Abstract
Purpose
The current study aims to evaluate bite force, perception of orofacial pain, and treatment satisfaction of patients with bruxism using two protocols of botulinum toxin A (BTX-A) injections.
Material and Methods
Two groups of patients seeking bruxism treatment and presenting bilateral orofacial pain of muscle origin were randomly created according to BTX-A injection sites: masseter muscle only, bilaterally (3 points in each muscle, 10 U per point), and masseter and temporal muscles (3 points in each masseter muscle and 2 points in each temporal muscle, 10 U per point). The patients were evaluated preoperatively and longitudinally at 15, 90, 120, and 180 days by the use of visual analog scales for pain and treatment satisfaction and a gnathodynamometer for bite force recording.
Results
The final sample included 10 participants in each group. Both groups presented mitigation of pain at 15, 90, 120, and 180 days in comparison with baseline; however, reduction in the posterior bite force was noted only at 15, 90, and 120 days. Quite high treatment satisfaction was reported from both groups at 15, 90, 120, and 180 days. No differences were observed between the groups in all evaluations and study periods.
Conclusion
In general, considering pain relief, reduction in bite force, and treatment satisfaction, both protocols of BTX-A seem to be somewhat equally effective in the short-term management (up to 120 days) of bruxism.
Keywords: Bruxism, Botulinum toxins, Bite force, Facial pain, Patient satisfaction
Abbreviations: BTX-A, botulinum toxin type A; VAS scale, visual analog scale
1. Introduction
Bruxism is a functional movement disorder of the masticatory muscles characterized by clenching or grinding teeth involuntarily and unconsciously. It occurs either during sleeping (“sleep bruxism”) or daytime (“awake bruxism”) and may lead to headaches, temporomandibular disorders, sleep disorders, depression, muscle pain and fatigue, and wear or fractures of teeth and restorations (Lobbezoo et al., 2018, Manfredini et al., 2013, Sateia, 2014). The etiology of bruxism remains unclear but is thought to be multifactorial and dependent upon dental, physiological, psychological, and neurological factors, which make the diagnosis and treatment very challenging (Manfredini et al., 2013, Sposito and Teixeira, 2014). Its prevalence is also uncertain; however, most people probably will experience bruxism episodes during life (Maluly et al., 2013, Manfredini et al., 2013).
Many therapeutic options for treating or controlling bruxism have been proposed (e.g., medications, physiotherapy, dentistry strategies) but the most common ones include the use of occlusal splints along with systemic drugs (myorelaxant, anti-inflammatory, antidepressant, or anxiolytic agents) (Yurttutan et al., 2019). On the other hand, as these conventional therapies may not be totally effective, there is an urgent need for research on more alternatives.
Botulinum toxin A (BTX-A), one of the seven serotypes of a toxin produced by the anaerobic gram-positive spore-forming rod Clostridium botulinum, is capable of inhibiting reversely acetylcholine release within the synaptic terminal of the neuromuscular junction (Freund and Schwartz, 1998, Suber et al., 2014). Given this therapeutic propriety, it has been suggested recently for the management of bruxism but scientific evidence is still scarce (Ågren et al., 2020, Cheng et al., 2022, Guarda-Nardini et al., 2008, Lobbezoo et al., 2018, Manfredini et al., 2017, Muñoz Lora et al., 2019, Pardo et al., 2022, Yurttutan et al., 2019).
Another matter of debate is the muscle sites of BTX-A applications for the management of bruxism. BTX-A injections into the masseter muscles only (Al-Wayli, 2017, Lee et al., 2010) or concomitantly with the temporal muscles (Alonso Navarro et al., 2011, Guarda-Nardini et al., 2008, Pardo et al., 2022) are the therapeutic protocols generally employed by clinicians for bruxism and related-orofacial pain; however, although both muscles contract synergistically to produce masticatory force, some authors question the need for approaching the temporal muscles (Al-Wayli, 2017, Shim et al., 2014).
