Abstract
Background:
Post-traumatic epilepsy (PTE) is a severe complication of traumatic brain injury (TBI). Electroencephalography aids early post-traumatic seizure diagnosis, but its optimal utility for PTE prediction remains unknown. We aim to evaluate the contribution of quantitative electroencephalograms to predict first-year PTE (PTE1).
Methods:
We performed a multicenter, retrospective case-control study of TBI patients. 63 PTE1 patients were matched with 63 non-PTE1 patients by admission Glasgow Coma Scale score, age, and sex. We evaluated the association of quantitative electroencephalography features with PTE1 using logistic regressions and examined their predictive value relative to TBI mechanism and Computed Tomography abnormalities.
Results:
In the matched cohort (n=126), greater epileptiform burden, suppression burden and beta variability were associated with 4.6 times higher PTE1 risk based on multivariable logistic regression analysis (area under the receiver-operating-characteristic curve, AUC [95% CI], 0.69 [0.60–0.78]). Among 116 (92%) patients with available Computed Tomography reports, adding quantitative electroencephalography features to a combined mechanism and Computed Tomography model improved performance (AUC [95% CI], 0.71 [0.61–0.80] vs 0.61 [0.51–0.72]).
Conclusions:
Epileptiform and spectral characteristics enhance covariates identified on TBI admission and Computed Tomography abnormalities in PTE1 prediction. Future trials should incorporate quantitative electroencephalography features to validate this enhancement of PTE risk stratification models.
INTRODUCTION
Post-traumatic epilepsy (PTE) is a devastating consequence of traumatic brain injury (TBI). Early stratification of PTE risk in TBI patients would facilitate targeted enrollment into anti-epileptogenesis treatment trials.1
While electroencephalography (EEG) is recommended to detect early post-TBI electrographic seizures (ESZs),2 whether and how it benefits later PTE prediction remains unclear.1,3,4 Early investigations suggest that classifying post-TBI (<3 month) EEG into normal/abnormal may not differentiate PTE risk.3 Recently, we found that the presence of epileptiform abnormalities (EAs, i.e., ESZs, sporadic epileptiform discharges [EDs], lateralized or generalized periodic discharges [LPDs, GPDs], lateralized rhythmic delta activity [LRDA]) and focal polymorphic slowing <1 month post-TBI is associated with first-year PTE (PTE1).1 Yet, accessible quantitative EEG (QEEG) tools5–8 remain unexplored in quantifying abnormalities1 relevant to PTE1.
Here, we propose a quantification scheme to automatically calculate QEEG characteristics ≤14 days post-TBI. We aim to evaluate the contribution of quantitative epileptiform and spectral features to PTE1 prediction beyond covariates identifiable on TBI admission and initial Computed Tomography (CT) head abnormalities.1,9–11
METHODS
In this case-control study, we collected data from nine centers of the Critical Care EEG Monitoring Research Consortium: Yale School of Medicine (New Haven, CT), Brigham and Women’s Hospital (Boston, MA), Duke University Medical Center (Durham, NC), Emory School of Medicine (Atlanta, GA), Henry Ford Health System (Detroit, MI), Massachusetts General Hospital (Boston, MA), University of Florida Health (Gainesville, FL), University of Miami School of Medicine/Jackson Memorial Health System (Miami, FL), and UT Southwestern Medical Center (Dallas, TX) between 2012 and 2019. The Institutional Review Board per center approved the study protocol (IRB#: 1405014045) and granted consent waivers because the study was retrospective.
Participants
TBI patients were included retrospectively if age ≥18 years, no seizure/epilepsy history, EEG monitoring data ≤14 days post-TBI, and ≥12-month follow-up or developed PTE1. Patients were excluded per signal quality inspection (Online Supplemental Methods I). Amongst included patients, one-to-one case-control (PTE1 vs non-PTE1) match was performed based on admission Glasgow Coma Scale (GCS) score, age, and sex (Online Supplemental Methods II).
Outcome and Exposures
We defined PTE1 according to our prior publication1 as an unprovoked seizure 1–12 months post-TBI. Eligible patients in this study commonly had protracted hospital courses. Hence a seizure >7 days post-TBI but during the acute hospitalization was likely provoked by subsequent complications.11 EEGs (21 channel, 10–20 system) were recorded for clinical indication.
