Abstract
Many viral infections cause acute and chronic neurologic diseases which can lead to degeneration of cortical functions. While neurotropic viruses that gain access to the central nervous system (CNS) may induce brain injury directly via infection of neurons or their supporting cells, they also alter brain function via indirect neuroimmune mechanisms that may disrupt the blood-brain barrier (BBB), eliminate synapses, and generate neurotoxic astrocytes and microglia that prevent recovery of neuronal circuits. Non-neuroinvasive, neurovirulent viruses may also trigger aberrant responses in glial cells, including those that interfere with motor and sensory behaviors, encoding of memories and executive function. Increasing evidence from human and animal studies indicate that neuroprotective antiviral responses that amplify levels of innate immune molecules dysregulate normal neuroimmune processes, even in the absence of neuroinvasion, which may persist after virus is cleared. In this review, we discuss how select emerging and re-emerging RNA viruses induce neuroimmunologic responses that lead to dysfunction of higher order processes including visuospatial recognition, learning and memory, and motor control. Identifying therapeutic targets that return the neuroimmune system to homeostasis is critical for preventing virus-induced neurodegenerative disorders.
Graphical Abstract
Introduction
A new paradigm in neuroimmunology has emerged whereby innate immune molecules are recognized as modulators of a variety of central nervous system (CNS) functions throughout life. These include the maintenance of blood-brain barrier (BBB) function (Jung et al., 2012; Wosik et al., 2007), synaptic networks (Filiano et al., 2016), microglial physiology (Butovsky & Weiner, 2018; Hickman et al., 2013; Yeh & Ikezu, 2019), astrogenesis and glial-mediated synapse remodeling (Kanski, Strien, Tijn, & Hol, 2013; Vainchtein et al., 2018), and repair (Amor & Woodroofe, 2014; Z. Chen & Palmer, 2008; Cossetti, Alfaro-Cervelló, Donegà, Tyzack, & Pluchino, 2012; Healy, Yaqubi, Ludwin, & Antel, 2019). Within the normal brain, innate immune molecules are expressed by resident cells, including perivascular myeloid and ependymal cells, microglia, astrocytes, oligodendrocytes, and neurons (Adelson et al., 2016; Blank et al., 2016; Cheng, Jin, Zhang, Tian, & Zou, 2011; Datwani et al., 2009; Dixon-Salazar et al., 2014, p.; Ejlerskov et al., 2015; Fourgeaud et al., 2010; Glynn et al., 2011; Green et al., 2012; Hirsch et al., 2009; Michelucci et al., 2015; Peferoen, Kipp, Valk, Noort, & Amor, 2014; Rey, Balschun, Wetzel, Randolf, & Besedovsky, 2013; C. Wang et al., 2020). The cellular source of these molecules, which include classical pattern recognition receptors (PRRs), complement proteins (C1q, C3, C4), major histocompatibility (MHC) I, cytokines (i.e. type I interferon (IFNαβ), interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF), transforming growth factor (TGF)-β), and chemokines (CXCL12, CXCL1), determine their effects on normal brain function. Some innate immune molecules, such as C1q and C3, are produced within the normal CNS (Veerhuis, Nielsen, & Tenner, 2011), contributing to CNS glial communication and synapse loss during normal forgetting, sleep and aging (Clark et al., 2021; Datta et al., 2020; Shi et al., 2015; Stephan et al., 2013; C. Wang et al., 2020), and increase in diseases with cognitive deficits (Hong et al., 2016; Michailidou et al., 2015; Vasek et al., 2016). Locations and levels of cytokine expression in the brain may also differentially impact their effects. For example, low basal levels of IFNβ promote neuronal survival and neurite outgrowth within the hippocampus (Ejlerskov et al., 2015), and disruptions to the IFNβ-IFNAR signaling promote Parkinson’s disease-like phenotypes (Magalhaes et al., 2021). Conversely, increased expression of IFNβ within the choroid plexus during aging is associated with decreased hippocampal neurogenesis (Baruch et al., 2014), which is critical for the formation of new memories. Similarly, IFNα treatment suppresses behavioral activity and reduced hippocampal neurogenesis in primates (Kaneko, Nakamura, & Sawamoto, 2020). These data suggest that the IFNAR signaling pathway may mediate both neurogenic and anti-neurogenic effects depending on the context. Factors that influence the effect of a cytokine signaling pathway could include variations in the cytokine and cellular milieu, the cytokine and receptor expression levels, and the microanatomical location of cytokine production within the CNS. This paradigm contrasts with previously held beliefs that innate immune responses within the CNS always induce pathology (Fig. 1). It also provides a framework for exploring mechanistic links between host-pathogen responses and neurodegenerative diseases, especially those that impact cognitive function.
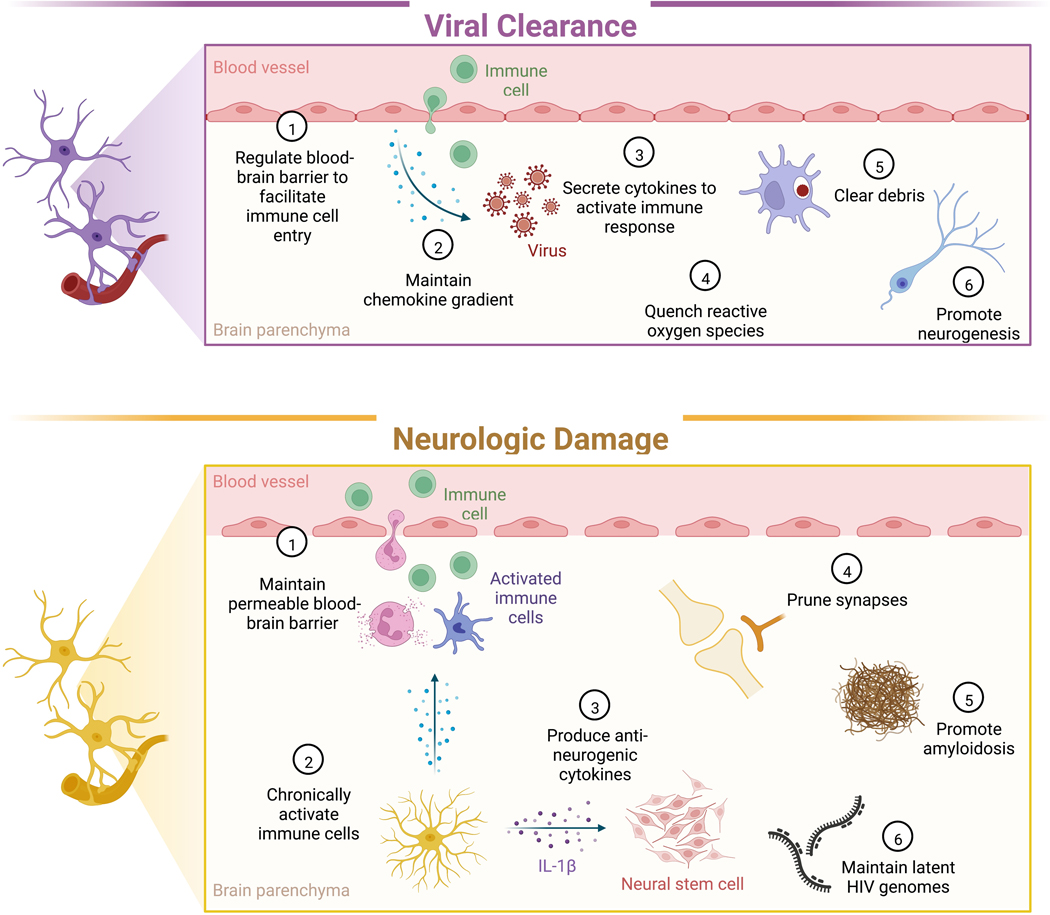
Figure 1. Glia facilitate clearance of CNS viral infections, but also mediate neurologic damage.
During neuroinvasive and neurovirulent viral infections, glia perform functions that are essential to viral clearance and neurocognitive recovery, such as regulating the BBB, maintaining chemokine gradients, and phagocytosing debris. However, these responses can also damage neuronal tissue by promoting chronic inflammation, impeding neurogenesis, and pruning synapses.
Viruses are an underappreciated factor in the development of neurodegenerative diseases, and many classes of viruses are associated with long-term neurologic symptoms (Table 1). A major example is the neuroinvasive RNA arboviruses, which include members of the Bornaviridae, Flaviviviridae, Phenuiviridae, Paramyxoviridae, Picornaviridae, and Togaviridae families (Table 1). The flaviviruses are transmitted by arthropod vectors and associated with neurocognitive impairments in the majority of survivors. These viruses may be associated with acute neuroinfectious diseases, such as meningitis and encephalitis, and include viruses that target neurons and/or glia. Members of the Flaviviridae family, West Nile (WNV) and Zika (ZIKV) viruses, invade the CNS via retrograde transport along neurons or cross the BBB within arthropod exosomes (Zhou et al., 2018). Diseases of pathological forgetting and dysexecutive function have also been reported in patient survivors of respiratory viruses that may not undergo neuroinvasion. Members of the Pnemoviridae, Orthomyxoviridae, and Coronaviridae families that cause infections of the upper and lower respiratory tract, including metapneumovirus, influenza A virus (IAV), and Severe acute respiratory syndrome coronavirus (SARS-CoV)-2, may also lead to long-term neuropsychiatric sequelae. Survivors of severe IAV, which may be neuroinvasive, may develop prolonged cognitive impairment and psychiatric disorders (Denke et al., 2018), although this has not been extensively studied and hypoxia is likely a contributing factor. Recovery of SARS-CoV-2 has been associated with a variety of neuropsychiatric diseases, even in those that do not experience severe symptoms acutely (Taquet, Geddes, Husain, Luciano, & Harrison, 2021).
Table 1.
RNA viruses associated with neurologic and cognitive sequelae.
| Virus | Family | Genome Structure | Neuroinvasion | Neurologic sequelae | Refs |
|---|---|---|---|---|---|
| West Nile virus (WNV) | Flaviviridae | Positive-strand RNA | + | Parkisonism, memory impairment | (Weatherhead et al., 2015) |
| Dengue virus (DENV) | Flaviviridae | Positive-strand RNA | + | Guillain-Barré syndrome | (Carod-Artal, Wichmann, Farrar, & Gascón, 2013) |
| Zika virus (ZIKV) | Flaviviridae | Positive-strand RNA | + | Guillain-Barré syndrome, memory impairment | (da Silva et al., 2017) |
| Tick-borne encephalitis virus (TBEV) | Flaviviridae | Positive-strand RNA | + | Effects on vision, balance, neuropsychiatric complaints | (Bogovic & Strle, 2015) |
| Japanese encephalitis virus (JEV) | Flaviviridae | Positive-strand RNA | + | Movement disorders | (Turtle & Solomon, 2018) |
| St. Louis encephalitis virus (SLEV) | Flaviviridae | Positive-strand RNA | + | Convulsive and motor disorders | (Palmer & Finley, 1956) |
| Eastern equine encephalitis virus (EEEV), Western equine encephalitis virus (WEEV), Venezuelan equine encephalitis virus (VEEV) | Togaviridae | Positive-strand RNA | + | Convulsions, somnolence, intellectual disability, confusion, emotional instability, behavioral changes | (Ronca et al., 2016) |
| Poliovirus (PV) | Picornaviridae | Positive-strand RNA | + | Motor neuron disorders, word finding difficulties, concentration difficulties, memory impairment | (Li Hi Shing et al., 2019) |
| Enterovirus 71 (EV71) | Picornaviridae | Positive-strand RNA | + | Acute flaccid paralysis | (Hu, Jiang, & Peng, 2015; P.-N. Huang & Shih, 2014) |
| Measles virus (MV) | Paramyxoviridae | Negative-strand RNA | + | Movement disorders, acute disseminated encephalomyelitis, subacute sclerosing panencephaltis (SSPE) | (Buchanan & Bonthius, 2012; Fisher, Defres, & Solomon, 2015) |
| HIV | Retroviridae | Positive-strand RNA | + | HIV-associated neurocognitive disorder (HAND) | (Holroyd, Vishnevetsky, Srinivasan, & Saylor, 2020) |
| Influenza A virus (IAV) | Orthomyxoviridae | Segmented RNA | Atypical | Seizures, altered mental states | (Ekstrand, 2012; Liang et al., 2018; Muhammad Ismail et al., 2015) |
| Mouse hepatitis virus (MHV) | Betacoronaviridae | Positive-strand RNA | + | In rodents, demyelinating disease resembling multiple sclerosis | (Bergmann, Lane, & Stohlman, 2006) |
| Middle Eastern respiratory syndrome coronavirus (MERS-CoV) | Betacoronaviridae | Positive-strand RNA | Reported in animal models | Guillain-Barré syndrome, movement disorders | (Jiang et al., 2021; J. E. Kim et al., 2017) |
| Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) | Betacoronaviridae | Positive-strand RNA | Atypical | Headache, brain fog, changes in brain morphology | (Solomon, 2021) |
| Respiratory syncytial virus (RSV) | Pneumoviridae | Negative-strand RNA | - | Seizures, lethargy, strabismus, swallowing difficulties | (Bohmwald et al., 2018) |
Neuroimmune mechanisms that lead to neurological sequelae in the setting of recovery from infections with emerging RNA viruses are not well understood. Given that these mechanisms may be generalizable to multiple diseases of cognition or motor dysfunction, and that there are no treatments that reverse deficits, there is a pressing need to define the mechanisms that promote cognitive recovery from neurotropic viral infections and to identify biomarkers that identify patients at risk for memory disorders. Building on prior reviews (Klein et al., 2019; A. Soung & Klein, 2018), we will discuss recent data on alterations in glial functions that underlie defects in higher order processes including visuospatial recognition, learning and memory and motor control that occur as a result of infections with emerging RNA viruses.
