Abstract
BACKGROUND
It seems that the time of performing cardiac rehabilitation is important in determining the risk of cardiac complications in patients with myocardial infarction (MI). The present study aimed to investigate the effects of a home-based cardiac rehabilitation program (HCRP) conducted in either the morning or evening on cardiometabolic risk factors in phase IV (maintenance) MI patients.
METHODS
In this randomized controlled clinical trial, 80 patients with MI were divided into 2 groups of intervention and control (40 individuals per group). Patients in each group were categorized into morning and evening subgroups (20 individuals per subgroup). The therapeutic regimen in the intervention group included HCRP, routine medications, and exercise and walking programs for 8 weeks. Patients in the control group received routine treatments for 8 weeks. Cardiovascular risk factors comprising of cardiac troponin I (cTnI), mean platelet volume (MPV), C-reactive protein (CRP), and cardiometabolic indicators including cholesterol (Cho), high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride (TG), and the maximum rate of oxygen consumption (VO2 max) were measured for all patients before and after the intervention.
RESULTS
Our results showed significant reductions in Cho, TG, HDL, LDL, VO2 max, CRP, and MPV (P < 0.05) in the group performing HCRP in the evening compared with the morning group.
CONCLUSION
Performing HCRP in the evening, compared with morning, can be significantly more effective in improving the levels of cardiometabolic risk factors in patients with MI. Therefore, it is recommended that rehabilitation programs be implemented in these patients in evening shifts.
Keywords: Sudden Cardiac Death, Cardiac Rehabilitation, Time, Myocardial Infarction, Risk Factors
Introduction
Coronary artery disease (CAD) is the leading cause of death in many societies.1 For instance, CADs lead to 500,000 deaths per year in the United States.1,2 Increased consumption of fast foods that are rich in saturated fats and reduced physical activity seem to increase the prevalence of obesity and diabetes, thus leading to the high incidence of cardiovascular risk factors in communities.3
CADs often present as angina pectoris and myocardial infarction (MI). Coronary artery bypass grafting (CABG) is still the main treatment for CAD worldwide. These patients also receive pharmaceutical therapies, but in order to be effective, these interventions must be accompanied by cardiac rehabilitation programs. As a complementary non-pharmacological therapy, cardiac rehabilitation plays an important role in the management of CAD patients.4 Rehabilitation programs include physical activities aiming to improve or manage cardiometabolic risk factors such as blood lipids.5
Generally, cardiac rehabilitation programs are divided into 4 phases. Phase I which is performed in patients with an acute disease (i.e., in-hospital) lasts 1 week. Phase II which is conducted after discharge lasts from 6 to 12 weeks. Phase III (i.e., supervised program) continues after the second phase until up to 6 months. Finally, phase IV is performed in a semi-supervised or unsupervised fashion for an indefinite time.6,7 Therefore, phase IV, which is usually a long-term program with undefined and variable durations, aims to increase and maintain patients’ physical fitness.7 The recent phase (IV) is recommended for low-risk patients who are able to properly control the intensity and frequency of exercise programs.6
According to the recommendations of the World Health Organization (WHO), patients with various diseases have the right to receive the most effective, low-cost, safest, and easiest medical treatments available.8 In comparison with hospital-based cardiovascular rehabilitation programs, cardiac tele-rehabilitation, as a home-based cardiovascular rehabilitation program (HCRP), presents a safe and effective method for the improvement of the physiological and physical health of patients with CADs.9 Considering that walking is the most common type of moderate physical activity in adults,10 most activity recorders such as pedometers are designed to count individuals’ steps. Actually, therapeutic objectives are set on a pedometer to motivate individuals to increase their physical activities in order to ultimately improve their physiological and psychological well-being. Additionally, the pedometer is useful for the monitoring of the level of physical activity and patients’ compliance with exercise programs for up to 12 months.11
Acute myocardial infarction (AMI), commonly known as the heart attack, generally occurs due to the loss of blood flow to a part of the heart, resulting in the death of cardiomyocytes. This arises from the blockage of a coronary artery after the rupture of a vulnerable atherosclerotic plaque [an unstable collection of lipids (cholesterol and fatty acids) and white blood cells (especially macrophages) on the vascular wall]. If left untreated for a sufficient amount of time, the resultant ischemia (limited blood flow) and the consequent shortage in tissue oxygenation can lead to damage in or the death (infarction) of the heart muscle.12 Tissue necrosis is an immediate acute phase reaction in AMI, and C-reactive protein (CRP) is an important indicator of heart necrosis. A study performed on lipid profiles of AMI patients showed a relationship between AMI and the CRP inflammatory marker.13 The results of a recent study also showed that changes in lipid profiles and inflammatory markers had important roles in the incidence of AMI.13
There is a complex interaction between the effects of physical activities and the day time of their performance. This interaction can somehow reflect the function of coagulation factors and platelets. However, no study has been carried out on the details of this issue in humans. It was shown in one study that mean platelet volume (MPV) insignificantly reduced following physical activity, especially in the evening. In accordance with the physiological changes of the human body, the rate of platelet aggregation has been shown to increase in the morning.14 Although the benefits of physical activities have been known for a long time, studies have indicated that performing physical activity at certain day-night times can cause sudden cardiac death (SCD) even in healthy individuals.15 This problem is even more concerning in patients with CAD. Most people and patients with CVDs do not know the best time for exercises to lower the risk of various complications to a minimum level. The present study was conducted with the aim to determine and compare the effects of HCRP (monitored using a pedometer as a remote electronic tool) performed in either the morning or evening on cardiometabolic risk factors (cardiac specific troponin I, lipid profile, CRP, and platelet count) in phase-IV MI patients.