So, considering that available information on the use of BTX-A for bruxism is quite heterogeneous due to both study design and methodology among the available papers (Ågren et al., 2020, Lee et al., 2010, Patel et al., 2019, Shim et al., 2014), the present study aims to evaluate perception pain of patients with bruxism using two protocols of BTX-A injections, as well as to assess treatment satisfaction and reduction in bite force as secondaries outcomes. The null hypothesis was that both protocols of BTX-A have similar clinical performance.
2. Material and methods
2.1. Study design, ethical issues, and recruitment
This two-arm parallel (1:1) single-blinded (patient) randomized clinical trial was conducted by experienced researchers; however, only one performed all the clinical procedures and analyses. The Research Ethics Committee of Ibirapuera University (#73845317.2.0000.0075) approved the study and then it was registered in the Brazilian Clinical Trials Register – ReBEC (U1111-1235-5386 [https://www.ensaiosclinicos.gov.br]). All participants read and signed the informed consent form before the beginning of the clinical procedures. The CONSORT Statement (Moher et al., 2010) was followed for reporting.
Patients seeking to treat bruxism were attendants in the Dental Clinic of Ibirapuera University (São Paulo, Brazil) between February to November 2019. Twenty participants, aged 18 years or older, with the chief complaint of either sleep or awake bruxism during at least 6 months and presenting bilateral orofacial pain of muscle origin (myofascial pain at rest, at clenching, or induced by palpation of the masseter and/or temporal muscles), were considered eligible for inclusion in this study. The following exclusion criteria were also considered: temporomandibular joint disorders (arthralgia, disc displacement disorders, degenerative joint disease, subluxation); known allergy to BTX-A or albumin; the presence of amyotrophic lateral sclerosis, motor neuropathy, myasthenia gravis, or Lambert-Eaton syndrome; pregnant or breastfeeding women; use of any medication that could alter neuromuscular function; use of any type of occlusal splint; individuals without most teeth (especially the first molar teeth) or wearing any type of total or partial removable dental prosthesis or under orthodontic treatment.
2.2. Study groups and randomization procedures
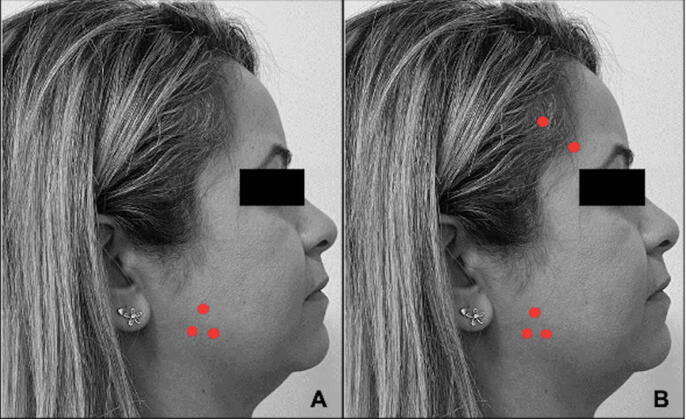
Patients were randomly assigned to receive BTX-A injections into different muscle sites: Group M – masseter muscle only, bilaterally (3 points in each muscle); and Group MT – masseter and temporal muscles (3 points in each masseter and 2 points in each temporal muscle) (Fig. 1). For that, randomization was performed by another researcher using a website (https://www.sealedenvelope.com), and the group code for each participant was allocated into a numbered, opaque, sealed envelope that was opened just before the clinical procedures.
Fig. 1.
Facial points for BTX-A injections. (A) Group M; (B) Group MT.
2.3. Interventions
The BTX-A for injection was obtained from 100 UI vials of Botulift™ (Medytox Inc., Cheongwon, South Korea) reconstituted with 1 mL of normal saline solution. The patients underwent the clinical procedures seated comfortably on a dental chair. Before the injections, the face was cleansed with alcohol-impregnated pads and a white pencil was used to mark the puncture dots. A 3-mL syringe attached to a 22-gauge needle was then used for injecting 1 mL into every facial point previously determined.
