Abstract
Ion-conductive hydrogels, with ions as signal carriers, have become promising candidates to construct functional ionotronics for sensing, actuating, and robotics engineering. However, rational modulation of ionic migration to mimic biological information processing, including learning and memory, remains challenging to be realized in hydrogel materials. Here, we develop a hybrid hydrogel with optically modulated ionic conductivity to emulate the functions of a biological synapse. Through a responsive supramolecular approach, optical stimuli can trigger the release of mobile ions for tuning the conductivity of the hydrogel, which is analogous to the modulation of synaptic plasticity. As a proof of concept, this hydrogel can be used as an information processing unit to perceive different optical stimuli and regulate the grasping motion of a robotic hand, performing logical motion feedback with “learning-experience” function. Our ionic hydrogel provides a valuable strategy toward developing bioinspired ionotronic systems and pushes forward the functional applications of hydrogel materials.
An ion-conductive hydrogel works as an information processor to regulate the robotic motion.
INTRODUCTION
Ion-conductive hydrogels show great promise in constructing bioinspired ionotronics due to their excellent ionic conductivity, versatile mechanical properties, and biocompatibility (1–3). Ion-conductive hydrogels are usually composed of cross-linked polymers as the mechanically supporting matrix and mobile ions as charge carriers for transmitting electrical signals (4, 5). Until now, huge efforts have been made to develop hydrogel ionotronics through designing the polymeric networks with diversified mechanical properties, including stretchability (6, 7), flexibility (8, 9), and self-healing ability (10, 11), among others. However, little emphasis has been paid to directly modulate the ions in hydrogels for realizing signal analysis and information processing (12). The modulation of charge carriers, such as electrons and holes, in semiconductor materials is the foundation for realizing various functions in modern electronic devices (13–15). Inspired by such principles, we can assume that rationally controlling the ion migration in conductive hydrogels would be of great importance in developing bioinspired information processing systems and would greatly expand the functionality and practicality of hydrogel materials (16–18).
Recently, ion-conductive hydrogels have been widely involved in fabricating functional electronic devices, including sensors (19), transistors (20), and synaptic devices (21), to achieve sensing, signal analysis, and information processing (22, 23). However, the electronic functions in these devices mainly rely on the modulation of electrons and holes in traditional semiconductive materials (24, 25), whereas hydrogel materials in the devices usually work as ionic conductors to simply deliver electric signals (26). Actually, the migration of ions plays an essential role in the neural systems for delivering biological information (27). Relying on the precise control of ions and small molecules (28), human beings and higher animals can execute intelligent neural activities, including learning, memory, and cognition, for realizing efficient interactions with external environment (Fig. 1A). In such neural activities, synapses as the basic processing units are vital to modulate the communication among neurons for achieving signal transmission, information processing, and regulation of body feedback (Fig. 1A). Notably, synaptic functions are controlled by a series of ion-dominated processes, including the transmission of action potentials, the transmembrane transport of ions, and the controlled release of neurotransmitters (29, 30). Therefore, the rational control of ion migration would be a promising strategy to mimic the information processing capability of the biological neural system (31), which is rarely realized in hydrogel materials. However, integrating the sensation of environmental information and transducing it into analyzable electrical signals for bioinspired information processing remains challenging in hydrogel materials, which needs to bridge the gap between chemical approaches and electronic functions.
Fig. 1. Schematic illustrations of biological visual perception and an optically modulated ion-conductive hydrogel for emulating the synaptic functions.
(A) Information transmission and processing of biological visual perception. Optical signals are detected by eyes and transduced into action potentials, which are transmitted through neurons and processed in synapses. When successive action potentials arrive at the axon terminal, calcium channels are activated and allow the influx of calcium ions, which further triggers the release of neurotransmitters to bind the receptors of the postsynaptic neuron and potentiate the membrane potential for modulating the synaptic plasticity, resulting in communication between two neurons. Following the all-or-none law, only when the synapse is stimulated to a strongly activated state does the synaptic weight reach the threshold and can the communication between neurons be realized for generating feedback. (B) Optically modulated ion-conductive (OMIC) hydrogel for emulating synaptic functions. The ionic conductivity of the OMIC hydrogel is attributed to the migration of azo-benzene functionalized imidazole (AZIM) ions, of which the amount is controlled by photothermally modulated reversible assembly process of AZIM ions. Therefore, optical pulses that functionally emulate the presynaptic spikes stimulate the conductance of the OMIC hydrogel, performing a similar response behavior with the modulation of synaptic weight in a biological synapse.
In this work, we innovatively develop a stimuli-responsive supramolecular strategy to realize optically mediated release of mobile ions from supramolecular aggregates in a hydrogel. By associating optical input with the signal of ionic conductivity, the dynamic assembly process of supramolecular aggregates can be used to emulate the modulation of synaptic plasticity (Fig. 1B), which enables the hydrogel itself to execute intelligent information processing functions without the assistance of software for logic programming. Here, we fabricated an optically modulated ion-conductive (OMIC) hydrogel consisting of Fe3O4 nanoparticles, polyacrylamide (PAAm) networks, and supramolecular assemblies made of azo-benzene functionalized imidazole (AZIM) salt. As shown in Fig. 1B, the near-infrared optical stimuli can be converted into heat by Fe3O4 nanoparticles to control the thermal-responsive reversible assembly process of AZIM ions. As the disassembly of supramolecular aggregates, the released AZIM ions will work as charge carriers to tune the conductance of the OMIC hydrogel, which is analogous to the function of neurotransmitters for modulating the synaptic weight in a biological synapse (32). Inspired by the biological visual perception (Fig. 1A), the OMIC hydrogel was used as an information processing unit to construct an optically modulated autonomous motion feedback system for logically regulating the grasping behavior of a robotic hand by perceiving different optical messages, performing the “all-or-none” principle and the brain-like “learning-experience” ability.
