Abstract
Quetiapine is an atypical antipsychotic drug that can be used to treat mental disorders, including schizophrenia, bipolar disorder and Alzheimer’s disease. Quetiapine is mainly converted into the active metabolites of norquetiapine and 7-hydroxyquetiapine by the liver enzymes CYP3A4 and CYP2D6. In this study, liquid-liquid extraction (LLE) was used as a sample pretreatment method to eliminate interferences in plasma. tert-Butyl methyl ether was chosen as the extraction solvent. Field-enhanced sample injection (FESS), an online preconcentration technique, was used to analyze quetiapine and its metabolites norquetiapine and 7-hydroxyquetiapine in plasma. The optimal separation condition was 120 mM phosphate (pH 4.0) containing 0.005% (w/v) polyvinyl pyrrolidone and 40% (v/v) methanol. The methanol plug was 0.3 psi for 6 s, the sample was electrokinetic injection by 10 kV for 60 s at positive polarity, and the separation voltage was set at 26 kV. In this experiment, quetiapine, norquetiapine and 7-hydroxyquetiapine were successfully extracted from plasma by the LLE method and stacking and separated by FESS within 15 min. The limits of detection (S/N = 3) of quetiapine, norquetiapine and 7-hydroxyquetiapine were 0.25 ng/mL, 0.50 ng/mL and 1.00 ng/mL, respectively. The linear ranges of quetiapine, norquetiapine and 7-hydroxyquetiapine were 3–120 ng/mL and the correlation coefficients were 0.999. Compared with that of the traditional capillary zone electrophoresis method, the sensitivity enrichment of analytes was 463-835-fold. The optimal experimental conditions were successfully applied to the analysis of plasma samples from patients taking quetiapine for the treatment of schizophrenia.
Keywords: Field-enhanced sample stacking, Liquid-liquid extraction, Metabolites, Plasma, Quetiapine
1. Introduction
Quetiapine (QTP), 2-[2-(4-dibenzo [b, f] [1, 4] thiazepin-11-yl-1-piperazinyl) ethoxy] ethanol, is a dibenzothiazepine derivative and a second-generation antipsychotic agent that is used to treat schizophrenia [1]. The mechanism of QTP is still unknown. However, preclinical research has shown that QTP exhibits antagonistic action on several receptors, such as serotonin 5-HT1A and 5- HT2, dopamine D1 and D2, histamine H1 and adrenergic α1 and α2 receptors [2]. QTP is metabolized by the liver, including hydroxylation, oxidation, dealkylation and sulfoxidation and produces more than 20 metabolites. The main metabolic pathways of QTP are shown in Fig. S1. QTP is metabolized to norquetiapine (NorQTP), quetiapine sulfoxide and 7-hydroxyquetiapine (7-QTP) via isoenzymes CYP3A4 and CYP2D6. NorQTP and 7-QTP are active metabolites [3,4]. NorQTP has moderate antagonism with 5-HT2 and dopamine D2 receptors [5]. Moreover, NorQTP can effectively inhibit norepinephrine transport in combination with norepinephrine. Insufficient concentrations of norepinephrine in the synaptic cleft may lead to depression. Therefore, blocking norepinephrine transport to prevent reuptake of norepinephrine can increase the concentration of norepinephrine in the synaptic cleft, which is the main mechanism to treat depression in bipolar disorder [6]. The metabolic half-life (t1/2) of QTP is 6 h, NorQTP is approximately 9–12 h, and for 7-QTP, it is has a very similar to QTP [7,8].
In a previous study, Mandrioli and Fanali et al. combined high-performance liquid chromatography (HPLC) with a UV detector to determine QTP in human plasma, and the LOD for this technique was 1.5 ng/mL [9]. In addition, Pucci and Mandrioli et al. detected QTP in pharmaceuticals by capillary electrophoresis (CE) with a UV detector. QTP was observed within 2 min in this capillary zone electrophoretic (CZE) mode, and the LOD for this technique was 50 ng/mL [10]. Compared with other analytical methods, the advantages of CE methods include few samples and solvent consumption, high separation efficacy and low cost of operating the instrument. However, unlike common HPLC measurements, low detection sensitivity was a disadvantage of CE. Therefore, capillary electrophoresis was combined with an online preconcentration technique for improving the detection sensitivity to estimate the concentration of QTP and its metabolites, NorQTP and 7-QTP, in human plasma.
In this study, an online preconcentration technique, field-enhanced sample stacking (FESS), was chosen to detect QTP and its metabolites in plasma to improve the detection sensitivity. The pKa values of QTP and its metabolites, NorQTP and 7-QTP, were 7.06, 8.83 and 7.06, respectively [11]. Hence, the analytes are positively charged in acidic environments. The sample stayed in a low conductivity solution and the background solution (BGS) was a high conductivity environment. When a high voltage was applied, the analytes move to cathode and stacked at the boundary of the sample solution and BGS by FESS [12,13]. Then, the analytes were separated by capillary zone electrophoresis.
