Abstract
Conducting polymers (CPs) are a category of polymeric materials with conjugated main chains. The characteristic electrical and optical properties of CPs can be fine-tuned through controlling the doping states of CPs. Because of their long-term stability in water, CPs have been demonstrated as electroactive biointerfaces and electrode materials especially in aqueous environments. Serving as multifunctional interfaces and organic electrodes for the integration bioelectronics and devices, CPs have been studied and applied in various biological applications. This paper provides a review of conducting polymer–based electrochemical sensors, particularly those used in biological fields. General conducting polymers and derivatives and their main electrochemical sensing platforms with different design of devices are introduced. Cyclic voltammetry, differential pulse voltammetry, chronoamperometry, electrochemical impedance spectroscopy, and quartz crystal microbalance methods and their features are then explored as detection methods for the analysis of drugs and food. To enhance the sensitivity and lower the detection limit of sensing platforms, various CP-based nanocomposites have been designed and developed. Although the electrodes made of CP-based nanocomposites usually outperform those made of pristine CPs, more systematic studies are required to provide insights into the design of nanocomposite-based electrodes. More applications of CP-based sensors for advanced food and drug analyses are expected.
Keywords: Conducting polymers, Electrochemical sensors, Food and drug analysis, Nanocomposites, Quartz crystal microbalance
1. Introduction
1.1. Conducting polymers
Conducting polymers (CPs) with effective electric conductivity features usually have conjugated backbones. CPs are synthesized by chemically or electrochemically oxidizing monomers to initiate polymerization. Furthermore, the electrical conductivity of CPs can be controlled by doping processes (p-doping or n-doping). For p-type doping, electrons are donated from CPs to the unfilled bands of dopants. On the contrary, for n-type doping, CPs gain electrons from dopants. There are several doping strategies, including [1–5]:
Chemical doping: Exposing CPs to strong oxidants or reductants to induce redox processes on the conjugated backbones.
Electrochemical doping: By applying the potential to induce redox reactions on CPs and control the doping states by electron transfer between CPs and the electrode. The doping and dedoping of ions take places reversibly in an electrolyte containing supporting salts. When an oxidation potential is applied, electrons are transferred from CPs to the electrode. When a reduction potential is applied, CPs receive electrons from the electrode. In aqueous solutions, the ions or electrolytes can move in or out from the CPs to compensate the charges formed during the redox process. The adding or losing of ions can further change the volume of CPs, which makes CPs good materials for actuator applications.
Solution doping: This type of doping method does not involve a redox reaction but an acid–base protonation process. Taking polyaniline (PANI) as an example, their acidic doping level depends on the pH value of the doping solutions.
After doping processes, the energy level rear-ranges, and the alternating single and double bonds make delocalized electrons/holes freely hop along and across the polymer chains to give the polymers conductor characteristics. Polyacetylene (PA) is the first conducting polymer after doped with I2, which was first discovered by Hideki Shirakawa, Alan Heeger, and Alan MacDiarmid [6]. Another example is polypyrrole (PPy), which has been used in many electronic devices due to its stability, easy fabrication with oxidants, and good electrical conductivity. By applying different polymerization methods, PPy of different morphologies can be fabricated. This characteristic provides more applications from PPy than PA, especially for tissue engineering [7]. Polythiophene (PTh), poly (3,4-ethylenedioxythiophene) (PEDOT) and their derivatives have also attracted numerous attention. Both of PTh and PEDOT have the advantages of non-toxic and environment friendly, which are ideal materials for organic electronics such as organic light-emitting devices and field effect transistor. Moreover, PEDOT also solve the insoluble problem of others CPs by integrating a water-soluble polyelectrolyte, poly (styrene sulfonic acid) (PSS), without sacrificing the electronic conductivity after making into film [8–10]. Several CPs have been widely used in organic electronics [11] (Fig. 1). For biomedical applications, the flexible mechanical properties of CPs present are particularly advantageous, as in this way they are similar to human tissues and have superior biocompatibility compared with metals and other conventional semiconductor materials. Having good electrical conductivity [12] and biocompatibility [13], CPs have attracted attention for potential applications in various bioelectronics for decades [14–16]. A key application in bioelectronics is electrochemical biosensors, which are used for the detection of biological analytes in vitro or in vivo by examining electrical signal output transduced from the devices.
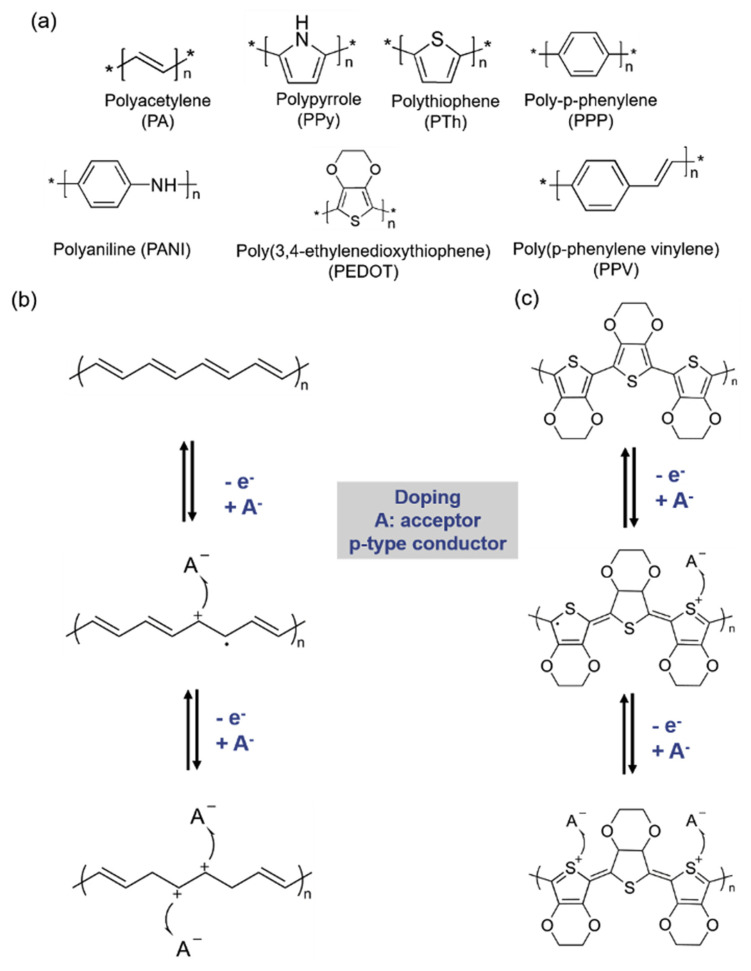
Fig. 1.
(a) Chemical structures of common conducting polymers. Examples of doping states of (b) PA and (c) PEDOT.
1.2. Conducting polymer-based biosensors
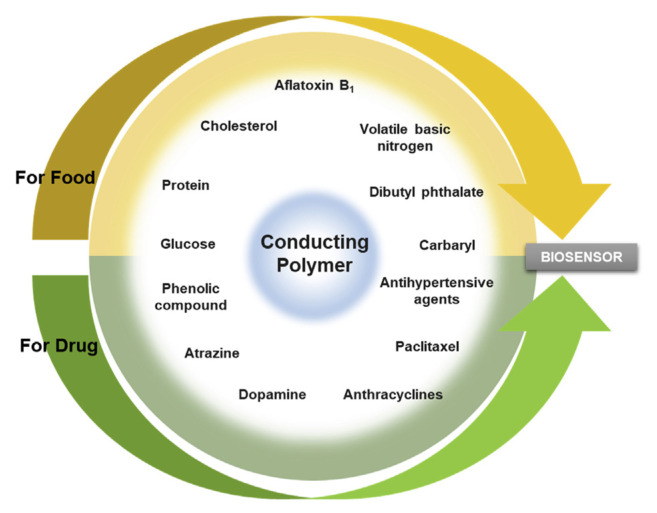
Electrochemical biosensors have played a prominent role in detecting harmful chemicals in food and agricultural environments, in monitoring biomarkers revealing abnormal cell behavior, and in examining drug overdose [17]. To improve detection performance, such as through higher sensitivity and lower detection limits, the electrodes are nano-fabricated. CPs have been demonstrated to be easily fabricated to nanoparticles, nanowires, or other nanostructures simply by tuning the chemical reaction and electrochemical processes [18,19]. The simple and cost-effective fabrication process makes CPs promising electrode materials for electrochemical biosensors. CP-based electrochemical biosensors have been widely used in various food and drug analyses (Fig. 2). CP thin films, which can be simply fabricated through electrochemical polymerization and deposited on devices, have been successfully used on several sensing platforms.
Fig. 2.
The application of conducting polymer biosensor in food and drug analysis.
1.2.1. Electrochemical DNA sensors/aptasensors
DNA sensors can be exploited for their specific binding between probe DNA and target DNA. A probe-modified surface act as a transducer to distinguish the target. The binding affinity and specificity between DNA probes and analytes are crucial to their capture efficiency. To enhance the output signals, the probes should be immobilized appropriately onto the sensing platform at a controlled probe density for efficient hybridization [20]. A probe can be immobilized on the platform through methods below [21,22]:
Physical adsorption: Physical adsorption is the simplest method without any pretreatment or modification of substrates. Generally, CPs stay in positively charged at doping state. Therefore, the electrostatic adsorption between a negatively charged DNA and positively charged CPs is feasible. To apply a positive potential through electrochemical procedures, the adsorption of DNA analytes on electrodes can also be enhanced, which is beneficial for promoting the signal readouts. However, this method might be lack of specificity due to the adsorption of all charged substances.
