Abstract
Background and Objectives
The clinical and physiologic time course for recovery following pediatric mild traumatic brain injury (pmTBI) remains actively debated. The primary objective of the current study was to prospectively examine structural brain changes (cortical thickness and subcortical volumes) and age-at-injury effects. A priori study hypotheses predicted reduced cortical thickness and hippocampal volumes up to 4 months postinjury, which would be inversely associated with age at injury.
Methods
Prospective cohort study design with consecutive recruitment. Study inclusion adapted from American Congress of Rehabilitation Medicine (upper threshold) and Zurich Concussion in Sport Group (minimal threshold) and diagnosed by Emergency Department and Urgent Care clinicians. Major neurologic, psychiatric, or developmental disorders were exclusionary. Clinical (Common Data Element) and structural (3 T MRI) evaluations within 11 days (subacute visit [SA]) and at 4 months (early chronic visit [EC]) postinjury. Age- and sex-matched healthy controls (HC) to control for repeat testing/neurodevelopment. Clinical outcomes based on self-report and cognitive testing. Structural images quantified with FreeSurfer (version 7.1.1).
Results
A total of 208 patients with pmTBI (age = 14.4 ± 2.9; 40.4% female) and 176 HC (age = 14.2 ± 2.9; 42.0% female) were included in the final analyses (>80% retention). Reduced cortical thickness (right rostral middle frontal gyrus; d = −0.49) and hippocampal volumes (d = −0.24) observed for pmTBI, but not associated with age at injury. Hippocampal volume recovery was mediated by loss of consciousness/posttraumatic amnesia. Significantly greater postconcussive symptoms and cognitive deficits were observed at SA and EC visits, but were not associated with the structural abnormalities. Structural abnormalities slightly improved balanced classification accuracy above and beyond clinical gold standards (∆+3.9%), with a greater increase in specificity (∆+7.5%) relative to sensitivity (∆+0.3%).
Discussion
Current findings indicate that structural brain abnormalities may persist up to 4 months post-pmTBI and are partially mediated by initial markers of injury severity. These results contribute to a growing body of evidence suggesting prolonged physiologic recovery post-pmTBI. In contrast, there was no evidence for age-at-injury effects or physiologic correlates of persistent symptoms in our sample.
Pediatric mild traumatic brain injury (pmTBI; used synonymously herein with concussion) is a major public health concern that negatively affects hundreds of thousands of children each year in terms of quality of life, normal development, and social and cognitive functioning.1 Emerging biomarker evidence suggests multifaceted pathologies following pmTBI that evolve over different timescales.2-4 Macroscopic changes in gray matter (GM; volume or cortical thickness) have been reported in some but not all adult mTBI studies, potentially reflective of atrophy or cortical reorganization. Deep GM structures may be particularly sensitive to the effects of mTBI due to the accumulation of shear strain forces in these regions,5 with the potential of increased adverse effects for hippocampi due to the spatial proximity of large, fluid-filled cavities (temporal horn).6 Two studies have prospectively assessed changes in GM following pmTBI,7,8 while others examined the potential effect of acute injury severity characteristics9,10 or age-at-injury effects11 on the expression of structural pathology.
The spectrum of TBI is renowned for heterogeneity both in terms of pathology and clinical presentation. Patients present with varying degrees of loss of consciousness [LOC], posttraumatic amnesia [PTA], and self-reported symptoms. These are then used to diagnose concussion, the presence of persistent postconcussive symptoms (PPCS), and even grade injury severity based on various expert consensus panel criteria.12,13 For example, approximately 20%–35% of patients with pmTBI experience PPCS, and there is great interest in identifying the physiologic underpinnings of prolonged recovery.1,2,14 However, self-reported postconcussive symptoms (PCS) temporally fluctuate in both patients with pmTBI and healthy controls (HC),15,16 which may decrease prediction ability for more objective injury markers. Similarly, LOC and PTA have traditionally not been predictive of subjective symptom resolution post-mTBI,17,18 but 2 recent studies independently replicated a dose-dependent relationship between LOC/PTA and glial fibrillary acidic protein levels in blood following sport-related concussion.9,10
Complicating matters further, there is also a need to disentangle the effects of injury on brain morphometry from the known reorganization of GM that occurs through typical neurodevelopment from middle childhood through adolescence.19,20 Preclinical data suggest that age at injury could moderate potential effects of pmTBI due to either age-related differences in plasticity or by adversely affecting critical periods of development.21 One study investigating the effect of age at injury on GM atrophy in a mixed injury severity sample reported both decreased injury severity and prolonged recovery for adolescents relative to younger children.11
The primary objective of the current study was therefore to determine whether pmTBI resulted in macroscopic changes in GM as a function of age at injury, potentially due to either neurodegeneration or altered neurodevelopmental trajectories. A total of 208 patients with pmTBI (age 8–18) and 176 age- and sex-matched HC were studied in a prospective cohort design. Patients were assessed at subacute (SA: within 11 days postinjury) and early chronic (EC: 4 months postinjury) injury phases. Based on previous work, it was hypothesized that pmTBI would exhibit reduced cortical thickness and hippocampal volume. It was also predicted that more objective injury severity characteristics (LOC and/or PTA) would be associated with cortical thickness changes and hippocampal volume loss, whereas null findings would be observed for total PPCS burden. Finally, we predicted that age at injury would be inversely associated with both clinical recovery1 and volume loss.11
Methods
Participants
Patients with pmTBI (age 8–18 years) were consecutively recruited from local emergency department and urgent care settings between July 2016 and February 2020 as part of an ongoing study. Enrollment criteria included a closed head injury with Glasgow Coma Score ≥13, LOC (if present) limited to 30 minutes, PTA (if present) limited to 24 hours, alteration in mental status at the time of injury, or at least 2 new PCSs. Inclusion criteria therefore represented a blend of the American Congress of Rehabilitation Medicine (upper injury limit threshold) and the Zurich Concussion in Sport Group (minimal criteria threshold). Age- and sex-matched HC, recruited through flyers posted in high foot traffic areas, staffing of community events, and word-of-mouth from existing participants (including pmTBI sibling recruitment), underwent identical assessments at similar time intervals to control for neurodevelopmental confounds and/or effects associated with repeat assessment.
Participants from both groups were excluded based on the history of (1) previous TBI with greater than 30-minute LOC, (2) any neurologic diagnosis, (3) any psychiatric disorders other than adjustment disorder, (4) developmental disorders (autism spectrum disorder or intellectual disability), (5) history of substance abuse/dependence, (6) contraindications for MRI including pregnancy, or (7) non–English speaking. Patients with braces were included as part of a nonquantitative study on radiologic common data elements,22 but were excluded from the current study due to MRI artifacts. Patients with pmTBI were excluded if injury affected the dominant hand or if general anesthesia was administered immediately postinjury for a surgical indication. HC were also excluded for diagnosis of attention-deficit/hyperactivity disorder or a learning disability. A positive urine drug screen (see eMethods, links.lww.com/WNL/C525) also resulted in exclusion.
