Abstract
Thiacloprid (TH) is a neonicotinoid insecticide employed in agriculture to protect fruits and vegetables against different insects. It showed different deleterious effects on the general health of non-target organisms including birds and animals, however, its developmental toxicity has yet to be fully elucidated. Chicoric (CA) and rosmarinic (RA) acids are polyphenolic compounds with a wide range of beneficial biological activities. In this study, the possible protective effects of CA and RA were investigated in chick embryos exposed in ovo to TH (1µg/egg) with or without CA (100 µg/egg) or RA (100 µg/egg) co-exposure. TH reduced the hatchling body weight, body weight/egg weight, and relative weight of bursa of Fabricius in the one-day-old hatchlings. Examination of the 7-day-old chicks revealed a decline in feed intake, daily weight gain, feed conversion ratio (FCR), and plasma levels of T3, T4, and growth hormone. Serum ALT, AST activities, and total cholesterol levels showed significant elevations. Hepatic MDA was increased with a reduction in SOD activity and GSH level and downregulation of the liver SOD and GST gene expression pattern. Serum IgG and IgM levels were reduced, and various histopathological alterations were noticed in the liver. Co-administration of CA or RA with TH mitigated the toxic effects on hatchlings. When both CA and RA are combined, they present a synergistic protective effect. CA and RA can be used as protective agents against TH toxicity as they improve growth performance and have hepatoprotective and immunostimulant effects in newly hatched chicks.
Key words: poultry, thiacloprid, chicoric acid, rosmarinic acid, growth performance
INTRODUCTION
Neonicotinoids (neonics) are the relatively newest class of insecticides, introduced in the 1990s, and in the last two decades, they have become the primary class of insecticides on the global market. They have replaced traditional insecticide classes, including organophosphates, carbamates, and pyrethroids, due to their flexibility, broad-spectrum, high potency of insecticidal activity, and relatively low toxicity to non-target organisms (Gibbons, et al., 2015; Han, et al., 2018; Thompson, et al., 2020; Xu, et al., 2022).
They are mainly used to control sucking and biting insects via their action on nicotinic acetylcholine receptors (nAChRs) and consequent central nervous system disruption (Han et al., 2018).
Neonics are mainly used for seed dressing due to their high-water solubility and small molecular weight, which allow their uptake by the roots of the developing plant, spread into foliage and vascular tissues, and thus protect against different insects. Therefore, several neonics were detected in fruits and vegetables, creating a potential hazard to humans and animals, as washing does not remove the residues before consumption (Wood and Goulson, 2017).
The persistence of neonics in the environment depends on several factors, including their relatively long half-lives in soil, excellent solubility in water, and low sorption in soil. The neonics persistence in soil, water, and biota constitutes a potential health concern. Neonics residues have been frequently detected in food, including rice, honey, and tea, in addition to fruits and vegetables (Thompson et al., 2020).
The wide usage of neonics has been attributed to their low toxicity to non-target vertebrates, such as mammals, birds, and fish, and relatively high toxicity to insects (Jeschke et al., 2011). Nevertheless, neonics were recently demonstrated to constitute a direct and indirect hazard to non-target species (Salvaggio et al., 2018; Velisek and Stara, 2018; Farag et al., 2021). Thus, the ecotoxicology of neonics has attracted increasing attention and interest from scientists who reported that neonics produce several harmful effects, including cytotoxicity, genotoxicity, impaired immune response, reduced growth and reproductive performance, and endocrine disruption (Thompson et al., 2020). In birds, neonics induced neurobehavioral abnormalities, immunotoxicity, and impaired growth and development (Gibbons et al., 2015; Gobeli, et al., 2017; Franzen-Klein et al., 2020).
Thiacloprid (TH) is a member of the neonics extensively used as an insecticide and, therefore, can be considered a potential hazard to non-target organisms. It has been demonstrated to be hepatotoxic and immunotoxic to rats (Abou-Zeid et al., 2021), zebrafish (Xie et al., 2022), reprotoxic to mice (Hartman et al., 2021), and embryotoxic to rabbits and mice (Babeľová et al., 2017). Alongside, U.S. Environmental Protection Agency (EPA) mentioned that TH is “likely to be carcinogenic to humans” depending upon the occurrence of ovarian luteoma in mice, uterine adenocarcinomas, and thyroid adenomas in rats (Rowland 2006).
Recently, our experimental studies demonstrated the in ovo neurotoxicity effects of TH during chick embryo development that affects brain development and hatching behavior (Farag et al. 2021; Alagawany and Farag 2022; Farag et al., 2022b). Preimplantation embryos of rabbits and mice exposed to TH in-vitro showed increased apoptosis incidence and decreased cell proliferation in the blastocysts (Babeľová et al., 2017; Arain et al., 2022). Fetal resorption, skeletal retardation, and changes in locomotor and motor activities in rat offspring have also been recorded (EPA, 2013). In zebrafish and carp, TH induced oxidative stress and delayed embryonic development (Velisek and Stara, 2018; Wang et al., 2020b).
Since the extent of the toxic developmental impacts of TH on humans and vertebrates is not yet fully established, our study could assume considerable importance in clarifying these effects on embryos. Using in-vivo animal models has some drawbacks, including ethical issues raised regarding the use of rodents and the species-specific metabolic pathways which lead to species-specific toxicological outcomes. In this sense, chicken embryos are an ideal model for embryotoxicity studies due to their morphological and molecular similarities with other vertebrates (Zosen et al., 2021). In addition, chicken embryos develop quickly without maternal influence and are easily manipulated under experimental conditions (Smith et al., 2012).
Thyroid hormones were reported to affect development and growth in conjunction with other hormones of the growth axis. In chickens, thyroid hormones are essential for growth regulation, tissue differentiation, and embryo hatchling. When the levels of circulating thyroid hormones are above or below the normal range, the growth of birds is inhibited (McNabb, 2007). Although other neonics, such as imidacloprid, were reported to disrupt the pituitary–thyroid axis of wildlife birds, the endocrine-disrupting potential of TH was not fully established (Pandey and Mohanty, 2015; Pandey and Mohanty, 2017). Moreover, the generation of ROS and damage of various cellular components, including lipids, protein, and DNA, play a significant role in the neonics-induced toxicity (Xu et al., 2022) and impairment of cellular macromolecules that ultimately affects the development, growth, and immunocompetence. In newly hatched chicks, the antioxidant system includes SOD, CAT, GPx, GSH, ascorbic acid, vitamin E, carotenoids, and selenium (Surai, 2015). Exposure to neonics during embryogenesis is expected to deplete those cellular antioxidants, which exposes the embryo to a greater risk of oxidative damage. Therefore, exogenous antioxidants administered in ovo are essential for improving performance and protecting chick embryos.
The use of synthetic growth promoters in food animals is restricted in many countries, which attracted more attention to using safe alternatives of natural origin. Phytochemicals were proposed as growth promoters in food animals, including poultry owing to their antioxidant and immunostimulant properties (Alagawany et al., 2015, 2016; Valenzuela-Grijalva et al., 2017; Farag et al., 2022a). Besides, chicoric acid (CA), also known as dicaffeoyl-L-tartaric acid, is a natural phenolic compound present in at least 63 plant genera and species, including Echinacea purpurea (purple coneflower) and many other plants such as Cichorium intybus (chicory) (Lee and Scagel, 2013; Guidetti et al., 2016). CA has been recently demonstrated to have antioxidant, anti-inflammatory, neuroprotective, anti-aging, and antiviral activities, in addition to maintaining glucose and lipid homeostasis (Farag et al., 2021; Yang et al., 2022). It has been reported to protect against the toxic effects of d-galactosamine (d-GalN) and lipopolysaccharide (LPS) (Li et al., 2020), polyinosinic-polycytidylic acid (Tráj, et al., 2022), and methotrexate (Hussein et al., 2020). This protective effect of CA has mainly been ascribed to its free radical scavenging activity (Zhu et al., 2018) and the anti-inflammatory effect through restraining MAPKs and NF-κB (Li et al., 2020).
Rosmarinic acid (RA) is a naturally occurring polyphenolic compound present in rosemary (Rosmarinus officinalis), Salvia officinalis, Melissa officinalis, and many other plants, especially those belonging to Boraginaeceae and Lamiaceae families (Alagawany et al., 2017; Iseppi et al., 2020; Guan et al., 2022). It has been demonstrated to have anticancer, antidiabetic, antimicrobial, cardioprotective, hepatoprotective, antidepressant, nephroprotective, antiaging, antiallergic, and neuroprotective activities (Nadeem et al., 2019).
RA was found as an effective antiradical scavenger and antioxidant compound by different in vitro bioassays such as inhibition of linoleic acid peroxidation, ABTS•+, O2•, DPPH•, and DMPD•+ scavenging activity, Cu2+ and Fe3+ reducing, and Fe2+ chelating assays (Topal and Gulcin, 2022). Furthermore, it has been reported that RA protects against the toxic effect of the acetaminophen (Yao et al., 2022), chromium (Khalaf et al., 2020), tert-butyl hydroperoxide (Yang et al., 2013), and LPS (Osakabe et al., 2002). Nevertheless, it remains unclear whether CA or RA can ameliorate the toxic impacts of TH on the performance, liver, and endocrine systems of chicken embryos.
Therefore, our study aimed to explore the potential developmental toxic effects of TH and the possible protective effects of CA and RA in hatchlings exposed in ovo, using the endpoints of growth performance, blood biochemistry, hepatic oxidant/antioxidant markers, serum immunoglobulins, and hormonal profile.
MATERIALS AND METHODS
Chemicals
Chemicals prepared in our study according to the highest purity. Thiacloprid (TH) (C10H9CIN4S) (Purity 98.6%, molecular weight, the analytical standard of 252.72 PESTANAL, CAS Number: 111988-49-9). Chicoric acid (CA) (C22H18O12) (Purity ≥ 95%, the molecular weight of 474.37, CAS Number: 70831-56-0). Rosmarinic acid (RA) (C18H16O8) (purity ≥ 98%, molecular weight 360.31, CAS Number: 20283-92-5). All chemicals were purchased from Sigma-Aldrich International GmbH (St. Louis, MO).
Eggs
Ross fertilized chicken eggs (n = 420, average weight 60 ± 5 g) were purchased from a commercial hatchery. Proper management and care were paid to eggs and the hatching chicks during the experiment.
Experimental Design
Management of Fertilized Eggs
After cleaning the egg's surface with povidone-iodine solution and drying with clean dry tissue papers, they were exposed to candling in a dark room to discard defective and broken eggs and to outline the air cell's exact location using a pencil. Four hundred twenty eggs were randomly divided into seven groups, each with triplicate subgroups. On the third day of incubation, thiacloprid (1 µg/egg), CA (100 µg/egg), RA (100 µg/egg) (each in 50 µL of sterile physiologic saline), and combinations of TH/CA, TH/RA, TH/CA+RA were inoculated in ovo, respectively under sterile conditions. At the same time, the control group was injected with 100 µg of saline solution. Doses selection of TH, CA, and RA, as well as procedures of air cell injections of embryos, were selected according to our previous study (Farag et al., 2021). TH was injected at the beginning of the embryogenesis process to allow its distribution within the entire organism. Finally, the injected eggs, after inoculation, were kept in an air sac up with holes to permit airflow around them and incubated at 55% humidity, a temperature of 37.8°C, and turned once per hour.
On the 19th day of incubation, eggs were transferred to the hatchery and placed in certain hatching boxes. At one-day post-hatching (PHD), the hatched chicks were transferred to a brooder (kept in a room at 35–38°C and 60% relative humidity).