RESULTS
Optically modulated ionic conductivity of the OMIC hydrogel
We design an ionic compound AZIM that could self-assemble into nanofibers and form a supramolecular hydrogel at room temperature (Fig. 2, A and B, and fig. S1). The assembly process is mainly driven by hydrogen bonding between the amide groups and π-π stacking interactions between the phenyl rings of azo-benzenes in the trans configuration (Fig. 2C and fig. S2 and S3). The characteristic absorption peaks of amide I and amide II occur at 1653 and 1545 cm−1 in the infrared spectrum (fig. S2), indicating that hydrogen bonding plays an important role in the assembly process (33). Meanwhile, as AZIM assemblies deaggregate at high temperature (Fig. 2A), the downfield shifts of AZIM protons (H1 to H5) in the 1H nuclear magnetic resonance (NMR) spectra with increasing temperature indicate that π-π stacking is an important driving force in the assembly process (Fig. 2C). Moreover, the small-angle x-ray reflection diffraction result shows that AZIM is patterned into a layered structure with a d spacing of 2.49 nm, which matches well with the molecular length of AZIM (fig. S4). These results clearly suggest that ionic compound AZIM assembles into nanofibers and gelates water via the synergistic effect of hydrogen bonding and π-π stacking interaction (Fig. 2D and fig. S5).
Fig. 2. Optically modulated ionic conductivity of the OMIC hydrogel.
(A) Molecular structure of AZIM and illustration of sol-gel transition of the AZIM supramolecular hydrogel. (B) Transmission electron microscopy image of AZIM assemblies. (C) Temperature-dependent 1H nuclear magnetic resonance spectra of AZIM assemblies in D2O. (D) Schematic representation of the self-assembly process of AZIM ions in water. (E) Dynamic responses of relative impedance of the AZIM supramolecular hydrogel to various temperatures. Impedance of the AZIM supramolecular hydrogel was measured by an LCR meter with the AC voltage of 0.2 V at 10 kHz. (F) Fabrication of the OMIC hydrogel by doping AZIM ions into PAAm/Fe3O4 xerogel. (G) Change of relative impedance of the OMIC hydrogel under various temperatures. (H) Temperature change of the OMIC hydrogel from 21.3°C to 39.8°C with the radiation of near-infrared light (808 nm, 390 mW/cm2). (I) Optically modulated impedance switching ability of the OMIC hydrogel in an AC circuit. The light-emitting diode bulb was off at the initial state and became bright after applying near-infrared light to the OMIC hydrogel for 180 s. (J) Real-time tracking of the relative impedance of the OMIC hydrogel with radiation of near-infrared light. After removal of optical stimulus, relative impedance of the OMIC hydrogel gradually increased to the initial state due to the recovery of temperature.
The assembly of AZIM ions is temperature sensitive, disassembling into free ions at high temperature (Fig. 2C and fig. S1). This phenomenon can be used to control the ionic conductivity of the AZIM hydrogel by tuning the temperature. As shown in fig. S6 and Supplementary Text, we analyzed the impedance change of the AZIM hydrogel at different environmental temperatures with an AC frequency of 10 kHz (34). Here, we defined the relative impedance as 100% (Z is the real-time reading of the impedance and Z0 is the initial impedance) to describe the conductivity of the AZIM hydrogel. When increasing temperature from 20°C to 40°C, the deaggregation of AZIM assemblies led to an obvious decrease in the relative impedance of the AZIM hydrogel (Fig. 2E). Reversely, the relative impedance of the AZIM hydrogel increased when temperature gradually decreased to 20°C. The temperature-dependent conductivity of the AZIM hydrogel can be attributed to the reversible assembly process of AZIM ions and the thermal motion of ions at different temperatures. By comparing to KBr solution as a reference, the temperature-induced impedance change of AZIM assemblies is obviously larger than KBr salt, which proved that the thermal-responsive assembly process of AZIM ions plays a dominant role in tuning the conductance of the AZIM hydrogel (fig. S7).
Because of the temperature-induced sol-gel transition, it is difficult to maintain the shape of the AZIM hydrogel while using its stimuli-responsive conductivity in practical applications. Therefore, we design a hybrid OMIC hydrogel, consisting of AZIM assemblies, PAAm networks, and Fe3O4 nanoparticles (fig. S10), to realize noncontact optical control of the ionic conductivity (Fig. 2F). PAAm networks serve as the polymer matrix to support the shape of the OMIC hydrogel (fig. S8 and S9), and Fe3O4 nanoparticles with a photothermal conversion efficiency of 45.3% (fig. S11) are used to transform optical stimuli into heat for modulating the assembly process of AZIM ions. In this hybrid hydrogel, the temperature-induced reversible assembly of AZIM ions endows the OMIC hydrogel with the thermal-responsive ionic conductivity (Fig. 2G). The near-infrared light with a wavelength of 808 nm was transduced into heat by Fe3O4 nanoparticles for controlling the temperature of the OMIC hydrogel (Fig. 2H). As a result, we can use the near-infrared light to modulate the conductance of the OMIC hydrogel via triggering the deaggregation of AZIM assemblies (Fig. 2J). Compared with the OMIC hydrogel, conductance of a PAAm/AZIM hydrogel without Fe3O4 nanoparticles showed only a little change under the near-infrared radiation, indicating the effective photothermal effect of Fe3O4 nanoparticles in the OMIC hydrogel (fig. S12 and fig. S13). To demonstrate the optically modulated ionic conductivity, we connected a piece of the OMIC hydrogel and a light-emitting diode (LED) bulb in series with an AC source of 2.6 V at a frequency of 100 Hz (Fig. 2I). The LED bulb turned on after the OMIC hydrogel was radiated by near-infrared light for 180 s, which indicated that the OMIC hydrogel can be used as a circuit element to modulate the electric current under optical radiation (fig. S14). This characteristic represents an essential ability of the OMIC hydrogel to be incorporated in a circuit to emulate a biological synapse for regulating information transmission.
