Abstract
Introduction
Chronic pain is one of the most frequent clinical problems after inguinal hernia surgery. Despite more than two decades of research and numerous publications, no evidence exists to allow for chronic postoperative inguinal pain (CPIP) specific treatment algorithms.
Methods
This narrative review presents the current knowledge of the non-surgical management of CPIP and makes suggestions for daily practice.
Results
There is a paucity for high-level evidence of non-surgical options for CPIP. Different treatment options and algorithms have been published for chronic pain patients in the last decades.
Discussion and conclusion
It is suggested that non-surgical treatment is introduced in the management of all CPIP patients. The overall approach to interventions should be pragmatic, tiered and multi-interventional, starting with least invasive and only moving to more invasive procedures upon lack of effect. Evaluation should be multidisciplinary and should take place in specialized centres. We strongly suggest to follow general guidelines for treatment of persistent pain and to build a database allowing for establishing CPIP specific evidence for optimal analgesic treatments.
Keywords: Chronic pain, Inguinal hernia repair, Groin hernia repair, Chronic postoperative inguinal pain
Introduction
Within the past decades, chronic postoperative pain has gone from sporadically reported, to being recognized as a common and complex problem after all types of surgery. Whereas the overall incidence ranges from 5 to 20% depending on the specific procedure, pain affecting everyday activities occurs in about 5–8% of patients across procedures [1–3]. The Global Burden of Disease Study 2016 showed a 15.3% increase in burden of disease due to abdominal wall hernias [4]. The majority of these hernias require repair, resulting in 20 million groin hernia repairs being performed annually [5, 6].
Chronic postoperative inguinal pain (CPIP) is defined as pain for at least 3 months, including a level of pain rated by the patient as at least moderate and impacting daily activities [7]. Since groin hernia repair has a 10–12% risk for CPIP, this means that two million people are at risk of sustained pain more than three months after groin hernia repair yearly, resulting in a significant global burden of disease [7–9]. Due to the low incidence of hernia recurrences, prevention of CPIP should be at least as high a priority for the hernia surgeon.
The optimal solution for CPIP would be prevention. However, despite several intra-operative strategies (e.g. laparoscopic technique, careful tissue handling, mesh selection, anaesthesiological and analgesic techniques, etc.), it is still impossible to avoid CPIP from occurring in specific patients. This is partially due to inpatient factors, such as patient’s genetics and nociceptive systems, making them susceptible to chronic pain. Thus, we as clinicians are left with the task of managing CPIP, which is difficult due to its complexity and heterogeneity, and the lack of clear evidence based guidelines.
Several approaches have been suggested for the management of CPIP, ranging from cognitive therapies or pharmacological interventions to re-operations with neurectomy and/or mesh removal. Treating CPIP with another surgical intervention sounds contradictory on itself, as surgery for pain could potentially aggravate pain. The essence is “do no further harm” or “doing less is best”. However, reoperation can produce significant improvements and is undoubtedly an effective treatment modality in selected patients [10, 11].
The management of patients with CPIP is complex, and we have to acknowledge that the evidence is still too sparse to allow firm recommendations for daily practice. Currently, studies on surgical interventions for CPIP have inadequate descriptions of preoperative triage processes to make firm conclusions on the actual risk/benefit profile relative to less invasive measures. Nevertheless, promising results are emerging and evidence from other areas within chronic pain can be drawn upon when treating these patients. The aim of this review was to present the current knowledge of the non-surgical management of CPIP, in the context of social, psychological and physical factors of the individual patient. Additionally, suggestions are made for the management of patients with CPIP and future research.
Methods
For this narrative review we were informed by studies that describe the non-surgical management of CPIP. We searched seven electronic databases (PubMed, PudMed Central, MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, Google Scholar and Springer Link). The following search terms were used: “inguinal hernia”, “groin hernia”, “hernia repair”, “mesh repair”, “CPIP”, “pain”, “inguinodynia”, “non-surgical treatment”, “capsaicin”, “lidocaine”, “pharmacological”, “radiofrequency”, “cryoablation”, “peripheral nerve stimulation”, “DRG”, “centralization”, “expertise”. All titles and abstracts were screened for eligibility by two authors (NVV, EA). In case of disagreement the other authors were consulted. Based on the literature and on personal experience suggestions were made.
Results
CPIP definition
Understanding the problem of CPIP renders a clear definition first. Throughout literature the definition of chronic postsurgical pain differs. The original definition of postsurgical pain by Macrae stipulates that the pain has developed after a surgical procedure. Furthermore, the definition includes at least two months duration, and that other causes for the pain as well as other pre-existing problems must have been excluded or solved [12]. According to the International Association for the Study of Pain (IASP), chronic postsurgical pain would be defined as “chronic pain that develops or increases in intensity after a surgical procedure or a tissue injury and persists beyond the healing process, i.e. at least 3 months” [13]. Although time thresholds are included in both definitions, caution should be taken when translating this definition for inguinal hernia surgery.
A time threshold of three months has been suggested by IASP because it provides for clear operationalization [14]. However, the inflammatory healing process in mesh-based inguinal hernia repairs may last longer [15]. A longer time frame of six months was suggested in the definition of CPIP in 2013 [16]. Additionally, it was stated that the postoperative pain should be different from the pre-operative pain in terms of frequency, intensity, location, and character. This is important, as it allows for the presence of pre-operative pain in CPIP, in contrast to the Macrae definition, recognizing that pre-existing pain itself is one of the most important risk factors for developing CPIP and other chronic postoperative pain syndromes [2, 3]. The two definitions are compared in Table 1.