RNA viruses linked to neurological sequelae
WNV, which emerged in North America in 1999, is the leading cause of domestically acquired arboviral disease in the United States (Sejvar, 2014), while ZIKV, the cause of a 2015 epidemic in South America, is still emerging (Weaver, 2017). WNV targets neurons throughout the adult human CNS, while ZIKV more specifically targets neurons in the hippocampus and cortex (Figueiredo et al., 2019). Both viruses are associated with neurological sequelae (Nicastri, Castilletti, Balestra, Galgani, & Ippolito, 2016; Sejvar, 2014; Zucker et al., 2017), which has been most extensively studied in WNV survivors. Studies evaluating rates of persistent memory impairment in patients previously diagnosed with WNV neuroinvasive disease (WNND) using neuropsychological testing report that 40–70% exhibit cognitive symptoms than continue to worsen for years after recovery from acute infection (Murray et al., 2018; Sadek et al., 2010; Weatherhead et al., 2015). Other neurotropic encephalitic arboviruses that lead to neurocognitive sequelae in US patients include LaCrosse, Eastern Equine Encephalitis, and Powassan viruses (Byrd, 2016; Hermance & Thangamani, 2017; Ronca, Dineley, & Paessler, 2016). Worldwide, neuroinvasive encephalitic arboviruses cause 50–100,000 cases/year with neurocognitive sequelae in most survivors (LaBeaud, Bashir, & King, 2011). Human immunodeficiency virus (HIV)-1, a retrovirus that invades the CNS via infected monocytes (Davis et al., 1992) leads to HIV-associated neurocognitive disorders (HAND), whose incidence has not decreased in the era of combination antiretroviral treatment (cART) (X. Chen, Zhang, & Zhang, 2020).There are currently no vaccines and treatments that mitigate persistent neurocognitive impairments associated with these neuroinvasive viral infections.
IAV and SARS-CoV-2, in particular, are the causes of worldwide pandemics with persistent neurocognitive diseases in survivors (Frontera et al., 2020; Heneka, Golenbock, Latz, Morgan, & Brown, 2020; Needham, Chou, Coles, & Menon, 2020; Serrano-Castro et al., 2020; Tansey et al., 2022). Pandemic H1N1 IAV RNA has been detected within the CNS of deceased patients who developed myelitis, meningitis, seizures, and acute necrotizing encephalopathy (Bohmwald, Gálvez, Ríos, & Kalergis, 2018; Liang, Yang, & Lin, 2018; Muhammad Ismail, Teh, & Lee, 2015; Ruisanchez-Nieva, Martinez-Arroyo, Gomez-Beldarrain, Bocos Portillo, & Garcia-Monco, 2017; Simon et al., 2013; Xia, Zhu, Hu, Wang, & Zhang, 2014). Patients that survive acute neurologic disease may also develop idiopathic Parkinson’s disease (Jang et al., 2009). Following acute SARS-CoV-2 infection, a majority of people recover within a few weeks; however >50% of recovered individuals continue to experience neurological diseases, which has been termed post-acute coronavirus syndrome (PACS) (Mehandru & Merad, 2022; Taquet, Luciano, Geddes, & Harrison, 2021; F. Wang, Kream, & Stefano, 2020). Vaccinated individuals continue to experience breakthrough infections, which reportedly reduces PACS by only 50% (Antonelli et al., 2022; Taquet, Luciano, et al., 2021). While there is little evidence of SARS-CoV-2 neuroinvasion (Espíndola et al., 2021; Paterson et al., 2020; Thakur et al., 2021), clinical studies report blood-brain barrier (BBB) disruption, and microglial activation in various brain regions upon post-mortem examination. In addition, a subset of individuals, including those with milder symptoms, suffer from a dysexecutive syndrome consisting of memory and learning impairments, inattention, disorientation, or poorly organized movements (Ortelli et al., 2021).
Higher cortical functions and systems impacted by infection with emerging neurotropic RNA viruses
Neuroimaging findings in patients with neuroinvasive viral diseases often reveal inflammation within various parts of the limbic system, including the cingulate gyrus/cortex, basal ganglia, thalami, and hippocampus (HPC). The HPC, in particular, exhibits higher susceptibility to viral infection, blood-brain barrier dysfunction, and inflammation (Chapman et al., 2012; Ivanidze et al., 2019; Johnston & Webster, 2009; Petito, 2004). The primary circuitry of the HPC includes trisynaptic sequential synapses between the entorhinal cortex (EC), the dentate gyrus (DG) granule cells, and pyramidal cells of the cornu ammoni (CA3 to CA1) (Basu & Siegelbaum, 2015; Chauhan, Jethwa, Rathawa, Chauhan, & Mehra, 2021). The HPC processes information derived from the EC and in turn influences cortical and subcortical areas that modulate complex behavioral processes involving recognition, formation and retrieval of declarative memories and spatial relationships. These circuits utilize excitatory amino acids aspartate or glutamate. Inhibitory GABAergic interneurons within the HPC modulate local synaptic transmission within this structure (Fazekas et al., 2022). Thus, alterations in the synthesis, storage, release, or inactivation of either the excitatory or inhibitory amino acids could lead to localized excitotoxicity with disruption of the circuit. Neural precursor cells that reside within the subgranular zone (SGZ) of the DG generate new neurons throughout life (Boldrini et al., 2018; Tartt et al., 2018). Studies in animal models indicate that adult neurogenesis is essential for visuospatial learning.
While most arboviruses infect neurons and/or astrocytes across brain regions, animal studies of ZIKV infection demonstrate high tropism to adult hippocampal neurons (Figueiredo et al., 2019; Garber et al., 2019), SGZ neural progenitor cells (H. Li et al., 2016), astrocytes (van den Pol, Mao, Yang, Ornaghi, & Davis, 2017), and glial progenitor cells (C. Li et al., 2018; Schultz et al., 2021), consistent with reports of damage and dysfunction of this brain region leading to neurocognitive sequelae (da Silva, Frontera, Bispo de Filippis, Nascimento, & for the RIO-GBS-ZIKV Research Group, 2017; Zucker et al., 2017). Damage to the HPC produces large and persistent effects on behavior, with cognitive impairments similar to those observed in human dementias. Consistent with this, survivors of arboviral infections exhibit features that overlap with neurodegenerative dementias such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). Magnetic resonance imaging (MRI) of patients that have recovered from WNV, including those without neuroinvasive disease, report atrophy of the posterior cingulate and insular cortices, and the hippocampus and entorhinal regions in the temporal lobe (Rev in (Borisow, Mori, Kuwabara, Scheel, & Paul, 2018), as observed in AD (Ledig, Schuh, Guerrero, Heckemann, & Rueckert, 2018). Involvement of the substantia nigra, which exhibits degeneration in patients with PD, has also been reported (Bosanko et al., 2003; Schafernak & Bigio, 2006) as has parkinsonism (Lenka, Kamat, & Mittal, 2019). While learning and memory disorders appear to worsen over time in WNV-recovered patients, parkinsonism and other movement disorders eventually resolve (Lenka et al., 2019). Recently, MRI-based studies examining 60 COVID-19-recovered patients at three months after acute infection reveal micro-structural alterations within gray matter areas involving the olfactory system, hippocampus and cingulate cortex, consistent with ongoing anosmia, tremors, impaired mobility and memory loss (Lu et al., 2020). Overall, these findings support the need for larger studies utilizing neuropsychological testing and neuroimaging in patients with post-infectious neurologic dysfunction after recovery from respiratory viruses.
Glia recognize neuroinvasive viral infections via pattern recognition, cytokine signaling, and damage sensing
Viruses that invade the CNS may infect glia, neurons, or both, leading to inflammation, tissue damage, or cell death (Fig. 2). Depending on the type of virus, glia use a variety of mechanisms to detect neuroinvasive viruses. One such mechanism is through the expression of pattern recognition receptors (PRRs), surface-expressed or cytosolic receptors that recognize common and conserved elements of pathogens that are not generally shared by host cells. PRR engagement generally leads to a downstream signaling cascade resulting in the expression of many host genes with antiviral effects. Neurons and glia express a range of PRRs in both humans and mice, as recently reviewed (Gern et al., 2021; L. Li, Acioglu, Heary, & Elkabes, 2021). Microglia especially express a wide breadth of PRRs, including toll-like receptors (TLRs) 1–10, allowing recognition of viral double-stranded RNA, single-stranded RNA, and viral envelope proteins (Gern et al., 2021; Martín-García & González-Scarano, 2009). Interestingly, astrocytic expression has also been reported for almost all of these TLRs (Furr & Marriott, 2012; Gern et al., 2021), albeit at lower levels than that observed in microglia. Oligodendrocytes, conversely, do not appear to express significant numbers or amounts of PRRs, but have been reported to express low levels of TLR-2 and TLR-3 (Martín-García & González-Scarano, 2009). The importance of TLRs in specifically protecting the CNS against viral encephalitides is clearly illustrated by the fact that TLR-3 deficiency in mice and humans confers heightened risk of herpes simplex virus 1 (HSV-1, a DNA virus) encephalitis without major alterations in peripheral infection severity (Reinert et al., 2012; Zhang et al., 2007). In fact, in the absence of TLR-3, astrocytes fail to mount an innate response to HSV-1 while microglia retain the ability to respond, likely reflecting differences in their PRR repertoires (Sato et al., 2018).
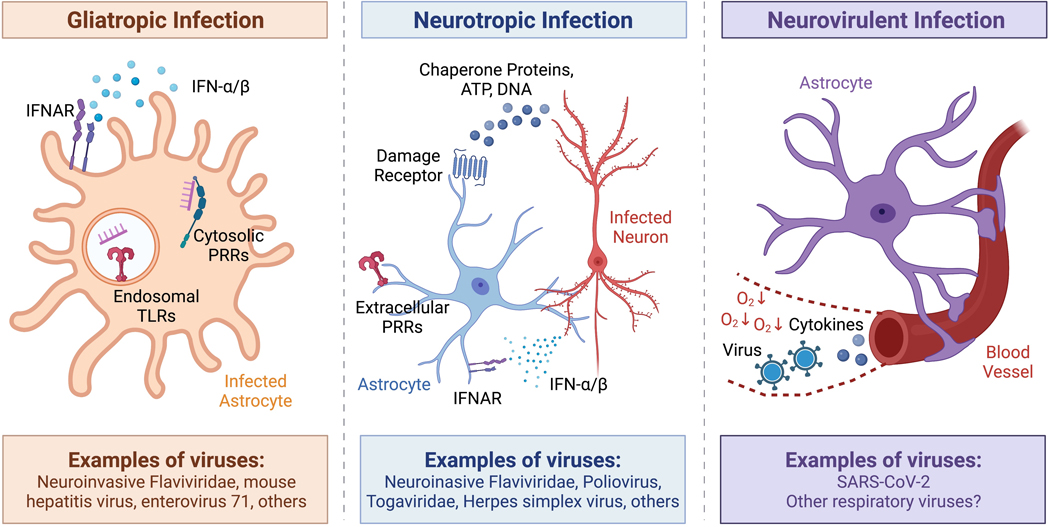
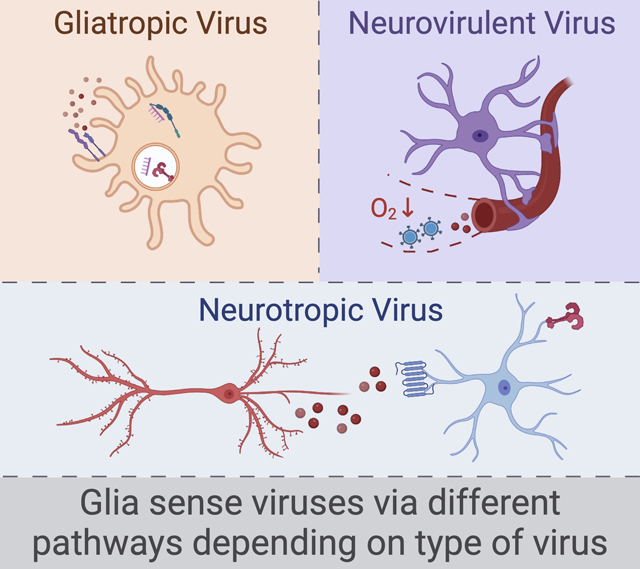
Figure 2. Glia use distinct detection pathways to respond to different types of viral infection.
