Background:
Approximately 3.5 million children and adolescents worldwide are chronically infected with HCV. This study uses pharmacokinetic modeling to identify pediatric doses of elbasvir/grazoprevir (EBR/GZR) that achieve plasma concentrations similar to those seen in adults receiving the approved fixed-dose combination regimen of EBR/GZR.
Patients and Methods:
We conducted a nonrandomized, single-arm, multicenter, open-label phase 2b trial in children and adolescents aged 3 to <18 years with chronic HCV genotype 1 or 4 infection (NCT03379506). Pharmacokinetic data were used to bridge efficacy and safety data from adults to children in a stepwise (oldest to youngest) manner. A total of 57 participants were enrolled: cohort 1 (aged 12 to <18 y), n=22; cohort 2 (aged 7 to <12 y), n=17; and cohort 3 (aged 3 to <7 y), n=18.
Results:
Steady-state plasma exposures were achieved by week 4 for EBR and GZR in all cohorts and daily dosing achieved geometric mean steady-state area under the concentration-time curve at 0–24 hours that fell within comparability bounds established for adults. All participants achieved sustained virologic response 12 weeks after completing treatment (ie, undetectable HCV RNA 12 wk following completion of treatment). Headache (n=4), fatigue (n=4), and nausea (n=2) were the most common treatment-related adverse events (all mild or moderate); no participant discontinued because of an adverse event.
Conclusions:
Pediatric EBR/GZR pharmacokinetic models were successfully developed based on complex adult population pharmacokinetic models. At appropriate age-related doses, EBR/GZR is safe and effective in pediatric and adolescent participants with HCV infection.
INTRODUCTION
Approximately 3.5 million children and adolescents worldwide are chronically infected with HCV1,2; however, until 2017, no direct-acting antiviral (DAA) agent was approved for use in the pediatric population. Subsequent clinical trials of DAAs have demonstrated high rates of sustained virologic response (SVR) coupled with acceptable safety profiles in children and adolescents.3–7 Comparisons with adult pharmacokinetic data have also confirmed that exposure within the equivalence boundaries of adults receiving DAAs can be achieved for adolescents receiving adult-approved doses and for children aged <12 years using weight-based dose adjustments.3–7 Guidance from the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver recommends the use of approved DAA treatment regimens for all children and adolescents with HCV infection aged ≥3 years, as they will benefit from antiviral therapy regardless of disease severity.8,9
Elbasvir/grazoprevir (EBR/GZR) is a fixed-dose combination (FDC) of 2 DAAs that is indicated for the treatment of adults with chronic HCV genotype 1 or 4 infection.10 EBR targets the HCV nonstructural (NS)5A protein to block viral RNA replication as well as virion assembly, and GZR inhibits the HCV NS3/4A protease to block viral RNA replication.11 EBR/GZR has a well-established efficacy and tolerability profile in adults with HCV genotype 1 or 4 infection.12–16 The aim of the present study was to use pharmacokinetic modeling to identify pediatric doses of EBR/GZR that achieve plasma concentrations similar to those seen in adults receiving the FDC regimen of EBR/GZR. We conducted a nonrandomized, single-arm, multicenter, open-label phase 2b trial to evaluate pharmacokinetics, safety, and efficacy of EBR/GZR in children and adolescents aged 3 to <18 years with chronic HCV genotype 1 or 4 infection. Pharmacokinetic data were used to bridge efficacy and safety data from adults to children in a stepwise (oldest to youngest) manner.
PATIENTS AND METHODS
This multicenter, nonrandomized, single-arm, open-label phase 2b study assessed the pharmacokinetics, safety, and efficacy of EBR/GZR combination therapy in participants aged 3 to <18 years with chronic HCV G1 or 4 infection (NCT03379506; Protocol PN079). The study was conducted in accordance with the provisions of the Declaration of Helsinki’s Good Clinical Practice requirements and other regulations governing clinical study conduct. All participants’ legally acceptable representatives provided written informed consent for the study. When applicable, the participant provided written informed assent. The protocol and any amendments, the written informed consent form, and any other written information provided to participants was reviewed and approved by the central institutional review board at each participating site.
Study design
The study employed a novel, stepwise, iterative, pharmacokinetic modeling approach to bridge efficacy and safety data from adults to children (oldest to youngest) and to predict the dose levels of EBR and GZR that would achieve optimal steady-state pharmacokinetics across different age cohorts (Appendix Figure 1, http://links.lww.com/HC9/A84). Participants were sequentially enrolled into 3 cohorts according to age: 12 to <18 years (cohort 1); 7 to <12 years (cohort 2); and 3 to <7 years (cohort 3).
