Abstract
Rationale:
Stress may contribute to relapse to alcohol use in part by enhancing reactivity to cues previously paired with alcohol. Yet, standard models of stress-induced reinstatement generally use contingent presentations of alcohol-paired cues to reinforce instrumental behaviors, making it difficult to isolate the ability of cues to invigorate alcohol-seeking.
Objective:
Here we sought to test the impact of stress on behavioral responses to alcohol-paired cues, using a model of stress-induced reinstatement of Pavlovian conditioned approach, inspired by Nadia Chaudhri’s work on context-induced reinstatement.
Methods:
Long Evans rats were trained to associate one auditory cue with delivery of alcohol or sucrose and an alternative auditory cue with no reward. Following extinction training, rats were exposed to a stressor prior to being re-exposed to the cues under extinction conditions. We assessed the effects of yohimbine, intermittent footshock and olfactory cues paired with social defeat on responses to alcohol-paired cues, and the effects of yohimbine on responses to sucrose-paired cues.
Results:
The pharmacological stressor, yohimbine, enhanced alcohol seeking in a Pavlovian setting, but not in a cue-selective manner. Intermittent footshock and social defeat cues did not enhance alcohol seeking in this paradigm.
Conclusions:
While yohimbine elicited reinstatement of reward seeking in a Pavlovian setting, these effects may be unrelated to activation of stress systems or to interactions with specific cues.
Keywords: cues, alcohol, stress, addiction, rat, footshock, social defeat, yohimbine
Introduction
Stress and alcohol-related cues contribute to alcohol use disorder in a variety of ways, including by triggering or exacerbating relapse of alcohol seeking and consumption (Brown et al. 1990; Lê et al. 1998; Sinha 2001; Logrip and Zorrilla 2012; Mantsch et al. 2015), and leading to escalated alcohol intake (Becker et al. 2011; Gomez et al. 2012; Hwa et al. 2015). Stress has been proposed to contribute to craving, consumption and relapse via many routes (Shaham et al. 2000; Sinha 2001), including by intensifying the motivational impact of drug-related cues (Robinson and Berridge 1993; Field and Powell 2007). Stress can also act as a contextual cue for the drug delivery or reward-seeking context. This stress context, like other reward-related environmental contexts (Zironi et al. 2006; Crombag et al. 2008), can then drive reinstatement of instrumental reward seeking (Schepers and Bouton 2019). Many studies of stress- or context-induced reinstatement of drug-seeking often involve response-contingent delivery of discrete drug-associated cues that are delivered throughout self-administration, extinction, and reinstatement testing (e.g. Tsiang and Janak 2006; Marinelli et al. 2007a; Bossert et al. 2007; Millan and McNally 2011). Because the same instrumental response was previously reinforced by simultaneous drug delivery and cue presentation, it is difficult in these studies to (1) determine the role of the cue in reinstatement per se and (2) to isolate the ability of cues to invigorate, as opposed to reinforce, reward-seeking actions.
A rich body of work led by Nadia Chaudhri and colleagues has shown that contextual cues can also renew responding for alcohol and other rewards in Pavlovian settings (Chaudhri et al. 2008b, 2013; Remedios et al. 2014; Sciascia et al. 2015; Valyear et al. 2017; Villaruel et al. 2017; Khoo et al. 2020; Segal et al. 2022). When rats undergo Pavlovian conditioning in one context and then have this Pavlovian association extinguished in a second context, placing rats back in the first context can drive reinstatement of responding to Pavlovian cues (Chaudhri et al. 2008b), similar to that seen for instrumental responding (Crombag et al. 2008; Chaudhri et al. 2008a; Janak and Chaudhri 2010). In addition to facilitating better understanding of the behavioral learning mechanisms that can lead to relapse-like behavior, this paradigm has facilitated neurobiological studies aimed at parsing the mechanisms underlying the invigoration of reward-seeking by environmental contexts, discrete cues, and interactions between these stimuli (Chaudhri et al. 2010, 2013; Valyear et al. 2020; Villaruel et al. 2022).
Inspired by these findings, and the possibility that stress potentiates alcohol use and relapse by potentiating the value of drug-paired cues, we sought to determine whether stress would also drive reinstatement of alcohol-seeking in a Pavlovian setting. We focused on stressors that have previously been shown to drive reinstatement of instrumental responding for alcohol. First, we assessed reinstatement of conditioned behavioral responses to alcohol and sucrose cues after exposure to a pharmacological stressor, yohimbine (Marinelli et al. 2007b; Lê et al. 2011; Bertholomey et al. 2016), an alpha-2 adrenoceptor antagonist. Next, we assessed the impact of a physical stressor, intermittent footshock, on behavioral responses to an extinguished alcohol cue (Lê et al. 1998). Finally, we evaluated the ability of a cue associated with a psychosocial stressor, social defeat in a resident-intruder paradigm (Funk et al. 2005; Manvich et al. 2015), to reinstate Pavlovian responding.
Materials and Methods
Subjects
Male and female Long Evans rats (n=44, 31 males and 13 females; Envigo) weighing 250-275 grams at arrival served as experimental subjects and were individually housed in a temperature- and humidity-controlled colony room on a 12 h light/dark cycle. A separate group of adult male Long Evans rats (n=4), weighing 500-750 at arrival served as resident aggressors in the social defeat experiment. These rats were each pair-housed with a sexually receptive, tubally-ligated adult female Long Evans rat (n=4 total) in a larger enclosure (approximately 3 X 2 ft) for nine days prior to social defeat conditioning. Rats trained with sucrose (n=8) were mildly food restricted to 90% of their free-feeding weight during training (~5% of their body weight in chow was provided per day). All other rats were fed ad libitum, and water was provided ad libitum to all rats. All experimental procedures were approved by the Institutional Animal Care and Use Committees at Johns Hopkins University or the University of Minnesota and were carried out in accordance with the guidelines on animal care and use of the National Institutes of Health of the United States.
Pavlovian conditioning with alcohol
Rats trained with alcohol reward (n=35) were first pre-exposed to ethanol in the homecage prior to training as described previously (Simms et al. 2008; Remedios et al. 2014; Millan et al. 2017). After one week of continuous access to 10% ethanol in the home cage, rats received chronic intermittent access to 20% ethanol in the homecage, for 24 hours at a time starting on Monday, Wednesday, and Friday, for a period of 7 weeks. As reported previously (Priddy et al. 2017; Aguirre et al. 2020), female rats consumed significantly more ethanol during intermittent access (Supplementary Figure 1; F(1,244)=12.723, p < 0.001). All rats included here consumed at least 2g/kg/day of ethanol during the last two weeks of pre-exposure. Prior to cue conditioning, rats underwent port training in which they received 30 deliveries of .065 mL 20% ethanol to a reward port. Next, they underwent Pavlovian conditioning (20-25 sessions; MWF) as described previously (Ottenheimer et al. 2019). Rats were randomly assigned one of the following cues as their conditioned stimulus (CS+) for training and testing: white noise or a 2900 Hz tone. Rats received the alternate auditory cue as their CS−. During conditioning sessions, the CS+ and CS−, each lasting 10s, were presented on a pseudorandom variable interval schedule with a mean inter-trial interval (ITI) of 80s. At 9s after the CS+ onset, 0.065 mL of 20% ethanol was delivered into the reward delivery port over a period of 1s. Rats then underwent extinction training and reinstatement testing with either yohimbine (n=17), intermittent footshock (n=11) or social defeat cues (n=7).
Pavlovian conditioning with sucrose
Prior to cue conditioning, rats trained with sucrose reward (n=8 males) underwent port training in which they received 30 deliveries of .13 mL 10% sucrose to a reward port. They then underwent 10 sessions of Pavlovian conditioning with sucrose as described previously (Richard et al. 2018; Ottenheimer et al. 2019). During sucrose conditioning sessions the CS+ and CS−, each lasting 10s, were presented on a pseudorandom variable interval schedule with a mean inter-trial interval (ITI) of 45s. At 8s after the CS+ onset, 0.13 mL of 10% sucrose was delivered into the reward delivery port over a period of 2s. Following Pavlovian conditioning with sucrose rats underwent extinction and reinstatement testing with yohimbine.
