Abstract
Silicon nanoparticles (Si-NPs) have shown their potential for use in farming under water-deficient conditions. Thus, the experiment was accomplished to explore the impacts of seed priming of Si-NPs on wheat (Triticum aestivum L.) growth and yield under different drought levels. The plants were grown in pots under natural ecological environmental conditions and were harvested on 25th of April, 2020. The results revealed that seed priming of Si-NPs (0, 300, 600, and 900 mg/L) suggestively improved, the spike length, grains per spike, 1000 grains weight, plant height, grain yield, and biological yield by 12–42%, 14–54%, 5–49%, 5–41%, 17–62%, and 21–64%, respectively, relative to the control. The Si-NPs improved the leaf gas trade ascribes and chlorophyll a and b concentrations, though decreased the oxidative pressure in leaves which was demonstrated by the diminished electrolyte leakage and upgrade in superoxide dismutase and peroxidase activities in leaf under Si-NPs remedies over the control. The outcomes proposed that Si-NPs could improve the yield of wheat under a dry spell. In this manner, the utilization of Si-NPs by seed priming technique is a practical methodology for controlling the drought stress in wheat. These findings will provide the basis for future research and helpful to improve the food security under drought and heat related challenges.
Subject terms: Plant sciences, Environmental sciences
Introduction
Drought is striking ecological stress among other abiotic stresses, which adversely influence crop growth and yield of the crops around the world1. Productivity of crops is diminishing because of restricted stock of water2, less intake of nutrients, and poor photosynthesis3. Even though drought effects wheat performance at all growth stages, it is more rudimentary during the blooming and grain-filling stages (terminal dry season), which results in considerable yield loss. The leading explanations behind these losses are the diminished rate of net photosynthesis due to metabolic limitations, oxidative impairment to chloroplasts and stomatal closing, and abandoned grain set and improvement4. Nonetheless, drought stress animates the creation of ethylene5,6 which delay and slow down the root growth and prolongation2,7. All things considered, plants have established different versatile mechanisms to acclimatize the water shortage by prompting a succession of physiological and biochemical reactions8. Among these components, antioxidants play a vital role in reducing oxidative damage caused by drought9.
Drought impact in Pakistan is more significant because of its massive reliance on agribusiness. Besides this, Pakistan become more vulnerable to drought due to a lack of stress awareness and control practices10, and high risk in the future due to recent changes in climate11,12. Wheat is a major rabi season crop which is contributing 8.7% of the worth expansion in agribusiness and 1.7% to Pakistan’s GDP. The territory under wheat growth in Pakistan during 2019–2020 was 8825 thousand hectares with a growth of 1.7% when contrasted with the space of a year ago which was 8678 thousand hectares. Moreover, the estimated production of wheat was 24.946 million tons in 2019 which was 2.5% higher than the previous year13,14.
Over half of the world’s wheat fields are influenced by periodic drought stress15. The rapid climate change and global warming have the worst impact on the crops in the future because it enhances the frequency and intensity of drought due to less rainfall and high temperature on earth16. Accordingly, for enhancing the yield and preserving the wheat from such stresses need to develop more understanding and approaches to overcome these issues17.
In the last decade, nanoparticles play an imperative role in agriculture due to their unique properties, such as greater absorption ability, higher surface area, efficient delivery method for a specific spot, and such technology used in many ways like additives, plant growth enhancers, nano-fertilizers, and plant protection agents18,19. Sedghi et al.20 stated that the utilization of silicon nanoparticles is assuming a significant part in the plant resistance against the shortage of water over expanding amino acids, chlorophyll b contents, lipids, and proteins. Among other NPs, the Si-NPs are helpful in elevation the plant growth and photosynthesis under an intensive atmosphere21,22.
Nanoparticles application expanded the growth, chlorophyll substances, and gas trade qualities in the wheat grain and diminished the water deficiency23. Between various nanoparticles, silica nanoparticles play a magnificent role by increasing the photosynthesis that enhances the yield of Cucurbita pepo L.24 and hawthorn seedlings25 under stress conditions. Many types of research indicated that plant growth, antioxidant activities, and nutrient uptake process under drought stress was faster with the application of nanoparticles because such particles enhance the resistance of plant21,26–28. Si-NPs improve photosynthesis parameters of certain plants during dry season and has a greater impact in agriculture as compared to other fertilizers29–31. The exogenous application of NPs provoke root endodermal silicification, and antioxidant system activity and manage the cell water stability32. Nano-silicon application has improved production and growth and enhanced plant water status with extraordinary changes in the ultrastructure of leaf organelles, initiation of plant protection and defense system and searching of unambiguous particles33. Besides, SiO2 NPs have been described as a plant growth inducer, which raises root endodermal silicification, upgrades antioxidant system under pressure, and improve resilience to abiotic stresses and the cell water balance32. The connection between SiO2 and plant cell wall has been reported in numerous monocots34.
Nano-particles can be applied in many ways under the biotic and abiotic conditions in the field of agriculture such as by seed priming, soil, and foliar application, but seed priming is the most efficient method23,35–37. Seed priming is a fruitful strategy in many ways, for example by eliminating the worst effect of abiotic stresses, stimulating the valuable physiological changes, helping at the initial stage of germination, enhancing seed germination rate, minimizing the seedling emergence time, and most importantly it is a cost-effective technique for farmers38–43. There is no literature regarding effect of Si-NPs on wheat morpho-physiological and biochemical attributes under drought at different growth stages. So, the objective of this study is to mitigate the catastrophic effects of drought on wheat under drought conditions by the application of silicon nanoparticles.
Materials and methods
Experimental design
The research work was carried out in the wirehouse, department of agronomy, The Islamia University of Bahawalpur, Pakistan (Latitude: 29° 23′ 60.00 N, Longitude: 71° 40′ 59.99 E). The experimental layout was a randomized complete block design with four replications of each treatment of silicon nanoparticles (0, 300, 600, and 900 mg/L). All pots were irrigated equally till complete emergence, after that water deficient condition was executed at critical growth stages, such as tillering, flowering, and grain filling stages, while complete irrigation was considered as control.
Silicon nanoparticles seed priming and drought imposition
The wheat seeds (Glaxy-2013) were sterilized with a solution of sodium hypochlorite (2.6% active chlorine) for 120 s and washed thrice with distilled water. Then the measured amount of silicon nanoparticles, Sigma-Aldrich supplied spherical Si-NPs with a particle size in the 40–80 nm range43, was mixed with deionized water and ultra-sonication of each Si-NPs treatment (0, 300, 600, and 900 mg/L) for thirty minutes for proper dispersion was done. The wheat seeds were then soaked in NPs solutions for 20 h in dark under room temperature. For control treatment seeds were soaked in deionized water. Finally, dried the primed seed of the wheat crop and stored at 4 °C for further experiment.
Crop husbandry
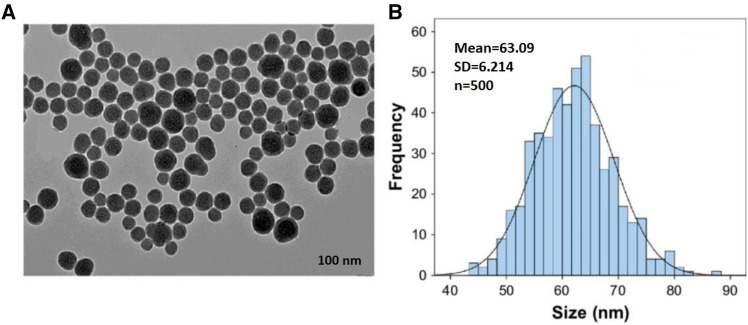
In the present study, Si-NPs was purchased from Sigma-Aldrich. In addition to having a purity of > 99%, Si-NPs had a specific surface area of > 80 m2g−1 and 28.08 molecular weight. Scanning Electron Microscope was used to measure the NPs’ sizes (Fig. 1). The mean size of the main particles was 63.09 ± 6.2 nm.
