Abstract
Enrolling patients in immunotherapy clinical trials is becoming increasingly competitive. Virtual clinical trials can help investigators answer key questions despite this. For example, pembrolizumab is the recommended first‐line treatment for non‐small cell lung cancer (NSCLC) with no driver alterations and a programmed death ligand 1 (PD‐L1) Tumor Proportion Score ≥50%. Salvage therapies for relapsed/refractory patients are limited. Retrospective studies suggest that a subset of patients may benefit from pembrolizumab beyond progression; these results have not been validated in a prospective study. We constructed digital twins of patients and simulated clinical trials to predict the best salvage therapy after progressive disease (PD) on pembrolizumab. Response dynamics were evaluated at the lesion level to represent patients who experience systemic PD while individual lesions continue shrinking. With >25,000 radiographic lesion measurements from >500 patients, we simulated responses to pembrolizumab, chemotherapy, and PD on pembrolizumab followed by either pembrolizumab beyond progression or salvage chemotherapy. Switching all progressors to salvage chemotherapy was suboptimal. Virtual trials predicted progression‐free survival (PFS) from pembrolizumab beyond progression to be comparable with salvage chemotherapy in patients whose PD was due to nontarget progression. A PFS‐optimized regimen may improve disease control rates ≥15%. Pembrolizumab beyond progression may benefit a subset of patients with PD‐L1‐high, driver alteration–free NSCLC, but prospective studies are warranted.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Virtual clinical trials are a pharmacometrics tool commonly used to inform clinical trial design. Within oncology, however, these methods primarily employ population‐level data without accounting for the within‐patient variability of metastatic disease. Pembrolizumab is a programmed cell death protein 1–targeting immunotherapy that elicits heterogenous responses in patients. Under current disease progression grading guidelines, this can result in patients being classified as treatment resistant even when many lesions (metastases) are still responding. Patients subsequently switched to salvage chemotherapy may be exposed to unnecessary toxicity.
WHAT QUESTION DID THIS STUDY ADDRESS?
Pembrolizumab monotherapy is the recommended first‐line treatment for non‐small cell lung cancer with no driver alterations and programmed death ligand 1 ≥50%. The best salvage therapy for patients who progress on pembrolizumab in this setting is unknown.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
We evaluated whether and for whom pembrolizumab beyond progression could be beneficial. We used or derived individual patient‐level lesion measurements to account for within‐patient heterogeneity in treatment response and conducted a series of virtual clinical trials. We predicted patients for whom pembrolizumab beyond progression could be beneficial.
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
Our study creates impetus to prospectively evaluate pembrolizumab treatment beyond progression in a subset of patients to determine the characteristics associated with response.
INTRODUCTION
Immunotherapy has revolutionized the treatment of many cancers. Unfortunately, the subsequent proliferation in the number of immunotherapy clinical trials has caused considerable competitiveness in patient enrollment. 1 Virtual clinical trials can be valuable tools for demonstrating proof of concept and generating hypotheses ex ante, thereby improving the success rates of real clinical trials.
Pembrolizumab is an antibody inhibitor of programmed cell death protein 1 (PD‐1) recommended for the first‐line treatment of advanced programmed death ligand 1 (PD‐L1) Tumor Proportion Score (TPS) ≥50% (PD‐L1‐high) non‐small cell lung cancer (NSCLC) without driver alterations. 2 However, within‐patient responses to pembrolizumab are heterogeneous, with frequent reports of spatiotemporally dissociated patterns of response and progression. 3 , 4 This leads to situations under the Response Evaluation Criteria in Solid Tumors (RECIST) Version 1.1, where patients with nontarget progression or new metastases can be classified as having progressive disease (PD) regardless of target lesion response. 4 , 5 As a result, patients who progress on pembrolizumab might be switched to salvage chemotherapy even while some or most of their lesions are still responding. 6 Determining the optimal salvage therapy after treatment failure in the PD‐L1‐high, driver alteration–free setting remains a challenge given the limited repertoire of approved therapeutics. This issue is also hard to address in clinical trials considering the competitive clinical trial landscape for immunotherapies.
Several retrospective studies have found that a subset of NSCLC patients who progress under immunotherapy can still benefit from the same treatment beyond PD. 7 , 8 , 9 , 10 Biomarkers of this subset have not been conclusively identified. We hypothesized that relevant knowledge might be found within the heterogeneity of individual lesion responses to pembrolizumab. This heterogeneity is well appreciated across anatomical sites: lesions in the liver, for example, respond poorly to pembrolizumab and correlate with inferior patient‐level responses in NSCLC. 3 Ethical considerations have precluded rigorous prospective studies of the relationship between treatment beyond progression and lesion‐level response heterogeneity, leaving biomarkers for patients likely to benefit from pembrolizumab beyond progression yet undiscovered. 11
To identify the patterns of PD most likely to respond to pembrolizumab beyond progression, we employed virtual clinical trials and in silico representations of individual patients—so‐called “digital twins.” 12 Digital twins are an important emerging tool used to derive clinical insights from patient data and test hypothetical clinical scenarios in a flexible, expedient manner. Within this study, we constructed digital twins from publicly available lesion growth dynamics of NSCLC treated with pembrolizumab or platinum doublet chemotherapy and enrolled them in virtual trials of hypothetical treatment regimens. Importantly, although we were able to directly obtain lesion‐level growth dynamics for NSCLCs treated with chemotherapy, we needed to develop a method to infer them from total tumor burden dynamics for NSCLCs treated with pembrolizumab due to a paucity of accessible data. These estimated growth dynamics were specific to the lesions' anatomical locations. We also adapted a published nontarget progression model for melanoma treated with immunotherapy to approximate nontarget progression rates under pembrolizumab. 5 Henceforth, we refer to PD caused by the appearance of new metastases or the growth of nontarget lesions collectively as “nontarget progression.”
Our final virtual cohort of digital twins comprised 1000 patients with realistic distributions of baseline tumor burden across anatomical sites. After demonstrating our model could robustly recapitulate monotherapy responses to chemotherapy and pembrolizumab, we examined patients who progressed on pembrolizumab and simulated treatment with either salvage chemotherapy or pembrolizumab beyond progression. This predicted a subset of patients who may benefit from pembrolizumab, supporting prospective clinical investigation as well as the further use of virtual clinical trials to generate testable hypotheses in oncology.