The OMIC hydrogel for the emulation of synaptic functions
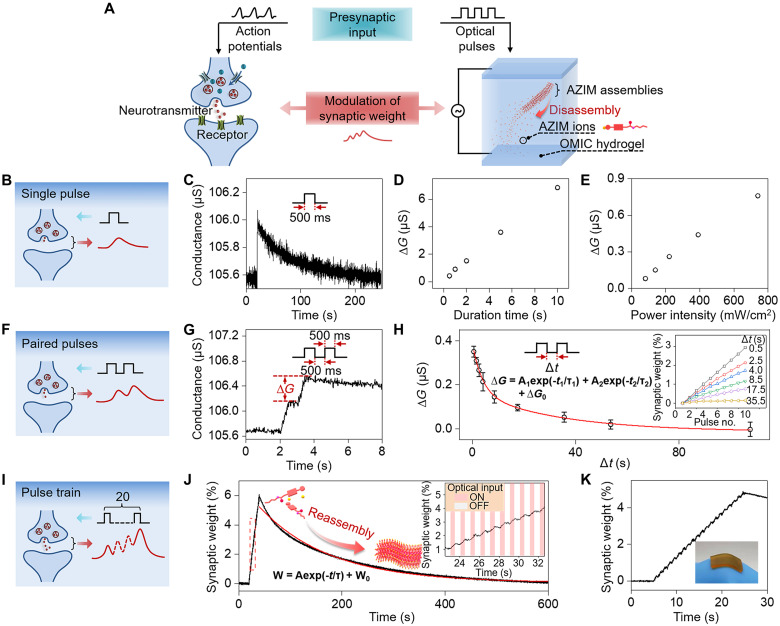
In neuroscience, synaptic plasticity refers to the change in synaptic weight, representing the strength of the connection between two neurons, which is the key characteristic of a biological synapse for modulating information transmission in neural systems (35). To emulate synaptic characteristics by our OMIC hydrogel, we used a piece of the OMIC hydrogel to construct a two-terminal artificial synapse and the near-infrared optical pulses as the presynaptic inputs (Fig. 3A). When applying a single optical pulse (390 mW/cm2, 500 ms) to the OMIC hydrogel, the conductance of the OMIC hydrogel spontaneously increased, which was mainly caused by photothermal-induced partial release of AZIM ions from AZIM assemblies. After removing the optical stimulus, the conductance of the OMIC hydrogel gradually decayed to the initial state, owing to the reversible assembly of AZIM ions (Fig. 3C). This optically modulated conductivity in the OMIC hydrogel is analogous to excitatory postsynaptic potential (EPSP) in a biological synapse (Fig. 3B), which is the essential process to fire an action potential in the postsynaptic neuron for transmitting neural information (36). The conductance change of the OMIC hydrogel is directly related to the operation parameters of the applied optical pulses. Increasing duration time and light intensity would effectively enhance the conductance change, because more heat was generated to promote the disassembly of AZIM assemblies (Fig. 3, D and E).
Fig. 3. Synaptic characteristics of the OMIC hydrogel.
(A) Biological information processing is realized through modulation of the synaptic weight, which originates from the release of neurotransmitters stimulated by action potentials. For emulating synaptic functions with the OMIC hydrogel, optical pulses are used to emulate the function of action potentials for triggering the release of AZIM ions through disassembly of AZIM assemblies, which mimics the function of neurotransmitters for modulating the synaptic weight. (B) Illustration of excitatory postsynaptic potential (EPSP) activated by a single pulse. (C) Change in conductance of the OMIC hydrogel activated by a single optical pulse (390 mW/cm2, 500 ms). Conductance change (ΔG) of the OMIC hydrogel caused by a single optical pulse potentiates with increasing duration time (390 mW/cm2) (D) and power intensity (500 ms) (E), respectively. (F) Illustration of EPSPs activated by two paired pulses. (G) The conductance of the OMIC hydrogel stimulated by two successive optical pulses with a time interval of 500 ms. (H) ΔG of the OMIC hydrogel stimulated by two consecutive optical pulses with different time intervals (Δt). Following the double exponential decay curve, two characteristic times τ1 and τ2 were calculated to be 3.82 s and 34.07 s, respectively. The inset illustrates the change of synaptic weight in the OMIC hydrogel stimulated by 10 optical pulses with different time intervals (Δt). (I) Illustration of optically modulated information processing of the OMIC hydrogel activated by a pulse train. (J) Modulation of synaptic weight in the OMIC hydrogel stimulated by 20 optical pulses (1.0 Hz). The inset is the magnified figure of the data plots in the red dashed box. (K) Modulation of synaptic weight in the OMIC hydrogel stimulated by 20 optical pulses (1.0 Hz) when attaching to a bending finger joint.
Paired pulse facilitation (PPF) is an essential characteristic of short-term synaptic plasticity in neuroscience, which represents an enhancement in EPSP evoked by the second spike that closely follows the first one (37). The reason is that the residual Ca2+ ions in the presynaptic terminal caused by the first action potential facilitate the release of neurotransmitters evoked by the second one (Figs. 1A and 3F). When applying two successive optical pulses with a time interval of 500 ms to the OMIC hydrogel, the conductance change caused by the second pulse was larger than the one evoked by the first pulse, which showed the characteristic of PPF (Fig. 3G). This phenomenon can be attributed to the fact that the released AZIM ions evoked by the first optical pulse are not able to totally reassemble when subjected to the second optical pulse, which will enhance the following EPSP. By increasing the time interval, the amount of conductance change (ΔG) caused by two successive optical pulses showed a gradual decrease (Fig. 3H). The change in ΔG fits well with a double exponential decay curve, which is similar to the decreasing trend of facilitation in a biological synapse (38). As a result, an optical pulse train with a shorter time interval efficiently facilitated the synaptic weight (, where G0 is the initial conductance of the OMIC hydrogel and Gn is the conductance facilitated by n optical pulses) of the OMIC hydrogel–based artificial synapse, whereas the synaptic weight showed a smaller change when applying an optical pulse train with a longer time interval (inset in Fig. 3H). This phenomenon demonstrated the spike rate–dependent plasticity, one of the essential characteristics of synaptic plasticity where the simulation of synaptic weight could be affected by the frequency of the presynaptic spikes (39).