Predictors
We recorded from CT reports the presence of intraparenchymal, subdural, subarachnoid, epidural hemorrhage (IPH, SDH, SAH, EDH), and skull fracture; and from EEG reports the presence of EA (i.e., ESZs, EDs, LPDs, GPDs, LRDA), generalized rhythmic delta activity (GRDA), suppression, focal slowing, and generalized slowing.
For QEEG analysis, we split each patient’s EEG into non-overlapping, one-hour windows of homogeneous duration (Online Supplemental Methods I). Each feature per patient was represented by the maximum (EA, GRDA burden) or median (spectra) values across all windows. We matched outputs from two algorithms for ESZ (“SPaRCNet”8, Persyst14®6) and ED (SpikeNet7, Persyst14®5) detection to reduce false-positive rates, and computed ESZ and ED presence.1,12 We analyzed EA burden (“SPaRCNet”8; hourly % EA presence), GRDA burden, suppression burden (hourly % signal with amplitude<3 μV lasting ≥0.5 seconds), global delta (1–4Hz), theta (4–8Hz), alpha (8–13Hz), and beta (13–20Hz) powers, theta/alpha/beta-over-delta ratios, power asymmetry (absolute hemispheric difference over global power), and power variability (hourly interquartile range) (Persyst14®).
Statistical Analysis
Univariable and multivariable (forward-selection algorithm applied) logistic regressions were used to evaluate the association of unmatched covariates and QEEG features with PTE1. To combat overfitting, ridge logistic regressions trained and tested via 8-fold nested cross-validation were applied to compare predictive values of different feature sets (Mechanism+CT, Mechanism+CT+QEEG, Mechanism+CT+EEG-report; Online Supplemental Methods III). Evaluation metrics were calculated by concatenating test sets.
Area Under the receiver-operating-characteristic Curve (AUC), accuracy (optimal operating point), odds ratio (OR), and calibration error were evaluated (Online Supplemental Methods IV). P=0.05 was the significance threshold. 95% confidence intervals (95% CIs) were generated by bootstrapping (n=1000). Analysis was performed using R3.6.1.
RESULTS
205 of 279 eligible patients with high-quality EEG were included. 63 PTE1 patients were matched with 63 non-PTE1 patients (Online Supplemental Table S1-2). 116 (90%) matched patients had CT reports.
TBI Mechanism, CT and QEEG Predictors of PTE1
We used univariable logistic regression to assess potential covariates, including QEEG features, that predict PTE1 risk independent of matched variables (Table 1; Figure 1A-B; Online Supplemental Figure S1). For TBI mechanism, penetrating injury was associated with an increased odd of PTE1 (OR=6.20, P=0.03) compared to acceleration/deceleration. For CT abnormalities, SDH (OR=3.34, P=0.01) and skull fracture (OR=2.48, P=0.03) were positively associated with PTE1 risk (n=116; Online Supplemental Table S3). For QEEG, ESZ presence (OR=2.79, P=0.02), greater EA burden (OR per 10%-increase [OR10%]=1.15, P=0.01; Figure 1A), LRDA burden (OR10%=1.13, P=0.02), and delta asymmetry (OR10%=1.29, P=0.047) were associated with increased odds of PTE1. Non-epileptiform GRDA burden1,13 (OR10%=0.77, P=0.01) was negatively associated with PTE1. QEEG findings generally agreed with EEG-report results (EA, OR=2.29, P=0.03; focal slowing, OR=2.18, P=0.04) except for ED presence (significant in EEG-report1 [OR=2.91, P=0.02] but non-significant in QEEG analysis) (Online Supplemental Table S3).
Table 1.