METHODS
Creating a virtual cohort with anatomically distributed baseline tumor burden
Lesion‐level response dynamics from Socinski et al. 13 were obtained from Project Data Sphere (registration number: LungNo_Celgene_2007_108, ClinicalTrials.gov identifier: NCT00540514). The study was approved by institutional review boards at each participating center and conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Conference on Harmonization. All patients provided written informed consent before study initiation. The control arm comprised 25,708 lesion diameter measurements from 524 patients with previously untreated advanced NSCLC. We cleaned these data such that each lesion's site corresponded to either one of seven anatomical sites reported by Osorio et al. 3 —adrenal, bone, liver, lung, lymph node, pleural, soft tissues—or “other.” Next, target lesion diameter measurements made before or within 1 day after treatment initiation were designated as baseline measurements. We constructed the final bootstrapped virtual cohort (1000 patients with 4109 lesions) from the cleaned data by sampling with replacement.
Obtaining lesion growth dynamics parameters under chemotherapy
Lesion‐level response dynamics from Socinski et al. 13 were segregated by anatomical site. We applied a nonlinear mixed‐effects population modeling approach to estimate lesion growth dynamics parameters f, d, and g for lesions in each anatomical site, 14 , 15
| (1) |
where N t represents tumor burden at time t, N 0 represents baseline tumor burden, f represents the fraction of treatment‐sensitive cells at baseline, d represents the death rate of treatment‐sensitive cells, and g represents the growth rate of treatment‐resistant cells. Default software recommendations in Monolix 2021 R1 (Lixoft) 16 were used to select error models and correlation models (Appendix S1). The best objective response (BOR) of each lesion was calculated by taking the nadir of non‐baseline measurements for each patient and stored alongside its corresponding f, d, and g parameters. The end result was a chemotherapy response matrix C , where each row contained values for f, d, g, and anatomical site s, and m is the number of lesions in Socinski et al. 13
| (2) |
Simulating chemotherapy
All treatment simulations were conducted in MATLAB R2020b (MathWorks). To simulate treatment, each lesion was assigned a random row vector of f, d, and g parameter values from the chemotherapy response matrix given an anatomical site s. These parameters were then used with Equation (1) to simulate lesion‐level response dynamics under 18 weeks of chemotherapy with radiographic assessments every 6 weeks. 13 Patient‐level tumor burden was calculated by summing the diameters of all lesions in a patient at any given time. Response and progression‐free survival (PFS) were calculated per RECIST Version 1.1 from tumor measurements at each simulated radiographic assessment. Of note, we classified PD as due to target, nontarget, or target and nontarget progression by adapting the total tumor burden‐based nontarget progression model in Kumar et al. 5 to NSCLC with data from Lee et al. 17 (Appendix S2). Objective response rate (ORR) was calculated by summing the proportion of patients who achieved partial response (PR) or complete response. Disease control rate (DCR) was calculated by summing the ORR with the proportion of patients who achieved stable disease (SD).
Obtaining lesion growth dynamics parameters under pembrolizumab
Patient‐level tumor burden dynamics of 88 driver alteration–free NSCLC patients treated with first‐line pembrolizumab monotherapy were reported in Nishino et al. 18 The majority (84%) of these patients had PD‐L1 TPS ≥50% before treatment initiation. Without access to the underlying data, we manually extracted the tumor burden dynamics of 77 patients with WebPlotDigitizer. 19 This provided sufficient data to use within Monolix 2021 R1 to perform nonlinear mixed‐effects population modeling and obtain parameter values for f, d, and g. We then calculated patient‐level BORs by taking the nadir of nonbaseline measurements. This was stored in an interim matrix M alongside its corresponding f, d, and g parameters. We also calculated a composite growth rate ϕ for each patient, 20
| (3) |
The interim matrix M of patient‐level pembrolizumab response parameters could be represented as follows, where n is the number of patients in Nishino et al., 18
| (4) |
Next, we extracted anatomical site‐specific best responses of 480 lesions treated with anti‐PD‐1 monotherapy in Osorio et al. 3 using WebPlotDigitizer. 19 From this matrix P′ , lesion‐level BORs were compared against the nearest patient‐level BORs in M and assigned their associated f, d, g, and ϕ parameters. Because the lesion BORs in Osorio et al. 3 were from a primarily pretreated population and M contained response parameters from first‐line pembrolizumab, 18 indexing within M was done after subtracting the difference in response rates r between the two studies. Letting q = number of lesions in Osorio et al. 3 and * denote all elements within a vector, we have
| (5) |
The end result was a scaled pembrolizumab response matrix P containing values for f, d, g, ϕ, and an anatomical site for each lesion in Osorio et al., 3
| (6) |
Simulating pembrolizumab monotherapy with intrapatient correlation in growth dynamics
We reasoned that systemic factors such as patient immune status might mediate diverse patterns of synchronous and dissociated response to pembrolizumab. The correlation in growth dynamics across lesions in each patient was accounted for by simulating with within‐patient response correlation anywhere from perfect (ρ = 1) to nonexistent (ρ = 0). A schematic representation of this process is provided in Figure S2. First, let Pt c denote a virtual patient c with u baseline lesions in certain anatomical sites,
To generate a parameter set that yielded within‐patient responses to pembrolizumab with a desired ρ, u vectors of were obtained and padded by replication until all were of identical length. Vectors were then horizontally concatenated into matrix X, where each column contained possible values of ϕ for a single lesion. Let z represent the unique anatomical sites harboring lesions in the patient, l(z) is the number of elements in z, represents the lowest common multiple of a vector, represents the number of elements in a vector, and represents k replications and vertical concatenations of vector ,
| (7) |
Values of ϕ were ranked within each column, denoted by f(X), for subsequent addition of Gaussian noise and reranking. Based on the observation in Osorio et al. 3 that ~20% of patients treated with pembrolizumab exhibit highly synchronous responses, the top 20% ϕ ranks were protected from this process; removal of these values yielded X' . A random amount of Gaussian‐distributed noise was added to X' , denoted by g(X). This was reranked to yield Y . Next, Y was reconcatenated to the values originally removed from X to yield Y′ ,
| (8) |
ρ was then calculated for Y′ . First, a correlation matrix Z was calculated, giving pairwise correlations between each column of Y′ . A Fisher Z‐transformation was performed on the matrix, after which the unique off‐diagonal pairwise correlations, represented by h(x), were averaged. An inverse Fisher transform was performed on this value to yield ρ,
| (9) |
In other words, the value of ρ represented the average pairwise correlation in growth dynamics between any two given lesions in a patient. If this average ρ deviated from the desired ρ by more than an acceptable level of tolerance, the magnitude of Gaussian noise was iteratively adjusted to increase or decrease ρ. Once the desired ρ was achieved, a random row vector v from the list was sampled such that a value of ϕ for each lesion was obtained,
| (10) |
Each lesion's ϕ value was used to assign it a corresponding set of f, d, and g parameter values from P . For tumors located in the “other” compartment, we sampled from the pooled parameters of all other sites,
| (11) |
We used f, d, and g parameters and Equation (1) to simulate 16 weeks of pembrolizumab treatment with radiographic assessment every 8 weeks. 18 Patient‐level tumor burden, response rates, and PFS were calculated as they were for chemotherapy. To determine the best value of ρ to use for further simulation and analysis, the simulated cohort's sum deviance in ORR and PD from Nishino et al. 18 was calculated. This process was repeated for all target values of ρ in [0, 1] in increments of 0.01. The ρ that minimized the simulated cohort's deviance in ORR and PD was carried forward.