Reinstatement testing with the yohimbine
Following conditioning with sucrose (n=8 males) or ethanol (n=17; 10 males and 7 females), rats underwent 5 extinction sessions in which cues were presented, but sucrose or ethanol reward was no longer delivered. Next, rats underwent a reinstatement session in which they either received an injection of yohimbine (2 mg/kg) or injection of saline vehicle, each given at a volume of 1ml/kg. Rats were placed in the operant chambers for 30 minutes prior to being re-exposed to 30 CS+ and CS− presentations under extinction conditions. A subset of rats trained with alcohol (n=7, 5 females and 2 males) were perfused following the first reinstatement test for assessment of c-fos immunofluorescence that is not included in this manuscript. Most rats (all sucrose, 10/17 alcohol rats) received a second reinstatement session 3-5 days later in which they received the opposite injection 30 minutes prior to being presented with 30 CS+ and CS− cues under extinction conditions.
Conditioned reinforcement testing with yohimbine
To assess whether yohimbine enhances the value of Pavlovian-conditioned sucrose cues, rats that were previously tested for yohimbine reinstatement with sucrose cues underwent 3 additional days of Pavlovian conditioning prior to conditioned reinforcement tests. Rats received injections of yohimbine (2 mg/kg) or injection of saline vehicle, each given at a volume of 1ml/kg. Following 30 minutes in their home cages, rats were placed in the operant chambers for the conditioned reinforcement test, which lasted 45 minutes. During each session, entries into one nosepoke port resulted in 2s presentations of the CS+ on a fixed-ratio-2 (FR2) schedule. Entries into the other nosepoke port resulted in 2s presentations of the CS− on an FR2 schedule. Nosepokes during cue presentations were recorded but had no programmed consequences. Rats underwent a second session with the opposite injection, counterbalanced for order. To limit extinction of the CS+ alcohol association, rats did not have the opportunity to nose poke for cue presentations prior to these two test sessions (Parkinson et al. 1999; Burke et al. 2007; Srey et al. 2015; Yager et al. 2015; DiFeliceantonio and Berridge 2016; Tabbara et al. 2016).
Reinstatement testing with intermittent footshock
Following Pavlovian conditioning with alcohol, rats (n=12; 6 males and 6 females) underwent 5 extinction sessions in which cue were presented but alcohol was no longer delivered. On the reinstatement test day rats received 10 minutes of intermittent footshock (ITI ~= 45 sec) (Le et al. 1999) or waited for 10 minutes in the operant chambers. The first cohort of rats (n=6; 4 males and 2 females) received 0.8 mA footshock intensity based on prior studies examining footshock reinstatement of instrumental alcohol seeking (Lê et al. 1998, 2000; Le et al. 1999). Because we observed suppression of responding in most rats, the remaining cohort (n=6; 2 males and 4 females), received 0.4 mA footshock intensity. At the end of the footshock or waiting period, rats were re-exposed to 30 CS+ and CS− presentations under extinction conditions. Rats were tested again 3-5 days later under the conditions they had not previously received (footshock versus waiting).
Social defeat conditioning and reinstatement testing
Following each of the final four Pavlovian conditioning sessions with ethanol, male rats (n=7) underwent four social defeat conditioning sessions using the resident-intruder paradigm as described previously (Funk et al. 2005; Manvich et al. 2015). Social defeat conditioning occurred 4-6 hours after the Pavlovian conditioning session. Pavlovian conditioned subjects (intruders) were placed inside a resident male aggressor’s home cage from which the female rat had been temporarily removed. Prior to the interaction the cage was prepared with either a lemon or banana odor on a cotton ball, counterbalanced across subjects. The resident rats’ home cages (approximately 3x2 feet) were constructed so that a perforated Plexiglas barrier could be inserted to bisect the box into two sides from which the rats could see, smell, and hear each other, but not physically interact. The interaction was terminated, and the barrier inserted between the two rats once (1) the intruder rat sustained 3 bites from the territorial rat, (2) the intruder engaged in a supine submissive posture for 4s, or (3) 4 minutes had elapsed. After the barrier was inserted between the rats, the rats stayed in the behavioral box for at least one more minute, or until 5 total minutes had elapsed. Intruder rats faced a different resident rat for each interaction but were exposed to the same scent for each social defeat session. After each social defeat session, the intruder rats spent five minutes alone in a separate behavioral box with a cotton ball saturated with the scent that was not their respective social defeat scent.
Social defeat training was followed by five days of extinction training, during which Pavlovian conditioned rats were placed in the operant chambers under conditions identical to training, but the cues no longer predicted the delivery of alcohol, or lack thereof. Next, rats underwent reinstatement tests. During each test rats were either exposed to their social defeat scent or the control scent via a scented cotton ball that was placed in the operant chamber. They were then presented with 30 presentations each of the CS+ and CS− cues. In the subsequent session rats were tested with the opposite scent. Only one scent (lemon or banana) was tested in the operant chambers each day.
Statistical Analysis
Our primary behavioral measure during Pavlovian conditioning and testing sessions was the number of port entries during each epoch (CS+, CS− and/or ITI). Our primary behavioral measures during the conditioned reinforcement sessions were active versus inactive nosepokes, in addition to the total number of port entries. To account for a mixture of within-subject and between-subjects data collection we analyzed the data using linear mixed effects models (fitlme function with maximum likelihood estimation) in MATLAB (Mathworks). To assess the development of cue discrimination across Pavlovian conditioning and extinction sessions, we fit models with fixed effects for cue type and session and a random effect for subject. To assess the impact of stress on responses to the cues after extinction learning, we fit models with fixed effects for cue type and stress condition, and a random effect for subject. For those experiments that included both male and female rats, we fit each model with and without sex as a factor to determine whether there were any significant main effect of sex or interaction of sex with other factors, or whether the addition of this variable improved the model fit, accounting for the increase in the number of parameters. Models were compared using the compare function in MATLAB, which conducts a likelihood ratio test to assess whether the addition of parameters significantly improves the model. Because adding sex as a variable did not significantly improve model fit, and did not significantly impact any behavioral outcomes during Pavlovian conditioning, extinction or reinstatement tests, we primarily report pooled data throughout the manuscript. When main effects or interactions of interest had non-significant effects, we followed this analysis by calculating a Bayes factor to assess support for the null versus the alternative hypothesis (bayesFactor package bf.anova function in MATLAB).
Results
Yohimbine reinstatement of responses to alcohol cues
Across 20-25 training sessions, male and female rats learned to discriminate between a CS+ paired with 20% ethanol delivery and an unpaired CS− cue. We observed no main effect of sex (F(1,590) = 1.1189, p = 0.29) or interaction of sex with other factors (Fs 0.14 to 2.20) during conditioning sessions. Port entries during the CS+, but not CS−, increased across training days (Figure 1A; main effect of session, F(1,594) = 85.616, p < 0.001; interaction of session and cue type, F(1,594) = 49.098, p < 0.001). Port entries during the intertrial interval decreased across training sessions (main effect of session, F(1,297)=9.092, p = 0.0028). Rats then underwent 5 extinction sessions in which ethanol was no longer delivered. We observed no main effect of sex (F(1,132) = 0.88, p = 0.35) or interaction of sex with other factors (Fs 0.078 to 0.42) during extinction learning. Across these sessions port entries decreased during both cue types (Figure 1B; main effect of session, F(1,136)= 39.735, p < 0.001), but more so during the CS+ (interaction of session and cue type, F(1,136) = 7.716, p = 0.0062). Despite the greater decrease in CS+ port entries, rats continued to discriminate between these cues, even during the final extinction session (CS+ versus CS−, F(1,10)=7.36, p = 0.022). Port entries during the intertrial interval also decreased across extinction training (main effect of session, F(1,69)=10.79, p = 0.0016).