Figure 1.
Si-NP characterization. TEM scans indicated well-dispersed spherical particles (A). (B) Normal distribution graph with 63.09 ± 6.21 nm mean diameter.
The seeds of the indigenous wheat cultivar (Galaxy-2013) were obtained from Regional Agricultural Research Institute (RARI) Bahawalpur, as it is lodging and heat resistant. Wheat seeds were primed with Si-NPs and planted in plastic pots (26 × 29 cm) filled with clayey loam soil on 9th November 2019 and a physio-chemical investigation of the soil was done before planting (Table 1). The wirehouse was covered with a transparent plastic sheet to preserve the plants from rain, when mandatory. The plant population was maintained as 4 plants per pot after 20 days of sowing and irrigated equally till complete emergence of seedlings. After that, 30% water holding capacity (WHC) was maintained in pots under drought at three observed stages and 80% WHC was considered as control.
Table 1.
Physical and chemical properties of the experimental soil.
| Parameters | Soil profile |
|---|---|
| Sand | 56.5% |
| Silt | 32.5% |
| Clay | 11% |
| Texture class | Sandy loam soil |
| Ph | 7.23 |
| Electric conductivity (dSm−1) | 2.55 |
| Ammoniac N (mg g−1) | 1.58 |
| Organic matter (%) | 0.92 |
| Available phosphorus (ppm) | 6.75 |
| Available potassium (ppm) | 112 |
Growth parameters and measurement
Wheat growth, yield, and yield attributes such as spike length (cm), number of grains per spike, 1000-grain weight (g), plant height (cm), organic yield per plant (g), grain yield per plant (g), and harvest index were calculated following the standard procedures and protocols. For yield, wheat plants were manually harvested after the completion of the life cycle. Similarly, for measuring the plant height, four plants were arbitrarily selected from each pot and measured the height from soil surface to spikelet with the help of a ruler having read in millimeters and centimeters. For measuring the spike length and grains per spike, used a centimeter scale measuring the length from base to terminal spikelet from 10 plants randomly and counted the grains from randomly selected ten clusters of seeds. Besides, to quantify 1000-grain weight, the digital balance was used due to its accuracy of 0.01 g.
Relative water content (RWC)
For measuring the RWC, 5 mature fresh flag leaves were taken randomly from each treatment and immediately placed in polyethylene bags and taken to the laboratory. Where, firstly, get the fresh weight of taken samples quickly before any dehydration. Then, the sample was placed in distilled water for getting a turgid weight. After 3 h, the sample was taken out from distilled water and measured its turgid weight. After that sample was placed in an oven for 2 days at 65 °C and obtained its dry weight. Finally, the relative water content of each treatment was measured by utilizing the below-given formula44:
where RWC, FW, DW, and TW are relative water contents, fresh weight, dry weight, and turgid weight of the leaf’s samples.
Superoxide dismutase and peroxidase in wheat leaves
The superoxide dismutase and peroxidase were measured with the help of a spectrophotometer. Fresh leaf material 0.5 g from each treatment was homogenized with 50 mM phosphate buffer with 7.5 pH and 1 mM dithiothreitol (DTT) under chilled conditions after washing and drying the sample with distal water45. SOD activities were measured by using the procedure published by Cakmak46,47, whereas POD was determined by the method of Zhang and Kirkham48.
Chlorophyll fluorescence
The photosynthetic pigments chlorophyll (Chl a, and Chl b) were extracted in 80% (v/v) acetone and measured spectrophotometrically (Spectronic 601, Milton Roy Company, Rochester, NY, USA) according to Metzner et al.49. Leaf Chl fluorescence was calculated utilizing a pulse amplitude modulation portable fluorometer (Handy PEA. Hansatech. Norfolk, UK). The leaves were dark and adapted in a leaf clip for 30 min. For all treatments, 30 measurements (three replicates of 10 leaves from various plants) were recorded. The data acquired were utilized to figure out the maximum efficiency of PS II (FV/FM) and performance index on an absorption basis (Plans)50 according to the equations reviewed by Stirbet51.
Statistical analysis
STATISTIX software (version 8.1) was run on present data to determine the analysis of variance (ANOVA) and 5% LSD was used as the probability for the mean comparison of data52. Data was then analyzed for principal component analysis (PCA) by R software (R Core Team, 2019) to check the association among different studied morpho-physiological attributes.
Plant guidelines
All the plant experiments were in compliance with relevant institutional, national, and international guidelines and legislations.
Results
The result of the combined analysis of variance showed that Silicon nanoparticles concentration, drought intervals, and control irrigation regimes significantly affected all measured traits. There was a critical two-way connection between NPs concentration and drought on enzyme action, SOD, and POD. Additionally, the interaction among nanoparticles concentration, drought, and irrigation regimes was significant for spike length, the number of grains per spike, 1000-grain weight, plant height, grain yield, and biological yield. Besides, there was significant correlation between NPs concentration and control, besides NPs concentration and drought interaction on photosynthesis rate, transpiration rate, stomatal conductance, and RWC content. Moreover, the percentage of nitrogen, phosphorus, and potassium was affected by the triple association between the drought, control, and nanoparticles treatment.
Effect of silicon nanoparticles and drought on plant height
Silicon nanoparticles played a major share in plant growth under drought conditions at critical periods of wheat growth, when analyzed with control treatments. Plant height was reduced by 38.25% in DTS, 9.07% in DFS, and 6.77% in DGFS compared to control watering. When compared to the control (0 mg/L NPs), applications of 300, 600, and 900 mg/L of silicon nanoparticles mitigate the negative effects of drought and increase plant height by 2.5%, 3.2%, and 6.9%, respectively. The results showed that seed priming with 900 mg/L Si-NPs was the most effective, whereas the 0 mg/L Si-NPs treatment resulted in the shortest plant heights.
Effect of silicon nanoparticles and drought on yield attributes
The yield attributes were significantly influenced by the application of Si-NPs under water-deficient conditions shown in Table 2. Drought stress decreased the spike length (27.81, 21.18, and 22.82%), the number of grains per spike (26.19, 25.43, and 22.9%), 1000 grain weight (48.22, 42.37, and 40.31%), biological yield (10.54, 10.17 and 6.04%), grain yield (83.59, 79.56 and 66.22%) and HI (67.7, 64.07 and 57.3%) in DGFS, DFS, and DTS, respectively, with compare to control treatment. Silicon nanoparticle applications alleviated the adverse effects of water deficiency under control and stress environments. The use of silicon nanoparticles enhanced the entire above-mentioned yield attributes fundamentally, contrasted with control treatment. Moreover, compared with the control application, most higher grain production (42.12%) was recorded with priming of 900 mg/L silicon nanoparticle.
Table 2.