2. Materials and methods
2.1. Chemicals and reagents
All chemicals and reagents were analytical grade. Quetiapine fumarate (QTP), norquetiapine hydrochloride (NorQTP) and 7-hydroxyquetiapine (7-QTP) were purchased from Sigma-Aldrich (TX, USA). Polyvinyl pyrrolidone (PVP) (Mw 1,300,000) and 4-aminopyridine (as internal standards) purchased from Sigma-Aldrich (St. Louis, MO, USA). Methanol was purchased from Macron Fine Chemicals (Center Valley, PA, USA). Ammonium hydroxide (30%) was purchased from J. T. Baker (Phillipsburg, NJ, USA). tert-Butyl methyl ether was purchased from Alfa Aesar (Ward Hill, MA, USA). Disodium hydrogen phosphate dehydrate (Na2H-PO4 · 2H2O), hydrochloric acid and sodium hydroxide were purchased from Merck (Darmstadt, Germany). The buffer and related aqueous solutions were prepared with deionized distilled water that was obtained with a Milli-Q System from Millipore (Bedford, MA, USA). The solutions were filtered through a 0.45 μm nylon filter from Jet Bio-Filtration (Guangzhou, China) prior to use. The QTP, NorQTP and 7-QTP were prepared in methanol at a concentration of 1 mg mL−1 and stored at −20 °C.
2.2. CE conditions
In this study, the CE apparatus was a P/ACE™ MDQ series capillary electrophoresis system equipped with a photodiode array (PDA) detector (Beckman Coulter Inc., Fullerton, CA, USA). The apparatus operation, electropherogram acquisition, and data organization were controlled using the Beckman Coulter 32-Karat software system (Fullerton). Uncoated fused silica capillaries (50 μm I.D.) were obtained from Polymicro Technologies (Phoenix, AZ, USA); the total capillary length was 50 cm, and the distance to the detector was 40 cm in the CE procedure. After each experimental run, the capillary was rinsed with hydrochloric acid (3 min) and deionized distilled water (3 min) at 30 psi. The BGS was 120 mM phosphate (pH 4.0) containing 0.005% (w/v) PVP and 40% (v/v) methanol. The methanol plug was 0.3 psi for 6 s, and it was hydrodynamically injected before the sample injection. The sample was injected electrokinetically at a positive voltage of 10 kV for 60 s, and a separation voltage of 26 kV was applied. The UV detector was set at 214 nm, and the temperature of separation was kept at 20 °C.
2.3. Sample preparation
Plasma samples were collected from patients taking QTP for the treatment of schizophrenia and were stored at −20 °C. (This study was approved by the Institutional Review Board (KMUHIRB-F(I)-20190014) at Kaohsiung Medical University, Taiwan). Liquid-liquid extraction was performed to eliminate interference present in the plasma. A 500 μL aliquot of each plasma sample was spiked with 70 μL 1 M ammonium hydroxide solution in a 2-mL microcentrifuge tube and extracted with 1000 μL tert-butyl methyl ether by vortexing for 3 min. After centrifugation at 12000 rpm for 10 min, 850 μL of the organic supernatant was separated and evaporated to dryness via a centrifugal vaporizer (EYELA UT-1000, Japan). The dried extracts were reconstituted with 100 μL 20 ng mL−1 internal standard and filtered through a nylon filter prior to CE analysis.
2.4. Nano-UPLC-MS/MS analysis and conditions
A nanoACQUITY UPLC system (Waters, MA, USA) was coupled with an LTQ orbitrap discovery hybrid Fourier transform mass spectrometer (Thermo Fisher Scientific, Inc. Bremen, Germany) equipped with a nanoelectrospray positive ionization (nano ESI) interface. The desalting column was equipped with a symmetric μm, 180 μm × 20 mm), and the analytical column was equipped with a BEH C18 column (1.7 μm, 75 μm × 150 mm). The mobile phase included 0.1% formic acid as solvent A and acetonitrile containing 0.1% formic acid as solvent B. The sample loading volume was 2 μL. The initial gradient elution conditions were solvent B at 1%, then conditions were adjusted to 100% after 5 min and maintained in isocratic mode for 45 min. Finally, solvent B was adjusted to 1% after 60 min. The flow rate was set at 300 nL min−1. Each plasma sample was pretreated and then analyzed with nano-UPLC-MS in positive ionization mode with MRM mode to identify the QTP at 384.17 (m/z), NorQTP at 296.12 (m/z) and 7-QTP at 400.16 (m/z).
3. Results and discussions
3.1. Sample pretreatment
In this study, the samples were pretreated with a liquid-liquid extraction (LLE) to concentrate and purify three targets, QTP, NorQTP and 7-QTP, and eliminate interferences in plasma. To obtain the optimal extraction conditions, several parameters were evaluated, including plasma pH values, extraction solvents and extraction solvent volumes.
