Abstract
Background
Adverse drug reactions (ADRs) and medication errors in children may result from a lack of appropriate drugs, dosages, and pharmaceutical forms. In addition, children may respond differently to drugs than adults. Reporting of ADRs in the pediatric population is therefore of importance in order to increase the amount of safety data. However, different methodological approaches are used to collect ADRs.
Objective
The aim of the present study was to analyze whether there were differences in the ADRs collected in the KiDSafe project (845 ADR reports) compared with the spontaneous ADR reports sent to EudraVigilance (697 reports) in the same time period. The strengths and limitations of these two different approaches should be discussed.
Methods
The same inclusion criteria were applied for the systematically collected ADRs in the KiDSafe project and the spontaneous reports from EudraVigilance, and only reports of ADRs coded with hospitalization were considered. In both datasets, the number of reports (related to number of hospitals), their documentation quality (VigiGrade), causal relationship (World Health Organization-Uppsala Monitoring Centre [WHO-UMC] criteria), most frequently reported drugs and ADRs, demographical parameters of the patients, reported medical histories, and the seriousness of ADR reports were analyzed descriptively. The results of the two analyses were compared.
Results
There was considerable underreporting of ADRs via the spontaneous reports (0.4 reports per hospital; 697/1902) compared with 70.4 reports per hospital (845/12) in the systematically collected KiDSafe reports. Documentation quality assessment yielded similar results in both datasets. Among the 10 most frequently reported drugs, anticonvulsants such as levetiracetam (6.6%), valproic acid (5.6%), oxcarbazepine (3.6%), and lamotrigine (3.4%) were mainly reported in the KiDSafe reports, while in the EudraVigilance reports, mite allergen extract (4.4%) and allergens (3.6%) were preferentially reported. Seizures were the most frequently reported clinically specific ADRs in the KiDSafe reports, whereas anaphylactic reactions and urticaria were prominent in the spontaneous reports from EudraVigilance. Notably, the proportion of reports referring to medication errors and other medication safety related issues were more prominent in KiDSafe than in the spontaneous reports (27.8% vs. 12.6% and 46.0% vs. 29.0%, respectively).
Conclusion
In general, reports from both data sources contributed to the identification of ADRs and dedicated issues related to drug therapy. However, these differed by nature and strength of the signal, likely due to the characteristics of the individual method. A combined approach could likely compensate for limitations inherent to the single approaches, but will most likely only be applied to dedicated pharmacovigilance topics or research objectives.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40272-022-00540-z.
Key Points
| Differences in the most frequently reported drugs and adverse drug reactions (ADRs) were observed between the systematically collected ADRs in the KiDSafe project and the spontaneous reports from EudraVigilance, which may be related to the characteristics of each individual method. |
| The systematic collection of ADRs particularly identified medication errors and issues related to medication safety compared with the spontaneous collection of ADRs. |
| A combined approach may compensate for the limitations of each single approach. |
Introduction
According to literature, 1.7% of children in Germany experienced adverse drug reactions (ADRs) under outpatient drug therapy [1] and 9.2–14.1% during a hospital stay [2, 3]. In addition, an estimated 3–5% of all hospital admissions of children are due to an ADR [4].
The reasons for this substantial risk of ADRs for children include, among others, the off-label administration of drugs, i.e. without dedicated data regarding safety and dosages in children [5]. Hence, postmarketing surveillance for drugs used in children is crucial.
Surveillance measures comprise, but are not limited to, the spontaneous reporting system, the analyses of electronic health records, registries, and systematic ADR collections as part of pharmacovigilance studies. Each of these instruments carries its inherent strengths and limitations [6].
The objective of the present study was to compare two different methodological approaches of pediatric postmarketing surveillance, i.e. solicited versus unsolicited reports [7]. Therefore, the results of a systematic collection of pediatric ADR reports as part of the KiDSafe project [8] (solicited) were compared with the results of analyses in a spontaneous reporting system (EudraVigilance reports; unsolicited). Based on this assessment, the strengths and limitations of these two different approaches were analyzed.
Material and Methods
Adverse Drug Reactions (ADRs)
An ADR is defined as a response to a medicinal product that is noxious and unintended [7]. In Germany, ADRs can be reported by health care professionals (HCP) and non-HCPs (e.g. patients), and these ADR reports are forwarded to the European ADR database (EudraVigilance) [9, 10]. Reporting obligations are reported elsewhere [10, 11]. The minimum criteria that should be provided in an ADR report include an identifiable patient, an identifiable reporter, one or more suspected drugs, and a suspected ADR [7].
ADR reports may either be unsolicited, i.e. they do not derive from a study or any organized data collection system, or they can be solicited, i.e. from organized data collection systems, including clinical trials, non-interventional studies, registries, etc. [7]. The two methodological approaches compared in the present study are representatives of unsolicited (EudraVigilance analysis) and solicited reports (KiDSafe project, see Sect. 2.2), respectively.
Within EudraVigilance, ADRs are coded in accordance with the terminology of the Medical Dictionary for Regulatory Activities (MedDRA) [12]. MedDRA terminology includes five hierarchical levels. The High Level Term (HLT) summarizes the Preferred Terms (PT) on a rather aggregated level of analysis, while the PT level describes the symptom, laboratory result, diagnosis or condition.
KiDSafe Project
The KiDSafe project was a large multicenter project aiming to improve pediatric drug safety.
One pillar of the project was the detection and reporting of ADRs leading to hospital admissions.
Study teams from 12 children’s hospitals in Germany screened all non-elective patients aged 0–17 years admitted to their hospital between 1 July 2018 and 30 June 2020. Notably, oncological patients who were admitted as inpatients with a suspected ADR and had been in inpatient oncological treatment in the previous 4 weeks were excluded from the screening. The screening was performed by applying a standardized algorithm [13], identifying patients with a potential relationship between hospitalization and drug administration prior to admission. Cases with suspected ADRs detected by this algorithm were then reported to an independent expert group (Adverse Drug Event Expert Group) if consent for data processing was obtained. In cases where no consent could be obtained, reports with suspected ADRs with a reasonable time relationship to drug use were sent directly to the Drug Commission of the German Medical Association (DCGMA) [as obliged by the professional conduct code; see Sect. 2.1]. Both groups (Adverse Drug Event Expert Group and the DCGMA) assessed the causal relationship between the reported ADR and the suspected drugs (see below). The reports presented within this manuscript comprise only ADR reports sent directly to the DCGMA (without consent), which were then forwarded to the BfArM for inclusion in EudraVigilance. Since these reports were generated ‘systematically’ by the application of the algorithm, they are designated as ‘systematically collected ADR reports’ from here on. Finally, these reports were identified in EudraVigilance based on their case identifier indicating a KiDSafe report.