2.4. Sample size
For sample size calculation, a mean reduction of pain of 4.6 (±0.66) in patients with bruxism after 1–3 months of BTX-A application into the masseter muscles only (Al-Wayli, 2017) was considered, as well as a mean reduction of pain of 1.2 (±3.15) when BTX-A was injected into the masseter and temporal muscles concomitantly (Jadhao et al., 2017). Thus, considering an effect size of 1.49, adopting a significance level of 0.05 and a power of 0.80, and using a two-tailed test, the final rounded number was 18 patients. After taking into account 10% of a possible drop-out, 20 patients were required.
2.5. Primary outcome
2.5.1. Perception of pain
A visual analog scale (VAS) composed of a horizontal line presenting values from 0 cm (no pain) to 10 cm (the worst pain) was given to the participants before BTX-A injections and they were asked to draw a vertical line corresponding to their perception of bruxism-related orofacial pain. The value obtained in centimeters was transformed into an absolute value (0–10) for data presentation. After 15, 90, 120, and 180 days, the same scale was applied and data was collected.
2.6. Secondaries outcomes
2.6.1. Treatment satisfaction
A VAS composed of a horizontal line presenting values from 0 (totally dissatisfied) to 10 (totally satisfied) was given to the participants before BTX-A injections and they were asked to mark a point corresponding to their satisfaction with the treatment. The value obtained in centimeters was transformed into an absolute value (0–10) for data presentation. After 15, 90, 120, and 180 days, the same scale was applied and data was collected.
2.6.2. Bite force
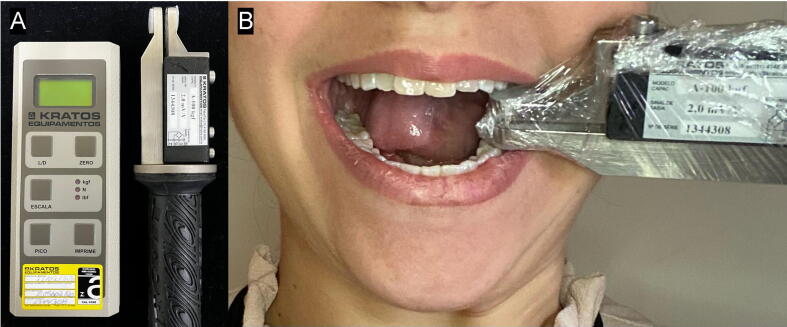
Bite force was assessed by placing the force transducer of a digital gnathodynamometer (DMD™, Kratos Equipamentos Industriais Ltda, Cotia, SP, Brazil) between the left mandibular and maxillary first molar teeth (Fig. 2), a region where 80% of the total bite force is exerted (Al-Zarea, 2015). For that, initially, the device was disinfected with 70% alcohol and inserted into plastic barriers before each measurement. Following, the patients were instructed to bite “as hard as they could” for 3 s, and the bite force was recorded in newtons considering the peak force shown on the device screen. This measurement process was performed twice further, with intervals of 1 min among them, and the arithmetic means of the measurements were calculated and considered. After 15, 90, 120, and 180 days, the same test was applied and data was computed and collected.
Fig. 2.
Bite force assessment. (A) Material: the digital gnathodynamometer used; (B) Procedure: note the force transducer placed between the left mandibular and maxillary first molar teeth.
2.7. Data synthesis
Data on the outcomes evaluated were organized using the Excel™ software (Microsoft, USA) and then submitted to both descriptive and inferential statistical analyses by the BioEstat 5.0™ software (Instituto Mamirauá, Brazil). The Shapiro-Wilk test was used to check data distribution and the Kruskal-Wallis test was applied to identify differences between groups, with a p-value <0.05 indicating statistical significance. Missing values were excluded only from the statistical evaluation to which they belonged.