Clinical Assessments
A Common Data Elements battery of tests was administered at SA/EC visits along with retrospective ratings (see eMethods and eTable 1, links.lww.com/WNL/C525, for status as primary vs secondary outcomes). This included a modified version of the 5P risk score (see eMethods) and a semistructured pediatric interview to ascertain previous history of TBI.23 The Post-Concussion Symptom Inventory (PCSI; see eMethods), Conflict and Behavioral Questionnaire, and the Pediatric Quality of Life Inventory were selected as primary clinical outcome measures. Symptomatic vs asymptomatic PCS status was calculated based on HC data.16 Secondary measures consisted of scales from the Patient-Reported Outcomes Measurement Information System (sleep, anxiety, and depression), self-report pain and headache ratings, Glasgow Outcome Scale Extended, and parent-reported Strengths and Difficulties Questionnaire. Parental distress was measured with the Brief Symptom Inventory-18. A battery of neuropsychological tests was also administered to determine potential cognitive deficits in attention, processing speed, working memory, executive functioning, and long-term memory recall and to estimate reading ability and effort (see eMethods).
Image Acquisition
High-resolution T1-weighted (voxel size = 1.0 mm3), T2-weighted (voxel size = 1.15 × 1.15 × 1.5 mm), susceptibility-weighted (voxel size = 1.0 × 1.0 × 1.5 mm), and fluid-attenuated inversion recovery (voxel size = 0.8 × 0.8 × 3 mm) images were collected on a Siemens 3 T TrioTim scanner with a 32-channel head coil (see eMethods for full sequence details, links.lww.com/WNL/C525). All MRIs were reviewed by a board-certified neuroradiologist blinded to participant group. A subset of patients with pmTBI (N = 99) received CT scans as part of routine care in the hospital.
The FreeSurfer (version 7.1.1) package was used to generate individual cortical thickness surface maps and regions of interest (ROI) volumes using standard segmentation labels.24 Cortical thickness maps were transformed to the FreeSurfer average template (fsaverage) and spatially smoothed using a 10-mm full-width at half-maximum Gaussian kernel. Primary ROI included the bilateral hippocampi, whereas secondary subcortical regions included the nuclei accumbens, the amygdalae, the thalami, the pallida, the putamina, and the caudate nuclei.
Statistical Analysis
Primary and secondary clinical data were assessed using age at injury as a covariate and retrospective ratings when available. Clinical analyses were conducted with either generalized linear models (group effect only) or generalized estimating equations (GEE; group and visit effect), using Gaussian, gamma, or negative binomial distributions. Statistical analyses of the cortical thickness surface data were conducted using linear mixed effects (Analysis of Functional NeuroImages [AFNI] 3dLME) with data missing at random. FreeSurfer data were converted to GIFTI format for use in AFNI. A 2 (visit [SA vs EC]) × 2 (group [HC vs pmTBI]) mixed-effects model with a random intercept was selected with age (unit = month) as a covariate. The minimum cortical surface area needed to achieve cluster-wise significance was p = 0.025 per hemisphere (Bonferroni corrected) based on results from the slow_surf_clustsim.py AFNI program (p = 0.001; 176 mm2).
Clinical and cortical thickness results are reported for main effects of group, visit, group × visit, group × age, and group × visit × age interactions. Subcortical models also included significant effects/interactions associated with hemisphere, with total intracranial volume used as an additional nuisance covariate. Analyses examining the effects of injury severity (presence/absence of LOC/PTA or PPCS) were limited to primary outcome measures and group effects to reduce redundancy. Supplemental analyses were repeated with age treated as an integer (unit = year), with all primary imaging findings replicating.
Finally, a random forests approach25 was used to determine classification accuracy from both clinical (model 1) and structural MRI (model 2) data. The random forests approach was performed using R (randomForestSRC) with 10,000 trees. This statistical approach was selected due to robust external cross-validation, lack of distributional assumptions, protections against data overfitting, and outputs examining variable importance and clinical prediction accuracy (see eMethods, links.lww.com/WNL/C525).
Standard Protocol Approvals, Registrations, and Patient Consents
All procedures were approved by the University of New Mexico Health Sciences Institutional Review Board (IRB #07-272). All participants provided informed assent and/or consent.
Data Availability
All data will be openly available in FITBIR at fitbir.nih.gov, reference number FITBIR-STUDY0000339, at the conclusion of the study.
Results
Demographics
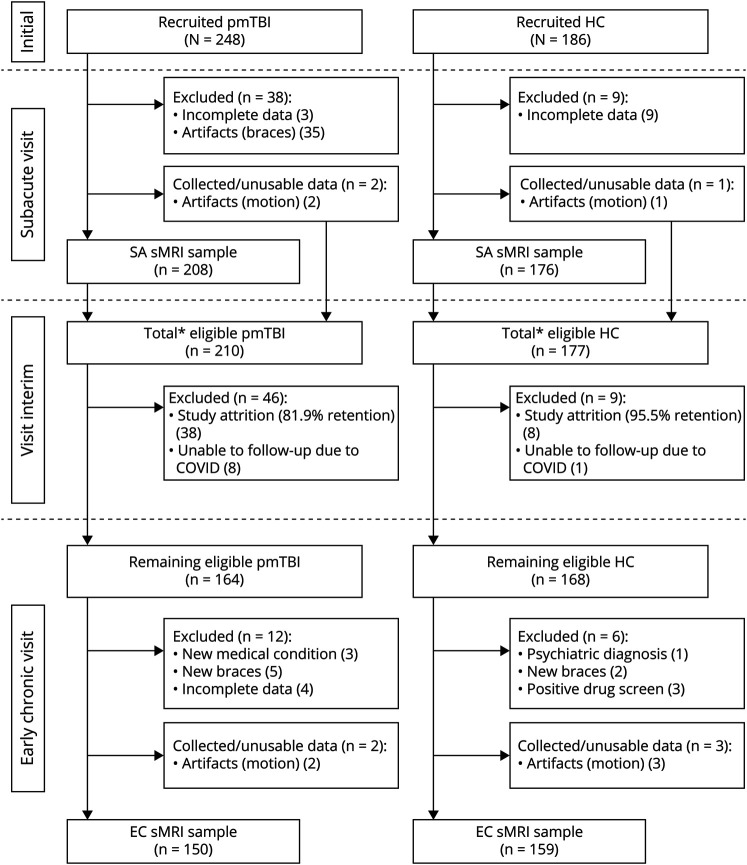
The final sample (see eResults, links.lww.com/WNL/C525; Figure 1) included 208 patients with pmTBI (84 females; age 14.4 ± 2.9; 7.4 ± 2.2 days postinjury) and 176 HC (74 females; age 14.2 ± 2.9) for the SA visit and 150 patients with pmTBI (62 females; 131.1 ± 14.5 days postinjury; 123.7 ± 14.4 days between visits; 81.5% retention without study exclusions) and 159 HC (66 females; 124.6 ± 15.7 days between visits; 95.5% retention without study exclusions) for the EC visit.
