Abstract
Background:
Clinicians and laboratories routinely use urinalysis (UA) parameters to determine if antimicrobial treatment and/or urine cultures are needed. Yet, the performance of individual UA parameters and common thresholds for action are not well defined and may vary across different patient populations.
Methods:
This retrospective cohort study included all encounters with UAs ordered 24 hours prior to a urine culture between 2015 and 2020 at three NC hospitals. We evaluated the performance of relevant UA parameters as potential outcome predictors, including sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV). We also combined 18 different UA criteria and used receiver operating curves to identify the top 5 performing models for predicting significant bacteriuria (≥100,000 colony forming units of bacteria/ml).
Results:
Of 221,933 encounters during the 6-year study period. No single UA parameter had both high sensitivity and high specificity in predicting bacteriuria. Absence of leukocyte esterase and pyuria had a high NPV for significant bacteriuria. Combined UA parameters did not perform better than pyuria alone with regards to NPV. The high NPV ≥0.90 of pyuria was maintained among most patient subgroups with the exception of females ≥65 and patients with indwelling catheters.
Conclusion:
UA parameters should be leveraged for their NPV instead of sensitivity, when used as a part of diagnostic workup. As many laboratories and hospitals use reflex urine culture algorithms, their workflow should include clinical decision support and or education to target symptomatic patients and focus on populations where absence of pyuria has high NPV.
Background
Urinalysis and urine cultures are routinely overused and misinterpreted in a variety of clinical settings.1 Inappropriate urine testing is a major driver of unnecessary antibiotic use, increased healthcare utilization, associated adverse events, and unnecessary costs. 2
Urinalysis parameters are often used by clinicians and laboratories to determine whether urine cultures should be performed or if treatment is indicated in cases of suspected urinary tract infection (UTI).3 On recent surveys of academic and community hospitals, almost 50% of hospitals and laboratories used reflex urine culture approaches (also referred to as urinalysis with reflex to culture).4,5 In this approach, when a urinalysis is ordered, the urine specimen is automatically processed for culture if specific urinalysis parameters (e.g., leukocyte esterase, white blood cells (WBC), or bacteria) are positive.6 Some hospitals used a combination of several different urinalysis criteria for reflexing, while others used a single criterion.4,5 There is no consensus on which urinalysis parameters should be used for reflexing or which populations are suited for this test.5 Most laboratories accept all urine specimens (e.g., catheterized specimens) for reflexing, which may be an inappropriate practice.4–6 Therefore, efforts are needed to identify and validate urinalysis criteria for use in reflex urine cultures and to identify populations best suited for reflex urine cultures.3
Our objectives were a) to compare the performance of different urinalysis parameters in predicting significant bacteriuria irrespective of symptoms and b) to assess the negative predictive value (NPV) of pyuria based on age, sex, setting, and presence of indwelling catheter. More importantly, the overall goal of this study is to provide guidance to existing laboratories that perform reflex urine cultures, related to urinalysis parameters and populations that are best suited for reflexing.
Methods:
Design:
This retrospective cohort study included all patient encounters (inpatient and outpatient) with paired urinalysis and urine cultures (urinalysis ordered <=24hrs prior to urine culture) between January 1, 2015 and December 31, 2020. The laboratory did not employ a reflex urine culture approach during the study. This study was considered exempt by Duke University Institutional Review Board (Protocol # 00107418).
Setting:
This study was conducted in North Carolina at three hospitals and over 150 outpatient clinic sites. The hospitals included one academic medical center with 1048 beds, and 2 community hospitals with 186 beds and 369 beds, respectively.
Outcomes and Definitions:
Our outcome of interest was “significant bacteriuria,” defined as a urine culture with ≥1 uropathogen growing at ≥100,000 colony forming units/mL. Negative cultures were defined as urine cultures with no bacterial growth. Mixed urine cultures were defined by Duke University Microbiology Laboratory as the presence of two or more organisms when all organisms were nonsignificant (not a known uropathogen) or when one of the organisms was considered a significant uropathogen but was in lesser quantity (approximately 10-fold fewer) than the concentration of the nonsignificant organisms (eg, 1000 colony forming units/milliliters (CFU/mL) of significant compared with 10,000 CFU/mL of nonsignificant organisms).7 Low-level pyuria on urinalysis (predictor variable of interest) was defined as WBC≥5 per high powered filed (hpf). Low-level bacteriuria on urinalysis was defined as 5–50 bacteria per hpf.