As discussed above, synaptic plasticity is determined by the synaptic weight, which can be continuously stimulated by the presynaptic spikes (35). Until the summation of the synaptic weight surpasses the firing threshold, action potentrials are generated at the postsynaptic terminal, leading to information transmission from a presynaptic neuron to a postsynaptic neuron (Fig. 1A). Similarly, synaptic plasticity of the OMIC hydrogel–based artificial synapse is closely related to the number of optical pulses as it continuously triggers the release of mobile AZIM ions from AZIM assemblies. Therefore, the synaptic weight increases as the number of optical pulses increases from 5 to 40 (fig. S15), which shows the characteristic of spike number–dependent plasticity (40). After being stimulated by the successive optical pulses (390 mW/cm2; fig. S16), the synaptic weight of the OMIC hydrogel–based artificial synapse showed a fast decay at first and then decreased slowly to the initial state (Fig. 3, I and J), which fitted well with stretched-exponential function (41). Such characteristic can be used to mimic the short-term plasticity of a synapse, which is analogous to the short-term memory behavior in psychology (42, 43). Furthermore, the characteristic relaxation time (τ) increased along with the pulse number change from 5 to 40, which showed the improved information retention capability when applying a strong optical stimulus containing more pulses (fig. S15). Because the change of the synaptic weight is modulated by the photothermal effect, the heat dissipation is closely related to the recovery rate of temperature, which is affected by the surrounding temperature of a hydrogel (fig. S17). Thus, we integrated a semiconductor cooler with our hydrogel-based device to control the surrounding temperature and modulate the heat dissipation of the OMIC hydrogel, which showed that the decay of the synaptic weight was obviously accelerated (fig. S17). As a result, we achieved to control the “forgetting” behavior of the hydrogel-based artificial synapse by the design of the overall device. The optically modulated synaptic plasticity of the OMIC hydrogel–based artificial synapse can be well maintained under different bending states (fig. S18) and stretching states (fig. S19) due to the intrinsic flexibility of the hydrogel, indicating that it can be adapted to the various curved surfaces (Fig. 3K). Meanwhile, the optically mediated switching of the ionic conductivity in the OMIC hydrogel–based artificial synapse is well maintained during multicycle operation (e.g., 100 times) (fig. S20). By sealing the hydrogel between a polyethylene terephthalate (PET) film and a silicone substrate, the synaptic functions of the hydrogel-based device can be well maintained for nearly 1 month (fig. S21), showing the great stability in the practical applications.
In neuroscience, memory is considered to be a behavioral change resulting from an experience, and learning is a process of acquiring memory (44). As the essential function for human intelligence, learning and memory are closely related to the dynamic change of the synaptic weight, which is affected by forgetting (45, 46). A typical behavior in learning experience is that relearning the forgotten memory becomes easier than the first learning process (47), leading to the “time-saving” effect to adapt to the changing environment (48). On the basis of the short-term synaptic plasticity discussed above, we used the OMIC hydrogel to emulate the learning-experience behavior by applying 20 consecutive optical pulses as a learning process; the synaptic weight of the hydrogel-based artificial synapse potentiated obviously and then decayed over time after removing the optical stimulus, which was analogous to the idea that partial information obtained from learning process would be forgotten spontaneously after a period of time (Fig. 4A). The decay curve fitted well with the Kohlrausch stretched-exponential function, consistent with the forgetting trend of acquired information in human brain (49). After removing the optical stimulus for 75 s, only 11 optical pulses were needed as a relearning process to recover the previous level of the synaptic weight in the first learning process, which was able to emulate the learning-experience behavior in the brain (fig. S22). These processes are attributed to the reversible assembly and disassembly of AZIM ions in the OMIC hydrogel induced by the near-infrared optical pulses (Fig. 4A). In the learning process, optically triggered release of AZIM ions from AZIM assemblies enhanced the ionic conductance of the OMIC hydrogel, representing the stimulation of the synaptic weight. After removing the optical stimulus, mobile AZIM ions gradually reassembled into nano aggregates. Before the released AZIM ions totally reassembled, fewer optical pulses were already able to trigger the release of enough AZIM ions to reach the previous level of conductance. These results show that, with the stimuli-responsive supramolecular design, our OMIC hydrogel can emulate the learning-experience behavior in human, performing a potential ability to modulate the incoming stimulus for exhibiting bioinspired feedback (49).
Fig. 4. Optically modulated autonomous motion feedback system with learning-experience capability.
(A) Learning-experience capability and corresponding illustration in the OMIC hydrogel. (B) Illustration of information processing with learning-experience capability. Four optical pulses (1.0 Hz) are described as a weak stimulus that cannot stimulate the synaptic weight to reach the firing threshold. Twenty optical pulses (1.0 Hz) are described as a strong stimulus that stimulates the synaptic weight to exceed the firing threshold. After experiencing a learning stage with a strong stimulus, each weak stimulus with a short time interval of forgetting process can recover the previous synaptic weight over the firing threshold. (C) Modulation of synaptic weight in the OMIC hydrogel stimulated by the weak stimulus with a time interval of 15 s. (D) Learning-experience capability emulated with the OMIC hydrogel. (E) Optically modulated motion feedback system consisting of the OMIC hydrogel as an information processor and a robotic hand for object grasping. (F) Illustrations of threshold-dependent motion feedback driven by optical stimuli. Once the OMIC hydrogel received the optical input and switched to a strongly activated state, the robotic hand would autonomously grasp the target object and move it into a box. In contrast, it would have no motion feedback when the postsynaptic neuron was at a weakly activated state that blocked the transmission of information. (G) Photographs of motion feedback of the robotic hand when applying different optical stimuli to the OMIC hydrogel. Without a learning process, weak stimulus could not make the artificial synapse generate a feedback command. After applying a strong stimulus as a learning process, each weak stimulus triggered by the sliding target object could control the robotic hand to successfully grasp and move it to a box.
Optically modulated autonomous motion feedback system
Information transmission from the presynaptic neuron to the postsynaptic neuron is determined by the change of the synaptic weight and the firing threshold of the postsynaptic membrane potential (50). As illustrated in Fig. 4B, only when the synapse is strongly activated does the synaptic weight reach the firing threshold and will the action potentials be triggered at the postsynaptic neuron for transmitting neural information and implementing neural activities, which exhibits an all-or-none principle (51, 52). As a proof of concept, we used a piece of the OMIC hydrogel as an information processor to build an optically modulated motion feedback system, which can autonomously generate robotic motions by perceiving various optical stimuli (Fig. 4E and figs. S23 and S24). In this setup, a piece of the OMIC hydrogel was attached to a robotic head model to receive and interpret optical stimuli containing different numbers of pulses. After being stimulated by optical pulse trains, the synaptic weight in the OMIC hydrogel–based artificial synapse was collected in a computer to compare with the given threshold for determining the generation of a feedback command (Fig. 4, B to D). Following an all-or-none principle, only when the synaptic weight surpassed the firing threshold would a command be generated to control the movement of the robotic hand (Fig. 4F). The connection of the meter and the robotic controller was achieved by a python program for delivering the data and commands. As a result, by perceiving different optical messages, our OMIC hydrogel will functionally emulate a biological synapse to regulate the robotic motion feedback in a logical way, performing the bioinspired learning and memory behavior without the help of the software (movie S1 and S2).