Figure 1. Participant Recruitment and Retention.
Flowchart of enrollment, inclusion, and data quality control from the subacute (SA) to early chronic (EC) visit for patients with pediatric mild traumatic brain injury (pmTBI) and matched healthy controls (HC). The asterisk denotes the total number of participants who were eligible to return, which is the sum of participants with usable structural MRI (sMRI) data and those whose data were excluded at the SA visit due to quality assurance issues.
The pmTBI and HC groups did not differ in terms of handedness, age, self-reported Tanner stage of development, or biological sex (all p's ≥ 0.05; see eTable 2, links.lww.com/WNL/C525). Conversely, significant group differences were observed for self-reported history of previous head injuries (χ2 = 9.01, p = 0.003; pmTBI = 17.6%, HC = 7.3%), parental self-reported psychopathology (Wald χ2 = 13.55; p ≤ 0.001; pmTBI > HC), premorbid reading ability (Wald χ2 = 26.70; p ≤ 0.001; pmTBI < HC), and effort (Wald χ2 = 23.17; p < 0.001; pmTBI < HC). The latter 2 measures were therefore included as covariates for cognitive analyses. eTable 2 provides additional information on mechanism of injury and injury characteristics for pmTBI.
Clinical Measures
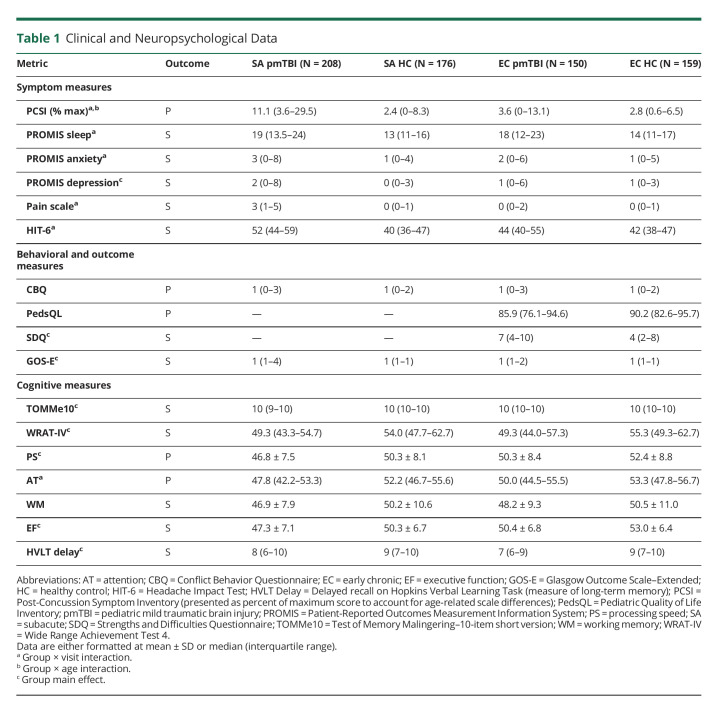
Table 1 presents clinical and neuropsychological results. Significant group × visit × age (Wald χ2 = 6.58; p = 0.010), group × visit (Wald χ2 = 21.24; p < 0.001), and group × age (Wald χ2 = 7.73; p = 0.005) interactions were observed for self-reported PCS severity. Follow-up tests conducted indicated that PCS severity was greater (pmTBI > HC) at the SA (Wald χ2 = 126.38; p < 0.001; 36.1% symptomatic) relative to EC visit (Wald χ2 = 11.50; p = 0.001; 20.7% symptomatic), suggesting partial but not full recovery. Moreover, the group × age interaction was significant at the SA (Wald χ2 = 27.40; p < 0.001) but not EC (p > 0.10) visit, with a positive association between PCS and age at injury within pmTBI at the SA visit (β = 0.061; p = 0.011) contrasted to a negative association in HC (β = −0.095; p < 0.001). Both primary quality of life and self-reported behavioral disturbances were not significant for group main effects or interactions (all p's > 0.0167 following Bonferroni correction).
Table 1.
Clinical and Neuropsychological Data
Tests examining secondary clinical measures (Bonferroni correction 0.05/7 = 0.007) demonstrated significant group × visit interactions for anxiety, sleep complaints, pain, and headaches (all p's ≤ 0.002), characterized by increased symptoms (pmTBI > HC) at the SA visit (all p's ≤ 0.001). There were no group-wise differences for anxiety, pain, and headache (all p's > 0.05) at the EC visit, with sleep complaints still higher for pmTBI but at a lower magnitude (p = 0.004). Worse outcomes (all p's ≤ 0.001) were also present for pmTBI for secondary measures of depression, a parent-rated multidimensional measure of behavioral functioning, and trauma-related functional outcome, but these effects did not statistically vary as a function of visit (i.e., main effect).
Cognitive Testing
Results for primary cognitive measures indicated a significant (Wald χ2 = 8.67; p = 0.003) main effect for group (HC > pmTBI) for processing speed and a significant group × visit interaction for attention (Wald χ2 = 6.17; p = 0.013) after controlling for effort and reading ability (Bonferroni corrected p's < 0.025). As expected, attentional performance deficits were observed at the SA (p = 0.012; HC > pmTBI) but not EC (p = 0.482) visit. Secondary analyses were significant for the main effect of group (HC > pmTBI) for both executive dysfunction (p = 0.007) and long-term memory deficits (p < 0.001). All group effects and interactions were null for working memory after correcting for multiple comparisons (all p's > 0.0167).
Radiologic Findings
A total of 8/99 (8.1%) pmTBI had positive day-of-injury CT scans for intracranial pathology or skull fracture (complicated pmTBI). Four pmTBI had negative CT scans but were diagnosed with probable trauma-related findings on MRI (eTable 2, links.lww.com/WNL/C525).22 Primary group findings were unchanged when these individuals were eliminated from analyses.