Univariable Analysis of QEEG Features Associated With PTE1 Developmenta
| Univariable Analysis | Univariable Logistic Regression | |||
|---|---|---|---|---|
| Variable, Descriptive Statistics, Unit | Non-PTE1 Patients (n=63) | PTE1 Patients (n=63) | OR (95% CI) | P value |
| Matched Variables | ||||
| Age at TBI, median (IQR), year | 49 (28–66) | 48 (28–65) | 1 (0.98–1.02) | 0.94 |
| Female, No. (%) | 18 (29) | 17 (27) | 1.09 (0.50–2.38) | 0.84 |
| Admission GCS Score, No. (%) | ||||
| 13–15 (Mild TBI) | 13 (21)b | 13 (21) | 1 [Reference] | |
| 9–12 (Moderate TBI) | 15 (24)b | 15 (24) | 1 (0.35–2.86) | 1 |
| 3–8 (Severe TBI) | 35 (56)b | 35 (56) | 1 (0.41–2.46) | 1 |
| Injury Mechanism, No. (%) | ||||
| Acceleration/Deceleration | 31 (49) | 20 (32) | 1 [Reference] | |
| Direct Impact to Head | 3 (5) | 6 (10) | 3.10 (0.69–13.83) | 0.14 |
| Fall from Standing | 16 (25) | 20 (32) | 1.94 (0.82–4.60) | 0.13 |
| Fall from >3ft | 11 (17) | 9 (14) | 1.27 (0.45–3.61) | 0.66 |
| Penetrating | 2 (3) | 8 (13) | 6.20 (1.19–32.23) | 0.03 |
| EEG Monitoring, median (IQR) | ||||
| Start Time Post-TBI, day | 2.3 (1.5–4.7) | 2.6 (1.6–4.8) | 1.01 (0.95–1.07) | 0.71 |
| Monitoring Duration, day | 0.7 (0.3–1.7) | 1.0 (0.7–1.8) | 1.21 (0.96–1.53) | 0.11 |
| QEEG Features, ≤14 Days Post-TBI | ||||
| ESZ Presence, No. (%) | 9 (14) | 20 (32) | 2.79 (1.15–6.75)c | 0.02 |
| ED Presence, No. (%) | 41 (65) | 45 (71) | 1.34 (0.63–2.85)c | 0.45 |
| Peak EA Burden, median (IQR), %1h | 8.2 (1.4–31.7) | 34.3 (2–81.3) | 1.15 (1.04–1.27)c | 0.01 |
| ESZ | 0 (0–0) | 0 (0–7.7) | 1.32 (0.99–1.75)c | 0.06 |
| ED | 0.1 (0–0.7) | 0.1 (0–0.8) | 1.04 (0.79–1.36)c | 0.80 |
| LPD | 0.1 (0–1.2) | 0.2 (0–3.3) | 1.09 (0.88–1.35)c | 0.42 |
| GPD | 0.1 (0–0.5) | 0.1 (0–1] | 11.76 (0.78->100)c | 0.08 |
| LRDA | 3.4 (0.5–24.2) | 9.1 (0.9–76.9) | 1.13 (1.02–1.25)c | 0.02 |
| Peak GRDA Burden, median (IQR), %1h | 8.3 (0.3–32.1) | 1.3 (0.1–9.5) | 0.77 (0.64–0.92)c | 0.01 |
| Suppression, median (IQR), %1h | 2.9 (0.6–7.1) | 3.8 (0.9–12.5) | 1.29 (0.96–1.73)c | 0.09 |