Covalent bonding: By covalently bonding a 5′-end modified oligonucleotide (ODN) onto CP functional groups. The end of ODN can be easily modified with a thiol group, an amine group (−NH2) or a carboxyl group (−COOH). In this method, the conducting polymer films should have functional groups, which allow bio-conjugation with modified ODN.
Avidin/streptavidin–biotin recognition: The interaction between avidin/streptavidin and biotin has been one of the essential bio-conjugation strategies for the immobilization of various biomolecules. Because of the high affinity, multivalent binding sites and stable complex, surface modification with avidin or streptavidin on CPs, gold nanoparticles and magnetics particles have been widely used.
To expand the applications for various targets, aptamers are widely used as gene-related capture probes. Aptamers can be short, single-stranded DNA or RNA or peptides that are selected from a large, random sequence pool called Systematic Evolution of Ligands by Exponential Enrichment (SELEX). SELEX is a combinatorial technology which pick random nucleic acid sequences from a library after several rounds of screening to obtain a high affinity aptamer for a specific target. Due to the intermolecular interaction between nucleotides, synthetic aptamers can be designed with different binding forms such as simple binding, folding, or structure-switching that can capture targets such as proteins, small molecules, toxins, living cells, and drugs [23].
Electrochemical DNA biosensors are regarded as general platforms using CPs as substrates for biosensor applications. The working mechanism of this sensing platform based on the electron transfer process across the environment through the surface of electrodes. DNA hybridization or the recognition events between aptamers and analytes will change the surface morphology compared to pristine electrodes, which may alter the path of electron transport. The biochemical signals are converted into an electrical readout through transducing mechanisms. These electrochemical biosensors are generally considered cost-effective, high precision and simple rapid detection tools. To detect a specific DNA sequence, Bizid et al. synthesized a CP film using ferrocene groups and carboxylic acid as functional groups on poly (p-phenylene) [24]. The amine groups on DNA-capture probes can covalently bond with carboxylic groups through NHS/EDC coupling. The films were fabricated on a gold electrode directly through electrochemical deposition, and the redox-active ferrocene groups enhanced the electron-transfer process, which is beneficial for improving electrochemical response for biosensors. Hui et al. presented a DNA biosensor to detect a breast cancer marker by grafting hydrophilic polyethylene glycol (PEG) onto polyaniline (PANI) nanofibers [25]. The biosensors use the antifouling properties of PEG and the large surface area of nanofiber structures to enhance sensitivity and the output signals. For a CP-based aptasensor, Chin et al. incorporated a peptide sequence that can capture calmodulin with maleimide-functionalized poly (3,4ethylenedioxythiophene), poly (EODT-MI) as the platform [26]. They also imported zwitterionic linker as their antifouling strategy to prevent nonspecific binding. Electrochemical impedance spectroscopy was applied for quantitative analysis of calmodulin concentration.
1.2.2. Hydrogel-based biosensors
Hydrogel, which is composed of hydrophilic cross-linked polymer chains, can form a water-containing three-dimensional network. Because hydrogels can be made by biopolymers or biocompatible polymers with a moderately mechanical property, they have been extensively used in several biomedical fields, including drug delivery and tissue regeneration [27]. One of the superiorities of hydrogels over other materials is their tunable mechanical properties to mimic biological tissue. Feig et al. has demonstrated a conducting interpenetrating network using PEDOT:PSS gel which has presented both stretchability and conductivity [28]. Because of its potential for modern bioelectronics, hydrogel-based biosensors have also been developed. The working strategies of hydrogel-based biosensors can be divided into two categories [29]; In the first category, bioreceptors are immobilized inside the hydrogel network for sensing purposes [30,31]; The second category includes stimulus-responsive hydrogels according to their intrinsic properties. Stimulus-responsive hydrogels may be sensitive to chemical factors, such as pH [32] and specific chemical agents [33], or physical factors, such as light, temperature, ionic concentration, electric field, and other external force [34].
CPs can be introduced to hydrogel-based sensing platforms through simple electropolymerization and coating on the hydrogel to make it conductive [35]. The conductive hydrogel acts as a flexible electrode to detect cell signals, such as neural recording, and can be implanted in a living creature. In hydrogel-based sensors, CPs usually play a key role in improving both electron conductivity and mechanical properties. To further improve the application of hydrogel-based sensors, researchers have also incorporated CPs with other polymers or nanomaterials, including graphene derivatives, through polymerization or physical blending. Wei et al. designed a near-infrared light-responsive electrochemical protein imprinted biosensor by incorporating graphene oxide (GO) and PANI with poly (N-isopropylacrylamide) hydrogels on a glassy carbon electrode [36]. Their results show that the presence of GO/PANI largely enhance the current signal. This enhancement is because the high surface area of PANI fibers promoted the charge transfer efficiency from the redox probes to the electrode surface. Hydrogel biosensors provide a large surface area and high multifunctionality, elasticity, and biocompatibility [37,38]. The dopants for CPs also contribute to electrical properties [39,40]. Through the incorporation of various additives into the network, CP-based hydrogels can be developed into versatile biosensor applications [41].
1.2.3. Field-effect transistor-based biosensors
Field-effect transistor (FET) devices have attracted attention for biosensing applications due to their advantages of simple miniaturization, low power consumption, high sensitivity, and quick response requiring few analytes [42]. Compared with a conventional three-electrode system, FET-based biosensors consist of two electrodes, namely source and drain terminals, and connect with a semiconducting material as a path. A bias potential is applied, and the current is modulated by a third electrode called a gate. Because biomolecules are usually dissolved in aqueous buffer solution, the buffer solution is used to control the gate in what is usually referred to as liquid-gated FET devices [43]. For biosensing, the surface of semiconducting materials is generally modified or immobilized with various biomolecules acting as capture probes, such as nucleic acids, cells, enzymes, antibodies, and aptamers, which have specificity and binding affinity with the target biomolecules [44]. Depending on the charges of the target molecules and the mechanism influencing the drain current, both n-type and p-type FETs have been successfully used as FET-based biosensors. Either physical or chemical interactions between target molecules and probe-modified surfaces can be utilized as the sensing mechanism. Generally, the gate potentials change depending on the concentrations of target molecules, which leads to the change in the drain current. Thus, the conductance functions as a readout in real time.
To create FET-based biosensors with high sensitivity and low detection limits, various nanomaterials have been intensely studied for the design of devices. Recently, the main research interest is the application of two-dimensional nanomaterials, of which graphene-based FETs are most common [45,46]. In FET performance, the stability of nanomaterials with a large surface area must be maintained, and they must possess high carrier mobility and conductivity. In this article, we focus on the CPs as the transistor channel and sensitive surface due to their facile functionalization, fabrication, mechanical property, and biocompatibility [47]. Polypyrrole (PPy), PANI, and poly (3,4-ethylenedioxythiophene) (PEDOT) are the most commonly used CPs for this application. Park's group demonstrated that dopamine (DA) receptors can be immobilized on carboxylated PEDOT nanofibers through covalent bonding with amine groups. This platform presented good sensitivity and real-time response to DA molecules [48]. PPy nanotubes which were immobilized with the cortisol antibodies were also coupled to a FET platform for the ultrasensitive stress biomarker detection. Nano-FET probes deposited with PPy has also been used for monitoring pH and ATP concentration to identify biochemical properties of a single living cell [49].
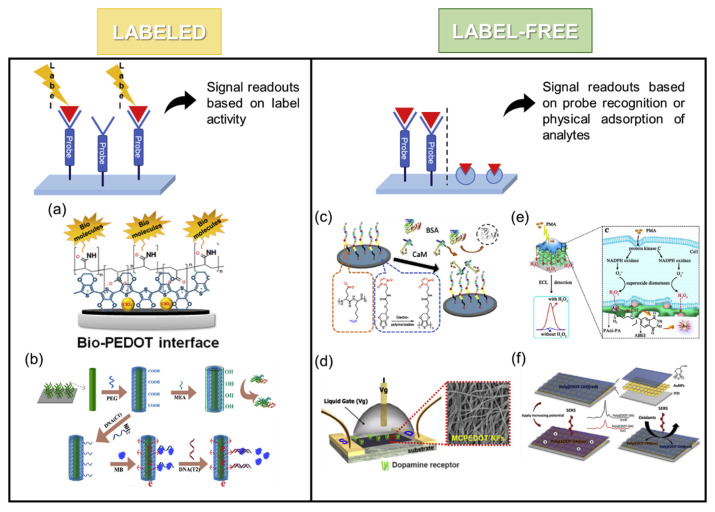
These biosensing platforms can be classified into two types: labeled and label-free biosensors (Fig. 3). In the labeled sensing technique, the platform relies on a tag to quantify target molecules such as fluorescent substances and electrochemically active probes [17]. By contrast, a label-free sensor can directly identify analytes through mass spectrometry, quartz crystal microbalance (QCM), or surface plasma resonance without labeling. The advantages of label-free methods are reduced time and costs of labeling and prevented interference from additional labels. Molecularly imprinting polymers is another method used to create a label-free platform [50]. By tuning the composition of EDOT monomers, the process of electropolymerization, and target concentrations during molecular imprinting by electrochemical polymerization, researchers can optimize the imprinting effectiveness of target molecules [51,52].
Fig. 3.