Table 1.
Definitions of post-surgical pain and chronic postoperative inguinal pain
| Chronic post-surgical pain | Chronic postoperative inguinal pain |
|---|---|
| 1. The pain developed after a surgical procedure | 1. Pain occurs after a herniotomy |
| 2. The pain is of at least 2 months duration | 2. Pain of at least 6 months duration |
| 3. Other causes for pain should have been excluded (e.g. continuing malignancy or chronic infection) | 3. Other causes of pain excluded |
| 4. The possibility that the pain is continuing from a pre-existing problem has been explored, and exclusion attempted | 4. Postoperative pain different from pre-operative pain (frequency, intensity, location, character) |
Despite the various definitions of chronic pain, the Hernia Surge guideline recommends using the widely accepted time period of at least three months to define CPIP, and we agree with that [7]. Additionally, it is recommended that the CPIP definition includes a level of discomfort rated by the patient as at least moderate and impacting daily activities. The instigation of treatment should assess to which extent the pain impacts the patient’s life, to discuss the pro and con’s when deciding whether and how to treat the patient.
CPIP: What do we already know?
Guidelines have gained much popularity in the last decades, by summarizing the best available evidence and providing recommendations for physicians. They will hopefully become more specific for CPIP in the future, when more evidence is gained, but as for now they are mainly overviews of topics that recommend more research.
Table 2 presents the evidence of the non-surgical treatment of CPIP. In 2018, the first international guideline on groin hernia management was published, including a thorough summary of the latest evidence regarding CPIP until January 2015 [7]. Until then, seven reviews had been published describing different treatment options and algorithms for chronic pain patients [8, 10, 17–21]. Neither any of these reviews or algorithms have been tested for the impact on patient outcomes in large series, nor have found that the evidence for CPIP treatment was solid enough to suggest changes to daily practice.
Table 2.
Evidence table of non-surgical management options of CPIP
| Intervention type | Author | Year | N = | Study design | Conclusion | Comment |
|---|---|---|---|---|---|---|
| Pharmacological topical therapy | ||||||
| Lidocaine patch (5%) | Bischoff [21] | 2013 | 21 | Randomized, double-blind, placebo-controlled, crossover trial: lidocaine patch (5%) versus placebo patch | No difference in summed pain intensity differences between lidocaine and placebo patch treatments in all patients | Small-volume study |
| Capsaicin patch | Bischoff [22] | 2014 | 46 | Randomized, double-blind, placebo-controlled, crossover trial: capsaicin 8% patch versus placebo patch | No statistically significant benefit of the capsaicin patch, although there was a trend toward less pain in the capsaicin group at one month | Small-volume study. Only one month follow-up |
| Pharmacological systemic therapy | ||||||
| Paracetamol, NSAID and gabapentinoid | No evidence | |||||
| Interventions | ||||||
| Nerve blocks and trigger point infiltrations | ||||||
| Ultrasound-guided nerve block | Voorbrood [23] | 2015 | 28 | Prospective study | Permanent pain reduction was achieved in 18 of 28 (62%) patients with neuropathic pain | |
| Ultrasound-guided nerve block | Bischoff [24] | 2012 | 12 | Randomized, double-blind, placebo-controlled, crossover trial: lidocaine versus placebo | 1 lidocaine responder, 6 non-responders, and 5 placebo responders. Ultrasound-guided ilioinguinal nerve and iliohypogastric nerve blocks did not produce pain relief | It is not clear from the study what percentage of patients had improperly-placed nerve blocks despite ultrasound guidance |
| Ultrasound-guided or nerve stimulator-guided nerve block | Thomassen [25] | 2013 | 43 | Retrospective study | Thirty-two percent of the patients were relieved of moderate-to-severe pain and nerve blocks 21 patients (55.3%) no longer reported neuropathic pain Ilioinguinal/iliohypogastric nerve blocks can be effective to treat chronic inguinal pain following surgery of the groin | Nerve stimulator-guided blocks were performed prior to January 2009, and thereafter, ultrasound-guided blocks |
| Ultrasound-guided or landmark-based nerve block | Trainor [26] | 2015 | 36 | Retrospective study | 14 patients (70%) in the landmark-based and 11 patients (79%) in the ultrasound-guided groups experienced at least a 50% reduction in VAS scores (p = 1.0) | Small-volume study. No information on follow-up |
| Ultrasound-guided tender point blockade | Wijaya-Singhe [29] | 2016 | 14 | Randomized, double-blind, placebo-controlled, crossover trial: bupivacaine 0.25% versus placebo | Median pain reduction of 63% (44.1 to 73.6%) after bupivacaine compared with 36% (11.6 to 49.7%; p = 0.003) after placebo | Small-volume study. Short follow-up of 14 days. No difference in movement related pain, summed pain intensity scores, or sleep quality scores |
| Tender point infiltration (TPI) | Verhagen [10] | 2018 | 54 | Randomized controlled trial: tender point infiltration versus neurectomy | TPI was successful in 6 patients (22%), a neurectomy was successful in 17 patients (71%). After unsuccessful TPI, 19 patients crossed over to neurectomy and their median VAS score dropped from 60 to 14 (p = 0.001). A step-up treatment strategy starting with tender point infiltration followed by a tailored neurectomy is advised | Although neurectomy seems superior in this trial, minimally invasive techniques are preferred in a step-based approach |