Analysis:
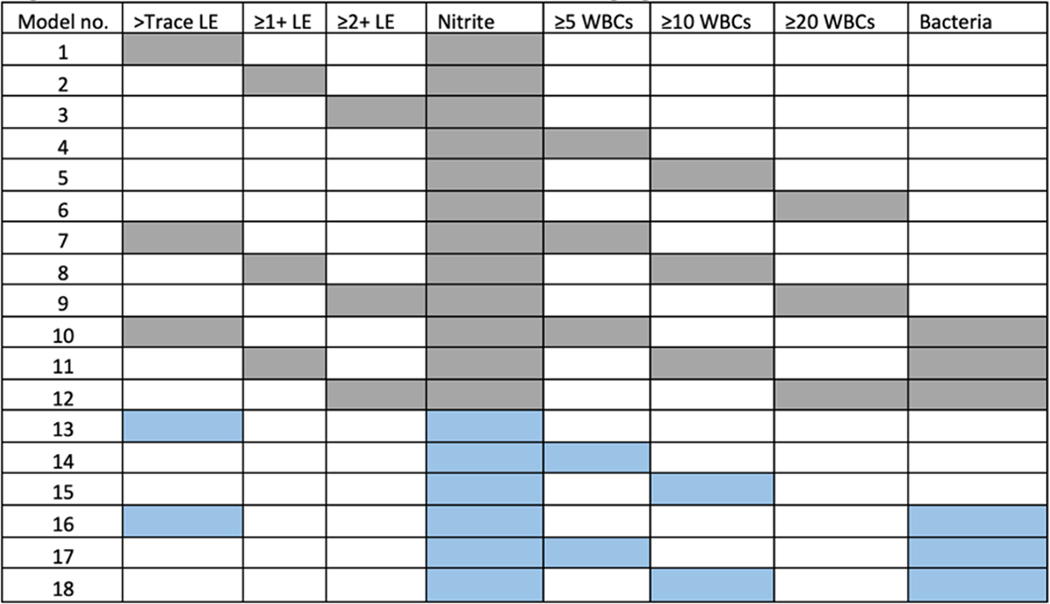
We evaluated the performance characteristics (i.e., sensitivity, specificity, NPV and positive predictive value (PPV)) of relevant urinalysis parameters (pyuria, leukocyte esterase, nitrite, and bacteria) for predicting significant bacteriuria based on prior evidence4,5. We further assessed the NPV of pyuria across different groups: catheterized vs non-catheterized patients, male vs. female, and <65 or ≥65 years. We also combined 18 different urinalysis criteria as shown in Figure 1 and used receiver operating characteristic (ROC) curves to identify the top five performing models for predicting significant bacteriuria (based on sensitivity and specificity).
Figure 1:
Different combinations of Urinalysis parameters in predicting significant bacteriuria
LE- Leukocyte esterase, WBC- White blood cells per high powered field
Grey squares (Models 1-12) - Use OR rule, Blue squares (Models 13-18) - Use AND rule
Results:
A total of 221,933 encounters met inclusion criteria in the study period. Of these, 84,334 (38%) specimens were obtained from outpatient clinics and 22,584 (10.2%) specimens were obtained from catheterized patients. Twenty-nine percent of urine cultures were positive, 30.7% were negative, and 46.9% grew mixed flora.
No single urinalysis parameter had both- high sensitivity and high specificity. Trace leukocyte esterase and low-level pyuria (WBC≥5) had low specificity (55%), but high sensitivity (87% and 78% respectively), however, sensitivity decreased with increasing degree of pyuria and leukocyte esterase. Similarly, bacteria on urinalysis had low specificity (37%) and high sensitivity (92%), but sensitivity decreased with increasing degree of bacteriuria (77%). Nitrite had low sensitivity (41%) but high specificity (95%, Table 1). When examining UA parameters for their NPV, trace leukocyte esterase, low-level pyuria and low-level bacteriuria had a high NPV (>90%, Table 1).