Identification of ADR Reports in the EudraVigilance Database
Reports from EudraVigilance [10] sent spontaneously served as a comparison group. All spontaneous reports of ADRs referring to children and adolescents aged 0–17 years from Germany received between 1 July 2018 and 30 June 2020 were identified. Notably, the ADRs from the KiDSafe project occurred during the study period 1 July 2018–30 June 2020, whereas the EudraVigilance reports were reported within this study period. For technical reasons, the occurrence date of the ADRs in EudraVigilance reports could not be accessed computer-based for the complete dataset. However, all reports were designated as serious (referring to the ADR) and were thus subject to a reporting obligation of 15 days [7]. Only reports in which the ADR led to or prolonged hospitalization were included (see Sect. 2.5.2 and Fig. 1). Vaccines were excluded since vaccination is a medical process that is different from drug administration and is linked to specific age intervals, which would affect the age- and sex-stratified analyses.
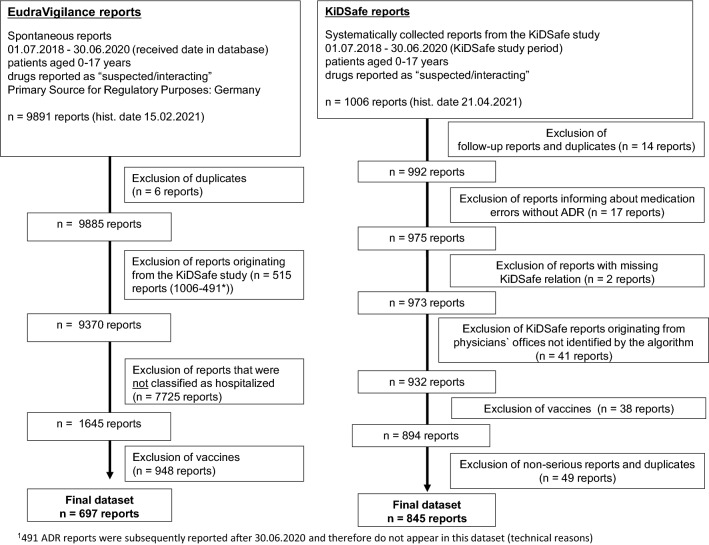
Fig. 1.
Generation of the final datasets. ADR adverse drug reaction, Hist. date historical date
Figure 1 shows the analyses criteria and number of ADR reports identified for both groups.
Documentation Quality and Causality Assessment of the Reports
Documentation quality was assessed by computer-based grading according to the VigiGrade score, a completeness score that measures the amount of information provided in structured formats [14]. Therefore, the VigiGrade score, which was developed for the VigiBase database [15], was slightly adapted to match the format and content of data in EudraVigilance. For assessment of the causal relationship, the World Health Organization–Uppsala Monitoring Centre (WHO-UMC) criteria [16] were used. The DCGMA conducted a causality assessment of 74.6% (630/845) of the KiDSafe reports as part of the KiDSafe project. A causality assessment was also available for 128 of the 697 spontaneous reports in the EudraVigilance final dataset (Table 1). With the exception of one report, this causality assessment was also performed by the DCGMA.
Table 1.
Assessment of the causal relationship between the administration of the drug and the ADR in the systematically collected versus spontaneous ADR reports
| Assessment of the causal association between the administration of the drug and the ADR (WHO-UMC criteria [16]) | KiDSafe reports (systematically collected ADR reports) [n = 845] 74.6% (n = 630) with available assessment |
EudraVigilance reports (spontaneous ADR reports) [n = 697] 18.4% (n = 128) with available assessment |
|---|---|---|
| Certain | 0.6% (4/630) | 0.8% (1/128) |
| Probable/likely | 3.5% (22/630) | 5.5% (7/128) |
| Possible | 88.4% (557/630) | 89.8% (115/128) |
| Unlikely | 1.4% (9/630) | 0% (0/128) |
| Conditional/unclassified | 0.6% (4/630) | 1.6% (2/128) |
| Unassessable/unclassifiable | 5.4% (34/630) | 2.3% (3/128) |
ADR adverse drug reaction, WHO-UMC World Health Organization–Uppsala Monitoring Centre
Also covers medication error-related ADRs. Due to the extension of the ADR definition in 2012, conditions such as overdose, misuse, abuse, medication errors, and occupational exposure are now included in the ADR definition [7, 21]. Although, theoretically, all such reports retrieved in our analyses should be associated with a suspected co-reported ADR, we cannot exclude the presence of reports not being associated with a suspected co-reported ADR among the reports (see Sect. 2.5)
Strategy of Analyses
All ADR reports were analyzed with regard to the distribution of age and sex, seriousness criteria, reporting sources, medical histories, quality of documentation, causality, frequently reported suspected drugs, and ADRs. Means and medians were calculated for the quality of documentation, and frequency distributions for all other variables.
Notably, not only were ‘conventional’ (substance-related) ADRs analyzed but also, according to a current ADR definition [7], medication safety-related [17] ADRs such as medication errors (e.g. product administration error), over- and underdoses (e.g. intentional overdose), and reports on abuses and misuses. As yet, there is no generally accepted definition for the term ‘medication safety’. In the context of this publication, medication safety means all measures that contribute to the optimization of the drug treatment process with the aim of reducing medication errors/preventable harm from drug use (German definition of ‘Arzneimitteltherapiesicherheit’) [17]. Medication safety-related issues describe problems during the drug treatment process rather than ‘conventional’ (substance-specific) ADRs. According to guidance [7], all reports describing such scenarios retrieved in our analyses should be associated with an additional co-reported suspected ADR; however, we cannot fully exclude the presence of reports not being associated with an additional suspected ADR among the reports. In order to identify the number of reports describing medication errors in the KiDSafe and EudraVigilance reports, the standardized MedDRA query (SMQ) ‘medication error (broad)’ was used [12]. Furthermore, we identified the reported PTs that described a scenario relating to medication safety and determined the number of reports reporting such scenarios in both datasets.
Our analyses also retrieved reports that describe a lack of efficacy of drug therapy. Notably, per definition, lack of efficacy is not an ADR. However, in certain circumstances (e.g. drugs used for life-threatening diseases), reports on lack of therapeutic efficacy with no co-reported suspected ADR may need to be submitted to EudraVigilance [7].
Primary Reporting Source
Both datasets were limited to reports coded with hospitalization. Per default, all ADR reports in the KiDSafe project were reported by HCPs due to the study design, whereas the EudraVigilance reports, reported in the same study period, included the reports of HCPs and non-HCPs (e.g. patients).
Seriousness Criteria
The designation ‘serious ADRs’ corresponds to the legal definition of an ADR and not to its clinical severity [7]. Accordingly, all ADRs that led to death, were life-threatening, required or prolonged hospitalization, conferred lasting or significant disability or incapacity, led to congenital abnormalities/birth defects, or endangered the patient and required medical or surgical intervention/treatment (‘other’) were considered as serious. Multiple assignments could be made in each ADR report. In order to differentiate whether the coding ‘hospitalization’ within the EudraVigilance reports was related to an outpatient-acquired ADR with subsequent hospitalization or an inpatient-acquired ADR that prolonged hospitalization, a random sample of 10% (68/697) of the EudraVigilance reports was analyzed in this regard.