| CT-guided nerve block | Parris [27] | 2010 | 1 | Case-report | CT-guided transpsoas genitofemoral nerve block is a viable option for safely and selectively blocking the genitofemoral nerve for diagnostic or therapeutic purposes | Case-report |
| CT-guided peri-neural injections | Poh [28] | 2019 | 58 | Retrospective study | Improvement was seen in 84% of the cases | Non-controlled, non-randomized study design and unclear duration of effect |
| Neuroablative techniques and stimulation techniques | ||||||
| Cryoablation | Fanelli [30] | 2003 | 10 | Case series | 77.5% mean overall pain reduction, average follow-up period of eight months | Small-volume study. Short follow-up |
| Peripheral nerve stimulation | Shaw [31] | 2016 | 6 | Retrospective study | Average improvement of 62% in the immediate post-operative follow-up. Eighty-five percent patients were completely satisfied with an average follow-up of 22 months | Small-volume study. Non-controlled, non-randomized study design |
| US-guided microwave ablation | Lee [32] | 2019 | 10 | Retrospective study | Immediate pain reduction in 92% of the subjects, and 69% pain reduction at 12 months follow-up. The average duration of clinically significant pain reduction was 10.5 months | Small-volume study. Non-controlled, non-randomized study design |
| Dorsal Root Gang-lion Stimulation | Schu [33] | 2014 | 12 | Case series | Mean VAS reduction of 76.8% ± 8.2%, pain relief in 10 (83%) patients, follow-up period of 17.4 ± 5.7 weeks | Small-volume study with various etiologies of groin pain. Short follow-up |
| Spinal Cord Stimulation | Yakovlev [34] | 2010 | 15 | Case series | Pain relief of > 75% and reduced pain medication intake, follow-up period of 12 months | Small-volume study |
| Other therapies | ||||||
| Physical therapy, psychotherapy, hypnosis, behavioural therapy, biofeedback, acupuncture, mind–body therapy | No evidence | |||||
Although limited evidence exists for systemic pharmacological treatments (e.g. acetaminophen/paracetamol, NSAIDs, TCAs, SSRIs, gabapentin, pregabalin, and opioids), a step-wise multidisciplinary approach starting with minimally invasive measures like systemic analgesics is advocated in the seven reviews and the Hernia Surge guideline [7]. Although low invasive, systemic opioids and other centrally acting drugs have significant side effects, and their effect and safety as a first line therapy, relative to minimally invasive interventions such as nerve blocks, are poorly described in studies.
Based on two small studies, lidocaine and capsaicin patches have not been proven to be effective in CPIP patients [22, 23].
Nerve blocks are another option in the management of CPIP that are advocated in all reviews, algorithms and the Hernia Surge guideline [7]. Although the evidence is scarce, nerve blocks are considered to be useful in the diagnostic and therapeutic management of CPIP [11, 24–30]. No evidence-based recommendations for preferred technique (ultrasound-guided, neuro-stimulator directed, anatomic landmark-guided) can be made based on the evidence available and it is left to the discretion of the individual physician (preferably a hernia expert since the optimal site of the block will depend on the surgical approach and anatomical mesh location). However, it is recommended to perform ultrasound-guided nerve blocks in order to obtain optimal visualization of the injection site. Similar to other interventions in CPIP, the studies on nerve blocks have poor descriptions of previous or failed interventional and medical therapies in the included patients.
In addition to the conventional nerve blocks, there is evidence on the diagnostic value of trigger point infiltrations [30]. These local injections are minimally invasive, safe and easy to perform. Therefore, trigger point infiltrations might be an appropriate modality in the early management of CPIP. In future studies trigger point infiltrations should be clearly differentiated from peripheral nerve blocks. Their therapeutic effect as first line therapy should be further studied.
All studies on more invasive conservative treatment modalities for CPIP (e.g. ablation techniques and neuromodulation) have significant limitations, such as small-volume studies, non-controlled and non-randomized designs, short follow-up periods and no report of adverse events or complications [31–35]. In addition, the selection of patients for these studies regarding previous therapeutic interventions is not consistent, precluding conclusions regarding its place in the therapeutic cascade. The initial positive results of these studies should, therefore, be interpreted with caution. Although very low evidence, early findings suggest that pulsed radio frequency ablation and neuromodulation of the Dorsal Root Ganglia (DRG) may be an effective treatment for chronic neuropathic pain conditions in the groin region [7]. Finally, there is no evidence for non-pharmacological and non-surgical interventions such as physiotherapy, psychotherapy, hypnosis, behavioural therapy, biofeedback, acupuncture or mind–body therapy [7].
In the Hernia Surge guideline it was already stated that there is a paucity of non-surgical options for CPIP. Despite many research questions that were raised, relatively few new insights were published in past decade. In future studies the role of trigger point infiltrations needs to be studied and clearly differentiated from peripheral nerve blocks. Additionally, it is suggested that the repetitive effect of more proximal nerve blocks in managing CPIP is explored. Furthermore, the role of central sensitization needs to be studied.