Materials and Methods
Participants: All the patients hospitalized because of MI in Shahid Madani Hospital of Khorramabad, Iran, from September to November 2018 were enrolled in this study after being discharged. All the methods employed in this research were registered at the Iranian registry for clinical trials under the code IRCT20181122041725N3. Based on clinical examinations by medical specialists and according to the New York Heart Association (NYHA) classification,16 all subjects had heart failure and were placed in NYHA classes II and III. This was a clinical trial in which neither patients nor researchers were aware of group assignments.
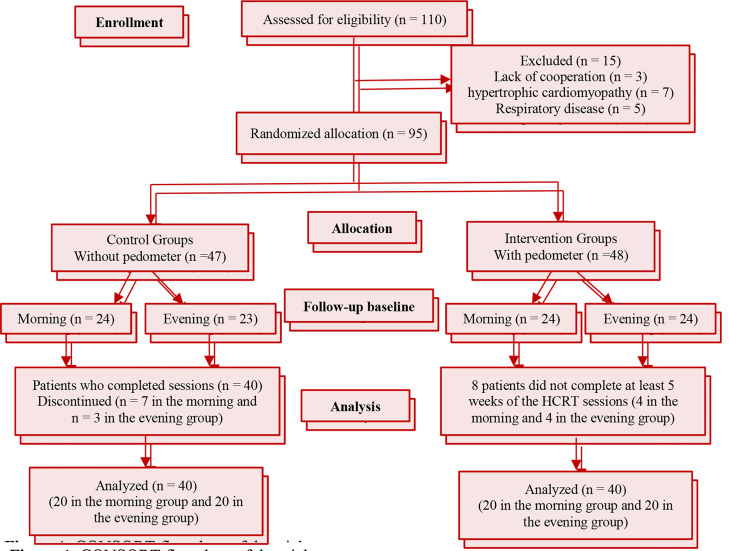
A total of 110 patients were evaluated for eligibility 95 of whom had the criteria to participate in the study. Randomization was implemented using block randomization with the block size of 4. The enrolled patients were randomly divided into intervention and control groups, and each of these groups was then categorized into two subgroups of morning and evening. In the control group, 6 participants were excluded from statistical analyses because they did not comply with the exercise program for at least 5 weeks (Figure 1).
Figure 1.
CONSORT flowchart of the trial
The intervention (with pedometer) subgroups [the morning (8-9 a.m.) and evening (5-6 p.m.)] included 40 patients. HCRP was designed based on a previous research17 and included walking at home using a waist-mounted pedometer (PA-S20, Switzerland). Patients were instructed to record the number of steps taken (i.e., shown on the step counter) in a sport notebook. The patients also consulted with a cardiologist during the 8 weeks of the study to manage cardiovascular risk factors and pharmaceutical therapies, and to adjust the extent of the home-based physical activities. The patients were also followed up by the research team through making phone calls every other day.
The control subgroups (morning and evening exercise) included 40 patients who only received counseling about routine physical activities. During this period, they neither attended exercise and educational sessions nor received any phone calls from the research team. The participants in the control group continued to receive routine care during the study.