3. Results
3.1. Baseline participants’ characteristics
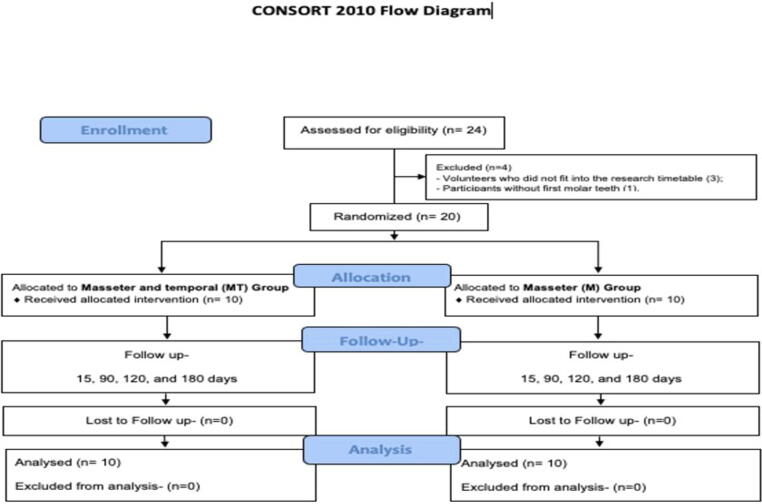
Each group comprised 10 participants. The average age in Group M was 39 years and in Group MT was 37 years. Both groups presented the same number of men (n = 2, 20%) and women (n = 8, 80%). Study stages are listed in the flowchart (Fig. 3).
Fig. 3.
Flow diagram of the progress through the phases of the present parallel randomized trial of two groups.
3.2. Patient's perception of pain
There was no statistically significant difference between the groups regarding pain in all study evaluation periods (Table 1). On the other hand, in comparison to baseline values, significant pain reduction was observed in both groups at 15 (Group M, p < 0.001; Group MT, p < 0.001), 90 (Group M, p < 0.001; Group MT, p < 0.001), 120 (Group M, p < 0.001; Group MT < 0.001), and 180 days (Group M, p < 0.001; Group MT, p < 0.001).
Table 1.
Detailed information on pain (scores from a visual analog scale).
| Period | Group | Average | Median | Standard-deviation | p-value |
|---|---|---|---|---|---|
| Baseline | MT | 7.6 | 8 | 1.84 | 0.125 |
| M | 6.5 | 6 | 1.65 | ||
| 15 days | MT | 2.2 | 3 | 1.81 | 0.642 |
| M | 1.9 | 1.5 | 1.792 | ||
| 90 days | MT | 0.9 | 0 | 1.73 | 0.815 |
| M | 0.3 | 0 | 0.483 | ||
| 120 days | MT | 1.5 | 1 | 1.43 | 0.099 |
| M | 0.5 | 0 | 0.707 | ||
| 180 days | MT | 2.5 | 2.5 | 2.27 | 0.349 |
| M | 1.3 | 1 | 1.567 |
There was no statistically significant difference between the groups (p > 0.05).
3.3. Treatment satisfaction
There was no statistically significant difference between the groups regarding treatment satisfaction at 15, 90, 120, and 180 days (Table 2). Quite high values for satisfaction were reported in both groups, ranging from 8.70 to 9.20 in Group MT and ranging from 9 to 9.78 in Group M (Table 2).
Table 2.
Detailed information on treatment satisfaction (scores from a visual analog scale).
| Period | Group | Average | Median | Standard-deviation | p-value |
|---|---|---|---|---|---|
| 15 days | MT | 9 | 10 | 2.21 | 0.358 |
| M | 9 | 9 | 1 | ||
| 90 days | MT | 9.20 | 10 | 1.62 | 0.597 |
| M | 9.78 | 10 | 0.441 | ||
| 120 days | MT | 8.70 | 9 | 1.25 | 0.096 |
| M | 9.56 | 10 | 0.726 | ||
| 180 days | MT | 8.40 | 8 | 1.26 | 0.066 |
| M | 9.44 | 10 | 0.882 |
There was no statistically significant difference between the groups (p > 0.05).