Effects of Si-NPs on different parameters of wheat under drought stress.
| Treatments | Si-NPs application | PH | SL | NGPS | GW | GY | BY | HI |
|---|---|---|---|---|---|---|---|---|
| CK | SiNP0 = 0 mg/L | 66.7 a | 6.43 bc | 33.2 a | 32.3 a | 22.1 b | 60.0 cdef | 37.0 a |
| SiNP1 = 300 mg/L | 65.9 a | 6.97 a | 33.0 a | 34.0 a | 24.5 ab | 65.0 abc | 37.8 a | |
| SiNP2 = 600 mg/L | 67.0 a | 6.73ab | 36.4 a | 35.1 a | 26.5a | 68.0 a | 38.9 a | |
| SiNP3 = 900 mg/L | 70.2 a | 7.13 a | 36.3 a | 34.4 a | 25.3 a | 67.0 ab | 37.8 a | |
| DTS | SiNP0 = 0 mg/L | 44.9 def | 4.90 h | 22.3 cd | 18.5 d | 10.5 gh | 55.0 fg | 19.1 de |
| SiNP1 = 300 mg/L | 47.8 cde | 5.50 efg | 26.2 bc | 23.6 c | 12.5 fgh | 58.0 defg | 21.5 cde | |
| SiNP2 = 600 mg/L | 47.8 cde | 5.87 de | 27.3 b | 25.4 bc | 16.4 cd | 62.0 bcde | 26.5 bc | |
| SiNP3 = 900 mg/L | 54.5 b | 5.93 de | 27.8 b | 27.9 b | 15.5 cdef | 61.0 cde | 25.4 bc | |
| DFS | SiNP0 = 0 mg/L | 43.3 ef | 5.07 gh | 25.2 bcd | 19.9 d | 11.0 gh | 57.33 efg | 19.3 de |
| SiNP1 = 300 mg/L | 43.9 ef | 5.80 de | 26.3 bc | 24.2 c | 13.5 defg | 60.0 cdef | 22.7 bcde | |
| SiNP2 = 600 mg/L | 48.1 cde | 5.57 ef | 26.4 bc | 24.9 c | 17.7 c | 63.0 abcd | 28.1 b | |
| SiNP3 = 900 mg/L | 55.3 b | 6.07 cd | 27.8 b | 27. 9b | 17.0 c | 65.0 abc | 26.4 bc | |
| DGFS | SiNP0 = 0 mg/L | 41.5 f | 5.13 fgh | 21.5 d | 18.0 d | 9.5h | 53.0g | 17.9 e |
| SiNP1 = 300 mg/L | 45.0 def | 5.07 gh | 24.6 bcd | 23.5 c | 13.0efg | 60.0cdef | 21. cde | |
| SiNP2 = 600 mg/L | 50.7 bcd | 5.2 3fgh | 27.7 b | 23.9 c | 15.0cdef | 62.0bcde | 24.2 bcd | |
| SiNP3 = 900 mg/L | 53.1 bc | 5.90d e | 28.7 b | 26.3 bc | 16.0cde | 60.0cdef | 26.7 bc | |
| LSD (P ≤ 0.05) | 5.0977 | 0.4666 | 4.2912 | 2.9595 | 299.18 | 1267.3 | 5.6913 | |
Plant height (PH, cm), spike length (SL, cm), number of grains per spike (NGPS), 1000-Grain Weight (GW, g), Grain Yield per Plant (GY, g), Biological Yield per Plant (BY, g), Harvest Index (HI) affected by Si-NPs application under drought stress conditions at different growth stages of wheat (CK Control, DTS Drought at tillering stage, DFS drought at flowering stage, and DGFS Drought at grain filling stage).
Effect of silicon nanoparticles and drought on chlorophyll contents
Seed preparation with silicon nanoparticles beneficially influenced the chlorophyll contents of wheat leaves grown under drought stress (Fig. 2A,B). Compared to well-watered plants (control), drought causes a significant reduction in chlorophyll a (30.2, 38.7, and 39.8%) and b (30.8. 40.1 and 39.2%) contents in DGFS, DFS, and DTS, respectively. Chlorophyll a and b substance was expanded significantly when nanoparticles were applied. However, the seeds were primed with a 900 mg/L solution of Si-NPs showing the highest amount of chlorophyll contents.
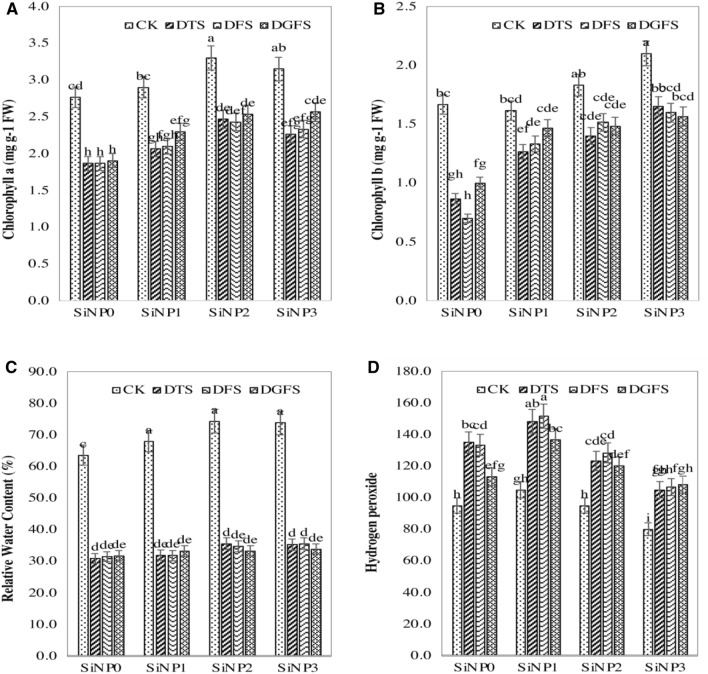
Figure 2.
Chlorophyll contents measurements. (A,B) Chlorophyll a and b contents (C,D) Relative water contents and H2O2affected by Si-NPs (SINP0 = 0, SINP1 = 300, SINP2 = 600, and SINP3 = 900 mg/L) application under drought stress at critical growth stages (CK = control, DTS = drought at tillering stage, DFS = drought at flowering stage, DGFS = drought at grain filling stage) of wheat.
Effect of silicon nanoparticles and drought on RWC and H2O2
Drought stress caused a significant reduction in RWC (Fig. 2C) as the crop goes to maturity. Usage of NPs, particularly 900 mg/L, increased RWC compared with the control (NPs = 0 mg/L). Application of 900 mg/L Si-NPs to plants under normal conditions (without stress) and the treatment without Si-NPs at tillering stage in wheat plants under water-deficient conditions caused the highest RWC (74.3%) and the lowest RWC (31.8%), respectively. On the other hand, drought positively reduced the mean of RWC compared with normal irrigation, while the utilization of NPs in plants under drought stress beneficially enhanced the mean of RWC of leaves (4.6–13.5%) compared with the control. H2O2 is also affected as the crop goes to later stages (Fig. 2D).
Effect of silicon nanoparticles and drought on gas exchange attributes
Transpiration and photosynthesis rates were significantly dropped by 63.6, 57.8, 61.0, 44.2, 31.7, and 29.3% under drought stress at DTS, DFS, and DGFS, respectively, as compared to the control treatment. Moreover, stomatal conductance decreased positively under water stress by 37.8, 30.0, and 29.1% at DTS, DFS, and DGFS. Si-NPs treatment had a substantial effect on the gas exchange attributes of wheat leaves subjected to drought stress (Fig. 3A,B). Seed preparation of 300, 600, and 900 mg/L of Si-NPs significantly enhanced the transpiration and photosynthesis rate by 27.8, 34.6%,40.6 and 20.3, 26.8, 34.2%, respectively when compared with control. Seed preparation with 900 mg/L of Si-NPs application performed the best result than other treatments under drought.