Inclusion and exclusion criteria: In order to control the various factors effective on the research outcomes, a set of conditions was considered in the inclusion of participants in the study. The inclusion criteria included history of angioplasty and/or angiography, mild-moderate cardiovascular disorders (mild CAD), urban residency, the passage of at least 8 weeks since MI, age of 45 to 60 years, functional capacity of 4 < [metabolic equivalents (METs)] ≤ 13, lack of regular physical activity during the 3 months prior to the intervention, 40% < ejection fraction > 55%, and lack of a pacemaker.
The exclusion criteria were ventricular arrhythmias, renal and uncontrolled metabolic diseases, hypertrophic cardiomyopathy, or respiratory and endocrine diseases. The present study was approved by the Ethics Committee of Lorestan University of Medical Sciences, Iran (approval ID: IR.LUMS.REC.1399.200). The protocols were conducted according to the guidelines of the Declaration of Helsinki.18
The home-based cardiovascular rehabilitation program protocol: The cardiac rehabilitation program included walking at home for almost 45-60 min (5 min warming-up, 40 min walking, and 5 min cooling-down and stretching exercises). The Borg rating of perceived exertion (RPE) of 11-13 was determined. The participants of the intervention group performed the program using a pedometer for 8 weeks (5 sessions per week) as previously noted.17 From the 2nd to 8th week, the number of steps gradually increased by 10% per week (i.e., 100 steps/day to a total of 500 steps/week). A pilot study was conducted to standardize the way of performing the protocol and to determine the number of steps at the beginning of the study (3500 steps/day).
The Bruce Protocol and Termination Criteria: The protocol was implemented in the framework of a 2-phase project (i.e., before and after the eight-week HCRP). During aerobic exhaustive exercise, an advanced treadmill test was used employing the Bruce protocol (i.e., 3 km/hour increasing by 1.4 km/hour after 3 minutes followed by boosting the grade by 3% at a constant speed) to determine cardiovascular and respiratory functional indicators in each phase.19 VO2 max was measured using the Bruce Treadmill Test which is an indirect test that estimates VO2 max using the formula: VO2max = 14.76 - (1.379. T) + (0.451. T2) - (0.012. T3) where T is the total time on the treadmill measured in a minute.20 Patients were initially examined by a physician to determine their heights and weights (Seca 769 Scale, Germany) using standard methods (i.e., light clothes on and shoes off). Body mass index (BMI) was calculated as body weight/height2. The participants were asked to avoid alcohol, caffeine, and smoking 24 hours prior to the exercise test and not consume beta blockers (propranolol, metoprolol, atenolol, and carvedilol), calcium blockers (verapamil and diltiazem), and nitroglycerin. The exercise test termination criteria included the development of any unexpected heart abnormalities, BP of higher than 180 mm Hg (systolic) and/or 100 mm Hg (diastolic), and HRrest of higher than 100 bpm.21 Researchers were blinded to the participants’ assignments. Immediately after the exercise test, blood samples (10 ml) were taken from the antecubital vein and transferred into tubes containing ethylenediaminetetraacetic acid (EDTA) for biochemical measurements. It should be noted that to minimize the risk of unexpected complications, all exercise tests were performed under the supervision of researchers and a cardiologist.
Mean platelet volume and other blood cell count parameters were assessed using a flow cytometry (Sysmex, XE-2100L, Japan). In order to determine plasma fibrinogen level, blood samples were centrifuged (3000 rpm, 5 minutes, 22°C), and the parameter was measured using enzyme-linked immunosorbent assay (ELISA; Stago, France). Serum cholesterol (Cho), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and very low-density lipoprotein (VLDL) were also measured using standard ELISA kits with 1 mg/dL sensitivity (Bionic, Iran). Serum CRP concentration was measured using a standard specific kit, and serum troponin level was determined using an ELISA sandwich method (Mono Kit, USA).
Randomization: This study was a clinical trial in which eligible patients were assigned to the study groups through the stratified randomization method. They were first divided into 2 strata of control and intervention. Then, the subjects were selected from the strata through the block randomization method and divided into the morning and evening subgroups according to the order of the numbers and internal arrangements of the selected blocks. The block number was selected through lottery. To blind the researcher and prevent any predictions of the allocations, sample numbers and the size of the blocks were hidden.