Table 1:
Performance of Individual Urinalysis Parameters for Predicting Significant Bacteriuria
| Leukocyte Esterase | ≥Trace | ≥1+ | ≥2+ |
| Sensitivity | 0.87 | 0.78 | 0.58 |
| Specificity | 0.55 | 0.67 | 0.83 |
| PPV | 0.43 | 0.48 | 0.57 |
| NPV | 0.91 | 0.89 | 0.84 |
| WBC Count/hpf | ≥5 | ≥10 | ≥20 |
| Sensitivity | 0.78 | 0.61 | 0.41 |
| Specificity | 0.55 | 0.73 | 0.86 |
| PPV | 0.32 | 0.38 | 0.44 |
| NPV | 0.90 | 0.87 | 0.84 |
| Nitrite | Positive | ||
| Sensitivity | 0.41 | ||
| Specificity | 0.95 | ||
| PPV | 0.75 | ||
| NPV | 0.80 | ||
| Bacteria Count/hpf | 5–50 | >50 | |
| Sensitivity | 0.93 | 0.77 | |
| Specificity | 0.37 | 0.74 | |
| PPV | 0.40 | 0.57 | |
| NPV | 0.92 | 0.88 | |
| Yeast Count/hpf | Positive | ||
| Sensitivity | 0.60 | ||
| Specificity | 0.95 | ||
| PPV | 0.53 | ||
| NPV | 0.96 |
WBC- white blood cell, hpf- high powered field, PPV-positive predictive value, negative predictive value
Combined urinalysis parameters did not perform much better than leukocyte esterase or pyuria in terms of NPV (Table 2). However, NPV of pyuria differed significantly between age and sex groups, with best performance in males <65 and worst performance in females ≥65 (Table 3). Additionally, NPV of pyuria (WBC ≥5) was lower in catheterized vs non-catheterized samples (0.87 vs 0.91, Table 3), but was ≥90 in both inpatient and outpatient settings (Table 3, supplemental tables 1, 2)
Table 2:
Complete Parameter Estimates for Models with Top 5 Area Under the Receiver Operating Characteristic curve (AUROC) Performance
| Model | Test Rule | AUROC |
|---|---|---|
| 1 | ≥trace leukocyte esterase OR positive nitrites | 0.72 |
| 2 | ≥1+ leukocyte esterase OR positive nitrites | 0.75 |
| 3 | ≥2+ leukocyte esterase OR positive nitrites | 0.76 |
| 4 | ≥5 WBCs OR positive nitrites | 0.70 |
| 5 | ≥10 WBCs OR positive nitrites | 0.75 |
| 6 | ≥20 WBCs OR positive nitrites | 0.77 |
| 7 | ≥5 WBCs OR ≥trace leukocyte esterase OR positive nitrites | 0.64 |
| 8 | ≥10 WBCs OR ≥1+ leukocyte esterase OR positive nitrites | 0.69 |
| 9 | ≥20 WBCs OR ≥2+ leukocyte esterase OR positive nitrites | 0.75 |
| 10 | ≥5 WBCs OR ≥Trace leukocyte esterase OR positive nitrites OR ≥5 bacteria/hpf | 0.58 |
| 11 | ≥10 WBCs OR ≥1+ leukocyte esterase OR positive nitrites OR ≥5 bacteria/hpf | 0.61 |
| 12 | ≥20 WBCs OR ≥2+ leukocyte esterase OR positive nitrites OR ≥5 bacteria/hpf | 0.63 |
| 13 | ≥trace leukocyte esterase AND positive nitrite | 0.66 |
| 14 | ≥5 WBCs AND positive nitrite | 0.61 |
| 15 | ≥10 WBCs AND positive nitrite | 0.59 |
| 16 | ≥trace leukocyte esterase AND positive nitrite PLUS ≥5 bacteria/hpf | 0.63 |
| 17 | ≥5 WBC AND positive nitrite PLUS ≥5 bacteria/hpf | 0.61 |
| 18 | ≥10 WBC AND positive nitrite PLUS ≥5 bacteria/hpf | 0.59 |
| Model | Test Rule | AUROC | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| 6 | ≥20 WBCs OR positive nitrites | 0.77 | 0.79 | 0.75 | 0.60 | 0.88 |
| 9 | ≥20 WBCs OR ≥2+ leukocyte esterase OR positive nitrites | 0.75 | 0.86 | 0.65 | 0.56 | 0.90 |
| 3 | ≥2+ leukocyte esterase OR positive nitrites | 0.76 | 0.73 | 0.80 | 0.59 | 0.88 |
| 5 | ≥10 WBCs OR positive nitrites | 0.75 | 0.86 | 0.64 | 0.53 | 0.90 |
| 2 | ≥1+ leukocyte esterase OR positive nitrites | 0.75 | 0.85 | 0.65 | 0.49 | 0.91 |
WBC- white blood cell, hpf- high powered field, PPV-positive predictive value, Negative predictive value
Table 3:
Comparing Negative Predictive Value of Pyuria based on Age, Sex, and Source of Collection.