On the basis of the aforementioned working principle of the optically modulated feedback system, we used a pulse train with 20 pulses as a strong stimulus that could stimulate the OMIC hydrogel to reach the firing threshold and a pulse train with 4 pulses as a weak stimulus for delivering an operating command (Fig. 4B). In this design, a line of toy cubes as target objects moved from the slide to the platform one by one every 15 s. When the target object arrived at the platform, the weak stimulus containing four optical pulses was triggered and applied to the OMIC hydrogel (Fig. 4E and fig. S24). As the stimulated synaptic weight failed to reach the threshold, the OMIC hydrogel–based artificial synapse remained at a weakly activated state (Fig. 4C). Following the all-or-none principle, the optically modulated motion feedback system could not generate a grasping motion to any weak stimulus, causing the target objects stranded at the platform (Fig. 4G and movie S1).
In contrast, when the hydrogel-based artificial synapse experienced a learning process by being subjected to the strong optical stimulus, this artificial synapse was stimulated to the strongly activated state, which enabled the transmission of incoming information for generating the robotic motion feedback to grasp the target object and drop it into the box (Fig. 4G). When the target objects slid to the platform with a time interval of 15 s, the triggered weak stimulus could recall the previous memory level and keep the artificial synapse at a strongly activated state, which maintained the synaptic connection in this system and controlled the robotic hand to complete the grasping motion repeatedly (Fig. 4, D and G; fig. S15; and movie S2). Compared with the hydrogel-based artificial synapse without a strong optical stimulus, the artificial synapse experienced a learning stage that was able to generate the reliable and repeated motion feedback to the weak optical stimuli. Such phenomena indicate that, when receiving the same optical inputs, the artificial synapse with different degrees of learning can result in the different motion feedback, performing the learning-experience behavior. Therefore, the OMIC hydrogel–based autonomous feedback system shows an effective simplification and energy conversation when performing the cyclical tasks. Our designed OMIC hydrogel performs a good collaboration of bioinspired optical sensation and information processing ability, which provides a promising strategy for designing the bioinspired autonomous devices.
DISCUSSION
Inspired by the information perception and processing in biological systems, we design an effective strategy to combine supramolecular approaches and device functions to convert environmental messages into processable information and generate logical and intelligent feedback, which greatly improved the applications of hydrogel materials. In addition, compared with the limited options of photoactive semiconductor materials used in the optoelectronic synaptic devices, such as photosensitive metal oxide semiconductors and perovskites, our ion-conductive hydrogel provides a different mechanism to emulate various functions of a biological synapse just through modulating ions, which simplified the structural design and expanded the choices of materials for synaptic emulation. As a demonstration, we designed an optically modulated autonomous motion feedback system consisting of the OMIC hydrogel as an information processing unit to logically regulate the motion feedback of a robotic hand. Meanwhile, the intrinsic flexibility and stretchability of PAAm network and ion-dominant synaptic functions make the hydrogel-based artificial synapse withstand mechanical deformation, which is vital for developing flexible electronics. These findings prove that rational designs of ionic modulation enable hydrogels as truly intelligent materials, which will surely push forward the applications of hydrogel materials in functional ionotronics and bring valuable insights into developing bioinspired functions.
MATERIALS AND METHODS
Materials
All solvents were purchased from Tianjin Fuyu Fine Chemical Co. Ltd. and used without further purification. Unless otherwise mentioned, all chemicals were purchased from Shanghai Aladdin Biochemical Technology Co. Ltd. and used without further purification. The starting material 4-[(4′-hydroxy)phenylazo]benzyl alcohol was synthesized according to a previous report (53). The details for preparing AZIM are shown in the Supplementary Materials.
Fabrication of the AZIM supramolecular hydrogel
To fabricate an AZIM supramolecular hydrogel, AZIM powder (4.0 mg) was dispersed in 0.2 ml of deionized (DI) water, which was heated to 70°C for 10 min. Then, an orange hydrogel was obtained when the mixture was cooled down to room temperature.
Fabrication of the OMIC hydrogel
For a typical OMIC hydrogel sample, 36.00 mg of AAm, 0.40 mg of N,N′-methylenebisacrylamide, 1.16 mg of photoinitiator (Irgacure, 2959), and 5.0 μl of 20 weight % aqueous dispersion of Fe3O4 nanoparticles were dispersed into 0.2 ml of DI water. Then, the mixture was radiated under violet light (365 nm) for 3 min to obtain a brown PAAm/Fe3O4 hydrogel, which was placed into a vacuum drying box for 3 hours at room temperature. After removing water, the PAAm/Fe3O4 xerogel was kept in the hot solution of AZIM (70°C) for 1 hour to obtain the OMIC hydrogel.
Fabrication of the OMIC hydrogel for emulating the synaptic functions
Two Cu electrodes with a “T”-shaped structure were adhered parallel on a PET film, and the distance between two electrode edges was set as 4 mm. Then, a piece of the OMIC hydrogel (8 mm by 7 mm by 2 mm) was placed on the Cu electrodes to fabricate a two-terminal artificial synapse. The contacting area between electrodes and the OMIC hydrogel was set as 20 mm2. This OMIC hydrogel–based artificial synapse was sealed with PE film to avoid the evaporation of water during testing.