Cortical Thickness Analyses for Effects of Group
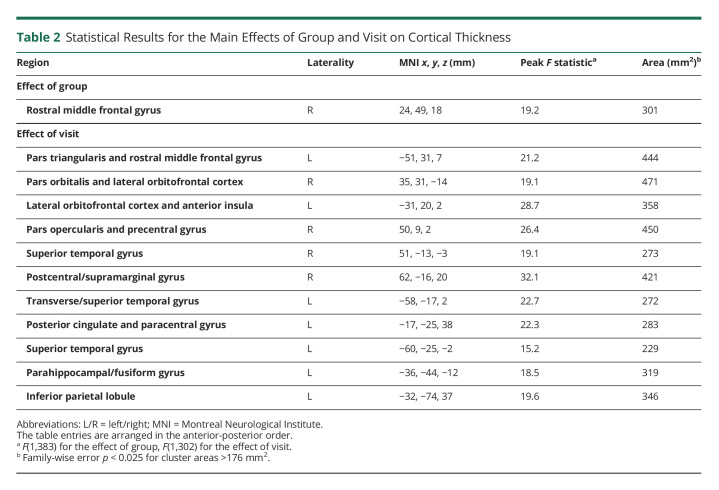
Results from the 2 × 2 (group [HC vs pmTBI] × visit [SA vs EC]) 3dLME indicated significant main effects of group, visit, and age, with no significant interactions. The main effect of group was significant for the right rostral middle frontal gyrus (Figure 2; Table 2), with pmTBI exhibiting reduced cortical thickness compared with HC (Cohen d = −0.49). Group effect size maps are presented in eFigure 1 (links.lww.com/WNL/C525). The main effect of visit was significant for 7 cortical areas on the left and 4 regions in the right hemisphere (summarized in Table 2; eFigure 2). All regions demonstrated reduced cortical thickness for the EC visit compared with the SA visit. The effect of age was widespread in both hemispheres (eFigure 3) and indicated decreased cortical thickness as a function of increasing age.
Figure 2. Group Effects in Cortical Thickness Analysis.
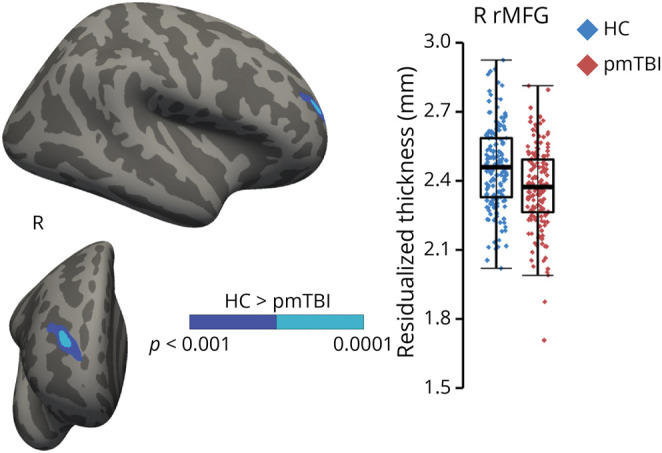
Main effect of group from the cortical thickness analysis for patients with pediatric mild traumatic brain injury (pmTBI; red diamonds) and healthy controls (HCs; blue diamonds). An inflated brain rendering presents a significant cluster within the right (R) rostral middle frontal gyrus (rMFG), with pmTBI exhibiting decreased cortical thickness relative to HC (dark blue: p < 0.001; cyan: p < 0.0001). Data points in the box-and-scatter plot have been residualized (Resid.) to remove the effect of age.
Table 2.
Statistical Results for the Main Effects of Group and Visit on Cortical Thickness
Subcortical Volumetric Analyses for Effects of Group
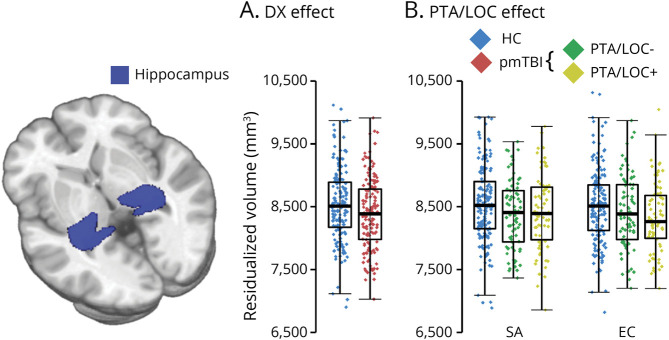
Separate 2 × 2 × 2 (group [HC vs pmTBI] × visit [SA vs EC] × hemisphere [right vs left]) GEE models examined subcortical volume loss with age at injury and total intracranial volume as covariates. There were no outliers for any of the subcortical volume measurements. Primary analyses (Figure 3A) indicated significantly reduced hippocampal volumes for pmTBI relative to HC (Wald χ2 = 7.32; p = 0.007; Cohen d = −0.24), along with a main effect for hemisphere (Wald χ2 = 552.73; p < 0.001; right > left).
Figure 3. Group Effects in Subcortical Volume Analysis.
Panel A presents subcortical volumetric results for primary analyses examining differences between diagnoses (DX: healthy controls [HC] = blue diamonds; patients with pediatric mild traumatic brain injury [pmTBI] = red diamonds). Panel B presents pmTBI subgroups based on injury characteristics (no reported posttraumatic amnesia or loss of consciousness [PTA/LOC-] = green diamonds; reported PTA/LOC = yellow diamonds). A glass brain rendering of the hippocampus (blue) is present to the left of the panels. All data points in box-and-scatter plots have been residualized (Resid.) to remove the effects of total intracranial volume and age. Hippocampal volumes were reduced in pmTBI relative to HC at both visits (Panel A), but recovery was greater for pmTBI who reported no PTA/LOC at the time of injury. eFigure 4 (links.lww.com/WNL/C525) depicts data for secondary subcortical volumes.
Secondary analyses for amygdalae, thalami, pallida, putamina, and caudate nuclei (eFigure 4, links.lww.com/WNL/C525) were nonsignificant for any main effects of group or interactions with group following Bonferroni correction (0.05/6 = 0.008). The nuclei accumbens indicated a significant group × visit × age × hemisphere interaction (Wald χ2 = 21.72; p < 0.001), but none of the main effects or interactions with group were significant when a follow-up GEE was performed examining each hemisphere separately. Age exhibited significant negative associations with amygdalae (β = −0.005; Wald χ2 = 8.74; p = 0.003), caudate nuclei (β = −0.007; Wald χ2 = 12.96; p < 0.001), and nuclei accumbens (β = −0.007; Wald χ2 = 34.03; p < 0.001) volume. See eResults for main effects associated with hemisphere and visit.
Cortical Thickness and Volumetric Analyses for Effects of Injury Characteristics
Primary analyses were repeated with the pmTBI group further divided by more traditional injury severity characteristics (positive LOC and/or PTA; full sample) and the presence of PPCS at 4 months postinjury (limited to participants who returned for the EC visit). Results from a 3 × 2 (group [HC vs pmTBI+LOC/PTA vs pmTBI-LOC/PTA] × visit [SA vs EC]) cortical thickness analyses indicated null findings for the main effect of group and nonsignificant group × visit and group × visit × age interactions. Null results were also obtained from a 3 × 2 (group [HC vs pmTBI+PPCS vs pmTBI-PPCS] × visit [SA vs EC]) PPCS analysis for PPCS cortical thickness analyses.