Viruses associated with neurocognitive sequelae may be gliatropic, neurotropic, neurovirulent, or some combination of each. Glia can recognize gliatropic viruses (left) via intracellular innate sensing receptors, such as endosomal Toll-like receptors or cytosolic pattern-recognition receptors that bind to viral nucleic acids. Infected glia can further amplify their innate immune response via autocrine cytokine signaling. Glia may recognize neurotropic viral infection (middle) via paracrine cytokine signaling. Infected neurons may also signal to glia via the release of damage-associated molecules such as chaperone proteins, host DNA, or ATP. Finally, glia may mediate neuroinflammation in response to neurovirulent viruses (right) via the sensing or translocation of viral particles or cytokines from the circulation. Hypoxia resulting from respiratory disease could amplify these signals via alterations to the blood-brain barrier.
Upon PRR engagement, microglia and astrocytes produce cytokines and other inflammatory mediators such as TNF-α, IL-12, CXCL2, CXCL10, and cyclooxygenase-2 (Chung & Benveniste, 1990; Esen & Kielian, 2006; J.-I. Kim et al., 2007; Phares, Stohlman, Hinton, & Bergmann, 2013; Walsh, Perry, & Minghetti, 2000). Likewise, TLR engagement can also trigger the type I interferon pathway (Zheng, Chen, Chu, Zhu, & Jin, 2019), leading to secretion of interferon-α and interferon-β (IFN-α/β). These cytokines signal through the interferon-α receptor (IFNAR) in both an autocrine and paracrine fashion, initiating a regional state of antiviral alarm by causing the upregulation of hundreds of interferon stimulated genes (Schneider, Chevillotte, & Rice, 2014). Therefore, TLR signaling in CNS cells can directly lead to neuroinflammation, with impacts on cognition and behavior. Mice treated systemically or intracranioventricularly with lipopolysaccharide, a potent bacterial TLR-4 ligand, displayed microglial activation and acute underperformance in Morris water maze, passive avoidance, and pole climb tests of memory and motor coordination (Zhao et al., 2019). In an in vitro assay of BBB permeability, exposure to agonists for TLR7 or the intracellular PRRs MDA5 or RIG-I increased barrier permeability in an IFNAR-dependent fashion (Daniels et al., 2014).
The neuroinflammatory effects of neural cell PRR engagement can contribute to neurologic disease. For example, TLR-7 contributed to pathogenesis in an animal model of enterovirus 71 (EV71) in which neonatal mice were infected intracranially (Luo et al., 2019). In this study, mice deficient in TLR-7 exhibited enhanced infection of astrocytes, enhanced neural cell apoptosis, and reduction in the integrity and amount of neurofilament protein (Luo et al., 2019). Interestingly, these changes in viral tropism and neuronal damage occurred in the absence of overall changes in viral titer in the brain (Luo et al., 2019).
In cases in which glia do not directly play host to viral infections, glia may rely on similar signals from infected neurons to initiate an immune response (Fig. 2). Like glia and immune cells, neurons also express PRRs that can lead to the production of cytokines that signal to nearby glia. Infected cells may suffer injury and consequently release proteases, complement factors, and damage-associated molecular patterns (DAMPs) such as host DNA, ATP, and chaperone proteins (Karve, Taylor, & Crack, 2016). These factors can signal to glial cells through complement receptors, purinergic receptors, TLRs, double-stranded DNA receptors, and others (G. Y. Chen & Nuñez, 2010; Davalos et al., 2005; Kanmogne & Klein, 2021). DAMPs are produced by damaged or dying cells during infection as well as other sources of CNS insult, such as neurodenerative disease, sterile inflammation, ischemic stroke, or traumatic injury (Bradbury & Burnside, 2019; Kigerl, de Rivero Vaccari, Dietrich, Popovich, & Keane, 2014; Yates, Anthony, Ruitenberg, & Couch, 2019). Therefore, damage-detection receptors may be one mechanism explaining similarities between the downstream cognitive sequelae of diverse types of CNS insults. This principle is illustrated by studies of traumatic spinal cord injury that link acute damage to TLR-4 signaling, astrocytic production of IL-1β and other cytokines, and leukocyte recruitment (Dickens et al., 2017; Didangelos et al., 2016).
Finally, mammalian mechanisms of viral detection are still being discovered. A recent report found that monocytes can respond to HSV-1 independent of PRRs via viral-mediated degradation of a negative regulator of the type I interferon pathway (Gaidt et al., 2021). This so-called “self-guarding” could play a similar role in allowing cells of the CNS to detect viral infection and other types of neuronal injury.
Astrocytes regulate the blood-brain barrier and respond to viral infection via cytokine signaling
Glia cooperate with endothelial and mural cells to regulate the blood-brain barrier (BBB) (Daneman & Prat, 2015). In the CNS, BBB regulation is essential to the immune response to viruses; a permeable BBB may allow pathogen entry to the CNS via passive diffusion or host cell transport (Ayala-Nunez & Gaudin, 2020), but is also necessary to allow peripheral immune cells to enter the CNS to aid in pathogen clearance. In response to signals from neurons and immune cells, astrocytes regulate BBB permeability via their cellular processes, which ensheath blood vessels and vascular tubes (Daneman, 2012; Daneman & Prat, 2015).
Neuroinvasive infection may either precede or follow a change in BBB permeability. Once a viral infection has been established in the brain, cytokine signals derived from within the CNS can lead to alterations in BBB permeability. For example, IFNAR signaling in cerebellar astrocytes leads to an decrease in BBB permeability after intracranial inoculation of West Nile virus (Daniels et al., 2017). Astrocytic type I interferon signaling is also necessary to activate microglia and recruit peripheral immune cells after vesicular stomatitis virus (Chhatbar et al., 2018), and is critical for protection and herpesvirus encephalitis (Hayes, Giraldo, Wilcox, & Longnecker, 2022). Similarly, interferon lambda (IFNλ) signaling via the interferon lambda receptor (IFNLR1) is critical for BBB integrity during WNV infection (Lazear et al., 2015), but the role astrocytes play in this signaling pathway has not yet been fully elucidated.
In the opposite case of peripheral or systemic viral infection preceding neuroinvasive infection, cytokines in serum may signal via the luminal side of the BBB to cause an increase in permeability, facilitating viral entry. In vitro models of the BBB suggest that exposure to human serum may increase BBB permeability, and the extent of this effect was correlated with levels of chemokine ligands 12 and 7 (CCL12 and CCL7), interleukin 13 (IL-13), and interleukin 1β (IL-1β) (Ho & Kelly, 2020). In SIV infection of non-human primates, changes in microglial interleukin-6 (IL-6) were found to precede viral invasion into the CNS (Gopalakrishnan et al., 2021). Proper regulation of leukocyte extravasation through the BBB is essential for viral control and prevention of serious encephalopathies, as indicated by the association between Natulizumab blocking leukocyte extravasation into the brain and risk of serious opportunistic CNS infections by human polyomavirus 2 (Bloomgren et al., 2012).
Astrocytes have a variety of roles during viral infection of the CNS beyond regulating permeability of the BBB. Notably, astrocytes undergo proliferation and other reactogenic changes following viral infection and other insults to the CNS, collectively referred to as astrogliosis or reactive astrogenesis (Escartin et al., 2021). In the context of viral infection, reactive astrogenesis may be triggered by direct infection of astrocytes or by pro-inflammatory signals from neighboring neurons, glia, or infiltrating immune cells. Reactive astrocytes produce cytokines with complex roles including promoting viral clearance as well as mediating adverse neurologic changes. After infection with a gliotropic variant of the betacoronavirus mouse hepatitis virus (MHV), astrocytic CXCL9 and CXCL10 promotes the accumulation of virus-specific IgG and antibody secreting cells within the CNS (Phares et al., 2013), supporting viral clearance. Conversely, astrocyte-derived IL-1β during a model of West Nile neuroinvasive disease is required for synapse elimination and post-infectious deficits in hippocampal neurogenesis and spatial learning (Garber et al., 2018). In this example, astrocyte IL-1β may signal directly to neural stem cells, promoting further generation of IL-1β+ reactive astrocytes at the expense of normal neurogenesis (A. L. Soung et al., 2022). Likewise, astrocyte NFκB signaling promotes astrogliosis, α-synuclein aggregation, and neuronal loss during recovery from Western equine encephalitis virus (Bantle et al., 2021). In a mouse model of encephalitis caused by the DNA virus herpes simplex virus, astrocytes produced CXCL1, recruiting neutrophils that further enhanced disease severity (Michael et al., 2020). Conversely, after intracranial infection with the RNA virus human enterovirus A71 (a cause of hand, foot, and mouth disease with occasional severe neuropathology), mouse astrocytes produced high levels of CXCL1, mimicking results observed in cerebrospinal fluid of patients, without significant neutrophil recruitment (Gunaseelan et al., 2022). Pharmacologic perturbation of this pathway lessened disease severity, suggesting that astrocyte-derived CXCL1 could promote neuropathogenesis either by direct effects on neurons or by recruitment of pro-inflammatory immune cells (Gunaseelan et al., 2022). The interferon-stimulated gene viperin is induced in astrocytes and other CNS cell types during viral infection, and restricts replication of the neurotropic flaviviruses TBEV, ZIKV, and Langat virus, although it remains unclear if astrocytic expression specifically is necessary for this protective effect (Lindqvist, Kurhade, Gilthorpe, & Överby, 2018; Lindqvist et al., 2016). These studies illustrate the complex context-dependence of astrocyte cytokine responses.
Astrocytes also participate in the phagocytosis and clearance of debris during viral encephalitides, a role shared with microglia. This process may be imperative for recovery but may also damage synapses. In the context of microglial depletion during ZIKV infection of the adult mouse brain, astrocytes appeared to increase their phagocytic capacity, suggesting a compensatory mechanism with unknown effects on neuronal health (Enlow et al., 2021). In another study of neonatal mice infected with ZIKV, convalescent mice maintained small parenchymal foci of viral material surrounded by activated astrocytes and microglia up to one year after recovery (Ireland et al., 2020). Astrocytes appear to regionally upregulate superoxide dismutase in response to viral infection in a non-human primate model of HAND, suggesting a role for astrocytes in quenching reactive oxygen species and reducing neuroinflammation (Sullivan et al., 2020), yet also contribute to amyloidosis in a similar model (Sil et al., 2020). Together, these studies highlight that reactive astrocytes affect both response and recovery during viral infections in a manner that is highly dependent on the host context and specific viral pathogen (Fig. 1).
Microglial activation affects neuroinvasive viral clearance and neurocognitive recovery
Microglia are resident myeloid cells that enter the brain during early development (Ginhoux et al., 2010). While they are not glia, they exhibit many neuroprotective functions similar to astrocytes, especially with regard to synaptic plasticity (Colonna & Butovsky, 2017; Nguyen et al., 2020; Sipe et al., 2016; Waltl & Kalinke, 2022). Microglia are key players during viral infection of the CNS, with both therapeutic and pathogenic roles depending on the context (Chhatbar & Prinz, 2021). For example, microglia are essential to the early immune response to MHV infection; mice succumb to intracerebral infection if microglia are depleted prior to infection using an inhibitor of colony stimulating factor 1 receptor (Wheeler, Sariol, Meyerholz, & Perlman, 2018). Likewise, microglial depletion affects virologic control in mouse models of DENV, ZIKV, WNV, and JEV (Enlow et al., 2021; Funk & Klein, 2019; Seitz, Clarke, & Tyler, 2018; Tsai et al., 2016). During vesicular stomatitis virus infection, microglia initiate a type I interferon response that limits transsynaptic viral spread (Drokhlyansky et al., 2017).
