Abstract
BACKGROUND
Obesity is associated with several metabolic disorders and myocardial apoptosis. The aim of this study was to determine the effect of high-intensity interval training (HIIT) and coenzyme Q10 (CoQ10) supplementation on myocardial apoptotic proteins in obese male rats.
METHODS
Forty-eight male Wistar rats were randomly assigned to 6 groups including non-obese control (NOC), baseline obese control (BOC), HIIT, CoQ10, HIIT + CoQ10, and control. NOC and BOC groups were sacrificed at the beginning of the study. Exercise groups were subjected to a HIIT program over 12 weeks. Rats in CoQ10 group were gavage-fed with 500 mg/kg-1 CoQ10 supplement throughout the study. The heart muscle was removed 48 hours after the last training session. The B-cell lymphoma-2 (Bcl-2), Bcl-2-associated X protein (Bax), cytochrome c, BH3-interacting domain death agonist (Bid), caspase-8, and caspase-3 protein expressions were analyzed using western blotting. Data were analyzed using independent t-test and two-way analysis of variance (ANOVA).
RESULTS
The Bax, Bid, cytochrome c, caspase-8, caspase-3 proteins, and Bax/Bcl-2 were significantly higher in BOC group compared to NOC (P = 0.025, P = 0.0001, P = 0.013, P = 0.017, P = 0.010, P = 0.180, respectively). Moreover, Bcl-2 protein was lower in BOC compared to NOC group (P = 0.025). HIIT program decreased Bax/Bcl-2 ratio (P = 0.012), Bid (P = 0.0001), cytochrome c (P = 0.003), caspase-8 (P = 0.006), and caspase-3 (P = 0.002) proteins and increased Bcl-2 (P = 0.001) proteins in heart muscle. CoQ10 supplementation significantly increased Bcl-2 protein content (P = 0.001).
CONCLUSION
HIIT exerts an anti-apoptotic effect in heart muscle of obese rats. Although the administration of CoQ10 increased Bcl-2 anti-apoptotic protein, it did not show a potent synergistic effect along with HIIT to reduce obesity-related myocardial apoptosis.
Keywords: High-Intensity Interval Training, Coenzyme Q10, Myocardium, Apoptosis, Obesity
Introduction
Obesity is the major cause of several metabolic disorders including hyperlipidemia, hyperglycemia, and insulin resistance.1 It is associated with structural and functional changes in adipose tissue, enhanced oxidative stress, and elevated release of various cytokines that are contributed in chronic inflammation. Review of the literature indicates that obesity-related oxidative stress and inflammation up-regulates pro-apoptotic proteins and down-regulates anti-apoptotic proteins in all tissues such as myocardium.2 In addition, myocardium apoptosis has been recently associated with overload of unsaturated fatty acid (FA).3 The consumption of high-calorie diet and obesity are associated with elevated circulating levels of inflammatory factors such as tumor necrosis factor alpha (TNF-α),3 which is a potential stimulator of extrinsic apoptotic pathway.
The consumption of high-calorie diet and obesity are associated with elevated circulating levels of inflammatory factors such as tumor necrosis factor alpha (TNF-α),3 which is a potential stimulator of extrinsic apoptotic pathway. In addition, the expression of apoptosis-related genes including B-cell lymphoma-2 (Bcl-2), Bcl-2-associated X protein (Bax), Fas ligand (FasL), and Fas-associated death domain (FADD) has been illustrated to be altered by obesity.4 Heo et al., for instance, indicated that cytochrome c protein level and Bax gene expression were increased in obese mice, while Bcl-2 gene expression was decreased.4 Overall, previous studies conclude that obesity, consequent to consumption of high-calorie diet, stimulates a number of proteins involved in both extrinsic and intrinsic pathways of apoptosis.5 Hence, any intervention to alter the regulation of apoptosis at molecular level to ameliorated myocardium apoptosis may be of high implication to obese patients.
Apoptosis is a complex process of programmed cell death that can lead to serious disorders in the heart muscle.6 Although scarce in health status (approximately 0.0001%-0.001%), apoptosis in cardiomyocytes can increase by 0.12%-0.70% in heart failure (HF). Approximately, a 0.1% apoptosis can lead to a 37% loss in cardiomyocyte within a year.6 It can be triggered by both intrinsic and extrinsic pathways in cardiomyocyte that is regulated by various signaling pathways. It is established that the two pathways are linked and activation of one can trigger the other pathway and eventually, augment myocardial apoptosis. Some critical proteins involved in the procession of apoptosis are caspases and Bcl-2 family.7 For instance, caspase-8 is the initiator caspase in extrinsic pathway that is activated by death receptor. Caspase-3 is also the most important executioner that can be activated by initiator caspases such as caspase-8.7 Bcl-2 family includes several pro and anti-apoptotic genes that are involved in intrinsic pathway of apoptosis. For example, increased expression of Bcl-2 and decreased expression of Bax were associated with reduced myocardial apoptosis. Thus, any intervention to activate anti-apoptotic proteins and inhibit pro-apoptotic proteins may have the potential to inhibit apoptosis during obesity.