3.1.1. Plasma pH
The pKa values of QTP and its metabolites, NorQTP and 7-QTP, were 7.06, 8.83 and 7.06, respectively. When the pH value of plasma is more than 10.83, QTP and its metabolites are present primarily in their uncharged forms, which means that it is easier to extract them in the organic layer. Four pH values of plasma were selected, pH 8.0, pH 9.0, pH 10.0, and pH 11.0, to evaluate the extraction efficiency. The three analytes can be successfully extracted under these four pH conditions. Their relative response was determined by comparing the ratio of the maximum peak areas of the analytes with the peak area of the internal standard, which was defined as 100%. As the pH values of plasma increased, the peak areas of QTP and NorQTP increased slightly. Fig. S2 shows that the relative response of 7-QTP at pH 11.0 was lower than that at pH 10.0. When the pH value of plasma is more than 10.48, the phenol group of 7-QTP (pKa value is 8.48) completely dissociates into a negatively charged form, which leads to low extraction efficiency. Hence, the optimal pH value of plasma was 10.0.
3.1.2. Extraction solvents
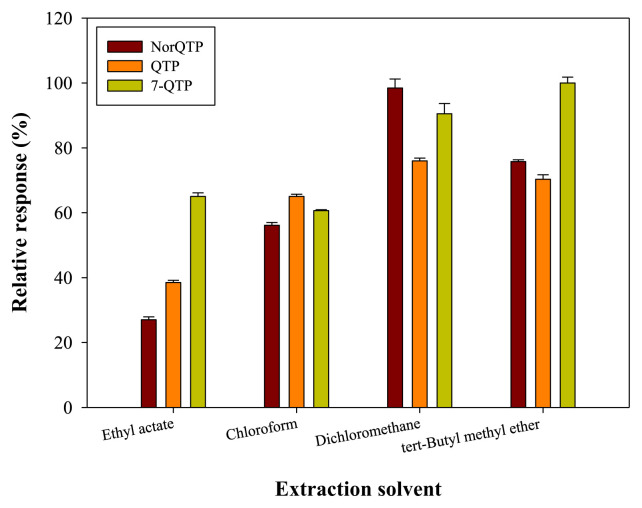
The log P values of QTP, NorQTP and 7-QTP were 2.81, 3.16 and 2.33, respectively, [14]. Therefore, medium- and low-polarity organic solvents were chosen for evaluating the extraction effect. In this study, four organic solvents with different polarities, ethyl acetate, chloroform, dichloromethane and tertbutyl methyl ether, were tested.
The log P values of the four extraction solvents are: chloroform (1.97) > dichloromethane (1.25) > tert-butyl methyl ether (0.94) > ethyl acetate (0.73) [14]. According to the electro-pherograms, all four solvents successfully extracted the three analytes. The extraction efficiency results shown in Fig. 1. Dichloromethane and tert-butyl methyl ether had a better extraction effect than the other two solvents. However, the densities of dichloromethane and chloroform were greater than that of plasma, which made it difficult to collect the extraction phase. In addition, an emulsion formed at the interface between plasma and two extraction solvents, which decreased the available volume of solvent. The extraction recoveries of different extraction solvents are summarized in Table S1. The extraction recoveries of tert-Butyl methyl ether were 55.3%, 20.57% and 52.9% for QTP, NorQTP and 7-QTP, respectively. Finally, tert-Butyl methyl ether was selected as the optimal extraction solvent.
Fig. 1.
Effect of different extraction solvents in LLE procedure. Analytes concentrations: NorQTP: 50 ng/mL, QTP: 50 ng/mL, 7-QTP: 50 ng/mL.
3.1.3. Solvent volume
To assess the effect of extract solvent volume, four different volume of tert-butyl methyl ether (500 μL, 750 μL, 1000 μL and 1250 μL) were analyzed and extraction recoveries are shown in Table S2. The extraction recoveries increased as the solvent volume increased from 500 μL to 1000 μL, and then decreased for 1250 μL. This may be because more matrix in the plasma was extracted in the highest solvent volume (1250 μL) and the matrix substrates compete with analytes during electrokinetic injection leading to the capillary. Hence, the optimal volume of tert-butyl methyl ether was set as 1000 μL.