| Global Band Power, mean (SD) | ||||
| Delta (1–4 Hz) | 9.4 (2.2) | 9.2 (2.4) | 0.97 (0.83–1.13) | 0.69 |
| Theta (4–8 Hz) | 7.3 (1.8) | 7.0 (1.9) | 0.92 (0.76–1.11) | 0.38 |
| Alpha (8–13 Hz) | 6.3 (1.2) | 6.1 (1.4) | 0.94 (0.72–1.22) | 0.63 |
| Beta (13–20 Hz) | 6.0 (1.3) | 6.0 (1.5) | 0.99 (0.76–1.27) | 0.92 |
| Global X-Over-Delta ratios, mean (SD) | ||||
| Theta-over-Delta | 0.8 (0.1) | 0.8 (0.1) | 0.47 (0.02–10.91) | 0.64 |
| Alpha-over-Delta | 0.7 (0.1) | 0.7 (0.1) | 1.82 (0.13–25.3) | 0.66 |
| Beta-over-Delta | 0.7 (0.1) | 0.7 (0.2) | 2.42 (0.28–21.02) | 0.42 |
| Power Asymmetry, median (IQR), % | ||||
| Delta | 8.4 (5.7–18.7) | 13.5 (7.3–33.1) | 1.29 (1–1.66)c | 0.047 |
| Theta | 9.0 (4.8–15.2) | 11.7 (6.7–28.3) | 1.25 (0.98–1.58)c | 0.07 |
| Alpha | 8.4 (5.6–14.3) | 11.7 (5.3–23.8) | 1.25 (0.98–1.59)c | 0.07 |
| Beta | 8.0 (4.6–13.8) | 9.7 (5.3–22.1) | 1.27 (0.98–1.64)c | 0.07 |
| Power Variability, median (IQR) | ||||
| Delta | 0.6 (0.4–0.8) | 0.7 (0.3–0.8) | 0.96 (0.39–2.31) | 0.92 |
| Theta | 0.3 (0.2–0.4) | 0.3 (0.2–0.5) | 3.08 (0.53–17.87) | 0.21 |
| Alpha | 0.3 (0.2–0.4) | 0.3 (0.2–0.4) | 3.77 (0.42–33.93) | 0.24 |
| Beta | 0.2 (0.2–0.3) | 0.3 (0.2–0.4) | 7.24 (0.70–74.49) | 0.096 |
Abbreviations: QEEG, quantitative electrocochleography; TBI, traumatic brain injury; PTE1, posttraumatic epilepsy within first-year post-TBI; OR, odds ratio; GCS, Glasgow Coma Scale; ESZ, electrographic seizure; ED, epileptiform discharge; EA, epileptiform abnormality; LPD, lateralized periodic discharge; GPD, generalized periodic discharge; LRDA, lateralized rhythmic delta activity; GRDA, generalized rhythmic delta activity.
Predictors were included in forward-selection algorithm if P<0.10
Numbers may not sum to 100% due to rounding issue
Odds ratio associated with 10 unit increase of features
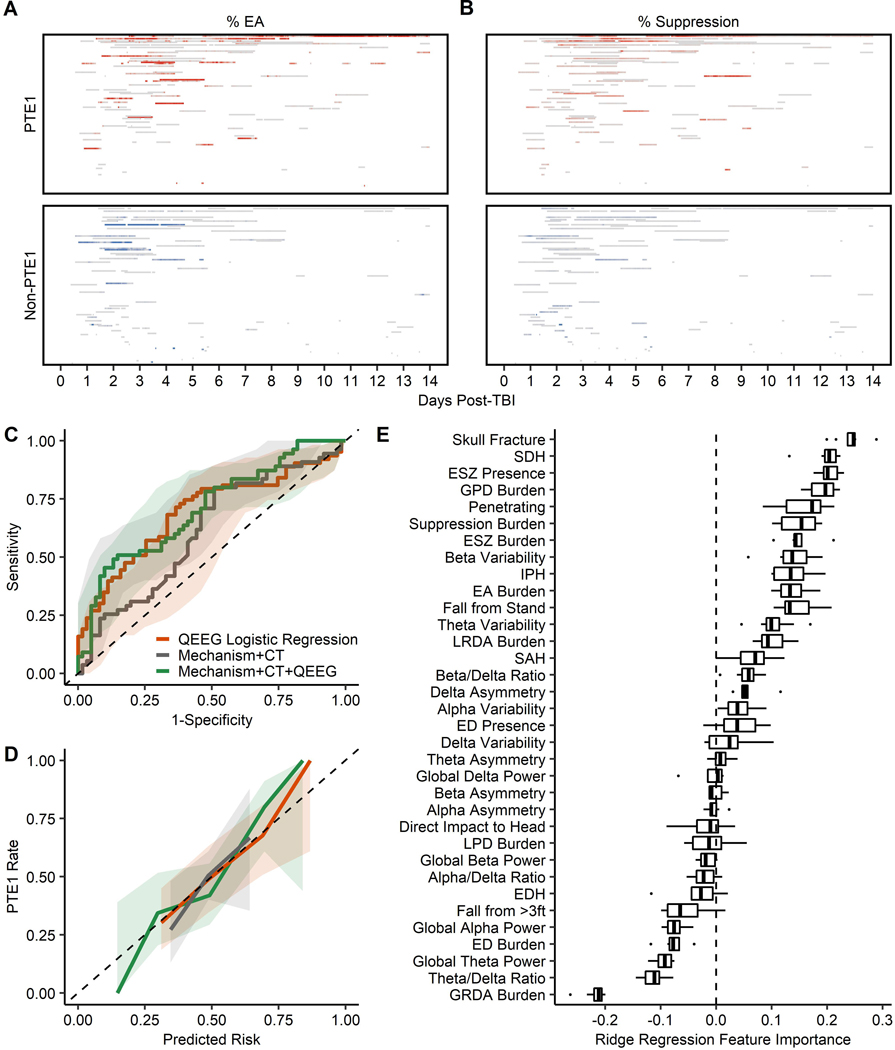
Figure 1: Quantitative Electroencephalography (QEEG) Prediction Models for First-year Post-traumatic Epilepsy (PTE1).