Chicks were fed daily on water and seeds (corn-based starter mash prepared at the Poultry Department, Zagazig University, Zagazig, Egypt) and kept throughout the experiment at a 16/8 h light/dark cycle till the 7 PHD.
The animal study was reviewed and approved by the institutional Ethics Committee of Zagazig University, Egypt (Approval number: ZUIACUC/2/F/56/2021).
Morphological and Performance Parameters
After egg hatching, the hatchability percentage, egg weight, body weight of hatchlings, and body weight/egg weight were recorded. After that, three newly hatched chicks were randomly dissected from each replicate, and the weights of internal organs such as the liver, spleen, and bursa of Fabricius were measured. The food intake and body weight were registered daily from 1 PHD to 7 PHD to calculate the daily weight gain and feed conversion ratio (FCR) [FCR = Feed intake, g ∕ Weight gain, g].
Blood Chemistry
Blood samples were collected in sterilized tubes from five broiler chicks randomly chosen from each experimental group. After clotting, samples were centrifuged at 3,500 rpm (2,328.24 g) for 15 min, and serum was collected and saved at −20°C until analysis.
Other biochemical variables were determined, such as total protein (TP), globulin (GLU), albumin (ALB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol (TC), and triglyceride (TG) utilizing trade diagnostic tools from Biodiagnostic Co., (Giza, Egypt).
Antioxidant Status and Oxidative Injury Assays in Liver Tissue
Dissected liver specimens were randomly chosen from each group and homogenized in cold PBS pH 7.5, in 1:5 w/v ratios using a Teflon Homogenizer in an ice-cold water bath. After samples centrifugation for 15 min at 10,000 rpm (at 0–4°C), the collected supernatant was used to estimate the activity of antioxidants enzymes, including superoxide dismutase (SOD), catalase (CAT), and the level of reduced glutathione (GSH) using the colorimetric method that described previously by (Beutler, 1963; Nishikimi et al., 1972; Aebi, 1984).
The lipid peroxidation marker malondialdehyde (MDA) was determined according to the manufacturer's instructions (Ohkawa et al., 1979).
Immunoglobulins Analysis
Serum immunoglobulin G (IgG) and immunoglobulin M (IgM) levels were determined using commercial diagnostic kits from Biodiagnostic Co. (Giza, Egypt).
Hormonal Profile
For growth hormone (GH), triiodothyronine (T3), and thyroxin (T4) evaluation, blood samples were collected from the wing vein in a heparinized syringe. The plasma was separated by centrifuge at 3,000 rpm/15 min using commercial ELISA kits from MyBiosource.com (Catalog No.: MBS266317, MBS701857, and MBS705497, respectively) under the manufacturer's instructions, San Diego, CA.
RNA Extraction and Quantitative Real-Time PCR
Isolated mRNA was extracted from the liver homogenates of each group (n = 6) using RNeasy Mini Kit (Qiagen, Heidelberg, Germany). RNA quality and concentration were evaluated by gel electrophoresis and NanoDrop2000. Then, the cDNA was obtained by reverse transcription of the total mRNA using a QuantiTect Reverse Transcription kit (Qiagen, Heidelberg, Germany) according to the manufacturer's instructions. The qRT-PCR analysis for SOD1, CAT, and GST-α mRNA expression levels were evaluated using QuantiTect SYBR Green PCR kits (Qiagen, Heidelberg, Germany) on the Rotor-Gene Q cycler (Qiagen, Germany). The thermal cycling conditions were 95 °C for 15 min, followed by 40 cycles of 94°C for 15 s, 60°C for 10 s, and 72 °C for 15. Primers used in the present study were listed in Table 1, and β-actin was used as an internal reference to normalize target gene expression levels. The comparative 2−ΔΔCt (Ct: cycle threshold) method was used to calculate the relative fold changes in the expression of target genes (Livak and Schmittgen, 2001).
Table 1.
Primer sequences for studied genes used for RT-qPCR.
| Gene | Sequences (5′→3′) | Accession number |
|---|---|---|
| SOD1 | F: AAAATTACCGGCTTGTCTGATG R: CGCTGGTACACCCATTTG |
NM_205064 |
| CAT | F: TGCAAGGCGAAAGTGTTTGA R: CAGATTCTCCAGCAACAGTGGA |
NM_001031215 |
| GST-α | F: GGAGAGAGCCTGGATTGATATG R: GGTTGTAGCTCGTTCAGTGAT |
NM_001001776 |
| β-actin | F: CCCAAAGCCAACAGAGAGAA R: CCATCACCAGAGTCCATCAC |
NM_205518 |
Assessments of Liver Histopathology
For histopathological analysis, liver specimens were dissected and cut into small pieces (3–5 mm), then fixed into 10% Neutral-Buffered Formalin solution for 48 h. After total fixation, the specimens were dehydrated in graded series of ethanol solution (70–100%) and cleared with xylene, followed by embedding in paraffin wax. Sections were obtained at 5 µm thickness and stained with hematoxylin and eosin (H&E) for light microscope investigations (Olympus BX51 Microscope) (Suvarna, et al., 2018). The degree of sinusoidal dilation, inflammation, blood infiltration, necrosis, and hepatocyte glycogen areas were estimated in 10 randomly selected 100X fields, following the manufacturer's recommendations (MacAulay et al., 2017).
Statistical Analysis
All experiments were performed in triplicates; Data were analyzed using a one-way-ANOVA procedure. Duncan's Multiple Range test was conducted to compare mean values between groups. Data were expressed as mean ± SEM. A value of *P < 0.05 was considered statistically significant.
RESULTS
Growth Performance and Organ Weight in One-Day-Old Hatchlings
Table 2 presents the effects of in-ovo inoculation of thiacloprid (TH), chicoric and (CA), and rosmarinic acids (RA) on the performance and organ weights of one-day-old hatchlings. TH significantly (*p < 0.05) reduced the hatchling body weight, body weight/egg weight, and relative weight of bursa of Fabricius. In contrast, the relative weights of liver and spleen showed no changes compared to the control group. In addition, the hatchability % revealed a non-significant reduction compared to the control. Compared to the TH group, the coadministration of CA or RA with TH failed to improve the body weight while improved the body weight/egg weight and the bursa weight. The protective effects of the phytochemicals were more evident in the TH/CA+RA group, where the control values were restored, reflecting the synergistic action of both phytochemicals.
Table 2.
Effect of in ovo exposure to thiacloprid (TH), chicoric (CA), and rosmarinic acids (RA) on performance criteria and carcass traits of hatchlings.
| Experimental groups |
||||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Control | TH | CA | RA | TH/CA | TH/RA | TH/CA+RA | P-value |
| Body weight (g) | 41.34 ± 0.83ab | 34.60 ± 1.86c | 44.74 ± 1.20a | 44.41 ± 0.49a | 34.11 ± 1.47c | 33.47 ± 0.89c | 38.63 ± 0.76bc | <0.001 |
| Egg weight (g) | 60.50 ± 0.36 | 60.48 ± 0.30 | 60.51 ± 0.35 | 60.53 ± 0.35 | 60.61 ± 0.18 | 60.48 ± 0.30 | 60.65 ± 0.15 | 0.999 |
| Body weight/egg weight | 68.13 ± 0.48ab | 52.49 ± 1.45d | 69.69 ± 0.38a | 70.09 ± 0.02a | 61.24 ± 0.63c | 61.33 ± 1.11c | 65.53 ± 0.35b | <0.001 |
| Relative liver weight % | 1.90 ± 0.02 | 1.90 ± 0.03 | 1.91 ± 0.03 | 1.91 ± 0.02 | 1.92 ± 0.02 | 1.89 ± 0.02 | 1.89 ± 0.05 | 0.991 |
| Relative spleen weight % | 0.09 ± 0.00b | 0.06 ± 0.00b | 0.12 ± 0.00a | 0.12 ± 0.01a | 0.07 ± 0.00b | 0.07 ± 0.00b | 0.08 ± 0.012b | <0.001 |
| Relative bursa weight % | 0.16 ± 0.01b | 0.08 ± 0.01d | 0.21 ± 0.01a | 0.23 ± 0.01a | 0.12 ± 0.00c | 0.14 ± 0.00bc | 0.16 ± 0.01b | <0.001 |
| Hatchability % | 84.15 ± 1.55ab | 80.17 ± 0.03b | 84.20 ± 1.64ab | 85.51 ± 0.05a | 82.26 ± 0.48ab | 81.97 ± 0.25ab | 84.04 ± 1.45ab | 0.042 |
Values are mean ± SEM, values not sharing a joint superscript letter (a - d) differ significantly at *P < 0.05.
Growth Performance and Hormonal Profile of the 7-Day-Old Hatchlings
The impacts of different treatments on growth performance and the hormonal profile of the 7-day-old hatchlings were mentioned in Table 3. The daily weight gain, feed intake, and FCR were significantly (*P < 0.05) decreased in the TH-exposed group.
Table 3.
Effect of in ovo exposure to thiacloprid (TH), chicoric (CA), and rosmarinic acids (RA) on growth performance and plasma hormonal profile of 7-day-old hatchlings.
| Experimental groups |
||||||||
|---|---|---|---|---|---|---|---|---|
| Control | TH | CA | RA | TH/CA | TH/RA | TH/CA+RA | P-value | |
| Performance | ||||||||
| Daily weight gain (g) | 13.49 ± 0.26a | 10.52 ± 0.94b | 13.61 ± 0.19a | 13.48 ± 0.27a | 12.24 ± 0.40ab | 12.24 ± 0.38ab | 13.65 ± 0.008a | < 0.002 |
| Daily feed intake (g) | 23.78 ± 0.29a | 17.69 ±1.92b | 24.00 ± 0.58a | 24.31 ± 0.73a | 19.71 ± 0.60b | 20.12 ± 0.37b | 23.99 ± 0.17a | < 0.001 |
| FCR (g feed/g gain) | 1.76 ± 0.02ab | 1.67 ± 0.03bc | 1.75 ± 0.01ab | 1.79 ± 0.02a | 1.61 ± 0.01c | 1.63 ± 0.02c | 1.75 ± 0.02ab | < 0.001 |
| Hormonal profile | ||||||||
| T3 (ng/ml) | 1.41 ± 0.00a | 1.07 ± 0.05b | 1.41 ± 0.00a | 1.41 ± 0.00a | 1.33 ± 0.02a | 1.35 ± 0.01a | 1.40 ± 0.00a | < 0.001 |
| T4 (ng/ml) | 9.51 ± 0.00a | 5.12 ± 0.05d | 9.66 ± 0.10a | 9.83 ± 0.03a | 6.57 ± 0.11c | 6.63 ± 0.20c | 7.41 ± 0.09b | < 0.001 |
| GH (ng/ml) | 102.83 ± 0.74a | 90.15 ± 2.48b | 102.77 ± 0.64a | 102.78 ± 0.77a | 100.51 ± 0.34a | 100.77 ± 0.38a | 102.60 ± 0.80a | < 0.001 |
Values are mean ± SE, values not sharing a joint superscript letter (a - d) differ significantly at *P < 0.05. P- overall treatment.
Upon the CA and RA co-administration with TH, a non-significant improvement was noticed in daily weight gain and feed intake compared to the TH group. The TH/CA+RA group presented significant protective effects, where control values were attained for the 3 variables.
Concerning the hormonal profile, the obtained data indicated a significant decline in plasma T3, T4, and GH levels in the TH group, compared to the control group (*P < 0.05).
