Abstract
Neurologists have long recognized the importance of the visual system in the diagnosis and monitoring of neurologic disorders. This is particularly true because approximately 50% of the brain's pathways subserve afferent and efferent aspects of vision. During the past 30 years, researchers and clinicians have further refined this concept to include investigation of the visual system for patients with specific neurologic diagnoses, including multiple sclerosis (MS), concussion, Parkinson disease (PD), and conditions along the spectrum of Alzheimer disease (AD, mild cognitive impairment, and subjective cognitive decline). This review highlights the visual “toolbox” that has been developed over the past 3 decades and beyond to capture both structural and functional aspects of vision in neurologic disease. Although the efforts to accelerate the emphasis on structure-function relationships in neurologic disorders began with MS during the early 2000s, such investigations have broadened to recognize the need for outcomes of visual pathway structure, function, and quality of life for clinical trials of therapies across the spectrum of neurologic disorders. This review begins with a patient case study highlighting the importance using the most modern technologies for visual pathway assessment, including optical coherence tomography. We emphasize that both structural and functional tools for vision testing can be used in parallel to detect what might otherwise be subclinical events or markers of visual and, perhaps, more global neurologic decline. Such measures will be critical because clinical trials and therapies become more available across the neurologic disease spectrum.
Approximately 50% of the brain's pathways subserve visual function, and numerous neurologic conditions initially present with visual symptoms.1,2 Many neurologic diseases have manifestations that localize to the pathways for afferent and efferent visual function. At the same time, tools to capture visual pathway structure and function have only recently become incorporated into therapeutic trials, research and clinical assessment for patients with neurologic conditions.1,3
As such, the past 30 years have represented a time of great interest in vision as an important window into neurologic function. A rapidly growing literature has aimed to better characterize visual manifestations in multiple sclerosis (MS), concussion, Parkinson disease (PD), and cognitive conditions along the spectrum of Alzheimer disease (AD), such as mild cognitive impairment (MCI). The Optic Neuritis Treatment Trial (ONTT) introduced both neurologists and ophthalmologists to the concept that high-contrast visual acuity (black letters on a white background) may not adequately capture visual symptoms and dysfunction in patients with neurologic disease.4,5 This needs to expand the visual “toolbox” to include contrast sensitivity (recognized as early as the 1970s as being abnormal in patients with PD, low-contrast letter acuity, optical coherence tomography (OCT, a measure of visual pathway structure) is well-illustrated by the case presentation that follows.7 Anchoring of the more recently developed structural, functional, and performance measures (such as rapid automatized naming [RAN] tasks) to vision-specific quality-of-life scales has brought to the forefront the role of the visual pathway as a model for investigating structure-function relationships in the central nervous system.8-10 This model has most recently been used for the study of remyelinating therapies and agents with the potential to provide neuroprotection, particularly in the case of acute ON.11
Case Presentation
A 30-year-old woman was referred to neuro-ophthalmology for right eye pain. The patient had an established diagnosis of relapsing MS and had presented 10 years prior with acute ON in the right eye. Surveillance MRI scans of the brain had demonstrated stable white matter lesions that were typical for MS. The patient reported compliance with intramuscular interferon β-1a. One day before evaluation by neuro-ophthalmology, the patient developed pain on movement of the right eye; she described this pain as “the same” as the pain she had experienced 10 years earlier during the initial acute ON episode. The pain was exacerbated by pressure on the globe, such when applying eye makeup. The patient did not note symptoms of visual acuity loss or color desaturation.
Neuro-ophthalmologic examination revealed high-contrast Snellen visual acuities of 20/15-2 in both eyes at distance. The pupils measured 4 mm in dim illumination and were briskly reactive to light and near without an afferent defect. Visual fields were full. She perceived 10/10 Ishihara color plates correctly and briskly with each eye; there was no red desaturation. Color fundus photographs of the posterior poles (Figure 1A) suggested subtle elevation of the superior optic nerve head in the right eye. This was confirmed by OCT scanning for which peripapillary retinal nerve fiber layer (RNFL) average thickness was elevated in the right eye to 115 μm. This was compared with an RNFL thickness of 108 μm (normal range) in the asymptomatic left eye (Figure 1B). MRI of the brain and orbits with gadolinium demonstrated enhancement of the right intraorbital optic nerve. She was treated with intravenous methylprednisolone at a dose of 1 gram daily for 3 days; there was complete resolution of symptoms. At a 3-month follow-up visit, OCT scans demonstrated normal RNFL and macular ganglion cell layer/inner plexiform layer (GCIPL) thickness values without intereye asymmetry.
Figure 1. Color Fundus Photos and OCT Output for Right and Left Eyes.
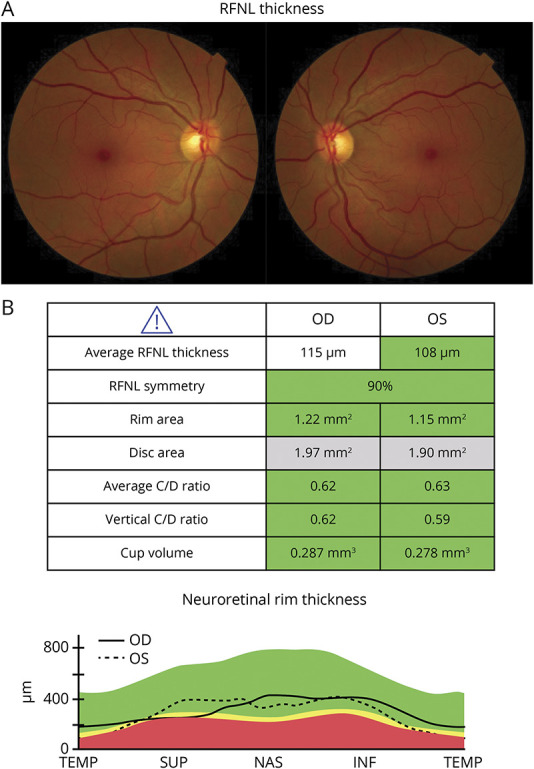
OCT = optical coherence tomography.