Figure 3.
Photosynthesis rate (Pr, A), transpiration rate (Tr, B), Water use efficiency (WUE, C), and stomatal conductance (SC, D) contents affected by Si-NPs application (0, 300, 600, and 900 mg/L) under drought stress at critical growth stages (CK Control, DTS Drought at tillering stage, DFS drought at flowering stage, DGFS drought at grain filling stage) of wheat.
Water use efficiency and stomatal conductance
Si-NPs significantly affected the water use efficiency in wheat both under control and drought conditions, as indicated in Fig. 3C,D. WUE dropped by 43, 47, and 35% under drought stress at DTS, DFS, and DGFS, respectively, compared to the control treatment. Seed preparation of 300, 600, and 900 mg/L of Si-NPs significantly reduced the drought impact by 34%. 42% and 56%, respectively, as compared to no Si-NPs treatment. Similarly, stomatal conductance (m mol m−2 s−1) dropped by37.8, 30.0, and 29.2% under drought stress at DTS, DFS, and DGFS, respectively, compared to the control treatment. Moreover, seed preparation of 300, 600 and 900 mg/L of Si-NPs significantly improved stomatal conductance by 15.1, 31.6, and 41.6% as compared to control. In a drought condition, the highest results were achieved by seed priming with 900 mg/L of Si-NPs treatment.
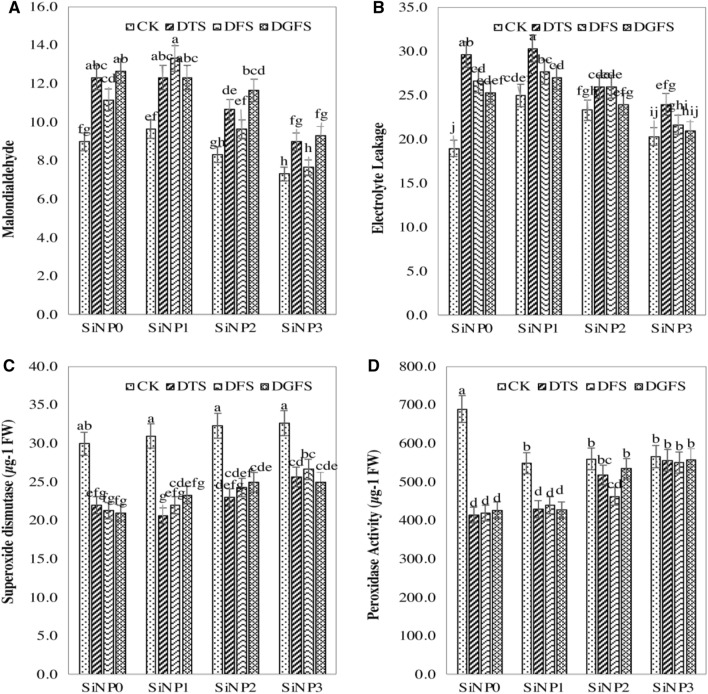
Effect of silicon nanoparticles and drought on enzymatic antioxidant
To observe the oxidative injury in wheat leaves, the effect of Si-NPs was evaluated by estimating the concentration of ROS and antioxidant enzyme activities in Fig. 4. For this reason, the activities of SOD, POD, MDA, EL, and H2O2 were measured and higher amounts were seen in control plants. Moreover, at a higher dose of Si-NPs values of these above-mentioned parameters were reduced (Figs. 2D, 4A–D). The EL contents were diminished by 8.95 and 20%: MDA contents were diminished by 5.5, 16.2, and 31.7%; and the H2O2 were diminished by 11, 18, and 26% in seed preparing of 300, 600, and 900 mg/L of Si-NPs over the respective controls. The values of SOD and POD activities were measured in leaves to determine the activity of antioxidant enzymes. The treatments applied widely improved the ingredient of antioxidant enzymes, and the most extreme improvement was measured in the highest treatment of NPs. The SOD activity was expanded by 2, 10, and 16%, and POD action expanded by 5, 11, and 17% in 300, 600, and 900 mg/L NPs priming.
Figure 4.
Effects of Si-NPs (0, 300, 600 and 900 mg/L) under drought stress on MDA (A), EL% (B), SOD (C) and POD (D). Critical growth stages of wheat were (CK Control, DTS drought at tillering stage, DFS Drought at the flowering stage, and DGFS Drought at the grain filling stage) of wheat.
Effect of silicon nanoparticles and drought on seed NPK %
The seed NPK values were significantly influenced by the application of Si-NPs under water-deficient conditions shown in Fig. 5. Drought stress decreased the wheat seed nitrogen% (11.2, 21.9 and 17.83%), P % (35.5, 28.8 and 16.3%), and K % (32.1, 23.2 and 14.3%) at different growth DTS, DFS and DGFS, respectively, when compared to control treatment. Silicon nanoparticle applications alleviated the adverse effects of water deficiency under control and stress environments. Seed preparing of 300, 600 and 900 mg/L of Si-NPs significantly enhanced the nitrogen% (13.1, 23.0 and 26.9%), P % (24.2, 35.8 and 40.4%), and K % (7.8, 19.7 and 26.4%), respectively when compared with control. The use of silicon nanoparticles enhanced the entire above-mentioned nutrient percentages, contrasted with the control treatment.
Figure 5.
Effect of Si-NPs applications (0, 300, 600, and 900 mg/L) on seed (A) nitrogen percentage (N%), (B) phosphorus percentage (P%), and (C) Potassium percentage (K%) under drought stress at critical growth stages (CK control, DTS Drought at tillering stage, DFS drought at flowering stage, and DGFS drought at grain filling stage) of wheat.
Principal components analysis (PCA)
A principal component analysis (PCA) was performed to analyze the variations and associations among different morpho-physiological parameters of wheat under the application of Silicon nano-particles and drought stress. The treatments were scattered in different biplots, while the parameters were represented as vectors (Fig. 6). The first two PCs (PC1 86% and PC2 5.1%) covered the 91.1% variability of the total variation from the 22 parameters. The first PC was related to all the studied parameters except EL%, H2O2, and MDA which were attributed to PC2.All the treatments of Si-NPs (SiNP0 = 0, SiNP1 = 300, SiNP2 = 600, and SiNP3 = 900 mg/L) at different stages (CK = control, DTS = drought at tillering stage, DFS = flowering stage, and DGFS = grain filling stage) of wheat under drought stress showed variability among the treatments and growth stages. The amount of H2O2 was boosted under drought with negative eigenvalues (Fig. 6). Similarly, the combined effect of SiNPs and drought also reduced the MDA.
Figure 6.
Principal component analysis (PCA) based on morpho-physiological and agronomic parameters of wheat under Si-NPs and drought stress. Plant height (PH, cm), spike length (SL, cm), number of grains per spike (NGPS), 1000-Grain Weight (GW, g), Grain Yield per Plant (GY, g), Biological Yield per Plant (BY, g), Harvesting Index (HI), Chlorophyll a (Chl a), Chlorophyll b (Chl b), Photosynthesis rate (PR), transpiration rate (Tr), Water use efficiency (WUE), stomatal conductance (SC), hydrogen peroxide (H2O2), malondialdehyde (MDA), superoxide dismutase (SOD), peroxidase (POD), and Electroleakage percentage (EL%). Application of Si-NPs (0, 300, 600 and 900 mg/L) under drought stress condition at different growth stages CK Control, DTS Drought at tillering stage, DFS drought at flowering stage, and DGFS drought at grain filling stage).