Age and Sex Distribution
The age stratification of the National Association of Statutory Health Insurance Physicians [18] was modified according to the physiological development of children and adolescents (i.e. 0–1 month, 2 months–1 year, 2–3 years, 4–6 years, 7–12 years, and 13–17 years). Reports in the 0–1 month age group were additionally analyzed to determine whether the ADR was related to exposure to the drugs in utero or via breast milk. All age groups were also stratified with regard to sex.
Most Frequently Reported Drugs and Their Most Frequently Reported ADRs
The final datasets of the two groups were analyzed with regard to the 10 drugs most frequently reported as suspected. In addition, age-stratified analyses of the most frequently reported drugs were performed. Furthermore, the three most frequently reported ADRs were analyzed for each of the five most frequently suspected drugs.
Medical History
In order to provide a complete description of the ADR reports for both groups, the medical history, if reported, was also evaluated (PT level of the MedDRA terminology [12]).
Compliance with Ethical Standards and Ethics Approval
Due to data privacy requirements and the EudraVigilance access policy [19], the complete individual pseudonymized case reports were not available to the readership. Although different levels of access are granted for different stakeholders, even with the lowest level of access an analysis of aggregated data is possible. For further information regarding the processing of personal data in the context of the operation of EudraVigilance Human, we refer to the European Medicines Agency’s Data Protection Notice for EudraVigilance Human [20].
Approval for the protocol of the KiDSafe project was obtained from the Ethics Committee of the Friedrich-Alexander University Erlangen-Nürnberg under reference number 351_17 B, and approval for the analysis of the spontaneous reports from EudraVigilance was obtained from the local Ethics Committee of the Medical Faculty of Bonn (009/17 and 100/21).
Results
Number of Reports
A total of 845 reports were reported via the 12 participating KiDSafe hospitals, and 697 reports were reported via the spontaneous reporting system in the same time period (Fig. 1).
Documentation Quality and Causal Relationship
Documentation quality based on calculation of the mean and median VigiGrade score was comparable between the KiDSafe (mean 0.64 ± 0.25) and EudraVigilance (mean 0.63 ± 0.25) reports (median 0.61 vs. 0.61). However, the relative share of reports rated with a VigiGrade score ≤ 0.3, suggesting rather poorly documented reports, was smaller in the systematically collected reports compared with the spontaneous reports (1.3% vs. 6.0%) [electronic supplementary material (ESM) Table 1].
Assessment of the causal relationship [16] was available for 74.6% (630/845) of the systematically collected reports and 18.4% (128/697) of the spontaneous reports (see the Sect. 4 for further information). In these reports, an ‘at least possible’ causal relationship was noted in 92.5% (583/630) of the systematically collected reports and 96.1% (123/128) of the spontaneous reports (Table 1). No substantial differences between the two groups in relation to the different categories of causality could be observed.
Reporting Sources
Reflecting the study design, 100% (845/845) of the systematically collected reports were sent by HCPs compared with 87.4% (609/697) of the spontaneous reports; 12.6% (88/697) of spontaneous reports were from non-HCPs (see ESM Table 2).
Seriousness Criteria
Since hospitalization is one of the legally defined seriousness criteria, all of the systematically collected reports and spontaneous reports were classified as serious. A lower number of reports was classified as ‘life-threatening’ and ‘fatal’ in the systematically collected reports than in the spontaneous reports (life-threatening: 8.3% [70/845] vs. 13.7% [95/692]; fatal: 0.1% [1/845] vs. 2.3% [16/692]) [ESM Table 3]. None of the random sample reports from the EudraVigilance reports described a hospital-acquired ADR.
Age and Sex Distribution
The largest proportion for both systematically collected (47.6% [402/845]) and spontaneous reports (40.0% [279/697]) referred to the 13–17 years age group. The 0–1 month age group accounted for only 0.7% (6/845) of the systematically collected reports but 6.9% (48/697) of the spontaneous reports. Among the six systematically collected reports for this age group, only one (16.7%) referred to the mother taking the drug and the child experiencing the ADR, compared with 34/48 (70.8%) of the spontaneous reports.
In both datasets, a higher proportion of reports for the 13–17 years age group referred to females than males, with a similar ratio of approximately 2:1 (systematically collected: 70.4% [283/402] vs. 29.1% [117/402]; spontaneous reports: 62.0% [173/279] vs. 36.9% [103/279]). In the younger age groups (0–6 years) more reports referred to males than to females in both datasets (ESM Table 4).
Reported ADRs
Clinically, rather unspecific ADRs (vomiting, nausea, and dizziness) were among the 10 most commonly reported ADRs in both datasets (Table 2). It was striking that in the systematically collected reports, product administration errors ranked first and were clearly more often reported in KiDSafe reports than in EudraVigilance reports. In summary, 27.8% (235/845) of the KiDSafe reports included medication errors compared with 12.6% (88/697) of the EudraVigilance reports. Furthermore, the proportion of reports referring to scenarios relating to medication safety in the KiDSafe reports (46.0%, 389/845) was higher than in the EudraVigilance reports (29.0%, 202/697).
Table 2.
The 10 most frequently reported ADRs for both data sources (PT level)
| KiDSafe reports (systematically collected reports) [n = 845] | EudraVigilance reports (spontaneous reports) [n = 697] | ||
|---|---|---|---|
| Product administration errora, | 18.1 (153) | Vomitingc | 10.0 (70) |
| Vomitingc | 15.6 (132) | Anaphylactic reactiond | 6.0 (42) |
| Intentional overdoseb | 13.0 (110) | Dyspneac | 5.9 (41) |
| Toxicity to various agentsb | 11.4 (96) | Pyrexiac | 5.5 (38) |
| Treatment non-complianceb | 9.9 (84) | Nauseac/urticariad | 5.0 (35) |
| Seizured | 8.8 (74) | Drug ineffectivee/off-label useb | 4.2 (29) |
| Nauseac | 7.6 (64) | Dizzinessc | 3.7 (26) |
| Dizzinessc | 6.3 (53) | Fetal exposure during pregnancy/headachec/tachycardiadd | 3.6 (25) |
| Accidental exposure to the product by a childb | 5.8 (49) | Abdominal painc/product administration errora,b | 3.4 (24) |
| Abdominal painc | 5.7 (48) | Rashd/somnolencec | 3.2 (23) |
Data are expressed as % (n)
ADRs adverse drug reactions, PT Preferred Term, SMQ standardized MedDRA query
aConditions describing a medication error coded in the SMQ medication errors (broad) [12]. Although theoretically all such reports retrieved in our analyses should be associated with a co-suspected ADR [7], we cannot exclude the presence of reports not being associated with a co-suspected ADR among the reports (see Sect. 2.5)
bConditions describing a scenario relating to medication safety. Although, theoretically, all such reports retrieved in our analyses should be associated with a co-suspected ADR [7], we cannot exclude the presence of reports not being associated with a co-suspected ADR among the reports (see Sect. 2.5)
cClinically, rather unspecific ADRs that may occur as a symptom of many diseases compared with more specific ADRs that are suggestive of a specific disease. The classification of ‘unspecific’ versus ‘specific’ ADRs is arbitrarily based on the subjective medical and pharmaceutical expertise and judgment of the project team, since no respective guidance, applicable for this constellation, could be found
dClinically specific ADRs. See explanation above referring to unspecific ADRs
eDespite ‘drug ineffective/lack of efficacy’ not being considered an ADR per definition, in certain circumstances submission of these reports to EudraVigilance, even if not associated with an ADR, is required [7] (see Sect. 2.5). Hence, these reports are included in the present analyses and are depicted in the table
Seizure (8.8%) was the most frequently reported clinically specific ADR in the systematically collected reports, compared with allergic-like reactions in the spontaneous reports (anaphylactic reaction [6.0%] and urticaria [5.0%]). In summary, the ADR profile between the systematically collected reports and the spontaneous reports differed.