Characterization
1H NMR and 13C NMR spectra were measured on a Bruker AVANCE III HD 400-MHz spectrometer with the residual solvent or tetramethylsilane as a reference. Elemental analysis was performed on an Elementar Vario EL cube elemental analyzer. The Fourier transform infrared spectra were measured using a Bruker Tensor II spectrometer. Ultraviolet-visible spectra were recorded on a SHIMADZU UV-2550 spectrophotometer. Powder x-ray diffraction was achieved on a Rigaku SmartLab x-ray diffractometer with Cu/Kα radiation (λ = 0.15406 nm), which was operated at 9 kW, 200 mA. Transmission electron microscopy (TEM) images were collected on a JEOL JEM-1400 electron microscope. The samples prepared for TEM tests were dropped on the copper grid and air-dried. Scanning electron microscopy images were collected by a Zeiss G300 scanning electron microscope. Atomic force microscopy characterization was performed on a Bruker Bioscope Resolve atomic force microscope. The temperature of the OMIC hydrogel was recorded by a FLUKE VT04A visible infrared detector. The optical pulses were generated through a near-infrared light source (808 nm) with an optical chopper (Scitec, model-300 CD). Demonstration for lighting the white bulb under optical stimulus was executed by a signal generator (Tektronix, AFG 1062) as power supplier. Impedances of the AZIM supramolecular hydrogel at various temperatures were measured by CHI 760B electrochemical workstation (Chenhua, China) with a frequency range from 10−2 to 105 Hz. For relative impedance changes of KBr solution, the AZIM hydrogel and the OMIC hydrogel were tracked by a Keysight E4980AL LCR [Inductance (L), Capacitance (C), and Resistance (R)] meter (0.2 V, 10 kHz). We also used a signal generator as power supplier with an output voltage of 0.2 V, an operating frequency of 10 kHz, and a data acquisition system (Keithley DAQ6510) to monitor the current when operating the OMIC hydrogel–based devices.
Demonstration design of optically modulated autonomous motion feedback system
A piece of the OMIC hydrogel was attached to the head of the robotic model to perceive the optical stimuli. To monitor the conductance of the OMIC hydrogel, an LCR meter was connected to the OMIC hydrogel (fig. S23). Then, a python program was operated to convert the conductance into the synaptic weight. In this process, the synaptic weight and the optically responsive conductance followed the relationship
G0 represents the initial conductance of the OMIC hydrogel. Gn represents the conductance facilitated by n optical pulses. Gth represents the given threshold of the conductance. When the normalized synaptic weight in the OMIC hydrogel–based artificial synapse reaches the given threshold, the robotic motion would be triggered by a feedback command. The python program is used to connect the LCR meter with the robotic controller for transforming the data read by the LCR meter into a command to trigger the robotic motion feedback.
Acknowledgments
We thank the technical support from Analytical Center for Structural Constituent and Physical Property, Shandong University.
Funding: This work was supported by the Qilu Young Scholarship Funding of Shandong University, the Natural Science Foundation of Jiangsu Province (BK20200231), the National Natural Science Foundation of China (Nos. 22002073), and the Natural Science Foundation of Shandong Province (ZR2020QB064).
Author contributions: Y.L. conceived the concept and supervised the work. H.T. designed the test protocol and performed the experiments. C.W., Y.C., and L.Z. analyzed the experimental data. H.J., L.X., and X.W. participated in the fabrications of device and circuit diagram. H.T., Y.L., and J.H. cowrote the paper. All the authors discussed the results and commented on the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 to S30
Legends for movies S1 and S2
References
Other Supplementary Material for this : manuscript includes the following:
Movies S1 and S2
REFERENCES AND NOTES
- 1.J. Yeom, A. Choe, S. Lim, Y. Lee, S. Na, H. Ko, Soft and ion-conducting hydrogel artificial tongue for astringency perception. Sci. Adv. 6, eaba5785 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.R. Yang, G. Li, C. Zhuang, P. Yu, T. Ye, Y. Zhang, P. Shang, J. Huang, M. Cai, L. Wang, W. Cui, L. Deng, Gradient bimetallic ion-based hydrogels for tissue microstructure reconstruction of tendon-to-bone insertion. Sci. Adv. 7, eabg3816 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.P. Li, Y. F. Poon, W. Li, H.-Y. Zhu, S. H. Yeap, Y. Cao, X. Qi, C. Zhou, M. Lamrani, R. W. Beuerman, E.-T. Kang, Y. Mu, C. M. Li, M. W. Chang, S. S. Jan Leong, M. B. Chan-Park, A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat. Mater. 10, 149–156 (2011). [DOI] [PubMed] [Google Scholar]
- 4.C. Yang, Z. Suo, Hydrogel ionotronics. Nat. Rev. Mater. 3, 125–142 (2018). [Google Scholar]