In some cases, microglia appear to have beneficial roles not just in viral clearance, but also in promoting neurologic and cognitive recovery. Depletion of microglia during MHV clearance impaired remyelination, suggesting that microglia guide oligodendrocyte remyelination after viral infection by clearing myelin debris and upregulating factors that promote oligodendrocyte maturation, such as galectin-3 and insulin-like growth factor 1 (Sariol et al., 2020). During ZIKV infection of adult mice, microglia undergo activation, correlate with virologic control, and appear to phagocytose debris (Enlow et al., 2021). Depletion of microglia during TMEV infection similarly allowed increased viral spread and exacerbated post-infectious seizures and hippocampal damage (Waltl et al., 2018). However, genetic deletion of CCR2 or CX3CR1 (which impacts both microglial and other myeloid responses) prevented hippocampal damage but did not affect seizure development, illustrating the complexity of the role of microglia in viral infection of the CNS (Käufer et al., 2018).
Microglia have also been implicated in detrimental neurologic outcomes of CNS viral infection. In a mouse model of post-infectious West Nile neurologic disease, microglia contribute to aberrant synaptic pruning after viral clearance via complement proteins C1q and C3 (Vasek et al., 2016). Microglia responding to T cell-derived IFNγ were also implicated in learning deficits and elimination of neuronal nuclei and post-synaptic terminals after ZIKV infection (Garber et al., 2019). In mice that recovered from West Nile neurologic disease, single cell RNA sequencing revealed that microglia underwent sustained changes, with transcriptional profiles that resembled those found in neurodegenerative diseases (Rosen et al., 2021). Similarly, unique microglial transcriptomic profiles were also observed after neurotropic MHV infection and glycoprotein-deleted Rabies virus infection (K. W. Huang & Sabatini, 2020; Syage et al., 2020). These studies illustrate how advances in single-cell transcriptomics have facilitated the differentiation of microglia from infiltrating macrophages, allowing more in-depth characterization of microglia in infected animals.
Microglia can also be directly infected by viruses, and serve as an important latent reservoir for HIV as well as a potential barrier to HIV cure strategies (Cosenza, Zhao, Si, & Lee, 2002). Astrocytes may similarly contribute to the cellular HIV reservoir (Lutgen et al., 2020; Valdebenito, Castellano, Ajasin, & Eugenin, 2021). The emerging RNA viruses Oropouche virus and Middle Eastern respiratory syndrome coronavirus infect neurons and glia in humanized mice or human brain slice cultures, leading to complement-mediated BBB changes and microglial activation (Almeida et al., 2021; Jiang et al., 2021). Future studies will determine whether these effects are also observed in human patients.
A Potential Role for Glia in Post-Acute Coronavirus Syndrome
Infection with SARS-CoV-2 has been associated with a variety of post-infectious syndromes including the development of new autoimmune conditions, ongoing respiratory deficiencies, and adverse cognitive and neurologic sequelae. Collectively, these conditions have been termed “long COVID” or post-acute coronavirus syndrome (PACS). Notably, these post-infectious syndromes can include stark changes to the CNS, including splenial alterations to the corpus collosum, reduction in grey matter thickness, diffuse edema, gliosis, microglial and astrocyte activation, and reductions in brain size (Abdel-Mannan et al., 2020; Douaud et al., 2022; Pajo, Espiritu, Apor, & Jamora, 2021; Schwabenland et al., 2021). Current evidence largely suggests that SARS-CoV-2 does not typically infect or invade the central nervous system and viral material is very rarely found in the brain parenchyma of patients (Pajo et al., 2021; Poloni et al., 2021; Solomon, 2021). However, unique clusters of activated microglia, astrocytes, and CD8+ T cells have been identified in the brain stem and olfactory bulb of deceased patients (Schwabenland et al., 2021). These cellular “nodules” differed from those observed in multiple sclerosis or non-viral severe respiratory failure patients, and correlated with axonal damage as well as the presence of SARS-CoV-2 viral components in the vascular compartments of the CNS (Schwabenland et al., 2021). There are many open questions about how SARS-CoV-2 infection leads to neurologic and cognitive sequelae. A variety of potential explanations have been proposed, including hypoxia, cytokine storm, low levels of viral invasion into the CNS, or induction of autoimmunity (Franke et al., 2021; Kumar et al., 2021; Meinhardt et al., 2021; Vargas et al., 2020). Glia could play a role in several of these potential pathways (Fig. 2), and early results from single nucleus RNA sequencing studies of COVID-19 patient brains indeed point to glial alterations associated with SARS-CoV-2 infection (Yang et al., 2021).
Hypoxia after non-infectious injury (cardiac arrest, ischemic stroke, and traumatic brain injury) is associated with adverse neurologic and cognitive sequelae including neuronal dysfunction and death, cerebral edema, and Parkinson’s disease (Brownlee, Wilson, Curran, Lyttle, & McCann, 2020; Nalivaeva & Rybnikova, 2019; Sekhon, Ainslie, & Griesdale, 2017). Even in the absence of infection, hypoxia has diverse effects on both the blood-brain barrier and CNS cytokine profiles. Experimentally-induced hypoxia leads to vascular remodeling and an increase in vascular permeability, an effect attributed to pericytic rather than astrocytic hypoxia inducible factor 1 (Baumann et al., 2022). In patients recovering from cardiac arrest, hypoxia but not normoxia was associated with cerebral release of IL-6, glial fibrillary acidic protein, tau, and other biomarkers of neuroglial injury (Hoiland et al., 2021). Respiratory distress, hypoxia, and induction of a pro-thrombotic state are associated with severe COVID-19 (Mehandru & Merad, 2022; Serebrovska, Chong, Serebrovska, Tumanovska, & Xi, 2020), and evidence from other non-neuroinvasive viral infections such as respiratory syncytial virus supports the theory that virus-mediated lung pathology can lead to increased BBB permeability and neuroinflammation (Bohmwald et al., 2021). In PACS, symptoms such as brain fog occur even following mild cases (Bliddal et al., 2021), suggesting that severe hypoxia is not critical to the development of PACS. Subclinical hypoxia could still play a role in post-infectious sequelae.
Altered soluble mediator or immune cell levels in the circulation following infection with SARS-CoV-2 could also underly the development of neurologic and cognitive symptoms associated with PACS. Severe COVID-19 is characterized by cytokine storm, a condition in which levels of circulating immune mediators, including TNF-α, IL-1, and IL-6, reach levels elevated enough to be life-threatening (Fajgenbaum & June, 2020; Kumar et al., 2021; Merad, Blish, Sallusto, & Iwasaki, 2022). Cytokine storm is not necessary for the development of PACS (Taquet, Geddes, et al., 2021). However, even in mild SARS-CoV-2 infection, mild hypoxia combined with circulating immune factors could synergize in their signaling to pericytes and astrocytes, leading to opening of the BBB (Reynolds & Mahajan, 2021) and resulting cytokine and immune cell infiltration, neuroinflammation, and adverse effects on neuronal circuits.
Future Outlook
Glial cells play diverse roles during viral infections, functioning as target cells, pro-inflammatory bystanders, mediators of neurologic damage, and facilitators of neurologic recovery depending on the host, immune context, and pathogen. Many open questions remain regarding the functions of glia during viral infections, including how they interact with infiltrating immune cells, how they promote neuronal survival vs. synaptic damage, and how they participate in brain recovery after viral clearance. In addition, the COVID-19 pandemic has firmly demonstrated that even in the absence of viral neuroinvasion, viral infections can still lead to significant cognitive impairments, a process which likely involves glia. Future research into these topics must employ a mixture of patient sample analysis, ex vivo organoid models, and animal models, as no single approach can fully shed light on the complex relationship between glia, neurons, and immune cells in the human brain. As research into the role of glia during viral infections continues, we anticipate the advent of pharmacologic interventions that will modulate glial functions during viral infections to promote neuronal and cognitive health.
Main Points.
Neuroinvasive and neurovirulent viral infections lead to acute and chronic neurologic sequelae
Glia use distinct viral sensing pathways depending on the tropism of the virus
Glial immune responses can both protect and damage neuronal tissues
Acknowledgments:
This work was supported by NIH grants R35 NS122310 (R.S.K.), T32 AI007172 (V.D.), and F32 NS126238 (V.D.). Figures were created with BioRender.com with a license to publish.
Footnotes
The authors declare no conflicts of interest.
References
- Abdel-Mannan O, Eyre M, Löbel U, Bamford A, Eltze C, Hameed B, … Hacohen Y. (2020). Neurologic and Radiographic Findings Associated With COVID-19 Infection in Children. JAMA Neurology, 77(11), 1440–1445. doi: 10.1001/jamaneurol.2020.2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelson JD, Sapp RW, Brott BK, Lee H, Miyamichi K, Luo L, … Shatz CJ (2016). Developmental Sculpting of Intracortical Circuits by MHC Class I H2-Db and H2-Kb. Cerebral Cortex, 26 4, 1453–1463. doi: 10.1093/cercor/bhu243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida GM, Souza JP, Mendes ND, Pontelli MC, Pinheiro NR, Nogueira GO, … Sebollela A. (2021). Neural Infection by Oropouche Virus in Adult Human Brain Slices Induces an Inflammatory and Toxic Response. Frontiers in Neuroscience, 15, 674576. doi: 10.3389/fnins.2021.674576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor S, & Woodroofe MN (2014). Innate and adaptive immune responses in neurodegeneration and repair. Immunology, 141. doi: 10.1111/imm.12134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli M, Penfold RS, Merino J, Sudre CH, Molteni E, Berry S, … Steves CJ (2022). Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: A prospective, community-based, nested, case-control study. The Lancet Infectious Diseases, 22(1), 43–55. doi: 10.1016/S1473-3099(21)00460-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala-Nunez NV, & Gaudin R. (2020). A viral journey to the brain: Current considerations and future developments. PLoS Pathogens, 16(5), e1008434. doi: 10.1371/journal.ppat.1008434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantle CM, Rocha SM, French CT, Phillips AT, Tran K, Olson KE, … Tjalkens RB (2021). Astrocyte inflammatory signaling mediates α-synuclein aggregation and dopaminergic neuronal loss following viral encephalitis. Experimental Neurology, 346, 113845. doi: 10.1016/j.expneurol.2021.113845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch K, Deczkowska A, David E, Castellano JM, Miller O, Kertser A, … Schwartz M. (2014). Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science, 346, 89–93. doi: 10.1126/science.1252945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu J, & Siegelbaum SA (2015). The Corticohippocampal Circuit, Synaptic Plasticity, and Memory. Cold Spring Harbor Perspectives in Biology, 7(11), a021733. doi: 10.1101/cshperspect.a021733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann J, Tsao C-C, Patkar S, Huang S-F, Francia S, Magnussen SN, … Ogunshola OO (2022). Pericyte, but not astrocyte, hypoxia inducible factor-1 (HIF-1) drives hypoxia-induced vascular permeability in vivo. Fluids and Barriers of the CNS, 19(1), 6. doi: 10.1186/s12987-021-00302-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann CC, Lane TE, & Stohlman SA (2006). Coronavirus infection of the central nervous system: Host-virus stand-off. Nature Reviews. Microbiology, 4(2), 121–132. doi: 10.1038/nrmicro1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank T, Detje CN, Spiess A, Hagemeyer N, Brendecke SM, Wolfart J, … Prinz M. (2016). Brain Endothelial- and Epithelial-Specific Interferon Receptor Chain 1 Drives Virus-Induced Sickness Behavior and Cognitive Impairment. Immunity, 44 4, 901–912. doi: 10.1016/j.immuni.2016.04.005 [DOI] [PubMed] [Google Scholar]