Discussion
Current practical suggestions for non-surgical handling of the patient with CPIP
Due to the lack of established evidence-based treatment options for CPIP, we suggest to apply the following strategy for handling CPIP:
Any hernia center should have a formulated policy for how to deal with the inevitable occurrence of CPIP. These include preoperative information of the patient on the risk, assessment of CPIP during postoperative follow-up including use of analgesics (opioids and gabapentinoids in particular) and established collaboration and referral to expert pain centres ideally with an interest in CPIP and/or other chronic postoperative pain syndromes.
Information: The patient assessed for a first-time hernia repair should be advised that there is a about 10–12% risk of persistent pain at three months follow-up and a 0.5–6% risk of pain affecting everyday life at one year follow-up, including sexual function [7]. The discussion should be nuanced with the evidence that for the pain-free hernia there is the option of watchful waiting [36]. The patient assessed for a re-operation for CPIP should be informed that although the evidence suggests a positive outcome in a proportion of patients, it is difficult to predict who will benefit and there is a risk for pain intensification [37, 38]. The patient should be advised on non-surgical treatment options (see below).
-
Assessment: As for now we suggest that patients with CPIP are referred to dedicated hernia expert centres that should collect core data in a standardized manner to allow for combining these later on with other centres [39]. This will also allow for updated knowledge on potential effective treatments to be offered for the patient, preferably as part of prospective studies. Thus, in line with the earlier suggestion we recommend that the following data are collected as a first step to assess the likelihood of CPIP, monitor treatment efficacy of the individual patient, and collect data for scientific progress overall.
History: As with any chronic pain patient the first approach should be to gather fulfilling information on the surgical and pain history of the particular patient, including previous treatment attempts and results. The intensity, frequency and impact of pain on everyday activities and sexual life should be registered, using validated questionnaires such as the activity assessment scale [40]. Additionally, the psychosocial history and risk factors of patients should be addressed, to identify those at increased risk of developing chronic pain [41].
Physical examination: A physical examination should be performed, describing the painful area including the surgical scar, surrounding skin, genitalia for testicular affection in particular, contralateral side, other potential differential causes (spinal disc herniation, hip arthrosis, hernia recurrence, etc.). These may include radiological investigations, and effect from diagnostic nerve blocks or trigger point infiltrations [30].
Sensory examination: Assessing the occurrence of sensory disturbances such as hypoesthesia, hyperalgesia, allodynia, etc., should be performed in a standardized but everyday clinical feasible way. The precise methodology is not agreed upon, but a relatively simple bedside testing protocol has been suggested with good agreement with the far more elaborate and time consuming Quantitative Sensory Testing methodology (QST), i.e. with cotton swaps, finger pressure, etc. [42]. The suggested test takes less than 5 minutes, and as previously suggested, we recommend that the findings are marked on a standardized body map (Fig. 1). We recommend sensory testing acknowledging that painful and painless neuropathies exist in CPIP [43], as in other conditions [44], but recognizing the emerging evidence that sensory profiling may potentially allow for identifying initial pharmacological treatment with a higher rate of success depending on the individual patients’ characteristics of loss or gain of sensory function [45].
-
3.
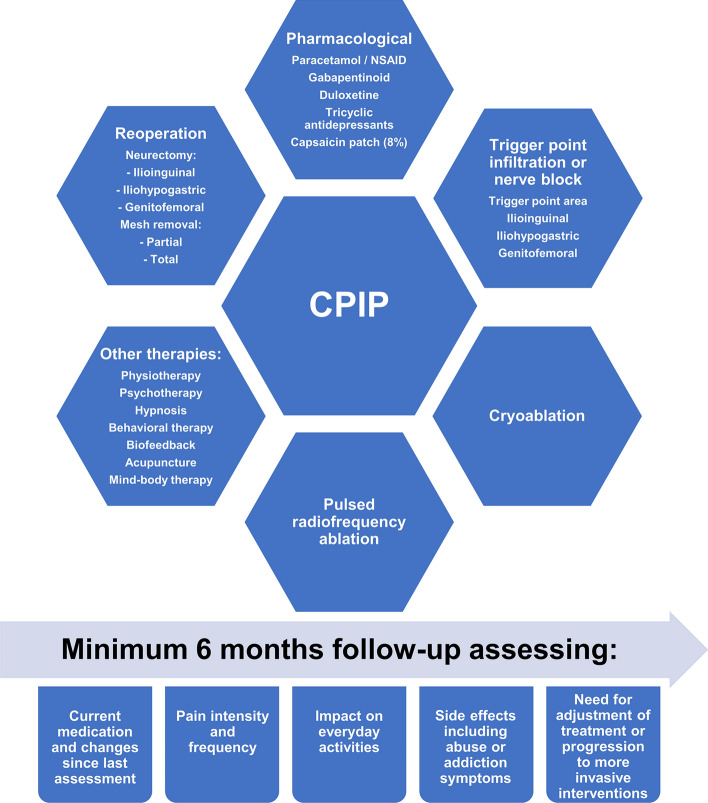
Treatment: Based on all literature it is clear that we have not yet formed solid evidence to give specific recommendations on the treatment of CPIP patients [7]. Despite the promising results of the surgical interventions for CPIP, we agree that caution should be taken to minimize the risk of pain aggravation by remedial surgery [7]. On the other hand, we must also stress that long-term analgesic treatment, especially opioids and gabapentinoids, have a high risk of adverse effects, including transition into abuse with well-documented increased mortality [46, 47]. Thus, we recommend the overall principle of starting out with the least invasive strategy and advancing into surgical procedures when conservative treatments are to no avail (Fig. 2). Ideally, a future joint effort may identify patients who are eligible for safe and effective surgical interventions early on, and those with a high risk for unsuccessful surgery who should be diverted into other treatments. For now, we recommend establishing a dedicated pain clinic at the hernia institution or collaborating with such to allow referral of patients. We recommend that the overall approach to interventions should be pragmatic, tiered and multi-interventional, starting with least invasive and only moving to more invasive treatments upon lack of effect. This will have the potential to minimize high-intervention procedures and potentially be more cost-effective with fewer side effects. Treatment of patients should start out by following the overall guidelines for persistent (neuropathic) pain treatment, including gabapentine, duloxetine and tricyclic antidepressants [48, 49]. However, as with all pain conditions there is an inflammatory component in CPIP and the benefit of paracetamol in combination with NSAIDs or COX-II inhibitors should not be excluded. This also implies attention to the well-known caveats in case of renal or cardiac failure and especially the risk of GI bleeding from long-term treatment, which can be prevented by proton-pump inhibitors. The initial step can also include treatment with capsaicin patches which in contrast to local-analgesic patches have shown effect on localized neuralgias [34] and is of particular interest due to the non-systemic effect. Second line treatments include tramadol due to the serotonine-noradrenaline reuptake inhibitory effect, whereas pure opioid agonists (i.e. morphine and oxycodone) are not recommended due to the moderate effect in (neuropathic) CPIP and high risk for adverse effects, including abuse.