3.2. Separation conditions of CE
3.2.1. Buffer pH
According to the pKa values of QTP (7.06), NorQTP (8.83) and 7-QTP (7.06), the analytes are weakly alkaline compounds. 4-Aminopyridine was as internal standard with pKa 9.17 and log P 0.32 [11,14]. Thus, a weakly acidic buffer was selected since the analytes were positively charged during the analysis. Four pH values of 120 mM phosphate buffer, pH 3.0, pH 3.5, pH 4.0, and pH 5.0, were studied. The electropherograms are shown in Fig. S3. The results show that QTP and 7-QTP could not be separated in 120 mM phosphate buffer at pH 3.0 and pH 3.5. At pH 4.0 and pH 5.0, the three analytes were completely separated. When the pH value is higher, the analytes moves more slowly. The reason is that the chargeability of the analytes was weaker and the electrophoretic mobility of analytes were smaller at higher pH. Considering the analysis time and separating efficiency, the pH 4.0 phosphate buffer was chosen as optimal condition.
3.2.2. Methanol proportion in BGS
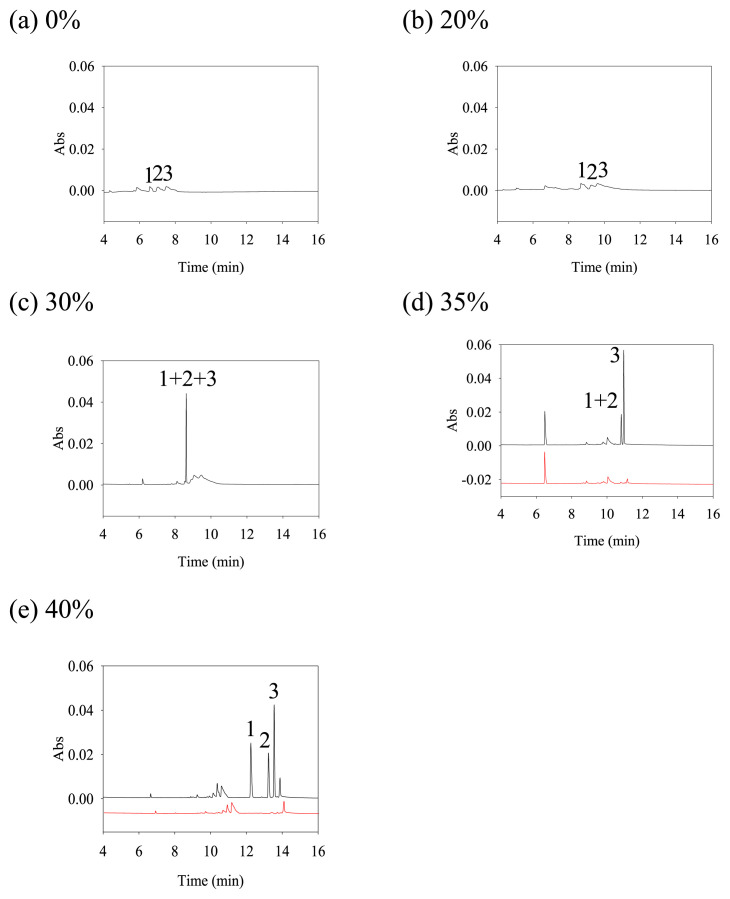
Adding methanol to phosphate buffer could effectively reduce the polarity of BGS and EOF [15,16]. Hence, methanol was proposed to improve the separation and accumulation of analytes in this study and four different proportions of methanol (0%, 20%, 30%, 35%, 40% (v/v)) were investigated. The electropherograms are shown in Fig. 2. Three peaks could not stack when there was no methanol or only 20% methanol in the BGS. Because of the larger EOF, it was not possible to accumulate the analytes at boundary of sample zone and BGS. With 30% or more methanol in the phosphate buffer, the polarity of BGS and EOF were effectively reduced [15,16], and the three analytes gradually stacked into sharp peaks. However, a high proportion of methanol (such as 50% methanol) in the solution can more easily cause volatilization, which leads to a dramatic change in the ratio of the buffer over time and causes poor reproducibility. With 50% organic solvent, the pH value of BGS may be 1–1.5 units higher than the measured pH [17]. The addition of methanol to BGS may increase the pH and suppress the ionization of analytes. In this study, all analytes still exhibited a positive charge when 40% (v/v) methanol was added to BGS. When the methanol ratio was increased to 40% (v/v), the three analytes could be fully separated. It was indicated that 40% (v/v) methanol was the optimal condition.
Fig. 2.
Electropherograms of different methanol proportion in BGS. (a) 0%, (b) 20%, (c) 30%, (d) 35%, (e) 40%. Analytes concentrations: peak 1: NorQTP (50 ng/mL), peak 2: QTP (50 ng/mL), peak 3: 7-QTP (50 ng/mL). CE condition: separation buffer, 120 mM phosphate (pH 4.0) containing 0.005% (w/v) PVP and methanol; methanol plug: 0.3 psi, 6 s; sample injection, +10 kV, 60 s; separation voltage, +26 kV; detection wavelength, 214 nm; temperature: 20 °C.