Physical activity and dietary interventions are the major factors to prevent the progression of several diseases and mortality.8 Exercise training has an anti-inflammatory effect that inhibits the production of TNF-α, a potential stimulus of extrinsic pathway of apoptosis.9 Besides, exercise training seems to modulate the balance between anti-apoptotic and pro-apoptotic proteins, so that Cechella et al. reported that exercise training decreased cleaved caspase-3/caspase-3 ratio and increased Bcl-2 expression.10 Literature review indicates that the adaptive response to exercise training is a complex process which depends on exercise mode, intensity, and duration.11 Aerobic exercise training is the most common type of exercise implemented in the literature. High-intensity interval training (HIIT) has recently received attention, because it is a time-efficient mode of exercise that brings about several benefits. Recent studies indicate that HIIT can exhibit cardioprotective effects, as it has been associated with reduced cardiac apoptosis.12 Besides, coenzyme Q10 (CoQ10) is an electron transporter in mitochondrial respiratory chain that has free-radical scavenging properties. CoQ10 has been indicated to prevent apoptosis induced by oxidative stress. Moreover, CoQ10 prevents cell death induced by mitochondrial permeability transition13 and inhibiting mitochondrial depolarization.14 Although some in vitro studies report positive effects of CoQ10 on cell death, its effect on proteins involved in myocardial apoptosis remains to be understood.
Overall, HIIT has been reported to exert cardioprotective effects; yet, little is known about its effects on myocardial apoptosis. Besides, it cannot be concluded from the literature that whether combination of HIIT and CoQ10 administration can exert synergistic effect to inhibit myocardial apoptosis during obesity. Thus, we investigated the effect of HIIT along with CoQ10 administration on myocardial apoptosis in obese male Wistar rats.
Materials and Methods
Animals: In an experimental study, 48 male Wistar rats (age: 21 days, mean weight: 40 ± 5 g) were purchased from the Pasteur Institute Animal Care Center (Karaj, Iran). Animals were maintained in an ambient temperature of 22 ± 2 °C, humidity of 50% ± 5%, and under 12-hour light-dark cycle. Animals were provided with standard chow and water ad libitum. The study was conducted according to the codes of Declaration of Helsinki and was approved by the Ethics Committee of Medical Sciences of the Islamic Azad University, Tabriz Branch, Tabriz, Iran (IR.IAU.TABRIZ.REC.1398. 806).
Diet and obesity induction: After week-long acclimatization, eight rats were randomly separated as the non-obese control (NOC) group (n = 8) and 42 rats were fed with high-calorie diet over 6 weeks. Over this period, rats were provided with high-fat chocolate milk (960 kcal/l, 14% protein, 20% fat, and 68% carbohydrates) ad libitum.15 The consumption of chocolate milk and weight changes were recorded every week. The obesity was confirmed by Lee index [(weight-3/height) × 1000] assessed by final weight and height (nasoanal length).16 Following this, eight rats were randomly separated as baseline obese control (BOC) group (n = 8). These two control groups were sacrificed at age of 11 weeks before the interventions to evaluate the effect of diet-induced obesity on myocardial apoptosis.
HIIT: Following the period of obesity induction, 32 animals were adapted to exercise protocol on a motorized rodent treadmill (instrument) for a week (incline: 0%, speed 10-25 m/min, duration: 5-10 minutes). Then, rats were subjected to an exhaustive exercise test to determine maximum running speed. For this, exercise was initiated at 10 m/min-1 with a gradual increase of 3 m/min-1 every 2 minutes until failure. Following this, rats were randomized to weight-matched groups of HIIT (n = 8), CoQ10 (n = 8), HIIT + CoQ10 (n = 10), and control (n = 8). HIIT groups were subjected to a HIIT program performed 5 sessions per week over 12 weeks (Table 1). HIIT program consisted of 2-minute running at 85%-90% of maximum speed followed by 2-minute running at 45%-50% of maximum speed at 15% incline with 5-16 repetitions.17 Each exercise session included a brief warm-up and cool-down.
Table 1.
Description of the high-intensity interval training (HIIT) protocol in the study (5 sessions per week)
| Week of training | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High-intensity interval (85%-90% speed max) | Speed (m/min) | 26 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 |
| Time (minute) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| Low-intensity interval (45%-50% speed max) | Speed (m/min) | 10 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Time (minute) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| No. of repetitions | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
| Treadmill grade (%) | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | |
| Total workout time (min/day) | 20 | 24 | 28 | 32 | 36 | 40 | 44 | 48 | 52 | 56 | 60 | 64 |
Supplementation: Rats in CoQ10 group were gavage-fed with 500 mg/kg-1 CoQ10 supplements (Golden Life, Iran) before and following each exercise session (~ 150 mg/day). The other groups received equal volume of normal saline.