| WBC Count/hpf | ≥5 |
|---|---|
| Outpatient specimen | 0.92 |
| Inpatient specimen | 0.90 |
| Male, <65 years of age | 0.94 |
| Male, ≥65 years of age | 0.92 |
| Female, <65 years of age | 0.90 |
| Female, ≥65 years of age | 0.86 |
| Non-catheterized specimen | 0.91 |
| Catheterized specimen | 0.87 |
Discussion:
Our findings show that absence of leukocyte esterase and pyuria had a high NPV for significant bacteriuria, and combination urinalysis parameters did not perform better than pyuria or leukocyte esterase alone in terms of NPV. Additionally, this is the first study, to our knowledge to highlight that performance of pyuria as a clinically relevant predictor differs by age and sex. Specifically, we show that the high NPV of pyuria was maintained in males and in non-catheterized specimens. These findings also suggested that reflex urine culture strategies may not be best suited for use in older females and persons with indwelling catheters.
Low-level bacteriuria on urinalysis also had a high NPV, however, presence of bacteria on urinalysis cannot discriminate between viable or nonviable organisms and pathogenic or non-pathogenic organisms.8 Hence, presence of bacteria on urinalysis, despite its high NPV, should not be used as a single inclusion or exclusion criteria in reflex urine cultures. Additionally, as combination urinalysis parameters did not perform better than pyuria alone, it is unclear what utility, if any, bacteria on urinalysis will serve in the future.
Based on these findings, how can reflex urine culture practices be optimized in hospitals and laboratories? First, our prior work has shown that reflex urine cultures should not be used to diagnose UTIs, but rather to reduce laboratory burden.3,5,9 Discrete orderables for urinalysis, reflex urine cultures, and direct urine cultures should be created in the EMR, and appropriate patient selection should be done in the ordering/pre-analytic phase. Reflex urine cultures should be directed towards symptomatic patients, either through clinical decision support or by educating ordering clinicians.9 To this effect, urinalysis parameters in a reflex urine culture should be based on their NPV.5 Secondly, our data suggest that low-level pyuria (WBC≥5) or trace leukocyte esterase may be adequate as single criterion in reflex urine culture algorithms if leveraged for its NPV to avoid unnecessary urine cultures. Deciding between pyuria and leukocyte esterase will depend on types of urinalysis tests available (microscopic vs dipstick). Third, laboratories should avoid the use of reflex urine culture strategies in specific patient populations like older women and catheterized patients due to poor NPV of pyuria. Neonates or patients who are neutropenic, pregnant, or undergoing urologic procedures should not undergo reflex testing as these populations may either need treatment for ASB or may not display pyuria on urinalysis.5,9 Last, laboratories should validate these findings on their own data to better optimize the local performance of reflex urine cultures. We recommend that institutions at least use include pyuria or leukocyte esterase (depending on availability of microscopic vs dipstick urinalysis) and nitrite when assessing the local performance of reflex urine cultures within their populations.