3.2.3. Polymers
Analytes were cations under acidic conditions, which can easily adsorb on the capillary wall and cause poor reproducibility. Adding polymers to the buffer can dynamically attach to the SiO− adsorption sites on the capillary wall to improve the reproducibility [18,19]. Three polymers, 0.005% (w/ v) hydroxyethyl cellulose (HEC) (Mw: 250,000), 0.005% (w/v) polyvinyl alcohol (PVA) (Mw: 31,000–50,000) and 0.005% (w/v) polyvinyl pyrrolidone (PVP) (Mw: 1, 3000, 000), were selected in this experiment. Among them, PVA exhibited poor solubility and it was necessary to heat to increase its solubility. PVP had better solubility than HEC [20]. Hence, PVP was selected in this study.
To evaluate the effect of different concentrations of PVP in BGS for reproducibility and separation efficiency, four concentrations of PVP were selected: 0%, 0.001%, 0.005% and 0.01% (w/v). The electropherograms are shown in Fig. S4 and these four concentrations of PVP in BGS were not affect the separation efficiency. At 0% PVP in BGS, the peak area of the analytes showed a significant deviation in three repeatability tests (n = 3), and the RSD values of the peak areas were all greater than 29%. This illustrated that without PVP in BGS, the partial analytes were adsorbed on the capillary wall. According to the results, it is indicated that a small amount of PVP in the separation buffer can avoid the adsorption of the analytes on the capillary wall. However, 0.01% PVP increases the viscosity of the solution, which causes poor resolution of the 7-QTP and blank peak. Thus, the optimal condition was set as 0.005% (w/v) PVP in BGS.
3.2.4. The effect different kinds of plugs
The principle of FESS was to initially rinse the capillary with the high-conductivity separation buffer and then inject a low-conductivity solvent plug. After applying a high voltage across the capillary, the analytes moved from a low-conductivity, fast-moving environment to a high-conductivity, slow-moving environment. Then, the analytes accumulated at interfaces that can improve the sensitivity of this assay [18]. In this study, the experiment was evaluated without a plug and injecting three solvent plugs (water, acetonitrile, and methanol). The conductivities of the phosphate buffer (120 mM phosphate (pH 4.0) containing 0.005% PVP and 40% (v/v) methanol), water, acetonitrile, and methanol plug were 2.62 mS, 0.6 μS, 0.0 μS, and 0.0 μS, respectively. The electropherograms are shown in Fig. S5. Three analytes can be separated under all the above conditions. A better stacking effect was obtained with the methanol plug than others. The conductivities of acetonitrile and methanol were the same; however, the viscosity of methanol was higher than that of acetonitrile. This causes the stacking efficiency to be better with a methanol plug than an acetonitrile plug. Among the candidates, a methanol plug was selected as the optimal condition.
3.2.5. The effect of sample injection time
In this study, the electrokinetic injection method was used to evaluate the influence of injection volume on peak area and migration time of analytes. The injection voltage was set at 10 kV with a positive voltage, four different injection times (50 s, 60 s, 70 s, and 80 s) were compared. The electropherograms are shown in Fig. S6. When the sample injection time was longer, the analysis time was shorter with good separation. Additionally, when the sample injection time was longer, the peak areas of the analytes were larger. However, in the three repeat tests of 60 s, 70 s, and 80 s sample injection times, the peak area RSD values of QTP, NorQTP, and 7-QTP were 3% in 60 s, 11–12% in 70 s, and 6–7% in 80 s, respectively. Considering the reproducibility, the shortest injection time of the sample (60 s) was selected as the optimal injection condition.
3.3. Analytical method
3.3.1. Selective experiment
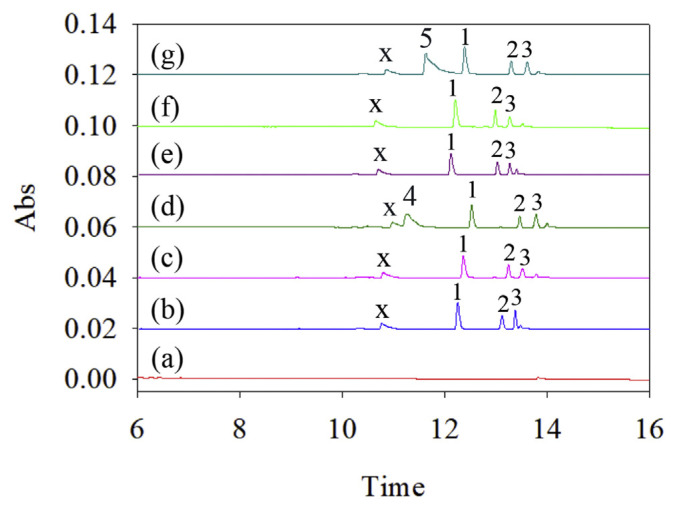
QTP and its metabolites, NorQTP and 7-QTP, were investigated in this study. However, it may be necessary for a patient who has several diseases to use multiple drugs. Thus, an experiment was performed to evaluate this method for quantifying analytes without interference by other drugs. Several drugs, such as Bupropion, sertraline, bromazepam, brotizolam, clonazepam, and lorazepam, are used to treat mental disorders and have been tested. The CE electrophergrams are shown in Fig. 3. The results showed that bupropion and sertraline, which are weak alkali compounds, were positively charged in the sample solution (Fig. 3 (d) and (g)). Others, such as bromazepam, brotizolam, clonazepam and lorazepam, had no charge or a negative charge in the sample solution, and no peak was observed. Additionally, peak x as the pharmaceutical excipient was speculated. Furthermore, these findings were supported by UV absorption spectra. The CE signals for QTP, NorQTP and 7-QTP were not influenced by the selected drugs.