This patient's story not only highlights the role of structural tools such as OCT in the confirmation and early diagnosis of acute ON but also emphasizes how modern and more sensitive techniques can complement functional visual outcomes in cases for which clinical findings are subtle. Our patient did not undergo low-contrast letter acuity testing; this may have also revealed an asymmetry not shown by high-contrast visual acuity, pupillary, and color testing. Taken together, the findings of this patient emphasize that acute ON in MS may occur more often than clinically recognized and that there are likely to be “interictal” events between obvious MS visual exacerbations that result in RNFL and GCIPL thinning over time. Such events can result in intereye differences that could, in fact, represent a marker of optic nerve demyelination and could validate lesions for dissemination in space or time.12
MS
The pathophysiology of MS includes inflammation, demyelination, and axonal degeneration that may affect both the afferent and efferent visual systems. ON is often the first clinical manifestation of MS, yet the most recent McDonald criteria do not include the optic nerve as a CNS region by imaging or other paraclinical test to qualify for dissemination in space.3 In the ONTT, patients with acute ON characteristically presented with unilateral, subacute painful vision loss that worsened over the course of 1 week.13 Fifty percent of patients enrolled in the ONTT were ultimately diagnosed with clinically definite MS, defined as occurrence of a second clinical demyelinating event, over the next 15 years.14 This trial was important in establishing the relation between acute ON and MS and in demonstrating the potential utility of corticosteroids for treatment. Although intravenous high-dose methylprednisolone treatment was not associated with long-term differences in high-contrast visual acuity outcomes, it was shown that this therapy may hasten visual recovery by several weeks and also could delay the occurrence of a second clinical demyelinating event.15 Today, the optic nerve is still not a CNS region that is used to determine radiologic dissemination in space by the McDonald criteria for MS diagnosis as of 2017.3 This is particularly problematic in high-risk patients with acute ON (particularly those with ON who have additional brain or spinal cord lesions) who are at substantial risk for a new clinical attack or MRI lesion.16-18 Delays in treatment initiation may be associated with increased T2 lesion burden, increased volume of gadolinium-enhancing lesions, and increased brain atrophy.16-18 Although vision loss is common and can be debilitating in demyelinating disease, clinical trials did not adequately assess visual outcome measures beyond the level of high-contrast visual acuity until the early 2000s.11
Low-Contrast Letter Acuity
After the use of contrast sensitivity in the ONTT, low-contrast letter acuity testing (gray letters on a white or retroilluminated background) was subsequently found to distinguish patients from normal controls based on subtle visual symptoms that may otherwise have been subclinical and not captured by high-contrast (black and white) visual acuity testing.14 Low-contrast Sloan letter charts have a format that is similar to the Early Treatment Diabetic Retinopathy Study charts that have remained a standard for high-contrast visual acuity testing; these charts have 5 Sloan letters per line and proportional spacing between lines.19 Investigations by Balcer et al.5 demonstrated that low-contrast letter acuity, compared with high-contrast visual acuity, contrast sensitivity and color vision testing, best distinguished patients with MS vs controls. Associations were established between worse low-contrast acuity scores and worse scores for the following measures that capture other dimensions of neurologic function in MS: Expanded Disability Status Scale (EDSS); MS Functional Composite components of timed 25-foot walk, 9-hole peg test, and Paced Serial Auditory Addition Task; peripapillary RNFL thickness and GCIPL thickness by OCT; brain MRI lesion burden; visual evoked potential changes; electroretinography findings; and pupillary function. A 7-letter change in low-contrast acuity scores was found to be clinically meaningful based on the above associations and based on the fact that a difference of 7 letters represents 2 standard deviations on the mean interrater difference from reliability studies.20 In parallel with the above studies, low-contrast letter acuity was incorporated as an exploratory outcome measure for the phase-3 trials of natalizumab for MS. Natalizumab is a humanized monoclonal antibody against the cell adhesion molecule alpha-4 integrin (Figure 2, Balcer et al.).21 Although high-contrast visual acuity in these trials was not able to demonstrate treatment effects, low-contrast acuity scores at 2.5% and 1.25% contrast captured treatment benefit for natalizumab regarding reductions in sustained visual loss and for sustained visual improvements over time. Unrecognized visual disability was also detected in up to a quarter of patients in these cohorts by vision-specific quality-of-life scales (Figure 3).19,22
Figure 2. Kaplan-Meier Plots of the Time to Sustained Worsening of Vision Scores From Baseline Among Patients Receiving Natalizumab Compared With Placebo for AFFIRM.