Results for hippocampi indicated a significant group × visit interaction (Wald χ2 = 6.02; p = 0.049) for the presence of LOC/PTA (Figure 3B). The main effect of group was significant at the SA visit (p = 0.038) but only a trend at the EC (p = 0.078) visit. Simple effect testing indicated that the pmTBI-LOC/PTA group exhibited reduced hippocampal volumes at the SA (p = 0.019) but not EC (p = 0.176) visit, whereas in pmTBI+LOC/PTA demonstrated a trend for reduced hippocampal volume at the SA visit (p = 0.061) that remained significantly decreased at the EC (p = 0.033) visit. There were no differences between the 2 pmTBI groups at either visit (p's ≥ 0.384). No interactions or main effect of interest were significant (all p's > 0.05) for hippocampi analyses examining PPCS.
Structural Abnormalities and Clinical Correlations
The next set of analyses examined the relationship between clinical indices of cognitive dysfunction (independent variables: measures of attention, processing speed, working memory, executive functioning, and long-term memory recall) and structural abnormalities (cortical thickness and hippocampi volumes) and was limited to the pmTBI group. However, both regression models were nonsignificant (Bonferroni corrected p's > 0.025).
A supervised machine learning algorithm investigated the diagnostic accuracy of the 5P risk score and PCSI (model 1) vs the diagnostic accuracy when the right rostral frontal gyrus thickness and hippocampal volume were included in the model (model 2). HC served as the reference group. Results from model 1 indicated approximately 70.7% balanced classification accuracy (Figure 4; sensitivity = 72.9%; specificity = 68.6%), with variable importance scores of 13.5% for the 5P risk score and 2.3% for the PCSI. Balanced classification accuracy increased to 74.6% when structural abnormalities were included in model 2, with a greater increase in specificity (sensitivity = 73.2%; specificity = 76.1%). However, the 5P score maintained the highest variable importance score at 11.4%.
Figure 4. Group Classification Using Random Forest Machine Learning Algorithm.
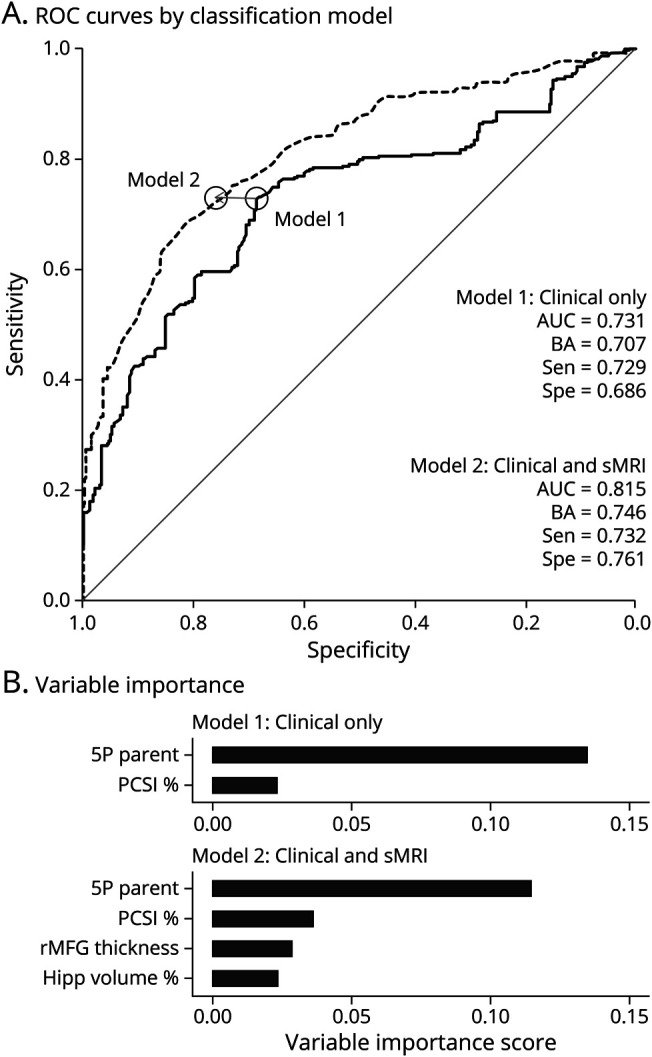
Receiver operating characteristic (ROC; A) results from the random forest supervised machine learning algorithm for classifying patients with pediatric mild traumatic brain injury relative to healthy controls. Diagnostic accuracy was compared based on a model that included current clinical gold standards (model 1: 5P risk score [5P] and Post-Concussion Symptom Inventory total score [PCSI]; solid line) or clinical gold standards in conjunction with significant structural MRI (sMRI) findings (model 2; dashed line). Structural abnormalities included reduced cortical thickness of the rostral middle frontal gyrus [rMFG] and the volume of the hippocampi (Hipp) relative to total intracranial volume. Estimates for area under the curve (AUC), balanced accuracy (BA), sensitivity (Sen), and specificity (Spe) are provided for each model, with the intersection of optimal sensitivity/specificity denoted with a circle for each model. Panel B lists the variable importance score for variables in each model.
Discussion
The current study prospectively examined quantitative changes in the GM structure over a 4-month period in a large cohort of patients with pmTBI relative to a sex- and age-matched HC. Results indicated small but significant differences in postinjury emotional, physical, and cognitive symptoms that persisted up to 4 months post-TBI. In addition, pmTBI was associated with reduced prefrontal cortical thickness in the right hemisphere and smaller hippocampal volumes, with the latter being mediated by traditional indicators of injury severity (LOC/PTA) in concussion research. These structural deficits improved the specificity of injury classification above and beyond clinical gold standards. However, there were no associations between cognitive deficits/symptom burden and structural abnormalities, and age at injury similarly did not account for any significant variance in terms of GM differences or recovery.
Similar to previous studies,26 results from our extensive clinical battery indicated small but statistically significant residual deficits in multiple emotional, physical, and cognitive domains. Specifically, processing speed, executive functioning, and long-term memory remained below the control group performance at 4 months postinjury even when controlling for premorbid reading abilities, whereas attentional functioning showed evidence of recovery. However, neither cortical thickness nor reduced hippocampal volumes were associated with cognitive deficits, which may be reflective of the small effect sizes observed for both cognitive testing and structural pathology, as well as the potential nonspecific nature of cognitive deficits.27 Thus, even if pmTBI results in long-standing physiologic changes, it may be challenging to relate physiologic changes to clinical measurements (cognitive, behavioral, or somatic symptoms). Similarly, several (pain, headache, and anxiety) physical and emotional symptoms demonstrated more complete resolution, whereas others (postconcussive symptoms, sleep, and depression) remained elevated at 4 months postinjury in the pmTBI group. Collectively, current and previous findings highlight the need for multidimensional clinical assessments multiple months postinjury.27
Current results indicate that hippocampal volume was reduced following pmTBI and that this reduction was further mediated by the presence of LOC/PTA but not PPCS. Smaller hippocampal volumes have been reported in children with mild, moderate, and severe TBI years following injury,28,29 in young adult athletes with a high exposure (concussions and subconcussive blows) history,30-32 in retired American football players with a high exposure history,33 and in adults decades after their most recent mTBI.34 Recent modeling studies suggest that the hippocampi may be more susceptible to injury due to the proximity of the fluid-filled temporal horns.6 Null findings for hippocampal volume have also been reported in a previous prospective pmTBI (N = 15) study,7 but this difference may be a result of the relatively small sample size based on current effect size estimates.