On the other hand, TH/CA and TH/RA co-exposed groups showed significant improvements in T3 and GH relative to the TH-exposed group, and control values were restored. Although the 2 phytochemicals improved the values of T4 compared to the TH group, control values still needed to be achieved. In the TH/CA+RA group, the protective effect on T4 was more evident than in other treated groups.
Effects on Blood Biochemistry Variables in 7-Day-Old Hatchlings
Regarding the effects of in-ovo exposure to TH, significant elevations (*P < 0.05) were observed in serum ALT and AST, and the level of TC, compared to the control. At the same time, protein profile and TG showed no significant variations. In the combined exposure groups (TH/CA, TH/RA, and TH/CA+RA), significant improvements were recorded in enzyme activities compared to the TH group. The control value of AST was restored in TH/CA+RA group. Concerning serum TC level, significant (*P < 0.05) refinement was noted in both TH/CA and TH/CA+RA groups, where control values were restored. On the other side, co-administration of RA with TH did not exert a protective effect on the TC level (Table 4).
Table 4.
Effect of in ovo inoculation of thiacloprid (TH), chicoric (CA), and rosmarinic acids (RA) on liver function markers and lipid profile in 7-day hatchlings.
| Experimental groups |
||||||||
|---|---|---|---|---|---|---|---|---|
| Control | TH | CA | RA | TH/CA | TH/RA | TH/CA+RA | P-value | |
| Liver function | ||||||||
| ALT (U/L) | 15.91 ± 2.15d | 78.90 ± 1.85a | 14.22 ± 0.48d | 15.80 ± 2.17d | 45.27 ± 2.88b | 45.09 ± 3.06b | 30.34 ± 2.92c | <0.001 |
| AST(U/L) | 61.81 ± 0.99d | 182.46 ± 2.05a | 54.52 ± 0.35d | 60.26 ±.09d | 108.96 ± 4.53c | 133.40 ± 3.58b | 64.30 ± 2.14d | <0.001 |
| TP (g/dl) | 4.58 ± 0.04 | 4.57 ± 0.03 | 4.58 ± 0.04 | 4.62 ± 0.03 | 4.55 ± 0.06 | 4.57 ± 0.05 | 4.60 ± 0.03 | 0.938 |
| ALB (g/dl) | 1.78 ± 0.04 | 1.77 ± 0.03 | 1.79 ± 0.04 | 1.80 ± 0.03 | 1.79 ± 0.04 | 1.77 ± 0.03 | 1.77 ± 0.03 | 0.996 |
| GLU (g/dl) | 2.86 ± 0.020 | 2.84 ± 0.01 | 2.86± 0.01 | 2.85 ± 0.01 | 2.86 ± 0.01 | 2.85 ± 0.02 | 2.85 ± 0.02 | 0.988 |
| Lipid profile | ||||||||
| TC (mg/dl) | 175.18 ± 0.56b | 190.34 ± 0.66a | 166.66 ± 1.80c | 165.55 ± 3.18c | 181.25 ± 0.57b | 189.61 ± 0.62a | 175.52 ± 0.05b | <0.001 |
| TG (mg/dl) | 49.15 ± 0.11 | 49.10 ± 0.10 | 48.08 ± 0.11 | 48.39 ± 0.58 | 48.81 ± 0.19 | 48.89 ± 0.28 | 48.99 ± 0.30 | 0.161 |
Values are mean ± SE, values not sharing a joint superscript letter (a - d) differ significantly at *P < 0.05.
Effects on Liver Antioxidant Status and Oxidative Stress Variables in 7-Day-Old Hatchlings
The effects of different treatments on liver antioxidant/oxidative status are displayed in Table 5. The in-ovo exposure to TH significantly (*p < 0.05) reduced the activity of hepatic SOD and level of GSH while increasing the level of MDA compared to the control. Co-exposure to either CA or RA with TH could not improve the SOD activity and MDA level but improved the GSH content relative to the TH group, and control values were not restored. In the TH/CA+RA group, the protective effect was more marked, where SOD and GSH were improved in addition to alleviating MDA increase, and control values were attained for both SOD and GSH.
Table 5.
Effect of in-ovo injection of thiacloprid (TH), chicoric (CA), and rosmarinic acids (RA) on liver antioxidants/oxidative stress biomarkers in the 7-day hatchlings.
| Experimental groups |
||||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Control | TH | CA | RA | TH/CA | TH/RA | TH/CA+RA | P-value |
| SOD (U/g) | 142.81 ± 1.22a | 112.57 ± 0.86b | 155.04 ± 3.76a | 153.61 ± 6.42a | 123.39 ± 0.97b | 124.10 ± 3.58b | 144.48 ± 4.41a | <0.001 |
| CAT (U/g) | 5.38 ± 0.08 | 5.36 ± 0.09 | 5.24 ± 0.18 | 5.38 ± 0.12 | 5.37 ± 0.07 | 5.37 ± 0.08 | 5.34 ± 0.16 | 0.986 |
| GSH (nmol/g) | 75.79 ± 0.21ab | 58.66 ± 1.71e | 78.22 ± 0.89a | 78.11 ± 0.31a | 64.02 ± 1.39d | 70.13 ± 0.01c | 73.53 ± 0.82bc | <0.001 |
| MDA (nmol/g) | 2.14 ± 0.1d | 3.62 ± 0.12a | 2.77 ± 0.19c | 2.80 ± 0.12c | 3.47 ± 0.14ab | 3.49 ± 0.14ab | 3.13 ± 0.02bc | <0.001 |
Values are mean ± SE, values not sharing a joint superscript letter (a - e) differ significantly at *P < 0.05.
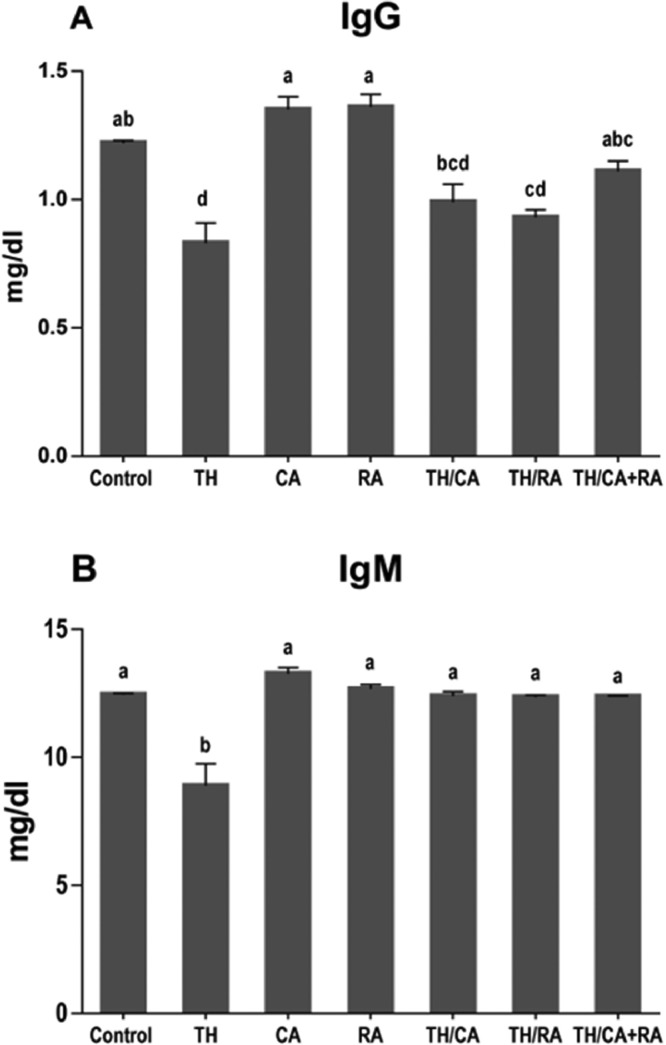
Effects on Serum Immunoglobulins in 7-Day-Old Hatchlings
Figure 1 shows a significant (*P < 0.05) decline in serum IgG and IgM levels in the TH group compared to the control group. TH/CA and TH/CA+RA groups presented significant improvements and restoration of control values. However, TH/RA group showed significant improvement in the IgM but not the IgG level.
Figure 1.
Effects of thiacloprid (TH), chicoric acid (CA), and/or rosmarinic acid (RA) in-ovo exposure on serum IgG (A) and IgM (B) levels in 7-day-old chick embryos. Values are mean ± SEM; bars are not sharing a common superscript letter (a-d) and differ significantly at *P < 0.05.
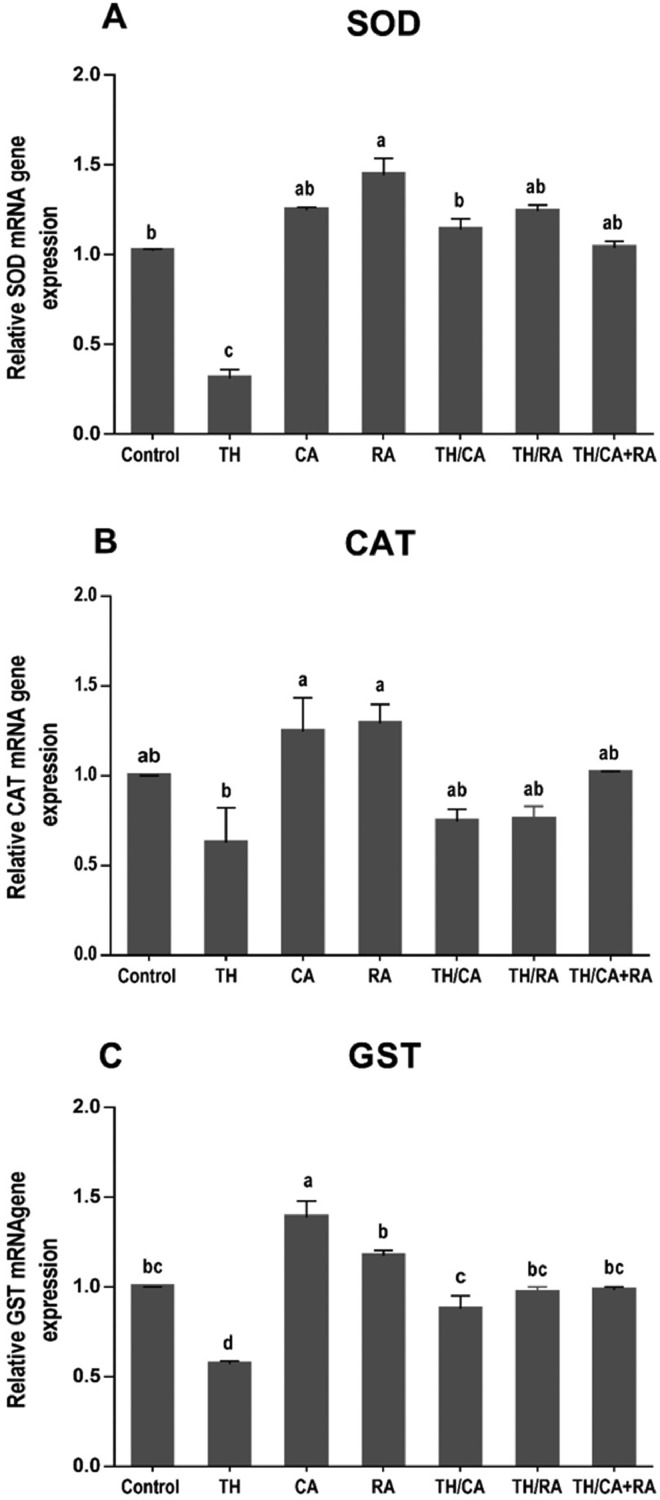
Transcriptional Levels of Antioxidant-Related Genes in the Liver of 7-Day-Old Hatchlings
Exposure to TH significantly (*P < 0.05) downregulated the hepatic expressions of SOD and GST genes relative to the control, while CAT gene expression showed no change. All co-exposed groups showed improved SOD and GST gene expression patterns and restored them to the control values (Figure 2).
