Abstract
Melanotic neuroectodermal tumors of infancy (MNTI) are a rare type of benign tumor that affects the head and neck region. MNTI represents (68–80%) of the tumors in the maxillary region. This tumor is usually located in the alveolar crest, skull, mandible, and brain. Although this tumor is considered benign, it can grow rapidly, with a high risk of recurrence and interference with functions of infancy, such as feeding and breathing. It is also frequently harmful to the surrounding soft and hard tissues or adjacent sensitive vital structures. This study aimed to review the pathological, clinical presentation, and treatment of melanotic neuroectodermal tumors in infancy and the role of dentists in these cases.
Keywords: MNTI, Infancy, Dental, Treatment, Complication
1. Introduction
Melanotic neuroectodermal tumors of infancy (MNTI) are an uncommon category of benign tumor that frequently affect the head and neck region mainly in the first year of life. Many other terms, including pigmented ameloblastoma, pigmented epulis of infancy, melanotic progonoma, and melanotic epithelial odontoma, are used to refer to MNTI. The alveolar crest of the maxilla is the most affected site (Fakuade et al. 2017; Rikhotso and Mohotlhoane, 2021, Goel et al., 2022, Kolokitha et al., 2022, Rustemeyer et al., 2021). In addition to the maxillary region (68–80 %), the skull (10.8 %), the mandible (5.8 %), and the brain (4.3 %) can also be affected (Kulkarni et al., 2020). Despite being benign, it is nonetheless vital to identify this tumor due to its rapid growth and risk of local recurrence (Rikhotso and Mohotlhoane, 2021, Kumar et al., 2022). MNTI can also grow rapidly and arises from the neural crest (Pontes et al., 2018, de Souza et al., 2013). This narrative review aims to illustrate the pathological, clinical presentation, and treatment of melanotic neuroectodermal tumors in infancy and the role of dentists in these cases.(See Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5).
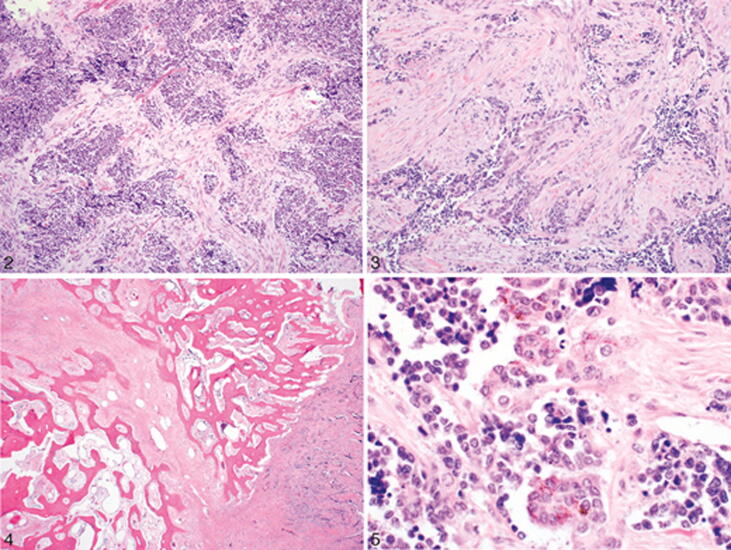
Fig. 1.
Image showing MNTI that resembles a small round blue cell tumor (Soles et al. 2018).