All participants received EBR/GZR FDC tablets or pediatric granules daily for 12 weeks and were followed for an additional 24 weeks after completing treatment. Dosing in each age cohort was initiated in a mini cohort of 7 participants, requiring ≥6 participants to have evaluable pharmacokinetic data before additional participants were enrolled into an expanded cohort. The adult FDC tablet was selected as the initial dose for the 12 to <18 years, cohort. The adult phase 2/3 clinical program comprised all participants, including those with body weight as low as 37 kg, who demonstrated favorable pharmacokinetic parameters, safety, and efficacy. Based on the known maturation of the relevant EBR/GZR metabolic and transporter pathways (CYP3A and OATP1B),17,18 participants in mini cohort 1 were administered the adult FDC of EBR/GZR, which was expected to result in comparable exposures to adults. Dose selection for the younger participants was determined based on cumulative pediatric pharmacokinetic data and pediatric population pharmacokinetic modeling and simulation to estimate the anticipated exposures. At each step, model-based simulations were used to derive a dosing regimen expected to provide similar exposures between pediatric and adult participants. Participants aged ≥12 years received EBR/GZR at the approved adult FDC tablet dosage of 50 mg/100 mg daily, whereas those aged 3 to <12 years received oral granule formulations of EBR and GZR. Dosing in mini cohorts was initiated at EBR 30 mg/GZR 60 mg for children aged 7 to <12 years. For children aged 3 to <7 years, dosing in the mini cohort was initiated at EBR 15 mg/GZR 30 mg for those with body weight <20 kg and EBR 15 mg/GZR 50 mg for those with body weight ≥20 kg (Appendix Table 1, http://links.lww.com/HC9/A84). Dosing in the expanded cohorts was started once dosing and pharmacokinetic analyses in the mini cohorts were completed. Pharmacokinetic parameters from the adult population pharmacokinetic model are shown in Appendix Table 2 (http://links.lww.com/HC9/A84).
Participants
Male and female children or adolescents aged 3 to <18 years with documented HCV GT1a, 1b, or 4 infection (HCV RNA ≥1000 IU/mL in peripheral blood) were enrolled. Participants were either HCV treatment-naive or had received previous treatment with pegylated interferon/ribavirin. Participants were excluded from the study if they had a history of chronic hepatitis not caused by HCV; evidence of Child-Pugh class B/C cirrhosis; decompensated liver disease; coinfection with hepatitis B virus or HIV; HCV GT1a infection and NS5A resistance-associated substitutions at amino acid position 28, 30, 31, or 93; or received previous treatment with a DAA agent for HCV infection. Female participants who were pregnant or breastfeeding or male participants with a pregnant partner were also excluded. Laboratory exclusion criteria are listed in the Supplemental Appendix Table 3 (http://links.lww.com/HC9/A84).
Assessments
The primary pharmacokinetic endpoints for EBR and GZR were steady-state area under the concentration-time curve at 0–24 hours (AUC0–24), maximum concentration (C max), concentration at 24 hours following the dose or immediately before the next dose (C 24; considered to be trough plasma concentration), and apparent clearance (CL/F) at week 4 for EBR. Plasma drug levels were measured by InVentiv Health Clinique using validated liquid chromatography-tandem mass spectrometric methods. The lower limits of quantification for EBR and GZR were 1.00 ng/mL (1.30 nM) and 0.25 ng/mL (0.283 nM), respectively.
Secondary endpoints included safety and tolerability of EBR/GZR [ie, the number of participants experiencing adverse events (AEs) and/or discontinuing treatment due to AEs] and efficacy [ie, the proportion of participants with an SVR 12 weeks after the end of treatment (SVR12)]. Sexual maturation was assessed using Tanner staging at baseline, treatment week 12, and follow-up week 24, and each subject completed a questionnaire on medication palatability and acceptability of treatment at treatment week 4.
Measurement of HCV RNA levels in plasma was performed using the COBAS AmpliPrep/COBAS TaqMan HCV Test v2.0 (Roche Diagnostics) on blood samples drawn at various time points before, during, and after dosing, according to the study treatment protocol.
Statistical analysis
The study planned to enroll ≥56 participants across the 3 age cohorts. With 22 participants in each cohort, it was considered ~80% likely that the lower and upper bounds of the 95% CI for EBR and GZR AUC0–24 would lie within 44% of the observed geometric mean.
The primary pharmacokinetic parameters of interest (AUC0–24, C max, C 24, and CL/F) of EBR and GZR at treatment week 4 are summarized separately by cohort and treatment as appropriate, with geometric mean and 95% CI based on natural log-transformed analysis and t distribution.
Data are presented by age cohorts (12 to <18 y, 7 to <12 y, and 3 to <7 y). Within each cohort, data are introduced separately for mini and expanded cohorts, unless the same dose was given to both cohorts within the age group. If all participants in a cohort received the same dose, then data from participants in both mini and expanded-age cohorts are reported together. To estimate the proportion of participants achieving SVR12, a 95% CI was calculated using the Clopper-Pearson method.