Simulating salvage chemotherapy or pembrolizumab beyond progression
Virtual patients were treated with pembrolizumab monotherapy for up to 16 weeks or until PD, at which point progressors were cloned in silico and given either salvage chemotherapy, pembrolizumab beyond progression, or a hypothetical PFS‐optimized regimen. This subsequent round of therapy was maintained for 18 weeks, with radiographic assessment simulated every 6 weeks. Salvage chemotherapy was simulated by replacing the pembrolizumab‐specific f, d, and g parameters of a patient's lesions with a random set of chemotherapy‐specific f, d, and g parameters.
In virtual patients treated with PFS‐optimized therapy, tumor burden was simulated pro forma under 18 weeks of either salvage chemotherapy or pembrolizumab beyond progression in parallel. The treatment that resulted in longer PFS was retroactively selected as the subsequent therapy. This virtual trial was repeated 1000 times for analysis.
RESULTS
Virtual trials recapitulate heterogeneous clinical responses to treatment
Baseline patient data from a clinical dataset were adapted to inform the tumor growth model (Figure 1a). 3 , 5 , 13 , 17 , 18 Patients had an average of ~4 target lesions, most commonly in the lung and lymph nodes (Figure 1b). Representative spider plots of individual lesion response dynamics are provided in Figure 1c. These lesion‐level responses were used to create a virtual cohort of 1000 patients with realistic baseline tumor burden, anatomical lesion distributions, nontarget progression rates, and site‐specific response dynamics.
FIGURE 1.
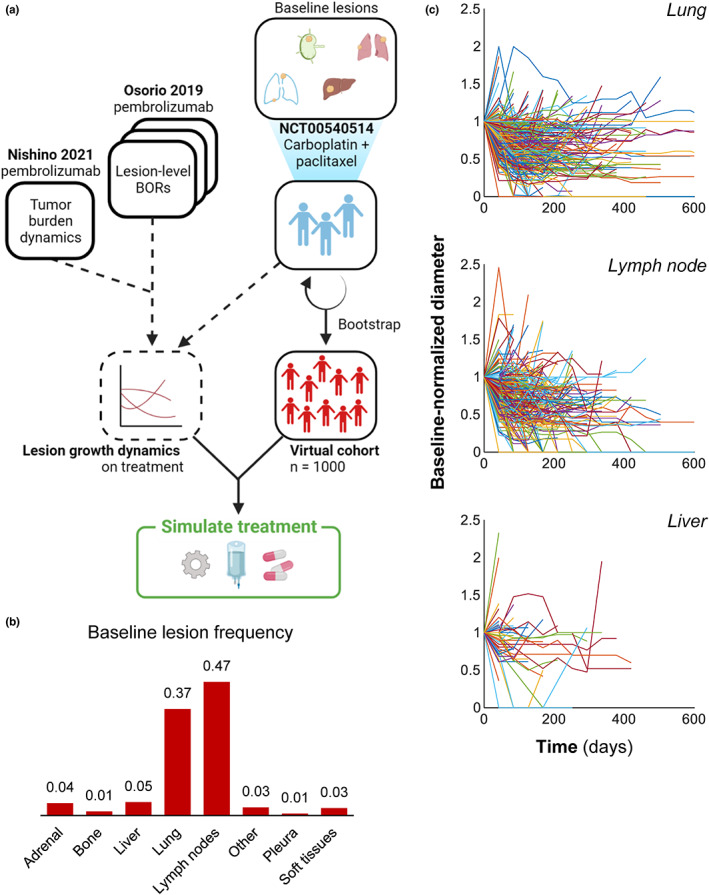
Digital twins were created from individual patient data. (a) Schematic of method for generating a virtual cohort, deriving lesion growth dynamics, and simulating treatment. Data in Socinski et al. 13 were obtained from Project Data Sphere and used to bootstrap a virtual cohort of 1000 patients. Anatomical site‐specific lesion size measurements of NSCLCs in Socinski et al. 13 were then used to fit a nonlinear mixed‐effects model of lesion‐level growth dynamics under platinum doublet therapy. Similarly, anatomical site‐specific lesion growth dynamics were obtained from Osorio et al. 3 and used in tandem with tumor burden dynamics from Nishino et al. 18 to fit a nonlinear mixed‐effects model for lesion growth dynamics under pembrolizumab (see Methods). (b) Baseline lesion distribution observed in the control arm of Socinski et al. 13 (c) Lesion‐level response dynamics of 524 patients treated with first‐line carboplatin and paclitaxel 13 obtained from Project Data Sphere. For clarity, only one lesion per patient is shown. Additional anatomical sites, individual lesion‐level model fits, and parameter estimates are provided in the Appendix S1. BOR, best objective response
Virtual patients were treated for 16–18 weeks with periodic radiographic assessment of tumor diameters (Figure 2a). We found that first‐line chemotherapy and pembrolizumab yielded comparable tumor control and PFS within this time frame, with 57% and 56.2% remaining progression‐free at the conclusion of treatment, respectively. This is consistent with clinical observations that the PFS advantage of pembrolizumab emerges over time 21 (Figure 2b,c). Simulated platinum doublet chemotherapy was able to recapitulate the heterogeneity and response rate of patients in the control arm of Socinski et al. 13 who received first‐line carboplatin and paclitaxel (29.7% vs. 31.4%; Figure 2d). Similarly, monotherapy simulations with moderate within‐patient correlation (ρ = 0.17; see Methods) were able to recapitulate the response rate of patients who received first‐line pembrolizumab (41.6% vs. 44.3%; Figure 2e). Importantly, these data show the ability of the model to recapitulate the significantly higher response rate of first‐line pembrolizumab compared with chemotherapy in this disease setting.