Most Frequently Suspected Drugs and Their Most Frequently Reported ADRs
Systematic ADR Collection
Overall, 7.5% (63/845) of the systematically collected reports referred to ibuprofen. In 46.0% (29/63) of these reports, the ADR intentional overdose was coded.
Two of the remaining top five drugs among the systematically collected reports were anticonvulsants (levetiracetam: 6.6% [n = 56]; valproic acid: 5.6% [n = 47]). In summary, 22% (182/845) of the systematically collected reports referred to the drug class of anticonvulsants and 5.5% (10/182) of these were coded as life-threatening. Anticonvulsants were strongly represented in all age groups, except for the 13–17 years age group (ESM Table 6). An individual assessment of these 182 reports revealed that the respective ADR (e.g. seizure) occurred during outpatient treatment and resulted in an inpatient admission for readjustment to the anticonvulsant therapy (Table 3).
Table 3.
The five most often suspected drugs and their three most often reported ADRs (PT level)
| KiDSafe reports (systematically collected reports) [n = 845] | % (n) | EudraVigilance reports (spontaneous reports) [n = 697] | % (n) | ||
|---|---|---|---|---|---|
| Ibuprofen [n = 63, 7.5%] | Intentional overdosea,b | 46.0 (29) | Insulin aspartd [n = 42, 6.0%] | Blood glucose increased | 38.1 (16) |
| Toxicity to various agentsa,b | 34.9 (22) | Product leakagea,b | 33.3 (14) | ||
| Vomiting/dizziness | 11.1 (7) | Diabetic ketoacidosis | 31.0 (13) | ||
| Levetiracetam [n = 56, 6.6%] | Seizure | 44.6 (25) | Mite allergen extract [n = 31, 4.4%] | Anaphylactic reaction | 58.1 (18) |
| Epilepsy/product administration errora,b,c | 23.2 (13) | Dyspnea | 25.8 (8) | ||
| Treatment non-compliancea,b | 17.9 (10) | Urticaria | 16.1 (5) | ||
| Insulin aspartd [n = 48, 5.7%] | Product administration errora,b,c/treatment non-compliance a,b | 58.3 (28) | Allergens [n = 25, 3.6%] | Anaphylactic reaction | 44.0 (11) |
| Blood glucose increased/ketoacidosis/vomiting | 31.3 (15) | Dyspnea/urticaria | 28.0 (7) | ||
| Hyperglycemia | 25.0 (12) | Anaphylactic shock/pruritus | 12.0 (3) | ||
| Valproic acid [n = 47, 5.6%] | Seizure | 38.3 (18) | Palivizumab [n = 25, 3.6%] | Respiratory syncytial virus infectione | 60.0 (15) |
| Epilepsy | 17.0 (8) | Respiratory failure | 16.0 (4) | ||
| Status epilepticus/thrombocytopenia | 12.8 (6) | Fluid intake reduced | 12.0 (3) | ||
| Methylphenidated [n = 35, 4.1%] | Headache/intentional overdosea/nausea vomiting | 17.1 (6) | Methylphenidated [n = 21, 3.0%] | Intentional overdosea/nausea/vision blurred | 19.0 (4) |
| Abdominal pain/seizure/weight decreased | 14.3 (5) | Headache/tachycardia/vomiting | 14.3 (3) | ||
| Dizziness/toxicity to various agentsa | 11.4 (4) | Agitation/decreased appetite/dizziness/electrocardiogram QT prolonged/feeling jittery/hallucination/mydriasis/personality change/suicide attempta | 9.5 (2) | ||
ADRs adverse drug reactions, PT Preferred Term, SmPc Summary of Product Characteristics, SMQ standardized MedDRA query
aConditions describing a scenario relating to the medication safety [17]. Although, theoretically, all such reports retrieved in our analyses should be associated with a co-suspected ADR [7], we cannot exclude the presence of reports not being associated with a co-suspected ADR among the reports (see Sect. 2.5)
bReactions related to the administration process of medicinal products but not explicitly mentioned in the SmPC. Please note that manufacturers are required to compile their SmPCs in accordance with the regulatory guidance [22], which also describes which information should be included in which section of the SmPC. In addition, besides the SmPC, other pharmacovigilance documents, e.g., risk management plans safeguarding a medicinal product’s safety, are required. Hence, certain reactions in the table above not mentioned in section 4.8 of the SmPC do not necessarily imply a safety concern.
cConditions describing a medication error coded in SMQ medication errors (broad) [12]. Although, theoretically, all such reports retrieved in our analyses should be associated with a co-suspected ADR [7], we cannot exclude the presence of reports not being associated with a co-suspected ADR among the reports (see Sect. 2.5).
dDrugs that are listed in the top five of both datasets
eLack of efficacy (following individual case assessment) not mentioned in the SmPC. Despite ‘drug ineffective/lack of efficacy’ not being considered an ADR per definition, in certain circumstances submission of these reports to EudraVigilance, even if not associated with an ADR, is required [7] (see Sect. 2.5). Hence, these reports are included in the present analyses and are depicted in Table 3
Spontaneous ADR Reports
Within the spontaneous reports, two of the top five drugs referred to allergens (mite allergen extract: 4.4% [n = 31]; allergens: 3.6% [n = 25]) [Table 3]. In summary, 11.5% (80/697) of the spontaneous reports related to allergens (Table 4). In these reports relating to allergens, mostly anaphylactic reactions and related symptoms were reported. Interestingly, if allergens were excluded from the spontaneous reports, anaphylactic reactions and related symptoms no longer appeared in the ranking of the 10 most frequently reported ADRs. The route of administration of these allergens was mostly subcutaneous (87.5% [70/80]) and rarely sublingual (12.5% [10/80]). 30.0% (24/80) of the EudraVigilance reports on these allergens were coded as life-threatening. Allergens were mainly reported for 7–12-year-olds (ESM Table 7).
Table 4.