Statistical methods: SPSS software (version 22; IBM Inc., Armonk, NY, USA) was used for statistical analysis. The normality of the data was investigated using the Shapiro-Wilk test. If the distribution of variables was normal, parametric methods were used, and if it was not normal, non-parametric methods were used. First, descriptive analysis was used to describe the variables. Quantitative variables were presented as mean ± SD. Frequency (number and percentage) was calculated and reported for qualitative variables. Then, for other study objectives, analytical analysis was performed. A 𝑃-value of ≤ 0.05 was considered as a statistically significant observation in all analyses. In order to determine any intergroup variation in the measured variables before and after HCRP sessions, the data was analyzed using paired samples Student t-test.
Moreover, we performed 2 ANCOVA tests. The first test was to compare the mean responses between the 2 control groups in the morning and evening when the values of the baseline phase of the morning were adjusted. The second test was to compare the mean responses between the 2 intervention groups in the evening in which the values of the baseline phase of the evening were adjusted using the ANCOVA test.
Results
The participants’ clinical and demographic characteristics including age, the type of, BMI, smoking, and were recorded at the time of hospitalization. Overall, 80 patients with CHDs were randomly divided into the control and intervention groups and the morning and evening subgroups (Figure 1). There were no significant differences between the groups and subgroups in the distributions of CHD types, addiction, hypertension, family histories of heart diseases or diabetes, coronary artery bypass grafting (CABG), non-ST segment elevation myocardial infarction (NSTEMI) and/or STEMI, as well as the mean values of age and BMI (Table 1).
Table 1.
Baseline characteristics of the subjects in this research (n = 20)
| Variables | Experimental-morning group | Control-morning group | P | Experimental-morning group | Control-morning group | P |
|---|---|---|---|---|---|---|
| Age (year) | 51.4 ± 7.97 | 51.1 ± 7.86 | 0.611 | 50.8 ± 8.09 | 48.1 ± 8.25 | 0.342 |
| BMI (Kg/m2) | 25.17 ± 1.68 | 25.23 ± 1.79 | 0.296 | 23.66 ± 1.58 | 24.6 ± 1.20 | 0.118 |
| Smoking | 2 (10) | 2 (10) | 0.653 | 1 (10) | - | 0.156 |
| Hypertension | 3 (15) | 4 (20) | 0.371 | 3 (20) | 2 (10) | 0.204 |
| Family history of heart disease | 3 (15) | 3 (15) | 0.211 | 3 (30) | 2 (10) | 0.131 |
| Diabetes mellitus | 1 (5) | 1 (5) | 0.756 | 2 (20) | 2 (10) | 0.698 |
| CABG | 3 (15) | 2 (10) | 0.149 | 2 (20) | 2 (10) | 0.254 |
| NSTEMI | 1 (5) | 2 (10) | 0.134 | 2 (20) | 2 (10) | 0.372 |
| STEMI | 2 (10) | 1 (5) | 0.347 | 2 (20) | 2 (10) | 0.251 |
Values are expressed as mean ± SD, and number and percentage. BMI: body mass index; CABG: coronary artery bypass grafting; NSTEMI: non-ST segment elevation myocardial infarction; STEMIZST: segment elevation myocardial infarction
Changes in Cardiovascular Risk Factors and Metabolic Parameters after Exercise Test: The effects of the HCRP on the subjects’ cardiovascular performance have been presented in table 2. In initial evaluation, there were no statistically significant differences between the groups regarding any of the studied variables.
Table 2.