FIGURE 2.
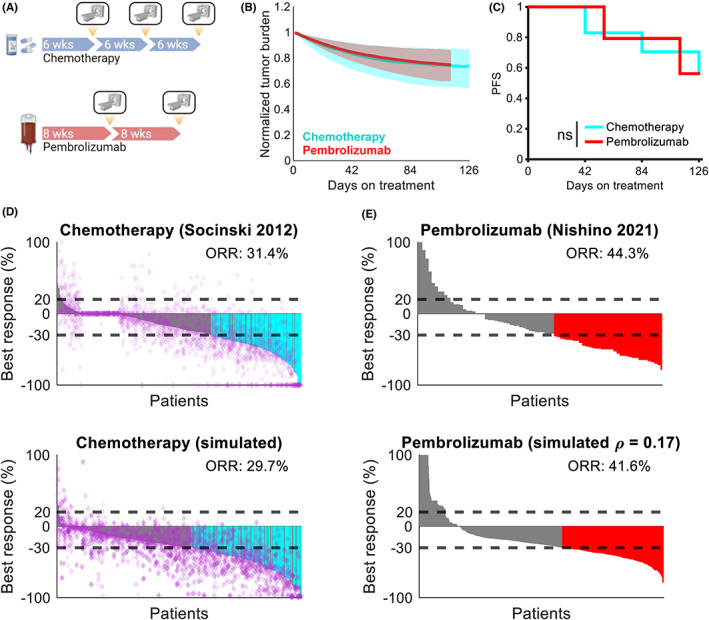
Digital twins recapitulate observed clinical responses to therapy. (a) Schematic of virtual clinical trial comparing pembrolizumab versus chemotherapy. Treatment with chemotherapy was simulated for 18 weeks with radiographic assessment for response every 6 weeks. Treatment with pembrolizumab was simulated for 16 weeks with radiographic assessment for response every 8 weeks. (b) Median tumor burden (Equation 1) under chemotherapy (cyan) and pembrolizumab (red). Shaded regions represent the interquartile range. (c) PFS under chemotherapy (cyan) and pembrolizumab (red). (d) Monotherapy responses to platinum doublet chemotherapy reported in Socinski et al. 13 (top) and simulated patients (bottom). Horizontal dashed lines indicate thresholds for PD and PR, purple diamonds represent individual lesions, cyan bars indicate patients with responses and no nontarget progression, and gray bars indicate patients with responses and simultaneous nontarget progression. Treatment was simulated for 18 weeks with radiographic assessment for response every 6 weeks. (e) Monotherapy responses to pembrolizumab in treatment‐naïve patients digitized from Nishino et al. 18 (top) and simulated patients (bottom). Horizontal dashed lines indicate thresholds for PD and PR, red bars indicate patients with PR or better based on target lesion dynamics only, and ρ represents the average correlation of responses among lesions within each patient (see Methods). Neither individual lesion response nor nontarget progression were assessed due to lack of availability in the published dataset. Ns, not significant (log‐rank test); ORR, objective response rate; PD, progressive disease; PFS, progression‐free survival; PR, partial response
Patterns of response vary with therapy and lesion location
Patients treated with virtual monotherapy were assessed for within‐patient heterogeneity in lesion responses across anatomical sites. At the patient level, baseline lesion distribution across anatomical sites did not dramatically affect lesion responses (Figure S3A,B). At the lesion level, lesions in the liver and soft tissues at high risk of nonresponse to pembrolizumab responded better to chemotherapy, with rates of increase in lesion diameters ≥20% of 39% versus 8% and 30% versus 0%, respectively (Figure 3a).
FIGURE 3.
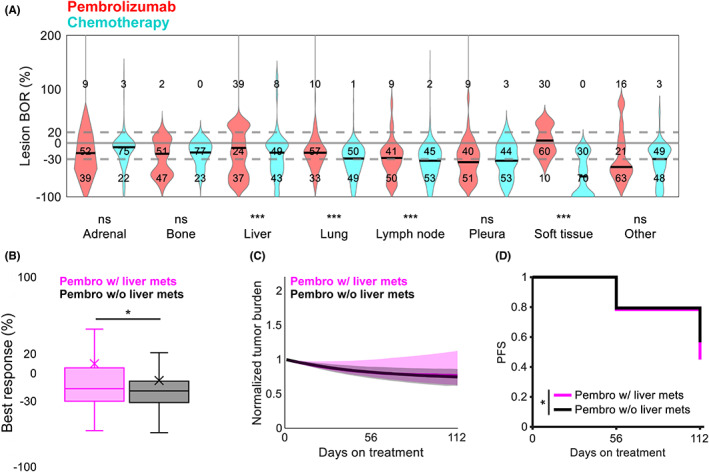
Responses to virtual monotherapy vary across anatomical sites. (a) Lesion‐level BORs under chemotherapy (cyan) and pembrolizumab (red) by anatomical location. Numbers indicate the proportion (%) of lesions with diameter changes under treatment that were ≥20%, −30% to 20%, and ≤−30%. Gray lines indicate cutoffs for 20% and −30%; solid black lines indicate medians. ***p < 0.001 (unpaired Student t test). (b) Best patient‐level reduction in tumor burden under pembrolizumab in patients with (pink) and without (black) baseline liver metastases. X, mean; *p < 0.05 (unpaired Student t test). (c) Median tumor burden under pembrolizumab in patients with (pink) and without (black) baseline liver metastases. Shaded regions represent the interquartile range at each timepoint. (d) PFS under pembrolizumab in patients with (pink) and without (black) baseline liver metastases. *p < 0.05 (log‐rank test). BOR, best objective response; Ns, not significant; PFS, progression‐free survival
Interestingly, previous studies have reported that liver metastases reduce the efficacy of anti‐PD‐1 therapy in NSCLC. 3 , 22 In our virtual cohort, patients with liver metastases before treatment initiation experienced shallower responses (mean [standard error of the mean, SEM]: 4.4% [7.9] vs. −14.1% [3.1]) and shorter PFS (week 16 surviving: 46% vs. 57%) under pembrolizumab than those without (Figure 3b–d). Conversely, liver metastases conferred no difference in PFS under chemotherapy, although they did slightly affect response depth (mean [SEM]: −24.9% [2.1] vs. −32% [0.8]) (Figure S3C–E).