Fig. 3.
Electropherogram of selective experiment in standard solution. (a) blank; QTP, NorQTP and 7-QTP with (b) Bromazepam, (c) Brotizolam, (d) Bupropion, (e) Clonazepam, (f) Lorazepam, (g) Sertraline. Analyte concentrations: peak 1: NorQTP (50 ng/mL), peak 2: QTP (50 ng/mL), peak 3: 7-QTP (50 ng/mL), peak 4: Bupropion (200 ng/mL), peak 5: Sertraline (800 ng/mL), peak x: Excipient. Other conditions were shown in Fig. 2.
3.3.2. Validation
The limit of detection (LOD) was defined by the signal-to-noise ratio (S/N = 3) for each analyte. In this method, the LODs of QTP, NorQTP, and 7-QTP were 0.25 ng/mL, 0.50 ng/mL and 1.00 ng/mL, respectively (Table S3). The linear range of the analytes was set at 3–120 ng/mL. Both the intraday correlation coefficients (n = 3) and the interday correlation coefficients (n = 5) were greater than 0.999, which implied that there was a good linear relationship with the quantitative range on the same day or between different days (Table S4). Three low, medium and high concentration in the linear range of the calibration curve were selected to evaluate the precision and accuracy of the proposed method; they were 5.0 ng/mL, 40.0 ng/mL and 100.0 ng/mL, respectively. In the intraday analysis, the RSD values of the three analytes were less than 15.11%, and the RE values were less than 13.09%. In the interday analysis, the RSD values were less than 13.23%, and the RE values were less than 14.05% (Table 1). This result indicated that the established method has good precision and accuracy.
Table 1.
Precision and accuracy analysis for intra-day (n=3) and interday (n=5) analysis of Quetiapine and its metabolites.
| Concentration (ng/mL) | Intraday (n=3) | Interday (n=5) | ||
|---|---|---|---|---|
|
|
|
|||
| RSD (%) | REa (%) | RSD (%) | REa (%) | |
| QTP | ||||
| 5 | 2.47 | 0.01 | 11.25 | −2.16 |
| 40 | 3.42 | 1.97 | 5.10 | 4.99 |
| 100 | 2.53 | 11.96 | 13.23 | 11.72 |
| NorQTP | ||||
| 5 | 2.51 | −1.11 | 6.74 | −3.70 |
| 40 | 2.99 | 4.37 | 5.44 | 8.76 |
| 100 | 3.18 | 13.09 | 6.71 | 13.79 |
| 7-QTP | ||||
| 5 | 15.11 | 0.13 | 6.12 | −8.85 |
| 40 | 1.05 | 0.15 | 2.29 | 14.05 |
| 100 | 4.78 | 0.00 | 10.36 | 10.94 |
RE:(concentration found-concentration known)/(concentration known)*100.
3.3.3. FESS sensitivity enhancement
The sensitivity of the online FESS preconcentration technique and capillary zone electrophoresis (CZE) were compared. In the FESS technique, the concentration of three analytes was set at 50 ng/mL, and in CZE, the concentration of three analytes was set at 1 μg/mL. The formula for the sensitivity improvement is shown in Eq. (1):
| (1) |
Peak area FESS: the peak area of analytes by FESS.
Peak area CZE: the peak area of analytes by CZE.
C CZE: the concentration of analytes by CZE.
C FESS: the concentration of analytes by FESS.
The electropherograms of CZE and FESS are shown in Fig. S7. After preconcentration of the three analytes, the sensitivity enhancements were calculated by Eq. (1). The increase in sensitivity of QTP, NorQTP and 7-QTP was 463-fold, 523-fold and 835-fold, respectively. The LODs in this study were superior to those of previously published CE and HPLC methods [9,10]. Therefore, this study combined the LLE method with the online preconcentration technique to analyze QTPs and metabolites with the advantage of enhanced sensitivity.