Discussion
The primary objective of this study was to determine the optimal doses of Si-NPs for seed priming in order to accelerate wheat development and mitigate the negative effects of drought. Significant enhancements in growth characteristics were seen in seed primed with 900 mg/L under stress. Seed germination and plant growth are both negatively impacted by drought stress47. It has been observed by several studies that drought stress reduces the germination rate of wheat23,35. Drought has been shown to reduce seed germination, growth, and wheat yield4,23,32. Seed priming of nanoparticles enhanced the germination, seedling, roots structure, nutrient uptakes, and minimized the effect of water deficient conditions18,20,37,41.
Under drought, the plant height of wheat decreased significantly at different critical stages reported by Raza et al.53 Correspondingly, Aslam et al.54 observed that plant height was reduced in quinoa plants under water-limited conditions, which is parallel with our study. Liu and Lal55 stated that nano-particles are providing one more method to provide nutrients to plants under drought situations which is beneficial for plant height. Similarly, Paparella et al.56 found that seedlings become more vigorous at the early stages due to seed priming which prompts the metabolic system of wheat seed. Moreover, plant height was enhanced by the application of Si because it performed as a barrier when it was deposited in leaf apoplasts and protect the plant from stresses57. Silicon nano-particle seed priming beneficially enhanced the plant height of wheat under water-deficient conditions at critical stages of wheat growth. Because, Si may improve the protein substance, uptake of nutrients, photosynthetic rate and decrease the cell membrane in various plants58,59. Another plausible explanation is that plant height and morphological activities were enhanced due to the accumulation of water and nutrient in the seed during the time of priming with nanoparticles60,61. In this study, role of Si-NPs in wheat growth with and without drought was also studied.
Moreover, our outcomes exhibited photosynthesis activity enhanced positively with the application of Si-NPs (Fig. 2). As research revealed that chlorophyll contents directly affect plant growth and development. In our research, under Si-NPs application significantly enhanced the chl. a chl. b, carotenoid contents, and gas exchange parameters with and without stress conditions (Fig. 2). In the latest experiments, researchers observed a similar increment in photosynthesis with the application of nanoparticles23,62,63. The Si might reduce the drought stress influence on photosynthetic pigments by increasing cytokinin endogenously, which restorative the chlorophyll formation and improve chloroplast ultrastructure64. Besides this, Asgari et al.36 stated that it might be a result of NPs enhancing the nutrients in plants that improve photosynthesis. Furthermore, Si-NPs increased the thickness of the cell wall and enhanced the transport of nutrients by the opening of the xylem which is a major cause of enhancing the photosynthesis rate36,65.
Drought stress decreased photosynthesis rate, transpiration rate, stomatal conductance, water use efficiency, and relative water contents of leaves in plants under water-deficient conditions. There was a close connection between the photosynthesis, transpiration rate, and total chlorophyll focus for wheat growth under drought stress66. Abaaszadeh et al.67 concluded that the production of peroxidase, phenolic, and chlorophyllase was reduced under water stress conditions due to that chlorophyll concentrations were badly effect. Additionally, reducing water use efficiency and RWC in plants under drought stress diminished turgor pressure, water retention, and plant size. Comparative outcomes related to our study were noticed by Farooq et al.68, Zhao et al.69, and Mamnouie et al.70. Contrary to this, photosynthesis rate, transpiration rate, stomatal conductance, and RWC expanded as the result of Si-NPs application in both irrigation regimes, particularly in plants under drought stress. Zhu and Gong71 stated that these increments in gas exchange parameters may be due to Si potential to preserve the water under drought. Moreover, Si is accumulated under leaves cuticle, framing a twofold layer of Si-cuticle, consequently, the accumulation of Si has diminished the transpiration72. Accordingly, it is recommended that a silica-cuticle twofold layer framed on leaf epidermal tissue is responsible for higher RWC of leaves. In agreement with our results, Gong et al.73 found that the use of Na2SiO3 enhanced LA, dry mass, RWC, and leaf thickness of wheat plants under water stress. Likewise, Silica NPs improved water use efficiency, RWC, and chlorophyll content in maize crops74,75.
Si-NPs application significantly enhanced the action of SOD and POD while, H2O2, EL, and MDA substances were decreased significantly shown in Fig. 2D and Fig. 4A–D. Furthermore, the significant decrease in reactive oxygen species was due to the maintenance and recovery of the cell membrane in wheat plants with the application of Si-NPs76,77. Similarly, many researchers reported that nano-particles rise the activity of antioxidants in many crops23,62,78,79. Consequently, the application of silicon enhances the activities of SOD, glutathione reductase80, and catalase81 moderately balancing the adverse effect of drought on plants.
The absorption of nutrients including N, P, and K by plants is also negatively impacted by drought stress. It has been shown in a number of studies that drought stress causes a decrease in both the content of N, K, and P in plant tissue and the rate of nutrient absorption from soil. It is well-documented that soybean N content drops significantly during drought stress78. A significant loss of nitrogen and phosphorus in plant tissues was documented by Rizwan et al.23 under situations of drought stress. The current research found that, compared to the control, Si-NPs significantly enhanced N, P, and K content under drought circumstances.
In drought conditions, wheat growth and development are diminished due to fluctuating in the balance of nutrients82. Wheat yield and biomass are enhanced by enhancing the number of Si-NPs (0–900 mg/L) shown in Table 2. The seed priming of Si-NPs demonstrates the advantageous impacts on wheat plants. As previous research reviled that molecular, biochemical, and physiological changes due to drought stress have negatively impacted plant growth and yield83. Such as dry matter production in plants decreases due to the limitation of photosynthesis and stomatal closure which reduced the carbon dioxide concentration under drought stress84. Moreover, Yazdanpanah et al.85 stated that under drought stress excessive accumulation of ROS which causing the oxidative damage of lipids, proteins, and DNA in plants which decreased plant growth and development. Similarly, other researchers reported that drought stress decreases the biomass, grain, and yield of wheat86,87.
Furthermore, the results exhibited that utilization of some concentrations of Si-NPs increased biomass, plant height, yield, and yield components in both irrigation regimes. Generally, the positive effect of Si application in plants is not too obvious under optimum conditions, but it is most evident when the plant is under suboptimal conditions88. However, Si-NPs improved the destructive effect of drought on the growth, yield, and its components due to a variation in transpiration, improvement in photosynthesis rate, and plant water status33,89. These findings are in line with Sharifi Rad et al.90 and Shallan et al.91. Si-NPs have the potential to boost the RWC, chlorophyll contents, ROS activities, growing conditions, and yield of crops34,92,93.
Conclusions
The results exposed that the Silicon nanoparticles (Si-NPs) seed priming under drought conditions significantly enhances the growth of wheat and reduces water stress. The biomass, plant height, and yield of the wheat crop were boosted due to Si-NPs seed priming. It can be concluded that enhancing the concentration of Si-NPs in wheat plants significantly enhanced the number of antioxidant enzymes, chlorophyll contents, and gas exchange parameters, and minimized the water stress. Moreover, seed priming Si-NPs enhanced the enzyme activities that minimized the concentrations of ROS in wheat leaves. Therefore, the results suggest that seed priming application of Si-NPs can be helpful to wheat plants either in normal irrigation or in drought stress. Though, further investigations at pot and field levels are still needed to see how nano-particles initiate this impact on drought stress in cereals by the priming application of NPs under drought conditions.