- 5.M. S. Sarwar, Y. Dobashi, C. Preston, J. K. M. Wyss, S. Mirabbasi, J. D. W. Madden, Bend, stretch, and touch: Locating a finger on an actively deformed transparent sensor array. Sci. Adv. 3, e1602200 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.H. Yuk, T. Zhang, G. A. Parada, X. Liu, X. Zhao, Skin-inspired hydrogel—Elastomer hybrids with robust interfaces and functional microstructures. Nat. Commun. 7, 12028 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Z. Han, P. Wang, Y. Lu, Z. Jia, S. Qu, W. Yang, A versatile hydrogel network-repairing strategy achieved by the covalent-like hydrogen bond interaction. Sci. Adv. 8, eabl5066 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.M. Baumgartner, F. Hartmann, M. Drack, D. Preninger, D. Wirthl, R. Gerstmayr, L. Lehner, G. Mao, R. Pruckner, S. Demchyshyn, L. Reiter, M. Strobel, T. Stockinger, D. Schiller, S. Kimeswenger, F. Greibich, G. Buchberger, E. Bradt, S. Hild, S. Bauer, M. Kaltenbrunner, Resilient yet entirely degradable gelatin-based biogels for soft robots and electronics. Nat. Mater. 19, 1102–1109 (2020). [DOI] [PubMed] [Google Scholar]
- 9.C. Keplinger, J.-Y. Sun, C. C. Foo, P. Rothemund, G. M. Whitesides, Z. Suo, Stretchable, transparent, ionic conductors. Science 341, 984–987 (2013). [DOI] [PubMed] [Google Scholar]
- 10.D. L. Taylor, M., Self-healing hydrogels. Adv. Mater. 28, 9060–9093 (2016). [DOI] [PubMed] [Google Scholar]
- 11.X. Li, K. Cui, T. Kurokawa, Y. N. Ye, T. L. Sun, C. Yu, C. Creton, J. P. Gong, Effect of mesoscale phase contrast on fatigue-delaying behavior of self-healing hydrogels. Sci. Adv. 7, eabe8210 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.H. Wang, C. N. Zhu, H. Zeng, X. Ji, T. Xie, X. Yan, Z. L. Wu, F. Huang, Reversible ion-conducting switch in a novel single-ion supramolecular hydrogel enabled by photoresponsive host–guest molecular recognition. Adv. Mater. 31, 1807328 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Y. H. Jung, B. Park, J. U. Kim, T. I. Kim, Bioinspired electronics for artificial sensory systems. Adv. Mater. 31, 1803637 (2019). [DOI] [PubMed] [Google Scholar]
- 14.K. Liu, Y. Bian, J. Kuang, X. Huang, Y. Li, W. Shi, Z. Zhu, G. Liu, M. Qin, Z. Zhao, X. Li, Y. Guo, Y. Liu, Ultrahigh-performance optoelectronic skin based on intrinsically stretchable perovskite-polymer heterojunction transistors. Adv. Mater. 34, 2107304 (2022). [DOI] [PubMed] [Google Scholar]
- 15.K. He, Y. Liu, J. Yu, X. Guo, M. Wang, L. Zhang, C. Wan, T. Wang, C. Zhou, X. Chen, Artificial neural pathway based on a memristor synapse for optically mediated motion learning. ACS Nano 16, 9691–9700 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Y. Zhang, C. K. Jeong, J. Wang, X. Chen, K. H. Choi, L.-Q. Chen, W. Chen, Q. M. Zhang, Q. Wang, Hydrogel ionic diodes toward harvesting ultralow-frequency mechanical energy. Adv. Mater. 33, 2103056 (2021). [DOI] [PubMed] [Google Scholar]
- 17.H.-R. Lee, J. Woo, S. H. Han, S.-M. Lim, S. Lim, Y.-W. Kang, W. J. Song, J.-M. Park, T. D. Chung, Y.-C. Joo, J.-Y. Sun, A stretchable ionic diode from copolyelectrolyte hydrogels with methacrylated polysaccharides. Adv. Funct. Mater. 29, 1806909 (2019). [Google Scholar]
- 18.X. Liu, J. Liu, S. Lin, X. Zhao, Hydrogel machines. Mater. Today 36, 102–124 (2020). [Google Scholar]
- 19.Z. Yin, M.-J. Yin, Z. Liu, Y. Zhang, A. P. Zhang, Q. Zheng, Solution-processed bilayer dielectrics for flexible low-voltage organic field-effect transistors in pressure-sensing applications. Adv. Sci. 5, 1701041 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.J. Hu, M.-J. Lu, F.-Z. Chen, H.-M. Jia, H. Zhou, K. Li, X. Zeng, W.-W. Zhao, P. Lin, Multifunctional hydrogel hybrid-gated organic photoelectrochemical transistor for biosensing. Adv. Funct. Mater. 32, 2109046 (2022). [Google Scholar]
- 21.D. Lai, E. Li, Y. Yan, Y. Liu, J. Zhong, D. Lv, Y. Ke, H. Chen, T. Guo, Gelatin-hydrogel based organic synaptic transistor. Org. Electron. 75, 105409 (2019). [Google Scholar]
- 22.I. Cunha, R. Barras, P. Grey, D. Gaspar, E. Fortunato, R. Martins, L. Pereira, Reusable cellulose-based hydrogel sticker film applied as gate dielectric in paper electrolyte-gated transistors. Adv. Funct. Mater. 27, 1606755 (2017). [Google Scholar]
- 23.L. Herlogsson, X. Crispin, S. Tierney, M. Berggren, Polyelectrolyte-gated organic complementary circuits operating at low power and voltage. Adv. Mater. 23, 4684–4689 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Y. Kim, A. Chortos, W. Xu, Y. Liu, J. Y. Oh, D. Son, J. Kang, A. M. Foudeh, C. Zhu, Y. Lee, S. Niu, J. Liu, R. Pfattner, Z. Bao, T.-W. Lee, A bioinspired flexible organic artificial afferent nerve. Science 360, 998–1003 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Y. van de Burgt, E. Lubberman, E. J. Fuller, S. T. Keene, G. C. Faria, S. Agarwal, M. J. Marinella, A. Alec Talin, A. Salleo, A non-volatile organic electrochemical device as a low-voltage artificial synapse for neuromorphic computing. Nat. Mater. 16, 414–418 (2017). [DOI] [PubMed] [Google Scholar]
- 26.M.-J. Yin, Z. Yin, Y. Zhang, Q. Zheng, A. P. Zhang, Micropatterned elastic ionic polyacrylamide hydrogel for low-voltage capacitive and organic thin-film transistor pressure sensors. Nano Energy 58, 96–104 (2019). [Google Scholar]
- 27.N. C. Spitzer, Activity-dependent neurotransmitter respecification. Nat. Rev. Neurosci. 13, 94–106 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.L. Mennel, J. Symonowicz, S. Wachter, D. K. Polyushkin, A. J. Molina-Mendoza, T. Mueller, Ultrafast machine vision with 2D material neural network image sensors. Nature 579, 62–66 (2020). [DOI] [PubMed] [Google Scholar]
- 29.L. Lagnado, F. Schmitz, Ribbon synapses and visual processing in the retina. Annu. Rev. Vis. Sci. 1, 235–262 (2015). [DOI] [PubMed] [Google Scholar]
- 30.S. E. Hyman, Neurotransmitters. Curr. Biol. 15, R154–R158 (2005). [DOI] [PubMed] [Google Scholar]