Several earlier (Cantu guidelines, American and Colorado Sports Concussion, American Academy of Neurology; Grade I, II, and III concussion grading) expert consensus panel guidelines recommend grading mTBI severity based on the presence of LOC/PTA or using LOC/PTA to stratify injury probability (possible, probable, and definitive).35 However, the inclusion of LOC/PTA for grading injury severity has generally not been adapted by more recent expert consensus panels.12 A large reason for this is because LOC/PTA has traditionally not been shown to be predictive of functional and symptom-based outcomes in mTBI,17,18 and LOC/PTA is more infrequent in sport-related concussion. However, LOC and PTA have higher test-retest reliability and specificity relative to postconcussive symptoms23 and have recently demonstrated a dose-dependent relationship with blood-based biomarkers in 2 independent studies.9,10 Thus, future research is necessary to determine whether LOC/PTA is a better predictor of objective biomarkers than subjective findings of injury severity (PPCS) across the spectrum of mTBI.
Current results indicated reduced cortical thickness in the right rostral middle frontal gyrus for pmTBI, with no evidence of recovery across a 4-month study period. Previous research suggests that neuronal degeneration occurs in the first few months of severe TBI and continues into more chronic injury phases, with rates of tissue loss ranging from 1% to 5% per year36,37 regardless or not of whether macroscopic lesions are present. In contrast, research on atrophy and/or neuropil loss following adult mTBI is more controversial, with mixed results across multiple adult studies.37-40 Similarly, previous cross-sectional studies in pmTBI have reported no differences in GM volume at 3 months post-pmTBI (N = 26) relative to sibling controls41 and at 6 months postinjury (age 8–15; N = 219) relative to orthopedically injured controls.42 Other cross-sectional studies have reported increased cortical thickness in the left parietal cortex for patients with pmTBI (age 8 to 16; N = 136) relative to orthopedically injured controls.43
One other prospective study of emergency department patients (age 10–17; N = 15) also reported reduced thickness values in several cortical regions from 2 weeks to 4 months post-pmTBI.7 Thus, it is possible that prospective designs may be more sensitive to smaller differences in cortical thinning than cross-sectional designs due to the decreased variance associated with repeat visits.7,39 Both current and previous7 prospective pmTBI studies also independently replicated findings of reduced cortical thickness in the right middle frontal gyrus. The prefrontal cortex is commonly affected by TBI pathology across all levels of severity and tended to exhibit the largest effect sizes in the current sample (eFigure 1, links.lww.com/WNL/C525). Importantly, effect size maps indicated that these potential differences are mostly small to moderate in magnitude and may therefore only be observed in large sample sizes. Nonetheless, the findings of reduced cortical thickness and hippocampal volume appeared to be clinically meaningful, as they improved the specificity of patient classification by approximately 8% even when using current clinical gold standards (5P risk score and total symptom burden).
Among the injured, patients with pmTBI with PPCS represent a greater public health burden due to costs associated with prolonged patient care and continued disruptions for return to normal activities.1 A minority of patients with pmTBI in the current study self-endorsed significant PCS at the SA visit in the current study (36.1%; N = 75), with 20.7% (N = 31) reporting PPCS at the EC visit. Clinically significant symptoms were determined using normative estimation methods that significantly reduce the rate of false positives.16 However, there were no significant differences in cortical thickness or hippocampal volumes for pmTBI with PPCS relative to either HC or pmTBI without PPCS.
Although 1 other study reported that adolescents with PPCS (age 10–14; N = 27) had smaller posterior cingulate cortex, caudal anterior cingulate cortex, and total brain volume relative to controls at 1 month postinjury,44 these findings did not replicate at 6 months postinjury in a similar sample when appropriate corrections for multiple comparisons were enacted.8 Null findings associated with PPCS may result from the nonspecific nature of postconcussive symptoms,15 their low to moderate test-retest reliability,16 and/or from reduced statistical power associated with smaller sample size of patients with PPCS (N = 31 in the current sample). Importantly, other recent larger sample studies have reported white matter microstructural abnormalities that varied as a function of PPCS presence (primarily affecting mean diffusivity) or absence (primarily affecting fractional anisotropy) relative to orthopedic injured controls,45 suggesting that additional work is needed to understand the potential biological basis of PPCS.14
In contrast to previous results,11 age at injury did not significantly interact with diagnosis in terms of cortical thickness or subcortical volume loss. Preclinical models suggest improved outcomes in the very young due to increased plasticity following TBI, although contrary evidence also exists for injuries occurring during critical developmental periods.21 Clinical studies have also reported worse outcomes for children who were injured before 7 years of age in mixed injury samples,46 whereas null age-at-injury findings have also been observed for diffusion47,48 and resting-state metrics of regional homogeneity and low-frequency fluctuations.49 Thus, current null findings could be the result of the older age range (8–18 years) and associated neurodevelopmental stages investigated in the current study, the highly prescribed subacute postinjury intervals at which participants were assessed, or due to the exclusion of more severely injured patients with TBI. As such, current null age-at-injury effects on structural abnormalities may not generalize to more severely injured or chronic TBI samples or to injuries that occur in the very young.
Cortical thickness was reduced both as a function of increasing age and study visit (SA > EC). Previous large-scale neurodevelopmental studies (N > 1,000) have also reported a reduction in cortical thickness as a function of age.19,20 However, it was unexpected to observe this effect at such a short time interval between visits (i.e., 4 months) during longitudinal analyses. Age-related reductions in cortical thickness have primarily been attributed to the combination of synaptic pruning of excessive connections in conjunction with increased myelination.19 Current findings of reductions in cortical thickness over a 4-month window highlight the relatively short time scale over which these neurodevelopment changes may occur and emphasize the need for large, prospective control samples to reduce the likelihood of spurious conclusions attributed to trauma rather than typical neurodevelopment in pmTBI studies.
Strengths of the current study include a large and homogeneous (same level of injury severity collected at similar time points postinjury) sample collected longitudinally at the same imaging center with a high retention rate for follow-up visits (>80%). This partially mitigates concerns regarding smaller effect sizes associated with pmTBI that may be further obscured by interscanner, intervendor, and even intrasoftware variances.48 However, this single-site recruiting strategy also increases the risk of sampling bias and limits generalization of findings to broader clinical contexts. Second, the current study did not include an orthopedically injured control cohort, which may control for nonspecific injury effects and potential differences in sampling strategies.42,43 Third, the current study may have had limited power to detect complex, nonlinear interactions between age at injury and quantitative structural abnormalities. Fourth, sexual dimorphisms have been reported as a function of both age19,20 and mTBI,50 but were not examined in the current study. Finally, although the current study exclusively focused on GM changes postinjury, several recent studies have also observed white matter pathology post-pmTBI in large samples that also persist multiple months postinjury.45,47 Thus, future studies that examine pathology from a multimodal perspective are needed, as well as the potential relationship between injury biomarkers and clinical outcomes.