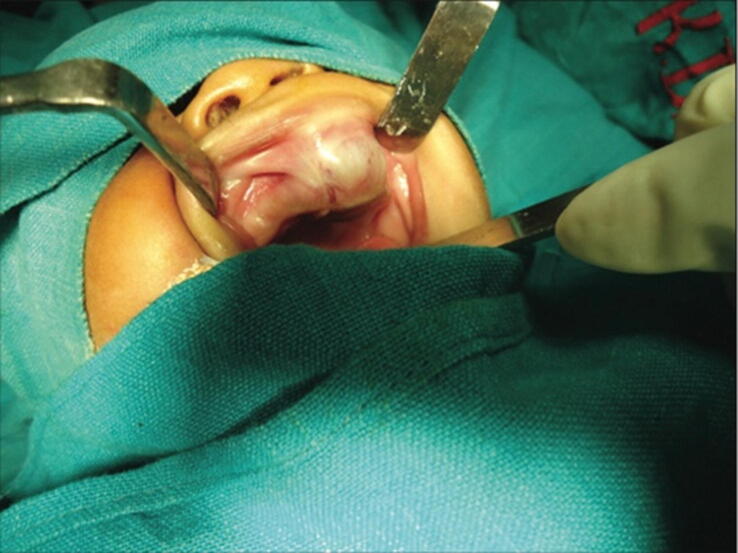
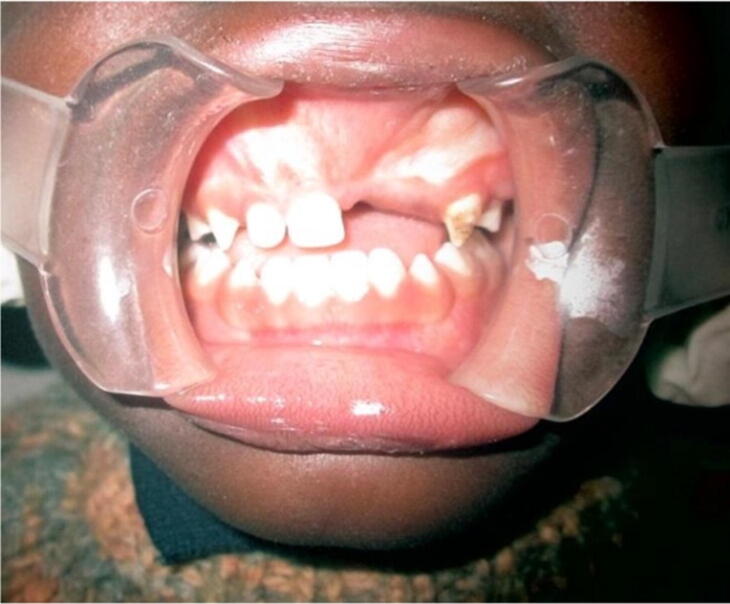
Fig. 2.
Image showing swelling in the pre-maxillary region (Chaudhary et al. 2014).
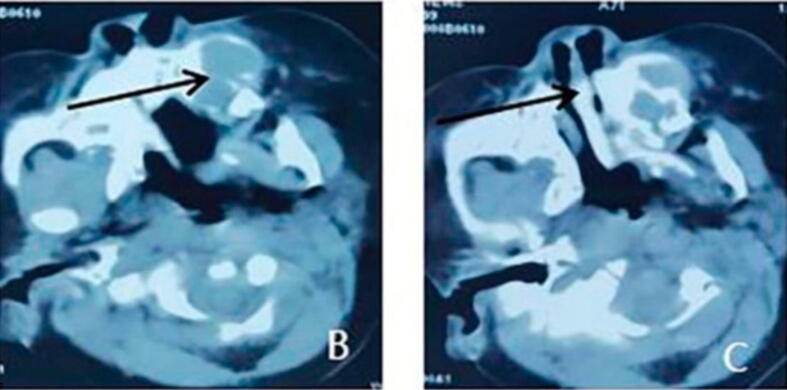
Fig. 3.
(A). The horizontal position of spiral CT, the low-density image of the anterior left maxilla, and the bone destruction (arrow). (B). The horizontal position of spiral CT showed multicentric growth in the lesion (Wang et al. 2022).
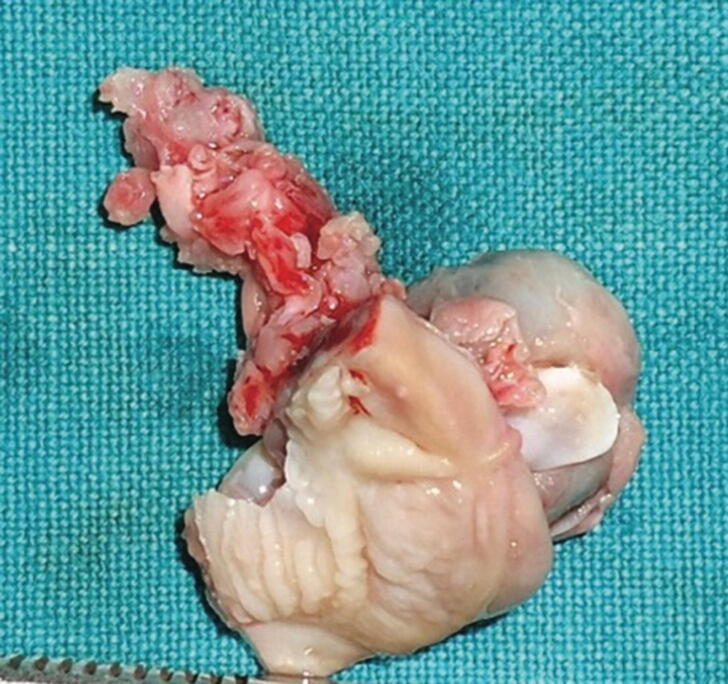
Fig. 4.