Tissue preparation: Rats were deeply anesthetized by intraperitoneal (IP) injection of ketamine (90 mg/kg-1) and xylazine (10 mg/kg-1). They were then dissected and heart tissues were extracted and transferred to cryotubes to be stored in liquid nitrogen under -80 °C for later analysis.
Western blotting: This technique was applied in order to evaluate the expression of Bax, Bcl-2, BH3-interacting domain death agonist (Bid), cytochrome c, caspase-8, and caspase-3 proteins in myocardial tissue. Protein lysates were isolated using lysis buffer supplemented with complete protease inhibitor cocktail and centrifuged at 12000 ×g for 15 minutes at 4 ºC. The protein concentration of the supernatant was determined by the Bradford method. Proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 8%-12% denatured ready gel and transferred onto a polyvinylidene difluoride (PVDF) membrane (Sigma Aldrich, USA). The membrane was blocked for 1 hour in 5% bovine serum albumin (BSA) in tris-buffered saline with 0.1% Tween 20 (TBST) to block nonspecific bindings. Subsequently, blots were incubated overnight at 4 ºC with primary antibodies: beta-actin (β-actin) (sc-47778), Bax (sc-7480), Bcl-2 (sc-492), Bid (ab10640), cytochrome c (sc-13156), caspase-8 (ab138485), caspase-3 (sc-7272) [purchased from Santa Cruz Biotechnology, CA, USA (1:500)], and acetyl-p53 [Lys382, purchased from Cell Signaling Technology Inc., MA, USA, (1:500)]. The membrane was then washed three times and incubated with the appropriate secondary antibody for 1 hour at room temperature in 5% milk in TBST. Protein bands were visualized with an enhanced chemiluminescence (ECL) reagent and radiographic film (Fujifilm, Tokyo, Japan) quantified by densitometry analysis with ImageJ software (National Institute of Health, Bethesda, Maryland, USA). Then, the density of the target protein bands was normalized against β-actin control loading.18 Finally, the results were presented as relative density (compared to the NOC group).
Statistical analysis: Results were presented as mean ± standard deviation (SD). Normal distribution of data was confirmed by Shapiro-Wilk test. Data were analyzed by independent samples t-test and two-way analysis of variance (ANOVA).
All statistical analyses were considered significant if P < 0.05. Statistical analysis was conducted by SPSS software (version 19, SPSS Inc., Chicago, IL, USA).
Results
Body weight, Lee index, heart weight, and ratio of heart weight to total body weight: There was a difference between NOC and BOC that body weight and Lee index increased following induction of obesity (P = 0.001 and P = 0.0001, respectively). Mean values of heart weight were higher in BOC group compared with NOC group (P = 0.34). However, the ratio of heart weight to total body weight was lower in BOC group compared with NOC group (P = 0.02). Total body weight and Lee index decreased following HIIT program (P = 0.001, η2 = 0.49 and P = 0.004, η2 = 0.37, respectively) and ratio of heart weight to total body weight increased following HIIT (P = 0.024, η2 = 0.28) (Table 2). η2 > 0.25 for these variables indicates a large main effect of HIIT on total body weight, Lee index, and heart weight/total weight ratio.19
Table 2.