3.4. Application
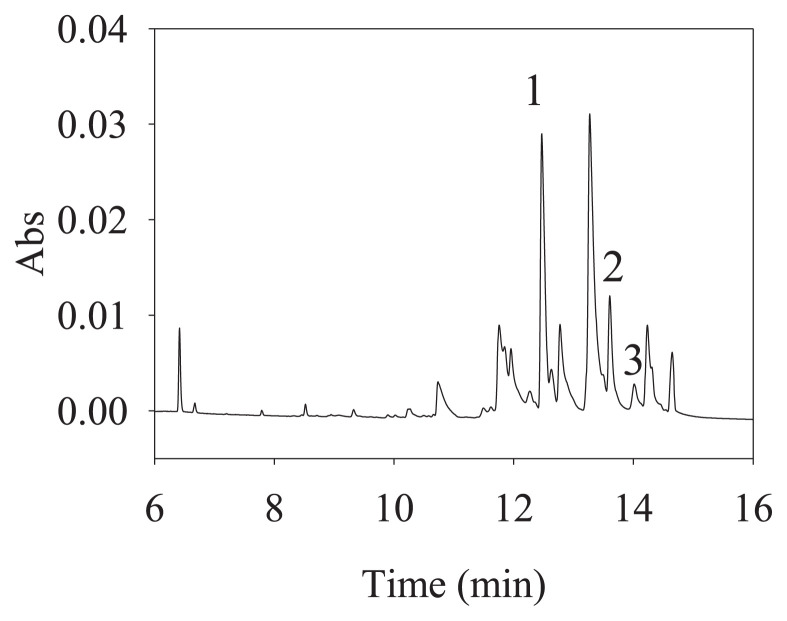
The analytical method established in this study was applied to the determination of plasma samples from a schizophrenia patient who was using QTP. Plasma samples were collected from a male patient (58 years old, 88.9 kg) who continued taking Seroquel ® (containing QTP/300 mg). A dose of 300 mg of QTP was administered once after dinner every day. Moreover, this patient also used ranitidine/150 mg, dimethylpolysiloxane/40 mg, domperidone/10 mg, zolpidem/10 mg, acetylsalicylic acid/100 mg, atorvastatin/ 40 mg, propafenone/150 mg, ramipril/ 10 mg, amlodipine/5 mg, flunitrazepam/1 mg, and duloxetine/30 mg. The plasma sample was collected 1 h after taking the QTP. The electropherograms are shown in Fig. 4 and the peaks of the QTP, NorQTP and 7-QTP were observed. In the CE analysis, the ratios of the peak areas of analytes and the peak areas of the internal standard was calculated by the calibration curve equation. Finally, the concentrations of QTP, NorQTP and 7-QTP in actual patients were 26.09 ± 0.18 ng/mL, 60.82 ± 0.17 ng/mL, and 5.29 ± 0.08 ng/mL, respectively. The sample was also analyzed by nano-UPLC-MS was to determine whether QTP and its metabolites were present. Although QTP, NorQTP and 7-QTP cannot be separated by nano-UPLC in present condition, MRM mode was successfully used to identify the QTP at 384.17 (m/z), NorQTP at 296.12 (m/z) and 7-QTP at 400.16 (m/z) (Fig. S8). The results were confirmed by the nano-UPLC–MS, which produced the same results.
Fig. 4.
Electropherogram of plasma sample 1 from patient in the treatment of schizophrenia by QTP in LLE coupled with FESS. I.S.: 4-aminopyridine (40 ng/mL), Peak 1: NorQTP, peak 2: QTP, peak 3: 7-QTP. Other condition were shown in Fig. 2.
4. Conclusions
In this study, the pretreatment technique and LLE were used to extract analytes and remove interference in plasma. The online pre-concentration method FESS was applied to improve the sensitivity of this assay. The validated method was rapid and had better sensitivity than the traditional CE mode; the new method can improve sensitivity 463-835-fold. The LODs of QTP, NorQTP and 7-QTP were 0.5 ng/mL, 0.25 ng/mL and 1 ng/mL, respectively. This method was also applied to a patient who was using QTP. The concentrations of QTP, NorQTP and 7-QTP in the patient’s plasma were determined, indicating that the findings could be used to evaluate the basis of pharmacokinetics for further establishing suitable personal medical treatments in the future (Supporting Information can be found at https://www.jfda-online.com/cgi/editor.cgi?article=3378&window=additional_files&context=journal).
Supplementary Information
Acknowledgements
This study was supported and funded by the Ministry of Science and Technology, Taiwan (MOST 109-2113-M-037-009).
Funding Statement
This study was supported and funded by the Ministry of Science and Technology, Taiwan (MOST 109-2113-M-037-009).
Footnotes
Conflict of interest
The authors have declared no conflicts of interest.