Third line treatments include (repeated) nerve blocks and trigger point infiltrations, whereas cryoablation and pulsed radiofrequency are still considered with low evidence for efficacy requesting further evidence. For patients without satisfactory effect, we believe that the possibility of re-operation with (partial) mesh removal and/or neurectomy can be discussed, but lies outside the scope of this review.
-
4.
Evaluation and follow-up: Regardless of the specific treatment chosen in the various steps described above, we consider it pivotal that follow-up should be standardized regarding assessment and time, with defined success criteria for continuation of treatment or initiation of other options. These also include assessing the side-effects from pharmacological treatments such as dizziness, cognitive impairment and potential abuse.
Fig. 1 .
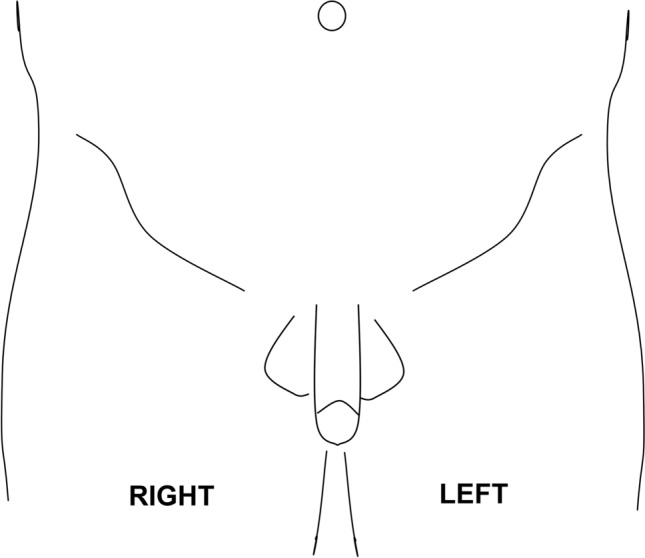
Suggested body map for recording of quantitative sensory testing findings
Fig. 2 .
Overview of potential CPIP treatment modalities, assessment, and follow-up
Future perspectives
Since there is a paucity of high-level evidence on best practices, more evidence needs to be created. Additionally, low-level evidence needs validation.
One of the major challenges in the data on both non-surgical and surgical interventions is the selection of patients in the individual trials. The scarce descriptions of any actual effect of previous interventions, as well as the lack of potential effects of less invasive interventions in the included patients, limits the ability to place the individual therapies in a rational staggered approach. This can only be solved by creating well-defined inclusion data and follow-up between interventions, ideally even standardizing the order of interventions.
Due to the scarcity of evidence, we suggest that a database including all treated patients is created. This can be a database on the local, national, or international level. Ideally a centralized database is created with core data collected at all sites, allowing merging of information and multicentre studies for large trials or in cases of rare patient findings. Such a database structure should also allow for individual centres to add local investigations and treatment strategies but informing about this as to make sure data are comparable. The database should not apply to research projects only, but collect data from all treatments being performed at the participating centres. It is crucial that all CPIP patients treated in these centres are included in this database to avoid selection bias. We suggest that the hernia-surgery community formulates an assessment algorithm with standardized treatment suggestions similar to the approach described above. This will allow for large data on specific treatment strategies regarding efficacy and side effects, as we believe the time has come to move away from the predominantly small single-centre studies. Besides describing the effect of the individual interventions, such a database will also allow the relative effects to be assessed, facilitating the establishment of an evidence-based treatment order of invasive interventions, optimizing benefit and minimizing harm. Formulating such an algorithm will undoubtedly be an advantage, also to local centres not participating, due to the constantly updated evidence-based best-practice being formulated.