Acknowledgements
The technical support for these studies was provided by the research area Department of Agronomy, the Islamia University of Bahawalpur, and Higher Education Commission, Pakistan (Award of Scholarship Under Indigenous 5000 Ph.D. Fellowship (Phase-II) Batch-VI. The soil analysis was performed cordially with the support of Soil and Water Testing Laboratory, Multan.
Author contributions
Conceptualization, M.A.S.R., B.Z., and R.I.; methodology, M.U.A..; software, R.I., B.Z., and M.U.; validation and formal analysis, F.M., J.A., M.A.I., M.N.M., M.U., and R.I.; resources, M.A.S.R.; data curation, R.I., M.N.M., M.H.-u.-R., J.A., B.Z., and F.M.; writing—original draft preparation, M.A.S.R., R.I., and B.Z.; writing—review and editing, M.A.S.R., M.U., M.H.-u.-R.,H.M.U.A., and B.Z.; visualization, M.U.; supervision, M.A.S.R., M.H.-u.-R. All authors have read and agreed to the published version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
All data generated or analysed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Muhammad Aown Sammar Raza, Email: aown.sammar@iub.edu.pk.
Muhammad Habib-ur-Rahman, Email: mhabibur@uni-bonn.de.
References
- 1.Farooq M, et al. Foliage-applied sodium nitroprusside and hydrogen peroxide improves resistance against terminal drought in bread wheat. J. Agron. Crop Sci. 2017;203:473–482. doi: 10.1111/jac.12215. [DOI] [Google Scholar]
- 2.Danish S, Zafar-ul-Hye M. Co-application of ACC-deaminase producing PGPR and timber-waste biochar improves pigments formation, growth and yield of wheat under drought stress. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-42374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fahad S, et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017;8:1147. doi: 10.3389/fpls.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farooq M, Mubshar H, Kadambot HMS. Drought stress in wheat during flowering and grain-filling periods. Crit. Rev. Plant Sci. 2014;33:331–349. doi: 10.1080/07352689.2014.875291. [DOI] [Google Scholar]
- 5.Johnson PR, Joseph RE. The ethylene gas signal transduction pathway: a molecular perspective. Annu. Rev. Genet. 1998;32:227. doi: 10.1146/annurev.genet.32.1.227. [DOI] [PubMed] [Google Scholar]
- 6.Glick BR, Donna MP, Li J. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Theor. Biol. 1998;190:63–68. doi: 10.1006/jtbi.1997.0532. [DOI] [PubMed] [Google Scholar]
- 7.Mayak S, Tsipora T, Bernard RG. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 2004;166:525–530. doi: 10.1016/j.plantsci.2003.10.025. [DOI] [Google Scholar]
- 8.Zulfiqar B, Raza MAS, Saleem MF, Aslam MU, Iqbal R, Muhammad F, Amin J, Ibrahim MA, Khan IH. Biochar enhances wheat crop productivity by mitigating the effects of drought: Insights into physiological and antioxidant defense mechanisms. PLoS ONE. 2022;17:4. doi: 10.1371/journal.pone.0267819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu S, et al. Drought stress tolerance mediated by zinc-induced antioxidative defense and osmotic adjustment in cotton (Gossypium hirsutum) Acta Physiol. Plant. 2015;37:1–9. doi: 10.1007/s11738-015-1919-3. [DOI] [Google Scholar]
- 10.Ahmad S, et al. Drought mitigation in Pakistan: Current Status and Options for Future Strategies. IWMI; 2004. [Google Scholar]
- 11.Adnan S, et al. Comparison of various drought indices to monitor drought status in Pakistan. Clim. Dyn. 2018;51:1885–1899. doi: 10.1007/s00382-017-3987-0. [DOI] [Google Scholar]
- 12.Ahmed K, et al. Characterization of seasonal droughts in Balochistan Province, Pakistan. Stoch. Env. Res. Risk Assess. 2016;30:747–762. doi: 10.1007/s00477-015-1117-2. [DOI] [Google Scholar]
- 13.Government of Pakistan, Economic survey 2019–20 finance division, Economic Advisor ‘s Wing, Islamabad, (2020).
- 14.Government of Pakistan. Economic Survey 2018, Finance Division, Economic Advisor’s Wing, Islamabad, Pakistan, (2018).
- 15.Habuš-Jerčić I, et al. Effect of terminal drought on yield and some physiological traits of winter wheat. Genetika. 2018;50:747–753. doi: 10.2298/GENSR1802747H. [DOI] [Google Scholar]
- 16.Zahra S, et al. Multivariate analysis of mutant wheat (Triticum aestivum L.) lines under drought stress. Turk. J. Agric. For. 2021;45:617–633. doi: 10.3906/tar-2106-73. [DOI] [Google Scholar]
- 17.Hasanuzzaman M, et al. Drought stress tolerance in wheat: Omics approaches in understanding and enhancing antioxidant defense. Abiotic Stress-Med Sens Sign Plants: Omics Perspect. 2018 doi: 10.1007/978-981-10-7479-0_10. [DOI] [Google Scholar]
- 18.Janmohammadi M, Naser S. Effect of pre-sowing seed treatments with silicon nanoparticles on germinability of sunflower (Helianthus annuus) Bot. Lith. 2015;21:13–21. [Google Scholar]
- 19.Rameshaiah GN, Pallavi J, Shabnam S. Nano fertilizers and nano sensors–an attempt for developing smart agriculture. Int. J. Eng. Res. Gen. Sci. 2015;3:314–320. [Google Scholar]
- 20.Sedghi M, Hadi M, Sahar GT. Effect of nano zinc oxide on the germination parameters of soybean seeds under drought stress. Ann. West Univ. Timis. Seri. Biol. 2013;16:73. [Google Scholar]
- 21.Cui J, et al. Silica nanoparticles alleviate cadmium toxicity in rice cells: Mechanisms and size effects. Environ. Pollut. 2017;228:363–369. doi: 10.1016/j.envpol.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Mousavi SM, et al. Geochemical fractions and phytoavailability of zinc in a contaminated calcareous soil affected by biotic and abiotic amendments. Environ. Geochem. Health. 2018;40:1221–1235. doi: 10.1007/s10653-017-0038-z. [DOI] [PubMed] [Google Scholar]
- 23.Rizwan M, et al. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere. 2019;214:269–277. doi: 10.1016/j.chemosphere.2018.09.120. [DOI] [PubMed] [Google Scholar]
- 24.Siddiqui MH, et al. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014;33:2429–2437. doi: 10.1002/etc.2697. [DOI] [PubMed] [Google Scholar]
- 25.Ashkavand, et al. Effect of SiO2 nanoparticles on drought resistance in hawthorn seedlings. Leśne Prace Badawcze. 2015;76(4):350–359. [Google Scholar]
- 26.Tripathi DK, et al. Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol. Biochem. 2017;110:70–81. doi: 10.1016/j.plaphy.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 27.Tripathi DK, et al. Silicon as a beneficial element to combat the adverse effect of drought in agricultural crops: Capabilities and future possibilities. Water Stress Crop Plants: Sustain. Appr. 2016;2:682–694. doi: 10.1002/9781119054450.ch39. [DOI] [Google Scholar]
- 28.Zhang W, et al. Silicon promotes growth and root yield of Glycyrrhiza uralensis under salt and drought stresses through enhancing osmotic adjustment and regulating antioxidant metabolism. Crop Prot. 2018;107:1–11. doi: 10.1016/j.cropro.2018.01.005. [DOI] [Google Scholar]
- 29.Ma, J. F. Silicon uptake and translocation in plants. In The Proceedings of the International Plant Nutrition Colloquium XVI, UC Davis, 1–6 (2009).