- Bliddal S, Banasik K, Pedersen OB, Nissen J, Cantwell L, Schwinn M, … Feldt-Rasmussen U. (2021). Acute and persistent symptoms in non-hospitalized PCR-confirmed COVID-19 patients. Scientific Reports, 11(1), 13153. doi: 10.1038/s41598-021-92045-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, … Bozic C. (2012). Risk of natalizumab-associated progressive multifocal leukoencephalopathy. The New England Journal of Medicine, 366(20), 1870–1880. doi: 10.1056/NEJMoa1107829 [DOI] [PubMed] [Google Scholar]
- Bogovic P, & Strle F. (2015). Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. World Journal of Clinical Cases, 3(5), 430–441. doi: 10.12998/wjcc.v3.i5.430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmwald K, Gálvez NMS, Ríos M, & Kalergis AM (2018). Neurologic Alterations Due to Respiratory Virus Infections. Frontiers in Cellular Neuroscience, 12, 386. doi: 10.3389/fncel.2018.00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmwald K, Soto JA, Andrade-Parra C, Fernández-Fierro A, Espinoza JA, Ríos M, … Kalergis AM (2021). Lung pathology due to hRSV infection impairs blood-brain barrier permeability enabling astrocyte infection and a long-lasting inflammation in the CNS. Brain, Behavior, and Immunity, 91, 159–171. doi: 10.1016/j.bbi.2020.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, … Mann JJ (2018). Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell, 22(4), 589–599.e5. doi: 10.1016/j.stem.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisow N, Mori M, Kuwabara S, Scheel M, & Paul F. (2018). Diagnosis and Treatment of NMO Spectrum Disorder and MOG-Encephalomyelitis. Frontiers in Neurology, 9, 888. doi: 10.3389/fneur.2018.00888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosanko CM, Gilroy J, Wang A-M, Sanders W, Dulai M, Wilson J, & Blum K. (2003). West Nile Virus Encephalitis Involving the Substantia Nigra: Neuroimaging and Pathologic Findings With Literature Review. Archives of Neurology, 60(10), 1448. doi: 10.1001/archneur.60.10.1448 [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, & Burnside ER (2019). Moving beyond the glial scar for spinal cord repair. Nature Communications, 10(1), 3879. doi: 10.1038/s41467-019-11707-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee NNM, Wilson FC, Curran DB, Lyttle N, & McCann JP (2020). Neurocognitive outcomes in adults following cerebral hypoxia: A systematic literature review. NeuroRehabilitation, 47(2), 83–97. doi: 10.3233/NRE-203135 [DOI] [PubMed] [Google Scholar]
- Buchanan R, & Bonthius DJ (2012). Measles Virus and Associated Central Nervous System Sequelae. Seminars in Pediatric Neurology, 19(3), 107–114. doi: 10/f37pr5 [DOI] [PubMed] [Google Scholar]
- Butovsky O, & Weiner HL (2018). Microglial signatures and their role in health and disease. Nature Reviews Neuroscience, 19, 622–635. doi: 10.1038/s41583-018-0057-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd BD (2016). La Crosse Encephalitis. North Carolina Medical Journal, 77, 330–333. doi: 10.18043/ncm.77.5.330 [DOI] [PubMed] [Google Scholar]
- Carod-Artal FJ, Wichmann O, Farrar J, & Gascón J. (2013). Neurological complications of dengue virus infection. The Lancet Neurology, 12(9), 906–919. doi: 10.1016/S1474-4422(13)70150-9 [DOI] [PubMed] [Google Scholar]
- Chapman TR, Barrientos RM, Ahrendsen JT, Hoover JM, Maier SF, & Patterson SL (2012). Aging and infection reduce expression of specific brain-derived neurotrophic factor mRNAs in hippocampus. Neurobiology of Aging, 33(4), 832.e1-832.e14. doi: 10.1016/j.neurobiolaging.2011.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan P, Jethwa K, Rathawa A, Chauhan G, & Mehra S. (2021). The Anatomy of the Hippocampus. In Laboratory of Ischemic and Neurodegenerative Brain Research, Mossakowski Medical Research Institute, Polish Academy of Sciences, Warsaw, Poland: & Ryszard P(Eds.), Cerebral Ischemia (pp. 17–30). Exon Publications. doi: 10.36255/exonpublications.cerebralischemia.2021.hippocampus [DOI] [Google Scholar]
- Chen GY, & Nuñez G. (2010). Sterile inflammation: Sensing and reacting to damage. Nature Reviews. Immunology, 10(12), 826–837. doi: 10.1038/nri2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang T, & Zhang Y. (2020). Endoplasmic reticulum stress and autophagy in HIV-1-associated neurocognitive disorders. Journal of NeuroVirology, 26(6), 824–833. doi: 10.1007/s13365-020-00906-4 [DOI] [PubMed] [Google Scholar]
- Chen Z, & Palmer TD (2008). Cellular repair of CNS disorders: An immunological perspective. Human Molecular Genetics, 17 R1, R84–92. doi: 10.1093/hmg/ddn104 [DOI] [PubMed] [Google Scholar]
- Cheng X, Jin G, Zhang X, Tian M, & Zou L. (2011). Stage-dependent STAT3 activation is involved in the differentiation of rat hippocampus neural stem cells. Neuroscience Letters, 493, 18–23. doi: 10.1016/j.neulet.2011.02.006 [DOI] [PubMed] [Google Scholar]
- Chhatbar C, Detje CN, Grabski E, Borst K, Spanier J, Ghita L, … Kalinke U. (2018). Type I Interferon Receptor Signaling of Neurons and Astrocytes Regulates Microglia Activation during Viral Encephalitis. Cell Reports, 25(1), 118–129.e4. doi: 10.1016/j.celrep.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatbar C, & Prinz M. (2021). The roles of microglia in viral encephalitis: From sensome to therapeutic targeting. Cellular & Molecular Immunology, 18(2), 250–258. doi: 10.1038/s41423-020-00620-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung IY, & Benveniste EN (1990). Tumor necrosis factor-alpha production by astrocytes. Induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. Journal of Immunology (Baltimore, Md.: 1950), 144(8), 2999–3007. [PubMed] [Google Scholar]
- Clark IC, Gutiérrez-Vázquez C, Wheeler MA, Li Z, Rothhammer V, Linnerbauer M, … Quintana FJ (2021). Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science (New York, N.Y.), 372(6540). doi: 10.1126/science.abf1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, & Butovsky O. (2017). Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annual Review of Immunology, 35, 441–468. doi: 10.1146/annurev-immunol-051116-052358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza MA, Zhao M-L, Si Q, & Lee SC (2002). Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathology (Zurich, Switzerland), 12(4), 442–455. doi: 10.1111/j.1750-3639.2002.tb00461.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossetti C, Alfaro-Cervelló C, Donegà M, Tyzack GE, & Pluchino S. (2012). New perspectives of tissue remodelling with neural stem and progenitor cell-based therapies. Cell and Tissue Research, 349, 321–329. doi: 10.1007/s00441-012-1341-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva IRF, Frontera JA, Bispo de Filippis AM, Nascimento OJM do, & for the RIO-GBS-ZIKV Research Group. (2017). Neurologic Complications Associated With the Zika Virus in Brazilian Adults. JAMA Neurology, 74(10), 1190. doi: 10.1001/jamaneurol.2017.1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R. (2012). The blood-brain barrier in health and disease. Annals of Neurology, 72(5), 648–672. doi: 10.1002/ana.23648 [DOI] [PubMed] [Google Scholar]
- Daneman R, & Prat A. (2015). The blood-brain barrier. Cold Spring Harbor Perspectives in Biology, 7(1), a020412. doi: 10.1101/cshperspect.a020412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels BP, Holman DW, Cruz-Orengo L, Jujjavarapu H, Durrant DM, & Klein RS (2014). Viral pathogen-associated molecular patterns regulate blood-brain barrier integrity via competing innate cytokine signals. MBio, 5(5), e01476–01414. doi: 10.1128/mBio.01476-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels BP, Jujjavarapu H, Durrant DM, Williams JL, Green RR, White JP, … Klein RS (2017). Regional astrocyte IFN signaling restricts pathogenesis during neurotropic viral infection. The Journal of Clinical Investigation, 127(3), 843–856. doi: 10.1172/JCI88720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D, Leslie S, Morozov YM, Duque A, Rakic P, Dyck CH van, … Arnsten AFT (2020). Classical complement cascade initiating C1q protein within neurons in the aged rhesus macaque dorsolateral prefrontal cortex. Journal of Neuroinflammation, 17. doi: 10.1186/s12974-019-1683-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datwani A, McConnell MJ, Kanold PO, Micheva KD, Busse B, Shamloo M, … Shatz CJ (2009). Classical MHCI Molecules Regulate Retinogeniculate Refinement and Limit Ocular Dominance Plasticity. Neuron, 64, 463–470. doi: 10.1016/j.neuron.2009.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, … Gan W-B (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nature Neuroscience, 8(6), 752–758. doi: 10.1038/nn1472 [DOI] [PubMed] [Google Scholar]
- Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, … Wiley CA (1992). Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology, 42(9), 1736-1736. doi: 10.1212/WNL.42.9.1736 [DOI] [PubMed] [Google Scholar]
- Denke C, Balzer F, Menk M, Szur S, Brosinsky G, Tafelski S, … Deja M. (2018). Long-term sequelae of acute respiratory distress syndrome caused by severe community-acquired pneumonia: Delirium-associated cognitive impairment and post-traumatic stress disorder. The Journal of International Medical Research, 46(6), 2265–2283. doi: 10.1177/0300060518762040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens AM, Tovar-Y-Romo LB, Yoo S-W, Trout AL, Bae M, Kanmogne M, … Haughey NJ (2017). Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Science Signaling, 10(473), eaai7696. doi: 10.1126/scisignal.aai7696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didangelos A, Puglia M, Iberl M, Sanchez-Bellot C, Roschitzki B, & Bradbury EJ (2016). High-throughput proteomics reveal alarmins as amplifiers of tissue pathology and inflammation after spinal cord injury. Scientific Reports, 6, 21607. doi: 10.1038/srep21607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon-Salazar TJ, Fourgeaud L, Tyler CM, Poole J, Park JJ, & Boulanger L. (2014). MHC Class I Limits Hippocampal Synapse Density by Inhibiting Neuronal Insulin Receptor Signaling. The Journal of Neuroscience, 34, 11844–11856. doi: 10.1523/JNEUROSCI.4642-12.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, … Smith SM (2022). SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. doi: 10.1038/s41586-022-04569-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drokhlyansky E, Göz Aytürk D, Soh TK, Chrenek R, O’Loughlin E, Madore C, … Cepko CL (2017). The brain parenchyma has a type I interferon response that can limit virus spread. Proceedings of the National Academy of Sciences of the United States of America, 114(1), E95–E104. doi: 10.1073/pnas.1618157114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejlerskov P, Hultberg JG, Wang J, Carlsson R, Ambjørn M, Kuss M, … Issazadeh-Navikas S. (2015). Lack of Neuronal IFN-β-IFNAR Causes Lewy Body- and Parkinson’s Disease-like Dementia. Cell, 163, 324–339. doi: 10.1016/j.cell.2015.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand JJ (2012). Neurologic complications of influenza. Seminars in Pediatric Neurology, 19(3), 96–100. doi: 10.1016/j.spen.2012.02.004 [DOI] [PubMed] [Google Scholar]