Description of animals in different groups
| Variable | Group |
||||||
|---|---|---|---|---|---|---|---|
| NOC | BOC | Control | HIIT | CoQ10 | HIIT + CoQ10 | ||
| Age (week) | Initial | 3 | 3 | 3 | 3 | 3 | 3 |
| After obesity induction | 11 | 11 | 11 | 11 | 11 | 11 | |
| After intervention | - | - | 24 | 24 | 24 | 24 | |
| Weight (g) | Initial | 40.00 ± 5.00 | 40.00 ± 5.00 | 40.00 ± 5.00 | 40.00 ± 5.00 | 40.00 ± 5.00 | 40.00 ± 5.00 |
| After obesity induction | 158.60 ± 22.20 | 302.40 ± 15.30* | 294.43 ± 11.67 | 300.22 ± 10.42 | 298.89 ± 12.57 | 301.12 ± 9.94 | |
| After intervention | - | - | 326.10 ± 18.30 | 304.20 ± 17.50 | 331.10 ± 22.10 | 307.30 ± 19.80 | |
| Lee index | After obesity induction | 284.20 ± 35.20 | 352.00 ± 29.70* | 349.47 ± 33.17 | 343.07 ± 36.45 | 351.57 ± 28.88 | 343.59 ± 35.20 |
| After intervention | - | - | 312.70 ± 27.60 | 304.00 ± 21.10 | 314.30 ± 30.70 | 306.30 ± 24.50 | |
| Heart weight (g) | After obesity induction | 0.61 ± 0.07 | 0.84 ± 0.06 | - | - | - | - |
| After intervention | - | - | 0.99 ± 0.11 | 1.02 ± 0.20 | 1.01 ± 0.12 | 1.10 ± 0.40 | |
| Heart/body weight (g/kg-1) | After obesity induction | 3.86 ± 0.35 | 2.91 ± 0.14* | - | - | - | - |
| After intervention | - | - | 3.03 ± 0.26 | 3.50 ± 0.21 | 3.05 ± 0.13 | 3.58 ± 0.43 | |
| Food intake (g/day-1) | During obesity induction | 19.00 ± 2.00 | 18.50 ± 1.00 | 18.00 ± 2.00 | 18.50 ± 2.00 | 18.00 ± 2.00 | 18.00 ± 1.00 |
| During intervention | - | - | 23.10 ± 5.00 | 22.30 ± 4.00 | 23.10 ± 5.00 | 22.60 ± 3.00 | |
| Chocolate milk intake (ml/day-1) | During obesity induction | - | 18.00 ± 4.50 | ||||
Data are shown as mean ± standard deviation (SD)
Independent t-test; significant difference with non-obese control (NOC), P < 0.05
NOC: Non-obese control; BOC: Baseline obese control; HIIT: High-intensity interval training; CoQ10: Coenzyme Q10
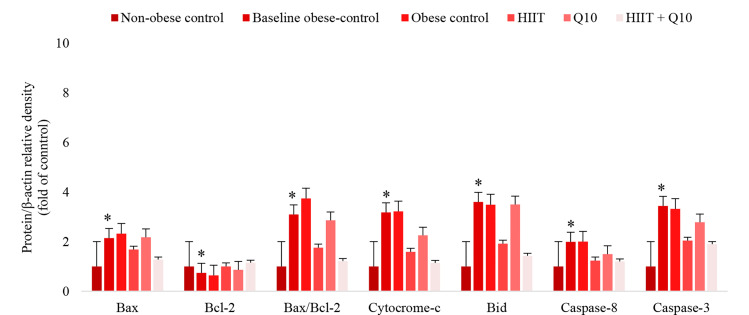
Myocardial apoptotic proteins: Data analysis indicated that protein content of Bax, Bid, cytochrome c, caspase-8, and caspase-3 was higher in BOC compared with NOC group (P = 0.025, P = 0.0001, P = 0.013, P = 0.017, and P = 0.01, respectively) (Figure 1). The ratio of Bax to Bcl-2 was also higher in BOC compared to NOC group (P = 0.018). Yet, Bcl-2 content was higher in BOC compared with NOC group (P = 0.018) (Figure 1).
Figure 1.
Mean ± standard deviation (SD) for myocardial apotosis variables after intervention in experimental groups *Independent t-test; significant difference with non-obese control (NOC), P < 0.05 HIIT: High-intensity interval training; Bcl-2: B-cell lymphoma-2; Bax: Bcl-2-associated X protein; Bid: BH3-interacting domain death agonist
Two-way ANOVA indicated that HIIT and CoQ10 supplementation increased Bcl-2 protein levels compared to control group (P = 0.001, η2 = 0.34 and P = 0.031, η2 = 0.15, respectively), but no interaction (HIIT × CoQ10) was observed (P = 0.64, η2 = 0.008). η2 > 0.25 indicates a large main effect of HIIT on Bcl-2 protein; yet, the effect size of HIIT and CoQ10 interaction was small.19 HIIT program and CoQ10 supplementation had no significant effect on myocardial Bax protein level in obese Wistar rats (P = 0.10, η2 = 0.093 and P = 0.77, η2 = 0.003). Data indicate a medium effect size for HIIT and a small effect size for CoQ10 supplementation. Moreover, HIIT program decreased protein levels of myocardial Bid, cytochrome c, caspase-8, caspase-3, and Bax to Bcl-2 ratio compared with control group (P = 0.0001, η2 = 0.47; P = 0.003, η2 = 0.27; P = 0.006, η2 = 0.23; P = 0.02, η2 = 0.15; and P = 0.012, η2 = 0.2, respectively). η2 > 0.25 indicates a large main effect of HIIT on Bid, cytochrome c, caspase-8, caspase-3, and Bax to Bcl-2 ratio.19 However, the effect of CoQ10 and HIIT interaction (HIIT × CoQ10) for these variables did not reach significance level (all Ps > 0.05) (Table 3, Figure 1).