Examples of labeled biosensors: (a) detection of Lactate (Reprinted with permission from ref [8]. Copyright 2020 American Chemical Society); (b) detection of breast cancer susceptibility gene (Reprinted with permission from ref [25]. Copyright 2017 American Chemical Society) and label-free biosensors: (c) detection of calmodulin (Reprinted with permission from ref [26]. Copyright 2020 American Chemical Society); (d) detection of DA (Reprinted with permission from ref [48]. Copyright 2016 American Chemical Society); (e) detection of H2O2 (Reprinted with permission from ref [53]. Copyright 2018 American Chemical Society); (f) detection of oxidants (Reprinted with permission from ref [54]. Copyright 2019 American Chemical Society).
2. Detection methods
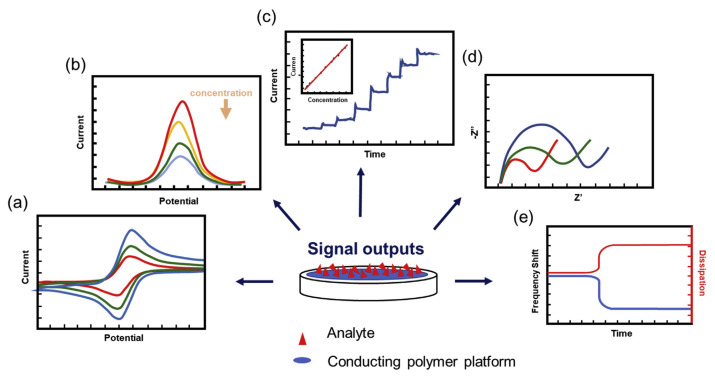
CP-decorated biosensors have become a powerful tool for detecting toxic chemicals in food and drugs such as antibacterial medications [55] and vasodilators [56]. Several detection methods have been regularly used to quantify the target analytes. In this review article, we summarized the most commonly used electrochemical techniques, including cyclic voltammetry (CV), differential pulse voltammetry (DPV), chronoamperometry (CA), and electrochemical impedance spectroscopy (EIS) [57], and an acoustic resonator QCM method (Fig. 4).
Fig. 4.
Five types of commonly used detection methods: (a) cyclic voltammetry (CV); (b) differential pulse voltammetry (DPV); (c) chronoamperometry (CA); (d) electrochemical impedance spectroscopy (EIS); (e) quartz crystal microbalance (QCM).
2.1. CV
CV is a basic, effective electrochemical method employed to monitor the redox process and electrochemical activity of analytes on electrode surfaces. The current is recorded when a cyclic potential (voltage) or repeated cyclic potential is applied to the electrode to modulate the electron-transfer process. The peak is considered an oxidation process at high potentials and a reduction process at low potentials. In a CV profile, information about the durability of the electrodes, the reversibility of the redox reaction, and the electron-transfer kinetics between the electrodes and the analytes can be obtained. Apart from investigation of the electrode condition, CV can be used to determine the concentration of analytes in solutions. The peak current increases with the concentration.
This simple method has been extensively used in food and drug analysis. For CP applications, CV is a common process to evaluate the electropolymerization process of CPs. By adjusting the electrochemical parameters and the composition of monomer solutions during the application of cyclic potentials, the film thickness and morphology of CPs can be well controlled. The detection of tamoxifen, which is a selective estrogen receptor modulator used to prevent breast cancer, was demonstrated by Radhapyari's group [58]. To achieve a moderate condition of thickness, they prepared PANI in acidic medium by applying cyclic potentials for electropolymerization. They successfully applied a PANI-modified platinum electrode to obtain a linear relationship between the tamoxifen concentration versus the peak current from the generation of hydrogen peroxide. Other applications of PANI electrodes include the detection of doxorubicin or chloramphenicol in eye drops [59,60]. The detection of acetylcholine (AChCl) in serum samples of individuals with Alzheimer disease was developed by Chauhan et al., who used a PEDOT composite-modified electrode. While bare fluorine-doped tin oxide electrode (FTO) presented no redox signals, Fe2O3/reduced-GO/PEDOT-coated electrode showed a vast increase in current intensity [61]. EIS was also used to obtain a smaller charge transfer resistance compared with bare FTO electrode. They used EIS to confirm the optimized composition of the nanocomposite for the detection of AChCl and used CV to display the stability of their electrode and present the linear relationship between AChCl concentration and peak current. Besides the immobilization of bioreceptors on the electrode, CPs also enhanced the signals due to their inherent charge transport properties and capability to maintain the bioactivity of analyst species [62].
2.2. DPV
In comparison with linear sweep voltammetry, DPV is considered a more sensitive method for identifying electroactive species at low concentrations, which is especially useful in biosensing. The principle of DPV is to apply a series of amplitude pulses increasing along a linear baseline. The pulse width and the waiting period of time interval are both fixed in the periods of the DPV method. By measuring each difference of the Faradaic current before and after the pulses in a short time interval. Namely, the readout of the experiment is a current difference versus the base potential. Instead of enhancing the electrical signals, the advantage of DPV is to reduce the background currents. Researchers can minimize the charging current from environment during this measurement. Beneficial in effectively reducing the background current, DPV is a popular electrochemical method used to detect analytes with slightly differing oxidation or reduction peaks and simultaneously quantify each substance. Piroxicam (PX) is a nonsteroidal anti-inflammatory drug which has been used in several therapeutic applications. Interferences, such as ascorbic acid (AA), tyrosine (Tyr), and uric acid (UA), are along with the monitoring of the PX concentration in urine. To deal with this multi-element analysis, DPV was utilized and PTh derivatives acted as a positively charged electrocatalytic film. With this coating, EIS demonstrated only a small charge transfer resistance. DPV also successfully identified three oxidation peaks for UA, PX and Tyr, respectively. It is because positively charged CP films can stabilize these negatively charged species and enhance the sensitivity [63]. An ionic liquid/CP composite was used to modify a carbon paste electrode (CPE), which exhibited high electroactive surface area and good conductivity for detecting the concentration of carbaryl. DPV results showed that poly-p-phenylenediamine/ionic liquid CPE of rough film morphology deliver two times higher current than pristine CPE. Besides, this composite electrode can be used for the detection of real samples in the presence of all kinds of ions. Furthermore, this electrode was stable for 4 weeks [64]. As mentioned, DPV can be used to analyze and determine the concentrations of multiple substances simultaneously. Functionalized PEDOT film can be electropolymerized into different morphology by alternating the solution systems. Besides, PEDOT films with carboxylic acid groups have also demonstrated a significant enhancement in DA detection and reduced interferences from UA and DA [65]. These examples came to a conclusion that the DPV method can be applied to CP-based electrode to achieve versatile sensing platforms of high sensitivity and low background signals, which are especially useful for quantitative analysis.
Analogous to DPV, square wave voltammetry (SWV) applied a square-wave pulse of amplitude E, and step height δE. Currents are measured twice in a scan cycle (forward and backward) of every symmetrical double pulse. Similar to DPV, SWV can minimize the background distribution as well. However, SWV can directly measure the current with a wide range of sweep potential without waiting time. As a result, SWV has been viewed as a time-saving method than other pulse voltammetry. SWV can also distinguish several analytes at the same time like DPV. Wang et al. used PPy 3D network hydrogels with Tartrazine (Tz) to detect the abnormality of UA, DA, and AA in real human urine [39]. By monitoring the Rct with EIS measurement, the optimized composition of PPy and Tz was decided. The SWV was then applied to analyze human urine and presented clear oxidation peaks with low detection limits.
2.3. CA
In CA, one or multiple potential steps are applied to the electrode, and the resulting current is then recorded as a function of time. The applied potential can affect the sensitivity, reliability, and selectivity of sensors. The performance of sensors can also be determined by observing the response time and the time intervals to reach a steady state. During the measurement, analytes are injected into the electrochemical cell when a potential is applied. Quantitative analysis can be simply decided by the resulting current. Therefore, CA can be used for continuous monitoring of the analytes. Pan et al. [66] presented a PANI-based hydrogel glucose sensor. CP-based hydrogels have become promising electrode materials for use as high-performance glucose oxidase sensors with fast response time and superior sensitivity when CA measurement is applied. A well-designed hydrogel can also be micropatterned by 3D fabrication, such as inkjet printing or spray coating. In this study, phytic acid was used as a dopant and crosslinker. After the gelation, a porous hydrogel was formed with high surface area and electrical conductivity. The working mechanism of this glucose biosensor was based on the glucose oxidase, which was immobilized in the hydrogel. The glucose can then be oxidized by this enzymatic-PANI hydrogel electrode. One advantage of applying hydrogel is to extend the lifetime of enzyme, which is protected by the hydrogel. However, the diffusion speed of analytes in hydrogel could limit the response time. With applying an oxidation potential, a large current rose instantly due to the oxidation of glucose around the electrode. The initial oxidation created a concentration gradient and induced a flux of glucose around the electrode surface. The current soon reached a steady state to form a plateau controlled by the diffusion rate of glucose. After more glucose was added, the current increased again to reach another plateau region. As a result, step growth signal could be observed with the increase of analyte concentration.