The analysis of safety parameters followed a tiered approach, with the tiers differing based on the analyses performed. The first tier included: (1) AEs of elevated laboratory values that are reported as events of clinical interest, (2) any AE, (3) a drug-related AE, (4) a serious AE, (5) a serious and drug-related AE, and (6) an AE leading to discontinuation. Point estimates and 95% CIs were provided for the proportion of participants with first-tier AEs. The 95% CIs for the safety parameters were estimated using the Clopper-Pearson method. The second tier included specific AEs by system organ class, vital signs, and standard laboratory safety tests at time points specified in the protocol. Point estimates were provided for the proportion of participants with second-tier AEs.
RESULTS
Participants
Participants were enrolled at 14 study sites in the US and Europe. The first participant received the first dose of study medication on February 27, 2018, and the final participant completed 24 weeks of follow-up on July 31, 2020. Seventy-eight participants were screened for inclusion in the study; 21 were excluded (withdrawal by parent, n=1; screen failure, n=20). Of the 21 excluded participants, 1 had evidence of decompensated liver disease and 10 had HCV GT1a infection and did not have documented absence of resistance-associated substitutions at NS5A positions 28, 30, 31, and/or 93.
Fifty-seven participants were enrolled in the study [22 in cohort 1 (aged 12 to <18 y), 17 in cohort 2 (aged 7 to <12 y), and 18 in cohort 3 (age 3 to <7 y)] and all completed their allocated treatment. Mean age (SD) was 14.1 years (1.9), 8.7 years (1.2), and 4.4 years (1.2) in cohorts 1, 2, and 3, respectively (Table 1). In all 3 cohorts, most participants were reported as White and naive to previous HCV treatment. HCV GT1a infection was present in 73%, 53%, and 33% of participants and HCV GT1b infection was present in 23%, 47%, and 61% of participants in cohorts 1, 2, and 3, respectively. Only 2 participants had HCV GT4 infection (one each in cohorts 1 and 3).
TABLE 1.
Participant demographics and baseline characteristics
| Variables | Age cohort 1: 12 to <18 y mini+expanded (n=22) | Age cohort 2: 7 to <12 y mini+expanded (n=17) | Age cohort 3: 3 to <7 y mini+expanded (n=18) |
|---|---|---|---|
| Male | 11 (50.0) | 10 (58.8) | 7 (38.9) |
| Age (mean±SD) (y) | 14.1±1.9 | 8.7±1.2 | 4.4±1.2 |
| Race | |||
| White | 21 (95.5) | 17 (100.0) | 18 (100.0) |
| Multiracial | 1 (4.5) | 0 | 0 |
| HCV genotype | |||
| GT1a | 16 (72.7) | 9 (52.9) | 6 (33.3) |
| GT1b | 5 (22.7) | 8 (47.1) | 11 (61.1) |
| GT4a | 0 | 0 | 1 (5.6) |
| GT4b | 1 (4.5) | 0 | 0 |
| Treatment history | |||
| Naive | 14 (63.6) | 15 (88.2) | 17 (94.4) |
| PR treatment-experienced | 8 (36.4) | 2 (11.8) | 1 (5.6) |
Note: All data except age are n (%). Abbreviations: GT, genotype; PR, peginterferon/ribavirin.
Dosing
Dosing of participants in mini cohorts 1 and 2 achieved EBR and GZR plasma levels that fell within comparability bounds established for adults. The same doses administered to the mini cohorts were also provided to the expanded cohorts 1 and 2 (Figure 1 and 2). However, EBR and GZR exposures during dosing in mini cohort 3 were suboptimal. Consequently, dosing for expanded cohort 3 was adjusted: the EBR dose was increased from 15 to 25 mg and the GZR dose was increased from 30 to 50 mg. Following these adjustments, EBR and GZR exposures in expanded cohort 3 were within comparability bounds established for adults (Figure 1 and 2).
Figure 1.

EBR and GZR AUC0–24 at steady state (week 4) for mini cohorts 1–3. Abbreviations: AUC0–24, area under the concentration-time curve at 0–24 hours; EBR, elbasvir; FDC, fixed-dose combination; Geo., geometric; GZR, grazoprevir; QD, once daily.
Figure 2.

EBR and GZR AUC0–24 at steady state (week 4) for mini cohorts 1 and 2 and expanded cohorts 1–3. EBR and GZR exposures following adjusted dosing in expanded cohort 3 are shown (EBR 25 mg; GZR 50 mg); initial exposures obtained in mini cohort 3 before dose adjustment are shown in Figure 1. Abbreviations: AUC0–24, area under the concentration-time curve at 0–24 hours; EBR, elbasvir; FDC, fixed-dose combination; Geo., geometric; GZR, grazoprevir; QD, once daily.