In summary, previous research suggests that pmTBI may result in delays in academic achievement, decreases in overall quality of health, and an increased incidence of neuropsychiatric conditions.14 Current clinical results suggest an impartial recovery at 4 months postinjury for clinical and cognitive functioning, with additional evidence of structural abnormalities within the right middle frontal gyrus and hippocampi. Recovery of hippocampal volume loss appeared to be partially mediated by injury severity as indexed by LOC/PTA, suggesting the need to reconsider the role of these injury markers in more objective outcomes.9,10 Current results highlight the need for more extended surveillance intervals to determine the potential long-term, physiologic effects of pmTBI on neurodevelopment.
Glossary
- AFNI
Analysis of Functional NeuroImages
- EC
early chronic
- GEE
generalized estimating equation
- GM
gray matter
- HC
healthy controls
- LOC
loss of consciousness
- PCS
postconcussive symptoms
- PCSI
Post-Concussion Symptom Inventory
- pmTBI
pediatric mild traumatic brain injury
- PPCS
persistent PCS
- PTA
posttraumatic amnesia
- ROI
region of interest
- SA
subacute
Appendix. Authors
Footnotes
See page e555
Study Funding
This research was supported by grants from the NIH (nih.gov; grant numbers NIH 01 R01 NS098494-01A1, R01 NS098494-03S1A1, and P30 GM122734) to A.R. Mayer.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Zemek R, Barrowman N, Freedman SB, et al. Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA. 2016;315(10):1014-1025. doi: 10.1001/jama.2016.1203. [DOI] [PubMed] [Google Scholar]
- 2.Mayer AR, Kaushal M, Dodd AB, et al. Advanced biomarkers of pediatric mild traumatic brain injury: progress and perils. Neurosci Biobehav Rev. 2018;94:149-165. doi: 10.1016/j.neubiorev.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mannix R, Levy R, Zemek R, et al. Fluid biomarkers of pediatric mild traumatic brain injury: a systematic review. J Neurotrauma. 2020;37(19):2029-2044. doi: 10.1089/neu.2019.6956. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt J, Hayward KS, Brown KE, et al. Imaging in pediatric concussion: a systematic review. Pediatrics. 2018;141(5):e20173406. doi: 10.1542/peds.2017-3406. [DOI] [PubMed] [Google Scholar]
- 5.Meaney DF, Smith DH. Biomechanics of concussion. Clin Sports Med. 2011;30(1):19-31. doi: 10.1016/j.csm.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Z, Li X, Domel AG, et al. The presence of the temporal horn exacerbates the vulnerability of hippocampus during head impacts. Front Bioeng Biotechnol. 2022;10:754344. doi: 10.3389/fbioe.2022.754344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer AR, Hanlon FM, Ling JM. Gray matter abnormalities in pediatric mild traumatic brain injury. J Neurotrauma. 2015;32(10):723-730. doi: 10.1089/neu.2014.3534. [DOI] [PubMed] [Google Scholar]
- 8.Mac Donald CL, Barber J, Wright J, et al. Longitudinal clinical and neuroimaging evaluation of symptomatic concussion in 10- to 14-year-old youth athletes. J Neurotrauma. 2019;36(2):264-274. doi: 10.1089/neu.2018.5629. [DOI] [PubMed] [Google Scholar]
- 9.McCrea M, Broglio SP, McAllister TW, et al. Association of blood biomarkers with acute sport-related concussion in collegiate athletes: findings from the NCAA and Department of Defense CARE Consortium. JAMA Netw Open. 2020;3(1):e1919771. doi: 10.1001/jamanetworkopen.2019.19771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meier TB, Huber DL, Bohorquez-Montoya L, et al. A prospective study of acute blood-based biomarkers for sport-related concussion. Ann Neurol. 2020;87(6):907-920. doi: 10.1002/ana.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ewing-Cobbs L, Johnson CP, Juranek J, et al. Longitudinal diffusion tensor imaging after pediatric traumatic brain injury: impact of age at injury and time since injury on pathway integrity. Hum Brain Mapp. 2016;37(11):3929-3945. doi: 10.1002/hbm.23286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer AR, Quinn DK, Master CL. The spectrum of mild traumatic brain injury: a review. Neurology. 2017;89(6):623-632. doi: 10.1212/wnl.0000000000004214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverberg ND, Iverson GL; ACRM Mild TBI Definition Expert Consensus Group and the ACRM Brain Injury Special Interest Group Mild TBI Task Force. Expert panel survey to update the American Congress of Rehabilitation Medicine definition of mild traumatic brain injury. Arch Phys Med Rehabil. 2021;102(1):76-86. doi: 10.1016/j.apmr.2020.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Rausa VC, Anderson V, Babl FE, Takagi M. Predicting concussion recovery in children and adolescents in the emergency department. Curr Neurol Neurosci Rep. 2018;18(11):78. doi: 10.1007/s11910-018-0881-z. [DOI] [PubMed] [Google Scholar]
- 15.Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr. 2015;169(12):1132-1140. doi: 10.1001/jamapediatrics.2015.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer AR, Stephenson DD, Dodd AB, et al. Comparison of methods for classifying persistent post-concussive symptoms in children. J Neurotrauma. 2020;37(13):1504-1511. doi: 10.1089/neu.2019.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNally KA, Bangert B, Dietrich A, et al. Injury versus noninjury factors as predictors of postconcussive symptoms following mild traumatic brain injury in children. Neuropsychology. 2013;27(1):1-12. doi: 10.1037/a0031370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Naalt J, Timmerman ME, de Koning ME, et al. Early predictors of outcome after mild traumatic brain injury (UPFRONT): an observational cohort study. Lancet Neurol. 2017;16(7):532-540. doi: 10.1016/s1474-4422(17)30117-5. [DOI] [PubMed] [Google Scholar]
- 19.Gennatas ED, Avants BB, Wolf DH, et al. Age-related effects and sex differences in gray matter density, volume, mass, and cortical thickness from childhood to young adulthood. J Neurosci. 2017;37(20):5065-5073. doi: 10.1523/jneurosci.3550-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brain Development Cooperative Group. Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI Study of Normal Brain Development. Cereb Cortex. 2012;22(1):1-12. doi: 10.1093/cercor/bhr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolb B, Teskey GC. Age, experience, injury, and the changing brain. Dev Psychobiol. 2012;54(3):311-325. doi: 10.1002/dev.20515. [DOI] [PubMed] [Google Scholar]
- 22.Mayer AR, Cohen DM, Wertz CJ, et al. Radiologic common data elements rates in pediatric mild traumatic brain injury. Neurology. 2020;94(3):e241-e253. doi: 10.1212/wnl.0000000000008488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hergert DC, Sicard V, Stephenson DD, et al. Test-retest reliability of a semi-structured interview to aid in pediatric traumatic brain injury diagnosis. J Int Neuropsychol Soc. 2021;28(7):687-699. doi: 10.1017/s1355617721000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968-980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Breiman L. Random forests. Machine Learn. 2001;45(1):5-32. doi: 10.1023/a:1010933404324. [DOI] [Google Scholar]