- Enlow W, Bordeleau M, Piret J, Ibáñez FG, Uyar O, Venable M-C, … Boivin G. (2021). Microglia are involved in phagocytosis and extracellular digestion during Zika virus encephalitis in young adult immunodeficient mice. Journal of Neuroinflammation, 18(1), 178. doi: 10.1186/s12974-021-02221-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C, Galea E, Lakatos A, O’Callaghan JP, Petzold GC, Serrano-Pozo A, … Verkhratsky A. (2021). Reactive astrocyte nomenclature, definitions, and future directions. Nature Neuroscience, 24(3), 312–325. doi: 10.1038/s41593-020-00783-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esen N, & Kielian T. (2006). Central role for MyD88 in the responses of microglia to pathogen-associated molecular patterns. Journal of Immunology (Baltimore, Md.: 1950), 176(11), 6802–6811. doi: 10.4049/jimmunol.176.11.6802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espíndola OM, Brandão CO, Gomes YCP, Siqueira M, Soares CN, Lima MASD, … Silva MTT (2021). Cerebrospinal fluid findings in neurological diseases associated with COVID-19 and insights into mechanisms of disease development. International Journal of Infectious Diseases, 102, 155–162. doi: 10.1016/j.ijid.2020.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajgenbaum DC, & June CH (2020). Cytokine Storm. New England Journal of Medicine, 383(23), 2255–2273. doi: 10.1056/NEJMra2026131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas CL, Szabó A, Török B, Bánrévi K, Correia P, Chaves T, … Zelena D. (2022). A New Player in the Hippocampus: A Review on VGLUT3+ Neurons and Their Role in the Regulation of Hippocampal Activity and Behaviour. International Journal of Molecular Sciences, 23(2), 790. doi: 10.3390/ijms23020790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo CP, Barros-Aragão FGQ, Neris RLS, Frost PS, Soares CP, Souza INO, … Ferreira ST (2019). Zika virus replicates in adult human brain tissue and impairs synapses and memory in mice. Nature Communications, 10. doi: 10.1038/s41467-019-11866-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano AJ, Xu Y, Tustison N, Marsh RL, Baker W, Smirnov I, … Kipnis J. (2016). Unexpected role of interferon-γ in regulating neuronal connectivity and social behavior. Nature, 535, 425–429. doi: 10.1038/nature18626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DL, Defres S, & Solomon T. (2015). Measles-induced encephalitis. QJM, 108(3), 177–182. doi: 10/f65drf [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Davenport CM, Tyler CM, Cheng TT, Spencer M, & Boulanger L. (2010). MHC class I modulates NMDA receptor function and AMPA receptor trafficking. Proceedings of the National Academy of Sciences, 107, 22278–22283. doi: 10.1073/pnas.0914064107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C, Ferse C, Kreye J, Reincke SM, Sanchez-Sendin E, Rocco A, … Prüß H. (2021). High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain, Behavior, and Immunity, 93, 415–419. doi: 10.1016/j.bbi.2020.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera JA, Mainali S, Fink EL, Robertson CL, Schober ME, Ziai WC, … Chou SH-Y (2020). Global Consortium Study of Neurological Dysfunction in COVID-19 (GCS-NeuroCOVID): Study Design and Rationale. Neurocritical Care, 1–10. doi: 10.1007/s12028-020-00995-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk KE, & Klein RS (2019). CSF1R antagonism limits local restimulation of antiviral CD8+ T cells during viral encephalitis. Journal of Neuroinflammation, 16(1), 22. doi: 10.1186/s12974-019-1397-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr SR, & Marriott I. (2012). Viral CNS infections: Role of glial pattern recognition receptors in neuroinflammation. Frontiers in Microbiology, 3, 201. doi: 10.3389/fmicb.2012.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidt MM, Morrow A, Fairgrieve MR, Karr JP, Yosef N, & Vance RE (2021). Self-guarding of MORC3 enables virulence factor-triggered immunity. Nature, 600(7887), 138–142. doi: 10.1038/s41586-021-04054-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber C, Soung A, Vollmer LL, Kanmogne M, Last A, Brown J, & Klein RS (2019). T cells promote microglia-mediated synaptic elimination and cognitive dysfunction during recovery from neuropathogenic flaviviruses. Nature Neuroscience, 22(8), 1276–1288. doi: 10.1038/s41593-019-0427-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber C, Vasek MJ, Vollmer LL, Sun T, Jiang X, & Klein RS (2018). Astrocytes decrease adult neurogenesis during virus-induced memory dysfunction via IL-1. Nature Immunology, 19(2), 151–161. doi: 10.1038/s41590-017-0021-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gern OL, Mulenge F, Pavlou A, Ghita L, Steffen I, Stangel M, & Kalinke U. (2021). Toll-like Receptors in Viral Encephalitis. Viruses, 13(10), 2065. doi: 10.3390/v13102065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, … Merad M. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science (New York, N.Y.), 330(6005), 841–845. doi: 10.1126/science.1194637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn MW, Elmer BM, Garay PA, Liu X-B, Needleman LA, El-Sabeawy F, & McAllister AK (2011). MHCI negatively regulates synapse density during the establishment of cortical connections. Nature Neuroscience, 14, 442–451. doi: 10.1038/nn.2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan RM, Aid M, Mercado NB, Davis C, Malik S, Geiger E, … Tan CS (2021). Increased IL-6 expression precedes reliable viral detection in the rhesus macaque brain during acute SIV infection. JCI Insight, 6(20), e152013. doi: 10.1172/jci.insight.152013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green HF, Treacy E, Keohane A, Sullivan AM, O’Keeffe GW, & Nolan YM (2012). A role for interleukin-1β in determining the lineage fate of embryonic rat hippocampal neural precursor cells. Molecular and Cellular Neuroscience, 49, 311–321. doi: 10.1016/j.mcn.2012.01.001 [DOI] [PubMed] [Google Scholar]
- Gunaseelan S, Ariffin MZ, Khanna S, Ooi MH, Perera D, Chu JJH, & Chua JJE (2022). Pharmacological perturbation of CXCL1 signaling alleviates neuropathogenesis in a model of HEVA71 infection. Nature Communications, 13(1), 890. doi: 10.1038/s41467-022-28533-z [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hayes CK, Giraldo D, Wilcox DR, & Longnecker R. (2022). The Astrocyte Type I Interferon Response Is Essential for Protection against Herpes Simplex Encephalitis. Journal of Virology, 96(4), e0178321. doi: 10.1128/JVI.01783-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy LM, Yaqubi M, Ludwin SK, & Antel JP (2019). Species differences in immune-mediated CNS tissue injury and repair: A (neuro)inflammatory topic. Glia, 68, 811–829. doi: 10.1002/glia.23746 [DOI] [PubMed] [Google Scholar]
- Heneka MT, Golenbock DT, Latz E, Morgan D, & Brown R. (2020). Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimer’s Research & Therapy, 12. doi: 10.1186/s13195-020-00640-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermance ME, & Thangamani S. (2017). Powassan Virus: An Emerging Arbovirus of Public Health Concern in North America. Vector Borne and Zoonotic Diseases, 17, 453–462. doi: 10.1089/vbz.2017.2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang L, Means T, & Khoury JBE (2013). The Microglial Sensome Revealed by Direct RNA Sequencing. Nature Neuroscience, 16, 1896–1905. doi: 10.1038/nn.3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M, Knight J, Tobita M, Soltys J, Panitch HS, & Mao-Draayer Y. (2009). The effect of interferon-beta on mouse neural progenitor cell survival and differentiation. Biochemical and Biophysical Research Communications, 388 2, 181–186. doi: 10.1016/j.bbrc.2009.07.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho DH, & Kelly GC (2020). Blood Serum Cytokines Induce Changes in Blood-Brain Barrier Permeability and Gene Expression in an In Vitro Blood-Brain Barrier Model. The FASEB Journal, 34(S1), 1-1. doi: 10.1096/fasebj.2020.34.s1.05527 [DOI] [Google Scholar]
- Hoiland RL, Ainslie PN, Wellington CL, Cooper J, Stukas S, Thiara S, … Sekhon MS (2021). Brain Hypoxia Is Associated With Neuroglial Injury in Humans Post-Cardiac Arrest. Circulation Research, 129(5), 583–597. doi: 10.1161/CIRCRESAHA.121.319157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd KB, Vishnevetsky A, Srinivasan M, & Saylor D. (2020). Neurologic Complications of Acute HIV Infection. Current Treatment Options in Infectious Diseases, 12(3), 227–242. doi: 10.1007/s40506-020-00228-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, … Stevens B. (2016). Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science, 352, 712–716. doi: 10.1126/science.aad8373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Jiang L, & Peng H. (2015). Clinical Analysis of 134 Children with Nervous System Damage Caused by Enterovirus 71 Infection. The Pediatric Infectious Disease Journal, 34(7), 718–723. doi: 10.1097/INF.0000000000000711 [DOI] [PubMed] [Google Scholar]
- Huang KW, & Sabatini BL (2020). Single-Cell Analysis of Neuroinflammatory Responses Following Intracranial Injection of G-Deleted Rabies Viruses. Frontiers in Cellular Neuroscience, 14, 65. doi: 10.3389/fncel.2020.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P-N, & Shih S-R (2014). Update on enterovirus 71 infection. Current Opinion in Virology, 5, 98–104. doi: 10.1016/j.coviro.2014.03.007 [DOI] [PubMed] [Google Scholar]
- Ireland DDC, Manangeeswaran M, Lewkowicz AP, Engel K, Clark SM, Laniyan A, … Verthelyi D. (2020). Long-term persistence of infectious Zika virus: Inflammation and behavioral sequela in mice. PLoS Pathogens, 16(12), e1008689. doi: 10.1371/journal.ppat.1008689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanidze J, Mackay M, Hoang A, Chi JM, Cheng K, Aranow C, … Sanelli PC (2019). Dynamic Contrast-Enhanced MRI Reveals Unique Blood-Brain Barrier Permeability Characteristics in the Hippocampus in the Normal Brain. American Journal of Neuroradiology, ajnr;ajnr.A5962v1. doi: 10.3174/ajnr.A5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H, Boltz D, Sturm-Ramirez K, Shepherd KR, Jiao Y, Webster R, & Smeyne RJ (2009). Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proceedings of the National Academy of Sciences, 106(33), 14063–14068. doi: 10.1073/pnas.0900096106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Chen Y, Sun H, Zhang X, He L, Li J, … Sun S. (2021). MERS-CoV infection causes brain damage in human DPP4-transgenic mice through complement-mediated inflammation. The Journal of General Virology, 102(10). doi: 10.1099/jgv.0.001667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston GR, & Webster NR (2009). Cytokines and the immunomodulatory function of the vagus nerve. British Journal of Anaesthesia, 102(4), 453–462. doi: 10.1093/bja/aep037 [DOI] [PubMed] [Google Scholar]
- Jung H-K, Ryu HJ, Kim M-J, Kim WI, Choi HK, Choi H-C, … Kang T-C (2012). Interleukin-18 attenuates disruption of brain-blood barrier induced by status epilepticus within the rat piriform cortex in interferon-γ independent pathway. Brain Research, 1447, 126–134. doi: 10.1016/j.brainres.2012.01.057 [DOI] [PubMed] [Google Scholar]
- Kaneko N, Nakamura S, & Sawamoto K. (2020). Effects of interferon-alpha on hippocampal neurogenesis and behavior in common marmosets. Molecular Brain, 13(1), 98. doi: 10.1186/s13041-020-00639-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmogne M, & Klein RS (2021). Neuroprotective versus Neuroinflammatory Roles of Complement: From Development to Disease. Trends in Neurosciences, 44(2), 97–109. doi: 10.1016/j.tins.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanski R, Strien ME van Tijn, van P, & Hol EM (2013). A star is born: New insights into the mechanism of astrogenesis. Cellular and Molecular Life Sciences, 71, 433–447. doi: 10.1007/s00018-013-1435-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karve IP, Taylor JM, & Crack PJ (2016). The contribution of astrocytes and microglia to traumatic brain injury. British Journal of Pharmacology, 173(4), 692–702. doi: 10.1111/bph.13125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käufer C, Chhatbar C, Bröer S, Waltl I, Ghita L, Gerhauser I, … Löscher W. (2018). Chemokine receptors CCR2 and CX3CR1 regulate viral encephalitis-induced hippocampal damage but not seizures. Proceedings of the National Academy of Sciences of the United States of America, 115(38), E8929–E8938. doi: 10.1073/pnas.1806754115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, & Keane RW (2014). Pattern recognition receptors and central nervous system repair. Experimental Neurology, 258, 5–16. doi: 10.1016/j.expneurol.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Heo JH, Kim HO, Song SH, Park SS, Park TH, … Choi JP (2017). Neurological Complications during Treatment of Middle East Respiratory Syndrome. Journal of Clinical Neurology (Seoul, Korea), 13(3), 227–233. doi: 10.3988/jcn.2017.13.3.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-I, Jin J-K, Choi E-K, Spinner D, Rubenstein R, Carp RI, & Kim Y-S (2007). Increased expression and localization of cyclooxygenase-2 in astrocytes of scrapie-infected mice. Journal of Neuroimmunology, 187(1-2), 74–82. doi: 10.1016/j.jneuroim.2007.04.013 [DOI] [PubMed] [Google Scholar]