Pharmacokinetics
In all cohorts, T max was achieved ∼2 hours after dosing and elimination followed a biexponential time course, with the terminal elimination phase occurring ∼10 hours after dosing; this was consistent with observations in adults (Figure 3). Steady-state plasma exposures were achieved by week 4 for EBR and GZR in all cohorts; this was supported by the fact that predose and 2-hour postdose exposures at week 8 were similar to those at week 4. Daily dosing of EBR/GZR in pediatric participants aged 3 to <18 years achieved geometric mean steady-state AUC0–24 that fell within comparability bounds established for adults, including dosing with the approved adult FDC in adolescent participants aged 12 to <18 years. It is notable that the adjusted dosing in the expanded cohort 3 also ensured that EBR and GZR exposures were within range for the safety and efficacy bounds established for FDC exposure in adults. For EBR, the geometric mean AUC0–24 at week 4 for each cohort ranged from 1.71 to 3.15 μM. h, which is 0.79–1.45 times the exposure in adults of 2.18 μM. h. For GZR, the geometric mean AUC0–24 at week 4 for each cohort ranged from 0.77 to 1.66 μM. h, which is 0.42–0.90 times the exposure in adults of 1.85 μM. h.
Figure 3.
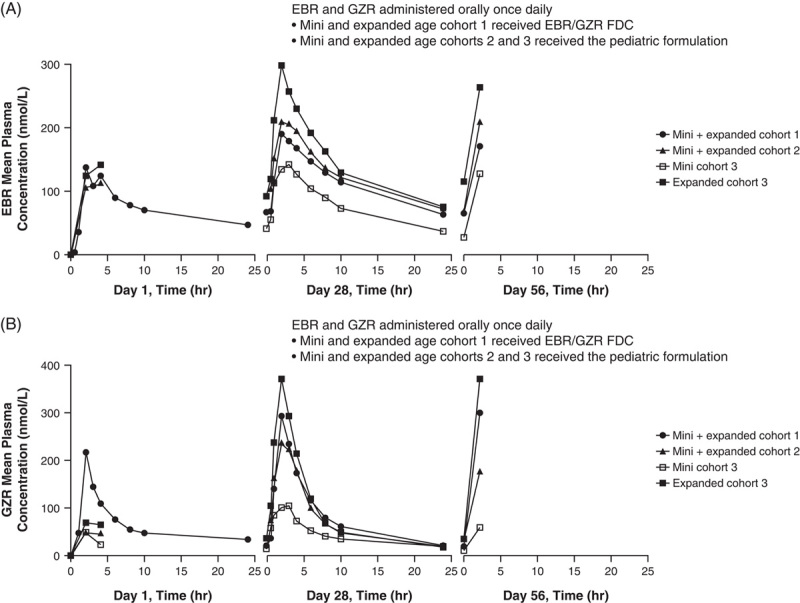
Arithmetic mean plasma concentrations of EBR (A) and GZR (B) at days 1, 28, and 56. Abbreviations: EBR, elbasvir; FDC, fixed-dose combination; GZR, grazoprevir; QD, once daily.
Efficacy
All 57 participants achieved SVR12, defined as undetectable HCV RNA 12 weeks following completion of treatment (Figure 4). One participant (aged 7 y) in cohort 2 did not return for the follow-up week 24 clinic visit because of fear of COVID-19 infection; therefore, SVR24 was achieved by 100% of participants in cohorts 1 (22/22) and 3 (18/18), and 94.1% (16/17) of those in cohort 2.
Figure 4.
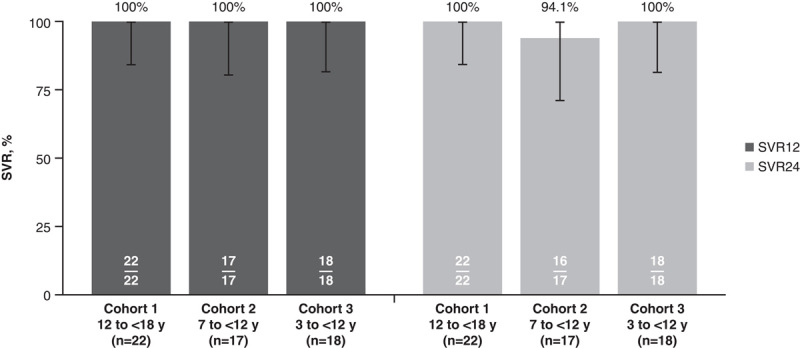
SVR12 and SVR24 with EBR/GZR in cohorts 1–3. Abbreviations: EBR, elbasvir; GZR, grazoprevir; SVR, sustained virologic response; SVR12, sustained virologic response at 12 weeks after completion of therapy; SVR24, sustained virologic response at 24 weeks after completion of therapy.