- 26.Babikian T, Satz P, Zaucha K, Light R, Lewis RS, Asarnow RF. The UCLA longitudinal study of neurocognitive outcomes following mild pediatric traumatic brain injury. J Int Neuropsychol Soc. 2011;17(5):886-895. doi: 10.1017/s1355617711000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polinder S, Cnossen MC, Real RGL, et al. A multidimensional approach to post-concussion symptoms in mild traumatic brain injury. Front Neurol. 2018;9:1113. doi: 10.3389/fneur.2018.01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beauchamp MH, Ditchfield M, Maller JJ, et al. Hippocampus, amygdala and global brain changes 10 years after childhood traumatic brain injury. Int J Dev Neurosci. 2011;29(2):137-143. doi: 10.1016/j.ijdevneu.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Wilde EA, Bigler ED, Hunter JV, et al. Hippocampus, amygdala, and basal ganglia morphometrics in children after moderate-to-severe traumatic brain injury. Dev Med Child Neurol. 2007;49(4):294-299. doi: 10.1111/j.1469-8749.2007.00294.x. [DOI] [PubMed] [Google Scholar]
- 30.Meier TB, Espana L, Nitta ME, et al. Positive association between serum quinolinic acid and functional connectivity following concussion. Brain Behav Immun. 2021;91:531-540. doi: 10.1016/j.bbi.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parivash SN, Goubran M, Mills BD, et al. Longitudinal changes in hippocampal subfield volume associated with collegiate football. J Neurotrauma. 2019;36(19):2762-2773. doi: 10.1089/neu.2018.6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh R, Meier TB, Kuplicki R, et al. Relationship of collegiate football experience and concussion with hippocampal volume and cognitive outcomes. JAMA. 2014;311(18):1883-1888. doi: 10.1001/jama.2014.3313. [DOI] [PubMed] [Google Scholar]
- 33.Misquitta K, Dadar M, Tarazi A, et al. The relationship between brain atrophy and cognitive-behavioural symptoms in retired Canadian football players with multiple concussions. Neuroimage Clin. 2018;19:551-558. doi: 10.1016/j.nicl.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.June D, Williams OA, Huang CW, et al. Lasting consequences of concussion on the aging brain: findings from the Baltimore Longitudinal Study of Aging. Neuroimage. 2020;221:117182. doi: 10.1016/j.neuroimage.2020.117182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kutcher JS, Giza CC. Sports concussion diagnosis and management. Continuum (Minneap Minn). 2014;20(6 Sports Neurology):1552-1569. doi: 10.1212/01.con.0000458974.78766.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cole JH, Jolly A, de Simoni S, et al. Spatial patterns of progressive brain volume loss after moderate-severe traumatic brain injury. Brain. 2018;141(3):822-836. doi: 10.1093/brain/awx354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris TC, de Rooij R, Kuhl E. The shrinking brain: cerebral atrophy following traumatic brain injury. Ann Biomed Eng. 2019;47(9):1941-1959. doi: 10.1007/s10439-018-02148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ling JM, Klimaj S, Toulouse T, Mayer AR. A prospective study of gray matter abnormalities in mild traumatic brain injury. Neurology. 2013;81(24):2121-2127. doi: 10.1212/01.wnl.0000437302.36064.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller AM, Panenka WJ, Lange RT, Iverson GL, Brubacher JR, Virji-Babul N. Longitudinal changes in brain parenchyma due to mild traumatic brain injury during the first year after injury. Brain Behav. 2021;11(12):e2410. doi: 10.1002/brb3.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Y, Kierans A, Kenul D, et al. Mild traumatic brain injury: longitudinal regional brain volume changes. Radiology. 2013;267(3):880-890. doi: 10.1148/radiol.13122542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeates KO, Luria J, Bartkowski H, Rusin J, Martin L, Bigler ED. Postconcussive symptoms in children with mild closed head injuries. J Head Trauma Rehabil. 1999;14(4):337-350. doi: 10.1097/00001199-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Bigler ED, Finuf C, Abildskov TJ, et al. Cortical thickness in pediatric mild traumatic brain injury including sports-related concussion. Int J Psychophysiol. 2018;132:99-104. doi: 10.1016/j.ijpsycho.2018.07.474. [DOI] [PubMed] [Google Scholar]
- 43.Ware AL, Goodrich-Hunsaker NJ, Lebel C, et al. Post-acute cortical thickness in children with mild traumatic brain injury versus orthopedic injury. J Neurotrauma. 2020;37(17):1892-1901. doi: 10.1089/neu.2019.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mac Donald CL, Barber J, Wright J, et al. Quantitative volumetric imaging and clinical outcome characterization of symptomatic concussion in 10- to 14-year-old adolescent athletes. J Head Trauma Rehabil. 2018;33(6):E1-E10. doi: 10.1097/htr.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 45.Ware AL, Yeates KO, Tang K, et al. Longitudinal white matter microstructural changes in pediatric mild traumatic brain injury: an A-CAP study. Hum Brain Mapp. 2022;43(12):3809-3823. doi: 10.1002/hbm.25885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson V, Moore C. Age at injury as a predictor of outcome following pediatric head injury: a longitudinal perspective. Child Neuropsychol. 1995;1(3):187-202. doi: 10.1080/09297049508400224. [DOI] [Google Scholar]
- 47.Mayer AR, Ling JM, Dodd AB, et al. Multicompartmental models and diffusion abnormalities in paediatric mild traumatic brain injury. Brain. 2022;145(11):4124-4137. doi: 10.1093/brain/awac221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodrich-Hunsaker NJ, Abildskov TJ, Black G, et al. Age- and sex-related effects in children with mild traumatic brain injury on diffusion magnetic resonance imaging properties: a comparison of voxelwise and tractography methods. J Neurosci Res. 2018;96(4):626-641. doi: 10.1002/jnr.24142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stephenson DD, Meier TB, Pabbathi Reddy S, et al. Resting-state power and regional connectivity after pediatric mild traumatic brain injury. J Magn Reson Imaging. 2020;52(6):1701-1713. doi: 10.1002/jmri.27249. [DOI] [PubMed] [Google Scholar]
- 50.Adamson MM, Main K, Harris OA, Kang X. Sex differences in cortical thickness and diffusion properties in patients with traumatic brain injury: a pilot study. Brain Inj. 2022;36(4):488-502. doi: 10.1080/02699052.2022.2034046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data will be openly available in FITBIR at fitbir.nih.gov, reference number FITBIR-STUDY0000339, at the conclusion of the study.