Comparison of Metabolic Markers, Aerobic Capacity and Risk Factors Before and After the Home-Based Cardiac Rehabilitation Program Performed in either the Morning or Evening by Patients with Myocardial Infraction
| Metabolic markers & risk factors | Time | Control groups |
Experimental groups |
Pc | Pd | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | After 8-week CR | Pa | Baseline | After 8-week HCRP | Pb | ||||
| Cho (mg/dl) | Morning | 177.6±44.44 | 185.6±41.42 | 0.07 | 177.6±44.36 | 173.25±50.11 | 0.47 | 0.65 | < 0.001*** |
| Evening | 190.40±63.19 | 188.10±36.50 | 0.83 | 203.2±54.36 | 145.75±26.68 | < 0.001*** | |||
| TG (mg/dl) | Morning | 192.20 ± 64.20 | 213.15±96.86 | 0.19 | 203.55±81.93 | 167.35±49.58 | 0.05* | 0.60 | 0.002** |
| Evening | 184.30±78.78 | 197.95±70.96 | 0.87 | 209.75±75.28 | 135.55±36.15 | < 0.001*** | |||
| HDL (mg/dl) | Morning | 37.8±7.35 | 39.65±8.18 | 0.33 | 35.65±6.06 | 39.10±4.55 | 0.05* | 0.57 | < 0.001*** |
| Evening | 38±8.32 | 40.5±9.39 | 0.08 | 35.35±6.34 | 43.30±7.37 | < 0.001*** | |||
| LDL (mg/dl) | Morning | 104.30±51.04 | 108.90±50.75 | 0.16 | 101.40±38.47 | 108.05±35.95 | 0.17 | 0.55 | < 0.001*** |
| Evening | 113.05±47.17 | 109.05±31.78 | 0.57 | 110.05±45.05 | 89.60±21.59 | < 0.001*** | |||
| VO2max (ml/kg/ml) | Morning | 34.67±7.96 | 33.19±9.22 | 0.08 | 33.01±7.23 | 36.04±8.77 | 0.05* | 0.52 | < 0.001*** |
| Evening | 31.80±9.48 | 32.76±9.49 | 0.21 | 28.67±7.21 | 38.96±7.42 | < 0.001*** | |||
| CRP (mg/dl) | Morning | 0.40±0.50 | 0.40±0.59 | > 0.999 | 0.35 ±0.58 | 0.50 ±0.60 | 0.37 | 0.77 | 0.001** |
| Evening | 0.35±0.48 | 0.30±0.47 | 0.66 | 0.35±0.48 | 0.15 ± 0.36 | 0.04* | |||
| MPV (fl) | Morning | 9.46±1.18 | 9.73±1.51 | 0.26 | 10.20±1.08 | 9.40±0.98 | < 0.001*** | 0.17 | < 0.001*** |
| Evening | 9.19±0.92 | 9.35±1.11 | 0.57 | 10.05±1.11 | 8.76±0.78 | < 0.001*** | |||
| cTnI (Ng/ml) | Morning | 0.10±0.00 | 0.10±0.00 | > 0.999 | 0.10±0.00 | 0.10±0.00 | > 0.999 | > 0.999 | > 0.999 |
| Evening | 0.10±.00 | 0.10±0.00 | > 0.999 | 0.10±0.00 | 0.10±0.00 | > 0.999 | |||
Mean ± standard deviation (SD) was reported
Cho: Cholesterol; TG: Triglyceride; HDL: high-density lipoprotein; LDL: low density lipoprotein; VO2max: Maximum oxygen consumption; CRP: C-reactive protein; MPV: Mean platelet volume; cTnI: Troponin I; HCRP: home-based cardiac rehabilitation program
*Significant difference: P ≤ 0.05. *P < 0.05, **P < 0.01, ***P < 0.001; Pa = P Value for comparison of control groups, and Pb = P Value for comparison of experimental groups using paired t-test between before and after the intervention; Pc = P Value for comparison of morning and evening control groups after 8 weeks of CR, and Pd = P Value for comparison of morning and evening experimental groups after 8 weeks HCRP using ANCOVA with baseline variable adjusted
In intragroup comparisons, paired t-test results showed significant differences in several cardiometabolic parameters including TG (P < 0.05), HDL (p < 0.05), and VO2max (P < 0.05), as well as cardiovascular risk factor MPV (P < 0.001) in the morning experimental group before and after the 8-week HCRP.
In intragroup comparisons, paired t-test results showed significant differences in several cardiometabolic parameters including Cho (P < 0.001), TG (p < 0.002), HDL (P < 0.001), LDL (P < 0.001), and VO2max (P < 0.001), and cardiovascular risk factors including CRP (P = 0.01) and MPV (P < 0.001) in the evening experimental group before and after the 8-week HCRP.
In intergroup comparisons, ANCOVA results showed significant differences in several cardiometabolic parameters including Cho (P < 0.001), TG (p < 0.001), HDL (P < 0.001), LDL (P < 0.001), and VO2max (P < 0.001), and cardiovascular risk factors including CRP (p < 0.01) and MPV (P < 0.001) between the morning and evening experimental groups after 8 weeks of HCRP. The comparison of the mentioned parameters between the experimental groups showed that the time of the rehabilitation program had a significant effect on some parameters in the morning and evening in the experimental groups, also in comparison with each other (P < 0.05). However, no significant differences were observed in the mentioned cardiometabolic parameters between the control group's morning and evening following the 8-week HCRP (P ≥ 0.05).
Discussion
In the present study, we investigated the impact of the time of performing HCRP on changes in cardiometabolic risk factors in phase-IV MI patients. To the best of our knowledge, this study is the first comprehensive assessment of the role of timing (i.e., morning or evening) of supervised cardiac rehabilitation programs in Iranian MI patients.