A food spoilage sensor was developed by using a PANI-based gas sensor [67]. The advantages of forming interconnected nanofibers of PANI offers a higher surface-to-volume ratio than bulk materials, which provides more reactive sites for gas adsorption to facilitate the electron transport process. After exposure to amine gas, the p-toluene sulfonate hexahydrate-doped PANI changed from conductive emeraldine salt form into insulating emeraldine base form, indicating that the sensor resistance raises as the increase of amine gas concentration. CA was applied to record the dynamic responses by converting the current readout to relative resistance. This study also demonstrated the reversibility that the signal approached its original baseline upon the exposure to air. The gas sensor integrated near-field communication (NFC) and micropatterned PANI on NFC tag to develop an easy-to-implant device was for detecting volatile basic nitrogen release from food, with the aim of developing a smarter food supply chain.
2.4. EIS
Impedance spectroscopy is a powerful tool for the analysis of electrode electron-transfer properties [68]. EIS is a complex electrochemical technique in which sinusoidal excitation potentials are applied to an electrochemical system. The measured currents usually require further analysis using the concept of equivalent circuit of resistors and capacitors, which represents same amplitude and phase angle with a real cell under a same excitation. The data can be plotted as a Nyquist diagram in which the real part of the impedance, Z′(ω), is the x-axis, and the imaginary part of the impedance, Z″(ω), is the y-axis. For most biosensor applications, researchers use a Randles circuit which combining the charge transfer resistance (Rct) and the differential capacitance (Cd) to explain their data. A normal impedance response usually exhibits a kinetically controlled semicircular region at high frequencies followed by a diffusion controlled linear region at low frequencies. With appropriate data analysis and modeling, EIS possesses potential for the study of the physiochemical behaviors of biomolecules attached to the electrode. The time required for EIS measurement is much shorter than that for polymerase chain reaction and enzyme-linked immunosorbent assay [69]. The addition of a redox probe, such as [Fe(CN)6]3−/4−, to electrolyte solutions, enables the determination of differences in the electron-transfer kinetics among the pristine electrode, modified electrode, and electrode after bio-recognition. Quantitative analysis can then be achieved by correlating the charge-transfer resistance with the concentration of target molecules in solutions [70].
Karimi-Maleh et al. developed an electrochemical sensor for the detection of 6-mercaptopurine (6-MP), which is an anticancer drug used to treat leukemia, a group of blood cancers. To increase the electron-transfer rate at the electrode surface, the electrode was coated with PPy and functionalized multiwalled carbon nanotubes (MWCNT). Characterization of the electrode surface and was revealed by using EIS. The EIS revealed that values of the Rct of a PPy-modified electrode decreased compared with a bare pencil graphite electrode [71]. In food analysis, food additives are closely monitored. Dibutyl phthalate (DBP) is a commonly used plasticizer in plastic products. However, the amount of DBP in food intake must be minimized due to the possibility to alter the expression of a number of genes [72]. In addition to analyzing the charge transfer process of the surface characterization, EIS plots can also be used to evaluate the binding behavior of analytes by observing the semicircular region which related to Rct. With increasing binding amount of analyte like DBP, the charge transfer process was blocked, which leads to the increase of the semicircular region [73]. Salmonella typhimurium (S. Typhimurium) is a pathogenic gram-negative bacteria. In poor sanitary conditions, this food-borne pathogen may spread through sewage contamination of food, water, and even person-to-person contact and cause gastrointestinal infections. Sheikhzadeh et al. developed a electrochemical biosensor with poly [pyrrole-co-3-carboxyl-pyrrole] copolymer and aptamer for detecting S. Typhimurium. This PPy derivative lowered the Rct at the copolymer/aptamer/electrolyte interface and skipped the requirement of additional redox probes. The response and concentration relationship was calculated by relative variation ΔRct/Rct0 with linear calibration cure [74].
2.5. QCM
QCM is a well-known analytical method with high sensitivity. It is used to measure weight changes at the nanogram to microgram level on the surface of a quartz crystal of known area in air or liquid. Unlike the aforementioned electrochemical methods, QCM affords the advantages of the piezo-electric effect of a thin quartz crystal between two electrodes. When an alternating electric field is applied, the quartz crystal starts to oscillate, and the vibration produces a transverse acoustic wave across the crystal. After foreign molecules deposited or adsorbed on the surface of the crystal, the resonant frequency and total energy of the system may change. QCM is a powerful instrument because this experimental setup can be used for real-time monitoring. In biosensor applications, it can be used to monitor the capture of various biomolecules, such as glucose, peptides, DNA, RNA, and proteins, on a probe-modified crystal [75–77].
Quantification is possible in a mass-frequency conversion relationship [78]. For conductive substrates such as CPs, a special QCM technique called electrochemical QCM (EQCM) can be applied. In EQCM, potentials can be simultaneously applied to the quartz during the measurement of mass changes, which broadens the application of QCM [79–81]. In food and drug analysis, QCM results are intuitive and readily understandable. By adopting adsorption models, observers can also estimate the binding affinity between the substrates and analytes. Tryptophan is an essential amino acid that is related to Alzheimer disease and Parkinson disease, and Prabakaran et al. developed a QCM sensor that allows the real-time monitoring of the association and dissociation of tryptophan levels in body fluids by using a molecularly imprinted polymer film [82]. By selecting an appropriate ODN as a probe, Truong et al. developed a MWCT-doped PPy as a DNA biosensor for the label-free QCM detection of exogenous gene sequences [83]. Salam et al. used QCM to detect S. typhimurium by using a mouse IgG antibody as a bioreceptor. This QCM sensor allows an LOD of approximately 10–20 colony forming units mL−1 [84].
Numerous materials have been incorporated with CPs to improve sensitivity and lower detection limits. Inorganic nanoparticles such as gold nanoparticles (AuNPs), silver nanoparticles (AgNPs), and nanocarbon materials are among the most popular candidates for these purposes. Other biocompatible polymers and biomolecules, including aptamers, DNA, and peptides, are also used to improve sensing performance in terms of selectivity and specificity [85–88]. We summarize recent applications of CP-based nanocomposites for the detection of various biomolecules and chemicals by using CV, DPV, CA, EIS, and QCM methods in Table 1.
Table 1.
Summary of recent developments of nanocomposites conducting polymer-based sensing platform for the detection of biomolecules and chemical.
| Polymeric system | Composite materials | Detection method | Target analyte | Limit of detection | Real sample | Ref. |
|---|---|---|---|---|---|---|
Polypyrrole (PPy)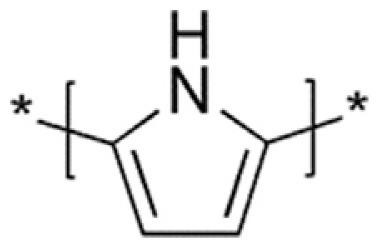
|
MWCNTs-PPy-DNA | QCM and EIS | subunit 35 S of ribosomal RNA of Cauliflower mosaic virus | 4 pM for QCM | – | [83] |
| Tz/PPy hydrogel | CV and SWV | AA, DA and UA | 1283 nM for AA 44 nM for DA 46 nM for UA |
human urine and Yinqiao tablet | [39] | |
| AuNPs–PPy–reduced GO | CA | Organophosphorus pesticides | 0.5 nM | paraoxon-ethyl dosed tap water | [89] | |
Polythiophene (PTh)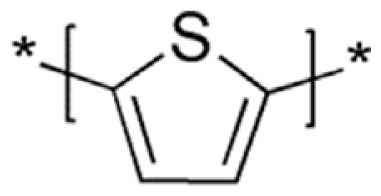
|
AuNPs/2,2′:5′,2″-terthiophene-3′ (p-benzoic acid) (TBA) | EIS | Chemokine ligand | 0.078 ng/mL | human serum sample | [90] |
| PTh–NH2-g-PEG/enzymes | CV and EIS | Phenolic compounds | 0.01 μM | artificial wastewater test | [91] | |
| mannose thiol/quinone-PTh | SWV and QCM | Bacterial detection (E. coli) | 25 cell/mL for SWV | – | [92] | |
Polyaniline (PANI)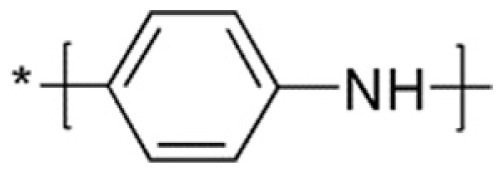
|
ABEI-Ag/PANI-ATMP hydrogel | electrochemiluminescence | Xanthine | 9.6 nM | fish meat with a cell disrupter | [40] |
| enzyme/PANI | CV | Tamoxifen | 0.07 ng mL−1 | 1.0 ng mL−1 tamoxifen tablet | [58] | |
| Melamine imprinted PANI/PAA film | DPV | Melamine | 0.0172 nM | infant formula milk samples | [93] | |
| Fe3O4/MWCNT/PANI/Nf | CV, DPV, CA | Urea | 67 μM | milk | [94] | |
| Graphene/polyvinylpyrrolidone/PANI/cholesterol oxidase | CV | Cholesterol | 1 μM | lyophilized human serum | [95] | |
| core–shell structure of NiCo2O4/PANI | CA | Glucose | 0.3833 μM | – | [96] | |
Poly (3,4-ethylene-dioxythiophene) (PEDOT)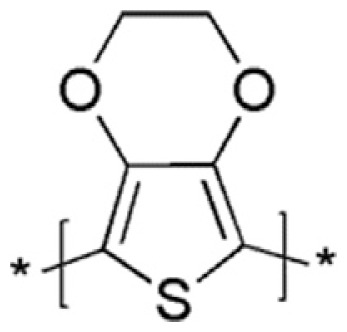
|
PEDOT-co-PEDOT-OH/peptide-imprinting nanotube | CV | α-synuclein gene | 4.0 pM | patient's midbrain-like organoids | [52] |
| phenylboronic acid-grafted PEDOT nanotube | QCM | Glucose | 50 μM | – | [76] | |
| iridium oxide/PEDOT/Tyrosinase | CA | Catechol and AZN | 0.017 μM for catechol 2.964 μM for AZN |
AZN in tap water, waste water, well water, human urine, serum | [97] | |
| Fe2O3/rGO/PEDOT/enzyme | CV and EIS | Acetylcholine | 4.0 nM | human serum | [61] | |
| PEDOT:PSS/AuNPs | CV | Xanthine | 30 nM | fish and meat samples | [98] | |
Poly (p-phenylene) (PPP)
|
ferrocenyl group/DNA/PPP | CV | OND of Hepatitis C | 30 fM | – | [99] |
(MWCNTs: multiwalled carbon nanotubes, Nf: Nafion, Tz: Tartrazine, rGO: reduced graphene oxide, AuNPs: gold nanoparticles, PAA: polyacrylic acid, AZN: azinphos methyl).