Image showing removed lesion with deciduous central incisor (Chaudhary et al. 2014).
Fig. 5.
2 years post-excision (Fakuade et al. 2017).
2. Literature review
2.1. Pathology and histopathologic features
The MNTI is a firm, lobed, well-circumscribed mass. Masses are usually superficially penetrated and compacted rather than deeply penetrated adjacent structures. Due to its pigmentation, tumors often have a bluish-black hue. In sectioned surfaces, tumors are likely to be gray to black depending on the quantity of the melanin pigment (Soles et al. 2018).
MNTIs consist of tiny, rounded islets of tumor cells enclosed by a fibrous collagen stroma. A biphasic cell population consisting of large epithelioid melanocytes and small primitive neurogenic cells (neuroblast-like) is the histological hallmark of this tumor. Cells can form cords, pseudo-glandular structures, sheets, nests, or sometimes pseudoalveolar spaces. These structures can typically contain both small neurogenic cells and large melanocytes. However, both populations can exist separated from each other (Soles et al. 2018).
In general, melanocytes are located peripherally, while neurogenic cells are centrally located within the nest or space. Melanocytes are epithelioid, polygonal, or cuboidal, medium to large. The nucleus is smooth with follicular chromatin and occasionally prominent nucleoli. These cells are rich with eosinophilic cytoplasm and typically contain melanin granules. Ultrastructurally, they are surrounded by a basement membrane and form desmosomes with neighboring cells. They have round, hyperstained nuclei with occasional 'salt and pepper' chromatin. Several cases show a neurofibrillary stoma surrounding neurogenic cells. The background is composed of a dense fibrous collagen stroma that is well-vascularized. Mitosis is usually rare, and necrosis is very rare unless the tumor behavior is clearly malignant. Bone tumor margins may be invasive, with clusters of cells located between the bone trabeculae. This infiltrative growth pattern and small neurogenic cells may give the false impression of a more malignant entity (Soles et al. 2018).
2.2. Clinical presentation and radiographic feature
Clinically, MNTI shows many manifestations such as swelling (Cui et al., 2015, Krishnamurthy et al., 2011), expansions, and rapidly growing projecting masses in the oral cavity raised on the mucosal surface and usually includes the alveolar ridge, maxilla, hard palate, and gingiva (Cui et al., 2015, Krishnamurthy et al., 2011).
The patients showed some similar manifestations such as no history of medications in the mothers during pregnancy (Agrawal et al., 2020, Chaudhary et al., 2014), bleeding (Han et al. 2019), swollen face, face deformity (Wang et al., 2022, Han et al., 2019), no fever or pain (Cui et al. 2015), some born with the MNTI, while some developed it a few months later. The problems caused by these projecting masses interfere with breathing and feeding, causing difficulties in breastfeeding and problems while eating; some patients had irritabilities (Sailukar et al. 2007); some of them had no history of airway feeding difficulties [14]. The swollen mass had common features (Cui et al., 2015, Krishnamurthy et al., 2011); it is dark, firm, bony hard, non-tender, non-ulcerated, non-pulsatile, non-fluctuant, non-mobile, non-erythematous, non-cystic, smooth, fixed, solid, round, pigmented; some specimens show brownish-black inner surface, their color usually ranges from pinkish red to purplish-blue, and the overlying mucosa/gingiva is always stretched, intact, and normal. It is also painless and resilient (Madrid et al. 2010).
The radiographic features are detected by the Computed Tomography (CT) scan due to the enlargement, swelling of the oral cavity, and bone involvement in the lesion. CT scans of some cases show an exophytic expansile mass of hard palate surrounded by a thin rim of sclerotic bone (Agrawal et al. 2020), low-density lesion (Cui et al., 2015, Krishnamurthy et al., 2011), expansion of both labial and palatal cortices, intact soft tissue, no ulceration, cystic shaped lacune, bone destruction, resorption, and irregularity (Wang et al. 2022).