3.4. Posterior bite force
There was no statistically significant difference between the groups regarding posterior bite force in all study evaluation periods (Table 3). On the other hand, in comparison to baseline values, a significant reduction in bite force was observed in both groups at 15 (Group M, p < 0.001; Group MT, p < 0.001), 90 (Group M, p < 0.001; Group MT, p < 0.001), and 120 days (Group M, p = 0.04; Group MT, p < 0.001). After 180 days, data did not differ significantly from the baseline (Group M, p = 0.544, Group MT, p = 0.771).
Table 3.
Detailed information on posterior bite force (in Newtons – N).
| Period | Group | Average | Median | Standard-deviation | p-value |
|---|---|---|---|---|---|
| Baseline | MT | 381 | 237 | 256 | 0.796 |
| M | 339 | 305 | 190.4 | ||
| 15 days | MT | 192 | 167 | 115 | 0.971 |
| M | 174 | 177 | 61.3 | ||
| 90 days | MT | 238 | 203 | 129 | 0.853 |
| M | 207 | 199 | 84.6 | ||
| 120 days | MT | 283 | 255 | 139 | 1 |
| M | 265 | 270 | 116.4 | ||
| 180 days | MT | 337 | 291 | 182 | 0.971 |
| M | 320 | 303 | 167.8 |
There was no statistically significant difference between the groups (p > 0.05).
3.5. Other relevant findings: Side effects
Two women of Group MT experienced side effects following the BTX-A applications. One presented a marked reduction of masticatory efficiency (dysmasesis) and another one showed smile asymmetry, both events lasting for some weeks.
4. Discussion
The current study evaluated two protocols of BTX-A for orofacial pain, treatment satisfaction, and reduction in bite force in patients with bruxism. It was conceptualized to address one of the identified gaps in this therapeutic field, that is, “Is there a need for BTX-A injections into temporal muscles in addition to masseter muscles for the management of bruxism?”. Some authors believe that both muscles are synergetic and activated during teeth grinding and clenching (Shim et al., 2014); however, others advocate that only the masseter muscles should be injected because they are the chief muscles involved in the repetitive grinding movements in bruxism (Al-Wayli, 2017).
To the best of the authors’ knowledge, there is no other paper with a similar approach, especially considering the use of a gnathodynamometer for bite force recording. Although interesting alternative diagnostic strategies for evaluating bite force (e.g., computerized occlusal analysis) have already been used in the literature (Pardo et al., 2022), most research has performed evaluations by electromyography (Al-Wayli, 2017, Lee et al., 2010, Shim et al., 2014). It is important to highlight, however, that the presence of hair in the temporal region may affect the electromyographic recording and the activity of adjacent muscles not related to bruxism may also lead to unreliable data.
Orofacial pain and discomfort are symptoms often reported by patients with bruxism, probably because of muscle hyperactivity (Ågren et al., 2020, Lobbezoo et al., 2018, Manfredini et al., 2013, Muñoz Lora et al., 2019, Sateia, 2014, Yurttutan et al., 2019). As BTX-A injections induce a transient condition of paralysis or neuromodulation of muscle contraction (Cheng et al., 2022, Suber et al., 2014), muscle relaxation and a subsequent decrease in pain levels may be achieved (Freund and Schwartz, 1998). BTX-A, furthermore, may also present two other mechanisms responsible for antinociceptive effects: (1) release inhibition of neurotransmitters specifically related to pain (e.g., substance P from the dorsal root ganglion) and (2) reduction of the delivery of the transient receptor potential to neuron cell membranes (Cheng et al., 2022).