(A) % Epileptiform Abnormalities (EAs) for all one-hour windows for all patients (0% corresponds to grey, higher % corresponds to darker red/blue); each block represents an one-hour window; y-axis represents individual patients sorted by total recording duration (top: longest duration). (B) Same as panel A but for % suppression distribution. (C) Area Under the receiver-operating-characteristic Curve (AUC) comparison; AUC for forward-selected QEEG logistic regression (orange): 0.69 (95% CI, 0.60–0.78); test AUC for cross-validated ridge logistic regression based on TBI mechanism and Computed Tomography (Mechanism+CT, grey): 0.61 (0.51–0.72), and test AUC for cross-validated ridge logistic regression based on TBI mechanism, Computed Tomography, and QEEG (Mechanism+CT+QEEG, green): 0.71 (0.61–0.80); shaded areas represent the bootstrapped (n=1000) 95% confidence intervals. (D) Same as panel C but showing calibration errors for QEEG logistic regression: 0.06 (0.02–0.12), Mechanism+CT ridge regression: 0.06 (0.02–0.12), Mechanism+CT+QEEG ridge regression: 0.08 (0.04–0.15). (E) Feature importance for Mechanism+CT+QEEG ridge regression; features were sorted by the importance measure; each boxplot visualizes the distribution of penalized coefficients across 8 folds.
To examine whether QEEG predicts PTE1 risk independent of significant covariates, we applied a forward-selection algorithm on QEEG features with P<0.1 in univariable analysis to construct a multivariable QEEG-only model, and then step-wise added penetrating injury, SDH, and skull fracture. EA burden (aOR10%=1.17, P<0.01), suppression burden (aOR10%=1.41, P=0.03), and beta variability (aOR=16.17, P=0.03) jointly predicted PTE1 with an AUC of 0.69 (95% CI, 0.60–0.78; Figure 1C). The association of forward-selected QEEG features with PTE1 remained significant relative to penetrating (n=126), and more importantly, relative to all penetrating, SDH, and skull fracture (n=116; Online Supplemental Table S4).
Benefits of QEEG in PTE1 Prediction
To avoid overfitting in small-sample cohort, we leveraged nested cross-validation and regularization (e.g., ridge) techniques to evaluate the additive benefits of QEEG beyond TBI mechanism and CT abnormalities in PTE1 prediction (n=116). Compared to the Mechanism+CT ridge regression, Mechanism+CT+QEEG demonstrated improved discrimination (Test AUC, 0.71 [0.61–0.80] vs 0.61 [0.51–0.72]) with a comparable calibration error (0.08 [0.04–0.15] vs 0.06 [0.02–0.12]) (Figure 1C-D; Online Supplemental Table S5). Per feature importance measures (Figure 1E), CT abnormalities (skull fracture, SDH) were the most important positive predictors, followed by QEEG epileptiform (ESZ presence, GPD burden, EA burden) and spectral (suppression burden, beta variability) features. Penetrating injury also had strong positive importance. Consistent with logistic regression, GRDA burden had strong negative importance. A ridge algorithm utilizing EEG-report abnormalities, instead of QEEG, (AUC [95% CI], 0.65 [0.54–0.75]; Online Supplemental Table S5) demonstrated modest, but less robust improvement upon the Mechanism+CT model.