- 30.Zhang C, et al. Foliar application of Sili-K® increases chestnut (Castanea spp) growth and photosynthesis, simultaneously increasing susceptibility to water deficit. Plant Soil. 2013;365:211–225. doi: 10.1007/s11104-012-1385-2. [DOI] [Google Scholar]
- 31.Jatav GK, Nirmal DE. Application of nanotechnology in soil-plant system. An. As. J. Soil Sci. 2013;8:176–184. [Google Scholar]
- 32.Siddiqui MH, et al. Role of nanoparticles in plants. Nanotechnol. Plant Sci. 2015 doi: 10.1007/978-3-319-14502-0_2. [DOI] [Google Scholar]
- 33.Nusrat P, Ashraf M. Role of silicon in mitigating the adverse effects of salt stress on growth and photosynthetic attributes of two maize (Zea mays L.) cultivars grown hydroponically. Pak. J. Bot. 2010;42:1675–1684. [Google Scholar]
- 34.Guerriero G, Jean-Francois H, Sylvain L. Silicon and the plant extracellular matrix. Front. Plant Sci. 2016;7:463. doi: 10.3389/fpls.2016.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nawaz F, Zulfiqar B, Ahmad KS, Majeed S, Shehzad MA, Javeed HMR, Tahir MN, Ahsan M. Pretreatment with selenium and zinc modulates physiological indices and antioxidant machinery to improve drought tolerance in maize (Zea mays L.) South Afr. J. Bot. 2021;138:209–216. doi: 10.1016/j.sajb.2020.12.016. [DOI] [Google Scholar]
- 36.Asgari F, et al. Effects of silicon nanoparticles on molecular, chemical, structural and ultrastructural characteristics of oat (Avena sativa L.) Plant Physiol. Biochem. 2018;127:152–160. doi: 10.1016/j.plaphy.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 37.Sundaria N, et al. Seed priming with iron oxide nanoparticles triggers iron acquisition and biofortification in wheat (Triticum aestivum L.) grains. J. Plant Growth Regul. 2019;38:122–131. doi: 10.1007/s00344-018-9818-7. [DOI] [Google Scholar]
- 38.Nawaz H, et al. Seed biopriming mitigates terminal drought stress at reproductive stage of maize by enhancing gas exchange attributes and nutrient uptake. Turk. J. Agric. For. 2020;44:250–261. doi: 10.3906/tar-1904-51. [DOI] [Google Scholar]
- 39.Lu MM, et al. Effects of nanosilica powder from rice hull ash on seed germination of tomato (Lycopersicon esculentum) Philipp. J. Appl. Res. Dev. (PeJARD) 2015;5:11–22. [Google Scholar]
- 40.Wojtyla Ł, et al. Molecular processes induced in primed seeds: increasing the potential to stabilize crop yields under drought conditions. J. Plant Physiol. 2016;203:116–126. doi: 10.1016/j.jplph.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Ibrahim EA. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016;192:38–46. doi: 10.1016/j.jplph.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Singh K, Gupta N, Dhingra M. Effect of temperature regimes, seed priming and priming duration on germination and seedling growth on American cotton. J. Environ. Biol. 2018;39:83–91. doi: 10.22438/jeb/39/1/MRN-446. [DOI] [Google Scholar]
- 43.Tian M, Wang YN, Wang R, Fane AG. Synthesis and characterization of thin film nanocomposite forward osmosis membranes supported by silica nanoparticle incorporated nanofibrous substrate. Desalination. 2017;401:142–150. doi: 10.1016/j.desal.2016.04.003. [DOI] [Google Scholar]
- 44.Barrs HD, Weatherley PE. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962;15:413–428. doi: 10.1071/BI9620413. [DOI] [Google Scholar]
- 45.Dixit V, Pandey V, Radhey S. Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad) J. Exp. Bot. 2001;52:1101–1109. doi: 10.1093/jexbot/52.358.1101. [DOI] [PubMed] [Google Scholar]
- 46.Cakmak I. Activity of ascorbate-dependent H2O2-scavenging enzymes and leaf chlorosis are enhanced in magnesium-and potassium-deficient leaves, but not in phosphorus-deficient leaves. J. Exp. Bot. 1994;45:1259–1266. doi: 10.1093/jxb/45.9.1259. [DOI] [Google Scholar]
- 47.Zulfiqar B, et al. Biochar enhances wheat crop productivity by mitigating the effects of drought: Insights into physiological and antioxidant defense mechanisms. PLoS ONE. 2022;17:e0267819. doi: 10.1371/journal.pone.0267819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, Kirkham MB. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol. 1996;132:361–373. doi: 10.1111/j.1469-8137.1996.tb01856.x. [DOI] [PubMed] [Google Scholar]
- 49.Metzner H, Rau H, Senger H. Investigations into the synchronization of individual pigment deficiency mutants of Chlorella. Planta. 1965;65:186–194. doi: 10.1007/BF00384998. [DOI] [Google Scholar]
- 50.Strasser RJ, Merope TM, Alaka S. Analysis of the Chlorophyll a Fluorescence Transient. Chlorophyll a Fluorescence Springer; 2004. pp. 321–362. [Google Scholar]
- 51.Stirbet A. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol., B. 2011;104:236–257. doi: 10.1016/j.jphotobiol.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 52.Steel, R. G .D., Torrie, J. H. & Dickey, D. Principles and procedure of statistics. A biometrical approach, in: McGraw hill book co, Inc, New York 3, pp. 352–358 (1997).