- Klein RS, Garber C, Funk KE, Salimi H, Soung A, Kanmogne M, … Cain M. (2019). Neuroinflammation During RNA Viral Infections. Annual Review of Immunology, 37, 73–95. doi: 10.1146/annurev-immunol-042718-041417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Jahan S, Khan A, Siddiqui AJ, Redhu NS, Wahajuddin null, … Alaidarous M. (2021). Neurological Manifestation of SARS-CoV-2 Induced Inflammation and Possible Therapeutic Strategies Against COVID-19. Molecular Neurobiology, 58(7), 3417–3434. doi: 10.1007/s12035-021-02318-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBeaud AD, Bashir F, & King CH (2011). Measuring the burden of arboviral diseases: The spectrum of morbidity and mortality from four prevalent infections. Population Health Metrics, 9, 1-1. doi: 10.1186/1478-7954-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Daniels BP, Pinto AK, Huang AC, Vick SC, Doyle SE, … Diamond MS (2015). Interferon-λ restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Science Translational Medicine, 7(284), 284ra59. doi: 10.1126/scitranslmed.aaa4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledig C, Schuh A, Guerrero R, Heckemann RA, & Rueckert D. (2018). Structural brain imaging in Alzheimer’s disease and mild cognitive impairment: Biomarker analysis and shared morphometry database. Scientific Reports, 8(1), 11258. doi: 10.1038/s41598-018-29295-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenka A, Kamat A, & Mittal SO (2019). Spectrum of Movement Disorders in Patients With Neuroinvasive West Nile Virus Infection. Movement Disorders Clinical Practice, 6(6), 426–433. doi: 10.1002/mdc3.12806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang Q, Jiang Y, Ye Q, Xu D, Gao F, … Xu Z. (2018). Disruption of glial cell development by Zika virus contributes to severe microcephalic newborn mice. Cell Discovery, 4, 43. doi: 10.1038/s41421-018-0042-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Saucedo-Cuevas L, Regla-Nava JA, Chai G, Sheets N, Tang W, … Gleeson JG (2016). Zika Virus Infects Neural Progenitors in the Adult Mouse Brain and Alters Proliferation. Cell Stem Cell, 19(5), 593–598. doi: 10.1016/j.stem.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Hi Shing S, Chipika RH, Finegan E, Murray D, Hardiman O, & Bede P. (2019). Post-polio Syndrome: More Than Just a Lower Motor Neuron Disease. Frontiers in Neurology, 10, 773. doi: 10.3389/fneur.2019.00773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Acioglu C, Heary RF, & Elkabes S. (2021). Role of astroglial toll-like receptors (TLRs) in central nervous system infections, injury and neurodegenerative diseases. Brain, Behavior, and Immunity, 91, 740–755. doi: 10.1016/j.bbi.2020.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C-Y, Yang C-H, & Lin J-N (2018). Focal Encephalitis, Meningitis, and Acute Respiratory Distress Syndrome Associated with Influenza A Infection. Medical Principles and Practice, 27(2), 193–196. doi: 10.1159/000487398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist R, Kurhade C, Gilthorpe JD, & Överby AK (2018). Cell-type- and region-specific restriction of neurotropic flavivirus infection by viperin. Journal of Neuroinflammation, 15(1), 80. doi: 10.1186/s12974-018-1119-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist R, Mundt F, Gilthorpe JD, Wölfel S, Gekara NO, Kröger A, & Överby AK (2016). Fast type I interferon response protects astrocytes from flavivirus infection and virus-induced cytopathic effects. Journal of Neuroinflammation, 13(1), 277. doi: 10.1186/s12974-016-0748-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Li X, Geng D, Mei N, Wu P-Y, Huang C-C, … Yin B. (2020). Cerebral Micro-Structural Changes in COVID-19 Patients - An MRI-based 3-month Follow-up Study. EClinicalMedicine, 25, 100484. doi: 10.1016/j.eclinm.2020.100484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Su R, Wang W, Liang Y, Zeng X, Shereen MA, … Wu J. (2019). EV71 infection induces neurodegeneration via activating TLR7 signaling and IL-6 production. PLoS Pathogens, 15(11), e1008142. doi: 10.1371/journal.ppat.1008142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgen V, Narasipura SD, Barbian HJ, Richards M, Wallace J, Razmpour R, … Al-Harthi L. (2020). HIV infects astrocytes in vivo and egresses from the brain to the periphery. PLOS Pathogens, 16(6), e1008381. doi: 10.1371/journal.ppat.1008381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes J, Tresse E, Ejlerskov P, Hu E, Liu Y, Marin A, … Issazadeh-Navikas S. (2021). PIAS2-mediated blockade of IFN-β signaling: A basis for sporadic Parkinson disease dementia. Molecular Psychiatry, 26(10), 6083–6099. doi: 10.1038/s41380-021-01207-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-García J, & González-Scarano F. (2009). Glial Responses to Virus Infection. In Encyclopedia of Neuroscience (pp. 861–869). Elsevier. doi: 10.1016/B978-008045046-9.01762-9 [DOI] [Google Scholar]
- Mehandru S, & Merad M. (2022). Pathological sequelae of long-haul COVID. Nature Immunology, 23(2), 194–202. doi: 10.1038/s41590-021-01104-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, … Heppner FL (2021). Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nature Neuroscience, 24(2), 168–175. doi: 10.1038/s41593-020-00758-5 [DOI] [PubMed] [Google Scholar]
- Merad M, Blish CA, Sallusto F, & Iwasaki A. (2022). The immunology and immunopathology of COVID-19. Science (New York, N.Y.), 375(6585), 1122–1127. doi: 10.1126/science.abm8108 [DOI] [PubMed] [Google Scholar]
- Michael BD, Bricio-Moreno L, Sorensen EW, Miyabe Y, Lian J, Solomon T, … Luster AD (2020). Astrocyte- and Neuron-Derived CXCL1 Drives Neutrophil Transmigration and Blood-Brain Barrier Permeability in Viral Encephalitis. Cell Reports, 32(11), 108150. doi: 10.1016/j.celrep.2020.108150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailidou I, Willems JGP, Kooi E, Eden CG van, Gold SM, Geurts JJG, … Ramaglia V. (2015). Complement C1q-C3-associated synaptic changes in multiple sclerosis hippocampus. Annals of Neurology, 77. doi: 10.1002/ana.24398 [DOI] [PubMed] [Google Scholar]
- Michelucci A, Bithell A, Burney MJ, Johnston C, Wong K-Y, Teng SW, … Buckley N. (2015). The Neurogenic Potential of Astrocytes Is Regulated by Inflammatory Signals. Molecular Neurobiology, 53, 3724–3739. doi: 10.1007/s12035-015-9296-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad Ismail HI, Teh CM, & Lee YL (2015). Neurologic manifestations and complications of pandemic influenza A H1N1 in Malaysian children: What have we learnt from the ordeal? Brain and Development, 37(1), 120–129. doi: 10.1016/j.braindev.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Murray KO, Nolan MS, Ronca SE, Datta S, Govindarajan KA, Narayana PA, … Hasbún R. (2018). The Neurocognitive and MRI Outcomes of West Nile Virus Infection: Preliminary Analysis Using an External Control Group. Frontiers in Neurology, 9. doi: 10.3389/fneur.2018.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalivaeva NN, & Rybnikova EA (2019). Editorial: Brain Hypoxia and Ischemia: New Insights Into Neurodegeneration and Neuroprotection. Frontiers in Neuroscience, 13, 770. doi: 10.3389/fnins.2019.00770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham E, Chou SH-Y, Coles AJ, & Menon DK (2020). Neurological Implications of COVID-19 Infections. Neurocritical Care, 1–5. doi: 10.1007/s12028-020-00978-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PT, Dorman LC, Pan S, Vainchtein ID, Han RT, Nakao-Inoue H, … Molofsky AV (2020). Microglial Remodeling of the Extracellular Matrix Promotes Synapse Plasticity. Cell, 182(2), 388–403.e15. doi: 10.1016/j.cell.2020.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicastri E, Castilletti C, Balestra P, Galgani S, & Ippolito G. (2016). Zika Virus Infection in the Central Nervous System and Female Genital Tract. Emerging Infectious Diseases, 22, 2228–2230. doi: 10.3201/eid2212.161280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortelli P, Ferrazzoli D, Sebastianelli L, Engl M, Romanello R, Nardone R, … Versace V. (2021). Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: Insights into a challenging symptom. Journal of the Neurological Sciences, 420, 117271. doi: 10.1016/j.jns.2020.117271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajo AT, Espiritu AI, Apor ADAO, & Jamora RDG (2021). Neuropathologic findings of patients with COVID-19: A systematic review. Neurological Sciences: Official Journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology, 42(4), 1255–1266. doi: 10.1007/s10072-021-05068-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RJ, & Finley KH (1956). Sequelae of encephalitis; report of a study after the California epidemic. California Medicine, 84(2), 98–100. [PMC free article] [PubMed] [Google Scholar]
- Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, … Zandi MS (2020). The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings. Brain, 143(10), 3104–3120. doi: 10.1093/brain/awaa240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peferoen LAN, Kipp M, Valk PV der, Noort JM, & Amor S. (2014). Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology, 141. doi: 10.1111/imm.12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito CK (2004). Human immunodeficiency virus type 1 compartmentalization in the central nervous system. Journal of Neurovirology, 10(s1), 21–24. doi: 10.1080/jnv.10.s1.21.24 [DOI] [PubMed] [Google Scholar]
- Phares TW, Stohlman SA, Hinton DR, & Bergmann CC (2013). Astrocyte-derived CXCL10 drives accumulation of antibody-secreting cells in the central nervous system during viral encephalomyelitis. Journal of Virology, 87(6), 3382–3392. doi: 10.1128/JVI.03307-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poloni TE, Medici V, Moretti M, Visonà SD, Cirrincione A, Carlos AF, … Ceroni M. (2021). COVID-19-related neuropathology and microglial activation in elderly with and without dementia. Brain Pathology (Zurich, Switzerland), 31(5), e12997. doi: 10.1111/bpa.12997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert LS, Harder L, Holm CK, Iversen MB, Horan KA, Dagnæs-Hansen F, … Paludan SR (2012). TLR3 deficiency renders astrocytes permissive to herpes simplex virus infection and facilitates establishment of CNS infection in mice. The Journal of Clinical Investigation, 122(4), 1368–1376. doi: 10.1172/JCI60893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. Balschun D, Wetzel W, Randolf A, & Besedovsky HO (2013). A cytokine network involving brain-borne IL-1β, IL-1ra, IL-18, IL-6, and TNFα operates during long-term potentiation and learning. Brain, Behavior, and Immunity, 33, 15–23. doi: 10.1016/j.bbi.2013.05.011 [DOI] [PubMed] [Google Scholar]
- Reynolds JL, & Mahajan SD (2021). SARS-COV2 Alters Blood Brain Barrier Integrity Contributing to Neuro-Inflammation. Journal of Neuroimmune Pharmacology: The Official Journal of the Society on NeuroImmune Pharmacology, 16(1), 4–6. doi: 10.1007/s11481-020-09975-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronca SE, Dineley KT, & Paessler S. (2016). Neurological Sequelae Resulting from Encephalitic Alphavirus Infection. Frontiers in Microbiology, 7. doi: 10.3389/fmicb.2016.00959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen SF, Soung AL, Yang W, Ai S, Kanmogne M, Davé VA, … Klein RS (2021). Single-cell RNA transcriptome analysis reveals CXCL16/CXCR6 as maintenance factors for CNS tissue resident T cells that drive synapse elimination during viral recovery [Preprint]. Immunology. doi: 10.1101/2021.10.08.463715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruisanchez-Nieva A, Martinez-Arroyo A, Gomez-Beldarrain M, Bocos Portillo J, & Garcia-Monco JC (2017). Influenza-associated seizures in healthy adults: Report of 3 cases. Epilepsy & Behavior Case Reports, 8, 12–13. doi: 10.1016/j.ebcr.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadek JR, Pergam SA, Harrington JA, Echevarria L, Davis LE, Goade DE, … Haaland KY (2010). Persistent neuropsychological impairment associated with West Nile virus infection. Journal of Clinical and Experimental Neuropsychology, 32, 81–87. doi: 10.1080/13803390902881918 [DOI] [PubMed] [Google Scholar]
- Sariol A, Mackin S, Allred M-G, Ma C, Zhou Y, Zhang Q, … Perlman S. (2020). Microglia depletion exacerbates demyelination and impairs remyelination in a neurotropic coronavirus infection. Proceedings of the National Academy of Sciences of the United States of America, 117(39), 24464–24474. doi: 10.1073/pnas.2007814117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato R, Kato A, Chimura T, Saitoh S-I, Shibata T, Murakami Y, … Miyake K. (2018). Combating herpesvirus encephalitis by potentiating a TLR3-mTORC2 axis. Nature Immunology, 19(10), 1071–1082. doi: 10.1038/s41590-018-0203-2 [DOI] [PubMed] [Google Scholar]
- Schafernak KT, & Bigio EH (2006). West Nile Virus Encephalomyelitis with Polio-like Paralysis & Nigral Degeneration. Canadian Journal of Neurological Sciences / Journal Canadien Des Sciences Neurologiques, 33(4), 407–410. doi: 10.1017/S0317167100005370 [DOI] [PubMed] [Google Scholar]