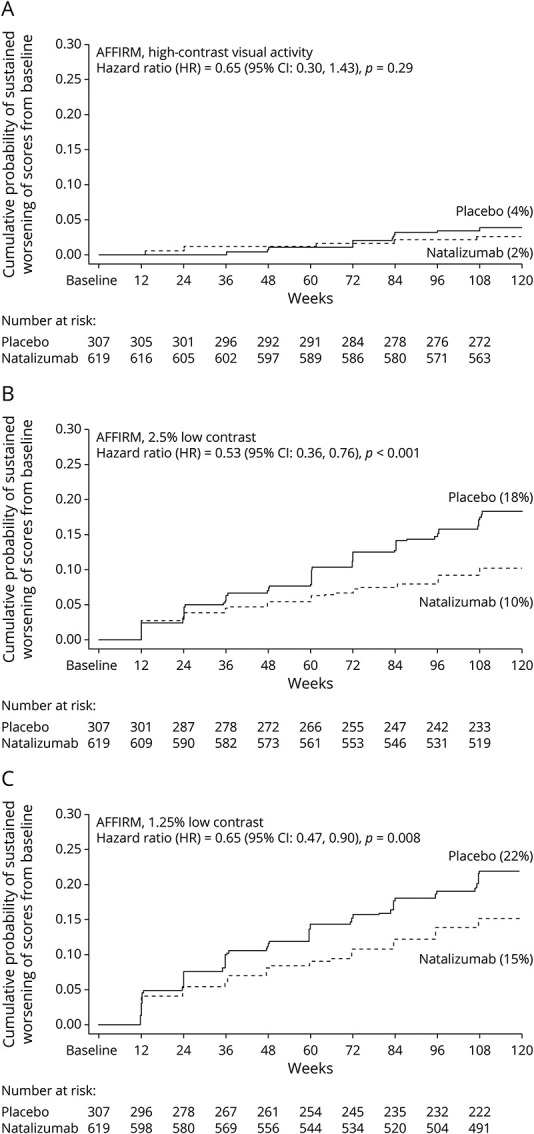
(A) High-contrast visual acuity (similar to Snellen acuity, the measure traditionally used in multiple sclerosis clinical trials) did not demonstrate substantial degrees of sustained worsening from baseline. Low-contrast letter acuity at the 2.5% (B) and 1.25% contrast levels (C) showed that natalizumab reduced the risk of sustained clinically significant worsening of vision (worsening by two or more lines [10 letters], sustained over 12 weeks). Significant differences in cumulative probabilities of sustained worsening of vision were noted at both the 2.5% and 1.25% contrast levels.
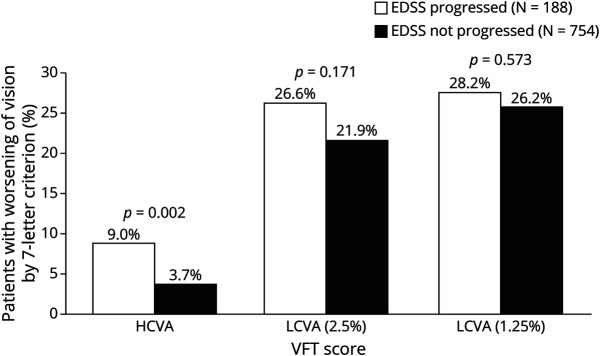
Figure 3. Proportions of Patients With Worsening Vision by EDSS Progression Status Over 2 Years.
Using the 7-letter criterion for clinically meaningful visual change, a significantly greater number of patients with EDSS progression had worsening in HCVA compared with those with no EDSS progression. Notably, there were no significant differences in LCVA worsening between patients with and without EDSS progression. p values are from the McNemar test. EDSS = Expanded Disability Status Scale; HCVA = high-contrast visual acuity; LCVA = low-contrast visual acuity; VFT = visual function testing.
Recent investigations have also highlighted the importance not only of monitoring the visual pathway integrity of patients with MS but also of examining the effects of early MS treatment with disease-modifying therapies.23 In a recent study of the MSBase Registry, among 935 patients presenting with acute ON, early MS treatment was associated with reduced risk and a delayed conversion to clinically definite MS (HR = 0.70, p < 0.001). Treatment was also associated with reduced risk of sustained EDSS worsening (HR = 0.46, p < 0.0001) and with reduced sustained worsening of visual function. It has been shown consistently that initiation of disease-modifying therapies, particularly early in disease, may delay onset and reduce risk of conversion to clinically definite MS, defined as a second clinical demyelinating event.23
OCT
OCT was first developed as a method to monitor retinal architecture and disease progression in glaucoma but was subsequently applied to study the optic nerve in neurologic conditions and to measure the anterior visual pathway structure.24 The most involved layers after optic nerve injury are the peripapillary RNFL and the macular GCIPL. The RNFL is the only part of the visual sensory system for which unmyelinated axons can be visualized noninvasively.25 Work by Frisen and Hoyt in the 1970s demonstrated the presence of insidious atrophy of retinal nerve fibers in MS by ophthalmoscopy.26 Other important studies demonstrated that, in patients with a clinical history of acute ON, approximately 75% had a 20% degree of peripapillary RNFL thinning compared with the fellow unaffected or control eyes; furthermore, this loss occurred early in the disease course.27
OCT has revolutionized the ability to monitor the structural aspects of visual pathway integrity in a real time, in vivo, and noninvasively. OCT imaging is quick, effective, and readily available in most neuro-ophthalmic clinics. The first studies of OCT technology in MS included 14 patients with a previous event of acute ON; the results of this study showed that peripapillary RNFL thickness was reduced by 46% in MS eyes affected by ON vs eyes of healthy control participants. In addition, there was a 28% reduction of RNFL thickness in affected eyes compared with unaffected fellow eyes of the same patients.8 Fisher et al. demonstrated that RNFL thickness was significantly reduced among all eyes of patients with MS in aggregate (average 92 μm) vs eyes of disease-free controls (average 105 μm). Eyes with a clinical history of acute ON among people with MS had even greater degrees of thinning (average 85 μm). These differences were statistically significant when accounting for age and for within patient intereye correlations using generalized estimating equation regression models (Figure 4, Fisher et al.). In addition, reduced average thicknesses in peripapillary RNFL were associated with lower visual function scores; each line of decrease in low-contrast letter acuity corresponded to a peripapillary RNFL decrease of 4 μm28. Costello et al.29 demonstrated that approximately 75% of patients with MS and a history of acute ON will have a 10-20-µm RNFL loss within 3–6 months (out of a usual control RNFL thickness of ∼100 µm by early OCT methods of time-domain imaging). By contrast, most healthy individuals will lose only 0.017% of their peripapillary RNFL per year by similar imaging methods (10–20 µm over 60 years).30 Many subsequent studies have examined associations of RNFL thinning and visual loss.31-33 These measurements of the RNFL can now aid in the diagnosis and differential diagnosis of ON and may be used to measure the effects of treatment both in the setting of acute ON and in MS in general.20,34 In 2012, Walter et al.35 demonstrated that GCIPL thinning in eyes of people with MS was significantly associated with reduced visual function (low-contrast letter acuity) and with vision-specific quality-of-life impairment by the 25-Item National Eye Institute Visual Functioning Questionnaire (NEI-VFQ-25) and the 10-Item Neuro-Ophthalmic Supplement (Figure 5, Walter et al.). OCT measurements are also able to identify mild, and even clinically undetectable, optic disc edema in the setting of acute ON, as illustrated by our case presentation. Given the changes in RNFL and GCIPL thickness that follow ON episodes and the fact that many patients may be disadvantaged by delays in the diagnosis and treatment of MS, there is an increasing need for the addition of the optic nerve to the McDonald criteria as a lesion site in space and time for the diagnosis of MS by imaging modalities.12 OCT can be used to monitor treatment outcomes with MS immunotherapy and lends further importance to including the optic nerve as a lesion site for revised diagnostic criteria of MS.36 As a more global measure, spectral domain OCT findings of peripapillary RNFL and GCIPL thinning have been found to be associated with the presence and degree of brain atrophy in MS.9
Figure 4. Mean Values for Overall Average RNFL Thickness (360° Around the Optic Disc) and for RNFL Thickness in Temporal, Superior, Nasal, and Inferior Quadrants for Patients With Multiple Sclerosis (MS; n = 90 [180 Eyes]) and Disease-Free Controls (n = 36 [72 Eyes]).