- 53.Aown M, et al. Foliar application of potassium under water deficit conditions improved the growth and yield of wheat (Triticum aestivum L.) J. Anim. Plant Sci. 2012;22:431–437. [Google Scholar]
- 54.Aslam MU, et al. Improving strategic growth stage-based drought tolerance in quinoa by rhizobacterial inoculation. Commun. Soil Sci. Plant Anal. 2020;51:853–868. doi: 10.1080/00103624.2020.1744634. [DOI] [Google Scholar]
- 55.Liu R, Rattan L. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015;514:131–139. doi: 10.1016/j.scitotenv.2015.01.104. [DOI] [PubMed] [Google Scholar]
- 56.Paparella S, et al. Seed priming: state of the art and new perspectives. Plant Cell Rep. 2015;34:1281–1293. doi: 10.1007/s00299-015-1784-y. [DOI] [PubMed] [Google Scholar]
- 57.Silva AJ, Nascimento CWA, Gouveia-Neto AS. Assessment of cadmium toxicities in potted garlic plants. Acta Physiol Plant. 2017;38:211. [Google Scholar]
- 58.Nazaralian S, et al. Comparison of silicon nanoparticles and silicate treatments in fenugreek. Plant Physiol. Biochem. 2017;115:25–33. doi: 10.1016/j.plaphy.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 59.Merwad A-RMA, El-Sayed MD, Mostafa MR. Response of water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci. Hortic. 2018;228:132–144. doi: 10.1016/j.scienta.2017.10.008. [DOI] [Google Scholar]
- 60.Mahakham W, et al. Environmentally benign synthesis of phytochemicals-capped gold nanoparticles as nanopriming agent for promoting maize seed germination. Sci. Total Environ. 2016;573:1089–1102. doi: 10.1016/j.scitotenv.2016.08.120. [DOI] [PubMed] [Google Scholar]
- 61.Abbas T, et al. Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicol. Environ. Saf. 2018;148:825–833. doi: 10.1016/j.ecoenv.2017.11.063. [DOI] [PubMed] [Google Scholar]
- 62.Hussain A, et al. Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ. Pollut. 2018;242:1518–1526. doi: 10.1016/j.envpol.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 63.Pullagurala VLR, et al. Finding the conditions for the beneficial use of ZnO nanoparticles towards plants-A review. Environ. Pollut. 2018;241:1175–1181. doi: 10.1016/j.envpol.2018.06.036. [DOI] [PubMed] [Google Scholar]
- 64.Liang YC. Effect of silicon on leaf ultrastructure, chlorophyll content and photosynthetic activity of barley under salt stress. Pedosphere. 1998;8:289–296. [Google Scholar]
- 65.Khan ZS, et al. Effects of silicon nanoparticles on growth and physiology of wheat in cadmium contaminated soil under different soil moisture levels. Environ. Sci. Pollut. Res. 2020;27:4958–4968. doi: 10.1007/s11356-019-06673-y. [DOI] [PubMed] [Google Scholar]
- 66.Ommen OE, et al. Chlorophyll content of spring wheat flag leaves grown under elevated CO2 concentrations and other environmental stresses within the ‘ESPACE-wheat’project. Eur. J. Agron. 1999;10:197–203. doi: 10.1016/S1161-0301(99)00011-8. [DOI] [Google Scholar]
- 67.Abaaszadeh P, et al. Effect of drought stress on proline, soluble sugars, chlorophyll and RWC level in Melissa oggicinalis. Iran. J. Med. Plants Res. 2007;23:504–513. [Google Scholar]
- 68.Farooq M, et al. Plant Drought Stress: Effects, Mechanisms and Management Sustainable Agriculture. Springer; 2009. pp. 153–188. [Google Scholar]
- 69.Zhao J, et al. Difference in response to drought stress among tibet wild barley genotypes. Euphytica. 2010;172:395–403. doi: 10.1007/s10681-009-0064-8. [DOI] [Google Scholar]
- 70.Mamnouie E, et al. The effects of water deficit on crop yield and the physiological characteristics of barley (Hordeum vulgare L.) varieties. J. Agric. Sci. Tech. 2006;8:211–219. [Google Scholar]
- 71.Zhu Y, Haijun G. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014;34:455–472. doi: 10.1007/s13593-013-0194-1. [DOI] [Google Scholar]
- 72.Ma JF, Yoshihiro M, Eiichi T. Silicon as a beneficial element for crop plants. Stud. Plant Sci. 2001;8:17–39. doi: 10.1016/S0928-3420(01)80006-9. [DOI] [Google Scholar]
- 73.Hai-jun G, et al. Effects of silicon on growth of wheat under drought. J. Plant Nutr. 2003;26:1055–1063. doi: 10.1081/PLN-120020075. [DOI] [Google Scholar]
- 74.Yuvakkumar R, et al. Influence of nanosilica powder on the growth of maize crop (Zea mays L.) Int. J. Green Nanotechnol. 2011;3:180–190. doi: 10.1080/19430892.2011.628581. [DOI] [Google Scholar]
- 75.Suriyaprabha R, et al. Growth and physiological responses of maize (Zea mays L.) to porous silica nanoparticles in soil. J. Nanopart. Res. 2012;14:1–14. doi: 10.1007/s11051-012-1294-6. [DOI] [Google Scholar]
- 76.Hussain A, et al. Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ. Sci. Pollut. Res. 2019;26:7579–7588. doi: 10.1007/s11356-019-04210-5. [DOI] [PubMed] [Google Scholar]
- 77.Alzahrani Y, et al. The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotoxicol. Environ. Saf. 2018;154:187–196. doi: 10.1016/j.ecoenv.2018.02.057. [DOI] [PubMed] [Google Scholar]
- 78.Jabborova D, et al. Co-inoculation of rhizobacteria promotes growth, yield, and nutrient contents in soybean and improves soil enzymes and nutrients under drought conditions. Sci. Rep. 2021;11(1):1–9. doi: 10.1038/s41598-021-01337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J, et al. Hydrogen sulfide regulates the activity of antioxidant enzymes through persulfidation and improves the resistance of tomato seedling to copper oxide nanoparticles (CuO NPs)-induced oxidative stress. Plant Physiol. Biochem. 2020;156:257–266. doi: 10.1016/j.plaphy.2020.09.020. [DOI] [PubMed] [Google Scholar]
- 80.Gong H, et al. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 2005;169:313–321. doi: 10.1016/j.plantsci.2005.02.023. [DOI] [Google Scholar]
- 81.Zarafshar M, et al. Toxicity assessment of SiO2 nanoparticles to pear seedlings. Int. J. Nanosci. Nanotechnol. 2015;11:13–22. [Google Scholar]
- 82.Taran N, et al. Effect of zinc and copper nanoparticles on drought resistance of wheat seedlings. Nanoscale Res. Lett. 2017;12:1–6. doi: 10.1186/s11671-017-1839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boutraa T. Improvement of water use efficiency in irrigated agriculture: a review. J. Agron. 2010;9(1):1–8. doi: 10.3923/ja.2010.1.8. [DOI] [Google Scholar]
- 84.Reddy AR, Kolluru VC, Munusamy V. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004;161:1189–1202. doi: 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 85.Yazdanpanah S, Baghizadeh A, Abbassi The interaction between drought stress and salicylic and ascorbic acids on some biochemical characteristics of Satureja hortensis. Afr. J. Agric. Res. 2011;6(4):798–807. [Google Scholar]
- 86.Shakeel AA, et al. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011;6:2026–2032. [Google Scholar]
- 87.Abdoli M, Saeidi M. Using different indices for selection of resistant wheat cultivars to post anthesis water deficit in the west of Iran. Ann. Biol. Res. 2012;3:1322–1333. [Google Scholar]
- 88.Henriet C, et al. Effects, distribution and uptake of silicon in banana (Musa spp.) under controlled conditions. Plant Soil. 2006;287:359–374. doi: 10.1007/s11104-006-9085-4. [DOI] [Google Scholar]
- 89.Sacala E. Role of silicon in plant resistance to water stress. J. Elementol. 2009;14:619–630. [Google Scholar]
- 90.Sharifi Rad J, et al. Evaluating SiO2 nanoparticles effects on developmental characteristic and photosynthetic pigment contents of Zeamays L. Bull. Environ. Pharm. Life Sci. 2014;3:194–201. [Google Scholar]
- 91.Shallan MA, et al. Biochemical and physiological effects of TiO2 and SiO2 nanoparticles on cotton plant under drought stress. Res. J. Pharm., Biol. Chem. Sci. 2016;7(4):1540–1551. [Google Scholar]
- 92.Siddiqui MH, et al. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014;33:2429–2437. doi: 10.1002/etc.2697. [DOI] [PubMed] [Google Scholar]
- 93.Behboudi F, et al. Improving growth and yield of wheat under drought stress via application of SiO2 nanoparticles. J. Agric. Sci. Technol. 2018;20:1479–1492. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.