Unconditionally applied salvage chemotherapy after progression on pembrolizumab yields suboptimal PFS
With successful recapitulation of responses to monotherapy, we simulated a pembrolizumab salvage therapy trial to identify which patients, if any, could benefit from pembrolizumab beyond progression (Figure 4a). Virtual patients were treated with pembrolizumab for up to 16 weeks or until PD, at which point they were computationally cloned. Each digital twin then received either pembrolizumab beyond progression or salvage chemotherapy for 18 additional weeks. Progressors were stratified by their initial cause of PD: target progression only, nontarget progression only, or simultaneous target and nontarget progression at the time of radiographic assessment. PFS under subsequent therapy (PFS2) was then assessed in each group.
FIGURE 4.
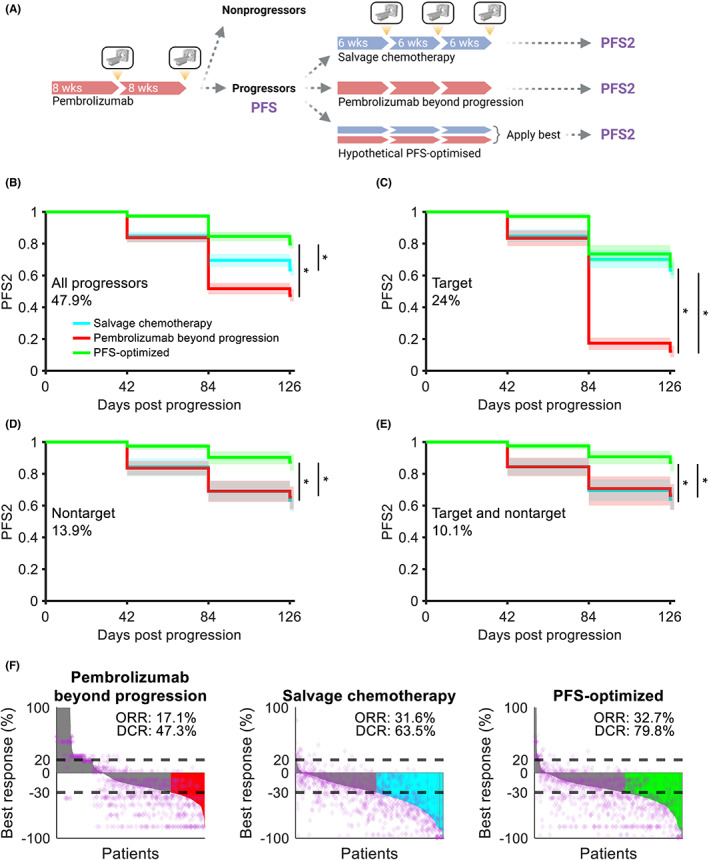
Pembrolizumab beyond progression can prolong PFS in nontarget progressors. (a) Schematic of a virtual clinical trial comparing pembrolizumab beyond progression versus salvage chemotherapy or a hypothetical PFS‐optimized regimen. Treatment with pembrolizumab was simulated for up to 16 weeks with radiographic assessment for response every 8 weeks. Progressors were cloned in silico and assigned to receive treatment for 18 weeks with radiographic assessment for response every 6 weeks. (b) PFS2 in patients receiving pembrolizumab beyond progression, salvage chemotherapy, or a hypothetical PFS‐optimized treatment (see Methods). Data are pooled across 100 trial replicates, with solid lines representing medians and shaded regions representing the 5th and 95th percentiles. (c) Same as (b) for patients with target progression without nontarget progression. (d) Same as (b) for patients with nontarget progression without target progression. (e) Same as (b) for patients with simultaneous target and nontarget progression at radiographic assessment. (f) Best response, ORR, and DCRs in patients after treatment. See the Methods for calculation. Data are pooled across 100 replicates. Horizontal dashed lines indicate thresholds for progressive disease and partial response, purple diamonds represent individual lesions from a representative replicate, and colored bars indicate patients with responses and no nontarget progression. Gray bars indicate patients with no response as well as patients with target lesion responses but simultaneous nontarget progression. *p < 0.0005 (log‐rank test). DCR, disease control rate; ORR, objective response rate; PFS, progression‐free survival; PFS2, progression‐free survival under subsequent therapy
We hypothesized that carefully selecting patients to maintain on pembrolizumab beyond progression would produce better cohort‐level outcomes than unconditionally selecting salvage chemotherapy. To investigate this, we also simulated a hypothetical “PFS‐optimized” regimen: whichever therapy yielded the longest PFS2 to the digital twins was provided to the virtual patient at initial progression. It is worth noting that this is currently impractical to implement in clinical practice given the lack of biomarkers guiding therapy selection in the pembrolizumab‐refractory, PD‐L1‐high, driver alteration–free setting. Nevertheless, if salvage chemotherapy were optimal for cohort‐level outcomes, we expected there to be no difference in PFS between the PFS‐optimized regimen and the salvage chemotherapy regimen. Instead, we found that salvage chemotherapy significantly underperformed the PFS‐optimized regimen in patients with nontarget progression, but not in patients with target‐only progression (Figure 4b–e). Interestingly, although salvage chemotherapy underperformed the PFS‐optimized regimen in DCR—BOR of SD or better (63.5% vs. 79.8%)—it produced a comparable response rate (31.6% vs. 32.7%). This indicated that the primary benefit of the PFS‐optimized regimen was through maintenance of SD in a greater proportion of patients as opposed to the generation of new, latent responses.