- 31.H. Yang, Z. Liu, B. K. Chandran, J. Deng, J. Yu, D. Qi, W. Li, Y. Tang, C. Zhang, X. Chen, Self-protection of electrochemical storage devices via a thermal reversible sol–gel transition. Adv. Mater. 27, 5593–5598 (2015). [DOI] [PubMed] [Google Scholar]
- 32.B. James, L. Darnet, J. Moya-Díaz, S.-H. Seibel, L. Lagnado, An amplitude code transmits information at a visual synapse. Nat. Neurosci. 22, 1140–1147 (2019). [DOI] [PubMed] [Google Scholar]
- 33.L. Ji, Q. He, D. Niu, J. Tan, G. Ouyang, M. Liu, Host-guest interaction enabled chiroptical photo-switching and enhanced circularly polarized luminescence. Chem. Commun. 55, 11747–11750 (2019). [DOI] [PubMed] [Google Scholar]
- 34.I. You, D. G. Mackanic, N. Matsuhisa, J. Kang, J. Kwon, L. Beker, J. Mun, W. Suh, T. Y. Kim, J. B.-H. Tok, Z. Bao, U. Jeong, Artificial multimodal receptors based on ion relaxation dynamics. Science 370, 961–965 (2020). [DOI] [PubMed] [Google Scholar]
- 35.A. Citri, R. C. Malenka, Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacology 33, 18–41 (2008). [DOI] [PubMed] [Google Scholar]
- 36.L. F. Abbott, W. G. Regehr, Synaptic computation. Nature 431, 796–803 (2004). [DOI] [PubMed] [Google Scholar]
- 37.P. Schulz, E. Cook, D. Johnston, Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation. J. Neurosci. Res. 14, 5325–5337 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R. S. Zucker, W. G. Regehr, Short-term synaptic plasticity. Annu. Rev. Physiol. 64, 355–405 (2002). [DOI] [PubMed] [Google Scholar]
- 39.S. Dai, Y. Zhao, Y. Wang, J. Zhang, L. Fang, S. Jin, Y. Shao, J. Huang, Recent advances in transistor-based artificial synapses. Adv. Funct. Mater. 29, 1903700 (2019). [Google Scholar]
- 40.Y. Lee, J. Y. Oh, W. Xu, O. Kim, T. R. Kim, J. Kang, Y. Kim, D. Son, J. B.-H. Tok, M. J. Park, Z. Bao, T.-W. Lee, Stretchable organic optoelectronic sensorimotor synapse. Sci. Adv. 4, eaat7387 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.T. Chang, S.-H. Jo, W. Lu, Short-term memory to long-term memory transition in a nanoscale memristor. ACS Nano 5, 7669–7676 (2011). [DOI] [PubMed] [Google Scholar]
- 42.R. S. Zucker, Short-term synaptic plasticity. Annu. Rev. Neurosci. 12, 13–31 (1989). [DOI] [PubMed] [Google Scholar]
- 43.T. Ohno, T. Hasegawa, T. Tsuruoka, K. Terabe, J. K. Gimzewski, M. Aono, Short-term plasticity and long-term potentiation mimicked in single inorganic synapses. Nat. Mater. 10, 591–595 (2011). [DOI] [PubMed] [Google Scholar]
- 44.H. Okano, T. Hirano, E. Balaban, Learning and memory. Proc. Natl. Acad. Sci. U.S.A. 97, 12403–12404 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.C. Hölscher, Synaptic plasticity and learning and memory: LTP and beyond. J. Neurosci. Res. 58, 62–75 (1999). [PubMed] [Google Scholar]
- 46.D. C. Rubin, A. E. Wenzel, One hundred years of forgetting: A quantitative description of retention. Psychol. Rev. 103, 734–760 (1996). [Google Scholar]
- 47.W. T. Greenough, J. E. Black, C. S. Wallace, Experience and brain development. Child Dev. 58, 539–559 (1987). [PubMed] [Google Scholar]
- 48.X.-B. Yin, R. Yang, K.-H. Xue, Z.-H. Tan, X.-D. Zhang, X.-S. Miao, X. Guo, Mimicking the brain functions of learning, forgetting and explicit/implicit memories with SrTiO3-based memristive devices. Phys. Chem. Chem. Phys. 18, 31796–31802 (2016). [DOI] [PubMed] [Google Scholar]
- 49.D. C. Rubin, S. Hinton, A. Wenzel, The precise time course of retention. J. Exp. Psychol. Learn. 25, 1161–1176 (1999). [Google Scholar]
- 50.A. M. Thomson, J. Deuchars, Temporal and spatial properties of local circuits in neocortex. Trends Neurosci. 17, 119–126 (1994). [DOI] [PubMed] [Google Scholar]
- 51.K. He, Y. Liu, M. Wang, G. Chen, Y. Jiang, J. Yu, C. Wan, D. Qi, M. Xiao, W. R. Leow, H. Yang, M. Antonietti, X. Chen, An artificial somatic reflex arc. Adv. Mater. 32, 1905399 (2020). [DOI] [PubMed] [Google Scholar]
- 52.M. Mukovski, S. Chauvette, I. Timofeev, M. Volgushev, Detection of active and silent states in neocortical neurons from the field potential signal during slow-wave sleep. Cereb. Cortex 17, 400–414 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Z.-X. Liu, Y. Feng, Z.-C. Yan, Y.-M. He, C.-Y. Liu, Q.-H. Fan, Multistimuli responsive dendritic organogels based on azobenzene-containing poly(aryl ether) dendron. Chem. Mater. 24, 3751–3757 (2012). [Google Scholar]
- 54.M. Itagaki, S. Suzuki, I. Shitanda, K. Watanabe, Electrochemical impedance and complex capacitance to interpret electrochemical capacitor. Electrochemistry 75, 649–655 (2007). [Google Scholar]
- 55.B. Yao, S. Wu, R. Wang, Y. Yan, A. Cardenas, D. Wu, Y. Alsaid, W. Wu, X. Zhu, X. He, Hydrogel ionotronics with ultra-low impedance and high signal fidelity across broad frequency and temperature ranges. Adv. Funct. Mater. 301, 2109506 (2021). [Google Scholar]
- 56.T. Chen, T. Yao, H. Peng, A. K. Whittaker, Y. Li, S. Zhu, Z. Wang, An injectable hydrogel for simultaneous photothermal therapy and photodynamic therapy with ultrahigh efficiency based on carbon dots and modified cellulose nanocrystals. Adv. Funct. Mater. 31, 2106079 (2021). [Google Scholar]
- 57.B. Liu, J. Sun, J. Zhu, B. Li, C. Ma, X. Gu, K. Liu, H. Zhang, F. Wang, J. Su, Y. Yang, Injectable and NIR-responsive DNA–Inorganic hybrid hydrogels with outstanding photothermal therapy. Adv. Mater. 32, 2004460 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 to S30
Legends for movies S1 and S2
References
Movies S1 and S2