2.3. Differential diagnosis
Differential diagnoses of an MNTI include melanogenic tumors such as clear cell sarcoma of soft tissue and malignant melanoma, peripheral neuroepithelioma, lymphoma, Ewing sarcoma, primitive neuroectodermal tumor, alveolar rhabdomyosarcoma, and some small round blue cell tumors that occur during childhood, such as neuroblastoma (Soles et al., 2018, Ünsal and Yalçin, 2018; Atarbashi et al. 2022; Ren et al., 2019, Yindeedej and Kittisangvara, 2021). Other differential diagnoses include inflammatory diseases, which can be ruled out by clinical and laboratory investigations (Manojlović et al. 2012).
The main morphologic features used to differentiate MNTI from others are the characteristic immunohistochemical findings, the biphasic population of epithelioid melanogenic cells, primitive neurogenic cells, and clinical presentation. A differential diagnosis is made depending on the typical imaging and clinical appearance of the melanotic neuroectodermal tumor and histopathologic markers, including the existence of pigment, epithelioid, and neuroblastoma-like biphasic differentiation of the cells (Ünsal et al. 2018). Increased VMA level is found to help make proper differential diagnoses in some cases (Ren et al. 2019). Small biopsies can pose a diagnostic challenge, for instance, leading to a misdiagnosis of MNTI as neuroblastoma.
This tumor lacks distinct diffuse expression of neuroendocrine markers and rosette formation. Neuroblastoma does not have any accumulated melanogenic cells with melanin pigment. Additionally, neuroblastoma can be negative for HMB-45 and cytokeratin. Ewing sarcoma contains a nest of small monomorphic sheets that are composed of round cells and does not include a large pigmented epithelioid melanogenic cell. Ewing sarcoma generally harbors EWSR1 gene rearrangement and exhibits diffuse solid membranous immunostaining for CD99. Ewing sarcoma cases commonly have t (11;22) (q24; q12) with EWSR1-FLI chimeric fusion, while unusual cases of melanotic neuroectodermal tumors react with CD99. However, the deficiency of the molecular signature of Ewing sarcoma is highly effective for distinction, especially when working with a small sample (Soles et al. 2018).
Alveolar rhabdomyosarcoma is sometimes present in the head and neck region and is more common in the extremities. Alveolar rhabdomyosarcoma contains a nest of poorly differentiated small hyperchromatic cells which are divided by fibrous septa. FISH and molecular analyses could be needed to prevent a false diagnosis of melanotic neuroectodermal tumor due to its well-described divergent muscular differentiation. Both alveolar rhabdomyosarcoma and Ewing sarcoma have extensive necrosis, which is not generally presented in MNTI. The benign and malignant hematolymphoid cells may resemble the neurogenic cells morphologically, alongside their crushing artifact in smaller biopsies. However, the immune profile of melanotic neuroectodermal tumors varies entirely from lymphomas, in which the hematolymphocytic markers are expressed but melanotic or epithelial markers are not. Meanwhile, the melanotic neuroectodermal tumor does not demonstrate any recurrent molecular alterations. However, cytogenetic and molecular analyses are highly recommended to differentiate it from others that have a well-recognized characteristic translocation (Soles et al. 2018).
Malignant melanoma is one of the main diseases that should be considered in the evaluation of possible differential diagnosis. Melanoma can be negative for cytokeratins. However, it is positive for S100 and melanoma markers. In addition, it presents with overtly malignant morphology. Clear cell sarcoma typically appears on the extremities in young patients and contains uniform, spindled to ovoid cells with clear to pale eosinophilic cytoplasm. A very important factor to distinguish this disease from the melanotic neuroectodermal tumor is the lack of primitive-appearing neurogenic cells. The presence of clear cell sarcoma is associated with recurrent characteristic translocations, mostly t (12;22) (q13; q12), which result in the EWS-ATF1 chimeric gene. MNTI can be confused with congenital granular cell tumors since they both occur during infancy; however, the histology characteristics could help distinguish the differences (Soles et al. 2018).
Odontogenic tumors in children can be distinguished clinically because of the presentation and location; however, these tumors hardly occur before 6 years of age (Soles et al. 2018). While examining any pigmented mass in the head and neck region, the differential diagnosis should include malignant melanoma, clear cell sarcoma of soft tissue, and lymphomas (Almomani et al. 2022). Several differential diagnoses of melanotic neuroectodermal tumors of infancy are demonstrated in (Table1).