Figure 2.
mRNA expression of antioxidant related genes given by qRT- PCR showing the effects of thiacloprid (TH), chicoric acid (CA), and/or rosmarinic acid (RA) in-ovo exposure on the expression of genes SOD (A), CAT (B), and GST‐α (C) in the liver of 7-day-old chick embryos. Values are mean ± SEM, and bars that are not sharing a common superscript letter (a–d) differ significantly at *P < 0.05.
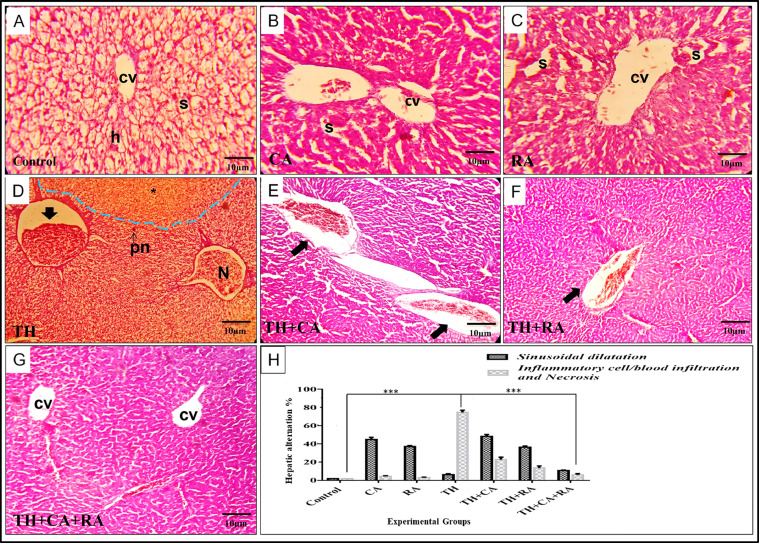
Histopathological Changes in the Liver of 7-Day-Old Hatchlings
Unlike the control liver, which exhibited normal hepatocyte architecture with distinct central vein and sinusoidal spaces (Figure 3A), exposure to CA and RA affected the sinusoidal spaces between hepatic cords. Herein, the dilation is typically characterized by a mottled, reticular architecture that gives a mosaic pattern of the liver and has been so far of unclear significance (Figures 3B and 3C). On the other side, TH pesticide increased the sensitivity of the liver toward inflammation, as central veins appeared dilated and engorged with inflammatory cell infiltration, besides small focal necrosis that was also detected (Figure 3D). In the co-exposure, CA relieved the toxic effect of TH by decreasing the inflammatory cell infiltration in the central vein. However, marked dilation and congestion of central veins were still engorged with blood cell aggregation (Figure 3E). At the same time, RA also reduced the inflammatory effect of TH in the same way as CA (Figure 3F). However, the dual injection of CA and RA with TH induced an ameliorative effect and restored the nearly normal liver structure with normal central veins and hepatic cords. However, mild sinusoidal dilatation and inflammatory cell infiltrations are still observed (Figure 3G). The lesion scoring of treated groups is represented in (Figure 3H).
Figure 3.
Photomicrographs of liver sections stained with H&E showing the histopathological alternations compared with the control group (A) that exhibited normal hepatic architecture with distinct hepatocyte (h), central vein (cv), and hepatic sinusoidal spaces (s). (B) Liver section of chicoric acid (CA) injected group showing moderate congestion in the central vein (cv) and mild sinusoidal dilation (s). (C) Liver sections of rosmarinic acid (RA) injected group showing moderate dilation and congestion of sinusoidal spaces (s) and congestion of hepatic parenchyma. (D) Liver section of thiacloprid (TH) treated group showing loss of hepatic cord architecture (blue dash line) with small focal necrosis (N), pyknotic nuclei (pn), congested central vein with mild fibrous tissue proliferation, and high inflammatory cells infiltrations (black arrow). (E) Liver section of the co-injected group with TH and CA showing dilation of the sinusoid and marked dilation and congestion of the central vein with small blood cell infiltration. (F) Liver section of the co-injected group of TH and RA showing moderate sinusoidal irregularity appearance and mild central vein dilation with little blood cell infiltration (black arrow). (G) Liver section of the group Co-injected with TH+CA+RA showing the near-normal architecture of liver tissue with distinct hepatocyte strands and normal central vein except for moderate sinusoidal dilation(s). (H) Quantification analysis of sinusoidal dilatation, inflammatory cell aggregation, and necrotic areas. Scale magnification is shown in pictures, and data are expressed as mean ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001.
DISCUSSION
The hypothesis that the in-ovo inoculation of antioxidants protects against the toxic effects of pesticides and has positive effects on embryonic development and hatchling growth performance has been supported by data from the current investigation. The findings of this study demonstrated that the in-ovo injection of chicoric (CA) and rosmarinic acids (RA) was beneficial to thiacloprid (TH)-exposed chick embryo at hatch and post-hatch, and the effects of both phytochemicals were synergistic.
Altered gene expression, DNA damage, and increased metabolism upon exposure to the pollutants can affect any aspect of development, including organ masses and embryo morphology (Gallo et al., 2017; Gobeli et al., 2017). This could explain the altered growth of hatchlings exposed to TH during embryonic development. In addition, the retarded growth could be attributed to the reduction in T3, T4, and GH levels recorded in this study.
Additionally, neonics-induced inhibition of food intake has been demonstrated in several birds, which might be associated with anorexia nervosa (Eng et al., 2019; Pan et al., 2022).
By our findings, the developmental delay was observed in chick embryos after in-ovo exposure to TH (Salvaggio et al., 2018). Reduction of feed intake and decrease in weight of hatchlings were also induced by other neonics such as imidacloprid in birds after in ovo exposure ( Hussein and Singh, 2016; Gobeli et al., 2017). TH was also reported to reduce the cell number and increase cell apoptosis in blastocysts of mice and rabbits (Babeľová et al., 2017) and decrease the body weight, weight gain, and food consumption in the rat and mice progeny after in-utero exposure (Hartman et al., 2021).
Moreover, Velisek and Stara documented that TH delayed the development and lowered the growth rate of common carp in the early life stages (Velisek and Stara, 2018). Interestingly, upon the co-administration of CA and RA with TH, a significant improvement in the body weight/egg weight in the one-day-old hatchling and a non-significant one in the daily weight gain and feed intake in 7-day-old chicks were noticed.
The protective effects of the phytochemicals seem to be complementary, as they were significant and more evident in the TH/CA+RA group. These findings indicate that both CA and RA are beneficial for improving the growth of broiler chickens at hatch and post-hatch. By our data, Saeed et al. (2018) and Shen et al. (2020) demonstrated that E. purpurea extract (containing CA as a principal constituent) promoted growth and improved meat quality in the broilers. Furthermore, RA restored normal growth and development in zebrafish embryos exposed to food colors (sunset yellow and crimson red) through its effect on aurora kinase A (Swarnalatha et al., 2017). In addition, rosemary-supplemented feed positively impacted body weight gain, FCR, deposition of fat, and cecal microbiological composition in the broilers (Petricevic et al., 2018; Farouk et al., 2022; Mawed et al., 2022). The growth-promoting effects of both phytochemicals may be attributed to the enhancement of the antioxidant activity and proliferation of the gut flora.
Environmental chemicals and pollutants may interfere with the functions of the thyroid gland via different mechanisms. They can act at the receptor level in binding to transport protein, cellular uptake mechanism, or in modifying the metabolism of thyroid hormones (Lacasaña et al., 2010). Thyroid hormones play essential functions in normal brain development, physiological status, organ functions, and control of metabolism in vertebrates (Tebourbi et al., 2010; Di Cerbo et al., 2017; Sechi et al., 2017). Endocrine disrupters can block right signals or send wrong signals, thereby throwing off the system. Therefore, endocrine disruptions can adversely affect function, morphology, behavior, and immune response in addition to specific malignancies (Du et al., 2010).
Results of the current work revealed that exposure of embryos to TH during their development induced significant decreases in both plasma T3 and T4 levels in hatchlings, suggesting the potential of TH to cause endocrine disruption. In agreement with our results, the endocrine-disrupting activity was recorded in the imidacloprid-treated wildlife bird Amandava amandava, showing reduced plasma T4 and T3 levels. In contrast, plasma TSH increased with altered thyroid weight and histology (Pandey and Mohanty, 2017). Similarly, thiamethoxam decreased body length and plasma T4 in the Chinese rare minnow fish (Zhu, et al., 2019). Furthermore, dinotefuran, thiamethoxam, and imidacloprid disrupted the hypothalamic–pituitary–thyroid (HPT) axis in treated lizards (Wang, et al., 2020d). Thyroid hormone levels may be reduced due to suppressed iodine intake and thyroid peroxidase activity in the epithelial cells (Miller et al., 2009).
Exposure to pesticides may (directly or indirectly) impair the hypothalamic TRH neurons with subsequent disruption of the HPT axis (Pandey and Mohanty, 2015). Herein, our findings revealed that in ovo, exposure to TH decreased the plasma growth hormone level in hatchlings, confirming the growth-inhibitory potential of TH.
Similar to our results, dinotefuran exposure reduced the growth hormone concentration in plasma and the ghr, igfbp2, and igf1 gene expressions in the lizard's (Wang et al., 2020d). The TH-endocrine disrupting effect could result from the increased generation of ROS as a response to TH exposure. It is well known that free radicals, besides their harmful impact on cellular macromolecules, can result in various pathological conditions and progressive deterioration in most endocrine functions (Kour and Bani, 2011a).
On the other hand, CA and RA, particularly in combination treatment, could reverse the adverse effects of TH on the hormonal profile of hatchlings, supporting their synergistic protective potential. RA exhibited a thyrotoprotective activity in rats treated with di-2-Ethylhexyl phthalate (DEHP, where it attenuated thyrocyte death and suppressed pro-inflammatory cytokine production and inflammasome activation (Wu et al., 2020).
The thyrotoprotective protective effect of CA and RA could be attributed to their antioxidant and anti-inflammatory properties.
Regarding the effects of in-ovo exposure to TH on blood biochemistry, significant elevations were observed in the serum activities of AST and ALT and the level of TC. In the same context, our data showed histopathological alterations in the liver of hatchlings.
In agreement with our data, elevated transaminase activities in response to TH treatment were previously demonstrated in rats ( Hendawi et al., 2016; Abou-Zeid et al., 2021). Moreover, the in-ovo treatment of chorioallantoic membranes of chicken embryos with imidacloprid elevated serum ALT, AST, and cholesterol levels (Khandia et al., 2020). Similarly, the administration of imidacloprid to white leghorn chicks has elevated the activities of ALT, AST, and ALP enzymes (Balani et al., 2008). The elevated ALT and AST activities reflect liver damage causing enzyme leakage into the bloodstream (Whalan, 2015; Di Cerbo et al., 2018). The increased blood cholesterol is in line with the recorded hypothyroidism and may also reflect liver damage, cholestasis, and renal affection (Öner et al., 2008).
Notably, in combined exposure groups (TH/CA, TH/RA, and TH/CA+RA), significant improvements were recorded in serum biochemical markers. In addition, the hepatic histopathological alterations were mitigated upon CA and RA co-administration with TH.