Table 3.
| F | P* | Eta squared | ||
|---|---|---|---|---|
| Bax protein/β-actin (fold of control) | HIIT | 2.190 | 0.1000 | 0.093 |
| CoQ10 | 0.680 | 0.7700 | 0.003 | |
| HIIT × CoQ10 | 2.640 | 0.2400 | 0.180 | |
| Bcl-2 protein/β-actin (fold of control) | HIIT | 9.620 | 0.0010 | 0.340 |
| CoQ10 | 4.490 | 0.0310 | 0.150 | |
| HIIT × CoQ10 | 1.050 | 0.6400 | 0.008 | |
| Bax/Bcl-2 | HIIT | 3.910 | 0.0120 | 0.200 |
| CoQ10 | 1.350 | 0.4300 | 0.032 | |
| HIIT × CoQ10 | 0.820 | 0.7400 | 0.003 | |
| Cytochrome c protein/β-actin (fold of control) | HIIT | 5.090 | 0.0030 | 0.270 |
| CoQ10 | 1.860 | 0.1300 | 0.160 | |
| HIIT × CoQ10 | 0.930 | 0.3700 | 0.004 | |
| Bid protein/β-actin (fold of control) | HIIT | 11.420 | 0.0001 | 0.470 |
| CoQ10 | 0.750 | 0.3900 | 0.043 | |
| HIIT × CoQ10 | 0.007 | 0.9300 | 0.001 | |
| Caspase-8 protein/β-actin (fold of control) | HIIT | 12.740 | 0.0060 | 0.230 |
| CoQ10 | 2.140 | 0.2900 | 0.180 | |
| HIIT × CoQ10 | 0.030 | 0.8700 | 0.004 | |
| Caspase-3 protein/β-actin (fold of control) | HIIT | 6.810 | 0.0200 | 0.150 |
| CoQ10 | 0.880 | 0.4900 | 0.005 | |
| HIIT × CoQ10 | 0.037 | 0.8600 | 0.002 |
Univariate analysis of variance (ANOVA)
HIIT: High-intensity interval training; CoQ10: Coenzyme Q10; Bcl-2: B-cell lymphoma-2; Bax: Bcl-2-associated X protein; Bid: BH3-interacting domain death agonist
Discussion
In the present study, we investigated the effect of HIIT and CoQ10 supplementation on apoptotic proteins in heart muscle of obese male Wistar rats. The effects of HIIT program on apoptosis of heart muscle have not been clearly identified and, to the best of our knowledge, this is the first study investigating simultaneous effect of HIIT and CoQ10 supplementation on apoptosis of heart muscle.
The main findings of the study can be summarized as follows: 1) obesity has the potential to trigger myocardial apoptosis, supported by increased concentration of pro-apoptotic proteins including Bax, Bid, cytochrome c, caspase-8, and caspase-3 and decreased level of anti-apoptotic protein Bcl-2, 2) HIIT decreased pro-apoptotic proteins including Bid, cytochrome c, caspase-8, caspase-3, and Bax/Bcl-2 ratio and increased anti-apoptotic protein Bcl-2 concentration in the heart muscle of obese rats, and 3) CoQ10 supplementation showed only a significant effect on anti-apoptotic protein Bcl-2.
We observed a remarkable increase in weight and Lee index, indicating that high-calorie regimen in this study was effective in inducing obesity. Body weight was almost 90% and Lee index was 23% higher in BOC compared with NOC group. Obesity is associated with a spectrum of disorders including metabolic impairments, chronic inflammation, oxidative stress, changes in cardiac phenotype, and elevated myocardial apoptosis.2 Long-term exposure to overproduction of reactive oxygen species (ROS) and activation of apoptotic pathways result in mitochondrial dysfunction and premature cell death in cardiomyocytes.20 Due to the limited capacity of cardiac tissue for regeneration, these abnormalities can impose serious health treats on obese patients.20 Dietary regimen applied in this study induced obesity and myocardial apoptosis as early as 6 weeks that was indicated by a significant increase in pro-apoptotic protein levels including Bax, Bid, cytochromes c, caspase-8, caspase-3 and a decrease in anti-apoptotic protein Bcl-2. These proteins have significant contribution in cell apoptosis. Bcl-2 family can be either pro-apoptotic (e.g., Bax) or anti-apoptotic (e.g., Bcl-2) that regulate intrinsic mitochondria-dependent pathway of apoptosis.7 In this study, elevated level of Bax protein in obese rats was accompanied by decreased Bcl-2 protein level and a marked increase of Bax/Bcl-2 ratio. This change in the balance between pro and anti-apoptotic proteins is assumed to be the underlying cause of elevated cytochrome c protein content in obese rats. It is also speculated that increased mitochondrial permeability and cytochrome c release during obesity can activate other apoptotic signaling such as caspase-dependent pathways. Another finding of the study was elevated levels of caspase-8 protein, activation of which triggers execution phase.7 This can be confirmed by elevated protein concentration of caspase-3 in obese rats compared to NOCs. Caspase-3 is the most important executioner caspase that can be activated by caspase-8 and cytochrome c.7 Thus, it might be speculated that obesity, caused by high-calorie diet, activates apoptosis in cardiomyocytes by a remarkable imbalance between pro and anti-apoptotic proteins, increased mitochondrial permeability and cytochrome c release, and triggering execution phase of apoptosis by up-regulating caspase-8 and caspase-3. Myocardial apoptosis has been indicated to contribute to cardiomyopathy and HF.6 Our findings support this concept, as elevated apoptotic protein level in obese rats was coupled with increased heart weight. Thus, obesity resulted from high-calorie diet over a short period provides a potential stimulus for myocardial apoptosis that is likely to result in cardiac dysfunctions. However, we did not assess cardiac functional measures in experimental groups that can be considered a limitation of the current study.