A subset of nontarget progressors may benefit from pembrolizumab beyond progression
Given the room to improve cohort‐level PFS2 above salvage chemotherapy, we next attempted to identify the progressors who stood to benefit most from pembrolizumab beyond progression. When considering all progressors or target‐only progressors, pembrolizumab beyond progression was inferior to salvage chemotherapy in PFS2 and control of tumor burden (Figures 4b,c and 5a,b). However, pembrolizumab beyond progression and salvage chemotherapy produced indistinguishable PFS2 and control of tumor burden in patients with nontarget progression (Figures 4d,e and 5c,d). As the PFS‐optimized regimen was significantly better than both pembrolizumab beyond progression and salvage chemotherapy in this group, we inferred the existence of a subset of nontarget progressors who indeed benefited more from pembrolizumab beyond progression than salvage chemotherapy. 8 In these patients, however, it is possible that pembrolizumab beyond progression maintains SD in more patients as opposed to eliciting deeper responses. This is reflected by the discrepancy in the response and DCRs of salvage chemotherapy and PFS‐optimized therapy (Figure 4f).
FIGURE 5.
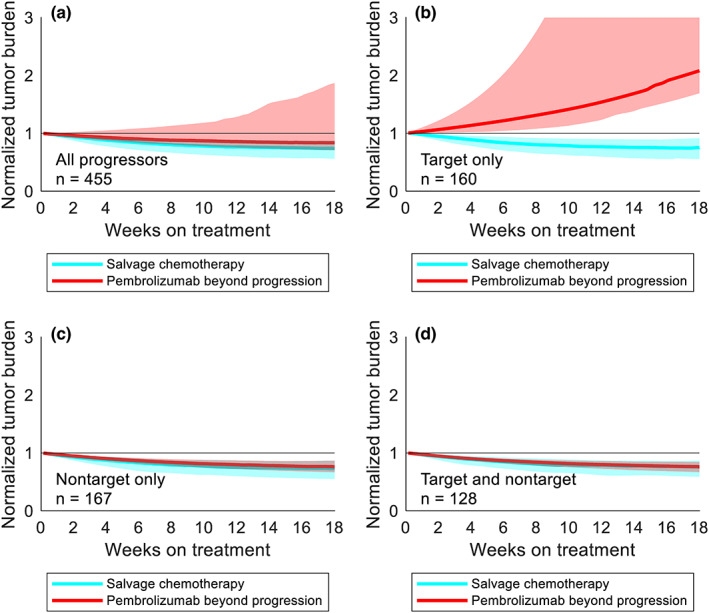
Treatment beyond progression may control tumor burden after nontarget progression on pembrolizumab. (a) Median tumor burden in patients from a representative trial replicate receiving pembrolizumab beyond progression (red) or salvage chemotherapy (cyan). Shaded regions represent the interquartile range at each timepoint. (b) Same as (a) for patients with target progression without nontarget progression. (c) Same as (a) for patients with nontarget progression without target progression. (d) Same as (a) for patients with simultaneous target and nontarget progression
DISCUSSION
Our study leveraged virtual trial simulation to explore the feasibility of treatment beyond progression with pembrolizumab in patients with PD‐L1 TPS ≥50%. 3 , 23 Several assumptions made throughout this study are summarized here:
Driver alteration status and PD‐L1 expression do not alter the anatomical distribution of tumor burden
Effects of variability in chemotherapy and pembrolizumab dose intensity are negligible across the population
Exponential, capacity‐less tumor growth
Differences in response rates to first‐line and second‐line treatments with pembrolizumab are reflected in different BORs
Baseline patient covariates in Socinski et al., 13 such as sex, weight, and immune status, are representative of the population who would receive pembrolizumab
Responses under carboplatin and paclitaxel are generally representative of platinum doublet chemotherapies
Patients whose target lesions progressed under pembrolizumab were unlikely to derive benefit from pembrolizumab beyond progression. Conversely, those who had nontarget progression were most likely to benefit. The population‐level underperformance of salvage chemotherapy compared with a hypothetical PFS‐optimized regimen was only noted in nontarget progressors. The rebaselining of patients at PD for the purpose of RECIST evaluation introduces the possibility that target lesions in patients whose initial PD was due to nontarget progression may, in fact, still be shrinking. Taken in the context of first‐line pembrolizumab's high response rate, this suggests that patients who are still responding favorably on pembrolizumab but progress from a new metastasis or nontarget lesion growth might be switched to less‐efficacious chemotherapies. This would then lead to an inferior population‐level PFS for salvage chemotherapy PFS when compared with a hypothetical PFS‐optimized regimen. Naturally, a qualification would be that the new metastasis did not appear in a terminal location such as the brain. Whether patient‐specific PFS‐ or survival‐optimized therapeutic selection is practically attainable is an active area of investigation.
Our simulations also yielded a modest increase in response rate to salvage chemotherapy after progression on pembrolizumab (31.6% vs. 29.7%), in agreement with previous reports that first‐line pembrolizumab enhances response rates to salvage chemotherapy compared with first‐line chemotherapy. 24 , 25 This may have been due to pembrolizumab and chemotherapy exerting differential efficacy in different anatomical sites, such that pembrolizumab “pretreatment” shrunk tumors in anatomical sites that otherwise would have responded poorly to chemotherapy—that is, one therapy covering the other's bases with respect to biodistribution or site‐specific efficacy. The differential biodistribution of chemotherapy, anti‐PD‐1 antibodies, and invigorated effector T cells remains underexamined in the setting of combination and sequential therapy.
Notably, although pembrolizumab plus pemetrexed and platinum chemotherapy is currently the recommended first‐line therapy for previously untreated advanced NSCLC with PD‐L1 TPS <50%, 2 , 26 we did not have access to lesion growth dynamics data for patients treated with this regimen. This is a clinically important population that we therefore did not investigate. We advocate for trial investigators to make lesion‐level data available whenever feasible, as these data are instrumental to robust studies of tumor growth dynamics under diverse treatment regimens.