Last, we suggest that the optimal management of CPIP patients should be centralized in regional, specialized hernia centres. Since the volume of hernia surgery in expert hernia centers reports lower incidences of CPIP, the treatment of CPIP patients should ideally be centralized as well. The management of CPIP has been proven to be very challenging, requiring a multidisciplinary team approach with dedicated professionals. Establishment of such expert centres forms the basis for creating evidence-based treatment algorithms for CPIP patients and initiating the databases mentioned before. We believe this is pivotal for the success of CPIP treatment in the future.
Limitations
It is difficult to draw conclusions on the management of CPIP given the scarcity of high-level evidence in this field. Literature has various definitions of CPIP; studies show heterogeneity of study populations and study cohorts are relatively small. This impacts the generalizability of results, limiting the applicability of study results in new CPIP patients.
Conclusion
CPIP is one of the most frequent clinical problems after inguinal hernia surgery and despite more than two decades of research and numerous publications, no evidence exists to allow for CPIP specific treatment algorithms. We suggest that non-surgical treatment is introduced in the management of all CPIP patients. The overall approach to interventions should be pragmatic, tiered and multi-interventional, starting with least invasive and only moving to more invasive upon lack of effect. Evaluation should be multidisciplinary and should take place in specialized centres. We strongly suggest to follow general guidelines for treatment of persistent pain and to build a database allowing for establishing CPIP specific evidence for optimal analgesic treatments.
Declarations
Conflict of Interest
NVV, NBF, MP, WARZ and EKA declare that they have no conflict of interest. MM received research grants from FEG Textiltechnik and Medtronic; received consulting fees from Consultancy Lifebond; and received honoraria for webinars for Bard Benelux NV and Medtronic AG. MM is a member of the European Commission Expert Panel in the field of Medical Devices for “General and plastic surgery and dentistry” and vice-chair of the subgroup “Surgical implants and general surgery.”
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Human and animal rights
For this narrative review, a human and animal right statement is not applicable.
Informed consent
For this narrative review, informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lavand'homme P. Transition from acute to chronic pain after surgery. Pain. 2017 doi: 10.1097/j.pain.0000000000000809. [DOI] [PubMed] [Google Scholar]
- 2.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 3.Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393:1537–1546. doi: 10.1016/S0140-6736(19)30352-6. [DOI] [PubMed] [Google Scholar]
- 4.GBD Disease and Injury Incidence and Prevalence Collaborators (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2016;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reistrup H, Fonnes S, Rosenberg J. Watchful waiting vs repair for asymptomatic or minimally symptomatic inguinal hernia in men: a systematic review. Hernia. 2021;25:1121–1128. doi: 10.1007/s10029-020-02295-3. [DOI] [PubMed] [Google Scholar]
- 6.Kingsnorth A, LeBlanc K. Hernias: inguinal and incisional. Lancet. 2003;362:1561–1571. doi: 10.1016/S0140-6736(03)14746-0. [DOI] [PubMed] [Google Scholar]
- 7.HerniaSurge Group International guidelines for groin hernia management. Hernia. 2018;22:1–165. doi: 10.1007/s10029-017-1668-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alfieri S, Amid PK, Campanelli G, Izard G, Kehlet H, Wijsmuller AR, Di Miceli D, Doglietto GB. International guidelines for prevention and management of post-operative chronic pain following inguinal hernia surgery. Hernia. 2011;15:239–249. doi: 10.1007/s10029-011-0798-9. [DOI] [PubMed] [Google Scholar]
- 9.Nienhuijs SW, Rosman C, Strobbe LJA, Wolff A, Bleichrodt RP. An overview of the features influencing pain after inguinal hernia repair. Int J Surg. 2008;6:351–356. doi: 10.1016/j.ijsu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Aasvang E, Kehlet H. Surgical management of chronic pain after inguinal hernia repair. Br J Surg. 2005;92:795–801. doi: 10.1002/bjs.5103. [DOI] [PubMed] [Google Scholar]
- 11.Verhagen T, Loos MJA, Scheltinga MRM, Roumen RMH. The groinpain trial: a randomized controlled trial of injection therapy versus neurectomy for postherniorraphy inguinal neuralgia. Ann Surg. 2018;267:841–845. doi: 10.1097/SLA.0000000000002274. [DOI] [PubMed] [Google Scholar]
- 12.Macrae WA. Chronic pain after surgery. Br J Anaesth. 2001;87:88–98. doi: 10.1093/bja/87.1.88. [DOI] [PubMed] [Google Scholar]