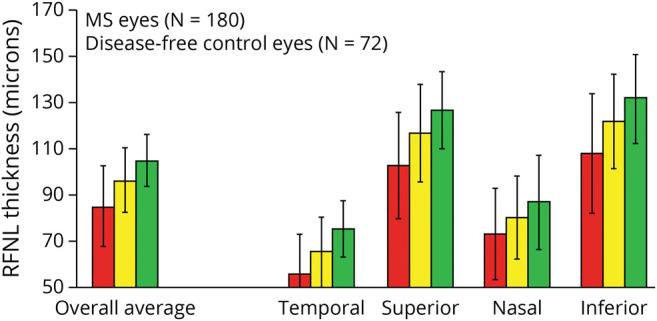
*Average overall RNFL thickness values were significantly lower for patients with MS vs controls (p < 0.001, generalized estimating equation [GEE] models accounting for age and adjusting for within-patient intereye correlations). †Mean RNFL thickness values varied significantly across retinal quadrants (p < 0.0001), with mean thickness greater in the superior and inferior quadrants. The mean thickness was greater for controls than for patients with MS in all quadrants, and the difference between patient groups was of the same magnitude in each quadrant (p = 0.34 for interaction terms, GEE models). GEE = generalized estimating equation; MS = multiple sclerosis; RNFL = generalized estimating equation.
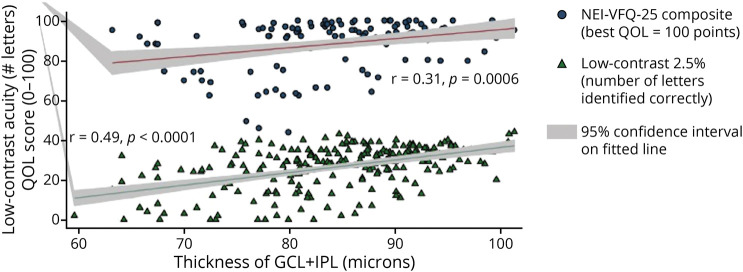
Figure 5. Scatter Plot and Fitted Linear Regression Line Showing Relation of Ganglion Cell Layer Plus Inner Plexiform Layer (GCL + IPL) Thickness to 25-Item National Eye Institute Visual Functioning Questionnaire (NEI-VFQ-25) Composite Scores and Low-Contrast Acuity at the 2.5% Level.
The regression lines represent fitted values for mean GCL + IPL thickness for each value of NEI-VFQ-25 or low-contrast acuity; the gray shaded areas show the 95% confidence intervals from the standard errors of the predictions for the fitted lines. This graph for all multiple sclerosis eyes illustrates that there are very few outliers with respect to quality of life (QOL) or low-contrast acuity. Linear correlations were significant. Accounting for age and adjusting for within-patient, intereye correlations, the relation of QOL and low-contrast acuity to GCL + IPL thickness was significant (p < 0.001, generalized estimating equation models).
RAN Tasks and the Mobile Universal Lexicon Evaluation System in MS
RAN tasks have been used for greater than 80 years as a method to assess aspects of vision, cognition, and language. To perform RAN tasks, participants are asked to rapidly name numbers or objects while minimizing errors in naming. In 2016, the Mobile Universal Lexicon Evaluation System (MULES) was introduced to assess functioning of brain pathways that are perhaps not entirely captured by rapid number naming or other naming tasks.37 These pathways include those underlying object recognition, color detection, and semantic categorization. MULES testing involves the rapid naming of photographs in the context of fruits, common objects, and animals.37 Compared with other alpha-numeric RAN tasks (number naming), the MULES requires color perception, semantic articulation, phonology, and object recognition. Neuroanatomically, MULES is believed to involve the bilateral fusiform gyri and lateral occipital lobe during rapid object naming, whereas the King-Devick (K-D) and Staggered Uneven Number (SUN) tests of rapid number naming primarily engage the primary left inferior temporal lobe, the left motor cortex, the left superior parietal gyrus, and the medial supplementary motor area cortex.38-41 Seay et al. studied 24 patients with MS compared with 22 disease-free controls using the MULES test. In this study, accounting for age, MS vs control status was a predictor of MULES test time (patients with MS had slower testing times, p = 0.01). Faster testing times, indicating better performance, were noted among patients with MS who had better scores for binocular low-contrast letter acuity at 2.5% contrast (p < 0.001). Thus, the MULES test may be of additional utility for detecting and assessing visual deficits in patients with MS and to measure outcomes in clinical trials. Such tests may be particularly helpful when used in combination with more sophisticated measurements of eye movements, such as high-resolution video-oculography (VOG).38
Assessment of Vision-Specific Quality-of-Life and Other Vision-Specific Modalities
Several related questionnaires exist to monitor patients with MS and other neurologic disorders for the more qualitative aspects related to vision. The most widely used and validated assessments include the NEI-VFQ-25 and the 10-Item Neuro-Ophthalmic Supplement to the NEI-VFQ-25. Although the NEI-VFQ-25 is a more generic measure that particularly captures aspects of afferent vision, the 10-Item Supplement was designed to assess information specifically in patients with MS and other neuro-ophthalmologic disorders.43 Both scales capture granular information related to activities of daily living and other symptoms that relate to scores for high-contrast visual acuity, low-contrast letter acuity and contrast sensitivity, visual field abnormalities, reduced color vision, and diplopia.44
Motion perception deficits may also persist in patients with ON who have recovered their high-contrast letter acuity. The association of motion perception deficits and VEP findings suggests that slow conduction through the optic nerve may disrupt the rapid transmission of visual input required for motion perception.45 In the phase-2 trial of opicinumab in patients with acute ON, there was a correlation of object form and number form motion with RGC/IPL loss. Nonetheless, low-contrast letter acuity loss was most strongly associated with changes in the GCIPL thickness in this study, emphasizing its importance as an outcome measure in patients with an ON history.46
Traumatic Brain Injury and Concussion
Patients with mild traumatic brain injury (mTBI), or concussion (the mildest form of mTBI), often note visual symptoms and vestibular dysfunction.47 With about half of the brain's circuits dedicated to vision and the control of eye movements, concussion often leads to symptomatic dysfunction referable to the visual pathways. Recent work from our group and others has demonstrated the utility of RAN tasks in the identification of athletes with sports-related concussion and with mTBI from other etiologies.