We also observed that HIIT program decreased pro-apoptotic proteins including Bid, cytochrome c, caspase-8, caspase-3, and Bax/Bcl-2 ratio and increased anti-apoptotic protein Bcl-2 in obese rats. These results suggest that increased mitochondria-dependent and caspase-dependent pathways in myocardial tissue of obese rats can be suppressed by HIIT program. In spite of lack of research investigating the effect of HIIT on myocardium apoptosis in obesity, our findings are partly in line with Soori et al., who reported that HIIT over 4 weeks increased Bcl-2 level in heart muscle.21 In the cardiomyocytes, the balance between cell survival and death is tightly controlled. The mitochondria-dependent pathway is regulated by Bcl-2 family members including Bax, Bid, and Bcl-2 proteins, cytochrome c, and caspase-3.7 Bax protein promotes caspase-dependent apoptosis by increasing cytochrome c release from mitochondria.22 However, Bcl-2 protein interacts with Bax to inhibit cytochrome c release.23 The exercise training program conducted in the current study was found to alleviate activated mitochondria-dependent pathway in obese rats. This is evidenced by decreased Bid, cytochrome c, and caspase-3 proteins and Bax/Bcl-2 ratio and significant increase of Bcl-2 following HIIT program. Exercise protocol implemented in this study is assumed to have the potential to influence extrinsic pathway of apoptosis, implied by decreased levels of caspase-8 protein following HIIT program. Caspase-8 is the initiator of extrinsic receptor-mediated apoptosis pathway that plays a significant role in Fas pathway.7 Caspase-8 can act upon Bid protein that is a pro-apoptotic member of Bcl-2 family, increasing mitochondrial permeability and cytochrome c release. Cleavage of Bid through Fas pathway mediated by caspase-8 can consequently damage mitochondria and activate mitochondria-dependent pathway.7 This is suggested to be an example of cross-talk between intrinsic death-receptor-mediated pathway and intrinsic mitochondrial pathway. Hence, HIIT program seems to have the potential to intervene the cross-talk between intrinsic and extrinsic pathways to decrease apoptosis during obesity. However, this is an example that requires to be confirmed by investigating further links. Following this, the decreased caspase-8 protein concentration was coupled with decreased caspase-3 level, indicating effectiveness of HIIT to suppress execution phase of apoptosis.
Our findings, regarding the effect of HIIT on pro and anti-apoptotic proteins in heart muscle, are inconsistent with a couple of previous studies in some aspects. Unlike our results, Holloway et al., for instance, reported that HIIT protocol increased pro-apoptotic caspase-3 and Bax proteins in healthy rats. However, other pro-apoptotic factors such as caspase-8 and apoptosis repressor with caspase recruitment domain (ARC) were not affected by HIIT protocol.24 This finding, when compared with other reports, indicates that pro and anti-apoptotic proteins, not always, exhibit the same response to exercise interventions. Thus, exercise training with different modalities may exert diverse effects on apoptosis pathways in heart muscle, that needs to be extensively investigated.