The patients herein evaluated showed a reduction of both bite force and orofacial pain (to some degree and irrespective of the study group). These results corroborate other studies that suggest that BTX-A is a useful treatment option for bruxism (Ågren et al., 2020, Manfredini et al., 2013, Muñoz Lora et al., 2019, Patel et al., 2019, Yurttutan et al., 2019). On the other hand, bite force was re-established faster than pain levels (120 days versus 180 days) probably because of the action of presynaptic mediators and the reduction of substances related to pain, such as substance P (Kwon et al., 2019). Anyway, treatment satisfaction was quite high in both groups during all the evaluation periods.
Bruxism can lead to tooth wear and impairment of oral basic functions, as well as sleep disorders, resulting in decreased quality of life (Lobbezoo et al., 2018). Although several studies have been reported on the prevalence, etiology, effects, and available treatments, there are no definitive guidelines or consensus up to now (Ågren et al., 2020, Alonso Navarro et al., 2011, Freund and Schwartz, 1998, Patel et al., 2019, Yurttutan et al., 2019). So, the use of BTX-A may be highly advantageous since it is not dependent on patients’ treatment adherence as other strategies such as physiotherapy and occlusal splint use (Ågren et al., 2020).
Although the current results are encouraging, they should be interpreted with caution owing to some study limitations. Polysomnography is considered the gold standard method for the diagnosis of sleep bruxism but the use of validated questionnaires concomitantly makes it possible to address the condition more comprehensively, as well as identify other associated factors (Maluly et al., 2013, Manfredini et al., 2013). In the present study, patients with awake bruxism were also enrolled, which made the inclusion criteria dependent on the patient complaint. Self-reported questionnaires about the presence of parafunctional habits (bruxism/clench) are of value in the diagnosis, based on an international consensus on the assessment of bruxism (Lobbezoo et al., 2018). From a clinical point of view, however, according to the American Academy of Sleep Medicine (2014) (Sateia, 2014), the best way to diagnose bruxism is from patient reports of teeth grinding, confirmed by a roommate or family member, in addition to other signs and symptoms identified in the Dentistry/Medicine office.
Another important limitation was the single-tooth method applied to measure bite force, which was adapted from other studies using both areas of first molar teeth to reach a single mean value (Al-Zarea, 2015, Calderon et al., 2006). Although most of the total bite force is dispensed at the molar regions (Al-Zarea, 2015), the authors evaluated only the left occlusal side (just a preference) in order to achieve reliable and standardized results and make the measurements easier and faster. Moreover, patients complaining of unilateral orofacial pain and those without most teeth or wearing any type of total or partial removable dental prosthesis were excluded from the present study, criteria which probably reduced sample heterogenicity (i.e., only patients with similar features of the orofacial conditions and disorders were included).
Last but not least, the limited sample size herein evaluated could have not provided sufficient power to detect some real statistically significant differences between both groups (type-2 error) (Shitsuka et al., 2020). Therefore, future RCTs using BTX-A for bruxism and related-orofacial pain should have larger sample sizes and even perform comparisons among other toxin protocols and placebo/control patients. Likewise, the methods applied for recording bite force should be better investigated.
5. Conclusion
Considering pain relief, reduction in bite force, and treatment satisfaction, BTX-A bilateral injections into masseter muscle only and in both masseter and temporal muscles seem to be somewhat equally effective in the short-term management (up to 120 days) of bruxism.
Ethical statement
The Research Ethics Committee of Ibirapuera University (#73845317.2.0000.0075) had approved the study and then it was registered in the Brazilian Clinical Trials Register – ReBEC (U1111-1235-5386 [http://www.ensaiosclinicos.gov.br]).
Conflicts of interest
None.