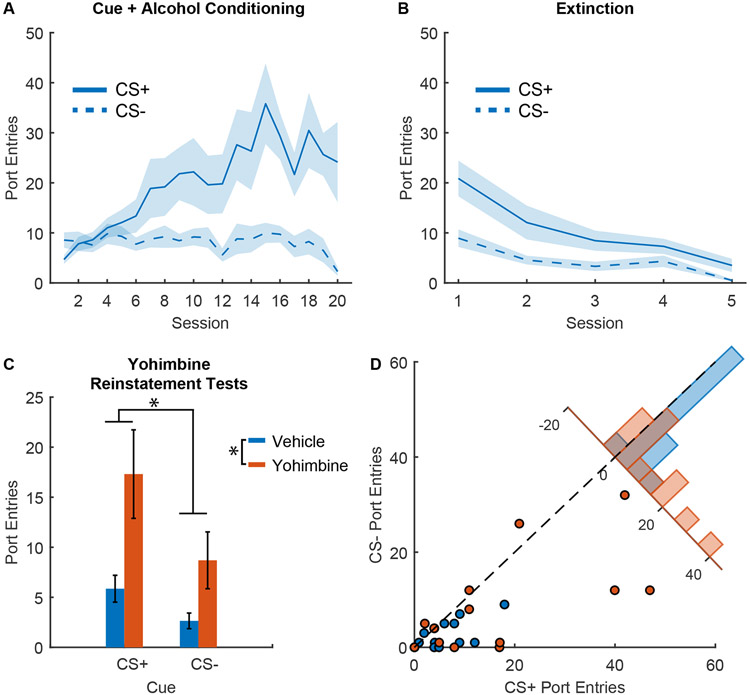
Figure 1. Yohimbine reinstatement of responses to alcohol cues.
A) Port entry number during the CS+ and CS− across Pavlovian conditioning with 20% ethanol reward (n=17; 10 males and 7 females), mean +/− SEM. B) Port entry number during the CS+ and CS− across extinction training, mean +/− SEM. C) Port entry number during reinstatement tests during the CS+ and CS− after injection of yohimbine (orange) or vehicle (blue), mean +/− SEM, *, p < 0.05. D) Scatterplot showing port entry number during the CS+ and CS− from individual reinstatement sessions after injections of yohimbine (orange) or vehicle. Inset histogram shows the distribution of sessions based on the difference in the number of port entries during the CS+ and the CS−.
We next assessed the ability of yohimbine to reinstate port entry behavior during ethanol cues. Because we observed no main effect of sex (F(1,46) = 0.0002, p = 0.99) and no interaction of sex with other factors (Fs 0.075 to 2.07) we pooled data across males and females for these analyses. In comparison to a saline control session, we found that yohimbine significantly enhanced port entries during the cues (Figure 1C; main effect of yohimbine, F(1,50)=14.429, p < 0.001), but that this response was not selective to the CS+ (interaction between yohimbine and cue type, F(1,50)=2.156, p = 0.148). Because the inclusion of a term for the interaction between yohimbine and cue type to the model did not improve the model fit in comparison to a model that only included the main effects of yohimbine and cue type (model comparison, p = 0.135; Bayes factor of 2.59 in favor of the model with no interaction term) we further assessed the impact of cue type without this term. We observed a significant effect of cue type during the reinstatement test (F(1,47) = 9.88, p = 0.0029). The difference between CS+ and CS− responses was also significantly different from zero (Figure 1D; F(1,25)=4.25, p = 0.049). Yohimbine did not produce any significant change in the difference between CS+ and CS− responses (F(1,25) = 2.598, p = 0.119; Bayes factor of 1.33 in favor of the null). Additionally, we also observed a significant effect of yohimbine on port entries during the intertrial interval (F(1,25)=6.698, p = 0.0158; Supplementary Figure 2A), suggesting that yohimbine increased port entries across the session, and not selectively during the CS+. Altogether our results suggest independent effects of cue type and yohimbine on entries into the alcohol port during the reinstatement test.
Yohimbine reinstatement of responses to sucrose cues
We next sought to determine whether yohimbine would also reinstate port entries after Pavlovian conditioning with 10% liquid sucrose. Across 10 sessions of training, male rats learned to discriminate between a CS+ paired with sucrose delivery, and an unpaired CS− (Figure 2A; main effect of session, F(1,156)=25.54, p < 0.001; interaction of session and cue type, F(1,156)=12.28, p < 0.001). Rats then underwent 5 extinction sessions in which sucrose was no longer delivered (Figure 2B). Across these sessions port entries decreased during the CS+ but not the CS− (main effect of session, F(1,76)=44.50, p < 0.001; interaction of session and cue type, F(1,76)=22.91, p < 0.001). By the final day of extinction rats no longer demonstrated significant discrimination between the CS+ and CS− (F(1,14) = 3.27, p = 0.092).
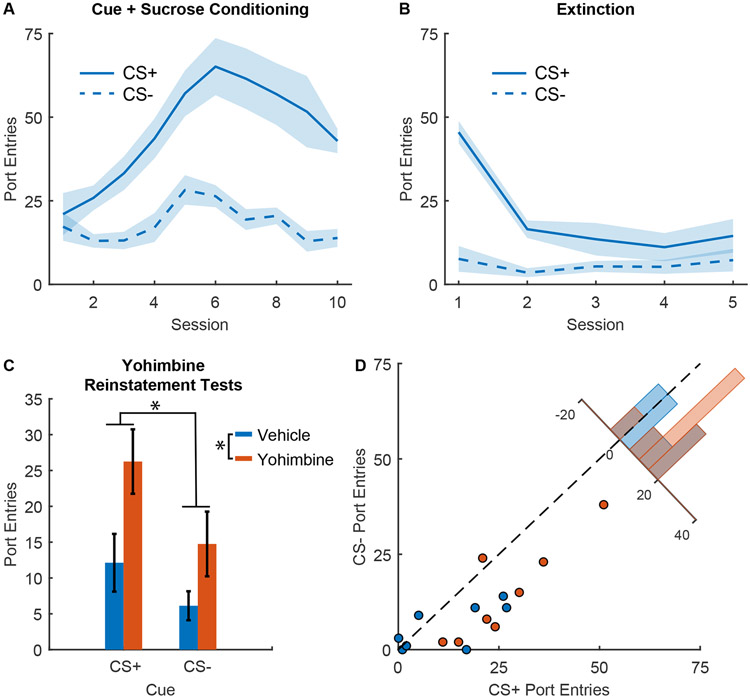
Figure 2. Yohimbine reinstatement of responses to sucrose cues.
A) Port entry number during the CS+ and CS− across Pavlovian conditioning with 10% sucrose reward (n=8 males), mean +/− SEM. B) Port entry number during the CS+ and CS− across extinction training, mean +/− SEM. C) Port entry number during reinstatement tests during the CS+ and CS− after injection of yohimbine (orange) or vehicle (blue), mean +/− SEM. D) Scatterplot showing port entry number during the CS+ and CS− from individual reinstatement sessions after injections of yohimbine (orange) or vehicle. Inset histogram shows the distribution of sessions based on the difference in the number of port entries during the CS+ and the CS−.
We next assessed the ability of yohimbine to reinstate port entry behavior during sucrose cues. During the reinstatement tests, we found that yohimbine significantly enhanced port entries during the cues (Figure 2C; main effect of yohimbine, F(1,28)=23.92, p < 0.001), but this effect was not selective to the CS+ (interaction of yohimbine and cue type, F(1,28) = 1.81, p = 0.11). Like the analysis of the prior experiment with alcohol cues, the inclusion of a term for the interaction between yohimbine and cue type did not significantly improve the model (model comparison, p = 0.19; Bayes factor of 2.52 in favor of the model without the interaction term). Port entries were significantly affected by cue type in both models (full model, F(1,28) = 4.32, p = 0.047; no interaction model, F(1,29) = 17.07, p < 0.001). We also assessed the impact of yohimbine on the difference between port entries during the CS+ versus CS−. Though we observed a numerical increase following yohimbine, this comparison was not statistically significant (Figure 2D; F(1,23) = 2.75, p = 0.11; Bayes factor of 1.32 in favor of the null). Additionally, we observed a significant effect of yohimbine on port entries during the intertrial interval (F(1,23) = 7.11, p = 0.013; Supplementary Figure 2B), suggesting that yohimbine increased port entries across the session, and not selectively during the CS+ or CS−. Altogether our results suggest independent effects of cue type and yohimbine on entries into the sucrose port during the reinstatement test.