Comparison of the main characteristics of the analyses
| Comparison criteria | KiDSafe reports (systematically collected reports) | EudraVigilance reports (spontaneous reports) |
|---|---|---|
| Number of ADR reports | 845 | 697 |
| Reporting rate per hospital | 70.4 reports (845/12a) | 0.4 reports (697/1902b) |
| Documentation quality (VigiGrade score) [14] [mean ± SD] | 0.63 ± 0.26 | 0.63 ± 0.25 |
| Causality assessment available (WHO-UMC criteria [16]) |
74.6% (630/845) 630 reports had been assessed by the DCGMA |
18.4% (128/697) 127 reports had been assessed by the DCGMA and, additionally, one external evaluation was performed |
| Most frequent rating | Possible: 88.4% (557/630) | Possible: 89.8% (115/128) |
| Information about medical history | 86.3% (729/845) | 74.5% (519/697) |
| ADR reports on 0–1 month | 0.7% (6/845) | 6.9% (48/697) |
| Thereof, reports that are due to the mother´s intake of the drug during breastfeeding/pregnancy | 16.7% (1/6) | 70.8% (34/48) |
| Highlighted drug classes in this datasetc |
Anticonvulsants 21.5% (182/845) |
Allergens 11.5% (80/697) |
| Number of reports describing medication errorsd | 27.8% (235/845) | 12.6% (88/697) |
| Number of reports describing issues related to the safety of drug therapy | 46.0% (389/845) | 29.0% (202/697) |
ADR adverse drug reaction, SD standard deviation, WHO-UMC World Health Organization-Uppsala Monitoring Centre, DCGMA Drug Commission of the German Medical Association, SMQ standardized MedDRA query
aNumber of participating hospitals in the KiDSafe project
bNumber of hospitals in Germany [23] minus the participating hospitals in the KiDSafe project
cHighlighted drug classes may not correspond to the most frequently reported drug classes but is based on analysis of the 10 most frequently suspected drugs. No analyses referring to drug classes were performed for the complete dataset
dNumber of reports that describe a condition coded in SMQ ‘medication error (broad)’[12]
Overall, 3.6% (25/697) of the spontaneous reports provided information about palivizumab. Individual case assessment revealed that the reported ADRs respiratory syncytial virus infection and respiratory failure were due to a lack of efficacy (for further explanation, see Sect. 2.5).
Drugs Occurring in Both Datasets
Insulin aspart and methylphenidate were among the five most frequently suspected drugs in both datasets. 5.7% (48/845) of the systematically collected reports related to insulin aspart. Here, product administration errors and treatment non-compliance were mainly reported (58.3%, n = 28) [Table 3]. According to an individual assessment of these reports, inconsistent adherence to therapy, fear of self-injection, and fear of hypoglycemia were mentioned, among others. 33.3% (14/42) of the spontaneous reports on insulin aspart informed about product leakage as an ADR, following an individual review. In 85.7% (12/14) of these reports, damage to the cartridge caused insulin to leak into the cartridge compartment of the pump, and in only 14.3% (2/14) of the reports a wrong technique in the product-use process was reported (Table 3).
Methylphenidate was reported in 4.1% (35/845) of the systematically collected reports and 3.0% (21/697) of the spontaneous reports. Reported methylphenidate-related ADRs were gastrointestinal complaints such as vomiting and nausea as well as headache and intentional overdoses (systematically collected reports: 17.1% (6/35); spontaneous reports: vomiting 14.3% (3/21), nausea 19.0% (4/21), headache 14.3% (3/21), intentional overdose 19.0% (4/21) [Table 3].
An overview of the top 10 most frequently reported drugs for both datasets (KiDSafe and EudraVigilance reports) is presented in ESM Table 5. Table 3 shows the five most often suspected drugs and their three most often reported ADRs in both datasets.
Medical History
Epilepsy and type 1 diabetes ranked among the top two reported medical histories in both databases (PT level) [ESM Table 8]. In summary, half of the medical histories were among the top 10 in both datasets; however, they differed in ranking and their relative share, e.g. 18.4% (134/729) of the systematically collected reports with medical history reported epilepsy compared with 6.9% (36/519) of those in the spontaneous reports.
Summary Comparison of the Two Datasets
Both datasets pointed to product administration errors associated with insulin aspart. Related to the reported medical history and to the reported drugs among the systematically collected reports, epilepsy appeared to be a prominent issue compared with allergens in the spontaneous reports.
The following table describes the main results for the two different approaches with regard to selected criteria (Table 4).
Discussion
This descriptive analysis compared ADR reports referring to children from Germany collected systematically within a pharmacovigilance project (KiDSafe reports) with those being spontaneously reported (EudraVigilance reports).
Characteristics of the Reports
Although the systematically collected ADR reports originated from only 12 participating hospitals from different geographic clusters of Germany, the number of reports (n = 845) exceeded the number of reports (n = 697) leading to hospitalization received via the spontaneous reporting system from all over Germany. Thus, the ADR rate per participating hospital was much higher for the systematically collected reports (70.4 reports [845/12]) than that of the spontaneous reports related to the number from any hospital in Germany (0.4 reports [697/1902 hospitals [23]]). Notably, this comparison is hampered, since, among others, the number of hospitals with a pediatric department in Germany was not available and the spontaneous reports could have been sent by HCPs working in a hospital, HCPs not working in a hospital, or non-HCPs. Nevertheless, these findings suggest a considerable underreporting for pediatric ADRs leading to hospitalization in the spontaneous reporting system, which was also observed in another study from The Netherlands [24]. Common reasons for underreporting are, among others, lack of time and not reporting of well-known ADRs [24, 25], which may also apply to pediatric ADRs. Although, in principle, every ADR can be reported, certain ADRs are of special interest from a pharmacovigilance point of view (e.g. serious ADRs, unknown ADRs [not listed in the SmPC], and ADRs of new drugs [marketed for < 5 years]) [26].
Interestingly, there were only small differences with regard to the quality of documentation between the KiDSafe (0.64 ± 0.25) and EudraVigilance reports (0.63 ± 0.25). However, the relative share of reports with a VigiGrade score ≤ 0.3, indicating a rather poor documentation quality, was higher in EudraVigilance reports versus KiDSafe reports (6.0% vs. 1.3%). Notably, whereas all KiDSafe reports originated from HCPs, 12.6% of the EudraVigilance reports originated from non-HCPs. However, the quality of documentation was only slightly higher for HCPs (mean 0.64) compared with non-HCPs (mean 0.57) in the EudraVigilance reports. It has to be noted that the VigiGrade score was calculated by means of a computer-based algorithm not considering any additional information provided in the free-text descriptions of the reports that may have upgraded the quality of documentation. This limitation applies to both datasets. In the original publication by Bergvall et al., reports with a VigiGrade score > 0.8 were defined as being well-documented, and this was achieved in 13% of the studied reports (median score 0.41) [14]. In our study, 30.4% of EudraVigilance reports and 31.3% of KiDSafe reports had a score > 0.8 (median score 0.61 in both groups). Thus, compared with the analysis by Bergvall et al., our data performed better. However, it has to be taken into account that the period of analyses differed and that our analysis only included reports from Germany, whereas Bergvall et al. included more than 100 countries. Bergvall et al. stated that there are countries with a higher proportion of well-documented reports, such as Germany [14].