Safety
Across all cohorts, ∼75% of participants experienced ≥1 AE during the study, and treatment-related AEs were reported by 27% (6/22), 18% (3/17), and 6% (1/18) of participants in cohorts 1, 2, and 3, respectively (Table 2). Headache (n=4; 7%), fatigue (n=4; 7%), and nausea (n=2; 4%) were the most common treatment-related AEs. All AEs were mild or moderate and resolved with ongoing study medication; no participants discontinued study medication because of AEs (Table 2).
TABLE 2.
AE and laboratory safety summary
| Variables | Age cohort 1: 12 to <18 y mini+expanded (n=22) | Age cohort 2: 7 to <12 y mini+expanded (n=17) | Age cohort 3: 3 to <7 y mini+expanded (n=18) |
|---|---|---|---|
| Any AE | 17 (77.3) | 13 (76.5) | 13 (72.2) |
| Treatment-related AE | 6 (27.3) | 3 (17.6) | 1 (5.6) |
| Headache | 3 (13.6) | 1 (5.9) | 0 |
| Fatigue | 1 (4.5) | 2 (11.8) | 1 (5.6) |
| Nausea | 2 (9.1) | 0 | 0 |
| Serious AEa | 1 (4.5) | 0 | 1 (5.6) |
| Death | 0 | 0 | 0 |
| Treatment discontinuation due to AEs | 0 | 0 | 0 |
Note: All data are n (%). aFractured fingertip (n=1); dyspepsia (n=1).
Abbreviation: AE, adverse event.
Alanine aminotransferase elevation occurred in 2 participants (grade 1, n=1; grade 3, n=1), aspartate aminotransferase elevation was observed in 3 participants (grade 1, n=2; grade 2, n=1), and bilirubin elevation occurred in 2 participants (grade 1, n=1; grade 2, n=1). It is notable that one participant (aged 7 y with HCV GT1b infection and without cirrhosis) experienced elevated on-treatment alanine aminotransferase levels that resolved with ongoing study medication (Appendix Table 4, http://links.lww.com/HC9/A84). This participant had a medical history of mild asthma and was receiving sodium fluoride for oral hygiene. After presenting healthy at treatment week 4 study visit, this participant subsequently experienced abdominal pain, diarrhea, vomiting, and decreased appetite of 2–3 days’ duration. Alanine aminotransferase/aspartate aminotransferase levels were elevated (218/123 U/mL). Follow-up laboratory tests at treatment week 5 showed normalizing values and attenuated symptoms. No specific cause was identified, and symptoms were attributed to a viral infection. Study medication was not interrupted, and the participant fully recovered and achieved SVR12.
Growth was unaffected during treatment and follow-up, with small changes in height, weight, and body mass index Z-scores occurring in each age cohort between baseline and follow-up week 12, consistent with normal growth (Table 3).
TABLE 3.
Change from baseline anthropometric measurements at follow-up week 12
| Height Z-score | Weight Z-score | BMI Z-score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | FW12 | Change | Baseline | FW12 | Change | Baseline | FW12 | Change | |
| Age cohort 1 (N=22) | −0.56 (1.07) | −0.57 (1.04) | −0.01 (0.15) | −0.12 (1.17) | −0.05 (1.21) | 0.08 (0.31) | 0.18 (0.92) | 0.25 (0.97) | 0.07 (0.27) |
| Age cohort 2 (N=17) | 0.45 (0.98) | 0.46 (0.94) | 0.01 (0.12) | 0.84 (1.11) | 0.95 (1.10) | 0.11 (0.20) | 0.84 (1.04) | 0.96 (1.06) | 0.12 (0.22) |
| Age cohort 3 | |||||||||
| Mini (n=7) | 0.60 (0.64) | 0.60 (0.50) | 0.00 (0.28) | 0.40 (0.69) | 0.37 (0.61) | −0.03 (0.23) | 0.02 (1.08) | −0.00 (1.07) | −0.02 (0.33) |
| Expanded (n=11) | 0.51 (1.14) | 0.49 (1.17) | −0.2 (0.28) | 0.60 (1.42) | 0.61 (1.59) | 0.01 (0.44) | 0.58 (1.24) | 0.52 (1.49) | −0.06 (0.62) |
Abbreviations: BMI, body mass index; FW12, follow-up week 12.
Palatability
Most participants in cohort 1 reported the taste of the EBR/GZR FDC tablet as “neither good nor bad” at treatment week 4 and did not report a problem taking the medication. Most participants in cohorts 2 and 3 reported the taste of EBR/GZR pediatric granules as either “good” or “neither good nor bad” at treatment week 4 and did not report a problem taking the medication.