- 13.Schug SA, Lavand'homme P, Barke A, Korwisi B, Rief W, Treede RD, IASP Taskforce for the Classification of Chronic Pain The IASP classification of chronic pain for ICD-11: chronic postsurgical or posttraumatic pain. Pain. 2019;160:45–52. doi: 10.1097/j.pain.0000000000001413. [DOI] [PubMed] [Google Scholar]
- 14.International Association for the Study of Pain. Subcommittee on Taxonomy Classification of chronic pain. Descript Pain Terms Pain Suppl. 1986;3:1–226. [PubMed] [Google Scholar]
- 15.Aasvang E, Kehlet H. Chronic postoperative pain: the case of inguinal herniorrhaphy. Br J Anaesth. 2005;95:69–76. doi: 10.1093/bja/aei019. [DOI] [PubMed] [Google Scholar]
- 16.Aasvang EK (2013) Clinical and neuropsychological characterization of persistent postherniotomy pain. Thesis, Copenhagen University, pp 12–14. ISBN-13:978–87–995763–0–2
- 17.Lichtenstein IL, Shulmana G, Amid PK, Montllor MM. Cause and prevention of postherniorrhaphy neuralgia: a proposed protocol for treatment. Am J Surg. 1988;155:786–790. doi: 10.1016/s0002-9610(88)80044-8. [DOI] [PubMed] [Google Scholar]
- 18.Ferzli GS, Edwards E, Al-Khoury G, Hardin R. Postherniorrhaphy groin pain and how to avoid it. Surg Clin North Am. 2008;88:203–216. doi: 10.1016/j.suc.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Lange JFM, Kaufmann R, Wijsmuller AR, Pierie JP, Ploeg RJ, Chen DC, Amid PK. An international consensus algorithm for management of chronic postoperative inguinal pain. Hernia. 2014 doi: 10.1007/s10029-014-1292-y. [DOI] [PubMed] [Google Scholar]
- 20.Bjurstrom MF, Nicol AL, Amid PK, Chen DC. Pain control following inguinal herniorrhaphy: current perspectives. J Pain Res. 2014;7:277–290. doi: 10.2147/JPR.S47005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Werner MU. Management of persistent postsurgical inguinal pain. Langenbecks Arch Surg. 2014;399:559–569. doi: 10.1007/s00423-014-1211-9. [DOI] [PubMed] [Google Scholar]
- 22.Bischoff JM, Petersen M, Uçeyler N, Sommer C, Kehlet H, Werner MU. Lidocaine patch (5%) in treatment of persistent inguinal postherniorrhaphy pain: a randomized, double-blind, placebo-controlled, crossover trial. Anesthesiology. 2013;119:1444–1452. doi: 10.1097/ALN.0b013e3182a2a243. [DOI] [PubMed] [Google Scholar]
- 23.Bischoff JM, Ringsted TK, Petersen M, Sommer C, Uçeyler N, Werner MU. A capsaicin (8%) patch in the treatment of severe persistent inguinal postherniorrhaphy pain: a randomized, double-blind, placebo-controlled trial. PLoS ONE. 2014 doi: 10.1371/journal.pone.0109144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voorbrood CE, Burgmans JP, Van Dalen T, Breel J, Clevers GJ, Wille F, Simmermacher RK. An algorithm for assessment and treatment of postherniorrhaphy pain. Hernia. 2015;19:571–577. doi: 10.1007/s10029-015-1387-0. [DOI] [PubMed] [Google Scholar]
- 25.Bischoff JM, Koscielniak-Nielsen ZJ, Kehlet H, Werner MU. Ultrasound-guided ilioinguinal/iliohypogastric nerve blocks for persistent inguinal postherniorrhaphy pain: a randomized, double-blind, placebo-controlled, crossover trial. Anesth Analg. 2012;114:1323–1329. doi: 10.1213/ANE.0b013e31824d6168. [DOI] [PubMed] [Google Scholar]
- 26.Thomassen I, van Suijlekom JA, van de Gaag A, Ponten JEH, Nienhuijs SW. Ultrasound-guided ilioinguinal/iliohypogastric nerve blocks for chronic pain after inguinal hernia repair. Hernia. 2013;17:329–332. doi: 10.1007/s10029-012-0998-y. [DOI] [PubMed] [Google Scholar]
- 27.Trainor D, Moeschler S, Pingree M, Hoelzer B, Wang Z, Mauck W, Qu W. Landmark-based versus ultrasound-guided ilioinguinal/iliohypogastric nerve blocks in the treatment of chronic postherniorrhaphy groin pain: a retrospective study. J Pain Res. 2015;23:767–770. doi: 10.2147/JPR.S86777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parris D, Fischbein N, Mackey S, Carroll I. A novel CT-guided transpsoas approach to diagnostic genitofemoral nerve block and ablation. Pain Med. 2010;11:785–789. doi: 10.1111/j.1526-4637.2010.00835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poh F, Xi Y, Rozen SM, Scott KM, Hlis R, Chhabra A. Role of MR neurography in groin and genital pain: ilioinguinal, iliohypogastric, and genitofemoral neuralgia. AJR Am J Roentgenol. 2019;212:632–643. doi: 10.2214/AJR.18.20316. [DOI] [PubMed] [Google Scholar]
- 30.Wijayasinghe N, Ringsted TK, Bischoff JM, Kehlet H, Werner MU. The role of peripheral afferents in persistent inguinal postherniorrhaphy pain: a randomized, double-blind, placebo-controlled, crossover trial of ultrasound-guided tender point blockade. Br J Anaesth. 2016;116:829–837. doi: 10.1093/bja/aew071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fanelli RD, DiSiena MR, Lui FY, Gersin KS. Cryoanalgesic ablation for the treatment of chronic postherniorrhaphy neuropathic pain. Surg Endosc Other Interv Tech. 2003;17:196–200. doi: 10.1007/s00464-002-8840-8. [DOI] [PubMed] [Google Scholar]