- Schneider WM, Chevillotte MD, & Rice CM (2014). Interferon-stimulated genes: A complex web of host defenses. Annual Review of Immunology, 32, 513–545. doi: 10.1146/annurev-immunol-032713-120231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz V, Barrie JA, Donald CL, Crawford CL, Mullin M, Anderson TJ, … Edgar JM (2021). Oligodendrocytes are susceptible to Zika virus infection in a mouse model of perinatal exposure: Implications for CNS complications. Glia, 69(8), 2023–2036. doi: 10.1002/glia.24010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabenland M, Salié H, Tanevski J, Killmer S, Lago MS, Schlaak AE, … Bengsch B. (2021). Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions. Immunity, 54(7), 1594–1610.e11. doi: 10.1016/j.immuni.2021.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz S, Clarke P, & Tyler KL (2018). Pharmacologic Depletion of Microglia Increases Viral Load in the Brain and Enhances Mortality in Murine Models of Flavivirus-Induced Encephalitis. Journal of Virology, 92(16), e00525–18. doi: 10.1128/JVI.00525-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejvar J. (2014). Clinical Manifestations and Outcomes of West Nile Virus Infection. Viruses, 6, 606–623. doi: 10.3390/v6020606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhon MS, Ainslie PN, & Griesdale DE (2017). Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: A “two-hit” model. Critical Care, 21(1), 90. doi: 10.1186/s13054-017-1670-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebrovska ZO, Chong EY, Serebrovska TV, Tumanovska LV, & Xi L. (2020). Hypoxia, HIF-1α, and COVID-19: From pathogenic factors to potential therapeutic targets. Acta Pharmacologica Sinica, 41(12), 1539–1546. doi: 10.1038/s41401-020-00554-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Castro PJ, Estivill-Torrús G, Cabezudo-García P, Reyes-Bueno JA, Petersen NC, Aguilar-Castillo MJ, … Fonseca FR de. (2020). Impact of SARS-CoV-2 infection on neurodegenerative and neuropsychiatric diseases: A delayed pandemic? Neurologia. doi: 10.1016/j.nrl.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Colodner KJ, Matousek SB, Merry K, Hong S, Kenison JE, … Lemere CA (2015). Complement C3-Deficient Mice Fail to Display Age-Related Hippocampal Decline. The Journal of Neuroscience, 35, 13029–13042. doi: 10.1523/JNEUROSCI.1698-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sil S, Hu G, Liao K, Niu F, Callen S, Periyasamy P, … Buch S. (2020). HIV-1 Tat-mediated astrocytic amyloidosis involves the HIF-1α/lncRNA BACE1-AS axis. PLoS Biology, 18(5), e3000660. doi: 10.1371/journal.pbio.3000660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M, Hernu R, Cour M, Casalegno J-S, Lina B, & Argaud L. (2013). Fatal Influenza A(H1N1)pdm09 Encephalopathy in Immunocompetent Man. Emerging Infectious Diseases, 19(6), 1005–1007. doi: 10.3201/eid1906.130062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe GO, Lowery RL, Tremblay M-È, Kelly EA, Lamantia CE, & Majewska AK (2016). Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nature Communications, 7, 10905. doi: 10.1038/ncomms10905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon T. (2021). Neurological infection with SARS-CoV-2—The story so far. Nature Reviews. Neurology, 17(2), 65–66. doi: 10.1038/s41582-020-00453-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soung A, & Klein RS (2018). Viral Encephalitis and Neurologic Diseases: Focus on Astrocytes. Trends in Molecular Medicine, 24(11), 950–962. doi: 10.1016/j.molmed.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soung AL, Davé VA, Garber C, Tycksen ED, Vollmer LL, & Klein RS (2022). IL-1 reprogramming of adult neural stem cells limits neurocognitive recovery after viral encephalitis by maintaining a proinflammatory state. Brain, Behavior, and Immunity, 99, 383–396. doi: 10.1016/j.bbi.2021.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan AH, Madison DV, Mateos JM, Fraser DA, Lovelett EA, Coutellier L, … Barres BA (2013). A Dramatic Increase of C1q Protein in the CNS during Normal Aging. The Journal of Neuroscience, 33, 13460–13474. doi: 10.1523/jneurosci.1333-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MN, Brill SA, Mangus LM, Jeong YJ, Solis CV, Knight AC, … Mankowski JL (2020). Upregulation of Superoxide Dismutase 2 by Astrocytes in the SIV/Macaque Model of HIV-Associated Neurologic Disease. Journal of Neuropathology and Experimental Neurology, 79(9), 986–997. doi: 10.1093/jnen/nlaa084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syage AR, Ekiz HA, Skinner DD, Stone C, O’Connell RM, & Lane TE (2020). Single-Cell RNA Sequencing Reveals the Diversity of the Immunological Landscape following Central Nervous System Infection by a Murine Coronavirus. Journal of Virology, 94(24), e01295–20. doi: 10.1128/JVI.01295-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey MG, Wallings RL, Houser MC, Herrick MK, Keating CE, & Joers V. (2022). Inflammation and immune dysfunction in Parkinson disease. Nature Reviews Immunology. doi: 10.1038/s41577-022-00684-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M, Geddes JR, Husain M, Luciano S, & Harrison PJ (2021). 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. The Lancet Psychiatry, 8(5), 416–427. doi: 10.1016/S2215-0366(21)00084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M, Luciano S, Geddes JR, & Harrison PJ (2021). Bidirectional associations between COVID-19 and psychiatric disorder: Retrospective cohort studies of 62 354 COVID-19 cases in the USA. The Lancet. Psychiatry, 8(2), 130–140. doi: 10.1016/S2215-0366(20)30462-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartt AN, Fulmore CA, Liu Y, Rosoklija GB, Dwork AJ, Arango V, … Boldrini M. (2018). Considerations for Assessing the Extent of Hippocampal Neurogenesis in the Adult and Aging Human Brain. Cell Stem Cell, 23(6), 782–783. doi: 10.1016/j.stem.2018.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur KT, Miller EH, Glendinning MD, Al-Dalahmah O, Banu MA, Boehme AK, … Canoll P. (2021). COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain, 144(9), 2696–2708. doi: 10.1093/brain/awab148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai T-T, Chen C-L, Lin Y-S, Chang C-P, Tsai C-C, Cheng Y-L, … Lin C-F (2016). Microglia retard dengue virus-induced acute viral encephalitis. Scientific Reports, 6, 27670. doi: 10.1038/srep27670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtle L, & Solomon T. (2018). Japanese encephalitis—The prospects for new treatments. Nature Reviews. Neurology, 14(5), 298–313. doi: 10.1038/nrneurol.2018.30 [DOI] [PubMed] [Google Scholar]
- Vainchtein ID, Chin G, Cho FS, Kelley KW, Miller JG, Chien EC, … Molofsky AV (2018). Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science, 359, 1269–1273. doi: 10.1126/science.aal3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdebenito S, Castellano P, Ajasin D, & Eugenin EA (2021). Astrocytes are HIV reservoirs in the brain: A cell type with poor HIV infectivity and replication but efficient cell-to-cell viral transfer. Journal of Neurochemistry, 158(2), 429–443. doi: 10.1111/jnc.15336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Mao G, Yang Y, Ornaghi S, & Davis JN (2017). Zika Virus Targeting in the Developing Brain. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 37(8), 2161–2175. doi: 10.1523/JNEUROSCI.3124-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas G, Medeiros Geraldo LH, Gedeão Salomão N, Viana Paes M, Regina Souza Lima F, & Carvalho Alcantara Gomes F. (2020). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and glial cells: Insights and perspectives. Brain, Behavior, & Immunity - Health, 7, 100127. doi: 10.1016/j.bbih.2020.100127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasek MJ, Garber C, Dorsey D, Durrant DM, Bollman B, Soung A, … Klein RS (2016). A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature, 534(7608), 538–543. doi: 10.1038/nature18283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerhuis R, Nielsen HM, & Tenner AJ (2011). Complement in the brain. Molecular Immunology, 48 14, 1592–1603. doi: 10.1016/j.molimm.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DT, Perry VH, & Minghetti L. (2000). Cyclooxygenase-2 is highly expressed in microglial-like cells in a murine model of prion disease. Glia, 29(4), 392–396. [PubMed] [Google Scholar]
- Waltl I, & Kalinke U. (2022). Beneficial and detrimental functions of microglia during viral encephalitis. Trends in Neurosciences, 45(2), 158–170. doi: 10.1016/j.tins.2021.11.004 [DOI] [PubMed] [Google Scholar]
- Waltl I, Käufer C, Gerhauser I, Chhatbar C, Ghita L, Kalinke U, & Löscher W. (2018). Microglia have a protective role in viral encephalitis-induced seizure development and hippocampal damage. Brain, Behavior, and Immunity, 74, 186–204. doi: 10.1016/j.bbi.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Yue H, Hu Z, Shen Y, Ma J, Li J, … Gu Y. (2020). Microglia mediate forgetting via complement-dependent synaptic elimination. Science, 367, 688–694. doi: 10.1126/science.aaz2288 [DOI] [PubMed] [Google Scholar]
- Wang F, Kream RM, & Stefano GB (2020). Long-Term Respiratory and Neurological Sequelae of COVID-19. Medical Science Monitor, 26. doi: 10.12659/MSM.928996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherhead JE, Miller VE, Garcia MN, Hasbún R, Salazar L, Dimachkie MM, & Murray KO (2015). Long-Term Neurological Outcomes in West Nile Virus-Infected Patients: An Observational Study. The American Journal of Tropical Medicine and Hygiene, 92, 1006–1012. doi: 10.4269/ajtmh.14-0616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC (2017). Emergence of Epidemic Zika Virus Transmission and Congenital Zika Syndrome: Are Recently Evolved Traits to Blame? MBio, 8. doi: 10.1128/mBio.02063-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DL, Sariol A, Meyerholz DK, & Perlman S. (2018). Microglia are required for protection against lethal coronavirus encephalitis in mice. The Journal of Clinical Investigation, 128(3), 931–943. doi: 10.1172/JCI97229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosik K, Cayrol R, Dodelet-Devillers A, Berthelet F, Bernard M, Moumdjian RA, … Prat A. (2007). Angiotensin II Controls Occludin Function and Is Required for Blood-Brain Barrier Maintenance: Relevance to Multiple Sclerosis. The Journal of Neuroscience, 27, 9032–9042. doi: 10.1523/JNEUROSCI.2088-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Zhu J, Hu J, Wang L, & Zhang H. (2014). H7N9 Influenza A-induced Pneumonia Associated with Acute Myelitis in an Adult. Internal Medicine, 53(10), 1093–1095. doi: 10.2169/internalmedicine.53.1801 [DOI] [PubMed] [Google Scholar]
- Yang AC, Kern F, Losada PM, Agam MR, Maat CA, Schmartz GP, … Wyss-Coray T. (2021). Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature, 595(7868), 565–571. doi: 10.1038/s41586-021-03710-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates AG, Anthony DC, Ruitenberg MJ, & Couch Y. (2019). Systemic Immune Response to Traumatic CNS Injuries-Are Extracellular Vesicles the Missing Link? Frontiers in Immunology, 10, 2723. doi: 10.3389/fimmu.2019.02723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H, & Ikezu T. (2019). Transcriptional and Epigenetic Regulation of Microglia in Health and Disease. Trends in Molecular Medicine, 25 2, 96–111. doi: 10.1016/j.molmed.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S-Y, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, … Casanova J-L (2007). TLR3 deficiency in patients with herpes simplex encephalitis. Science (New York, N.Y.), 317(5844), 1522–1527. doi: 10.1126/science.1139522 [DOI] [PubMed] [Google Scholar]
- Zhao J, Bi W, Xiao S, Lan X, Cheng X, Zhang J, … Zhu L. (2019). Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Scientific Reports, 9(1), 5790. doi: 10.1038/s41598-019-42286-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Chen J, Chu F, Zhu J, & Jin T. (2019). Inflammatory Role of TLR-MyD88 Signaling in Multiple Sclerosis. Frontiers in Molecular Neuroscience, 12, 314. doi: 10.3389/fnmol.2019.00314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Woodson M, Neupane B, Bai F, Sherman MB, Choi KH, … Sultana H. (2018). Exosomes serve as novel modes of tick-borne flavivirus transmission from arthropod to human cells and facilitates dissemination of viral RNA and proteins to the vertebrate neuronal cells. PLoS Pathogens, 14. doi: 10.1371/journal.ppat.1006764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker JE, Neu NM, Chiriboga CA, Hinton VJ, Leonardo M, Sheikh A, & Thakur KT (2017). Zika Virus-Associated Cognitive Impairment in Adolescent, 2016. Emerging Infectious Diseases, 23, 1047–1048. doi: 10.3201/eid2306.162029 [DOI] [PMC free article] [PubMed] [Google Scholar]