, no dose-dependent improvement in this ratio was observed as UPP doses increased (Fig. 4F).
In addition, the results showed that the abundances of pathogenic Acetatifactor and Tyzzerella, were higher in HFD group (Fig. 4G). They had been found to be significantly increased with systemic inflammation in HFD [17]. Further statistical analysis demonstrated that the HFD group had significantly higher abundances of Desulfovibrio and Ruminoccoccus_1. Inability to regulate levels of Desulfovibrio and Ruminoccoccus_1 in obese individuals has been associated with metabolic disorder and increased body adipose mass [18]. UPP supplementation remarkably decreased the abundance of Acetatifactor, Tyzzerella, Desulfovibrio, and Ruminoccoccus_1 in HFD-fed mice. In addition, compared to the HFD group, UPPM intervention resulted in enrichment of the abundances of Parasutterella, Faecalibaculum, and Bifidobacterium. It has been shown that Parasutterella and Faecalibaculum might possess an anti-obesity effect [19] and lead to an increase in levels of Bifidobacteria, which are depleted in inflammatory diseases and promote gut health [20].
4. Discussion
The dosages of the UPP were chosen based on studies that indicated that 300 and 500 mg/kg of sulphated polysaccharide from sea cucumber and Pacific abalone could ameliorate obesity [3,21]. In this study, we showed that body weight, body fat, and metabolic syndrome parameters were improved after intervention with UPP, as soluble fiber may affect lipid absorption. Furthermore, it has been shown that the water-holding capacity of polysaccharide is higher than insoluble fiber, and that a viscous solution was created within the gastrointestinal tract that could cause gastroparesis to create a postprandial satiety effect [22]. The digestion process was slowed down, and this might decrease the food intake. However, there was no significant difference in food intake between the HFD and treatment groups. This indicated that the higher energy density in the HFD group slowed down gastric emptying, prompting a lower food intake in this group.
In the study, we expected liver injury by HFD, but serum AST and ALT concentration no significant differences were observed between control and HFD groups. AST concentration did not vary significantly among control, HFD, and the UPP supplemented HFD groups. Intriguingly, the HFD-fed mice fed a medium and high dose UPP had a significantly higher ALT concentration to the UPPL group. Atherogenic index (AI) is a novel indicator involved in dyslipidemia [23]. An elevated of TC, LDL-C, and AI are the factors for the development of many lipid-related diseases such as atherosclerosis, cardiovascular disease and obesity. Moreover, study found that HFD induced hyperlipidemia in rats, results in a remarkable increase in serum TC and LDL-C. Treatment with UPP resulted in a declined lipid profile, such as TC and LDL-cholesterol, similar to the reference.
Adiponectin has recently been indicated as a treatment for obesity-associated metabolic syndromes. Yamauchi and colleagues indicated that the effects of adiponectin require AMPK activation [11]. Undeniably, AMPK plays an important role in adipocyte homeostasis. Stimulating β-oxidation by inhibiting ACC through phosphorylation via upregulation of adiponectin appeared to be primarily mediated by the activation of AMPK [24]. Our experiment demonstrated that the intervention of UPP reduced fatty acid synthesis gene expression in HFD-fed mice. SREBP is a key lipogenic transcription factor. Surprisingly, we found that SREBP-1c in 125 kDa gene expression was higher in the UPPM and UPPH groups than in the HFD group. According to Li et al., AMPK directly stimulated phosphorylation of SREBP-1c to prevent its cleavage and nuclear translocation, leading to reduced lipogenesis [25]. Moreover, we also assessed levels of PPARα, a master regulator of genes involved in triglyceride metabolism, particularly β-oxidation [16]. Song et al. found that CPT-1A was induced 2- to 3-fold by the addition of each PPARα ligand; these PPARα agonists enhanced β-oxidation [26]. Our results suggest that UPP might increase the expression of AMPK via adiponectin to effectively modulate lipogenic gene expression in HFD-fed mice.
Fiber reaches the colon undigested by human digestive enzymes; however, it can be fermented by colonic microflora that generate SCFAs. SCFAs exert multiple effects on host metabolism and physiology, such as mediating anti-inflammatory effects by inducing IL-10 secretion, stimulating insulin excretion, and lowering food intake [27]. It was also reported that short-term oral SCFA intervention could alleviate diet-induced obesity and insulin resistance in mice through activation of the adiponectin-mediated pathway [28]. Our results revealed that the administration of UPP could increase the SCFA concentration in the colons of HFD-fed mice, especially acetate and propionate. Hence, we conjecture that acetate and propionate exert antiobesity effects by stimulating the expression of adiponectin in adipose tissue.