DISCUSSION
Development of therapeutics for children and adolescents poses unique challenges that are not encountered when developing medications for adults. Difficulties in enrolling participants, ethical considerations, differences in physiology, and a limited number of participants can all hinder traditional approaches to drug testing in pediatric populations.19 Indeed, it has been estimated that the interval between product approval in adults and addition of a pediatric indication to the label can be as long as 9 years.20 To combat these deficiencies, regulatory guidance from the US Food and Drug Administration has been introduced that allows for the extrapolation of data from well-controlled adult studies to pediatric participants in situations where the course of disease and effects of the drug are considered similar in adult and pediatric populations.21 With regard to the treatment of HCV infection with DAA therapies, it is appropriate to assume that the cause of the disease and the effect of the drug directly on the virus will be the same in both adult and pediatric participants. Extrapolation of data from adult to the pediatric population is further warranted in this area due to the similar exposure–response profile expected in these populations.21
Data from the present study confirm that EBR/GZR exposures equivalent to those present in adult participants can be achieved in pediatric participants using a weight-based dosing approach. The reference exposure levels from adults are associated with a high rate of SVR in the adult population. Indeed, EBR/GZR exposures in adolescents aged 12 to <18 years receiving the approved FDC tablet were equivalent to those associated with high efficacy in the adult population pharmacokinetic model. Similar exposures were achieved in children aged 7 to <12 years receiving EBR 30 mg/GZR 60 mg and in children aged 3 to <7 years receiving EBR 25 mg/GZR 50 mg.
A limitation of this study was the enrollment of only 2 participants with HCV GT4 infection. However, data from the clinical development program in adult patients with HCV infection confirm a consistent efficacy profile in adult patients with HCV GT4 infection compared with those with GT1 infection. In a pooled analysis of patients from across the clinical development program, an SVR rate of 96% (97 of 101 participants) was achieved in treatment-naive adult patients with HCV GT4 infection receiving the FDC of EBR/GZR for 12 weeks.22 Furthermore, no difference in safety profile would be anticipated based on the type of genotype; therefore, a consistent safety profile of EBR/GZR can be expected regardless of genotype.12 These observations suggest that at the pediatric doses confirmed in this analysis, EBR/GZR is a safe and effective option for pediatric patients with HCV GT4 infection.
In this study, efficacy was high across all age cohorts. All participants achieved SVR12, confirming that high efficacy can be predicted when exposures remain within adult bioequivalence boundaries. Tolerability was also generally comparable between the pediatric and adult populations. All AEs were mild or moderate and resolved with ongoing study medication. Alanine aminotransferase/aspartate aminotransferase elevation, a known AE occurring in <1% of adult EBR/GZR recipients, was reported in one pediatric participant and resolved with ongoing treatment.12 Tanner staging indicated no impact on pubertal development during treatment, and most participants found medication palatability to be acceptable.
In conclusion, pediatric EBR/GZR pharmacokinetic models were successfully developed based on complex adult population pharmacokinetic models. Daily dosing of EBR/GZR in pediatric participants aged 3 to <18 years achieved geometric mean steady-state AUC0–24 that fell within comparability bounds established for adults. These data indicate that at appropriate age-related doses, EBR/GZR is safe and effective in children and adolescents with HCV GT1 or GT4 infection.
Supplementary Material
Acknowledgments
AUTHOR CONTRIBUTIONS
Concept and design: Barbara A. Haber, Anjana Grandhi, Regino P. Gonzalez-Peralta, Patricia Castronuovo, Luzelena Caro. Acquisition of data: Robert H. Squires, Philip Rosenthal, Rene Romero, Malgorzata Pawlowska, Frauke Mutschler, Ewa Majda-Stanislawska, Thomas Lang, Maureen M. Jonas, Barbara A. Haber, Anjana Grandhi, Regino P. Gonzalez-Peralta, Bjorn Fischler, Patricia Castronuovo, William Balistreri, Niviann Blondet, Stefan Wirth. Analysis and interpretation of data: Robert H. Squires, Philip Rosenthal, Daniel I.S. Rosenbloom, Rene Romero, Ewa Majda-Stanislawska, Thomas Lang, Maureen M. Jonas, Barbara A. Haber, Anjana Grandhi, Regino P. Gonzalez-Peralta, Lihong Du, Naim Alkhouri, William Balistreri, Luzelena Caro. Drafting the article or revising it critically for important intellectual content and final approval of the version to be published: All authors.
ACKNOWLEDGMENTS
The authors extend their gratitude to the participants, investigators, and site personnel who participated in this study. Thanks to the Extended Pediatric HCV Team at Merck & Co., Inc., (Rahway, New Jersey, USA). Medical writing and editorial assistance was provided by Tim Ibbotson, PhD, of ApotheCom (Yardley, Pennsylvania, USA) and funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co. Inc. (Rahway, New Jersey, USA).
FUNDING INFORMATION
This study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co. Inc. (Rahway, New Jersey, USA).