Yohimbine effects on conditioned reinforcement by a sucrose cue
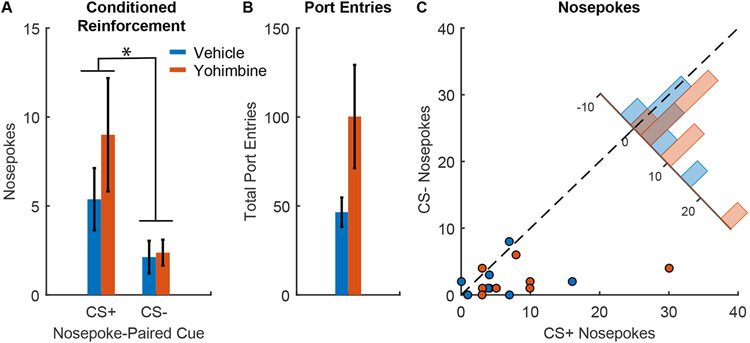
While yohimbine-induced increases in port entry behavior were not specific to the CS+ phase for rats trained with sucrose or alcohol, the cues may have still impacted port entry behavior during the session more generally. Therefore, we next sought to determine whether yohimbine would impact other aspects of cue-related behavior by assessing conditioned reinforcement. Yohimbine has previously been shown to increase conditioned reinforcement by cues predicting both 12% ethanol and 21.7% sucrose (Tabbara et al. 2020). Rats tested for yohimbine-induced reinstatement of Pavlovian sucrose-seeking were retrained with the CS+ paired with sucrose, and then underwent conditioned reinforcement testing. Overall we found that rats made more entries into a nose poke that produced CS+ presentations in comparison to a nose poke that produced CS− presentation (Figure 3A; main effect of cue type, F(1,28) = 8.207, p = 0.0078), but that yohimbine did not significantly impact this behavior (Figure 3C; main effect of yohimbine, F(1,28) = 2.447, p = 0.128; interaction between yohimbine and cue type, F(1,28) = 1.0649, p = 0.31). The addition of either a simple main effect for yohimbine, or an interaction term for yohimbine by nosepoke did not significantly improve the model versus a restricted model that included only cue type and the random effect of subject (model with main effect of yohimbine, p = 0.25, Bayes factor of 2.47 in favor of the restricted model; model with interaction term, p = 0.31, Bayes factor of 7.65 in favor of the restricted model). While we observed a numerical increase in port entries during the yohimbine session, this effect was not significant (Figure 3B; F(1,14) = 3.6929, p = 0.075; Bayes factor of 1.5392 in favor of the alternative). We also observed no relationship between CS+ nose pokes and total port entries (Figure 3B; F(1,12) = 0.879, p = 0.367, Bayes factor of 76.80 in favor of the null) or any effect of yohimbine on this relationship (F(1,12) = 0.204, p = 0.659, Bayes factor of 6.96 in favor of the null). Overall yohimbine did not produce a reliable increase in conditioned reinforcement.
Figure 3. Effects of yohimbine on conditioned reinforcement by sucrose cues.
A) Number of entries into nosepoke holes that resulted in presentations of the CS+ versus CS− following injections of yohimbine (orange) versus vehicle (blue) in rats that underwent Pavlovian conditioned with 10% sucrose reward (n=8 males), mean +/− SEM, *, p < 0.05. B) Total port entries during conditioned reinforcement sessions after yohimbine (orange) versus vehicle (blue), mean +/− SEM. C) Scatterplot showing entries into the CS+-paired nosepoke versus the CS− nosepoke from individual sessions after yohimbine (orange) or vehicle (blue). Inset histogram shows the distribution of sessions based on the difference in the number of CS+ versus CS− nosepokes.
Footshock reinstatement of responses to alcohol cues
We next sought to determine the impact of a physical stressor, intermittent footshock, on behavioral responses to an extinguished alcohol CS+. Following homecage pre-exposure, male and female rats learned to discriminate between a CS+ predicting ethanol and an unpaired CS− (Figure 4A; main effect of session, F(1,454) = 81.783, p < 0.001; main effect of cue type, F(1,454) = 0.10924, p = 0.74; interaction of session and cue type F(1,434)=44.989, p < 0.001). We observed no effect of sex (F(1,490) = 1.53, p = 0.22) or interaction of sex with other factors during conditioning (Fs 0.003 to 0.48). Rats then underwent extinction sessions in which ethanol was no longer delivered, and port entry behavior then decreased (Figure 4B; main effect of session, F(1,106) = 36.746, p < 0.001, main effect of cue type, F(1,106)=51.548, p<0.001; interaction of session and cue type, F(1,106) = 14.889, p < 0.001). While port entry behavior decreased selectively during the CS+ relative to the CS−, rats continued to discriminate between these cues even on the final day of extinction (F(1,20) = 10.493, p = 0.0041). Similarly to conditioning sessions, we observed no main effect of sex (F(1,112) = 0.29, = 0.59) or interaction of sex with other factors (Fs 0.012 to 0.86) during extinction learning.
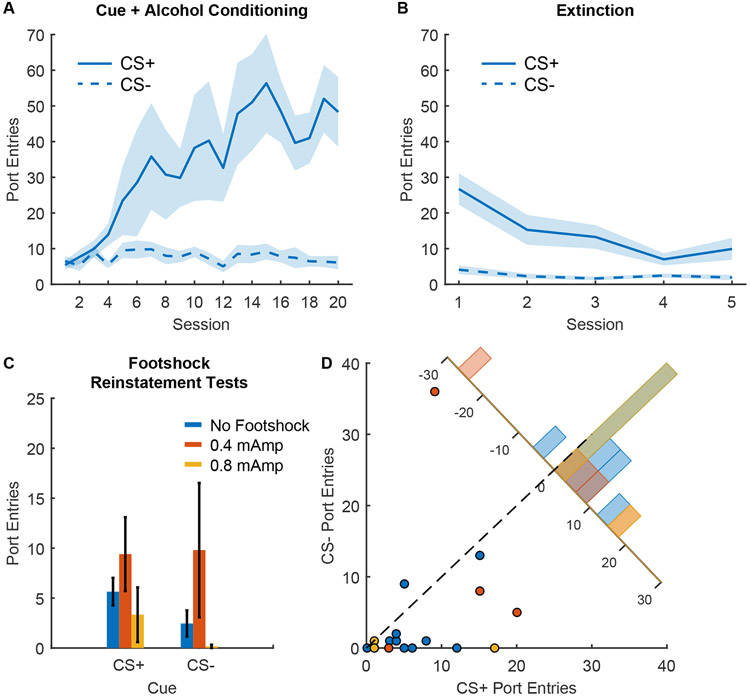
Figure 4. Footshock reinstatement of responses to alcohol cues.
A) Port entry number during the CS+ and CS− across Pavlovian conditioning with 20% ethanol reward (n=12; 6 males and 6 females), mean +/− SEM. B) Port entry number during the CS+ and CS− across extinction training, mean +/− SEM. C) Port entry number during reinstatement tests during the CS+ and CS− after 0.4 mAmp intermittent footshock (orange), 0.8 mAmp intermittent footshock (yellow) or a 10-min control period without footshock (blue), mean +/− SEM. D) Scatterplot showing port entry number during the CS+ and CS− from individual reinstatement sessions after 0.4 mAmp intermittent footshock (orange), 0.8 mAmp intermittent footshock (yellow) or a 10-min control period without footshock (blue). Inset histogram shows the distribution of sessions based on the difference in the number of port entries during the CS+ and the CS−.
We then assessed the impact of intermittent footshock on subsequent port entry behavior during the alcohol cues (Figure 4C). Since we observed no main effect of sex (F(1,36) = 0.034, p = 0.85) or interaction of sex and other factors (Fs 0.46 to 0.94), males and females were pooled for all analysis. In contrast to yohimbine, we found no effect of footshock on port entries during the cues (main effect of footshock, F(2,38) = 0.898, p = 0.415, Bayes factor of 1.32 in favor of the null) regardless of cue type (interaction of footshock and cue type, F(2,38) = 0.342, p = 0.71, Bayes factor of 29.52 in favor of the null). We also found no evidence of cue discrimination during the test (main effect of cue type, F(1,38) = 1.54, p = 0.22) or effect of footshock on cue discrimination (Figure 4D; F(2,19) = 0.473, p = 0.63, Bayes factor of 6.47 in favor of the null). While we observed a numerical increase in total port entries after the 0.4 mAmp footshocks, this effect did not reach statistical significance (F(2,19) = 2.80, p = 0.085, Bayes factor of 1.99 in favor of the null; Supplementary Figure 2C). Overall, intermittent footshock stress did not produce reliable increases in alcohol-seeking behavior, including in response to cues.