One vision-based assessment tool that has been investigated in-depth is the K-D test of rapid number naming.48 This RAN task consists of 3 cards with numbers arranged in rows with variable spacing horizontally and vertically. Participants read numbers on each of the 3 cards as quickly as possible; scores are primarily determined based on time to completion. Performance on the KD test in outpatients who had visual symptoms after concussion was predictive of the numbers of subspeciality referrals and total numbers of clinic visits with moderate to high reliability.10 In a meta-analysis of raw data from 15 studies that were focused on the utility of sideline KD testing, this tool demonstrated a high sensitivity (86%) and high specificity (90%) for distinguishing concussed athletes from controls.48
Similarly, the Mobile Lexicon Evaluation System (MULES), suggested by several decades of previous literature and also by recognized authorities in the field, involves picture naming and testing times that are longer (worse) after concussion.49 Additional image-based RAN tests include the modified versions of the Snodgrass and Vanderwart image bank, examined 58 participants of ages 10–22 years, 32 of whom suffered a concussion. This study demonstrated that concussed participants performed more slowly on object-naming tasks.
RAN tasks are functionally distinct and may have niche applications that depend on the disease process involved. However, eye movement data using eye tracking systems collected in the VOG laboratory enable more precise data on eye movements, gaze position, pupil position, and pupil size. Such data can be used to calculate fundamental parameters of eye movements including saccadic latency, saccadic velocity, amplitude, duration, accuracy of saccades, and directional errors.50 Specific eye movement abnormalities have been shown to correlate with impaired performance on the K-D test, such as increased intersaccadic intervals (ISIs); ISIs have been shown to be prolonged by oculography during KD test performance in patients with a history of concussion.e1 By contrast, formal eye movement recordings in chronically concussed patients performing the MULES test showed increased numbers of saccades during the naming of each picture, suggesting that individual RAN tasks may capture different aspects of visual dysfunction. Devices for tracking smooth pursuit eye movements have been able to track changes corresponding to fractional anisotropy on diffuse tensor imaging, an MRI marker of axonal injury.e2
RAN tasks offer ease of administration and accessibility for sideline assessment of athletic injuries, as well as the prospect monitoring patients through telemedicine and in office settings for other neurologic conditions, including MS, Parkinson disease (PD), and conditions along the spectrum of AD, such as MCI. The Mobile Integrated Cognitive Kit (MICK) app has been newly developed for research and presents the MULES and SUN tests on a tablet or computer device (Figures 6 and 7). This app calculates testing times and has capacity for voice recording and is designed and intended to be financially and logistically accessible for a broad range of ages and populations. A first study of the MICK application in office volunteers and in women's professional hockey players at preseason baseline demonstrated excellent agreement of time scores for MICK app–based MULES and SUN with paper/pencil versions of these tests. There were no significant differences observed for comparisons of mean best scores (p = 0.45 for MULES, p = 0.50 for SUN) and high intraclass correlation coefficients (ICCs) of 0.92 for MULES (95% CI 0.86, 0.95) and 0.94 (95% CI 0.89, 0.96) for SUN. These ICC values indicate excellent levels of interplatform agreement for the test versions, allowing accessibility and scalability for assessment of neurologic diseases and sports-related concussion.e3 When a concussion is suspected by athletic trainers, coaches, or parents, the MICK can be readily available to administer digitized versions of the MULES and SUN with the goal of supporting clinical impressions of a concussion diagnosis. Recent evidence has demonstrated that the time scores from the paper/pencil version of MULES and SUN have high levels of agreement with those obtained using the MICK app, rendering it valuable for clinical and athletic sideline settings. RAN tasks rely on precise coordination of saccadic eye movements, which require multiple cortical regions and neurologic tracts including the frontal eye fields, dorsolateral prefrontal cortex, supplementary motor area, posterior parietal cortex, middle temporal area, and the striate cortex.48 App-based tests can be used remotely and can be helpful in time-sensitive circumstances, such as athletic injury. Given the increasing role of telemedicine, remote practice physicians may be able to use these tools to accurately track treatment response to medications in MS or associated conditions. Tele-neurology will allow patients increased access to reliable, low-cost health care with objective outcome measures that are easily performed during a video visit.e4
Figure 6. Examples of Application Interface for Mobile Integrated Cognitive Kit (MICK) App for Mobile Universal Lexicon Evaluation System (MULES).