CONFLICT OF INTEREST
Regino P. Gonzalez-Peralta: consulting: Mirum and Alexion; advisory arrangements: Mirum, Albireo, Takeda, Orphalan, and Alexion; speakers bureau: Mirum and Albireo; and grants and research contracts: Gilead, AbbVie, and Merck. Thomas Lang: consulting: Univar and Alexion. Malgorzata Pawlowska: research grants and contracts: Merck. Wojciech Sluzewski: deceased. William Balistreri: speakers bureau: Mirum; grants and research contracts: Merck, AbbVie, and Gilead. Philip Rosenthal: research grants and contracts: AbbVie and Gilead. Naim Alkhouri: speakers bureau: AbbVie and Gilead; research grants: Gilead and Merck. Rene Romero: research grants and contracts: Merck and Gilead; consulting: Mirum and Albireo. Anjana Grandhi: employment: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and stock ownership or equity Merck & Co., Inc., Rahway, NJ, USA. Patricia Castronuovo: employment: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co. Inc. (Rahway, New Jersey, USA). Luzelena Caro: employment: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and stock ownership or equity Merck & Co., Inc., Rahway, NJ, USA. Lihong Du: employment: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co. Inc. (Rahway, New Jersey, USA). Daniel I. S. Rosenbloom: employment: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and stock ownership or equity Merck & Co., Inc., Rahway, NJ, USA. Barbara A. Haber: employment: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and stock ownership or equity Merck & Co., Inc., Rahway, NJ, USA.
DATA SHARING STATEMENT
The data sharing policy, including restrictions, of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA is available http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
Footnotes
Abbreviations: AE, adverse event; AUC0–24, area under the concentration-time curve at 0–24 hours; C 24, concentration at 24 hours following the dose or immediately before the next dose; CL/F, apparent clearance; DAA, direct-acting antiviral; EBR, elbasvir; FDC, fixed-dose combination; GZR, grazoprevir; SVR, sustained virologic response; SVR12, sustained virologic response after 12 weeks of treatment.
Wojciech Sluzewski: Deceased.
These data were presented in abstract form at the American Association for the Study of Liver Diseases (AASLD) Liver Meeting 2020; November 13–16, 2020.
Present address: Naim Alkhouri, Arizona Liver Health, Chandler, Arizona, USA.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.hepcommjournal.com.
Contributor Information
Regino P. Gonzalez-Peralta, Email: Regino.Gonzalez-Peralta.MD@AdventHealth.com.
Stefan Wirth, Email: stefan.wirth@helios-gesundheit.de.
Robert H. Squires, Email: rhsquires50@gmail.com.
Frauke Mutschler, Email: Mutschler.Frauke@mh-hannover.de.
Thomas Lang, Email: thomas.lang@klinikum-starnberg.de.
Malgorzata Pawlowska, Email: mpawlowska@cm.umk.pl.
Wojciech Sluzewski, Email: sluzewski@post.home.pl.
Ewa Majda-Stanislawska, Email: emajda@lodz.home.pl.
Bjorn Fischler, Email: bjorn.fischler@karolinska.se.
William F. Balistreri, Email: William.Balistreri@cchmc.org.
Maureen M. Jonas, Email: maureen.jonas@childrens.harvard.edu.
Niviann Blondet, Email: niviann.blondet@seattlechildrens.org.
Philip Rosenthal, Email: prosenth@ucsf.edu.
Naim Alkhouri, Email: nalkhouri@azliver.com.
Rene Romero, Email: rene.romero@choa.org.
Anjana Grandhi, Email: anjana.grandhi@merck.com.
Patricia Castronuovo, Email: patricia.castronuovo@merck.com.
Luzelena Caro, Email: luzelena_caro@merck.com.
Lihong Du, Email: lihong_du@merck.com.
Daniel I.S. Rosenbloom, Email: daniel.rosenbloom@merck.com.
Barbara A. Haber, Email: barbara.haber@merck.com.