Impact of a social defeat cue on behavioral responses to alcohol cues
Next, we sought to determine the impact of a psychosocial stressor on behavioral responses to alcohol cues. Because acute social defeat has been shown to reduce alcohol self-administration and responding in a reinstatement test (Funk et al. 2005), we elected to use odor cues paired with social defeat, which have been shown to produce reinstatement of instrumental responding for both alcohol and cocaine seeking (Funk et al. 2005; Manvich et al. 2015). Male rats first learned to discriminate between a CS+ auditory cue predicting alcohol delivery and an unpaired CS− (Figure 5A; main effect of session, F(1,276) = 63.425, p < 0.001; main effect of cue, F(1,276)=0.142, p = 0.706; interaction of session and cue type, F(1,276) = 28.517, p < 0.001). Following the final 4 sessions of Pavlovian conditioning, rats underwent 4 social defeat sessions paired with their assigned odor cue. Next, rats underwent extinction training, during which their port entry behavior decreased across sessions (Figure 5B; main effect of session, F(1,108) = 17.976 p < 0.001), moreso during the CS+ than the CS− (main effect of cue type, F(1,108) = 30.437, p < 0.001; interaction of session and cue type, F(1,108) = 9.062, p = 0.0032). While CS+ port entries decreased more than CS− port entries, port entries during the CS+ remained numerically higher on the final day of extinction, though this difference was not statistically significant (F(1,12) = 4.18, p = 0.063).
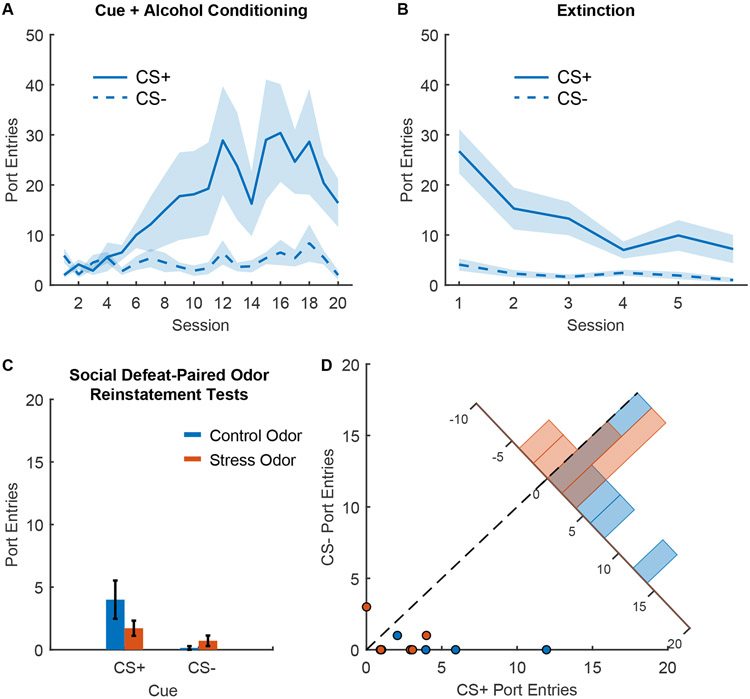
Figure 5. Reinstatement of responses to alcohol cues by olfactory cues associated with social defeat.
A) Port entry number during the CS+ and CS− across Pavlovian conditioning with 20% ethanol reward (n=7), mean +/− SEM. B) Port entry number during the CS+ and CS− across extinction training, mean +/− SEM. C) Port entry number during reinstatement tests during the CS+ and CS− in presence of an olfactory cue previously paired with social defeat (stress odor, orange), or a control odor (blue), mean +/− SEM. D) Scatterplot showing port entry number during the CS+ and CS− from individual reinstatement sessions in presence of an olfactory cue previously paired with social defeat (stress odor, orange), or a control odor (blue). Inset histogram shows the distribution of sessions based on the difference in the number of port entries during the CS+ and the CS−.
Finally, we assessed reinstatement behavior in the presence of each rat’s social defeat odor cue or a control cue. In contrast to prior work with instrumental self-administration, we found that the social defeat stress odor failed to enhance Pavlovian conditioned responses to alcohol cues (Figure 5C and D). If anything, the odor paired with social defeat reduced port entries during the cue, though this effect did not reach statistical significance (main effect of stress odor, F(1,24) = 4.2066, p = 0.0513) which was not surprising given the low number of port entries in the control condition. We also found no effect of cue type (F(1,24) = 0.80516, p = 0.38) or significant interaction between odor type and cue type (F(1,24) = 3.286, p = 0.082; Bayes factor of 1.08 in favor of the null). We also saw a numerical reduction in the difference between port entry number during the CS+ and the CS−, but this effect also did not reach statistical significance (main effect of stress odor, F(1,12) = 2.9661, p = 0.11; Bayes factor of 1.11 in favor of the alternative). We found no evidence of an effect on port entries during the intertrial interval (F(1,12) = 0.67, p = 0.43, Bayes factor of 2.47 in favor of the null; Supplementary Figure 2D). Overall, the social defeat cues did not drive reinstatement of alcohol-seeking in the presence or absence of the Pavlovian cues. Instead, the social defeat cues may have actually reduced behavior in this paradigm.
Discussion
Here we found that while yohimbine can drive reinstatement of alcohol seeking in a Pavlovian setting, these responses are not selective to the CS+ period, and this effect does not generalize to other stressors that have been shown to reinstate instrumental alcohol seeking. Specifically, we found that yohimbine drove increases in port entry behavior after extinction of either cue-alcohol or cue-sucrose associations. While port entry behavior was greater during the CS+ during these tests, increases were also seen during the control cue and ITI periods. When we assessed the effects of intermittent footshock and odor cues paired with social defeat, we failed to see a reinstatement-like effect, and even saw a slight suppression of port entry behavior in the case of social defeat cues. Overall, our results suggest that the effects of stress on Pavlovian behavioral responses are complex and Pavlovian approach may not be the best model through which to understand the ways in which certain stressors potentiate cue-driven reward-seeking behavior.
Yohimbine effects and their relationship to stress
One unanswered question from these studies is the relationship between the yohimbine-induced increased in alcohol seeking reported here and any stress-related effects of yohimbine. Yohimbine has been shown to elicit anxiety-like behaviors and increases in corticosterone in rodents (Davis et al. 1979; Johnston et al. 1988; Gill et al. 2013; Arrant et al. 2013), and yohimbine-induced reinstatement of instrumental alcohol and drug seeking behavior has been shown to depend on activation of extra-hypothalamic corticotropin-releasing factor receptors (Lê et al. 2000; Hansson et al. 2006; Marinelli et al. 2007b; Shalev et al. 2010). Despite this, the use of yohimbine as a stressor has been called into question in part due findings that suggest it is not aversive. Specifically, yohimbine does not increase the emission of 22 kHz vocalizations thought to indicate a negative affective state in rats (Mahler et al. 2013), and yohimbine produces a mild conditioned place preference (Chen et al. 2015). This critique conflates stress and aversion, and while stressors can certainly have negative affective qualities and long term negative affective consequences, stress is not defined by those negative affective qualities (Selye 1976). In some cases activation of stress systems may be rewarding; for instance, infusions of corticotropin releasing factor into the nucleus accumbens can also produce a conditioned place preference (Lemos et al. 2012).