Alterations in the phyla Firmicutes and Bacteroidetes were described in obese animals, and increased levels of Firmicutes and decreased levels of Bacteroidetes have been associated with obesity and metabolic disorders [29]. The Firmicutes/Bacteroidetes ratio has been considered a hallmark for obesity. However, Schwiertz and his collogue did not observe any modifications or even reported decreased Firmicutes/Bacteroidetes ratios in obese animals and humans [30]. Interestingly, Mendez-Salazar and colleagues found that Proteobacteria was substantially more abundant in the obese group and that there was a positive correlation between Proteobacteria and fat consumption [31]. Meanwhile, the relative abundance of Proteobacteria was 3% in mice from the HFD group compare to control group (1.87%) which is indicative of dysbiosis. Notably, taxonomic profiling demonstrated that treatment with UPPL, UPPM, and UPPH reduced the Proteobacteria phylum, with a 1.73, 1.61, and 2.05%, respectively in HFD-fed mice. Intervention with Enterobacter, a member of the Proteobacteria phylum, in germ-free mice resulted in the development of obesity and insulin resistance [32]. The abnormal growth of Proteobacteria may be a marker of disease risk. Besides, we observed an increase in Bifidobacterium in the colon after supplementation with UPP. In fact, some studies demonstrated that animals fed with the Bifidobacterium showed suppression of weight gain, ectopic fat accumulation, increased glucose tolerance, and a decrease in inflammatory diseases compared to placebo-treated control animals [20].
In conclusion, the present study (Fig. S3) indicates that UPP alleviates HFD-induced weight gain, reduction in adipose tissue hypertrophy and triglyceride concentration in liver. These effects are associated with mitigates of systemic low-grade inflammation, and improves FBG and lipid metabolism. In addition, UPP may increase the acetate and propionate concentration and improve gut microbiota composition to maintain colonic health. We suggest that oral administration with UPP represents potential prebiotic for ameliorate HFD-induced obesity and metabolic disorder.
Supplementary Figures
Effect of Ulva prolifera polysaccharide on body weight in high-fat diet-fed mice.
Chow-fed and high-fat diet (HFD)-fed mice were treated daily with normal saline or UPP (100, 300, or 500 mg/kg) for 15 weeks by intragastric gavage. A) Representative images of each group of mice at the end of week 15 are shown. B) Average body weight and C) body weight gain were measured throughout the 15-week period. Data are expressed as mean ± SD (n = 11 mice/group). The statistical significance of differences among the five groups were analyzed by one-way ANOVA and Duncan’s multiple range tests. The values with different letters (a–c) are significantly different ( p < 0.05) between each group.
Effect of Ulva prolifera polysaccharide on hepatic steatosis in high-fat diet-fed mice.
A) Representative liver images of each group of mice are shown at the end of week 15. B) Representative images of hematoxylin and eosin (H&E)-stained liver tissue in each group of mice.
Schematic diagram of molecular mechanisms by which Ulva prolifera polysaccharide exerts anti-obesity effects in high-fat diet-fed mice.
Administration of UPP to the high-fat diet-fed mice could prevent body weight gain, reduce metabolic disorder and inflammation, regulate lipid metabolism in adipose tissue, and modulate the composition of the gut microbiota.
Acknowledgements
This study was supported by the Ministry of Science and Technology (108-2320-B-002-016-MY3, 109-2320-B-002-012-MY3).
Abbreviations
- ACC
Acetyl-CoA carboxylase
- AI
Atherogenic index
- ALT
Alanine aminotransferase
- AMPK
5′-AMP-activated protein kinase
- AST
Aspartate aminotransferase
- C/EBP
CCAAT/enhancer binding protein
- CPT-1
Carnitine palmitoyltransferase-1
- FBG
Fasting blood glucose
- FER
Food efficiency ratio
- HDL-C
High-density lipoprotein cholesterol
- HPAEC
High performance anion-exchange chromatography
- HSL
Hormone-sensitive lipase
- IL-1β
Interleukin 1β
- IL-6
Interleukin 6
- LDL-C
Low-density lipoprotein cholesterol
- pAMPK
Phosphorylated AMPK
- pHSL
Phosphorylated Hormone-sensitive lipase
- PPARα
Peroxisome proliferator activated receptor α
- PPARγ
Peroxisome proliferator activated receptor γ
- SCFA
Short-chain fatty acid
- SREBP
Sterol regulatory element-binding protein
- TC
Total cholesterol
- TG
Triglyceride
- TNF-α
Tumor necrosis factor alpha
- WAT
White adipose tissue
Funding Statement
This study was supported by the Ministry of Science and Technology (108-2320-B-002-016-MY3, 109-2320-B-002-012-MY3).