Consistent with our results, CA protected hepatic cells in vitro against polyinosinic-polycytidylic acid, evidenced by reduced LDH leakage (Tráj et al., 2022). Moreover, CA mitigated the function of liver enzymes (AST, ALT, and ALP) and the histological alterations induced by methotrexate in rats (Hussein et al., 2020) and by LPS in mice (Li et al., 2020). RA showed a hepatoprotective potential represented by improving the serum AST and ALT activities and the liver histopathological alterations in tert-butyl hydroperoxide-treated rats (Yang et al., 2013) and mice treated with Concanavalin A (Wang et al., 2020c) or acetaminophen (Yao et al., 2022). In addition, it alleviated total cholesterol and triglyceride levels in estrogen-deficient rats (Zych et al., 2019).
The hepatoprotective effect seems to be mediated through the antioxidant and anti-inflammatory properties, as CA and RA restrained the MAPKs and NF-κB and enhanced the Nrf2 pathway (Scarano et al., 2017; Ding et al., 2019; Li et al., 2020).
The redox balance is considered a major determinant of hepatocyte functions; therefore, we evaluated the oxidant/antioxidant biomarkers in the liver of hatchlings after in ovo TH exposure. Our results revealed increased hepatic MDA levels with reduced SOD activity and GSH level, reflecting oxidative stress. In addition, downregulation of the liver SOD and GST gene expression patterns was recorded.
Elevation of MDA indicates high rates of lipid peroxidation in response to the overgeneration of the ROS (Nordberg and Arnér, 2001). Non-enzymatic antioxidants like GSH and antioxidant enzymes like CAT and SOD are responsible for the cellular defense against oxidative stress.
Depletion of liver GSH may reflect the failure of the capacity of antioxidant systems to neutralize the ROS oxidizing effect. Inhibition of hepatic SOD may reflect increased enzyme utilization in detoxicating the superoxide radical. This may result from the downregulation of translation and transcription processes, substrate depletion, and direct toxic impact on the enzyme by the free radicals (Yonar and Sakin, 2011). Our data agree with previous reports demonstrating hepatotoxic oxidative stress in response to TH-exposure. (Hendawi et al., 2016; Kammoun et al., 2019; Abou-Zeid et al., 2021).
Imidacloprid was also reported to induce a similar effect in songbird grayish baywing (Poliserpi et al., 2021) and rock pigeon (Zeid et al., 2019). Furthermore, Thiamethoxam produced oxidative stress-mediated hepatotoxicity in the Japanese quail (Pan et al., 2022).
Notably, co-inoculation of either CA or RA with TH could not improve the SOD activity and MDA level but the GSH level. In the TH/CA+RA group, the protective effect was more ore marked, where SOD and GSH were replenished in addition to alleviation of MDA increase, and control values were attained for both SOD and GSH. These data reflect a commentary antioxidant effect for both phytochemicals, making them promising for future applications in preventive and therapeutic strategies to slow the progression and exacerbation of oxidative stress.
In agreement with our data, CA ameliorated the oxidative damage and enhanced the antioxidant capacity in the liver of rats poisoned with methotrexate (Hussein et al., 2020) and in HepG2 cells treated with H2O2 and larval zebrafish model (Ma et al., 2018). Moreover, CA ameliorated the oxidative stress induced by TH in the brain of hatchlings (Farag et al., 2022b) and lungs of LPS-treated mice (Ding et al., 2019). The antioxidant activity of CA can be returned to its efficient free radical scavenging potential (Zhu et al., 2018) and via the activation of the Keap1/Nrf2 and HO-1 transcriptional pathways responsible for the expression of antioxidant proteins (Ma et al., 2018).
In several reports, RA has been shown to decline lipid peroxidation and to enhance the enzymatic and non-enzymatic antioxidants in the hepatic tissues of rats intoxicated with chromium (Khalaf et al., 2020), tert-butyl hydroperoxide (Yang et al., 2013), and mice treated with acetaminophen (Yu et al., 2021) and LPS (Osakabe et al., 2002). Similar action was recorded in vitro in rat liver cells (BRL-3A) treated with acrylamide (Hong et al., 2021).
The antioxidant property of RA could be returned to its ability to detoxicate a wide variety of free radicals demonstrated by different assays, including inhibition of linoleic acid peroxidation, ABTS•+, O2•, DPPH•, and DMPD•+ scavenging activity, Cu2+ and Fe3+ reducing, and Fe2+ chelating assays (Topal and Gulcin, 2022). It is suggested to be mediated through the upregulation of the Nrf2 signaling (Khalaf et al., 2020) and RACK1/TNF-α pathways (Iannitti et al., 2020; Yu et al., 2021).
Significant decline in serum IgG and IgM levels and relative weight of bursa of Fabricius were recorded in the TH-exposed group, reflecting affection of the humoral immune response. This action may result from the influence on B lymphocytes, T helper cells, or the antigen-presenting cells (APCs) (Ladics, 2005) secondary to oxidative stress, which may affect the proliferation of lymphocytes and co-operations between B and T cells required for the production of antibodies, and activation and signaling of B cell receptors (Tarazona et al., 2002). By our data, Abou-Zeid et al.(2002) demonstrated that TH inhibited the humoral immunity variables in rats. Moreover, imidacloprid suppressed antibodies against total immunoglobulins Newcastle disease vaccine and circulating immune complexes in broiler chicks (Kammon et al., 2012), and T-cell immune response in red-legged partridges (Lopez-Antia et al., 2015). Similarly, thiamethoxam inhibited both humoral and cellular immunity in domestic chickens (toxin reviews). Furthermore, imidacloprid adversely affected the developing immunity of rat pups exposed in utero, where the antibody titer and serum immunoglobulin levels were reduced (Gawade et al., 2013).
Significant improvements in IgM and IgG levels were noticed in the TH/CA and TH/CA+RA groups, while TH/RA group showed improvement in the IgM but not IgG level. This demonstrates that CA showed a more immunoprotective effect compared to RA. In addition, co-administration of either phytochemical with TH improved the relative weight of the bursa. This suggests that the immune components implicated in antibody production and the bursa development in hatchlings benefited from the antioxidant properties of CA and RA.
In agreement with our findings, (Kour and Bani, 2011b) demonstrated that CA restored the suppressed Th1/Th2 homeostasis and immune response in mice with chronic restraint stress and reversed the atrophy of the thymus and spleen. Furthermore, Echinacea purpurea extract enhanced the antibody titers in the infectious bursal (Ma et al., 2009) and Newcastle diseases in the broilers (Gurbuz et al., 2010).
Regarding RA, it doubled the monoclonal antibody titer in the CHO DG44 cell line culture (Xu et al., 2020). Farouk et al. (2022) declared that broilers infected with E. coli showed an increase in IgG and a decline in Il-6 level when fed on a rosemary-supplemented feed. Also, Al Sheyab et al. (2012) demonstrated that rosemary extract increased the IgM and IgG levels in mice.
The immunostimulant potential of CA and RA seems to be mediated through their antioxidant activities, as the antioxidant status was demonstrated to be a major determinant of the function of immune cells (Fuente et al., 2005). In addition, the enhancement of immunity by CA was reported to be due to increasing lymphocyte proliferation and upregulating the expressions of CD28 and CD80, downregulating CTLA-4, improving the leukotriene production (Kour and Bani, 2011b), and complement activation via the P-factor production (Hartwich, 2011).
Herein, examining the liver of hatchlings exposed in ovo to TH revealed histopathological alterations mainly in the form of inflammatory cell infiltration and necrosis, which might be secondary to oxidative damage and inflammatory responses in the hepatic tissues (Alarcan, et al., 2020; Abou-Zeid et al., 2021). Our results align with Salvaggio et al. (2018), wherein in ovo injection of TH to Gallus domesticus caused liver hemorrhages and steatosis. Goyal et al. (2010) reported that oral administration of TH to Gallus domesticus caused liver fatty changes, congestion, and degeneration of hepatocytes. Furthermore, other neonics were demonstrated to induce histopathological alterations in the liver of birds exposed in ovo (Khandia et al., 2020) or orally (Zeid et al., 2019; Pan et al., 2022). The histopathological alterations were alleviated when CA and RA were inoculated with TH, probably via inhibition of inflammation and necrotic development; however, the damaged blood cells that are still inside the central veins and small area of hemorrhages are due to the active immune response and phagocytic action of Kupffer cells (Lee et al., 2015; Mushtaq et al., 2015; Rahbardar et al., 2017; Elufioye and Habtemariam, 2019; Hussein et al., 2020; Luo et al., 2020). The protective effect was more evident in the group that received both phytochemicals together, reflecting synergistic hepatoprotective potential. Dilated hepatic sinusoids after CA and RA injection result from obstruction of hepatic venous outflows, which leads to vascular stasis and congestions of the hepatic parenchyma (Kakar et al., 2004).
The congestion of hepatic parenchyma results from the proliferative and anti-apoptotic effect of the chicoric acid (Wang et al., 2020a).
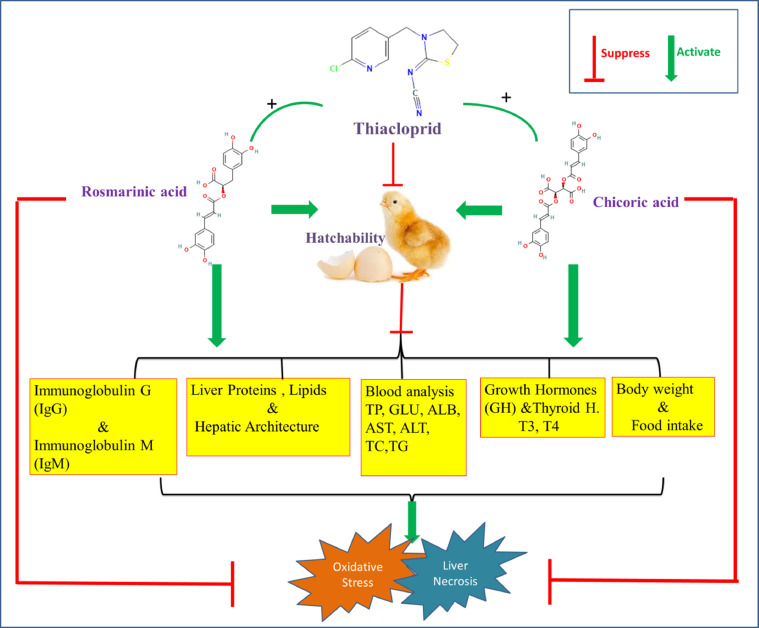
CONCLUSIONS
Overall, our data support a hypothesis that co-administration of RA or CA with TH in ovo mitigated the toxic impacts on growth performance and liver with augmentation of serum immunoglobulins, at least partially via the antioxidant and thryoprotective effects of both phytochemicals. Dual administration of CA and RA noteworthy augmented the protective effect. These data suggest that in ovo, inoculation of CA and RA positively affects the general health and productivity of poultry (Figure 4).
Figure 4.
A schematic diagram represents the possible effects of the three chemicals on the chick hatchability, body performance, and physiological parameters and the synergetic co-administration (+) of RA and CA with TH in-ovo could mitigate the toxic impacts of TH on the growth performance.
Acknowledgments
This work was supported by Researcher Supporting Project Number (RSPD2023R731), King Saud University (Riyadh, Saudi Arabia).