3. Recent applications of conducting polymer-based biosensors
3.1. For food analysis-milk products
Milk is among the most popular beverages globally and is a rich source of calcium, minerals, proteins, and other nutrients. Milk is also a raw material for many foods that promote health. Because it is so crucial for daily life, the market for high-quality milk is large. However, milk adulterants can pose a threat to human health, and detection techniques for these adulterants are crucial [97]. Urea is a metabolic end-product of proteins, and its concentration in milk is regarded as relevant to understanding the care and supervision of cows [98]. Melamine is an organic compound added to milk, infant formula, and other food products to falsify its protein content. The ingestion of melamine may increase the risk of infant fatality and induce kidney disease [99].
CP composite materials have been developed for electrochemical sensing platforms to detect urea and melamine. Das and Sarkar developed a urea sensor by coating PANI-based hydrogels with enzyme urease immobilized on the working electrode. The hydrogel gave the advantage of high surface area and PANI contributed electroactivity to make the sensor sensitive and applicable for urea detection in samples including milk, puffed rice, soil, and human blood [100]. Singh et al. presented a Fe3O4/MWCNT/PANI-Nafion nanocomposite film as a urea sensor that immobilized enzyme urease. Unlike other groups, CNT and Fe3O4 acted as two effective electron-transfer mediators that successfully enhanced their current signals [101]. For melamine, Regasa et al. developed a melamine-imprinted poly (aniline-co-acrylic acid) (PAA) composite thin film. The hydrogen bonds in this PANI-PAA composite template formed a donor–acceptor pattern with a spacing of 4.8 Å to precisely capture the melamine [102].
3.2. For drug analysis – phenolic compounds
Phenolic compounds are characterized by their chemical structures with one or more phenol units, and different functionalized phenols may have various effects on human health [103]. For example, isoxsuprine hydrochloride is used as a vasodilator. A sensitive sensor based on conductive nanocomposites consisting of PPy, metal nanoparticles, and chitosan has been developed [56]. Although some phenolic compounds have been reported as having antioxidant, anticancer, and immune system–promoting effects (useful for pharmaceutical and biomedical applications), other phenol-related compounds discharged during the production of plastic, drugs, pesticides, and herbicides are viewed as wastewater or environmental pollutants. Catechol is a toxic, bioreactive molecule that is also used as an antioxidant [104]. Because of the importance of this molecule and its potential risks, its qualitative and quantitative analysis is necessary. Electrochemical detection can be time efficient, with high sensitivity for effectively detecting minute quantities of chemicals. Tyrosinase is a natural enzyme also known as phenol oxidase; it enables the oxidization of catechol to form o-quinone. The production of o-quinone can be monitored to determine the concentration of catechol. With CP-based nanocomposites as electrode materials, the signals of biosensors are enhanced, and the signaling and activity of enzymes are retained [91,105].
4. Conclusion and future perspectives
CP-based biosensors have several advantages, including simple fabrication, mass production, light weight, and flexibility, making them suitable for portable devices. Their performance can be promoted by simply tuning the surface morphology and nanostructure of CP-based electrodes or by mixing them with other nanomaterials to increase conductivity and thus enhance the sensitivity of signals and lower the LOD. Additional studies regarding applications of CP-based sensors for various food and drug analyses are expected. Furthermore, new technologies may be integrated with current electrochemical sensors, such as in the application of antifouling surfaces to prevent nonspecific binding with proteins, which is crucial for the detection of target molecules in blood or serum samples with high specificity requirements [106]. Antifouling CPs have also demonstrated great potential for application in in vivo and real-time monitoring as implanted electrodes [107]. Surface-enhanced Raman spectroscopy (SERS) has been demonstrated as a powerful tool for various detection purposes, such as for food additives, drug analysis, and environmental monitoring. With an electrochemical setup, electrochemical SERS (EC-SERS) technology has the advantages of high sensitivity and rapid response time, and the setup can be fabricated to a portable device [108]. The quantification can also be achieved through an optimized mapping strategy to reduce the variation of signal measured from different spots on SERS-active substrates [109]. Researchers also developed a CP platform for detecting oxidants by monitoring their SERS signals [54]. The intensity of the Raman signal of CPs is highly dependent on their redox state. Quantitative analysis of oxidant concentration can be achieved by using this EC-SERS platform. For novel sensing instruments, CPs are promising materials for paper-based analytical devices that offer portable, user-friendly, and cost-effective features for on-site biochemical or chemical detection and point-of-care testing. High-conductivity CPs with a desired functional group can be modified on the interface of the electrode on a paper-based electrochemical device for effective lowering of the background signal, thereby enhancing the sensitivity of the device. By conjugating recognition sites such as an enzyme, antibody, or aptamer to the polymer, the CP modified paper-based device can be utilized for the detection of biomarkers such as metabolites, proteins, and nucleic acids for medical diagnosis, food safety, and environmental surveillance. The CP-based device can also contain grafts of nanomaterials that can assist catalysis and minimize deactivation for improving the sensitivity and long-term stability of the device, respectively. Moreover, the device can be integrated to have additional functionalities such as sample processing or preconcentration, signal amplification, and signal output into a “sample-to-answer” platform that helps simplify manual operation and minimize the use of sophisticated instrumentation [110]. We expect that incorporating the remarkable features of CP in a paper-based device can enable the exploration of a wide range of possibilities for accurate, low-cost measurements in resource-limited settings.
Acknowledgements
We gratefully acknowledge the financial support provided by the Ministry of Science and Technology of Taiwan under grant MOST 108-2113-M-002-013-MY3 and RIKEN-MOST Joint Research Project MOST 109-2327-B-002-009.
Funding Statement
We gratefully acknowledge the financial support provided by the Ministry of Science and Technology of Taiwan under grant MOST 108-2113-M-002-013-MY3 and RIKEN-MOST Joint Research Project MOST 109-2327-B-002-009.
Footnotes
Conflict of interest
The authors have no conflicts of interest.