Figure 7. Examples of Application Interface for Mobile Integrated Cognitive Kit (MICK) App for Staggered Uneven Number (SUN) Testing.
Neurodegenerative Diseases
Visual outcomes are becoming increasingly relevant in the investigation and clinical care of individuals with neurodegenerative conditions, including AD, and PD. In a study of 115,240 participants, of whom 1,438 (1.25%) were diagnosed with PD, there was an increased odds ratio in favor of having impaired vision among those with PD (OR: 2.67, 95%: 1.91–3.72).e5 Deficits in visual acuity, color vision, and visual fields have been suggested to be premotor symptoms for PD development, and visual impairment has been considered a consequence of PD progression.e6 Visual impairment has also been associated with negative PD-related outcomes, including incident hip fracture, depression, anxiety, dementia, and death.e7
Relation of Low-Contrast Letter Acuity to MULES Scores
Patients with MCI, AD, and PD have demonstrated worse scores on the MULES and low-contrast acuity testing.e8 In a study of 14 participants with MCI, Wu et al. demonstrated that these patients had worse binocular low-contrast letter acuity at 1.25% contrast compared with controls (p = 0.009). Patients also demonstrated worse MULES test times (p = 0.006), with more errors in naming images (p = 0.0009), compared with controls. Thus, rapid picture naming and LCLA testing may distinguish MCI because of AD from normal aging.e8 These scores also reflected vision-specific quality of life by the NEI-VFQ-25 in this cohort. Studies are underway to use visual outcome measures as exploratory in forthcoming clinical trials for primary neurodegenerative conditions.
Low-contrast letter acuity testing has been examined in a cohort of patients with Friedrich ataxia (FA); these patients had lower scores compared with similar-aged controls. In a manner similar to studies of MS, high-contrast visual acuity was not significantly different between FA and control participants.e9 LCLA impairment has also been demonstrated in Parkinson disease; these patients had lower scores for both low-contrast and high-contrast acuity scores compared with controls.e10
OCT and OCT Angiography in Neurodegenerative Disease
A growing literature is investigating roles for OCT and OCT angiography measurements as potential markers for AD and PD progression.7 Measures investigated include RNFL and GCIPL thickness as well as foveal and parafoveal macular thickness, with comparisons with control groups. However, the results remain mixed across studies, and there is a lack of consistency of findings, potentially explained by a naturally occurring wide range of thinning in patients.e11 Barrett-Young introduced OCT measurements of RNFL and GCL thickness as markers for declining cognitive performance.e12 In a study of 865 patients, thinner RNFL was indicative of a decline in processing speed between childhood and adulthood.62 GCIPL thickness was demonstrated to be best correlated with memory, global cognitive performance, clinical dementia rating, and hippocampal atrophy. At a cutoff score of 75 μm, GCIPL thickness was useful in discriminating cognitive performance status and was used to identify MCI and Alzheimer disease and related disorders.e13
Conclusions and Future Directions
We have summarized the methodologies that are currently in use and under investigation to measure vision-related outcomes for a variety of neurologic conditions. To date, the literature supports using noninvasive tools such as OCT for capturing data on structural integrity of the afferent visual pathway. Low-contrast letter acuity and rapid picture (MULES) and number naming (SUN) represent potentially effective visual outcomes across a spectrum of neurologic disorders, including MS, MCI, PD, and concussion. Neurodegenerative conditions within the AD spectrum also require further investigation, but evidence thus far supports LCLA and MULES as having worse scores in MCI with successful differentiation from those with normal aging. Recent evidence supports the use of mobile applications to make RAN tools such as MULES more accessible and available from the office to telemedicine and the athletic sideline. We are currently investigating the use of digitally accessible tools, such as the MICK app, to gather preseason baseline data for youth or collegiate athletes and to enable office-based or telemedicine-based assessments. As we enter the era of therapies that aim to alter the trajectory of disease progression in primary neurodegenerative disease, visual outcome measures, such as RAN tasks, OCT, and LCLA, will offer crucial insights into efficacy of such approaches.
Glossary
- AD
Alzheimer disease
- EDSS
Expanded Disability Status Scale
- ETDRS
Early Treatment Diabetic Retinopathy Study
- FA
Friedrich ataxia
- GCIPL
ganglion cell layer/inner plexiform layer
- GEE
generalized estimating equation
- ICCs
intraclass correlation coefficients
- ISI
intersaccadic interval
- KD
King-Devick
- MCI
mild cognitive impairment
- MICK
Mobile Integrated Cognitive Kit
- MS
multiple sclerosis
- MSFC
MS Functional Composite
- ON
optic neuritis
- mTBI
mild traumatic brain injury
- MULES
Mobile Universal Lexicon Evaluation System
- NEI-VFQ-25
25-Item National Eye Institute Visual Functioning Questionnaire
- OCT
optical coherence tomography
- ONTT
Optic Neuritis Treatment Trial
- PASAT
Paced Serial Auditory Addition Task
- PD
Parkinson disease
- RAN
rapid automatized naming
- RNFL
retinal nerve fiber layer
- SCD
subjective cognitive decline
- SUN
Staggered Uneven Number
- VOG
video-oculography
Appendix. Authors
Study Funding
The authors report no targeted funding.