Table 1.
Differential diagnosis of a melanotic neuroectodermal tumor of infancy (el-Saggan et al. 1998).
| Odontogenic cysts | Palatal and dental laminal cysts of the newborn and Eruption cysts and hematoma |
|---|---|
| Odontogenic tumors | Congenital gingival granular cell tumor, Neuroblastoma, Lymphangioma, Hemangioma, Pyogenic granuloma, Histocytosis X, Fibrosarcoma, Chondrosarcoma, and Rhabdomyosarcoma |
2.4. Treatment
According to the findings and explanation presented, the treatment of MNTI relies primarily on surgical procedures, with a commitment to conservative ones. Most clinicians prefer two ways of treatment, either surgical excision (local excision) or curettage (Moreau et al., 2018, Cui et al., 2015, Krishnamurthy et al., 2011, Selim et al., 2008), while some clinicians advocate surgical resection, en-bloc excision (Han et al., 2019, Béogo et al., 2013), enculation (Pinheiro et al. 2013), and dissection (Wang et al. 2022), with all procedures performed under general anesthesia (Cui et al., 2015, Krishnamurthy et al., 2011). Previous treatment modalities included the use of chemotherapy and radiotherapy, but proved to be ineffective in preventing tumor recurrence (Agrawal et al. 2020); further, they are not suitable for surgical patients or to be used as adjuvant therapies before or after surgery (Wang et al. 2022). So, as mentioned previously, complete surgical procedures are the most suitable treatment option. Post-operative periods are mostly uneventful, without any recurrence (Cui et al., 2015, Krishnamurthy et al., 2011).
2.5. Prognosis
Melanotic neuroectodermal tumor of infancy is generally benign and described as a painless, enlarging firm mass with an excellent prognosis (Takeuchi et al., 2021, Atarbashi-Moghadam et al., 2020). This mass is frequently covered by an intact epithelial surface. Yet, there is a high risk of recurrence, with frequent lymph node involvement (Takeuchi et al. 2021). This disease is typically considered benign. However, its biological behavior is not yet fully understood (Soles et al. 2018).
In general, the primarily benign lesion has a good prognosis (Emmerling et al. 2019). This tumor can be clinically disturbing due to the significant risk of local recurrence and its rapid growth (Soles et al. 2018). The recurrence rate was reported by previous studies to be in the range of 15 % to 27 %. In a previous study, the risk of recurrence of the melanotic neuroectodermal tumor in the skull was found to be higher than in the maxilla (Ebel et al. 2020). Recurrence of the neuroectodermal tumor could be fatal, particularly when involving vital structures or the central nervous system. Some authors reported that large cell components and a predominance of a neuroblast-like component were associated with a high risk of local recurrence and aggressive progression (Soles et al. 2018).
The age of diagnosis seems to be a prognostic marker of the recurrence of the disease. Studies show that infants diagnosed during the first 2 months of life are more prone to recurrence within 6 months. Infants diagnosed at age of 4.5 months or older have a minimal chance of recurrence, and infants diagnosed within 2–4.5 months have a moderate risk of recurrence (Soles et al. 2018). Metastasis was found to occur in 3 % of cases. Spread has been reported to the central nervous system and lymph nodes; these conditions are almost universally fatal (Soles et al., 2018, Emmerling et al., 2019). Although MNTI generally has no sex predilection, the mandible shows a slight male predominance (Krishnamurthy et al. 2011). MNTI was also reported to be present in the orbit as a quickly progressing mass (Wu et al. 2021).
2.6. Complications and dental consideration
One of the most challenging aspects of melanotic neuroectodermal tumors in infancy is the reconstruction of the structure, function, and occlusal region of the lost tissue after tumor removal (Takeuchi et al. 2021). There are high risks due to altered maxillofacial growth after surgical intervention, particularly after massive surgery. Moreover, maintaining a maxillofacial growth pattern requires long-term care and reconstructive needs. However, extensive resection is recommended in most cases, except if the melanotic neuroectodermal tumor is adjacent to sensitive regions which could affect the functional or developmental pattern in the future. Thus, in these cases, the clinician should consider a balanced and conservative approach (Chillal et al., 2021).