One potential limitation of our study was the absence of simulated growth dynamics for new lesions, for which diameters were not reported in our source dataset. It is possible that growth of these nontarget lesions in the sequential therapy trial might have caused some patients receiving subsequent therapy to progress more quickly. We considered this unlikely for two related reasons: first, these patients by definition have tumor burden of at least 20% greater than baseline, causing a second increase of 20% to require substantially greater additional tumor mass; second, new metastases are likely to be smaller than extant lesions. Metastatic lesion measurements might have helped us assess patients via the immune RECIST criteria and more accurately determine progression on pembrolizumab at radiographic assessments 4–8 weeks after initial unconfirmed PD. 27
As the field moves toward increasingly mechanistic, biology‐driven models, it is important to note that the mechanism of action of PD‐1 axis inhibitors is still emerging. 28 Although the originally implicated effector population in humans was exhausted tumor‐infiltrated lymphocytes, mounting evidence suggests a substantial role for peripherally expanded lymphocytes. 29 , 30 Furthermore, important immune covariates such as tumor infiltration, baseline lymphocyte and leukocyte counts, and effector phenotype distributions are highly variable, making many systems model parameters challenging to justify when simulating on an individual patient basis. 31 Our study therefore provides a complementary “top‐down” approach to predict patient response to either continued anti‐PD‐1 therapy or salvage chemotherapy upon partial progression. Despite our model being biologically coarse grained, it is able to integrate multiple sources of variability to make statistical predictions of patient response. However, one should interpret this statistical evidence with the aforementioned biological caveats in mind.
The intent of our work is not to identify specific biomarkers from biology (e.g., PD‐L1 expression or extent of tumor lymphocyte infiltration) but, rather, to evaluate macroscopic patterns of disease, such as metastatic sites and tumor burden, which are associated with therapeutic benefit by continued anti‐PD‐1 therapy beyond progression. Treatment beyond (partial) progression has become an interesting topic for immunotherapy, and many retrospective analyses of trials have maintained the field's continued interest. Concretely, we highlight that those who progress per RECIST from nontarget sources might still have benefit to gain from treatment beyond progression.
Our findings broadly aligned with those of Metro et al., who found pembrolizumab beyond progression to be beneficial for NSCLC patients with progression in ≤2 organ sites. 8 Overall, this work supports (1) the continued development of virtual clinical trials as a tool for generating clinical hypotheses and (2) the identification of better salvage therapies for patients with PD‐L1‐high, driver alteration–free NSCLC. Prospective studies in the subset of patients who experience nontarget progression may be warranted.
AUTHOR CONTRIBUTIONS
T.Q. and Y.C. wrote the manuscript. T.Q. and Y.C. designed the research. T.Q. performed the research. T.Q. analyzed the data. T.Q. contributed new reagents/analytical tools.
FUNDING INFORMATION
This work was supported by the National Institute of General Medical Sciences R35GM119661.
CONFLICT OF INTEREST
T.Q. is a contractor for Hatteras Venture Partners. Y.C. is a consultant for Janssen Research & Development. Initial study ideation was conducted with input from employees of Merck; however, all elements of study design, execution, analysis, and interpretation were conducted independently with no external input.
Supporting information
Appendix S1
Appendix S2
Figures S1‐S3 32
ACKNOWLEDGMENTS
We thank Brian Topp, Kapil Mayawala, and Dinesh de Alwis for their helpful comments during study ideation. Figures were prepared in BioRender.
Qi T, Cao Y. Virtual clinical trials: A tool for predicting patients who may benefit from treatment beyond progression with pembrolizumab in non‐small cell lung cancer. CPT Pharmacometrics Syst Pharmacol. 2023;12:236‐249. doi: 10.1002/psp4.12896
REFERENCES
- 1. Tran G, Harker M, Chiswell K, et al. Feasibility of cancer clinical trial enrollment goals based on cancer incidence. JCO Clin Cancer Inform. 2020;4:35‐49. doi: 10.1200/CCI.19.00088 [DOI] [PubMed] [Google Scholar]
- 2. Hanna NH, Schneider BJ, Temin S, et al. Therapy for stage IV non‐small‐cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol off J Am Soc Clin Oncol. 2020;38(14):1608‐1632. doi: 10.1200/JCO.19.03022 [DOI] [PubMed] [Google Scholar]
- 3. Osorio JC, Arbour KC, Le DT, et al. Lesion‐level response dynamics to programmed cell death protein (PD‐1) blockade. J Clin Oncol. 2019;37(36):3546‐3555. doi: 10.1200/JCO.19.00709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Topp BG, Thiagarajan K, De Alwis DP, Snyder A, Hellmann MD. Lesion‐level heterogeneity of radiologic progression in patients treated with pembrolizumab. Ann Oncol. 2021;32(12):1618‐1625. doi: 10.1016/j.annonc.2021.09.006 [DOI] [PubMed] [Google Scholar]
- 5. Kumar R, Thiagarajan K, Jagannathan L, et al. Beyond the single average tumor: understanding IO combinations using a clinical QSP model that incorporates heterogeneity in patient response. CPT Pharmacomet Syst Pharmacol. 2021;10(7):684‐695. doi: 10.1002/psp4.12637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ettinger DS, Wood DE, Aisner DL, et al. Non‐small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(4):504‐535. doi: 10.6004/jnccn.2017.0050 [DOI] [PubMed] [Google Scholar]
- 7. Reinhorn D, Jacobi O, Icht O, et al. Treatment beyond progression with immune checkpoint inhibitors in non‐small‐cell lung cancer. Immunotherapy. 2020;12(4):235‐243. doi: 10.2217/imt-2019-0131 [DOI] [PubMed] [Google Scholar]