Cardiac rehabilitation programs are known to improve cardiometabolic risk factors including blood lipids and aerobic capacity in patients with CVDs. The American Heart Association recommends the incorporation of cardiac rehabilitation programs in sport activities to improve exercise capacity in patients with CVDs.22
The role of regular exercises in the prevention of AMI can be related to the mitigating effects of these activities on numerous cardiac risk factors such as TG, LDL-C, and HDL-C.23 Furthermore, morning exercises have been shown to improve hemostatic function, inflammatory status, and oxidative stress, ultimately leading to the improvement of VO2max, cardiovascular parameters, and aerobic capacity.17,24 Investigators have suggested that hormonal responses may be influenced by the time, as well as the intensity, and duration of exercise.25
In a previous research, evidence has been found supporting the clinical significance of circadian variations in the onset of unstable angina, MI, and SCD.15 The incidence of unstable angina26 and MI27 peaks from 6 a.m. to noon. The Framingham Heart Study also showed that a definite or possible occurrence of SCD (with a peak incidence between 7 and 9 a.m.) preceded by pronounced variations in circadian level. Furthermore, MI occurs in about one-third of all SCD cases.26 It is unclear whether or not the effects of morning exercises are different from the physical activities performed at other times of the day. Murray et al.28 assigned patients with established CAD to either morning (07:30) or afternoon (15:00) training groups to study the effects of long-term submaximal exercises; nevertheless, they found no statistically significant differences in outcomes between the 2 exercise times. Zhao et al. reported that evening exercises had significantly greater protective effects than morning activities against CVDs.29 Similar to our study, Lian et al.30 observed greater improvements in lipid and inflammatory markers of patients with CADs who walked in the evening than the morning.
In the present study, the HCRP was performed both in the morning (8:00-9:00 a.m.) and evening (17:00-18:00 p.m.). Our findings showed that phase IV (maintenance) MI patients who performed the HCRP in the evening were at a lower risk of developing AMI compared with those who exercised in the morning. These effects were consistent with the results of other studies.31
The direct mechanisms underlying the observed preventive effects of evening exercises against AMI are unclear. Previous studies showed that the response of ambulatory blood pressure to everyday physical activity was the highest at 8 to 10 a.m.32 Furthermore, a greater shear stress was evident after morning exercises.33 While the tone of the large coronary artery is higher, its diameter is smaller in the early morning compared with the afternoon and evening.34 Flow-mediated vasodilation in patients with variant angina has been shown to deteriorate by early morning (6 a.m.) and improve by afternoon (2 p.m.) and evening (8 p.m.).35
In comparison with morning physical activities, evening exercises can result in higher improvements in fibrinogen, LDL, HDL, cholesterol, and high-sensitivity reactive protein (hsCRP) levels. Performing cardiac rehabilitation programs in the evening can prevent potential adverse changes in risk factors such as morning hypertension, platelet aggregation, fibrinogen level, MPV, morning hypercoagulability tendency, as well as plaque formation, vascular dysfunction, thrombosis, and intra-arterial pressure. Therefore, this can reduce the risk of AMI, SCD, acute coronary syndrome, and ventricular arrhythmias.
Conclusion
The results showed that the implementation of HCRP had significant beneficial effects in both morning and evening intervention groups. Moreover, the results of this study showed that performing an 8-week HCRP in the evening compared to the morning had significantly better effects on the improvement of cardiometabolic risk factors in patients with MI. Therefore, it is suggested that cardiac rehabilitation programs be performed for these patients in evening shifts.
Limitations: Two potential limitations of our study must be considered when interpreting the results. First, we did not obtain detailed information on the use of the medications predisposing to the risk of AMI; therefore, we cannot rule out the possibility of such medications interfering with the association between exercise activities and AMI occurrence. Second, as all of our patients were Iranians, the results should be extrapolated to other populations with caution.