DISCUSSION
We demonstrate that EA burden, suppression burden, and beta variability combined enhance PTE1 risk stratification in this case-control cohort. Furthermore, QEEG provides added benefit in PTE1 prediction beyond TBI mechanism and CT abnormalities, especially given our data suggesting potential collinearity between penetrating and skull fracture (Online Supplemental Table S4). Taking a PTE1 rate at 9.8% amongst moderate-to-severe TBI patients,10 our Mechanism+CT ridge model would identify patients with 15% PTE1, similar to the previously reported 1-year rates using clinical covariates.14 Our mechanism+CT+QEEG model increases this PTE1 identification nearly 2-fold to 27%; reducing the enrollments for anti-epileptogenesis trials by 50% (Online Supplemental Table S6).
We found that a greater EA burden was associated with PTE1, generating hypotheses on metabolic dysregulation. EA burden post-TBI may increase metabolic demand when there is decreased metabolic supply, leading to a mismatch triggering epileptogenesis. Whether interventions reducing EAs post-TBI prevent metabolic exhaustion and PTE development warrants exploration. The larger delta asymmetry for PTE1 vs Non-PTE1 patients suggests that focal/hemispheric network dysfunction may be relevant as reported previously.1,4 Suppression burden predicts PTE1, perhaps reflecting injury severity independent of GCS.
Together, our data highlight the benefits of EEG monitoring for moderate-to-severe TBI patients.1,2 With increased post-TBI EEG monitoring, our quantification scheme may reduce the cost of manually reviewing EEG reports without compromising PTE1 prediction accuracy. However, ED algorithms may need further improvement in specificity (Online Supplemental Table S4&7).
Limitations
First, medication and state changes (sleep, awake, sedated) may affect EEG. These differences are highly influenced by TBI severity, and thereby more comparable amongst patients matched by admission GCS. However, the impacts of these and other TBI severity measures (lesion location/type, craniectomy/craniotomy) on QEEG and PTE1 risk warrant further exploration. Second, our study is retrospective with possible selection bias toward moderate-to-severe TBI patients and/or those at risk for ESZ. Therefore, PTE1 incidences or prediction models here need further refinement to apply to the mild TBI population. Our findings should be validated in prospective studies. Third, some non-PTE1 patients here might develop >12-month PTE. If such patients had QEEG similar to those of PTE1 patients, their risk for PTE would be underestimated, and so would the contributions of QEEG in PTE prediction. Studies investigating the association of QEEG with PTE latency are warranted. Finally, combining QEEG with quantitative neuroimaging data15 may improve PTE prediction.
In summary, epileptiform and spectral features quantified by QEEG tools enhance covariates identifiable on TBI admission and CT abnormalities in PTE1 prediction. Future large-sample, prospective studies should validate our findings and could incorporate QEEG into PTE risk models.
Supplementary Material
ACKNOWLEDGEMENTS
KD received funding from the National Institute on Aging (NIA) (R34AG061304) and the National Institute of Neurological Disorders and Stroke (NINDS) (R01NS117904) of the National Institutes of Health (NIH). CS and CBM acknowledge the University of Florida Integrated Data Repository (IDR) and the UF Health Office of the Chief Data Officer for providing the analytic data set for this project. CS and CBM were supported by the National Center for Advancing Translational Sciences (NCATS) of the NIH under University of Florida Clinical and Translational Science Awards (UL1TR000064, UL1TR001427). CBM received funding from the American Heart Association (AHA). AA is supported by NCATS of the NIH through an institutional KL2 Career Development Award from the Miami Clinical and Translational Science Institute (UL1TR002736). MBD received funding from the NINDS of the NIH and the American Epilepsy Society. EJG received funding from NIH (R01NS117904). AFS received funding from the NINDS under the NIH (R01NS111022) and Ceribell. BE received funding from the NINDS (R21NS109627, RF1NS115268) and the Office of the Director (DP2HD101400) of the NIH, the James S. McDonnell Foundation, and the Tiny Blue Dot Foundation. MBW received funding from the Glenn Foundation for Medical Research, the American Federation for Aging Research (Breakthroughs in Gerontology), the American Academy of Sleep Medicine Strategic Research Award, and the NINDS (R01NS102190, R01NS102574, R01NS107291) and the NIA (RF1AG064312, R01AG062989, R01AG073410) of the NIH. JAK received funding from the NINDS (R25N065743, K23NS112596-01A1, R01NS117904), the American Academy of Neurology Clinical Research Training Scholarship, the AHA, and the Bee Foundation.