DISCLOSURES
There was no conflict of interest
REFERENCES
- Abou-Zeid S.M., Aljuaydi S.H., AbuBakr H.O., Tahoun E.A., Di Cerbo A., Alagawany M., Khalil S.R., Farag M.R. Astaxanthin mitigates thiacloprid-induced liver injury and immunotoxicity in male rats. Marine Drugs. 2021;19:525. doi: 10.3390/md19090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebi, H. 1984. Catalase in vitro. In Packer, L., (Ed.), Methods in Enzymology, Academic Press, San Diego, 105:121–126. [DOI] [PubMed]
- Al Sheyab F.M., Abuharfeil N., Salloum L., Hani R.B., Awad D.S. The effect of rosemary (Rosmarinus officinalis. L) plant extracts on the immune response and lipid profile in mice. J. Biol. Life Sci. 2012;3:26–29. [Google Scholar]
- Alagawany M., Abd El-Hack M.E., El-Kholy M.S. Productive performance, egg quality, blood constituents, immune functions, and antioxidant parameters in laying hens fed diets with different levels of Yucca schidigera extract. Environ. Sci. Pollut. Res. 2016;23:6774–6782. doi: 10.1007/s11356-015-5919-z. [DOI] [PubMed] [Google Scholar]
- Alagawany M., Abd El-Hack M.E., Farag M.R., Gopi M., Karthik K., Malik Y.S., Dhama K. Rosmarinic acid: Modes of action, medicinal values and health benefits. Anim. Health Res. Rev. 2017;18:167–176. doi: 10.1017/S1466252317000081. [DOI] [PubMed] [Google Scholar]
- Alagawany M., Farag M.R., Dhama K. Nutritional and biological effects of turmeric (Curcuma longa) supplementation on performance, serum biochemical parameters and oxidative status of broiler chicks exposed to endosulfan in the diets. Asian J. Anim. Sci. Vet. Adv. 2015;10:86–96. [Google Scholar]
- Alagawany M., Farag M.R. In Ovo Techniques and Treatments in Poultry Eggs. SERVET Publisher. Grupo Asís Biomedia S.L.; Zaragoza, Spain: 2022. ISBN 9788418498992. [Google Scholar]
- Alarcan J., Waizenegger J., Solano M.d.L.M., Lichtenstein D., Luckert C., Peijnenburg A., Stoopen G., Sharma R.P., Kumar V., Marx-Stoelting P. Hepatotoxicity of the pesticides imazalil, thiacloprid and clothianidin–individual and mixture effects in a 28-day study in female Wistar rats. Food Chem. Toxicol. 2020;140 doi: 10.1016/j.fct.2020.111306. [DOI] [PubMed] [Google Scholar]
- Arain M.A., Nabi F., Shah Q.A., Alagawany M., Fazlani S.A., Khalid M., Soomro F., Khand F.M., Farag M.R. The role of early feeding in improving performance and health of poultry: herbs and their derivatives. Worlds Poult. Sci. J. 2022;78:499–513. [Google Scholar]
- Babeľová J., Šefčíková Z., Čikoš Š., Špirková A., Kovaříková V., Koppel J., Makarevich A.V., Chrenek P., Fabian D. Exposure to neonicotinoid insecticides induces embryotoxicity in mice and rabbits. Toxicology. 2017;392:71–80. doi: 10.1016/j.tox.2017.10.011. [DOI] [PubMed] [Google Scholar]
- Balani T., Agrawal S., Thaker A. Effects of imidacloprid a neonicotinoid insecticide on the immune system of white leghorn cockerels. J. Vet. Pharm. Toxicol. 2008;7:27–30. [Google Scholar]
- Beutler E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- Di Cerbo A., Canello S., Guidetti G., Fiore F., Corsi L., Rubattu N., Testa C., Cocco R. Adverse food reactions in dogs due to antibiotic residues in pet food: a preliminary study. Vet. Ital. 2018;54:137–146. doi: 10.12834/VetIt.1357.7466.2. [DOI] [PubMed] [Google Scholar]
- Di Cerbo A., Sechi S., Canello S., Guidetti G., Fiore F., Cocco R. Behavioral disturbances: an innovative approach to monitor the modulatory effects of a nutraceutical diet. J. Vis. Exp. 2017;119 doi: 10.3791/54878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H., Ci X., Cheng H., Yu Q., Li D. Chicoric acid alleviates lipopolysaccharide-induced acute lung injury in mice through anti-inflammatory and anti-oxidant activities. Int. Immunopharmacol. 2019;66:169–176. doi: 10.1016/j.intimp.2018.10.042. [DOI] [PubMed] [Google Scholar]
- Du G., Shen O., Sun H., Fei J., Lu C., Song L., Xia Y., Wang S., Wang X. Assessing hormone receptor activities of pyrethroid insecticides and their metabolites in reporter gene assays. Toxicol. Sci. 2010;116:58–66. doi: 10.1093/toxsci/kfq120. [DOI] [PubMed] [Google Scholar]
- Elufioye T.O., Habtemariam S. Hepatoprotective effects of rosmarinic acid: Insight into its mechanisms of action. Biomed. Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.108600. [DOI] [PubMed] [Google Scholar]
- Eng M.L., Stutchbury B.J., Morrissey C.A. A neonicotinoid insecticide reduces fueling and delays migration in songbirds. Science. 2019;365:1177–1180. doi: 10.1126/science.aaw9419. [DOI] [PubMed] [Google Scholar]
- EPA U. Thiacloprid. Pesticide tolerances. Federal Register. 2013;70:8410–8416. [Google Scholar]
- Farag M.R., Alagawany M., Khalil S.R., Abd El-Aziz R.M., Zaglool A.W., Moselhy A.A., Abou-Zeid S.M. Effect of parsley essential oil on digestive enzymes, intestinal morphometry, blood chemistry and stress-related genes in liver of Nile tilapia fish exposed to Bifenthrin. Aquaculture. 2022;546 [Google Scholar]
- Farag M.R., Alagawany M., Moselhy A.A., Said E.N., Ismail T.A., Di Cerbo A., Pugliese N., Ahmed M.M. The neonicotinoid thiacloprid interferes with the development, brain antioxidants, and neurochemistry of chicken embryos and alters the hatchling behavior: modulatory potential of phytochemicals. Biology. 2022;11:73. doi: 10.3390/biology11010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag M.R., Khalil S.R., Zaglool A.W., Hendam B.M., Moustafa A.A., Cocco R., Di Cerbo A., Alagawany M. Thiacloprid induced developmental neurotoxicity via ROS-oxidative injury and inflammation in chicken embryo: the possible attenuating role of chicoric and rosmarinic acids. Biology. 2021;10:1100. doi: 10.3390/biology10111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farouk S.M., Abdel-Rahman H.G., Abdallah O.A., El-Behidy N.G. Comparative immunomodulatory efficacy of rosemary and fenugreek against Escherichia coli infection via suppression of inflammation and oxidative stress in broilers. Environ. Sci. Pollut. Res. 2022;29:40053–40067. doi: 10.1007/s11356-021-18358-6. [DOI] [PubMed] [Google Scholar]
- Franzen-Klein D., Jankowski M., Roy C.L., Nguyen-Phuc H., Chen D., Neuman-Lee L., Redig P., Ponder J. Evaluation of neurobehavioral abnormalities and immunotoxicity in response to oral imidacloprid exposure in domestic chickens (Gallus gallus domesticus) J. Toxicol. Environ. Health, Part A. 2020;83:45–65. doi: 10.1080/15287394.2020.1723154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuente M.D.L., Hernanz A., Vallejo M. The immune system in the oxidative stress conditions of aging and hypertension: favorable effects of antioxidants and physical exercise. Antioxid. Redox Signal. 2005;7:1356–1366. doi: 10.1089/ars.2005.7.1356. [DOI] [PubMed] [Google Scholar]
- Gallo A., Landi R., Rubino V., Di Cerbo A., Giovazzino A., Palatucci A.T., Centenaro S., Guidetti G., Canello S., Cortese L., Ruggiero G., Alessandrini A., Terrazzano G. Oxytetracycline induces DNA damage and epigenetic changes: a possible risk for human and animal health? PeerJ. 2017;5:e3236. doi: 10.7717/peerj.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawade L., Dadarkar S.S., Husain R., Gatne M. A detailed study of developmental immunotoxicity of imidacloprid in Wistar rats. Food Chem. Toxicol. 2013;51:61–70. doi: 10.1016/j.fct.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Gibbons D., Morrissey C., Mineau P. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ. Sci. Pollut. Res. 2015;22:103–118. doi: 10.1007/s11356-014-3180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobeli A., Crossley D., II, Johnson J., Reyna K. The effects of neonicotinoid exposure on embryonic development and organ mass in northern bobwhite quail (Colinus virginianus) Compar. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2017;195:9–15. doi: 10.1016/j.cbpc.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Goyal S., Sandhu H.S., Brar R.S. Histopathological alterations induced after oral sub-acute thiacloprid toxicity in Gallus domesticus. Vet. Arhiv. 2010;80:673–682. [Google Scholar]
- Guan H., Luo W., Bao B., Cao Y., Cheng F., Yu S., Fan Q., Zhang L., Wu Q., Shan M. A comprehensive review of rosmarinic acid: from phytochemistry to pharmacology and its new insight. Molecules. 2022;27:3292. doi: 10.3390/molecules27103292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidetti G., Di Cerbo A., Giovazzino A., Rubino V., Palatucci A.T., Centenaro S., Fraccaroli E., Cortese L., Bonomo M.G., Ruggiero G., Canello S., Terrazzano G. In vitro effects of some botanicals with anti-inflammatory and antitoxic activity. J. Immunol. Res. 2016;2016 doi: 10.1155/2016/5457010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gul S.T., Khan A., Ahmad M., Ahmad H., Saleemi M.K., Naseem M.N., Bilal M. Immuno-toxicological effects of different sub-lethal doses of thiamethoxam (TMX) in broiler birds. Toxin Rev. 2018;38:200–205. [Google Scholar]
- Gurbuz E., Balevi T., Kurtoglu V., Coskun B., Oznurlu Y., Kan Y., Kartal M. Effects of Echinacea extract on the performance, antibody titres, and intestinal histology of layer chicks. Br. Poult. Sci. 2010;51:805–810. doi: 10.1080/00071668.2010.528753. [DOI] [PubMed] [Google Scholar]
- Han W., Tian Y., Shen X. Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: an overview. Chemosphere. 2018;192:59–65. doi: 10.1016/j.chemosphere.2017.10.149. [DOI] [PubMed] [Google Scholar]
- Hartman C., Legoff L., Capriati M., Lecuyer G., Kernanec P.-Y., Tevosian S., D’Cruz S.C., Smagulova F. Epigenetic effects promoted by neonicotinoid thiacloprid exposure. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.691060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwich M. The importance of immunological studies on Rhodiola rosea in the new effective and safe herbal drug discovery. Cent. Eur. J. Immunol. 2011;35:263–266. [Google Scholar]
- Hendawi M.Y., Alam R., Abdellatief S.A. Ameliorative effect of flaxseed oil against thiacloprid-induced toxicity in rats: hematological, biochemical, and histopathological study. Environ. Sci. Pollut. Res. 2016;23:11855–11863. doi: 10.1007/s11356-016-6376-z. [DOI] [PubMed] [Google Scholar]