Acknowledgments
The authors would like to express their gratitude to the staff of the Specialized Heart Health Center at the Cardiovascular Research Center of Shahid Rahimi Hospital of Khoramabad, the Clinical Laboratory of Dr. Adeli, and all the patients who did their best to cooperate with the researchers.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
Authors’ Contribution
MD: The conception and design of the study
MN: Acquisition of data
MRK: Acquisition of data
BBA: Drafting the article
YM: Drafting the article
PN: Analysis and interpretation of data
MD: Data collection
MC: Final approval of the version to be submitted
REFERENCES
- 1.Khoshbin M, Ahmadi SAY, Cheraghi M, Nouryazdan N, Birjandi M, Shahsavari G. Association of E-Selectin gene polymorphisms and serum E-Selectin level with risk of coronary artery disease in lur population of Iran. Arch Physiol Biochem. 2020:1–6. doi: 10.1080/13813455.2020.1828481. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Sharif F, Shoul A, Janati M, Kojuri J, Zare N. The effect of cardiac rehabilitation on anxiety and depression in patients undergoing cardiac bypass graft surgery in Iran. BMC Cardiovasc Disord. 2012;12:40. doi: 10.1186/1471-2261-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavie CJ, Ozemek C, Carbone S, Katzmarzyk PT, Blair SN. Sedentary behavior, exercise, and cardiovascular health. Circ Res. 2019;124(5):799–815. doi: 10.1161/CIRCRESAHA.118.312669. [DOI] [PubMed] [Google Scholar]
- 4.Verwijmeren L, Noordzij PG, Daeter EJ, van Zaane B, Peelen LM, van Dongen EPA. Preoperative determinants of quality of life a year after coronary artery bypass grafting: A historical cohort study. J Cardiothorac Surg. 2018;13(1):118. doi: 10.1186/s13019-018-0798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goel K, Pack QR, Lahr B, Greason KL, Lopez-Jimenez F, Squires RW, et al. Cardiac rehabilitation is associated with reduced long-term mortality in patients undergoing combined heart valve and CABG surgery. Eur J Prev Cardiol. 2015;22(2):159–68. doi: 10.1177/2047487313512219. [DOI] [PubMed] [Google Scholar]
- 6.Umeda IIK . Manual of physical therapy in the cardiovascular rehabilitation. Sao Paulo, Brazil: Manole; In Portuguese. [Google Scholar]
- 7.Carvalho T, Cortez A, Ferraz A, Nobrega A, Brunetto A, Herdy A, et al. Cardiopulmonary and metabolic rehabilitation: Practical aspects and responsibilities. Rev Bras Med Esporte. 2005;11(6):313–8. [Google Scholar]
- 8.Braunwald E, Castellanos A, Sdrof P, Craige E. Heart disease. 7th. Philadelphia, PA: Saunders; 2004. p. 114. [Google Scholar]
- 9.Beatty AL, Fukuoka Y, Whooley MA. Using mobile technology for cardiac rehabilitation: a review and framework for development and evaluation. J Am Heart Assoc. 2013;2(6):e000568. doi: 10.1161/JAHA.113.000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munro J, Angus N, Leslie SJ. Patient focused Internet-based approaches to cardiovascular rehabilitation--a systematic review. J Telemed Telecare. 2013;19(6):347–53. doi: 10.1177/1357633X13501763. [DOI] [PubMed] [Google Scholar]
- 11.Anderson L, Thompson DR, Oldridge N, Zwisler AD, Rees K, Martin N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2016;(1):CD001800. doi: 10.1002/14651858.CD001800.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baharvand-Ahmadi B, Sharifi K, Namdari M. Prevalence of non-alcoholic fatty liver disease in patients with coronary artery disease. ARYA Atheroscler. 2016;12(4):201–5. [PMC free article] [PubMed] [Google Scholar]
- 13.Rathore V, Singh N, Rastogi P, Mahat RK, Mishra MK, Shrivastava R. Lipid profile and its correlation with C-reactive protein in patients of acute myocardial infarction. Int J Res Med Sci. 2017;5(5):2182–6. [Google Scholar]
- 14.Aldemir H, Kilic N. The effect of time of day and exercise on platelet functions and platelet-neutrophil aggregates in healthy male subjects. Mol Cell Biochem. 2005;280(1-2):119–24. doi: 10.1007/s11010-005-8238-8. [DOI] [PubMed] [Google Scholar]
- 15.Zhao S, Zhang Z, Long Q, Ma Y, Lian X, Yang Y, et al. Association between time of day of sports-related physical activity and the onset of acute myocardial infarction in a Chinese population. PLoS One. 2016;11(1):e0146472. doi: 10.1371/journal.pone.0146472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Criteria Committee of the New York Heart Association. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. Boston, MA: Little, Brown; 1994. pp. 253–256. [Google Scholar]