REFERENCES
- 1.Indolfi G, Easterbrook P, Dusheiko G, El-Sayed MH, Jonas MM, Thorne C, et al. Hepatitis C virus infection in children and adolescents. Lancet Gastroenterol Hepatol. 2019;4:477–487. [DOI] [PubMed] [Google Scholar]
- 2.Schmelzer J, Dugan E, Blach S, Coleman S, Cai Z, DePaola M, et al. Global prevalence of hepatitis C virus in children in 2018: a modelling study. Lancet Gastroenterol Hepatol. 2020;5:374–392. [DOI] [PubMed] [Google Scholar]
- 3.Balistreri WF, Murray KF, Rosenthal P, Bansal S, Lin CH, Kersey K, et al. The safety and effectiveness of ledipasvir-sofosbuvir in adolescents 12–17 years old with hepatitis C virus genotype 1 infection. Hepatology. 2017;66:371–378. [DOI] [PubMed] [Google Scholar]
- 4.Murray KF, Balistreri WF, Bansal S, Whitworth S, Evans HM, Gonzalez-Peralta RP, et al. Safety and efficacy of ledipasvir-sofosbuvir with or without ribavirin for chronic hepatitis C in children ages 6-11. Hepatology. 2018;68:2158–2166. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz KB, Rosenthal P, Murray KF, Honegger JR, Hardikar W, Hague R, et al. Ledipasvir-sofosbuvir for 12 weeks in children 3 to <6 years old with chronic hepatitis C. Hepatology. 2020;71:422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenthal P, Schwarz KB, Gonzalez-Peralta RP, Lin CH, Kelly DA, Nightingale S, et al. Sofosbuvir and ribavirin therapy for children aged 3 to <12 years with hepatitis C virus genotype 2 or 3 infection. Hepatology. 2020;71:31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonas MM, Squires RH, Rhee SM, Lin CW, Bessho K, Feiterna-Sperling C, et al. Pharmacokinetics, safety, and efficacy of glecaprevir/pibrentasvir in adolescents with chronic hepatitis C virus: Part 1 of the DORA Study. Hepatology. 2020;71:456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghany MG, Morgan TR. Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases–Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology. 2020;71:686–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Association for the Study of Liver Diseases. HCV in Children. American Association for the Study of Liver Diseases; 2020. [Google Scholar]
- 10.Zepatier (elbasvir and grazoprevir) tablets, for Oral Use [package insert]. Merck Sharp & Dohme Corp.; 2021. [Google Scholar]
- 11.Wang SJ, Huang CF, Yu ML. Elbasvir and grazoprevir for the treatment of hepatitis C. Expert Rev Anti Infect Ther. 2021;19:1071–1081. [DOI] [PubMed] [Google Scholar]
- 12.Nangia G, Vierling JM, Kwo P, Brown DD, Klopfer SO, Robertson MN, et al. Safety and tolerability of elbasvir/grazoprevir in chronic hepatitis C virus therapy: integrated analysis from clinical trials. J Viral Hepat. 2020;27:1222–1233. [DOI] [PubMed] [Google Scholar]
- 13.Zeuzem S, Ghalib R, Reddy KR, Pockros PJ, Ben AZ, Zhao Y, et al. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic HCV genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med. 2015;163:1–13. [DOI] [PubMed] [Google Scholar]
- 14.Kwo P, Gane E, Peng CY, Pearlman B, Vierling JM, Serfaty L, et al. Effectiveness of elbasvir and grazoprevir combination, with or without ribavirin, for treatment-experienced patients with chronic hepatitis C infection. Gastroenterology. 2017;152:64–175. [DOI] [PubMed] [Google Scholar]
- 15.Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour H, Jr, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386:1537–1545. [DOI] [PubMed] [Google Scholar]
- 16.Rockstroh JK, Nelson M, Katlama C, Lalezari J, Mallolas J, Bloch M, et al. Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised, open-label trial. Lancet HIV. 2015;2:e319–e327. [DOI] [PubMed] [Google Scholar]
- 17.Ince I, Knibbe CA, Danhof M, de Wildt SN. Developmental changes in the expression and function of cytochrome P450 3A isoforms: evidence from in vitro and in vivo investigations. Clin Pharmacokinet. 2013;52:333–345. [DOI] [PubMed] [Google Scholar]
- 18.Prasad B, Evers R, Gupta A, Hop CE, Salphati L, Shukla S, et al. Interindividual variability in hepatic organic anion-transporting polypeptides and P-glycoprotein (ABCB1) protein expression: quantification by liquid chromatography tandem mass spectroscopy and influence of genotype, age, and sex. Drug Metab Dispos. 2014;42:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bi Y, Liu J, Li L, Yu J, Bhattaram A, Bewernitz M, et al. Role of model-informed drug development in pediatric drug development, regulatory evaluation, and labeling. J Clin Pharmacol. 2019;59(suppl 1):S104–S111. [DOI] [PubMed] [Google Scholar]
- 20.Tanaudommongkon I, John Miyagi S, Green DJ, Burnham JM, van den Anker JN, Park K, et al. Combined pediatric and adult trials submitted to the US Food and Drug Administration 2012–2018. Clin Pharmacol Ther. 2020;108:1018–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Department of Health and Human Services. General clinical pharmacology considerations for pediatric studies for drugs and biological products. Guidance for Industry. Verified August 24, 2018. Accessed February 25, 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/general-clinical-pharmacology-considerations-pediatric-studies-drugs-and-biological-products [DOI] [PubMed]
- 22.Asselah T, Reesink H, Gerstoft J, de Ledinghen V, Pockros PJ, Robertson M, et al. Efficacy of elbasvir and grazoprevir in participants with hepatitis C virus genotype 4 infection: a pooled analysis. Liver Int. 2018;38:1583–1591. [DOI] [PubMed] [Google Scholar]