Footnotes
Author contributions
H.C. Pung, Y.C. Lo, and M.H. Pan designed the whole study. H.C. Pung, W.S. Lin, and M.H. Pan conceived the idea and wrote the manuscript. H.C. Pung performed most of the experiments. Hsu carried out the pDART-MS analysis. Y.C. Lo, C.C. Hsu, and M.H. Pan and C.T. Ho contributed comments and to the revision of the manuscript. All authors were involved in editing the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA, et al. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci. 2019;20:2358. doi: 10.3390/ijms20092358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Z, Zhu B, Sun Y, Ai C, Wang L, Wen C, et al. Sulfated polysaccharide from sea cucumber and its depolymerized derivative prevent obesity in association with modification of gut microbiota in high-fat diet-fed mice. Mol Nutr Food Res. 2018;62:e1800446. doi: 10.1002/mnfr.201800446. [DOI] [PubMed] [Google Scholar]
- 4.Kidgell JT, Magnusson M, de Nys R, Glasson CRK. Ulvan: a systematic review of extraction, composition and function. Algal Res. 2019:39. [Google Scholar]
- 5.Song W, Wang Z, Zhang X, Li Y. Ethanol extract from Ulva prolifera prevents high-fat diet-induced insulin resistance, oxidative stress, and inflammation response in mice. BioMed Res Int. 2018;2018:1374565. doi: 10.1155/2018/1374565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang YW, Lu TJ. Molecular characterization of polysaccharides in hot-water extracts of ganoderma lucidum fruiting bodies. J Food Drug Anal. 2004;12:59–67. [Google Scholar]
- 7.Chiou YS, Sang S, Cheng KH, Ho CT, Wang YJ, Pan MH. Peracetylated (−)-epigallocatechin-3-gallate (AcEGCG) potently prevents skin carcinogenesis by suppressing the PKD1-dependent signaling pathway in CD34+ skin stem cells and skin tumors. Carcinogenesis. 2013;34:1315–22. doi: 10.1093/carcin/bgt042. [In eng] [DOI] [PubMed] [Google Scholar]
- 8.Weng CY, Kuo TH, Chai LMX, Zou HB, Feng TH, Huang YJ, et al. Rapid quantification of gut microbial short-chain fatty acids by pDART-MS. Anal Chem. 2020;92:14892–7. doi: 10.1021/acs.analchem.0c03862. [DOI] [PubMed] [Google Scholar]
- 9.Lee PS, Teng CY, Kalyanam N, Ho CT, Pan MH. Garcinol reduces obesity in high-fat-diet-fed mice by modulating gut microbiota composition. Mol Nutr Food Res. 2019;63:e1800390. doi: 10.1002/mnfr.201800390. [DOI] [PubMed] [Google Scholar]
- 10.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 11.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 12.Chen SC, Brooks R, Houskeeper J, Bremner SK, Dunlop J, Viollet B, et al. Metformin suppresses adipogenesis through both AMP-activated protein kinase (AMPK)-dependent and AMPK-independent mechanisms. Mol Cell Endocrinol. 2017;440:57–68. doi: 10.1016/j.mce.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, et al. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008;22:2941–52. doi: 10.1101/gad.1709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daval M, Foufelle F, Ferré P. Functions of AMP-activated protein kinase in adipose tissue. J Physiol. 2006;574:55–62. doi: 10.1113/jphysiol.2006.111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreurs M, Kuipers F, van der Leij FR. Regulatory enzymes of mitochondrial beta-oxidation as targets for treatment of the metabolic syndrome. Obes Rev. 2010;11:380–8. doi: 10.1111/j.1467-789X.2009.00642.x. [DOI] [PubMed] [Google Scholar]
- 16.Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62:720–33. doi: 10.1016/j.jhep.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 17.Li LL, Wang YT, Zhu LM, Liu ZY, Ye CQ, Qin S. Inulin with different degrees of polymerization protects against diet-induced endotoxemia and inflammation in association with gut microbiota regulation in mice. Sci Rep. 2020;10:978. doi: 10.1038/s41598-020-58048-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao C, Yang C, Liu B, Lin L, Sarker SD, Nahar L, et al. Bioactive compounds from marine macroalgae and their hypoglycemic benefits. Trends Food Sci Technol. 2018;72:1–12. [Google Scholar]