- 32.Shaw A, Sharma M, Zibly Z, Ikeda D, Deogaonkar M. Sandwich technique, peripheral nerve stimulation, peripheral field stimulation and hybrid stimulation for inguinal region and genital pain. Br J Neurosurg. 2016;30:631–636. doi: 10.1080/02688697.2016.1199777. [DOI] [PubMed] [Google Scholar]
- 33.Lee KS, Sin JM, Patil PP, Hanna AS, Greenberg JA, Zea RD, Brace CL. Ultrasound-guided microwave ablation for the management of inguinal neuralgia: a preliminary study with 1-year follow-up. J Vasc Interv Radiol. 2019;30:242–248. doi: 10.1016/j.jvir.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 34.Schu S, Gulve A, ElDabe S, Baranidharan G, Wolf K, Demmel W, Rasche D, Sharma M, Klase D, Jahnichen G, Wahlstedt A, Nijhuis H, Liem L. Spinal cord stimulation of the dorsal root ganglion for groin pain-a retrospective review. Pain Pract. 2014;15:293–299. doi: 10.1111/papr.12194. [DOI] [PubMed] [Google Scholar]
- 35.Yakovlev AE, Al Tamimi M, Barolat G, Karasev SA, Merkulov YA, Resch BE, Yakovleva VE. Spinal cord stimulation as alternative treatment for chronic post-herniorrhaphy pain. Neuromodulation. 2010;13:288–290. doi: 10.1111/j.1525-1403.2010.00276.x. [DOI] [PubMed] [Google Scholar]
- 36.Cirocchi R, Burini G, Avenia S, Tebala G, Palumbo P, Cianci MC, Morabito A, Bruzzone P, Asymptomatic inguinal hernia: does it need surgical repair? A systematic review and meta-analysis. ANZ J Surg. 2022 doi: 10.1111/ans.17594. [DOI] [PubMed] [Google Scholar]
- 37.Valvekens E, Nijs Y, Miserez M. Long-term outcome of surgical treatment of chronic postoperative groin pain: a word of caution. Hernia. 2015;19:587–594. doi: 10.1007/s10029-013-1125-4. [DOI] [PubMed] [Google Scholar]
- 38.Zwaans WA, Verhagen T, Roumen RM, Scheltinga MR. Factors determining outcome after surgery for chronic groin pain following a Lichtenstein hernia repair. World J Surg. 2015;39:2652–2662. doi: 10.1007/s00268-015-3183-5. [DOI] [PubMed] [Google Scholar]
- 39.Kehlet H, Bay-Nielsen M, Kingsnorth A. Chronic postherniorrhaphy pain—a call for uniform assessment. Hernia. 2002;6:178–181. doi: 10.1007/s10029-002-0082-0. [DOI] [PubMed] [Google Scholar]
- 40.McCarthy M, Jr, Jonasson O, Chang CH, Pickard AS, Giobbie-Hurder A, Gibbs J, Edelman P, Fitzgibbons R, Neumayer L. Assessment of patient functional status after surgery. J Am Coll Surg. 2005;201:171–178. doi: 10.1016/j.jamcollsurg.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 41.Miller BT, Scheman J, Petro CC, Beffa LRA, Prabhu AS, Rosen MJ, Krpata DM. Psychological disorders in patients with chronic postoperative inguinal pain. Hernia. 2022 doi: 10.1007/s10029-022-02662-2. [DOI] [PubMed] [Google Scholar]
- 42.Reimer M, Forstenpointner J, Hartmann A, Otto JC, Vollert J, Gierthmühlen J, Klein T, Hüllemann P, Baron R. Sensory bedside testing: a simple stratification approach for sensory phenotyping. Pain Rep. 2020 doi: 10.1097/PR9.0000000000000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aasvang EK, Kehlet H. Persistent sensory dysfunction in pain-free herniotomy. Acta Anaesthesiol Scand. 2010;54:291–298. doi: 10.1111/j.1399-6576.2009.02137.x. [DOI] [PubMed] [Google Scholar]
- 44.Forstenpointner J, Ruscheweyh R, Attal N, Baron R, Bouhassira D, Enax-Krumova EK, Finnerup NB, Freynhagen R, Gierthmühlen J, Hansson P, Jensen TS, Maier C, Rice ASC, Segerdahl M, Tölle T, Treede RD, Vollert J. No pain, still gain (of function): the relation between sensory profiles and the presence or absence of self-reported pain in a large multicenter cohort of patients with neuropathy. Pain. 2021;162:718–727. doi: 10.1097/j.pain.0000000000002058. [DOI] [PubMed] [Google Scholar]
- 45.Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, Finnerup NB, Haanpää M, Hansson P, Hüllemann P, Jensen TS, Freynhagen R, Kennedy JD, Magerl W, Mainka T, Reimer M, Rice ASC, Segerdahl M, Serra J, Sindrup S, Sommer C, Tölle T, Vollert J, Treede RD, German Neuropathic Pain Research Network (DFNS), and the EUROPAIN, and NEUROPAIN consortia Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain. 2017;158:261–272. doi: 10.1097/j.pain.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larach DB, Hah JM, Brummett CM. Perioperative opioids, the opioid crisis, and the anesthesiologist. Anesthesiology. 2022;136:594–608. doi: 10.1097/ALN.0000000000004109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bykov K, Bateman BT, Franklin JM, Vine SM, Patorno E. Association of gabapentinoids with the risk of opioid-related adverse events in surgical patients in the United States. JAMA Netw Open. 2020 doi: 10.1001/jamanetworkopen.2020.31647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C, Kalso E, Bennett DL, Dworkin RH, Raja SN. Neuropathic pain. Nat Rev Dis Primers. 2017 doi: 10.1038/nrdp.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]