Disclosure
L.J. Balcer is editor-in-chief of the Journal of Neuro-Ophthalmology. S.L. Galetta has received consulting fees from Genentech. The remaining authors have no disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Graves JS, Oertel FC, Van der Walt A, et al. Leveraging visual outcome measures to advance therapy development in neuroimmunologic disorders. Neurol Neuroimmunol Neuroinflamm. 2022;9(2):e1126. doi: 10.1212/nxi.0000000000001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1-47. doi: 10.1093/cercor/1.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi: 10.1016/s1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 4.Balcer LJ, Baier ML, Pelak VS, et al. New low-contrast vision charts: reliability and test characteristics in patients with multiple sclerosis. Mult Scler. 2000;6(3):163-171. doi: 10.1191/135245800701566025. [DOI] [PubMed] [Google Scholar]
- 5.Balcer LJ, Baier ML, Cohen JA, et al. Contrast letter acuity as a visual component for the multiple sclerosis functional composite. Neurology. 2003;61(10):1367-1373. doi: 10.1212/01.wnl.0000094315.19931.90. [DOI] [PubMed] [Google Scholar]
- 6.Kenney RC, Liu M, Hasanaj L, et al. The role of optical coherence tomography criteria and machine learning in multiple sclerosis and optic neuritis diagnosis. Neurology. 2022;99(11):e1100-e1112. doi: 10.1212/wnl.0000000000200883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weil RS, Schrag AE, Warren JD, Crutch SJ, Lees AJ, Morris HR. Visual dysfunction in Parkinson's disease. Brain. 2016;139(11):2827-2843. doi: 10.1093/brain/aww175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parisi V, Manni G, Spadaro M, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci. 1999;40(11):2520-2527. [PubMed] [Google Scholar]
- 9.Shi Z, Zheng H, Hu J, et al. Retinal nerve fiber layer thinning is associated with brain atrophy: a longitudinal study in nondemented older adults. Front Aging Neurosci. 2019;11:69. doi: 10.3389/fnagi.2019.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyle Harrold G, Hasanaj L, Moehringer N, et al. Rapid sideline performance meets outpatient clinic: results from a multidisciplinary concussion center registry. J Neurol Sci. 2017;379:312-317. doi: 10.1016/j.jns.2017.06.038. [DOI] [PubMed] [Google Scholar]
- 11.Sakai RE, Feller DJ, Galetta KM, Galetta SL, Balcer LJ. Vision in multiple sclerosis: the story, structure-function correlations, and models for neuroprotection. J Neuroophthalmol. 2011;31(4):362-373. doi: 10.1097/wno.0b013e318238937f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nolan-Kenney RC, Liu M, Akhand O, et al. Optimal intereye difference thresholds by optical coherence tomography in multiple sclerosis: an international study. Ann Neurol. 2019;85(5):618-629. doi: 10.1002/ana.25462. [DOI] [PubMed] [Google Scholar]
- 13.Beck RW, Cleary PA, Anderson MM Jr., et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med. 1992;326(9):581-588. doi: 10.1056/NEJM199202273260901. [DOI] [PubMed] [Google Scholar]
- 14.Beck RW, Gal RL, Bhatti MT, et al. Visual function more than 10 years after optic neuritis: experience of the optic neuritis treatment trial. Am J Ophthalmol. 2004;137(1):77-83. doi: 10.1016/s0002-9394(03)00862-6. [DOI] [PubMed] [Google Scholar]
- 15.Stiebel-Kalish H, Hellmann MA, Mimouni M, et al. Does time equal vision in the acute treatment of a cohort of AQP4 and MOG optic neuritis? Neurol Neuroimmunol Neuroinflamm. 2019;6(4):e572. doi: 10.1212/nxi.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galetta SL. The controlled high risk Avonex multiple sclerosis trial (CHAMPS Study). J Neuroophthalmol. 2001;21(4):292-295. doi: 10.1097/00041327-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Comi G, Martinelli V, Rodegher M, et al. Effects of early treatment with glatiramer acetate in patients with clinically isolated syndrome. Mult Scler. 2013;19(8):1074-1083. doi: 10.1177/1352458512469695. [DOI] [PubMed] [Google Scholar]
- 18.Kappos L, Edan G, Freedman MS, et al. The 11-year long-term follow-up study from the randomized BENEFIT CIS trial. Neurology. 2016;87(10):978-987. doi: 10.1212/wnl.0000000000003078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balcer LJ, Galetta SL, Polman CH, et al. Low-contrast acuity measures visual improvement in phase 3 trial of natalizumab in relapsing MS. J Neurol Sci. 2012;318(1-2):119-124. doi: 10.1016/j.jns.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Balcer LJ, Raynowska J, Nolan R, et al. Validity of low-contrast letter acuity as a visual performance outcome measure for multiple sclerosis. Mult Scler. 2017;23(5):734-747. doi: 10.1177/1352458517690822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balcer LJ, Galetta SL, Calabresi PA, et al. Natalizumab reduces visual loss in patients with relapsing multiple sclerosis. Neurology. 2007;68(16):1299-1304. doi: 10.1212/01.wnl.0000259521.14704.a8. [DOI] [PubMed] [Google Scholar]
- 22.Chahin S, Balcer LJ, Miller DM, Zhang A, Galetta SL. Vision in a phase 3 trial of natalizumab for multiple sclerosis: relation to disability and quality of life. J Neuroophthalmol. 2015;35(1):6-11. doi: 10.1097/wno.0000000000000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenney R, Liu M, Patil S, et al. Long-term outcomes in patients presenting with optic neuritis: analyses of the MSBase registry. J Neurol Sci. 2021;430:118067. doi: 10.1016/j.jns.2021.118067. [DOI] [PubMed] [Google Scholar]
- 24.Galetta KM, Calabresi PA, Frohman EM, Balcer LJ. Optical coherence tomography (OCT): imaging the visual pathway as a model for neurodegeneration. Neurotherapeutics. 2011;8(1):117-132. doi: 10.1007/s13311-010-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levin LA, Sengupta M, Balcer LJ, Kupersmith MJ, Miller NR. Report from the national eye Institute workshop on neuro-ophthalmic disease clinical trial endpoints: optic neuropathies. Invest Ophthalmol Vis Sci. 2021;62(14):30. doi: 10.1167/iovs.62.14.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frisén L, Hoyt WF. Insidious atrophy of retinal nerve fibers in multiple sclerosis. Funduscopic identification in patients with and without visual complaints. Arch Ophthalmol. 1974;92(2):91-97. doi: 10.1001/archopht.1974.01010010097001. [DOI] [PubMed] [Google Scholar]