Footnotes
COMPETING INTERESTS
Nothing to report.
REFERENCES
- 1.Kim JA, Boyle EJ, Wu AC, et al. Epileptiform activity in traumatic brain injury predicts post-traumatic epilepsy. Ann Neurol. 2018;83(4):858–862. doi: 10.1002/ana.25211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee H, Mizrahi MA, Hartings JA, et al. Continuous Electroencephalography after Moderate to Severe Traumatic Brain Injury. Crit Care Med. 2019;47(4):574–582. doi: 10.1097/CCM.0000000000003639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jennett B, Van De Sande J. EEG prediction of post-traumatic epilepsy. Epilepsia. 1975;16(2):251–256. [DOI] [PubMed] [Google Scholar]
- 4.Tomkins O, Feintuch A, Benifla M, Cohen A, Friedman A, Shelef I. Blood-brain barrier breakdown following traumatic brain injury: A possible role in posttraumatic epilepsy. Cardiovasc Psychiatry Neurol. [Published online February 22, 2011]. doi: 10.1155/2011/765923 [DOI] [PMC free article] [PubMed]
- 5.Scheuer ML, Bagic A, Wilson SB. Spike detection: Inter-reader agreement and a statistical Turing test on a large data set. Clin Neurophysiol. 2017;128(1):243–250. doi: 10.1016/j.clinph.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 6.Scheuer ML, Wilson SB, Antony A, Ghearing G, Urban A, Bagid AI. Seizure Detection: Interreader Agreement and Detection Algorithm Assessments Using a Large Dataset. J Clin Neurophysiol. 2021;38(5):439–447. doi: 10.1097/WNP.0000000000000709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jing J, Sun H, Kim JA, et al. Development of Expert-Level Automated Detection of Epileptiform Discharges during Electroencephalogram Interpretation. JAMA Neurol. 2020;77(1):103–108. doi: 10.1001/jamaneurol.2019.3485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge W, Jing J, An S, et al. Deep active learning for Interictal Ictal Injury Continuum EEG patterns. J Neurosci Methods. 2021;351:108966. doi: 10.1016/j.jneumeth.2020.108966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu T, Yu X, Ou S, et al. Risk factors for posttraumatic epilepsy: A systematic review and meta-analysis. Epilepsy Behav. 2017;67:1–6. doi: 10.1016/j.yebeh.2016.10.026 [DOI] [PubMed] [Google Scholar]
- 10.Englander J, Bushnik T, Duong TT, et al. Analyzing risk factors for late posttraumatic seizures: a prospective, multicenter investigation. Arch Phys Med Rehabil. 2003;84(3):365–373. doi: 10.1053/apmr.2003.50022 [DOI] [PubMed] [Google Scholar]
- 11.Annegers JF, Hauser WA, Coan SP, Rocca WA. A population-based study of seizures after traumatic brain injuries. N Engl J Med. 1998;338(1):20–24. doi: 10.1056/NEJM199801013380104 [DOI] [PubMed] [Google Scholar]
- 12.Tubi MA, Lutkenhoff E, Blanco MB, et al. Early seizures and temporal lobe trauma predict post-traumatic epilepsy: A longitudinal study. Neurobiol Dis. 2019;123:115–121. doi: 10.1016/j.nbd.2018.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz AR, Vlachy J, Lee JW, et al. Association of periodic and rhythmic electroencephalographic patterns with seizures in critically ill patients. JAMA Neurol. 2017;74(2):181–188. doi: 10.1001/jamaneurol.2016.4990 [DOI] [PubMed] [Google Scholar]
- 14.Temkin NR, Dikmen SS, Wilensky AJ, Keihm J, Chabal S, Winn HR. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323(8):497–502. doi: 10.1056/NEJM199008233230801 [DOI] [PubMed] [Google Scholar]
- 15.Lutkenhoff ES, Shrestha V, Ruiz Tejeda J, et al. Early brain biomarkers of post-traumatic seizures: Initial report of the multicentre epilepsy bioinformatics study for antiepileptogenic therapy (EpiBioS4Rx) prospective study. J Neurol Neurosurg Psychiatry. 2020;91(11):1154–1157. doi: 10.1136/jnnp-2020-322780 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.