During melanotic neuroectodermal tumor removal, the clinician should also examine the surrounding structure, including sclerotin, inferior alveolar nerve, teeth, and bone. While removing the tumor periosteum, the clinician should be conservative to minimize any growth alteration or deficiency in the mandible. The associated teeth within the tumor must be completely removed, and regular follow-up visits to the dentist should be maintained to detect any future abnormal development. Following the removal of the tumor, inspection and examination of the sclerotin should be done. Sclerotin usually presents as dark brown-black spots around the tumor. If during the pathological examination the sclerotin is found to contain tumor cell remnants, it is indicative of incomplete tumor removal. This poses a high risk of recurrence, which needs further surgical intervention (Atarbashi-Moghadam et al., 2020, Cui et al., 2015, Hoshina et al., 2000).
3. Conclusion
Melanotic neuroectodermal tumors of infancy (MNTI) are rare and benign tumors. However, it shows some risks such as rapid growth, recurrence risk, conflict with biological functions, and possible harm to the surrounding vital structures. One of the main challenges that could face dentists while dealing with these patients is the altered growth and development of the maxillofacial structure after the surgical procedure, which requires long-term care needs. Hence, regular visits to the dentist in the first year of the infant’s life are necessary to detect any abnormal development and deal with this kind of tumor as soon as possible to avoid any undesired complications.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Contributor Information
Bader Fatani, Email: 438100923@student.ksu.edu.sa.
Abdulaziz Abdullah Alabood, Email: 438101727@student.ksu.edu.sa.
Rifal Fahad Almuaybid, Email: 439200363@student.ksu.edu.sa.
Reema Mohammed Alsubaie, Email: 441200290@student.ksu.edu.sa.
References
- Agrawal A., Joshi J., Agrawal D., Kumar P., Modi B. Oral melanotic neuroectodermal tumor of infancy: management of a case affecting the maxilla. J. Indian Soc. Pedodontics Prevent. Dent. 2020;38(3):319. doi: 10.4103/JISPPD.JISPPD_169_18. [DOI] [PubMed] [Google Scholar]
- Almomani M.H., Rentea R.M. StatPearls Publishing; In StatPearls: 2022. Melanotic Neuroectodermal Tumor Of Infancy. [PubMed] [Google Scholar]
- Atarbashi-Moghadam S., Lotfi A., Moshref M., Atarbashi-Moghadam F. Melanotic neuroectodermal tumor of infancy, a rapidly growing maxillary alveolar mass: a case report. J. Dent. (Shiraz, Iran) 2020;21(1):77–80. doi: 10.30476/DENTJODS.2019.44910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béogo R., Nikiéma Z., Traoré S.S., Bouletreau P. Maxillary melanotic neuroectodermal tumor of infancy management: is conservative surgery the best approach? J. Craniofac. Surg. 2013;24(4):e338–e340. doi: 10.1097/SCS.0b013e31828a7c4c. [DOI] [PubMed] [Google Scholar]
- Chaudhary S., Manuja N., Ravishankar C.T., Sinha A., Vijayran M., Singh M. Oral melanotic neuroectodermal tumor of infancy. J. Indian Soc. Pedodontics Prevent. Dent. 2014;32(1):71. doi: 10.4103/0970-4388.127064. [DOI] [PubMed] [Google Scholar]
- Chillal M., Menon S., Archana S., Sham M.E. Melanotic neuroectodermal tumor of infancy of maxilla: report of a case with review of literature. J. Oral Maxillofac. Pathol. : JOMFP. 2021;25(2):351–355. doi: 10.4103/0973-029X.325239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Mao Z.H.E., Liao C. Melanotic neuroectodermal tumor of infancy: a case report and review of the surgical treatment. Oncol. Lett. 2015;9(1):29–34. doi: 10.3892/ol.2014.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza, D. F. M., Sendyk, D. I., Seo, J., da Fonseca, E. V., Naclério-Homem, M. D. G., & Deboni, M. C. Z. (2013). Melanotic neuroectodermal tumor of infancy in the maxilla. Case Reports in Dentistry, 2013.https://www.hindawi.com/journals/crid/2013/726815/. [DOI] [PMC free article] [PubMed]