An additional issue that has been raised for the use of yohimbine as a stressor is that it has been shown to cause reinstatement of operant responding for food, or even for cues alone (Ghitza et al. 2005; Nair et al. 2006; Chen et al. 2015). Yohimbine has also been shown to increase conditioned reinforcement by cues previously paired with sucrose or ethanol (Tabbara et al. 2020). While we did not replicate these conditioned reinforcement effects here, this could be due to differences in the length of Pavlovian training, or the fact that rats in our experiments did not experience the new operant-cue contingency prior to testing, which may have blunted overall responding across the testing conditions. Prior extinction may have also altered the ability of the cues to robustly reinforce novel operant responses. That being said, we did find similar effects of yohimbine on alcohol- and sucrose-seeking in our paradigm. This lack of specificity to drug-seeking behavior and drug-paired cues has contributed to uncertainty over yohimbine’s actions via stress-related mechanisms.
If stress generally acts as a context cue to drive reinstatement of drug-seeking (Schepers and Bouton 2019), it follows that stressors that reinstate drug-seeking should not reinstate food-seeking, since drug but not food reinforcers drive activation of stress systems (Sinha 2008). This is the case for intermittent footshock (Ahmed and Koob 1997; Buczek et al. 1999). A counterpoint to this is that food restriction, such as that implemented during Pavlovian conditioning with sucrose in this study, can also produce activation of stress systems (Stewart et al. 1988; Schroff et al. 2004), as does anticipation of food (Kalsbeek et al. 2012). Whether we would see similar effect in ad libitum fed rats trained with sucrose reward remains to be determined. As far as we are aware, the previous studies demonstrating yohimbine-induced reinstatement of food-seeking behaviors were all conducted in food restricted rats (Nair et al. 2006, 2008; Pickens et al. 2012; Chen et al. 2015; Tabbara et al. 2020).
Stress may also act by activating dopamine systems and therefore increasing the motivational impact of reward-related cues (Robinson and Berridge 1993; Field and Powell 2007; Nair et al. 2011; Brown et al. 2012). If stress acts primarily via this mechanism to drive reinstatement, the question remains why many stressors fail to reinstate instrumental food-seeking behaviors, and why we failed to see reinstatement of Pavlovian approach after intermittent footshock or exposure to odors paired with social defeat.
Stress intensity and null effects of intermittent footshock and social defeat cues
Reinstatement of reward-seeking by stress-induced activation of dopaminergic systems and resulting changes in the motivational impact of cues may depend on both the intensity or aversiveness of the stressor (Janak et al. 2004; Mahler et al. 2013; Zhou et al. 2021) and the nature of the cue response being tested. This may explain in part why we failed to see an increase in Pavlovian approach after intermittent footshock and exposure to social defeat cues. Dopamine has been implicated preferentially in behavioral responses driven by the incentive value of cues, such as approach to a reward cue or “sign tracking”, in comparison to behaviors driven by the predictive value of cues, such as approach to a reward delivery location or “goal-tracking” (Flagel et al. 2011; Saunders and Robinson 2012). Here, we used auditory cues, which meant that the only measure of cue learning or motivated behavior we were able to monitor took the form of approach to the reward-delivery port, or goal tracking. Whether intermittent footshock or social defeat cues would potentiate or reinstate sign tracking behavior following Pavlovian conditioning remains unclear. Entry to a reward port where the reward is normally consumed may result in behavioral responses that are more closely tied to reward consumption, rather than reward-seeking. The effects of acute stress on alcohol consumption are complex (Becker et al. 2011). While it is difficult to identify specific rules that govern whether stress results in increases, decreases, or no change in alcohol consumption, more intense stressors may result in decreased consummatory behavior (Bond 1978; Champagne and Kirouac 1987; Van Erp and Miczek 2001; Darnaudéry et al. 2007), which could also affect goal-tracking tracking behavior in response to cues. Therefore, even if intermittent footshock and social defeat cues can act to potentiate the motivational value of cues, these effects may be competing with opposing effects on consummatory behavior in our paradigm. It is also important to note that intense or prolonged stressors can also suppress even sign-tracking and reinstatement of instrumental drug-seeking behavior (Funk et al. 2005; Fitzpatrick et al. 2018). Finally, the timing of each stressor relative to the reinstatement test was based on the prior literature showing reinstatement of instrumental responding for each stressor. This resulted in a delay of 30 minutes between yohimbine injection and cue presentations, and a more limited (1 to 2-minute delay) between the intermittent footshock session (lasting 10 minutes) or exposure to social defeat cues, and subsequent presentations of alcohol cues.
Potential sex differences in the impact of stressors on alcohol-seeking behavior
While our goal here was not to specifically assess sex differences, we are cognizant of the rich body of literature indicating the male and female rats differ on some measures of alcohol seeking and conditioned responding (Becker and Koob 2016; Segal et al. 2022; Mineur et al. 2022). For instance, prior work has demonstrated the female rats are more sensitive to yohimbine-induced reinstatement of instrumental alcohol seeking behavior (Bertholomey et al. 2016; Bertholomey and Torregrossa 2019). Here, we found no evidence of sex differences in the impact of yohimbine or intermittent footshock on alcohol seeking in a Pavlovian setting. Several differences between instrumental and Pavlovian settings could explain this difference. One feature of the Pavlovian setting is that the rats do not control the number of alcohol deliveries, limiting baseline differences in alcohol seeking and consumption that have been observed in female rats prior to reinstatement. It is also possible that there are sex differences in a Pavlovian setting that our experiment is insufficiently powered to detect. In the case of the social defeat experiment using the resident-intruder paradigm, we used only male subjects as experimental animals and intruders because that is the basis for the model that has been shown to drive reinstatement of alcohol and cocaine seeking instrumental settings (Funk et al. 2005; Manvich et al. 2015). Social defeat experiments in female rats would require the use of an entirely different paradigm, such as the use of lactating dams as aggressive stimulus rats (Holly et al. 2012; Shimamoto et al. 2011). As far as we know the effect of social stress in this model have not been tested in an instrumental reinstatement paradigm. Finally, we only assessed yohimbine-induced reinstatement of sucrose seeking in male rats. While we observed similar effect of yohimbine on sucrose and alcohol seeking, regardless of sex, it is possible that yohimbine might differentially impact sucrose seeking in a Pavlovian setting in female rats. Prior work on reinstatement of instrumental food-seeking by yohimbine in male and female rats suggests this is not likely (Pickens et al. 2012).
Conclusions
Here we found divergent effects of yohimbine, intermittent footshock and social defeat cues on reinstatement of alcohol seeking in a Pavlovian setting. Yohimbine potentiated alcohol seeking behaviors, whereas the other stressors failed to increase alcohol-seeking and may have been reduced alcohol-seeking behavior. The results highlight the multiple behavioral mechanisms via which stress may act to increase or decrease drug-seeking behaviors. In some models of reward-seeking these stress effects and behaviors may be in conflict, which is distinct from the effects of a reward-paired context (Valyear et al. 2017). Pavlovian-conditioned approach may be a useful model for studying the excitatory effects of mild stressors, like yohimbine, on responses to drug-paired cues, but may be less useful to understanding that mechanisms by which more intense stressors potentiate the motivational value of drug-paired cues. Future work examining the effects of these stressors in other paradigms in which cues are presented non-contingently to drive incentive motivated behaviors is warranted.
Supplementary Material
Acknowledgements:
This work was supported in part by National Institutes of Health grants K99AA025384 and R00AA025384 to JMR, and a Provost’s Undergraduate Research Award to AA.