Another finding of the current study was a statistically significant increase in Bcl-2 protein following CoQ10 consumption. However, CoQ10 has no remarkable effect on other variables including Bax, Bid, cytochrome c, caspase-8, and caspase-3 proteins. CoQ10 has been suggested to have anti-oxidant properties and prevent cell death induced by oxidative stress.13 Li et al. reported that CoQ10 supplementation decreased suppression of caspase-3 and Bax proteins and increased the expression of Bcl-2.25 Evidence from in vivo and in vitro studies indicates that CoQ10 can inhibit mitochondrial permeability transition pore (PTP) opening that plays a critical role in triggering apoptosis.13,14 Although CoQ10 supplementation did not alter pro-apoptotic proteins in our study, it might have influenced mitochondrial permeability by other factors and mechanisms. Proteins including voltage-dependent anion channel (VDAC), cyclophilin-D, and adenine nucleotide translocator (ANT) are shown to play a significant role in PTP formation. We did not assess these proteins, that can be considered a limitation of the current study. However, it has been suggested that Bcl-2 protein can affect PTP opening.7 A significant increase in Bcl-2 following CoQ10 supplementation indicates that it has a minor potential to affect apoptosis pathways. The increase of Bcl-2 by CoQ10 consumption was not as potent to cause significant changes in cytochrome c and pro-apoptotic proteins. These results indicate that CoQ10 has neither substantial synergistic nor isolated impact on pro-apoptotic proteins in myocardium of obese rats. Thus, CoQ10 might regulate above-mentioned proteins involved in mitochondrial permeability that were not determined in this study. CoQ10 supplementation along with HIIT protocol applied in the current study is assumed to have trivial impact on apoptotic proteins and longer durations or higher doses of supplementation may be required to observe remarkable changes. The present study had some limitations to be acknowledged. First, the number of selected samples was not very high; therefore, more samples will be necessary for future studies. Second, there was a lack of direct measurement of cardiomyocyte cell death and determination of the mechanism of death. Therefore, changes in the expression of proteins involved in apoptosis may not confirm the outcome.
Conclusion
Our findings indicated that obesity induced by a high-calorie diet over a short duration increased pro-apoptotic proteins and decreased anti-apoptotic Bcl-2 protein in heart muscle. In contrast, HIIT exerted an anti-apoptotic effect against apoptosis in heart muscle of obese rats. The consumption of CoQ10 had only a trivial impact on anti-apoptotic protein Bcl-2 but did not show a potent synergistic effect beside HIIT to reduce obesity-related apoptosis.
Acknowledgments
This article was extracted from a PhD thesis in exercise physiology by Kameleh Astani (dissertation code: 1024815308027171398162293400). The authors gratefully appreciate all of the coworkers that participated in this study. All ethical considerations are observed in this research. This study did not receive any grants from any organizations.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
Authors’ Contribution
Study design and literature search: KA, HP
Data collection and statistical analysis: KA, JB, MAN
Data interpretation: KA, HP, MAN
Manuscript preparation: KA, JB, HP
REFERENCES
- 1.Pinheiro-Castro N, Silva LBAR, Novaes GM, Ong TP. Hypercaloric diet-induced obesity and obesity-related metabolic disorders in experimental models. Adv Exp Med Biol. 2019;1134:149–61. doi: 10.1007/978-3-030-12668-1_8. [DOI] [PubMed] [Google Scholar]
- 2.Sbierski-Kind J, Mai K, Kath J, Jurisch A, Streitz M, Kuchenbecker L, et al. Association between subcutaneous adipose tissue inflammation, insulin resistance, and calorie restriction in obese females. J Immunol. 2020;205(1):45–55. doi: 10.4049/jimmunol.2000108. [DOI] [PubMed] [Google Scholar]
- 3.Nakandakari SCBR, Munoz VR, Kuga GK, Gaspar RC, Sant'Ana MR, Pavan ICB, et al. Short-term high-fat diet modulates several inflammatory, ER stress, and apoptosis markers in the hippocampus of young mice. Brain Behav Immun. 2019;79:284–93. doi: 10.1016/j.bbi.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Heo JW, Yoo SZ, No MH, Park DH, Kang JH, Kim TW, et al. Exercise training attenuates obesity-induced skeletal muscle remodeling and mitochondria-mediated apoptosis in the skeletal muscle. Int J Environ Res Public Health. 2018;15(10) doi: 10.3390/ijerph15102301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuang HL, Baskaran R, Hsuan DC, Lin YM, Ho CC, Ho TJ, et al. Role of potato protein hydrolysate and exercise in preventing high-fat diet-induced hepatocyte apoptosis in senescence-accelerated mouse. J Food Biochem. 2020;44(12):e13525. doi: 10.1111/jfbc.13525. [DOI] [PubMed] [Google Scholar]