- 17.Dehghani M, Cheraghi M, Namdari M, Dabidi Roshan. Effects of phase IV pedometer feedback home-based cardiac rehabilitation on cardiovascular functional capacity in patients with myocardial infarction: A randomized controlled trial. Int J Basic Sci Med. 2019;4(2):75–80. [Google Scholar]
- 18. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 19.Dimeo F, Pagonas N, Seibert F, Arndt R, Zidek W, Westhoff TH. Aerobic exercise reduces blood pressure in resistant hypertension. Hypertension. 2012;60(3):653–8. doi: 10.1161/HYPERTENSIONAHA.112.197780. [DOI] [PubMed] [Google Scholar]
- 20.Gonulates S. Analysis of difference between the VO 2 max values in field and laboratory tests. Univers J Educ Res. 2018;6(9):1938–41. [Google Scholar]
- 21.British Association. The BACPR standards and core components for cardiovascular disease prevention and rehabilitation. 2nd. London, UK: BACPR; 2012. [DOI] [PubMed] [Google Scholar]
- 22.Valkeinen H, Aaltonen S, Kujala UM. Effects of exercise training on oxygen uptake in coronary heart disease: A systematic review and meta-analysis. Scand J Med Sci Sports. 2010;20(4):545–55. doi: 10.1111/j.1600-0838.2010.01133.x. [DOI] [PubMed] [Google Scholar]
- 23.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347(19):1483–92. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 24.Dehghani M, Cheragi M, Namdari M, Roshan VD, Dehghani M. The effectiveness of home-based cardiac rehabilitation program on cardiovascular stress indices in men and women with myocardial infarction: A randomised controlled clinical trial. Rev Colomb Cardiol. 2021;28(2):128–35. [Google Scholar]
- 25.Seo DY, Lee S, Kim N, Ko KS, Rhee BD, Park BJ, et al. Morning and evening exercise. Integr Med Res. 2013;2(4):139–44. doi: 10.1016/j.imr.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeda N, Maemura K. Circadian clock and the onset of cardiovascular events. Hypertens Res. 2016;39(6):383–90. doi: 10.1038/hr.2016.9. [DOI] [PubMed] [Google Scholar]
- 27.Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313(21):1315–22. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 28.Murray PM, Herrington DM, Pettus CW, Miller HS, Cantwell JD, Little WC. Should patients with heart disease exercise in the morning or afternoon? Arch Intern Med. 1993;153(7):833–6. [PubMed] [Google Scholar]
- 29.Zhao H, Chu XQ, Lian XQ, Wang ZM, Gao W, Wang LS. Relationship between time of day physical exercise and the reduced risk of coronary artery disease in a Chinese population. Int J Sport Nutr Exerc Metab. 2014;24(2):139–47. doi: 10.1123/ijsnem.2012-0226. [DOI] [PubMed] [Google Scholar]
- 30.Lian XQ, Zhao D, Zhu M, Wang ZM, Gao W, Zhao H, et al. The influence of regular walking at different times of day on blood lipids and inflammatory markers in sedentary patients with coronary artery disease. Prev Med. 2014;58:64–9. doi: 10.1016/j.ypmed.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Noites A, Freitas CP, Pinto J, Melo C, Vieira A, Albuquerque A, et al. Effects of a phase IV home-based cardiac rehabilitation program on cardiorespiratory fitness and physical activity. Heart Lung Circ. 2017;26(5):455–62. doi: 10.1016/j.hlc.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Jones H, Pritchard C, George K, Edwards B, Atkinson G. The acute post-exercise response of blood pressure varies with time of day. Eur J Appl Physiol. 2008;104(3):481–9. doi: 10.1007/s00421-008-0797-4. [DOI] [PubMed] [Google Scholar]
- 33.Jones H, Green DJ, George KP, Black MA, Atkinson G. Evidence for a greater elevation in vascular shear stress after morning exercise. Med Sci Sports Exerc. 2009;41(6):1188–93. doi: 10.1249/MSS.0b013e318195109c. [DOI] [PubMed] [Google Scholar]
- 34.Hung MJ, Hu P, Hung MY. Coronary artery spasm: Review and update. Int J Med Sci. 2014;11(11):1161–71. doi: 10.7150/ijms.9623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawano H, Motoyama T, Yasue H, Hirai N, Waly HM, Kugiyama K, et al. Endothelial function fluctuates with diurnal variation in the frequency of ischemic episodes in patients with variant angina. J Am Coll Cardiol. 2002;40(2):266–70. doi: 10.1016/s0735-1097(02)01956-3. [DOI] [PubMed] [Google Scholar]