The share of ADR reports with an ‘at least possible’ causal relationship was high and comparable in both datasets (systematic ADR collection: 88.4% [557/630]; spontaneous reports: 96.1% [123/128]). However, in only 18.4% (128/697) of the spontaneous reports, a causality assessment was available compared with 74.6% (630/845) of the systematically collected ADR reports. This difference reflects that the systematic ADR collection according to the protocol required a causality assessment by the DCGMA. Systematically collected reports without causality assessment (215/845; 25.4%) referred to reports informing about, for example, accidental intoxication or overdoses without clinically manifest ADR, which were thus not assessed for causality. In contrast, only those (127/128) spontaneous reports that were forwarded to BfArM by the DCGMA contained a causality assessment, whereas reports not provided by the DCGMA usually do not contain a causality assessment. Due to the huge number of reports, no causality assessment is routinely performed by the competent authorities, and marketing authorization holders are not obliged to provide a causality assessment when submitting the report to EudraVigilance [9]. However, it should be considered that these reports without causality assessment still carry an ‘implied causal association’ since, as one minimum criterion (as required by EudraVigilance), there was suspicion of an ADR. The high proportion of reports with a possible causal relationship was also observed in other studies from our group [27, 28], as well as in other ADR database analyses [29]. This finding may be related to the fact that often not all information (e.g. comedications, histories) required to exclude that the reported ADR could also be related to other drugs or diseases is provided. The latter also applies to the KiDSafe reports explaining why the causality assessment performed worse compared with other studies of similar design. In addition, it has to be taken into account that in other studies, different methods for the assessment of the causality, i.e. not WHO-UMC [16], may have been applied, which could explain a higher proportion of ADRs assessed as probable in these studies compared with KiDSafe.
Reporting Sources and Seriousness Criteria
Because of the study design [13], all KiDSafe reports originated from HCPs compared with 87.4% (609/697) of the EudraVigilance reports. More spontaneous than systematically collected reports were designated as life-threatening (13.7% vs. 8.3%), fatal (2.3% vs. 0.1%) and disabling (1.3% vs. 0.1%) [ESM Table 3]. This may to some extent reflect a considerable underreporting of non-serious ADRs since physicians may tend to preferentially report serious ADRS [30]. In summary, the spontaneous ADR collection included consumer reports but inherently may carry a selection bias towards more serious ADRs.
Age and Sex Distribution
The high proportion of ADR reports for 7- to 12- and 13- to 17-year-olds in both datasets underlines the importance of intensive monitoring of drug administration in these age groups. It may however also reflect a higher drug exposure in these age groups, which we did not assess in the present study. According to a study from Germany, the latter was at least applicable for 14- to 17-year-olds [31]. In contrast, two other prospective studies performed at a university hospital in Germany also showed a higher ADR incidence for 11- to 18-year-olds compared with 0- to 10-year-olds [32].
Medical History
Interestingly, a much higher proportion of epilepsy was reported in the medical histories of the systematically collected reports than the spontaneous reports. In summary, depending on the inclusion and exclusion criteria, systematic ADR reporting may include different patients (e.g. with different medical histories) than spontaneous ADR reporting and may therefore be more focused on a specific group of patients, whereas spontaneous reporting may principally cover all patient groups.
Reported ADRs and Drugs
The analyses of the reported ADRs from both datasets showed similarities and differences. Whereas the spontaneous reports draw attention to anaphylactic ADRs related to allergens, the systematic ADR collection pointed to anticonvulsant-related ADRs. This might be due to the fact that patients with epilepsy are often readmitted to hospitals due to unsatisfactory seizure control, while at the same time, seizures may occur as ADRs of some anticonvulsants. Whereas this could deter physicians from reporting suspected ADRs via the spontaneous reporting system, the KiDSafe team members were explicitly asked to report any ADR to the DCGMA with an assumed, at least reasonable, time relationship to the administration of the drug. In contrast, allergens were prominent in the spontaneous reports, indicating that ADRs associated with allergens used for hyposensitizations, such as ADRs associated with ‘conventional’ drugs, are also reported. These spontaneous reports mostly described hyposensitizations that resulted in anaphylactic reactions and patients being hospitalized for treatment or monitoring. The latter finding may also reflect that patients who receive hyposensitization are intensively monitored and explicitly asked about the occurrence of ADRs before they are allowed to leave the doctor’s office. Hence, the physicians may be confronted with more ADRs for this particular drug class, which may increase the likelihood of allergen-related ADRs being reported by physicians. Notably, in our analysis, almost all of the spontaneous reports referring to allergens originated from physicians (97.5% [78/80]).
In other studies, antibiotics were frequently reported as the drug class most often associated with ADRs in children and adolescents [32–35]. Please note that our analysis was performed at the drug ingredient level and not at an aggregated drug class level, which may have impacted on the order of the most frequently reported drugs or drug classes compared with other studies. Furthermore, the previous studies were performed before 2017 and antibiotic prescriptions in Germany decreased enormously between 2010 and 2019 [36], which may have also impacted on the number of ADR reports referring to antibiotics in our study. However, if antiepileptics and allergens were excluded, antibiotics would rank sixth (amoxicillin) and tenth (cefuroxime), respectively, in the ranking of the most frequently reported drugs in the KiDSafe and EudraVigilance reports.
Notably, oncological patients were not considered in the KiDSafe project (see the Sect. 2). In consequence, respective drugs were not among the suspected drugs in the KiDSafe reports. With regard to the EudraVigilance reports, these were also reported as suspected drugs, in particular in the age-stratified analysis (see ESM Table 7); however, they did not rank among the first 10 suspected drugs in the overall analysis.
In summary, these are two different pharmacovigilance issues originating from the two different approaches; however, they both illustrate the necessity to closely monitor patients during drug therapy.
Reports on insulin aspart describing application problems during treatment were contained in both datasets. Children, adolescents, and their parents need to be well-educated on how to correctly use the medical device [37, 38], and both parents and HCPs should assist in this process until it can be applied unassisted. This education should also convey the importance of insulin therapy for the health of the patient.
Remarkably, more KiDSafe reports described medication errors and other medication safety related issues compared with EudraVigilance reports. In other words, the systematic ADR collection pointed to a pharmacovigilance issue that is crucial for drug safety and that has not been identified in comparable strength in the spontaneous reports. The reasons for its assumed underreporting in daily routine may relate to the limited time and personnel resources.
With regard to medication errors and other scenarios relating to medication safety, the fear of legal consequences may also play a role. In the literature, a considerable number of medication errors or scenarios relating to the safety of drug therapy were also reported for children and adolescents admitted to hospitals [33, 34, 39, 40].
Conclusion
In summary, both approaches carry inherent advantages and disadvantages from a pharmacovigilance point of view that should be considered when interpreting the results of the respective analyses. Systematic ADR collection may be helpful if there is a focus on a particular ADR, drug, or region, and may also point to dedicated safety issues not emerging from the spontaneous reports. It is thus applied both when a signal has already occurred, e.g. as part of the spontaneous reporting system requiring further investigation, and for signal generation.
Although possibly providing a broader spectrum of ADR reports, the spontaneous ADR collection will, conversely, most likely deliver fewer spontaneous reports to a particular ADR or drug of interest compared with a targeted systematic ADR report collection. In fact, its main purpose is to deliver signals of rare, serious, and as yet unknown ADRs, typically based on disproportionality analysis [41].