Our study has limitations. Our study hospitals and clinics are based in North Carolina. Hence, findings may have limited generalizability. Over forty percent of our samples grew mixed urine cultures, but this is consistent with rates of mixed urine cultures reported by other large academic medical centers and outpatient clinics.7 Our data on risk factors for mixed urine cultures have been previously described, but did not have data on delays in collection or transport across the health system.7 We could not include all urinalysis orders in our analyses, instead we paired urinalysis with urine cultures ordered within a 24-hour period to replicate how clinicians and laboratories would use reflex urine cultures in real life. Lastly, we were not able to assess for ASB vs. UTI and instead used significant bacteriuria as our outcome for two reasons. There is no consistent definition for clinical UTI across national infectious disease and urologic societies, and the definition of clinical UTI has been evolving over the last decade.3,10–12
In conclusion, future reflex urine culture workflows and diagnostic stewardship algorithms should focus on populations where absence of pyuria has a high NPV. Our next steps will include incorporating population specific criteria into reflex urine culture algorithms and examining the impact on urine cultures and antibiotic use. More data are needed to better understand how to incorporate specific WBC thresholds into reflex workflows for populations, specimen types (catheterized vs non catheterized), and test type (microscopic vs dipstick). As we move away from one size fits all model for interpretation of urinalysis criteria, our findings will help optimize reflex urine cultures by using a population specific approach to diagnostic stewardship.
Supplementary Material
Acknowledgements:
S.D.A. reports grants from the Centers for Disease Control and Prevention and Duke Claude D. Pepper Older Americans Independence Center (NIA P30AG028716), consulting fees from Locus Biosciences, Sysmex America, GSK, IDSA, bioMérieux, is a co-owner of IPEC Experts, LLC (unrelated to this grant)
Funding:
This work is funded by SHEA Research Scholar Award awarded to SDA. Part of SDA’s time is also funded by National Institutes of Health (NIDDK K12DK100024).
Footnotes
Previous Presentation: These data were presented as a poster at IDWeek abstract # 1261422
References
- 1.Advani SD, Gao CA, Datta R, et al. Knowledge and Practices of Physicians and Nurses Related to Urine Cultures in Catheterized Patients: An Assessment of Adherence to IDSA Guidelines. Open Forum Infect Dis. 2019;6(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petty LA, Vaughn VM, Flanders SA, et al. Risk Factors and Outcomes Associated With Treatment of Asymptomatic Bacteriuria in Hospitalized Patients. JAMA Intern Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fakih MG, Advani SD, Vaughn VM. Diagnosis of urinary tract infections: need for a reflective rather than reflexive approach. Infect Control Hosp Epidemiol. 2019:1–2. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan KV, Morgan DJ, Leekha S. Use of diagnostic stewardship practices to improve urine culturing among SHEA Research Network hospitals. Infect Control Hosp Epidemiol. 2019;40(2):228–231. [DOI] [PubMed] [Google Scholar]
- 5.Ling D, Seidelman J, Dodds-Ashley E, et al. Navigating reflex urine culture practices in community hospitals: Need for a validated approach. Am J Infect Control. 2020. [DOI] [PubMed] [Google Scholar]
- 6.Humphries RM, Dien Bard J. Point-Counterpoint: Reflex Cultures Reduce Laboratory Workload and Improve Antimicrobial Stewardship in Patients Suspected of Having Urinary Tract Infections. J Clin Microbiol. 2016;54(2):254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whelan P, Nelson A, Kim CJ, et al. Investigating Risk Factors for Urine Culture Contamination in Outpatient Clinics: A New Avenue for Diagnostic Stewardship. Antimicrob Steward Healthc Epidemiol. 2022;2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Advani SD, Polage CR, Fakih MG. Deconstructing the urinalysis: A novel approach to diagnostic and antimicrobial stewardship. Antimicrob Steward Healthc Epidemiol. 2021;1(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Advani S, Vaughn VM. Quality Improvement Interventions and Implementation Strategies for Urine Culture Stewardship in the Acute Care Setting: Advances and Challenges. Curr Infect Dis Rep. 2021;23(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Advani SD, Lee RA, Schmitz M, Camins BC. Impact of Changes to the National Healthcare Safety Network (NHSN) Definition on Catheter-Associated Urinary Tract Infection (CAUTI) Rates in Intensive Care Units at an Academic Medical Center. Infect Control Hosp Epidemiol. 2017;38(5):621–623. [DOI] [PubMed] [Google Scholar]
- 11.Trautner BW. Urinary Tract Infections as a Continuum: Implications for Diagnostic and Antibiotic Stewardship. Clin Infect Dis. 2021;72(8):1339–1341. [DOI] [PubMed] [Google Scholar]
- 12.CDC. National Healthcare Safety Network (NHSN) Patient Safety Component Manual. https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf. Published January 2017. Accessed January 1, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.