The authors of the JBC research article entitled “A potent complement factor C3–specific nanobody inhibiting multiple functions in the alternative pathway of human and murine complement” stated as follows.
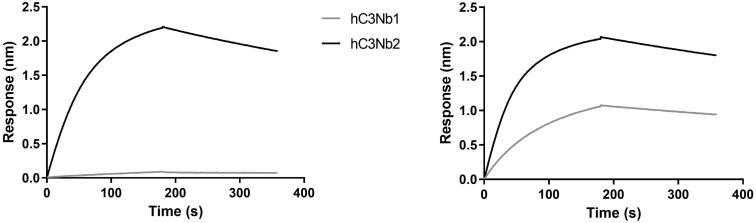
In figure 5 of the published paper, we provided evidence that the nanobody hC3Nb1 could bind with low-nanomolar affinity to multiple functional states of complement factor C3, including non-activated native C3 and its protease-activated form C3b (1). In agreement with this finding, we later described the crystal structure of hC3Nb1 bound to native C3 (2). However, later attempts to reproduce the interaction with native C3 were unsuccessful (Figure 1). Despite thorough analysis, we have not been able to identify the underlying reason for this discrepancy. Possibly minor unknown differences in the procedure for preparation of hC3Nb1 can result in subtle structural differences in the N-terminal residues of hC3Nb1 that can induce large differences in the affinity for native C3 (3). It is, therefore, advisable to test thoroughly for a given batch of hC3Nb1 whether it binds tightly to native C3. Importantly, the ability of hC3Nb1 to act as a powerful inhibitor of the alternative pathway of complement in vitro, as originally reported (1), does not require high affinity binding to native C3, as demonstrated in (3).
Figure 1. The hC3Nb1 nanobody does not interact with native complement C3, but does interact with protease activated complement C3b. To analyze the interaction between hC3Nb1 and the control nanobody hC3Nb2 (4) with C3 or C3b, nanobody at 10 μg/mL was immobilized on anti-penta his (His1K) biosensors (Sartorius). The sensors were transferred into 50 nM C3 (left) or C3b (right) for 180 seconds, followed by a 180 seconds dissociation period. The sensorgrams were normalized by subtracting sensorgrams recorded without C3 and C3b.
VOLUME 293 (2018) PAGES 6269–6281
References
- 1.Jensen R.K., Pihl R., Gadeberg T.A.F., Jensen J.K., Andersen K.R., Thiel S., Laursen N.S., Andersen G.R. A potent complement factor C3-specific nanobody inhibiting multiple functions in the alternative pathway of human and murine complement. J Biol Chem. 2018;293:6269–6281. doi: 10.1074/jbc.RA117.001179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedersen D.V., Gadeberg T.A.F., Thomas C., Wang Y., Joram N., Jensen R.K., Mazarakis S.M.M., Revel M., El Sissy C., Petersen S.V., Lindorff-Larsen K., Thiel S., Laursen N.S., Fremeaux-Bacchi V., Andersen G.R. Structural Basis for Properdin Oligomerization and Convertase Stimulation in the Human Complement System. Front Immunol. 2019;10:2007. doi: 10.3389/fimmu.2019.02007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen H., Jensen R.K., Hansen A.G., Petersen S.V., Thiel S., Laursen N.S., Andersen G.R. Structure-Guided Engineering of a Complement Component C3-Binding Nanobody Improves Specificity and Adds Cofactor Activity. Frontiers in Immunology. 2022;13 doi: 10.3389/fimmu.2022.872536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedersen H., Jensen R.K., Hansen A.G., Gadeberg T.A.F., Thiel S., Laursen N.S., Andersen G.R. A C3-specific nanobody that blocks all three activation pathways in the human and murine complement system. J Biol Chem. 2020;295:8746–8758. doi: 10.1074/jbc.RA119.012339. [DOI] [PMC free article] [PubMed] [Google Scholar]