- 19.Morrison KE, Jašarević E, Howard CD, Bale TL. It’s the fiber, not the fat: significant effects of dietary challenge on the gut microbiome. Microbiome. 2020;8:15. doi: 10.1186/s40168-020-0791-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schroeder BO, Birchenough GMH, Ståhlman M, Arike L, Johansson MEV, Hansson GC, et al. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe. 2018;23:27–40.e7. doi: 10.1016/j.chom.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ai C, Duan M, Ma N, Sun X, Yang J, Wen C, et al. Sulfated polysaccharides from pacific abalone reduce diet-induced obesity by modulating the gut microbiota. J Funct Foods. 2018;47:211–9. [Google Scholar]
- 22.Capuano E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit Rev Food Sci Nutr. 2017;57:3543–64. doi: 10.1080/10408398.2016.1180501. [DOI] [PubMed] [Google Scholar]
- 23.Wu T-T, Gao Y, Zheng Y-Y, Ma Y-T, Xie X. Atherogenic index of plasma (AIP): a novel predictive indicator for the coronary artery disease in postmenopausal women. Lipids Health Dis. 2018;17:197. doi: 10.1186/s12944-018-0828-z. [In eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55:2562–70. doi: 10.2337/db05-1322. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metabol. 2011;13:376–88. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song S, Attia RR, Connaughton S, Niesen MI, Ness GC, Elam MB, et al. Peroxisome proliferator activated receptor alpha (PPARalpha) and PPAR gamma coactivator (PGC-1alpha) induce carnitine palmitoyltransferase IA (CPT-1A) via independent gene elements. Mol Cell Endocrinol. 2010;325:54–63. doi: 10.1016/j.mce.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Shimizu Y, Kimura I. Gut microbial metabolite short-chain fatty acids and obesity. Biosci Microbiota Food Health. 2017;36:135–40. doi: 10.12938/bmfh.17-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong J, Jia Y, Pan S, Jia L, Li H, Han Z, et al. Butyrate alleviates high fat diet-induced obesity through activation of adiponectin-mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice. Oncotarget. 2016;7:56071–82. doi: 10.18632/oncotarget.11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim B, Choi HN, Yim JE. Effect of diet on the gut microbiota associated with obesity. J Obes Metab Syndr. 2019;28:216–24. doi: 10.7570/jomes.2019.28.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18:190–5. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 31.Méndez-Salazar EO, Ortiz-López MG, Granados-Silvestre M, Palacios-González B, Menjivar M. Altered gut microbiota and compositional changes in Firmicutes and Proteobacteria in Mexican undernourished and obese children. Front Microbiol. 2018;9:2494. doi: 10.3389/fmicb.2018.02494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2013;7:880–4. doi: 10.1038/ismej.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of Ulva prolifera polysaccharide on body weight in high-fat diet-fed mice.
Chow-fed and high-fat diet (HFD)-fed mice were treated daily with normal saline or UPP (100, 300, or 500 mg/kg) for 15 weeks by intragastric gavage. A) Representative images of each group of mice at the end of week 15 are shown. B) Average body weight and C) body weight gain were measured throughout the 15-week period. Data are expressed as mean ± SD (n = 11 mice/group). The statistical significance of differences among the five groups were analyzed by one-way ANOVA and Duncan’s multiple range tests. The values with different letters (a–c) are significantly different ( p < 0.05) between each group.
Effect of Ulva prolifera polysaccharide on hepatic steatosis in high-fat diet-fed mice.
A) Representative liver images of each group of mice are shown at the end of week 15. B) Representative images of hematoxylin and eosin (H&E)-stained liver tissue in each group of mice.
Schematic diagram of molecular mechanisms by which Ulva prolifera polysaccharide exerts anti-obesity effects in high-fat diet-fed mice.
Administration of UPP to the high-fat diet-fed mice could prevent body weight gain, reduce metabolic disorder and inflammation, regulate lipid metabolism in adipose tissue, and modulate the composition of the gut microbiota.