- 27.Frohman E, Costello F, Zivadinov R, et al. Optical coherence tomography in multiple sclerosis. Lancet Neurol. 2006;5(10):853-863. doi: 10.1016/s1474-4422(06)70573-7. [DOI] [PubMed] [Google Scholar]
- 28.Fisher JB, Jacobs DA, Markowitz CE, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113(2):324-332. doi: 10.1016/j.ophtha.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 29.Costello F, Coupland S, Hodge W, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59(6):963-969. doi: 10.1002/ana.20851. [DOI] [PubMed] [Google Scholar]
- 30.Kanamori A, Escano MF, Eno A, et al. Evaluation of the effect of aging on retinal nerve fiber layer thickness measured by optical coherence tomography. Ophthalmologica. 2003;217(4):273-278. doi: 10.1159/000070634. [DOI] [PubMed] [Google Scholar]
- 31.Trip SA, Schlottmann PG, Jones SJ, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005;58(3):383-391. doi: 10.1002/ana.20575. [DOI] [PubMed] [Google Scholar]
- 32.Zaveri MS, Conger A, Salter A, et al. Retinal imaging by laser polarimetry and optical coherence tomography evidence of axonal degeneration in multiple sclerosis. Arch Neurol. 2008;65(7):924-928. doi: 10.1001/archneur.65.7.924. [DOI] [PubMed] [Google Scholar]
- 33.Al-Mujaini AS, Al-Mujaini MS, Sabt BI. Retinal nerve fiber layer thickness in multiple sclerosis with and without optic neuritis: a four-year follow-up study from Oman. BMC Ophthalmol. 2021;21(1):391. doi: 10.1186/s12886-021-02158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iorga RE, Moraru A, Ozturk MR, Costin D. The role of Optical Coherence Tomography in optic neuropathies. Rom J Ophthalmol. 2018;62(1):3-14. doi: 10.22336/rjo.2018.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walter SD, Ishikawa H, Galetta KM, et al. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology. 2012;119(6):1250-1257. doi: 10.1016/j.ophtha.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pro MJ, Pons ME, Liebmann JM, et al. Imaging of the optic disc and retinal nerve fiber layer in acute optic neuritis. J Neurol Sci. 2006;250(1-2):114-119. doi: 10.1016/j.jns.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Cobbs L, Hasanaj L, Amorapanth P, et al. Mobile Universal Lexicon Evaluation System (MULES) test: a new measure of rapid picture naming for concussion. J Neurol Sci. 2017;372:393-398. doi: 10.1016/j.jns.2016.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seay M, Akhand O, Galetta MS, et al. Mobile Universal Lexicon Evaluation System (MULES) in MS: evaluation of a new visual test of rapid picture naming. J Neurol Sci. 2018;394:1-5. doi: 10.1016/j.jns.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 39.Aggleton JP, Albasser MM, Aggleton DJ, Poirier GL, Pearce JM. Lesions of the rat perirhinal cortex spare the acquisition of a complex configural visual discrimination yet impair object recognition. Behav Neurosci. 2010;124(1):55-68. doi: 10.1037/a0018320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cummine J, Szepesvari E, Chouinard B, Hanif W, Georgiou GK. A functional investigation of RAN letters, digits, and objects: how similar are they? Behav Brain Res. 2014;275:157-165. doi: 10.1016/j.bbr.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 41.Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32(5):1055-1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Sabadia SB, Nolan RC, Galetta KM, et al. 20/40 or better visual acuity after optic neuritis: not as good as we once thought? J Neuroophthalmol. 2016;36(4):369-376. doi: 10.1097/wno.0000000000000421. [DOI] [PubMed] [Google Scholar]
- 43.Raphael BA, Galetta KM, Jacobs DA, et al. Validation and test characteristics of a 10-item neuro-ophthalmic supplement to the NEI-VFQ-25. Am J Ophthalmol. 2006;142(6):1026-1035.e2. doi: 10.1016/j.ajo.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 44.Balcer LJ, Frohman EM. Evaluating loss of visual function in multiple sclerosis as measured by low-contrast letter acuity. Neurology. 2010;74(Issue 17, Suppl 3):S16-S23. doi: 10.1212/wnl.0b013e3181dbb664. [DOI] [PubMed] [Google Scholar]
- 45.Raz N, Dotan S, Chokron S, Ben-Hur T, Levin N. Demyelination affects temporal aspects of perception: an optic neuritis study. Ann Neurol. 2012;71(4):531-538. doi: 10.1002/ana.22692. [DOI] [PubMed] [Google Scholar]
- 46.Galetta SL, Balcer LJ, Altincatal A, Jiang X, Aktas O, Naylor ML. Retinal structure and function as a predictor of long-term visual outcomes: a post hoc analysis of RENEW and RENEWED (1945). Neurology. 2021;96:1945. [Google Scholar]
- 47.Sen N. An insight into the vision impairment following traumatic brain injury. Neurochem Int. 2017;111:103-107. doi: 10.1016/j.neuint.2017.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galetta KM, Liu M, Leong DF, Ventura RE, Galetta SL, Balcer LJ. The King-Devick test of rapid number naming for concussion detection: meta-analysis and systematic review of the literature. Concussion. 2016;1:CNC8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akhand O, Galetta MS, Cobbs L, et al. The new Mobile Universal Lexicon Evaluation System (MULES): a test of rapid picture naming for concussion sized for the sidelines. J Neurol Sci. 2018;387:199-204. doi: 10.1016/j.jns.2018.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hutton SB. Cognitive control of saccadic eye movementsor Health Outcomes. Brain Cogn Mov Disord. 2008;68(3):327-340. or Health Outcomes. Mov Disord 2020;35:1542-1549. [DOI] [PubMed] [Google Scholar]
- eReferences e1–e13 are available at links.lww.com/WNL/C524