Footnotes
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Aguirre CG, Stolyarova A, Das K, et al. (2020) Sex-dependent effects of chronic intermittent voluntary alcohol consumption on attentional, not motivational, measures during probabilistic learning and reversal. PLoS One 15:e0234729. 10.1371/JOURNAL.PONE.0234729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF (1997) Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology (Berl) 132:289–295. 10.1007/S002130050347 [DOI] [PubMed] [Google Scholar]
- Arrant AE, Schramm-Sapyta NL, Kuhn CM (2013) Use of the light/dark test for anxiety in adult and adolescent male rats. Behav Brain Res 256:119–127. 10.1016/J.BBR.2013.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL (2011) Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl) 218:131–56. 10.1007/s00213-011-2443-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF (2016) Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev 68:242–263. 10.1124/pr.115.011163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholomey ML, Nagarajan V, Torregrossa MM (2016) Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure. Psychopharmacology (Berl) 233:2277–2287. 10.1007/s00213-016-4278-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholomey ML, Torregrossa MM (2019) Gonadal hormones affect alcohol drinking, but not cue+yohimbine-induced alcohol seeking, in male and female rats. Physiol Behav 203:70–80. 10.1016/J.PHYSBEH.2017.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond NW (1978) Shock induced alcohol consumption in rats: role of initial preference. Pharmacol Biochem Behav 9:39–42. 10.1016/0091-3057(78)90010-2 [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, et al. (2007) Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci 27:12655–12663. 10.1523/JNEUROSCI.3926-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Vik PW, McQuaid JR, et al. (1990) Severity of psychosocial stress and outcome of alcoholism treatment. J Abnorm Psychol 99:344–8 [DOI] [PubMed] [Google Scholar]
- Brown ZJ, Kupferschmidt DA, Erb S (2012) Reinstatement of cocaine seeking in rats by the pharmacological stressors, corticotropin-releasing factor and yohimbine: role for D1/5 dopamine receptors. Psychopharmacology (Berl) 224:431–440. 10.1007/S00213-012-2772-3 [DOI] [PubMed] [Google Scholar]
- Buczek Y, Lê AD, Wang A, et al. (1999) Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology (Berl) 144:183–188. 10.1007/S002130050992 [DOI] [PubMed] [Google Scholar]
- Burke KA, Franz TM, Miller DN, Schoenbaum G (2007) Conditioned reinforcement can be mediated by either outcome-specific or general affective representations. Front Integr Neurosci 1:2. 10.3389/neuro.07.002.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Kirouac G (1987) Effects of unavoidable electric shocks on voluntary alcohol consumption in the rat. Percept Mot Skills 64:335–338. 10.2466/PMS.1987.64.1.335 [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Cone JJ, Janak PH (2008a) Reinstated ethanol-seeking in rats is modulated by environmental context and requires the nucleus accumbens core. Eur J Neurosci 28:2288–98. 10.1111/j.1460-9568.2008.06517.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH (2008b) Context-induced relapse of conditioned behavioral responding to ethanol cues in rats. Biol Psychiatry 64:203–10. 10.1016/j.biopsych.2008.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, Janak PH (2010) Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology 35:783–91. 10.1038/npp.2009.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Woods CA, Sahuque LL, et al. (2013) Unilateral inactivation of the basolateral amygdala attenuates context-induced renewal of Pavlovian-conditioned alcohol-seeking. Eur J Neurosci 38:2751–61. 10.1111/ejn.12278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-W, Fiscella KA, Bacharach SZ, et al. (2015) Effect of yohimbine on reinstatement of operant responding in rats is dependent on cue contingency but not food reward history. Addict Biol 20:690–700. 10.1111/adb.12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y (2008) Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci 363:3233–43. 10.1098/rstb.2008.0090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnaudéry M, Louvart H, Defrance L, et al. (2007) Impact of an intense stress on ethanol consumption in female rats characterized by their pre-stress preference: modulation by prenatal stress. Brain Res 1131:181–186. 10.1016/J.BRAINRES.2006.11.005 [DOI] [PubMed] [Google Scholar]
- Davis M, Redmond DE, Baraban JM (1979) Noradrenergic agonists and antagonists: effects on conditioned fear as measured by the potentiated startle paradigm. Psychopharmacology (Berl) 65:111–118. 10.1007/BF00433036 [DOI] [PubMed] [Google Scholar]
- DiFeliceantonio AG, Berridge KC (2016) Dorsolateral neostriatum contribution to incentive salience: Opioid or dopamine stimulation makes one reward cue more motivationally attractive than another. Eur J Neurosci. 10.1111/ejn.13220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Powell H (2007) Stress increases attentional bias for alcohol cues in social drinkers who drink to cope. Alcohol Alcohol 42:560–6. 10.1093/alcalc/agm064 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick CJ, Jagannathan L, Lowenstein E, et al. (2018) Single prolonged stress decreases sign-tracking and cue-induced reinstatement of cocaine-seeking. Behav Brain Res. 10.1016/j.bbr.2018.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, et al. (2011) A selective role for dopamine in stimulus-reward learning. Nature 469:53–59. 10.1038/NATURE09588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Harding S, Juzytsch W, Lê AD (2005) Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 183:341–9. 10.1007/s00213-005-0194-1 [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, et al. (2005) The Anxiogenic Drug Yohimbine Reinstates Palatable Food Seeking in a Rat Relapse Model: a Role of CRF(1) Receptors. Neuropsychopharmacology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill MJ, Ghee SM, Harper SM, See RE (2013) Inactivation of the lateral habenula reduces anxiogenic behavior and cocaine seeking under conditions of heightened stress. Pharmacol Biochem Behav 111:24–29. 10.1016/J.PBB.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Lewis MJ, Luine VN (2012) The interaction of chronic restraint stress and voluntary alcohol intake: effects on spatial memory in male rats. Alcohol 46:499–504. 10.1016/j.alcohol.2011.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, et al. (2006) Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A 103:15236–41. 10.1073/pnas.0604419103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Holly EN, DeBold JF, Miczek KA (2015) Social stress-escalated intermittent alcohol drinking: modulation by CRF-R1 in the ventral tegmental area and accumbal dopamine in mice. Psychopharmacology (Berl). 10.1007/s00213-015-4144-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Chaudhri N (2010) The Potent Effect of Environmental Context on Relapse to Alcohol-Seeking After Extinction. Open Addict J 3:76–87. 10.2174/1874941001003010076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Chen M-T, Caulder T (2004) Dynamics of neural coding in the accumbens during extinction and reinstatement of rewarded behavior. Behav Brain Res 154:125–35. 10.1016/j.bbr.2004.02.003 [DOI] [PubMed] [Google Scholar]
- Johnston AL, Baldwin HA, File SE (1988) Measures of anxiety and stress in the rat following chronic treatment with yohimbine. J Psychopharmacol 2:33–38. 10.1177/026988118800200106 [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, van der Spek R, Lei J, et al. (2012) Circadian rhythms in the hypothalamo–pituitary–adrenal (HPA) axis. Mol Cell Endocrinol 349:20–29. 10.1016/J.MCE.2011.06.042 [DOI] [PubMed] [Google Scholar]
- Khoo SY- S, Sciascia JM, Brown A, Chaudhri N (2020) Comparing ABA, AAB, and ABC Renewal of Appetitive Pavlovian Conditioned Responding in Alcohol- and Sucrose-Trained Male Rats. Front Behav Neurosci 14:. 10.3389/fnbeh.2020.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Funk D, Juzytsch W, et al. (2011) Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology (Berl) 218:89–99. 10.1007/s00213-011-2178-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Harding S, Juzytsch W, et al. (2000) The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 150:317–24 [DOI] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, et al. (1999) Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology 21:435–444. https://doi.org/S0893-133X(99)00024-X [pii] 10.1016/S0893-133X(99)00024-X [doi] [DOI] [PubMed] [Google Scholar]
- Lê AD, Quan B, Juzytch W, et al. (1998) Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl) 135:169–74 [DOI] [PubMed] [Google Scholar]