CRediT authorship contribution statement
Juliana Alves da Silva Ramalho: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Luiz Felipe Palma: Data curation, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. Karen Muller Ramalho: Conceptualization, Data curation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. Tamara Kerber Tedesco: Data curation, Methodology, Software, Validation, Writing – review & editing. Susana Morimoto: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Acknowledgements
The authors would like to thank Dental Cremer (São Paulo, Brazil). Funding was provided by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2017/24153-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
References
- Ågren M., Sahin C., Pettersson M. The effect of botulinum toxin injections on bruxism: A systematic review. J. Oral Rehabil. 2020;47:395–402. doi: 10.1111/joor.12914. [DOI] [PubMed] [Google Scholar]
- Alonso Navarro H., Jiménez Jiménez F.J., Plaza Nieto J.F., Pilo De la Fuente B., Navacerrada F., Arroyo Solera M., Calleja M. Tratamiento del bruxismo grave con toxina botulínica tipo A. Rev. Neurol. 2011;53:73. doi: 10.33588/rn.5302.2011017. [DOI] [PubMed] [Google Scholar]
- Al-Wayli H. Treatment of chronic pain associated with nocturnal bruxism with botulinum toxin. A prospective and randomized clinical study. J. Clin. Exp. Dent. 2017;9:e112–e117. doi: 10.4317/jced.53084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zarea B.K. Maximum Bite Force following Unilateral Fixed Prosthetic Treatment: A Within-Subject Comparison to the Dentate Side. Med. Princ. Pract. 2015;24:142–146. doi: 10.1159/000370214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon P. dos S., Kogawa E.M., Lauris J.R.P., Conti P.C.R. The influence of gender and bruxism on the human maximum bite force. J. Appl. Oral Sci. 2006;14:448–453. doi: 10.1590/S1678-77572006000600011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Yuan L., Ma L., Pang F., Qu X., Zhang A. Efficacy of botulinum-A for nocturnal bruxism pain and the occurrence of bruxism events: a meta-analysis and systematic review. Br. J. Oral Maxillofac. Surg. 2022;60:174–182. doi: 10.1016/j.bjoms.2021.03.005. [DOI] [PubMed] [Google Scholar]
- Freund B., Schwartz M. The use of botulinum toxin for the treatment of temporomandibular disorder. Oral Health. 1998;88:32–37. [PubMed] [Google Scholar]
- Guarda-Nardini L., Manfredini D., Salamone M., Salmaso L., Tonello S., Ferronato G. Efficacy of Botulinum Toxin in Treating Myofascial Pain in Bruxers: A Controlled Placebo Pilot Study. CRANIO®. 2008;26:126–135. doi: 10.1179/crn.2008.017. [DOI] [PubMed] [Google Scholar]
- Jadhao V., Lokhande N., Habbu S., Sewane S., Dongare S., Goyal N. Efficacy of botulinum toxin in treating myofascial pain and occlusal force characteristics of masticatory muscles in bruxism. Indian J. Dent. Res. 2017;28:493. doi: 10.4103/ijdr.IJDR_125_17. [DOI] [PubMed] [Google Scholar]
- Kwon K.-H., Shin K.S., Yeon S.H., Kwon D.G. Application of botulinum toxin in maxillofacial field: part I. Bruxism and square jaw. Maxillofac. Plast. Reconstr. Surg. 2019;41:38. doi: 10.1186/s40902-019-0218-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.J., McCall W.D., Kim Y.K., Chung S.C., Chung J.W. Effect of botulinum toxin injection on nocturnal bruxism: a randomized controlled trial. Am. J. Phys. Med. Rehabil. 2010;89:16–23. doi: 10.1097/PHM.0b013e3181bc0c78. [DOI] [PubMed] [Google Scholar]