- Hong Z., Minghua W., Bo N., Chaoyue Y., Haiyang Y., Haiqing Y., Chunyu X., Yan Z., Yuan Y. Rosmarinic acid attenuates acrylamide induced apoptosis of BRL-3A cells by inhibiting oxidative stress and endoplasmic reticulum stress. Food Chem. Toxicol. 2021;151 doi: 10.1016/j.fct.2021.112156. [DOI] [PubMed] [Google Scholar]
- Hussein M., Singh V. Effect on chick embryos development after exposure to neonicotinoid insecticide imidacloprid. J. Anat. Soc. India. 2016;65:83–89. [Google Scholar]
- Hussein O.E., Hozayen W.G., Bin-Jumah M.N., Germoush M.O., El-Twab A., Sanaa M., Mahmoud A.M. Chicoric acid prevents methotrexate hepatotoxicity via attenuation of oxidative stress and inflammation and up-regulation of PPARγ and Nrf2/HO-1 signaling. Environ. Sci. Pollut. Res. 2020;27:20725–20735. doi: 10.1007/s11356-020-08557-y. [DOI] [PubMed] [Google Scholar]
- Iannitti T., Di Cerbo A., Loschi A.R., Rea S., Suzawa M., Morales-Medina J.C. Repeated administration of a flavonoid-based formulated extract from citrus peels significantly reduces peripheral inflammation-induced pain in the rat. Food Sci. Nutr. 2020;8:3173–3180. doi: 10.1002/fsn3.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseppi R., Di Cerbo A., Aloisi P., Manelli M., Pellesi V., Provenzano C., Camellini S., Messi P., Sabia C. In vitro activity of essential oils against planktonic and biofilm cells of extended-spectrum beta-lactamase (ESBL)/carbapenamase-producing gram-negative bacteria involved in human nosocomial infections. Antibiotics (Basel) 2020;9 doi: 10.3390/antibiotics9050272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke P., Nauen R., Schindler M., Elbert A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2011;59:2897–2908. doi: 10.1021/jf101303g. [DOI] [PubMed] [Google Scholar]
- Kakar S., Kamath P.S., Burgart L.J. Sinusoidal dilatation and congestion in liver biopsy: is it always due to venous outflow impairment? Arch. Pathol. Lab. Med. 2004;128:901–904. doi: 10.5858/2004-128-901-SDACIL. [DOI] [PubMed] [Google Scholar]
- Kammon A., Brar R., Banga H., Sodhi S. Ameliorating effects of vitamin E and selenium on immunological alterations induced by imidacloprid chronic toxicity in chickens. J. Environ. Anal. Toxicol. S. 2012;4 2161-0525. [Google Scholar]
- Kammoun I., Sellem I., Ben Saad H., Boudawara T., Nasri M., Gharsallah N., Mallouli L., Amara I.B. Potential benefits of polysaccharides derived from marine alga Ulva lactuca against hepatotoxicity and nephrotoxicity induced by thiacloprid, an insecticide pollutant. Environ. Toxicol. 2019;34:1165–1176. doi: 10.1002/tox.22818. [DOI] [PubMed] [Google Scholar]
- Khalaf A.A., Hassanen E.I., Ibrahim M.A., Tohamy A.F., Aboseada M.A., Hassan H.M., Zaki A.R. Rosmarinic acid attenuates chromium-induced hepatic and renal oxidative damage and DNA damage in rats. J. Biochem. Mol. Toxicol. 2020;34:e22579. doi: 10.1002/jbt.22579. [DOI] [PubMed] [Google Scholar]
- Khandia R., Pathe C.S., Vishwakarma P., Dhama K., Munjal A. Evaluation of the ameliorative effects of Phyllanthus niruri on the deleterious insecticide imidacloprid in the vital organs of chicken embryos. J. Ayurv. Integ. Med. 2020;11:495–501. doi: 10.1016/j.jaim.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kour K., Bani S. Augmentation of immune response by chicoric acid through the modulation of CD28/CTLA-4 and Th1 pathway in chronically stressed mice. Neuropharmacology. 2011;60:852–860. doi: 10.1016/j.neuropharm.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Kour K., Bani S. Chicoric acid regulates behavioral and biochemical alterations induced by chronic stress in experimental Swiss albino mice. Pharmacol. Biochem. Behav. 2011;99:342–348. doi: 10.1016/j.pbb.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Lacasaña M., López-Flores I., Rodríguez-Barranco M., Aguilar-Garduño C., Blanco-Muñoz J., Pérez-Méndez O., Gamboa R., Bassol S., Cebrian M.E. Association between organophosphate pesticides exposure and thyroid hormones in floriculture workers. Toxicol. Appl. Pharmacol. 2010;243:19–26. doi: 10.1016/j.taap.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Ladics G. In: Encyclopedic Reference of Immunotoxicology. Vohr HW, editor. Springer; New York, NY: 2005. Assays for antibody production; pp. 50–53. Ed. [Google Scholar]
- Lee J., Scagel C.F. Chicoric acid: chemistry, distribution, and production. Front. Chem. 2013;1:40. doi: 10.3389/fchem.2013.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N.Y., Chung K.-S., Jin J.S., Bang K.S., Eom Y.-J., Hong C.-H., Nugroho A., Park H.-J., An H.-J. Effect of chicoric acid on mast cell-mediated allergic inflammation in vitro and in vivo. J. Nat. Prod. 2015;78:2956–2962. doi: 10.1021/acs.jnatprod.5b00668. [DOI] [PubMed] [Google Scholar]
- Li Z., Feng H., Han L., Ding L., Shen B., Tian Y., Zhao L., Jin M., Wang Q., Qin H. Chicoric acid ameliorate inflammation and oxidative stress in lipopolysaccharide and d-galactosamine induced acute liver injury. J. Cell. Mol. Med. 2020;24:3022–3033. doi: 10.1111/jcmm.14935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lopez-Antia A., Ortiz-Santaliestra M.E., Mougeot F., Mateo R. Imidacloprid-treated seed ingestion has lethal effect on adult partridges and reduces both breeding investment and offspring immunity. Environ. Res. 2015;136:97–107. doi: 10.1016/j.envres.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Luo C., Zou L., Sun H., Peng J., Gao C., Bao L., Ji R., Jin Y., Sun S. A review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front. Pharmacoll. 2020;11:153. doi: 10.3389/fphar.2020.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma A., Shi W., Niu X., Wang M., Zhong X. Effects of Echinacea purpurea extract on the immunological response to infectious bursal disease vaccine in broilers. Front. Agric. China. 2009;3:452–456. [Google Scholar]
- Ma J., Li M., Kalavagunta P.K., Li J., He Q., Zhang Y., Ahmad O., Yin H., Wang T., Shang J. Protective effects of cichoric acid on H2O2-induced oxidative injury in hepatocytes and larval zebrafish models. Biomed. Pharmacother. 2018;104:679–685. doi: 10.1016/j.biopha.2018.05.081. [DOI] [PubMed] [Google Scholar]
- MacAulay C., Keyes M., Hayes M., Lo A., Wang G., Guillaud M., Gleave M., Fazli L., Korbelik J., Collins C. Quantification of large scale DNA organization for predicting prostate cancer recurrence. Cytometry Part A. 2017;91:1164–1174. doi: 10.1002/cyto.a.23287. [DOI] [PubMed] [Google Scholar]
- Mawed S.A., Marini C., Alagawany M., Farag M.R., Reda R.M., El-Saadony M.T., Elhady W.M., Magi G.E., Di Cerbo A., El-Nagar W.G. Zinc oxide nanoparticles (ZnO-NPs) suppress fertility by activating autophagy, apoptosis, and oxidative stress in the developing oocytes of female zebrafish. Antioxidants (Basel) 2022;11 doi: 10.3390/antiox11081567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNabb F.A. The hypothalamic-pituitary-thyroid (HPT) axis in birds and its role in bird development and reproduction. Crit. Rev. Toxicol. 2007;37:163–193. doi: 10.1080/10408440601123552. [DOI] [PubMed] [Google Scholar]
- Miller M.D., Crofton K.M., Rice D.C., Zoeller R.T. Thyroid-disrupting chemicals: interpreting upstream biomarkers of adverse outcomes. Environ. Health Perspect. 2009;117:1033–1041. doi: 10.1289/ehp.0800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq N., Schmatz R., Ahmed M., Pereira L.B., da Costa P., Reichert K.P., Dalenogare D., Pelinson L.P., Vieira J.M., Stefanello N. Protective effect of rosmarinic acid against oxidative stress biomarkers in liver and kidney of strepotozotocin-induced diabetic rats. J. Physiol. Biochem. 2015;71:743–751. doi: 10.1007/s13105-015-0438-4. [DOI] [PubMed] [Google Scholar]
- Nadeem M., Imran M., Aslam Gondal T., Imran A., Shahbaz M., Muhammad Amir R., Wasim Sajid M., Batool Qaisrani T., Atif M., Hussain G. Therapeutic potential of rosmarinic acid: a comprehensive review. Appl. Sci. 2019;9:3139. [Google Scholar]
- Nishikimi M., Rao N.A., Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. and Biophys. Res. Commun. 1972;46:849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- Nordberg J., Arnér E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Rad. Biol. Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Annal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Öner M., Atli G., Canli M. Changes in serum biochemical parameters of freshwater fish Oreochromis niloticus following prolonged metal (Ag, Cd, Cr, Cu, Zn) exposures. Environ. Toxicol. Chem. Int. J. 2008;27:360–366. doi: 10.1897/07-281R.1. [DOI] [PubMed] [Google Scholar]
- Osakabe N., Yasuda A., Natsume M., Sanbongi C., Kato Y., Osawa T., Yoshikawa T. Rosmarinic acid, a major polyphenolic component of Perilla frutescens, reduces lipopolysaccharide (LPS)-induced liver injury in D-galactosamine (D-GalN)-sensitized mice. Free Rad. Biol. Med. 2002;33:798–806. doi: 10.1016/s0891-5849(02)00970-x. [DOI] [PubMed] [Google Scholar]
- Pan Y., Chang J., Wan B., Liu Z., Yang L., Xie Y., Hao W., Li J., Xu P. Integrative analysis of transcriptomics and metabolomics reveals the hepatotoxic mechanism of thiamethoxam on male Coturnix japonica. Environ. Pollut. 2022;293 doi: 10.1016/j.envpol.2021.118460. [DOI] [PubMed] [Google Scholar]
- Pandey S.P., Mohanty B. The neonicotinoid pesticide imidacloprid and the dithiocarbamate fungicide mancozeb disrupt the pituitary–thyroid axis of a wildlife bird. Chemosphere. 2015;122:227–234. doi: 10.1016/j.chemosphere.2014.11.061. [DOI] [PubMed] [Google Scholar]
- Pandey S.P., Mohanty B. Disruption of the hypothalamic-pituitary-thyroid axis on co-exposures to dithiocarbamate and neonicotinoid pesticides: Study in a wildlife bird, Amandava amandava. Neurotoxicology. 2017;60:16–22. doi: 10.1016/j.neuro.2017.02.010. [DOI] [PubMed] [Google Scholar]
- Petricevic V., Lukic M., Skrbic Z., Rakonjac S., Doskovic V., Petricevic M., Stanojkovic A. The effect of using rosemary (Rosmarinus officinalis) in broiler nutrition on production parameters, slaughter characteristics, and gut microbiological population. Turkish J. Vet. Anim. Sci. 2018;42:658–664. [Google Scholar]