References
- 1. MacDiarmid AG, Epstein AJ. The concept of secondary doping as applied to polyaniline. Synth Met. 1994;65:103–16. [Google Scholar]
- 2. Snook GA, Kao P, Best AS. Conducting-polymer-based supercapacitor devices and electrodes. J Power Sources. 2011;196:1–12. [Google Scholar]
- 3. Warren LF, Walker JA, Anderson DP, Rhodes CG, Buckley LJ. A study of conducting polymer morphology: the effect of dopant anions upon order. J Electrochem Soc. 1989;136:2286–95. [Google Scholar]
- 4. KN, Rout CS. Conducting polymers: a comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021;11:5659–97. doi: 10.1039/d0ra07800j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bai H, Shi G. Gas sensors based on conducting polymers. Sensors. 2007:7. [Google Scholar]
- 6. Etemad S, Heeger AJ. Polyacetylene, (CH)x: the prototype conducting polymer. Annu Rev Phys Chem. 1982;33:443–69. [Google Scholar]
- 7. Ateh DD, Navsaria HA, Vadgama P. Polypyrrole-based conducting polymers and interactions with biological tissues. J R Soc Interface. 2006;3:741–52. doi: 10.1098/rsif.2006.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Promsuwan K, Meng L, Suklim P, Limbut W, Thavarungkul P, Kanatharana P, et al. Bio-PEDOT: modulating carboxyl moieties in poly(3,4-ethylenedioxythiophene) for enzyme-coupled bioelectronic interfaces. ACS Appl Mater Interfaces. 2020;12:39841–9. doi: 10.1021/acsami.0c10270. [DOI] [PubMed] [Google Scholar]
- 9. Groenendaal L, Jonas F, Freitag D, Pielartzik H, Reynolds JR. Poly(3,4-ethylenedioxythiophene) and its derivatives: past, present, and future. Adv Mater. 2000;12:481–94. [Google Scholar]
- 10. McCullough RD. The chemistry of conducting polythiophenes. Adv Mater. 1998;10:93–116. [Google Scholar]
- 11. Simon DT, Gabrielsson EO, Tybrandt K, Berggren M. Organic bioelectronics: bridging the signaling gap between biology and technology. Chem Rev. 2016;116:13009–41. doi: 10.1021/acs.chemrev.6b00146. [DOI] [PubMed] [Google Scholar]
- 12. Rivnay J, Owens RM, Malliaras GG. The rise of organic bioelectronics. Chem Mater. 2013;26:679–85. [Google Scholar]
- 13. Luo S-C. Conducting polymers as biointerfaces and biomaterials: a perspective for a special issue of polymer reviews. Polym Rev. 2013;53:303–10. [Google Scholar]
- 14. Guo J, Fang G, Wang S, Wang J. Quartz crystal microbalance sensor based on 11-mercaptoundecanoic acid self-assembly and amidated nano-titanium film for selective and ultrafast detection of phosphoproteins in food. Food Chem. 2021;344:128656. doi: 10.1016/j.foodchem.2020.128656. [DOI] [PubMed] [Google Scholar]
- 15. Hsiao YS, Luo SC, Hou S, Zhu B, Sekine J, Kuo CW, et al. 3D bioelectronic interface: capturing circulating tumor cells onto conducting polymer-based micro/nanorod arrays with chemical and topographical control. Small. 2014;10:3012–7. doi: 10.1002/smll.201400429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inal S, Rivnay J, Suiu AO, Malliaras GG, McCulloch I. Conjugated polymers in bioelectronics. Acc Chem Res. 2018;51:1368–76. doi: 10.1021/acs.accounts.7b00624. [DOI] [PubMed] [Google Scholar]
- 17. Tao X, Peng Y, Liu J. Nanomaterial-based fluorescent biosensor for veterinary drug detection in foods. J Food Drug Anal. 2020;28:576–95. doi: 10.38212/2224-6614.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu JG, Lee CY, Wu SS, Luo SC. Ionic liquid-assisted electropolymerization for lithographical perfluorocarbon deposition and hydrophobic patterning. ACS Appl Mater Interfaces. 2016;8:22688–95. doi: 10.1021/acsami.6b07578. [DOI] [PubMed] [Google Scholar]
- 19. Luo SC, Yu HH, Wan AC, Han Y, Ying JY. A general synthesis for PEDOT-coated nonconductive materials and PEDOT hollow particles by aqueous chemical polymerization. Small. 2008;4:2051–8. doi: 10.1002/smll.200800033. [DOI] [PubMed] [Google Scholar]
- 20. Luo SC, Xie H, Chen NY, Yu HH. Trinity DNA detection platform by ultrasmooth and functionalized PEDOT biointerfaces. ACS Appl Mater Interfaces. 2009;1:1414–9. doi: 10.1021/am900117e. [DOI] [PubMed] [Google Scholar]
- 21. Rashid JIA, Yusof NA. The strategies of DNA immobilization and hybridization detection mechanism in the construction of electrochemical DNA sensor: a review. Sens Bio-Sens Res. 2017;16:19–31. [Google Scholar]
- 22. Peng H, Zhang L, Soeller C, Travas-Sejdic J. Conducting polymers for electrochemical DNA sensing. Biomaterials. 2009;30:2132–48. doi: 10.1016/j.biomaterials.2008.12.065. [DOI] [PubMed] [Google Scholar]
- 23. Arya SK, Zhurauski P, Jolly P, Batistuti MR, Mulato M, Estrela P. Capacitive aptasensor based on interdigitated electrode for breast cancer detection in undiluted human serum. Biosens Bioelectron. 2018;102:106–12. doi: 10.1016/j.bios.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 24. Bizid S, Blili S, Mlika R, Said AH, Korri-Youssoufi H. Direct Electrochemical DNA Sensor based on a new redox oligomer modified with ferrocene and carboxylic acid: application to the detection of Mycobacterium tuberculosis mutant strain. Anal Chim Acta. 2017;994:10–8. doi: 10.1016/j.aca.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 25. Hui N, Sun XT, Niu SY, Luo XL. PEGylated polyaniline nanofibers: antifouling and conducting biomaterial for electrochemical DNA sensing. ACS Appl Mater Interfaces. 2017;9:2914–23. doi: 10.1021/acsami.6b11682. [DOI] [PubMed] [Google Scholar]
- 26. Chin M, Tada SI, Tsai MH, Ito YH, Luo SC. Strategy to immobilize peptide probe selected through in vitro ribosome display for electrochemical aptasensor application. Anal Chem. 2020;92:11260–7. doi: 10.1021/acs.analchem.0c01891. [DOI] [PubMed] [Google Scholar]
- 27. Ahmed EM. Hydrogel: preparation, characterization, and applications: a review. J Adv Res. 2015;6:105–21. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feig VR, Tran H, Lee M, Bao Z. Mechanically tunable conductive interpenetrating network hydrogels that mimic the elastic moduli of biological tissue. Nat Commun. 2018;9:2740. doi: 10.1038/s41467-018-05222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tavakoli J, Tang Y. Hydrogel based sensors for biomedical applications: an updated review. Polymers. 2017:9. doi: 10.3390/polym9080364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qu F, Zhang Y, Rasooly A, Yang M. Electrochemical biosensing platform using hydrogel prepared from ferrocene modified amino acid as highly efficient immobilization matrix. Anal Chem. 2014;86:973–6. doi: 10.1021/ac403478z. [DOI] [PubMed] [Google Scholar]
- 31. Lian M, Chen X, Lu Y, Yang W. Self-assembled peptide hydrogel as a smart bio-interface for enzyme-based electrochemical biosensing and cell monitoring. ACS Appl Mater Interfaces. 2016;8:25036–42. doi: 10.1021/acsami.6b05409. [DOI] [PubMed] [Google Scholar]
- 32. Yang ZH, Zhuo Y, Yuan R, Chai YQ. Amplified impedimetric aptasensor combining target-induced DNA hydrogel formation with pH-stimulated signal amplification for the heparanase assay. Nanoscale. 2017;9:2556–62. doi: 10.1039/c6nr08353f. [DOI] [PubMed] [Google Scholar]
- 33. Goh KB, Li H, Lam KY. Development of a multiphysics model to characterize the responsive behavior of urea-sensitive hydrogel as biosensor. Biosens Bioelectron. 2017;91:673–9. doi: 10.1016/j.bios.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 34. Wei Y, Zeng Q, Hu Q, Wang M, Tao J, Wang L. Self-cleaned electrochemical protein imprinting biosensor basing on a thermo-responsive memory hydrogel. Biosens Bioelectron. 2018;99:136–41. doi: 10.1016/j.bios.2017.07.049. [DOI] [PubMed] [Google Scholar]
- 35. Kim DH, Wiler JA, Anderson DJ, Kipke DR, Martin DC. Conducting polymers on hydrogel-coated neural electrode provide sensitive neural recordings in auditory cortex. Acta Biomater. 2010;6:57–62. doi: 10.1016/j.actbio.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 36. Wei Y, Zeng Q, Wang M, Huang J, Guo X, Wang L. Near-infrared light-responsive electrochemical protein imprinting biosensor based on a shape memory conducting hydrogel. Biosens Bioelectron. 2019;131:156–62. doi: 10.1016/j.bios.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 37. Tomczykowa M, Plonska-Brzezinska ME. Conducting polymers, hydrogels and their composites: preparation, properties and bioapplications. Polymers. 2019:11. doi: 10.3390/polym11020350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang L, Wang H, Lu H, Hui N. Phytic acid functionalized antifouling conducting polymer hydrogel for electrochemical detection of microRNA. Anal Chim Acta. 2020;1124:104–12. doi: 10.1016/j.aca.2020.05.025. [DOI] [PubMed] [Google Scholar]
- 39. Wang M, Cui M, Liu W, Liu X. Highly dispersed conductive polypyrrole hydrogels as sensitive sensor for simultaneous determination of ascorbic acid, dopamine and uric acid. J Electroanal Chem. 2019;832:174–81. [Google Scholar]