- Ebel F., Thieringer F.M., Kunz C., Klein-Franke A., Scheinemann K., Guzman R., Soleman J. Melanotic neuroectodermal tumor of infancy to the skull: case-based review. Child's Nervous Syst. : ChNS : Off. J. Int. Soc. Pediatr. Neurosurg. 2020;36(4):679–688. doi: 10.1007/s00381-020-04509-6. [DOI] [PubMed] [Google Scholar]
- el-Saggan, A., Bang, G., & Olofsson, J. (1998). Melanotic neuroectodermal tumour of infancy arising in the maxilla. The Journal of Laryngology & Otology, 112(1), 61-64. https://www.cambridge.org/core/journals/journal-of-laryngology-and-otology/article/abs/melanotic- neuroectodermal-tumour-of-infancy-arising-in-the-maxilla/3C5F2D92C69F02EDB2B2B5276B9C0539. [DOI] [PubMed]
- Emmerling M.R., York T.A., Caccamese J.F. Melanotic neuroectodermal tumor of infancy: case report and review of management. J. Oral Maxillofac. Surg. : Off. J. Am. Assoc. Oral Maxillofac. Surg. 2019;77(2):315–320. doi: 10.1016/j.joms.2018.09.033. [DOI] [PubMed] [Google Scholar]
- Fakuade B.O., Adeoye J.B. Melanotic neuroectodermal tumor of infancy: a rare presentation of an extremely rare neoplasm and diagnostic implications in Gombe, Nigeria. Pan Afr. Med. J. 2017;28(1) doi: 10.11604/pamj.2017.28.5.9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel, D., Qayoom, S., Goel, M. M., & Rawa, J. (2022). Melanotic neuroectodermal tumor of infancy (MNTI)–A rare entity. Journal of Cancer Research and Therapeutics, 18(3), 784. https://www.cancerjournal.net/article.asp? issn=0973-1482;year=2022;volume=18;issue=3;spage=784;epage=787;aulast=Goel. [DOI] [PubMed]
- Han D., Gordon J., Nguyen S., Turner M.D. Melanotic neuroectodermal tumor of infancy with a negative VMA: a case report and review of the presentation, etiology, and management. Craniomaxillofac. Trauma Reconstr. Open. 2019;3(1):s-0039. [Google Scholar]
- Hoshina Y., Hamamoto Y., Suzuki I., Nakajima T., Ida-Yonemochi H., Saku T. Melanotic neuroectodermal tumor of infancy in the mandible: report of a case. Oral Surg., Oral Med., Oral Pathol., Oral Radiol., Endodontol. 2000;89(5):594–599. doi: 10.1067/moe.2000.105519. https://www.sciencedirect.com/science/article/abs/pii/S1079210400350776 [DOI] [PubMed] [Google Scholar]
- Kolokitha O.E., Kotsiomiti E., Lazaridis K., Lazaridis N. Mandibular melanotic neuroectodermal tumor of infancy: interdisciplinary treatment from 2 months to 19 years of age. J. Maxillofac. Oral Surg. 2022;21(1):105–111. doi: 10.1007/s12663-021-01571-8. https://link.springer.com/article/10.1007/s12663-021-01571-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy A., Vaidhyanathan A., Majhi U. Malignant melanotic neuroectodermal tumor of infancy arising in the mandible. J. Cancer Res. Ther. 2011;7(3):368. doi: 10.4103/0973-1482.87018. [DOI] [PubMed] [Google Scholar]
- Kulkarni T.M., Nagpal D.J., Shete A.V., Hande P.S., Shete M.V. Melanotic neuroectodermal tumor of infancy: a rare case report. Contemporary Clin. Dent. 2020;11(2):168–170. doi: 10.4103/ccd.ccd_119_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K.S., Naleer M.H., Visweswaran V., Krishnamurthy G. Melanotic neuroectodermal tumor of infancy: a rare case report. Asian J. Neurosurg. 2022 doi: 10.1055/s-0042-1750299. https:// www.thieme-connect.com/products/ejournals/html/10.1055/s-0042-1750299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid,C.,Aziza,J.,Hlali,A.,Bouferrache,K.,&Abarca,M.(2010).Melanoticneuroectodermal tumour of infancy: a case report and review of the aetiopathogenic hypotheses. [DOI] [PubMed]