- Lemos JC, Wanat MJ, Smith JS, et al. (2012) Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. https://doi.org/nature11436 [pii] 10.1038/nature11436 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Zorrilla EP (2012) Stress history increases alcohol intake in relapse: relation to phosphodiesterase 10A. Addict Biol 17:920–33. 10.1111/j.1369-1600.2012.00460.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Feltenstein MW, et al. (2013) A rodent “self-report” measure of methamphetamine craving? Rat ultrasonic vocalizations during methamphetamine self-administration, extinction, and reinstatement. Behav Brain Res 236:78–89. 10.1016/j.bbr.2012.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, et al. (2015) Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology. 10.1038/npp.2015.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manvich DF, Stowe TA, Godfrey JR, Weinshenker D (2015) A method for psychosocial stress-induced reinstatement of cocaine seeking in rats. Biol Psychiatry. 10.1016/j.biopsych.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, et al. (2007a) Effects of opioid receptor blockade on the renewal of alcohol seeking induced by context: relationship to c-fos mRNA expression. Eur J Neurosci 26:2815–2823. 10.1111/J.1460-9568.2007.05898.X [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, et al. (2007b) The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 195:345–55. 10.1007/s00213-007-0905-x [DOI] [PubMed] [Google Scholar]
- Millan EZ, Kim HA, Janak PH (2017) Optogenetic activation of amygdala projections to nucleus accumbens can arrest conditioned and unconditioned alcohol consummatory behavior. Neuroscience. 10.1016/j.neuroscience.2017.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, McNally GP (2011) Accumbens shell AMPA receptors mediate expression of extinguished reward seeking through interactions with basolateral amygdala. Learn Mem 18:414–21. 10.1101/lm.2144411 [DOI] [PubMed] [Google Scholar]
- Mineur YS, Garcia-Rivas V, Thomas MA, et al. (2022) Sex differences in stress-induced alcohol intake: a review of preclinical studies focused on amygdala and inflammatory pathways. Psychopharmacology (Berl) 239:2041–2061. 10.1007/S00213-022-06120-W [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Golden SA, Shaham Y (2008) Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. Br J Pharmacol 154:406–416. 10.1038/BJP.2008.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Gray SM, Ghitza UE (2006) Role of food type in yohimbine- and pellet-priming-induced reinstatement of food seeking. Physiol Behav 88:559–566. 10.1016/J.PHYSBEH.2006.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Navarre BM, Cifani C, et al. (2011) Role of dorsal medial prefrontal cortex dopamine D1-family receptors in relapse to high-fat food seeking induced by the anxiogenic drug yohimbine. Neuropsychopharmacology 36:497–510. 10.1038/npp.2010.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenheimer DJ, Wang K, Haimbaugh A, et al. (2019) Recruitment and disruption of ventral pallidal cue encoding during alcohol seeking. Eur J Neurosci 50:3428–3444. 10.1111/ejn.14527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, et al. (1999) Dissociation in Effects of Lesions of the Nucleus Accumbens Core and Shell on Appetitive Pavlovian Approach Behavior and the Potentiation of Conditioned Reinforcement and Locomotor Activity byd-Amphetamine. J Neurosci 19:2401–2411. 10.1523/JNEUROSCI.19-06-02401.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Cifani C, Navarre BM, et al. (2012) Effect of fenfluramine on reinstatement of food seeking in female and male rats: implications for the predictive validity of the reinstatement model. Psychopharmacology (Berl) 221:341–353. 10.1007/S00213-011-2585-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priddy BM, Carmack SA, Thomas LC, et al. (2017) Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol Biochem Behav 152:61–67. 10.1016/j.pbb.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remedios J, Woods C, Tardif C, et al. (2014) Pavlovian-conditioned alcohol-seeking behavior in rats is invigorated by the interaction between discrete and contextual alcohol cues: implications for relapse. Brain Behav 4:278–89. 10.1002/brb3.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Stout N, Acs D, Janak PH (2018) Ventral pallidal encoding of reward-seeking behavior depends on the underlying associative structure. Elife 7:e33107. 10.7554/eLife.33107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18:247–91 [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE (2012) The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci 36:2521–2532. 10.1111/j.1460-9568.2012.08217.x [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers ST, Bouton ME (2019) Stress as a context: Stress causes relapse of inhibited food seeking if it has been associated with prior food seeking. Appetite 132:131–138. 10.1016/J.APPET.2018.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroff KC, Cowen MS, Koch S, Spanagel R (2004) Strain-specific responses of inbred mice to ethanol following food shortage. Addict Biol 9:265–271. 10.1080/13556210412331292596 [DOI] [PubMed] [Google Scholar]
- Sciascia JM, Reese RM, Janak PH, Chaudhri N (2015) Alcohol-Seeking Triggered by Discrete Pavlovian Cues is Invigorated by Alcohol Contexts and Mediated by Glutamate Signaling in the Basolateral Amygdala. Neuropsychopharmacology 40:2801–12. 10.1038/npp.2015.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal D, Valyear MD, Chaudhri N (2022) The role of context on responding to an alcohol-predictive cue in female and male rats. Alcohol 99:70–81. 10.1016/J.ALCOHOL.2021.10.004 [DOI] [PubMed] [Google Scholar]
- Selye H (1976) Stress without Distress. Psychopathol Hum Adapt 137–146. 10.1007/978-1-4684-2238-2_9 [DOI] [Google Scholar]
- Shaham Y, Erb S, Stewart J (2000) Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Rev 33:13–33 [DOI] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y (2010) Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res 1314:15–28. 10.1016/j.brainres.2009.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, et al. (2008) Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res 32:1816–23. 10.1111/j.1530-0277.2008.00753.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 158:343–359 [DOI] [PubMed] [Google Scholar]
- Sinha R (2008) Chronic Stress, Drug Use, and Vulnerability to Addiction. Ann N Y Acad Sci 1141:105–130. 10.1196/ANNALS.1441.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srey CS, Maddux J- MN, Chaudhri N (2015) The attribution of incentive salience to Pavlovian alcohol cues: a shift from goal-tracking to sign-tracking. Front Behav Neurosci 9:54. 10.3389/fnbeh.2015.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, Meaney MJ, Aitken D, et al. (1988) The Effects of Acute and Life-Long Food Restriction on Basal and Stress-Induced Serum Corticosterone Levels in Young and Aged Rats. Endocrinology 123:1934–1941. 10.1210/ENDO-123-4-1934 [DOI] [PubMed] [Google Scholar]
- Tabbara RI, Maddux J-MN, Beharry PF, et al. (2016) Effects of sucrose concentration and water deprivation on Pavlovian conditioning and responding for conditioned reinforcement. Behav Neurosci 130:231–42. 10.1037/bne0000138 [DOI] [PubMed] [Google Scholar]
- Tabbara RI, Rahbarnia A, Lê AD, Fletcher PJ (2020) The pharmacological stressor yohimbine, but not U50,488, increases responding for conditioned reinforcers paired with ethanol or sucrose. Psychopharmacology (Berl) 237:3689–3702. 10.1007/S00213-020-05647-0 [DOI] [PubMed] [Google Scholar]
- Tsiang MT, Janak PH (2006) Alcohol seeking in C57BL/6 mice induced by conditioned cues and contexts in the extinction-reinstatement model. Alcohol 38:81–8. 10.1016/j.alcohol.2006.05.004 [DOI] [PubMed] [Google Scholar]
- Valyear MD, Glovaci I, Zaari A, et al. (2020) Dissociable mesolimbic dopamine circuits control responding triggered by alcohol-predictive discrete cues and contexts. Nat Commun 11:1–14. 10.1038/s41467-020-17543-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valyear MD, Villaruel FR, Chaudhri N (2017) Alcohol-seeking and relapse: A focus on incentive salience and contextual conditioning. Behav Processes 141:26–32. 10.1016/j.beproc.2017.04.019 [DOI] [PubMed] [Google Scholar]
- Van Erp AMM, Miczek KA (2001) Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiol Behav 73:301–311. 10.1016/S0031-9384(01)00458-9 [DOI] [PubMed] [Google Scholar]
- Villaruel FR, Lacroix F, Sanio C, et al. (2017) Optogenetic Activation of the Infralimbic Cortex Suppresses the Return of Appetitive Pavlovian-Conditioned Responding Following Extinction. Cereb Cortex 1–12 [DOI] [PubMed] [Google Scholar]
- Villaruel FR, Martins M, Chaudhri N (2022) Corticostriatal Suppression of Appetitive Pavlovian Conditioned Responding. J Neurosci 42:834–849. 10.1523/JNEUROSCI.1664-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Pitchers KK, Flagel SB, Robinson TE (2015) Individual variation in the motivational and neurobiological effects of an opioid cue. Neuropsychopharmacology 40:1269–77. 10.1038/npp.2014.314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Xiang W, Huang M (2021) Inactivation of Zona Incerta Blocks Social Conditioned Place Aversion and Modulates Post-traumatic Stress Disorder-Like Behaviors in Mice. Front Behav Neurosci 15:. 10.3389/FNBEH.2021.743484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zironi I, Burattini C, Aicardi G, Janak PH (2006) Context is a trigger for relapse to alcohol. Behav Brain Res 167:150–155. 10.1016/J.BBR.2005.09.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.