- 6.van Empel VP, Bertrand AT, Hofstra L, Crijns HJ, Doevendans PA, De Windt LJ. Myocyte apoptosis in heart failure. Cardiovasc Res. 2005;67(1):21–9. doi: 10.1016/j.cardiores.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 7.D'Arcy MS. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43(6):582–92. doi: 10.1002/cbin.11137. [DOI] [PubMed] [Google Scholar]
- 8.Gronek P, Wielinski D, Cyganski P, Rynkiewicz A, Zajac A, Maszczyk A, et al. A review of exercise as medicine in cardiovascular disease: Pathology and mechanism. Aging Dis. 2020;11(2):327–40. doi: 10.14336/AD.2019.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henrique JS, Franca EF, Cardoso FDS, Serra FT, de Almeida AA, Fernandes J, et al. Cortical and hippocampal expression of inflammatory and intracellular signaling proteins in aged rats submitted to aerobic and resistance physical training. Exp Gerontol. 2018;110:284–90. doi: 10.1016/j.exger.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Cechella JL, Leite MR, Pinton S, Zeni G, Nogueira CW. Neuroprotective benefits of aerobic exercise and organoselenium dietary supplementation in hippocampus of old rats. Mol Neurobiol. 2018;55(5):3832–40. doi: 10.1007/s12035-017-0600-9. [DOI] [PubMed] [Google Scholar]
- 11.MacInnis MJ, Gibala MJ. Physiological adaptations to interval training and the role of exercise intensity. J Physiol. 2017;595(9):2915–30. doi: 10.1113/JP273196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gahramani M, Azarbayjani MA, Peeri M, Raoufi A. Interval training intensity and the expression of caspase-9 in obese rats with myocardial infarction. Iran J diabetes Obes. 2016;8(3):115–20. [Google Scholar]
- 13.Belliere J, Devun F, Cottet-Rousselle C, Batandier C, Leverve X, Fontaine E. Prerequisites for ubiquinone analogs to prevent mitochondrial permeability transition-induced cell death. J Bioenerg Biomembr. 2012;44(1):207–12. doi: 10.1007/s10863-012-9406-7. [DOI] [PubMed] [Google Scholar]
- 14.Papucci L, Schiavone N, Witort E, Donnini M, Lapucci A, Tempestini A, et al. Coenzyme q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J Biol Chem. 2003;278(30):28220–8. doi: 10.1074/jbc.M302297200. [DOI] [PubMed] [Google Scholar]
- 15.Archer ZA, Corneloup J, Rayner DV, Barrett P, Moar KM, Mercer JG. Solid and liquid obesogenic diets induce obesity and counter-regulatory changes in hypothalamic gene expression in juvenile Sprague-Dawley rats. J Nutr. 2007;137(6):1483–90. doi: 10.1093/jn/137.6.1483. [DOI] [PubMed] [Google Scholar]
- 16.Molz P, Molz WA, Dallemole DR, Weber AF, Salvador M, Pra D, et al. Potential ameliorative effects of chromium supplementation on glucose metabolism, obesity, and genomic stability in prediabetic rat model. Biol Trace Elem Res. 2021;199(5):1893–9. doi: 10.1007/s12011-020-02299-1. [DOI] [PubMed] [Google Scholar]
- 17.Pourrazi H, Asgharpour-Arshad M, Gholami F, Abbasi S. Effect of high-intensity interval training on apoptotic gene expression in rat myocardial tissue. Gene Cell Tissue. 2020;7(2):e101963. [Google Scholar]
- 18.Tolouei AJ, Habibi MA, Moshari S, Razi M. The effect of different types of exercise training on diet-induced obesity in rats, cross-talk between cell cycle proteins and apoptosis in testis. Gene. 2020;754:144850. doi: 10.1016/j.gene.2020.144850. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson CJ. An effect size primer: A guide for clinicians and researchers. Prof Psychol Res Pract. 2009;40(5):532–8. [Google Scholar]
- 20.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301(6):H2181–H2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 21.Soori R, Ghram A, Zare Shahneh M, Choobineh S, Costa PB, Voltarelli FcA. Effects of high intensity interval training and aging on cardiac muscle apoptosis markers in C57BL/6 Mice. Sport Sci Health. 2021;17(1):173–9. [Google Scholar]
- 22.Tarantino G, Scopacasa F, Colao A, Capone D, Tarantino M, Grimaldi E, et al. Serum Bcl-2 concentrations in overweight-obese subjects with nonalcoholic fatty liver disease. World J Gastroenterol. 2011;17(48):5280–8. doi: 10.3748/wjg.v17.i48.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen M, Tan M, Jing M, Liu A, Liu Q, Wen S, et al. Berberine protects homocysteic acid-induced HT-22 cell death: involvement of Akt pathway. Metab Brain Dis. 2015;30(1):137–42. doi: 10.1007/s11011-014-9580-x. [DOI] [PubMed] [Google Scholar]
- 24.Holloway TM, Bloemberg D, da Silva ML, Simpson JA, Quadrilatero J, Spriet LL. High intensity interval and endurance training have opposing effects on markers of heart failure and cardiac remodeling in hypertensive rats. PLoS One. 2015;10(3):e0121138. doi: 10.1371/journal.pone.0121138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Zhan J, Hou Y, Hou Y, Chen S, Luo D, et al. Coenzyme Q10 regulation of apoptosis and oxidative stress in h2o2 induced bmsc death by modulating the nrf-2/nqo-1 signaling pathway and its application in a model of spinal cord injury. Oxid Med Cell Longev. 2019;2019:6493081. doi: 10.1155/2019/6493081. [DOI] [PMC free article] [PubMed] [Google Scholar]