- 8. Metro G, Addeo A, Signorelli D, et al. Outcomes from salvage chemotherapy or pembrolizumab beyond progression with or without local ablative therapies for advanced non‐small cell lung cancers with PD‐L1 ≥50% who progress on first‐line immunotherapy: real‐world data from a European cohort. J Thorac Dis. 2019;11(12):4972‐4981. doi: 10.21037/jtd.2019.12.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stinchcombe TE, Miksad RA, Gossai A, Griffith SD, Torres AZ. Real‐world outcomes for advanced non‐small cell lung cancer patients treated with a PD‐L1 inhibitor beyond progression. Clin Lung Cancer. 2020;21(5):389‐394.e3. doi: 10.1016/j.cllc.2020.04.008 [DOI] [PubMed] [Google Scholar]
- 10. Ge X, Zhang Z, Zhang S, et al. Immunotherapy beyond progression in patients with advanced non‐small cell lung cancer. Transl Lung Cancer Res. 2020;9(6):2391‐2400. doi: 10.21037/tlcr-20-1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spagnolo F, Boutros A, Cecchi F, Croce E, Tanda ET, Queirolo P. Treatment beyond progression with anti‐PD‐1/PD‐L1 based regimens in advanced solid tumors: a systematic review. BMC Cancer. 2021;21(1):425. doi: 10.1186/s12885-021-08165-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hernandez‐Boussard T, Macklin P, Greenspan EJ, et al. Digital twins for predictive oncology will be a paradigm shift for precision cancer care. Nat Med. 2021;27(12):2065‐2066. doi: 10.1038/s41591-021-01558-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab‐paclitaxel in combination with carboplatin versus solvent‐based paclitaxel plus carboplatin as first‐line therapy in patients with advanced non‐small‐cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30(17):2055‐2062. doi: 10.1200/JCO.2011.39.5848 [DOI] [PubMed] [Google Scholar]
- 14. Chatterjee MS, Elassaiss‐Schaap J, Lindauer A, et al. Population pharmacokinetic/Pharmacodynamic modeling of tumor size dynamics in Pembrolizumab‐treated advanced melanoma. CPT Pharmacomet Syst Pharmacol. 2017;6(1):29‐39. doi: 10.1002/psp4.12140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model‐based drug development. CPT Pharmacomet Syst Pharmacol. 2012;1:e6. doi: 10.1038/psp.2012.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Antony F. Monolix Version 2021R1. Lixoft SAS; 2019. [Google Scholar]
- 17. Lee VHF, Chan WWL, Lee EYP, et al. Prognostic significance of standardized uptake value of lymph nodes on survival for stage III non‐small cell lung cancer treated with definitive concurrent Chemoradiotherapy. Am J Clin Oncol. 2016;39(4):355‐362. doi: 10.1097/COC.0000000000000070 [DOI] [PubMed] [Google Scholar]
- 18. Nishino M, Hong F, Ricciuti B, Hatabu H, Awad MM. Tumor response dynamics during first‐line Pembrolizumab therapy in patients with advanced non‐small‐cell lung cancer. JCO Precis Oncol. 2021;5:501‐509. doi: 10.1200/PO.20.00478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rohatgi A. WebPlotDigitizer. 2022. Available from: https://automeris.io/WebPlotDigitizer
- 20. Stein WD, Figg WD, Dahut W, et al. Tumor growth rates derived from data for patients in a clinical trial correlate strongly with patient survival: a novel strategy for evaluation of clinical trial data. Oncologist. 2008;13(10):1046‐1054. doi: 10.1634/theoncologist.2008-0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reck M, Rodríguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375(19):1823‐1833. doi: 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 22. Tumeh PC, Hellmann MD, Hamid O, et al. Liver metastasis and treatment outcome with anti‐PD‐1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017;5(5):417‐424. doi: 10.1158/2326-6066.CIR-16-0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reuben A, Cooper ZA, Amaria RN, et al. Intra‐patient intra‐tumoral immune heterogeneity is evident at progression on targeted therapy and immunotherapy for melanoma. J Immunother Cancer. 2014;2(Suppl 3):P200. doi: 10.1186/2051-1426-2-S3-P200 [DOI] [Google Scholar]
- 24. Park SE, Lee SH, Ahn JS, Ahn M‐J, Park K, Sun J‐M. Increased response rates to salvage chemotherapy administered after PD‐1/PD‐L1 inhibitors in patients with non‐small cell lung cancer. J Thorac Oncol. 2018;13(1):106‐111. doi: 10.1016/j.jtho.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 25. Rothschild SI, Leger P, Castellanos EL, Pillai RN, York SJ, Horn L. Response to salvage chemotherapy following exposure to PD‐1/PD‐L1 inhibitors in patients with NSCLC. Ann Oncol. 2017;28(Suppl 2):II33. doi: 10.1093/annonc/mdx091.011 [DOI] [Google Scholar]
- 26. Gadgeel S, Rodríguez‐Abreu D, Speranza G, et al. Updated analysis from KEYNOTE‐189: pembrolizumab or placebo plus Pemetrexed and platinum for previously untreated metastatic nonsquamous non‐small‐cell lung cancer. J Clin Oncol. 2020;38(14):1505‐1517. doi: 10.1200/JCO.19.03136 [DOI] [PubMed] [Google Scholar]
- 27. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143‐e152. doi: 10.1016/S1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Borst J, Busselaar J, Bosma DMT, Ossendorp F. Mechanism of action of PD‐1 receptor/ligand targeted cancer immunotherapy. Eur J Immunol. 2021;51(8):1911‐1920. doi: 10.1002/eji.202048994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang J, Ji Z, Caushi JX, et al. Compartmental analysis of T‐cell clonal dynamics as a function of pathologic response to Neoadjuvant PD‐1 blockade in Resectable non‐small cell lung cancer. Clin Cancer Res. 2020;26(6):1327‐1337. doi: 10.1158/1078-0432.CCR-19-2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yost KE, Satpathy AT, Wells DK, et al. Clonal replacement of tumor‐specific T cells following PD‐1 blockade. Nat Med. 2019;25(8):1251‐1259. doi: 10.1038/s41591-019-0522-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Diem S, Schmid S, Krapf M, et al. Neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) as prognostic markers in patients with non‐small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176‐181. doi: 10.1016/j.lungcan.2017.07.024 [DOI] [PubMed] [Google Scholar]
- 32. Palmer AC, Sorger PK. Combination cancer therapy can confer benefit via patient‐to‐patient variability without drug additivity or synergy. Cell. 2017;171(7):1678‐1691.e13. doi: 10.1016/j.cell.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Figures S1‐S3 32