- Poliserpi M.B., Cristos D., Pérez-Iglesias J.M., Brodeur J.C. Tissue distribution and sublethal effects of imidacloprid in the South American grayish baywing (Agelaioides badius) Chemosphere. 2021;284 doi: 10.1016/j.chemosphere.2021.131327. [DOI] [PubMed] [Google Scholar]
- Rahbardar M.G., Amin B., Mehri S., Mirnajafi-Zadeh S.J., Hosseinzadeh H. Anti-inflammatory effects of ethanolic extract of Rosmarinus officinalis L. and rosmarinic acid in a rat model of neuropathic pain. Biomed. Pharmacother. 2017;86:441–449. doi: 10.1016/j.biopha.2016.12.049. [DOI] [PubMed] [Google Scholar]
- Rowland J. Office of Pesticide Programs; Washington, DC: 2006. Chemicals Evaluated for Carcinogenic Potential by the Office of Pesticide Programs. [Google Scholar]
- Saeed M., Babazadeh D., Arain M., Naveed M., Shah Q., Kamboh A., Moshaveri A., Modarresi-Ghazani F., Hejazi V., Chao S. The use of chicoric acid from Echinacea purpurea as a feed additive in poultry nutrition. Worlds Poult. Sci. J. 2018;74:69–78. [Google Scholar]
- Salvaggio A., Antoci F., Messina A., Ferrante M., Copat C., Ruberto C., Scalisi E.M., Pecoraro R., Brundo M.V. Teratogenic effects of the neonicotinoid thiacloprid on chick embryos (Gallus gallus domesticus) Food Chem. Toxicol. 2018;118:812–820. doi: 10.1016/j.fct.2018.06.026. [DOI] [PubMed] [Google Scholar]
- Scarano A., Murmura G., Vantaggiato G., Lauritano D., Silvestre-Rangil J., A D.I.C., Lorusso F. Delayed expansion of atrophic mandible (deam): a case report. Oral Implantol (Rome) 2017;10:190–196. doi: 10.11138/orl/2017.10.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechi S., Di Cerbo A., Canello S., Guidetti G., Chiavolelli F., Fiore F., Cocco R. Effects in dogs with behavioural disorders of a commercial nutraceutical diet on stress and neuroendocrine parameters. Vet Rec. 2017;180:18. doi: 10.1136/vr.103865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C., Li S., Cai Z., Man R., Wang X. Effect of echinacea purpurea extract given in drinking water on performance, slaughter variables, and meat quality of broilers. ES Food Agroforestry. 2020;2:42–49. [Google Scholar]
- Smith S.M., Flentke G.R., Garic A. Avian models in teratology and developmental toxicology. Dev Toxicol. 2012:85–103. doi: 10.1007/978-1-61779-867-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surai P.F. Antioxidant systems in poultry biology: superoxide dismutase. J. Anim. Res. Nutrit. 2015;1:1–8. [Google Scholar]
- Suvarna K.S., Layton C., Bancroft J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book. Elsevier health sciences; Amsterdam, Netherlands: 2018. [Google Scholar]
- Swarnalatha Y., Joseph I.J., Jayakrishna T. Rosmarinic acid plays a protective role in the embryogenesis of zebrafish exposed to food colours through its influence on aurora kinase A level. Biomed. Pharmacother. 2017;89:1166–1171. doi: 10.1016/j.biopha.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Tarazona R., Solana R., Ouyang Q., Pawelec G. Basic biology and clinical impact of immunosenescence. Exp. Gerontol. 2002;37:183–189. doi: 10.1016/s0531-5565(01)00182-6. [DOI] [PubMed] [Google Scholar]
- Tebourbi O., Hallègue D., Yacoubi M.T., Sakly M., Rhouma K.B. Subacute toxicity of p, p′-DDT on rat thyroid: hormonal and histopathological changes. Environ. Toxicol. Pharmacol. 2010;29:271–279. doi: 10.1016/j.etap.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Thompson D.A., Lehmler H.-J., Kolpin D.W., Hladik M.L., Vargo J.D., Schilling K.E., LeFevre G.H., Peeples T.L., Poch M.C., LaDuca L.E. A critical review on the potential impacts of neonicotinoid insecticide use: current knowledge of environmental fate, toxicity, and implications for human health. Environ. Sci. Processes Impacts. 2020;22:1315–1346. doi: 10.1039/c9em00586b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topal M., Gulcin İ. Evaluation of the in vitro antioxidant, antidiabetic and anticholinergic properties of rosmarinic acid from rosemary (Rosmarinus officinalis L.) Biocatalysis Agric. Biotechnol. 2022;43 [Google Scholar]
- Tráj P., Herrmann E.M., Sebők C., Vörösházi J., Mackei M., Gálfi P., Kemény Á., Neogrády Z., Mátis G. Protective effects of chicoric acid on polyinosinic-polycytidylic acid exposed chicken hepatic cell culture mimicking viral damage and inflammation. Vet. Immunol. Immunopathol. 2022;250 doi: 10.1016/j.vetimm.2022.110427. [DOI] [PubMed] [Google Scholar]
- Valenzuela-Grijalva N.V., Pinelli-Saavedra A., Muhlia-Almazan A., Domínguez-Díaz D., González-Ríos H. Dietary inclusion effects of phytochemicals as growth promoters in animal production. J. Animal Sci. Technol. 2017;59:1–17. doi: 10.1186/s40781-017-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velisek J., Stara A. Effect of thiacloprid on early life stages of common carp (Cyprinus carpio) Chemosphere. 2018;194:481–487. doi: 10.1016/j.chemosphere.2017.11.176. [DOI] [PubMed] [Google Scholar]
- Wang M.-j., Raza S.H.A., Wu Q., Xue C.-h., Liu J.-h., Zhang L.-f., Zhang W.-y., Wang A.-c., Wu H. Cichoric acid from extracted Echinacea purpurea induces the proliferation and apoptosis of peripheral blood mononuclear cells from yaks. Electron. J. Biotechnol. 2020;47:17–28. [Google Scholar]
- Wang Y., Li X., Yang G., Weng H., Wang X., Wang Q. Changes of enzyme activity and gene expression in embryonic zebrafish co-exposed to beta-cypermethrin and thiacloprid. Environ. Pollut. 2020;256 doi: 10.1016/j.envpol.2019.113437. [DOI] [PubMed] [Google Scholar]
- Wang Y., Meng J., Men L., An B., Jin X., He W., Lu S., Li N. Rosmarinic acid protects mice from concanavalin A-induced hepatic injury through AMPK signaling. Biol. Pharmaceut. Bull. 2020;43:1749–1759. doi: 10.1248/bpb.b20-00477. [DOI] [PubMed] [Google Scholar]
- Wang Y., Xu P., Chang J., Li W., Yang L., Tian H. Unraveling the toxic effects of neonicotinoid insecticides on the thyroid endocrine system of lizards. Environ. Pollut. 2020;258 doi: 10.1016/j.envpol.2019.113731. [DOI] [PubMed] [Google Scholar]
- Whalan J.E. Springer International Publishing; Switzerland: 2015. A Toxicologist's Guide to Clinical Pathology in Animals. Hematology, Clinical Chemistry, Urinalysis. [Google Scholar]
- Wood T.J., Goulson D. The environmental risks of neonicotinoid pesticides: a review of the evidence post 2013. Environ. Sci. Pollut. Res. 2017;24:17285–17325. doi: 10.1007/s11356-017-9240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Ma K., Na X. Rosmarinic acid alleviates di-2-ethylhexyl phthalate (DEHP)-induced thyroid dysfunction via multiple inflammasomes activation. J. Toxicol. Sci. 2020;45:373–390. doi: 10.2131/jts.45.373. [DOI] [PubMed] [Google Scholar]
- Xie Z., Lu G., Zhou R., Ma Y. Thiacloprid-induced hepatotoxicity in zebrafish: activation of the extrinsic and intrinsic apoptosis pathways regulated by p53 signaling pathway. Aquatic Toxicol. 2022;246 doi: 10.1016/j.aquatox.2022.106147. [DOI] [PubMed] [Google Scholar]
- Xu J., Rehmann M.S., Tian J., He Q., Chen J., Lee J., Borys M.C., Li Z.J. Rosmarinic acid, a new raw material, doubled monoclonal antibody titer in cell culture manufacturing. Biochem. Eng. J. 2020;160 [Google Scholar]
- Xu X., Wang X., Yang Y., Ares I., Martínez M., Lopez-Torres B., Martínez-Larrañaga M.-R., Wang X., Anadón A., Martinez M.-A. Neonicotinoids: mechanisms of systemic toxicity based on oxidative stress-mitochondrial damage. Arch. Toxicol. 2022;96:1493–1520. doi: 10.1007/s00204-022-03267-5. [DOI] [PubMed] [Google Scholar]
- Yang M., Wu C., Zhang T., Shi L., Li J., Liang H., Lv X., Jing F., Qin L., Zhao T. Chicoric acid: natural occurrence, chemical synthesis, biosynthesis, and their bioactive effects. Front. Chem. 2022;10 doi: 10.3389/fchem.2022.888673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.-Y., Hong C.-O., Lee G.P., Kim C.-T., Lee K.-W. The hepatoprotection of caffeic acid and rosmarinic acid, major compounds of Perilla frutescens, against t-BHP-induced oxidative liver damage. Food Chem. Toxicol. 2013;55:92–99. doi: 10.1016/j.fct.2012.12.042. [DOI] [PubMed] [Google Scholar]
- Yao Y., Li R., Liu D., Long L., He N. Rosmarinic acid alleviates acetaminophen-induced hepatotoxicity by targeting Nrf2 and NEK7-NLRP3 signaling pathway. Ecotoxicol. Environ. Saf. 2022;241 doi: 10.1016/j.ecoenv.2022.113773. [DOI] [PubMed] [Google Scholar]
- Yonar M.E., Sakin F. Ameliorative effect of lycopene on antioxidant status in Cyprinus carpio during pyrethroid deltamethrin exposure. Pesticide Biochem. Physiol. 2011;99:226–231. [Google Scholar]
- Yu Y., Wu Y., Yan H.-z., Xia Z.-r., Wen W., Liu D.-y., Wan L.-h. Rosmarinic acid ameliorates acetaminophen-induced acute liver injury in mice via RACK1/TNF-α mediated antioxidant effect. Pharmaceut. Biol. 2021;59:1284–1291. doi: 10.1080/13880209.2021.1974059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeid E.H.A., Alam R.T., Ali S.A., Hendawi M.Y. Dose-related impacts of imidacloprid oral intoxication on brain and liver of rock pigeon (Columba livia domestica), residues analysis in different organs. Ecotoxicol. Environ. Saf. 2019;167:60–68. doi: 10.1016/j.ecoenv.2018.09.121. [DOI] [PubMed] [Google Scholar]
- Zhu L., Li W., Zha J., Li N., Wang Z. Chronic thiamethoxam exposure impairs the HPG and HPT axes in adult Chinese rare minnow (Gobiocypris rarus): docking study, hormone levels, histology, and transcriptional responses. Ecotoxicol. Environ. Saf. 2019;185 doi: 10.1016/j.ecoenv.2019.109683. [DOI] [PubMed] [Google Scholar]
- Zhu X., Huang F., Xiang X., Fan M., Chen T. Evaluation of the potential of chicoric acid as a natural food antioxidant. Exp. Ther. Med. 2018;16:3651–3657. doi: 10.3892/etm.2018.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zosen D., Hadera M.G., Lumor J.S., Andersen J.M., Paulsen R.E. Chicken embryo as animal model to study drug distribution to the developing brain. J. Pharmacol. Toxicolo. Methods. 2021;112 doi: 10.1016/j.vascn.2021.107105. [DOI] [PubMed] [Google Scholar]
- Zych M., Kaczmarczyk-Sedlak I., Wojnar W., Folwarczna J. Effect of rosmarinic acid on the serum parameters of glucose and lipid metabolism and oxidative stress in estrogen-deficient rats. Nutrients. 2019;11:267. doi: 10.3390/nu11020267. [DOI] [PMC free article] [PubMed] [Google Scholar]