- 40. Xu LH, Li JJ, Zeng HB, Zhang XJ, Cosnier S, Marks RS, et al. ATMP-induced three-dimensional conductive polymer hydrogel scaffold for a novel enhanced solid-state electrochemiluminescence biosensor. Biosens Bioelectron. 2019;143:111601. doi: 10.1016/j.bios.2019.111601. [DOI] [PubMed] [Google Scholar]
- 41. Guo B, Ma Z, Pan L, Shi Y. Properties of conductive polymer hydrogels and their application in sensors. J Polym Sci, Part B: Polym Phys. 2019;57:1606–21. [Google Scholar]
- 42. Sarkar D, Liu W, Xie XJ, Anselmo AC, Mitragotri S, Banerjee K. MoS2 field-effect transistor for next-generation label-free biosensors. ACS Nano. 2014;8:3992–4003. doi: 10.1021/nn5009148. [DOI] [PubMed] [Google Scholar]
- 43. Lowe BM, Sun K, Zeimpekis I, Skylaris CK, Green NG. Field-effect sensors - from pH sensing to biosensing: sensitivity enhancement using streptavidin-biotin as a model system. Analyst. 2017;142:4173–200. doi: 10.1039/c7an00455a. [DOI] [PubMed] [Google Scholar]
- 44. Dai X, Vo R, Hsu HH, Deng P, Zhang Y, Jiang X. Modularized field-effect transistor biosensors. Nano Lett. 2019;19:6658–64. doi: 10.1021/acs.nanolett.9b02939. [DOI] [PubMed] [Google Scholar]
- 45. Cai BJ, Wang ST, Huang L, Ning Y, Zhang ZY, Zhang GJ. Ultrasensitive label-free detection of PNA-DNA hybridization by reduced graphene oxide field-effect transistor biosensor. ACS Nano. 2014;8:2632–8. doi: 10.1021/nn4063424. [DOI] [PubMed] [Google Scholar]
- 46. Wang S, Hossain MZ, Shinozuka K, Shimizu N, Kitada S, Suzuki T, et al. Graphene field-effect transistor biosensor for detection of biotin with ultrahigh sensitivity and specificity. Biosens Bioelectron. 2020;165:112363. doi: 10.1016/j.bios.2020.112363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xie H, Luo SC, Yu HH. Electric-field-assisted growth of functionalized poly(3,4-ethylenedioxythiophene) nanowires for label-free protein detection. Small. 2009;5:2611–7. doi: 10.1002/smll.200900312. [DOI] [PubMed] [Google Scholar]
- 48. Park SJ, Lee SH, Yang H, Park CS, Lee CS, Kwon OS, et al. Human dopamine receptor-conjugated multidimensional conducting polymer nanofiber membrane for dopamine detection. ACS Appl Mater Interfaces. 2016;8:28897–903. doi: 10.1021/acsami.6b10437. [DOI] [PubMed] [Google Scholar]
- 49. Zhang Y, Clausmeyer J, Babakinejad B, López Córdoba A, Ali T, Shevchuk A, et al. Spearhead nanometric field-effect transistor sensors for single-cell analysis. ACS Nano. 2016;10:3214–21. doi: 10.1021/acsnano.5b05211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. BelBruno JJ. Molecularly imprinted polymers. Chem Rev. 2019;119:94–119. doi: 10.1021/acs.chemrev.8b00171. [DOI] [PubMed] [Google Scholar]
- 51. Luo S-C, Thomas JL, Guo H-Z, Liao W-T, Lee M-H, Lin H-Y. Electrosynthesis of nanostructured, imprinted poly(-hydroxymethyl 3,4-ethylenedioxythiophene) for the ultrasensitive electrochemical detection of urinary progesterone. ChemistrySelect. 2017;2:7935–9. [Google Scholar]
- 52. Lee M-H, Liu KT, Thomas JL, Su ZL, O’Hare D, van Wuellen T, et al. Peptide-imprinted poly(hydroxymethyl 3,4-ethylenedioxythiophene) nanotubes for detection of α synuclein in human brain organoids. ACS Appl Nano Mater. 2020;3:8027–36. [Google Scholar]
- 53. Jiang XY, Wang HJ, Yuan R, Chai YQ. Functional three-dimensional porous conductive polymer hydrogels for sensitive electro-chemiluminescence in situ detection of H2O2 released from live cells. Anal Chem. 2018;90:8462–9. doi: 10.1021/acs.analchem.8b01168. [DOI] [PubMed] [Google Scholar]
- 54. Tsai MH, Lin YK, Luo SC. Electrochemical SERS for in situ monitoring the redox states of PEDOT and its potential application in oxidant detection. ACS Appl Mater Interfaces. 2019;11:1402–10. doi: 10.1021/acsami.8b16989. [DOI] [PubMed] [Google Scholar]
- 55. Aydogdu G, Gunendi G, Zeybek DK, Zeybek B, Pekyardimci S. A novel electrochemical DNA biosensor based on poly-(5-amino-2-mercapto-1,3,4-thiadiazole) modified glassy carbon electrode for the determination of nitrofurantoin. Sensor Actuator B Chem. 2014;197:211–9. [Google Scholar]
- 56. Hassanein A, Salahuddin N, Matsuda A, Kawamura G, Elfiky M. Fabrication of biosensor based on Chitosan-ZnO/ Polypyrrole nanocomposite modified carbon paste electrode for electroanalytical application. Mater Sci Eng C-Mater Biol Appl. 2017;80:494–501. doi: 10.1016/j.msec.2017.04.101. [DOI] [PubMed] [Google Scholar]
- 57.Bard AJ, Faulkner LR. Electrochemical methods: fundamentals and applications. Wiley; 2000. [Google Scholar]
- 58. Radhapyari K, Kotoky P, Khan R. Detection of anticancer drug tamoxifen using biosensor based on polyaniline probe modified with horseradish peroxidase. Mater Sci Eng C-Mater Biol Appl. 2013;33:583–7. doi: 10.1016/j.msec.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 59. Kulikova TN, Porfireva AV, Shamagsumova RV, Evtugyn GA. Voltammetric sensor with replaceable polyaniline-DNA layer for doxorubicin determination. Electro-analysis. 2018;30:2284–92. [Google Scholar]
- 60. Mao Y, Guo L, Ning X, Li J, Zheng J. The signal amplification in electrochemical detection of chloramphenicol using sulfonated polyaniline-chitosan composite as redox capacitor. Electroanalysis. 2018;30:2085–93. [Google Scholar]
- 61. Chauhan N, Chawla S, Pundir CS, Jain U. An electrochemical sensor for detection of neurotransmitter-acetylcholine using metal nanoparticles, 2D material and conducting polymer modified electrode. Biosens Bioelectron. 2017;89:377–83. doi: 10.1016/j.bios.2016.06.047. [DOI] [PubMed] [Google Scholar]
- 62. Ates M. A review study of (bio)sensor systems based on conducting polymers. Mater Sci Eng C. 2013;33:1853–9. doi: 10.1016/j.msec.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 63. Noh HB, Revin SB, Shim YB. Voltammetric analysis of anti-arthritis drug, ascorbic acid, tyrosine, and uric acid using a graphene decorated-functionalized conductive polymer electrode. Electrochim Acta. 2014;139:315–22. [Google Scholar]
- 64. Salih FE, Oularbi L, Halim E, Elbasri M, Ouarzane A, El Rhazi M. Conducting polymer/ionic liquid composite modified carbon paste electrode for the determination of carbaryl in real samples. Electroanalysis. 2018;30:1855–64. [Google Scholar]
- 65. Chen CH, Luo SC. Tuning surface charge and morphology for the efficient detection of dopamine under the interferences of uric acid, ascorbic acid, and protein adsorption. ACS Appl Mater Interfaces. 2015;7:21931–8. doi: 10.1021/acsami.5b06526. [DOI] [PubMed] [Google Scholar]
- 66. Pan L, Yu G, Zhai D, Lee HR, Zhao W, Liu N, et al. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc Natl Acad Sci U S A. 2012;109:9287–92. doi: 10.1073/pnas.1202636109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ma Z, Chen P, Cheng W, Yan K, Pan L, Shi Y, et al. Highly sensitive, printable nanostructured conductive polymer wireless sensor for food spoilage detection. Nano Lett. 2018;18:4570–5. doi: 10.1021/acs.nanolett.8b01825. [DOI] [PubMed] [Google Scholar]
- 68. Eugenii Katz, Willner I. Probing biomolecular interactions at conductive and semiconductive surfaces by impedance spectroscopy: routes to impedimetric immunosensors, DNA-sensors, and enzyme biosensors. Electroanalysis. 2003;15:913–47. [Google Scholar]
- 69. Qi H, Wang C, Cheng N. Label-free electrochemical impedance spectroscopy biosensor for the determination of human immunoglobulin G. Microchimica Acta. 2010;170:33–8. [Google Scholar]
- 70. Ohno R, Ohnuki H, Wang H, Yokoyama T, Endo H, Tsuya D, et al. Electrochemical impedance spectroscopy biosensor with interdigitated electrode for detection of human immunoglobulin A. Biosens Bioelectron. 2013;40:422–6. doi: 10.1016/j.bios.2012.07.052. [DOI] [PubMed] [Google Scholar]
- 71. Karimi-Maleh H, Tahernejad-Javazmi F, Atar N, Yola ML, Gupta VK, Ensafi AA. A novel DNA biosensor based on a pencil graphite electrode modified with polypyrrole/functionalized multiwalled carbon nanotubes for determination of 6-mercaptopurine anticancer drug. Ind Eng Chem Res. 2015;54:3634–9. [Google Scholar]
- 72. Zeng Q, Wei C, Wu Y, Li K, Ding S, Yuan J, et al. Approach to distribution and accumulation of dibutyl phthalate in rats by immunoassay. Food Chem Toxicol. 2013;56:18–27. doi: 10.1016/j.fct.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 73. Bolat G, Yaman YT, Abaci S. Molecularly imprinted electrochemical impedance sensor for sensitive dibutyl phthalate (DBP) determination. Sensor Actuator B Chem. 2019:299. [Google Scholar]
- 74. Sheikhzadeh E, Chamsaz M, Turner APF, Jager EWH, Beni V. Label-free impedimetric biosensor for Salmonella Typhimurium detection based on poly [pyrrole-co-3-carboxyl-pyrrole] copolymer supported aptamer. Biosens Bioelectron. 2016;80:194–200. doi: 10.1016/j.bios.2016.01.057. [DOI] [PubMed] [Google Scholar]