- Manojlović S., Virag M., Lukšić I., Müller D. Melanotic neuroectodermal tumour of infancy: report of two cases and review of the literature. J. Cranio-Maxillo-Facial Surg. : Off. Publi. Eur. Assoc. Cranio-Maxillo-Facial Surg. 2012;40(4):e103–e107. doi: 10.1016/j.jcms.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Moreau A., Galmiche L., Minard-Colin V., Rachwalski M., Belhous K., Orbach D., Joly A., Picard A., Kadlub N. Melanotic neuroectodermal tumor of infancy (MNTI) of the head and neck: a French multicenter study. J. Cranio-Maxillo-Facial Surg. : Off. Publ. Eur. Assoc. Cranio-Maxillo-Facial Surg. 2018;46(2):201–206. doi: 10.1016/j.jcms.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Pinheiro, T. P. D. S., Carneiro, J. T., de Melo Alves, S., de Jesus Viana Pinheiro, J., & Tuji, F. M. (2013). Melanotic neuroectodermal tumor of infancy in an African indigenous patient from the Amazon: a case report. Head & Face Medicine, 9(1), 1-4 [DOI] [PMC free article] [PubMed]
- Pontes, F., de Souza, L. L., Uchôa, D., Joaquim, A., do Nascimento, L. S., da Mata Rezende, D., & Pontes, H. (2018). Melanotic neuroectodermal tumor of infancy of the jaw bones: Update on the factors influencing survival and recurrence. Head & neck, 40(12), 2749–2756. https://doi.org/10.1002/hed.25514 [DOI] [PubMed]
- Ren Q., Chen H., Wang Y., Xu J. Melanotic neuroectodermal tumor of infancy arising in the skull and brain: a systematic review. World Neurosurg. 2019;130:170–178. doi: 10.1016/j.wneu.2019.07.017. https:// www.sciencedirect.com/science/article/abs/pii/S1878875019319138 [DOI] [PubMed] [Google Scholar]
- Rikhotso R.E., Mohotlhoane G.P. Melanotic neuroectodermal tumour of infancy in the maxilla: A case report. Oral Maxillofac. Surg. Cases. 2021;7(4) 100235.https:// www.sciencedirect.com/science/article/pii/S2214541921000298 [Google Scholar]
- Rustemeyer J., Günther L., Junker K., Thieme V., Busch A., Okcu Y., Siegmund B.J. Melanotic neuroectodermal tumour of infancy: clinical courses and therapeutic options-a review of three cases. J. Maxillofac. Oral Surg. 2021;20(2):219–226. doi: 10.1007/s12663-019-01324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailukar M., Bhagwat R., Seth T. Melanocytic neuroectodermal tumor of infancy. J. Indian Assoc. Pediatric Surg. 2007;12(3):136. [Google Scholar]
- Selim H., Shaheen S., Barakat K., Selim A.A. Melanotic neuroectodermal tumor of infancy: review of literature and case report. J. Pediatr. Surg. 2008;43(6):E25–E29. doi: 10.1016/j.jpedsurg.2008.02.068. [DOI] [PubMed] [Google Scholar]
- Soles B.S., Wilson A., Lucas D.R., Heider A. Melanotic neuroectodermal tumor of Infancy. Arch. Pathol. Lab. Med. 2018;142(11):1358–1363. doi: 10.5858/arpa.2018-0241-RA. [DOI] [PubMed] [Google Scholar]
- Takeuchi R., Funayama A., Oda Y., Abé T., Yamazaki M., Maruyama S., Hayashi T., Tanuma J.I., Kobayashi T. Melanotic neuroectodermal tumor of infancy in the mandible: a case report. Medicine. 2021;100(50):e28001. doi: 10.1097/MD.0000000000028001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ünsal H.Y., Yalçin M. Melanotic neuroectodermal tumor of infancy in the maxilla. J. Craniofac. Surg. 2018;29(1):e28–e30. doi: 10.1097/SCS.0000000000003993. [DOI] [PubMed] [Google Scholar]
- Wang,S.,Song,C.,Yang,X.,Yang,Y.,&Wei,J.(2022).Melanoticneuroectodermaltumorof infancy: Case report and literature review. Ear, Nose & Throat Journal, 01455613221112353 [DOI] [PubMed]
- Wu Y.X., Ren M.Y. Zhonghua yan ke za zhi. Chinese J. Ophthalmol. 2021;57(5):372–374. doi: 10.3760/cma.j.cn112142-20201120-00765. [DOI] [PubMed] [Google Scholar]
- Yindeedej V., Kittisangvara L. Melanotic neuroectodermal tumor of infancy at skull: rare and rapid-growing tumor but histologically Benign. Pediatr. Neurosurg. 2021;56(3):306–311. doi: 10.1159/000515686. [DOI] [PubMed] [Google Scholar]