Beyond these considerations, the spontaneous reporting system is more cost effective and available without a time delay, but limited by considerable underreporting and the unknown amount of drug-exposed individuals.
Systematic ADR collection is more cost expensive and personnel intensive and may be subject to biases depending on the protocol of the individual study. Additionally, investigating rare ADRs may require sufficiently large, and thus expensive and logistically complex, studies.
A combined approach could likely compensate for limitations inherent to the single approaches. However, in clinical practice, since the systematic ADR collection is more expensive and complex, such a combined approach will most likely only be applied to dedicated pharmacovigilance topics or research objectives.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the ADR database research team of the Pharmacovigilance Division of the Federal Institute for Drugs and Medical Devices (BfArM) for their excellent support, and Claudia Kayser from the Pharmacovigilance Division for critically reviewing and commenting on any medication error and medication safety related aspects of the manuscript. Finally, the authors would also like to thank Norbert Paeschke, Tania Meier, Dennis Lex, Thomas Grüger, Martin Huber, and Simone Bergner from the Pharmacovigilance Division for critically reading and commenting on the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Funding
Sarah Leitzen received funding from the KiDSafe project. KiDSafe was funded by the German innovation fund of the Federal Joint Committee (G-BA) under the fund mark 01NVF16021. Antje Neubert received funding from the German Ministry of Health and German innovation fund of the Federal Joint Committee (G-BA).
Conflict of interest
Sarah Leitzen, Diana Dubrall, Irmgard Toni, Julia Stingl, Patrick Christ, Ursula Köberle, Matthias Schmid, Antje Neubert, and Bernhardt Sachs have no conflicts of interest to declare.
Ethics approval
Approval for the protocol of the KiDSafe project was obtained from the Ethics Committee of the Friedrich-Alexander University Erlangen-Nürnberg under reference number 351_17 B, and approval for the analysis of the spontaneous reports from EudraVigilance was obtained from the local Ethics Committee of the Medical Faculty of Bonn (009/17 and 100/21).
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Data availability
Due to data protection requirements and the EudraVigilance access policy, the individual pseudonymized case reports are not accessible to the readership since different levels of access are granted for different stakeholders [16]. The Federal Institute for Drugs and Medical Devices (BfArM), as the competent authority in Germany, is granted the highest access level; however, even with the lowest level of access researchers can perform the same analyses in EudraVigilance with aggregated data (public access: http://www.adrreports.eu/en/index.html).
Code availability
Not applicable.
Authors' contributions
Sarah Leitzen, Diana Dubrall and Bernhardt Sachs contributed to the conception and design of the analysis. Irmgard Toni and Antje Neubert contributed to the design of the analysis of the KiDSafe data and provided input to the interpretation and discussion of the data. Patrick Christ and Diana Dubrall contributed to data evaluation. Ursula Köberle gave advice regarding ADR reporting and supervised the recording and assessing within the DCGMA. The first drafts of this manuscript were written by Sarah Leitzen and Bernhardt Sachs. All authors commented on previous versions of the manuscript, and read and approved the final version.
Footnotes
Disclaimer
The information and views set out in this manuscript are those of the authors and do not necessarily reflect the official opinion of the Federal Institute for Drugs and Medical Devices.
Sarah Leitzen, Diana Dubrall and Bernhardt Sachs contributed equally to this work.
References
- 1.Knopf H, Du Y. Perceived adverse drug reactions among non-institutionalized children and adolescents in Germany. Br J Clin Pharmacol. 2010;70:409–417. doi: 10.1111/j.1365-2125.2010.03713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haffner S, von Laue N, Wirth S, Thürmann PA. Detecting adverse drug reactions on paediatric wards: intensified surveillance versus computerised screening of laboratory values. Drug Saf. 2005;28(5):453–464. doi: 10.2165/00002018-200528050-00008. [DOI] [PubMed] [Google Scholar]
- 3.Rashed AN, Wong IC, Cranswick N, Hefele B, Tomlin S, Jackman J, et al. Adverse Drug Reactions in Children-International Surveillance and Evaluation (ADVISE): a multicentre cohort study. Drug Saf. 2012;35(6):481–494. doi: 10.2165/11597920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Smyth RL, Peak M, Turner MA, Nunn AJ, Williamson PR, Young B, et al. ADRIC: adverse drug reactions in children: a programme of research using mixed methods. NIHR J Libr. 2014 doi: 10.3310/pgfar02030. [DOI] [PubMed] [Google Scholar]
- 5.Wimmer SN, Neubert A, Rascher W. Arzneimitteltherapiesicherheit bei Kindern. Dtsch Arztebl Int. 2015;112:781–787. doi: 10.3238/arztebl.2015.0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aurich B, Apele-Freimane D, Banaschewski T, Chouchana L, Day S, Kaguelidou F, et al. c4c: paediatric pharmacovigilance: methodological considerations in research and development of medicines for children: a c4c expert group white paper. Br J Clin Pharmacol. 2021 doi: 10.1111/bcp.15119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Medicines Agency. Module VI—Collection, management and submission of reports of suspected adverse reactions to medicinal products (Rev 2). https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guideline-good-pharmacovigilance-practices-gvp-module-vi-collection-management-submission-reports_en.pdf.
- 8.Neubert A, Urschitz M, Schwab M, Rascher W. KiDSafe—Verbesserung der Arzneimitteltherapiesicherheit bei Kindern und Jugendlichen. Kinder und Jugendarzt. 2019;50(2/19):30–33. [Google Scholar]
- 9.European Medicines Agency (EMA). EudraVigilance—European database of suspected adverse drug reaction reports. https://www.adrreports.eu/.
- 10.Kommas E, Lex D, Huber M, Paeschke N. Verdachtsfälle melden—Warum am behördlichen Nebenwirkungsmelde-System kein Weg vorbeiführt. Deutsche ApothekerZeitung. 2019;37.
- 11.Dubrall D, Schmid M, Alesik E, Paeschke N, Stingl J, Sachs B. Frequent adverse drug reactions, and medication groups under suspicion. Dtsch Arztebl Int. 2018;115(23):393–400. doi: 10.3238/arztebl.2018.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medical Dictionary for Regulatory Activities. 2022 (08.02.2022). https://www.meddra.org/.
- 13.Schulze C, Toni I, Moritz K, Eberl S, Rascher W, Neubert A. Development and adjustment of an algorithm for identifying drug-related hospital admissions in pediatrics. J Patient Saf. 2022;18(5):421–429. doi: 10.1097/PTS.0000000000000951. [DOI] [PubMed] [Google Scholar]
- 14.Bergvall T, Norén GN, Lindquist M. vigiGrade: a tool to identify well-documented individual case reports and highlight systematic data quality issues. Drug Saf. 2014;37(1):65–77. doi: 10.1007/s40264-013-0131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uppsala Monitoring Center. VigiBase. https://who-umc.org/vigibase/.