References
- 1. Arida H, Robaian MA, Fataftah A, Al-Sllami G. A new coated wire selective electrode for quetiapine in biological and pharmaceutical analysis. Int J Electrochem Sci. 2017;12:4120–33. [Google Scholar]
- 2.Product Information: SEROQUEL(R) oral tablets, quetiapine fumarate oral tablets. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2013. [Google Scholar]
- 3. Bakken GV, Rudberg I, Christensen H, Molden E, Refsum H, Hermann M. Metabolism of quetiapine by CYP3A4 and CYP3A5 in presence or absence of cytochrome B5. Drug Metab Dispos. 2009;37:254–8. doi: 10.1124/dmd.108.023291. [DOI] [PubMed] [Google Scholar]
- 4. DeVane CL, Nemeroff CB. Clinical pharmacokinetics of quetipaine. Drug Metab Dispos. 2001;40(7):509–22. doi: 10.2165/00003088-200140070-00003. [DOI] [PubMed] [Google Scholar]
- 5. Nyberg S, Grimm S, Gulyas D, McCarthy D, Lee C, Goldstein J, et al. PET-measured D2, 5-HT2, and norepinephrine transporter (NET) occupancy by quetiapine and N-desalkyl-quetiapine in non-human primates. Eur Neuro-psychopharmacol. 2007:S253–5. [Google Scholar]
- 6. Cross AJ, Widzowski D, Maciag C, Zacco A, Hudzik T, Liu J, et al. Quetiapine and its metabolite norquetiapine: translation from in vitro pharmacology to in vivo efficacy in rodent models. Br J Pharmacol. 2016;173:155–66. doi: 10.1111/bph.13346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Winter HR, Earley WR, Hamer-Maansson JE, Davis PC, Smith MA. Steady-state pharmacokinetic, safety, and tolerability profiles of quetiapine, norquetiapine, and other quetiapine metabolites in pediatric and adult patients with psychotic disorders. J Child Adolesc Psychopharmacol. 2008;18:81–98. doi: 10.1089/cap.2007.0084. [DOI] [PubMed] [Google Scholar]
- 8. Kim DW, Weon KY, Hong EP, Chung EK, Lee KT. Comparative physicochemical and pharmacokinetic properties of quetiapine and its active metabolite norquetiapine. Chem Pharm Bull. 2016;64(11):1546–54. doi: 10.1248/cpb.c16-00223. [DOI] [PubMed] [Google Scholar]
- 9. Silva Gracia M, Koppl A, Unholzer S, Haen E. Development and validation of an HPLC-UV method for the simultaneous determination of the antipsychotics clozapine, olanzapine and quetiapine, several beta-blockers and their metabolites. Biomed Chromatogr. 2017;31:1–11. doi: 10.1002/bmc.3968. [DOI] [PubMed] [Google Scholar]
- 10. Pucci V, Mandrioli R, Ferranti A, Furlanetto S, Augusta Raggi M. Quality control of commercial tablets containing the novel antipsychotic quetiapine. J Pharm Biomed Anal. 2003;32:1037–44. doi: 10.1016/s0731-7085(03)00206-1. [DOI] [PubMed] [Google Scholar]
- 11.Chemicalize. [Accessed 16 April 2020]. http://chemicalize.com .
- 12. Jiang TF, Lv ZH, Cui XY, Wang YH. Analysis of malachite green, gentian violet and their Leuco metabolites in catfish and carp by micellar electrokinetic capillary chromatography. J Food Drug Anal. 2012;20(1):94–100. [Google Scholar]
- 13. Chen YL, Jong YJ, Wu SM. Capillary electrophoresis combining field-amplified sample stacking and electroosmotic flow suppressant for analysis of sulindac and its two metabolites in plasma. J Chromatogr A. 2006;1119(1–2):176–82. doi: 10.1016/j.chroma.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 14.Pubchem. https://pubchem.ncbi.nlm.nih.gov .
- 15. Guo XF, Wang ZH, Zhou SP. The separation and determination of nitrophenol isomers by high-performance capillary zone electrophoresis. Talanta. 2004;64(1):135–9. doi: 10.1016/j.talanta.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 16. Chou YW, Huang WS, Chen CC, Lin SJ, Wu HL, Chen SH. Trace analysis of zotepine and its active metabolite in plasma by capillary electrophoresis with solid phase extraction and head-column field-amplified sample stacking. J Chromatogr A. 2005;1087:189–96. doi: 10.1016/j.chroma.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 17.Watson DG, editor. Pharmaceutical analysis. third ed. Elsevier; New Yorrk: p. 311. [Google Scholar]
- 18. Horvath J, Dolník V. Polymer wall coating for capillary electrophoresis. Electrophoresis. 2001;22:644–55. doi: 10.1002/1522-2683(200102)22:4<644::AID-ELPS644>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19. Iki N, Yeng ES. Non-bonded poly(ethylene oxide) polymer-coated column for protein separation by capillary electrophoresis. J Chromatogr A. 1996;731:273–82. [Google Scholar]
- 20.Chemical book. [Accessed 19 July 2019]. https://www.chemicalbook.com/ProductIndex_EN.aspx .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.