- 75. Wang R, Li Y. Hydrogel based QCM aptasensor for detection of avian influenza virus. Biosens Bioelectron. 2013;42:148–55. doi: 10.1016/j.bios.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 76. Huang P-C, Shen M-Y, Yu H-h, Wei S-C, Luo S-C. Surface engineering of phenylboronic acid-functionalized poly(3,4-ethylenedioxythiophene) for fast responsive and sensitive glucose monitoring. ACS Appl Bio Mater. 2018;1:160–7. [Google Scholar]
- 77. Wu JG, Wei SC, Chen Y, Chen JH, Luo SC. Critical study of the recognition between C-reactive protein and surface-immobilized phosphorylcholine by quartz crystal microbalance with dissipation. Langmuir. 2018;34:943–51. doi: 10.1021/acs.langmuir.7b02724. [DOI] [PubMed] [Google Scholar]
- 78. Sauerbrey G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z Phys. 1959;155:206–22. [Google Scholar]
- 79. Buttry DA, Ward MD. Measurement of interfacial processes at electrode surfaces with the electrochemical quartz crystal microbalance. Chem Rev. 1992;92:1355–79. [Google Scholar]
- 80. Wu JG, Wei SC, Luo SC. In situ probing unusual protein adsorption behavior on electrified zwitterionic conducting polymers. Adv Mater Inter. 2020:7. [Google Scholar]
- 81. Chen Y, Luo SC. Synergistic effects of ions and surface potentials on antifouling poly(3,4-ethylenedioxythiophene): comparison of oligo(ethylene glycol) and phosphorylcholine. Langmuir. 2019;35:1199–210. doi: 10.1021/acs.langmuir.8b02122. [DOI] [PubMed] [Google Scholar]
- 82. Prabakaran K, Jandas PJ, Luo J, Fu C, Wei Q. Molecularly imprinted poly(methacrylic acid) based QCM biosensor for selective determination of L-tryptophan. Colloid Surface Physicochem Eng Aspect. 2021:611. [Google Scholar]
- 83. Truong TN, Tran DL, Vu TH, Tran VH, Duong TQ, Dinh QK, et al. Multi-wall carbon nanotubes (MWCNTs)-doped polypyrrole DNA biosensor for label-free detection of genetically modified organisms by QCM and EIS. Talanta. 2010;80:1164–9. doi: 10.1016/j.talanta.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 84. Salam F, Uludag Y, Tothill IE. Real-time and sensitive detection of Salmonella Typhimurium using an automated quartz crystal microbalance (QCM) instrument with nanoparticles amplification. Talanta. 2013;115:761–7. doi: 10.1016/j.talanta.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 85. Zare EN, Makvandi P, Ashtari B, Rossi F, Motahari A, Perale G. Progress in conductive polyaniline-based nanocomposites for biomedical applications: a review. J Med Chem. 2020;63:1–22. doi: 10.1021/acs.jmedchem.9b00803. [DOI] [PubMed] [Google Scholar]
- 86. Kucherenko IS, Soldatkin OO, Kucherenko DY, Soldatkina OV, Dzyadevych SV. Advances in nanomaterial application in enzyme-based electrochemical biosensors: a review. Nanoscale Adv. 2019;1:4560–77. doi: 10.1039/c9na00491b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Prajapati DG, Kandasubramanian B. Progress in the development of intrinsically conducting polymer composites as biosensors. Macromol Chem Phys. 2019;220:1800561. doi: 10.1002/macp.201800561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wongkaew N, Simsek M, Griesche C, Baeumner AJ. Functional nanomaterials and nanostructures enhancing electrochemical biosensors and lab-on-a-chip performances: recent progress, applications, and future perspective. Chem Rev. 2019;119:120–94. doi: 10.1021/acs.chemrev.8b00172. [DOI] [PubMed] [Google Scholar]
- 89. Yang Y, Asiri AM, Du D, Lin Y. Acetylcholinesterase biosensor based on a gold nanoparticle-polypyrrole-reduced graphene oxide nanocomposite modified electrode for the amperometric detection of organophosphorus pesticides. Analyst. 2014;139:3055–60. doi: 10.1039/c4an00068d. [DOI] [PubMed] [Google Scholar]
- 90. Chung S, Chandra P, Koo JP, Shim YB. Development of a bifunctional nanobiosensor for screening and detection of chemokine ligand in colorectal cancer cell line. Biosens Bioelectron. 2018;100:396–403. doi: 10.1016/j.bios.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 91. Akbulut H, Bozokalfa G, Asker DN, Demir B, Guler E, Odaci Demirkol D, et al. Polythiophene-g-poly(ethylene glycol) with lateral amino groups as a novel matrix for biosensor construction. ACS Appl Mater Interfaces. 2015;7:20612–22. doi: 10.1021/acsami.5b04967. [DOI] [PubMed] [Google Scholar]
- 92. Ma F, Rehman A, Liu H, Zhang J, Zhu S, Zeng X. Glycosylation of quinone-fused polythiophene for reagentless and label-free detection of E. coli. Anal Chem. 2015;87:1560–8. doi: 10.1021/ac502712q. [DOI] [PubMed] [Google Scholar]
- 93. Regasa MB, Refera Soreta T, Femi OE, PCR Development of molecularly imprinted conducting polymer composite film-based electrochemical sensor for melamine detection in infant formula. ACS Omega. 2020;5:4090–9. doi: 10.1021/acsomega.9b03747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Singh AK, Singh M, Verma N. Electrochemical preparation of Fe3O4/MWCNT-polyaniline nanocomposite film for development of urea biosensor and its application in milk sample. J Food Meas Char. 2019;14:163–75. [Google Scholar]
- 95. Ruecha N, Rangkupan R, Rodthongkum N, Chailapakul O. Novel paper-based cholesterol biosensor using graphene/ polyvinylpyrrolidone/polyaniline nanocomposite. Biosens Bioelectron. 2014;52:13–9. doi: 10.1016/j.bios.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 96. Yu Z, Li H, Zhang X, Liu N, Tan W, Zhang X, et al. Facile synthesis of NiCo2O4@Polyaniline core-shell nanocomposite for sensitive determination of glucose. Biosens Bioelectron. 2016;75:161–5. doi: 10.1016/j.bios.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 97. Erkmen C, Kurbanoglu S, Uslu B. Fabrication of poly(3,4-ethylenedioxythiophene)-iridium oxide nanocomposite based Tyrosinase biosensor for the dual detection of catechol and azinphos methyl. Sensor Actuator B Chem. 2020:316. [Google Scholar]
- 98. Khan MZH, Ahommed MS, Daizy M. Detection of xanthine in food samples with an electrochemical biosensor based on PEDOT:PSS and functionalized gold nanoparticles. RSC Adv. 2020;10:36147–54. doi: 10.1039/d0ra06806c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bizid S, Mlika R, Haj Said A, Chemli M, Korri Youssoufi H. Investigations of poly(p-phenylene) modified with ferrocene and their application in electrochemical DNA sensing. Sensor Actuator B Chem. 2016;226:370–80. [Google Scholar]
- 100. Azad T, Ahmed S. Common milk adulteration and their detection techniques. Int J Flow Contr. 2016:3. [Google Scholar]
- 101. Albaaj A, Foucras G, Raboisson D. Changes in milk urea around insemination are negatively associated with conception success in dairy cows. J Dairy Sci. 2017;100:3257–65. doi: 10.3168/jds.2016-12080. [DOI] [PubMed] [Google Scholar]
- 102. Peng Y-H, Wu T, Lin Y-W. Detection of melamine based on the suppressed anodic response of uric acid by a Au-Ag nanoparticles modified glassy carbon electrode. J Food Drug Anal. 2020:28. doi: 10.38212/2224-6614.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Das J, Sarkar P. Enzymatic electrochemical biosensor for urea with a polyaniline grafted conducting hydrogel composite modified electrode. RSC Adv. 2016;6:92520–33. [Google Scholar]
- 104. Ge L, Li SP, Lisak G. Advanced sensing technologies of phenolic compounds for pharmaceutical and biomedical analysis. J Pharmaceut Biomed Anal. 2020;179:112913. doi: 10.1016/j.jpba.2019.112913. [DOI] [PubMed] [Google Scholar]
- 105. Chichirau A, Flueraru M, Chepelev LL, Wright JS, Willmore WG, Durst T, et al. Mechanism of cytotoxicity of catechols and a naphthalenediol in PC12-AC cells: the connection between extracellular autoxidation and molecular electronic structure. Free Radic Biol Med. 2005;38:344–55. doi: 10.1016/j.freeradbiomed.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 106. Russo MJ, Han M, Desroches PE, Manasa CS, Dennaoui J, Quigley AF, et al. Antifouling strategies for electrochemical biosensing: mechanisms and performance toward point of care based diagnostic applications. ACS Sens. 2021;6:1482–507. doi: 10.1021/acssensors.1c00390. [DOI] [PubMed] [Google Scholar]
- 107. Wu JG, Chen JH, Liu KT, Luo SC. Engineering antifouling conducting polymers for modern biomedical applications. ACS Appl Mater Interfaces. 2019;11:21294–307. doi: 10.1021/acsami.9b04924. [DOI] [PubMed] [Google Scholar]
- 108. Robinson AM, Harroun SG, Bergman J, Brosseau CL. Portable electrochemical surface-enhanced Raman spectroscopy system for routine spectroelectrochemical analysis. Anal Chem. 2012;84:1760–4. doi: 10.1021/ac2030078. [DOI] [PubMed] [Google Scholar]
- 109. Lin YK, Tai RJ, Wei SC, Luo SC. Electrochemical SERS on 2D mapping for metabolites detection. Langmuir. 2020;36:5990–6. doi: 10.1021/acs.langmuir.0c00863. [DOI] [PubMed] [Google Scholar]
- 110. Chen CA, Yuan H, Chen CW, Chien YS, Sheng WH, Chen CF. An electricity- and instrument-free infectious disease sensor based on a 3D origami paper-based analytical device. Lab Chip. 2021;21:1908–15. doi: 10.1039/d1lc00079a. [DOI] [PubMed] [Google Scholar]