- 16.World Health Organization. The use of the WHO-UMC system for standardised case causality assessment. 2013 (08.02.2022). https://cdn.who.int/media/docs/default-source/medicines/pharmacovigilance/whocausality-assessment.pdf?sfvrsn=5d8130bb_2&download=true.
- 17.Arzneimittelkommission der deutschen Ärzteschaft (Akdae). Arzneimitteltherapiesicherheit. https://www.akdae.de/fileadmin/user_upload/akdae/Kommission/Presse/DAe/20141031.pdf.
- 18.Kassenärztliche Bundesvereinigung. Altersgruppen 2022 (08.02.2022). https://www.kbv.de/tools/ebm/html/4.3.5_162395004446927562274884.html.
- 19.European Medicines Agency. Access to EudraVigilance data 2022. https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance/access-eudravigilance-data.
- 20.European Medicines Agency (EMA). European Medicines Agency’s Data Protection Notice for EudraVigilance Human (EV). https://www.ema.europa.eu/en/documents/other/european-medicines-agencys-data-protection-notice-eudravigilance-human-ev_en.pdf.
- 21.Kaumanns K, Kayser C, Paeschke N, von Mallek D, Stingl J, Köberle U, et al. Medikationsfehler im Fokus der Forschung und Pharmakovigilanz. Bulletin zur Arzneimittelsicherheit; 2015. https://www.bfarm.de/SharedDocs/Downloads/DE/Arzneimittel/Pharmakovigilanz/Bulletin/2015/2-2015.pdf?__blob=publicationFile.
- 22.A guideline on summary of products characteristics. September 2009. https://ec.europa.eu/health/system/files/2016-11/smpc_guideline_rev2_en_0.pdf.
- 23.Statista. Anzahl der Krankenhäuser in Deutschland in den Jahren 2000 bis 2019; 2022 (08.02.2022). https://de.statista.com/statistik/daten/studie/2617/umfrage/anzahl-der-krankenhaeuser-in-deutschland-seit-2000/.
- 24.Dittrich ATM, Draaisma JMT, van Puijenbroek EP, et al. Analysis of reporting adverse drug reactions in paediatric patients in a university hospital in the Netherlands. Pediatr Drugs. 2020;22:425–432. doi: 10.1007/s40272-020-00405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta R, Malhotra A, Malhotra P. A study on determinants of underreporting of adverse drug reactions among resident doctors. Int J Res Med Sci. 2018;6(2).
- 26.Arzneimittelkommission der deutschen Ärzteschaft (AkdÄ). Nebenwirkungen melden—Ein Leitfaden für Ärzte. 2019 (15.02.2022). https://www.akdae.de/Arzneimitteltherapie/LF/PDF/Nebenwirkungen_melden.pdf.
- 27.Dubrall D, Just KS, Schmid M, et al. Adverse drug reactions in older adults: a retrospective comparative analysis of spontaneous reports to the German Federal Institute for Drugs and Medical Devices. BMC Pharmacol Toxicol. 2020;21:25. doi: 10.1186/s40360-020-0392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubrall D, Leitzen S, Toni I, et al. Descriptive analysis of adverse drug reaction reports in children and adolescents from Germany: frequently reported reactions and suspected drugs. BMC Pharmacol Toxicol. 2021;22:56. doi: 10.1186/s40360-021-00520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stammschulte T, Ludwig WD, Mühlbauer B, et al. Metamizole (dipyrone)-associated agranulocytosis. An analysis of German spontaneous reports 1990–2012. Eur J Clin Pharmacol. 2015;71:1129–1138. doi: 10.1007/s00228-015-1895-y. [DOI] [PubMed] [Google Scholar]
- 30.Schneeweiss S, Göttler M, Hasford J, Swoboda W, Hippius M, Hoffmann A, et al. First results from an intensified monitoring system to estimate drug related hospital admissions. Br J Clin Pharmacol. 2001;52(2):196–200. doi: 10.1046/j.0306-5251.2001.01425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knopf H, Sarganas G, Grams D, et al. Application of medicines and nutritional supplements in childhood and adolescence in Germany. Bundesgesundheitsblatt. 2020;63:1287–1296. doi: 10.1007/s00103-020-03128-5. [DOI] [PubMed] [Google Scholar]
- 32.Oehme AK, Rashed AN, Hefele B, et al. Adverse drug reactions in hospitalised children in Germany are decreasing: results of a nine year cohort-based comparison. PLoS One. 2012;7(9):e44349. doi: 10.1371/journal.pone.0044349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smyth RMD, Gargon E, Kirkham J, et al. Adverse drug reactions in children—a systematic review. PLoS One. 2012;7(3):e24061. doi: 10.1371/journal.pone.0024061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zed PJ, Haughn C, Black KJL, et al. Medication-related emergency department visits and hospital admissions in pediatric patients: a qualitative systematic review. J Pediatr. 2013;163(2):477–483. doi: 10.1016/j.jpeds.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 35.Vazquez-Alvarez AO, Brennan-Bourdon LM, Rincon-Sanchez AR, et al. Improved drug safety through intensive pharmacovigilance in hospitalized pediatric patients. BMC Pharmacol Toxicol. 2017;18:79. doi: 10.1186/s40360-017-0186-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holstiege J, Bätzing J, Akmatov MK, et al. Rückgang der ambulanten Antibiotikaverordnungen bei Kindern und Jugendlichen in Deutschland 2010–2019. Regionale Entwicklung in den deutschen KV-Regionen. Monatsschr Kinderheilkd. 2022;170:392–402. doi: 10.1007/s00112-021-01276-9. [DOI] [Google Scholar]
- 37.Baharvand P, Hormozi M. Can parents' educational level and occupation affect perceived parental support and metabolic control in adolescents with type 1 diabetes? J Educ Health Promot. 2019;8:11. doi: 10.4103/jehp.jehp_215_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zysberg L, Lang T. Supporting parents of children with type 1 diabetes mellitus: a literature review. Patient Intell. 2015;7:21–31. doi: 10.2147/PI.S77566. [DOI] [Google Scholar]
- 39.Sutherland A, Phipps DL, Tomlin S, et al. Mapping the prevalence and nature of drug related problems among hospitalised children in the United Kingdom: a systematic review. BMC Pediatr. 2019;19:486. doi: 10.1186/s12887-019-1875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallagher RM, Mason JR, Bird KA, et al. Adverse drug reactions causing admission to a paediatric hospital. PLoS One. 2012;7(12):e50127. doi: 10.1371/journal.pone.0050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raschi E, Moretti U, Salvo F, Pariente A, Antonazzo IC, De Ponti F, et al. Evolving roles of spontaneous reporting systems to assess and monitor drug safety. In: Kothari CS, Shah M, Patel RM, et al., editors. Pharmacovigilance. London: IntechOpen; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to data protection requirements and the EudraVigilance access policy, the individual pseudonymized case reports are not accessible to the readership since different levels of access are granted for different stakeholders [16]. The Federal Institute for Drugs and Medical Devices (BfArM), as the competent authority in Germany, is granted the highest access level; however, even with the lowest level of access researchers can perform the same analyses in EudraVigilance with aggregated data (public access: http://www.adrreports.eu/en/index.html).