- Lobbezoo F., Ahlberg J., Raphael K.G., Wetselaar P., Glaros A.G., Kato T., Santiago V., Winocur E., De Laat A., De Leeuw R., Koyano K., Lavigne G.J., Svensson P., Manfredini D. International consensus on the assessment of bruxism: Report of a work in progress. J. Oral Rehabil. 2018;45:837–844. doi: 10.1111/joor.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maluly M., Andersen M.L., Dal-Fabbro C., Garbuio S., Bittencourt L., de Siqueira J.T.T., Tufik S. Polysomnographic Study of the Prevalence of Sleep Bruxism in a Population Sample. J. Dent. Res. 2013;92:S97–S103. doi: 10.1177/0022034513484328. [DOI] [PubMed] [Google Scholar]
- Manfredini D., Winocur E., Guarda-Nardini L., Paesani D., Lobbezoo F. Epidemiology of Bruxism in Adults: A Systematic Review of the Literature. J. Orofac. Pain. 2013;27:99–110. doi: 10.11607/jop.921. [DOI] [PubMed] [Google Scholar]
- Manfredini D., Serra-Negra J., Carboncini F., Lobbezoo F. Current Concepts of Bruxism. Int. J. Prosthodont. 2017;30:437–438. doi: 10.11607/ijp.5210. [DOI] [PubMed] [Google Scholar]
- Moher D., Hopewell S., Schulz K.F., Montori V., Gøtzsche P.C., Devereaux P.J., Elbourne D., Egger M., Altman D.G. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340 doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz Lora V.R.M., Del Bel Cury A.A., Jabbari B., Lacković Z. Botulinum Toxin Type A in Dental Medicine. J. Dent. Res. 2019;98:1450–1457. doi: 10.1177/0022034519875053. [DOI] [PubMed] [Google Scholar]
- Pardo N.B., Kerstein R.B., Júnior M.C., Ferreira L.S., Abrahão M. Botulinum toxin type A for controlling bruxism assessed with computerized occlusal analysis: A pilot study. Cranio. 2022;40:207–216. doi: 10.1080/08869634.2020.1724458. [DOI] [PubMed] [Google Scholar]
- Patel J., Cardoso J.A., Mehta S. A systematic review of botulinum toxin in the management of patients with temporomandibular disorders and bruxism. Br. Dent. J. 2019;226:667–672. doi: 10.1038/s41415-019-0257-z. [DOI] [PubMed] [Google Scholar]
- Sateia M.J. International Classification of Sleep Disorders-Third Edition. Chest. 2014;146:1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- Shim Y.J., Lee M.K., Kato T., Park H.U., Heo K., Kim S.T. Effects of Botulinum Toxin on Jaw Motor Events during Sleep in Sleep Bruxism Patients: A Polysomnographic Evaluation. J. Clin. Sleep Med. 2014;10:291–298. doi: 10.5664/jcsm.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitsuka C., Palma L.F., Pedron I.G., Polotow T.G.G., de Barros M.P., Leite M.F., CorrÊa M.S.N.P. Salivary profile of children with erosive tooth wear: a transversal study. Braz. Oral Res. 2020;34:e115. doi: 10.1590/1807-3107bor-2020.vol34.0115. [DOI] [PubMed] [Google Scholar]
- Sposito M.M de M., Teixeira S.A.F. Botulinum Toxin A for bruxism: a systematic review. Acta Fisiátrica. 2014;21:201–204. doi: 10.5935/0104-7795.20140039. [DOI] [Google Scholar]
- Suber J.S., Dinh T.P., Prince M.D., Smith P.D. OnabotulinumtoxinA for the Treatment of a “Gummy Smile”. Aesthetic Surg. J. 2014;34:432–437. doi: 10.1177/1090820X14527603. [DOI] [PubMed] [Google Scholar]
- Yurttutan M.E., Tütüncüler Sancak K., Tüzüner A.M. Which Treatment Is Effective for Bruxism: Occlusal Splints or Botulinum Toxin? J. Oral Maxillofac. Surg. 2019;77:2431–2438. doi: 10.1016/j.joms.2019.06.005. [DOI] [PubMed] [Google Scholar]