Abstract
Copper offers unique capability as catalyst for multicarbon compounds production in the electrochemical carbon dioxide reduction reaction. In lieu of conventional catalysis alloying with other elements, copper can be modified with organic molecules to regulate product distribution. Here, we systematically study to which extent the carbon dioxide reduction is affected by film thickness and porosity. On a polycrystalline copper electrode, immobilization of porous bipyridine-based films of varying thicknesses is shown to result in almost an order of magnitude enhancement of the intrinsic current density pertaining to ethylene formation while multicarbon products selectivity increases from 9.7 to 61.9%. In contrast, the total current density remains mostly unaffected by the modification once it is normalized with respect to the electrochemical active surface area. Supported by a microkinetic model, we propose that porous and thick films increase both local carbon monoxide partial pressure and the carbon monoxide surface coverage by retaining in situ generated carbon monoxide. This reroutes the reaction pathway toward multicarbon products by enhancing carbon–carbon coupling. Our study highlights the significance of customizing the molecular film structure to improve the selectivity of copper catalysts for carbon dioxide reduction reaction.
Subject terms: Electrocatalysis, Pollution remediation, Materials for energy and catalysis, Surface assembly
This study explores how the activity and selectivity of Cu electrodes for CO2 reduction can be tuned with organic films of different porosity and thickness. As a result, the films increase the local CO partial pressures and surface coverages.
Introduction
The electrochemical carbon dioxide reduction reaction (CO2RR) constitutes a carbon-neutral method for manufacturing carbon chemical feedstock and storing electricity from renewable sources such as wind and solar1,2. Cu is by far the best electrocatalyst for forming hydrocarbons and oxygenated products3,4, although recently low selectivity of longer carbon chains was also observed on nickel-based catalysts5. The modest adsorption energy of carbon monoxide (CO), a critical CO2RR intermediate, makes it feasible for this intermediate to be further reduced to hydrocarbons on Cu electrodes. This gives rise to numerous distinct products, including both single carbon (C1) products [e.g., formate (HCOO−), CO, and methane (CH4)], and multicarbon (C2+) products [e.g., acetate (CH3COO−), ethylene (C2H4), ethanol (C2H5OH), and isopropanol (C3H7OH)]6,7. The C2+ products are particularly interesting because of their larger energy density and wider applicability8. To switch the reaction pathway from C1 to C2+, as well as suppress the competitive hydrogen (H2) evolution reaction (HER), considerable research has been conducted on adjusting the Cu morphology (e.g., size9, grain boundaries10,11, and facets12–15), oxidation states16–18, controlling the dopants (e.g., alloy)19–22, and electrolyte (e.g., cations23,24 and anions25).
In addition, the concept of steering activity and selectivity toward C2+ products during CO2RR by introducing molecular catalysts or organic compounds containing specific functional groups to tailor the Cu surface is well established26–30. These studies ascribe the improved CO2RR performance to various phenomena, including electric field strength31, stabilization of CO2 reduction intermediate, hydrophobicity32,33, or higher CO2 concentration in the vicinity of the electrode31,34. For instance, Sargent and co-workers demonstrated the correlation between the selectivity of C2H4 and the ratio of atop-bound CO to bridge-bound CO on Cu surfaces by modifying Cu with a library of N-arylpyridinium salts with different substituents35. Züttel and co-workers revealed that the distinct hydrophobic properties of polymers could change the transport of H2O and CO2, thus greatly affecting the CO2RR activity36. Nonetheless, recently, Grubbs and Goddard III found that a tricomponent copolymer-modified Cu electrode rivaled the best performance of existing modified polycrystalline Cu foil catalysts due to not only the electric field induced by the polymer but also the increased porosity of the polymer film for CO2 diffusion31. This underlines the significance of the physical structure of organic films and animates to further research into understanding the impact of film thickness and porosity of organic films. Such knowledge is pertinent as it would better enable customization of the chemical microenvironment surrounding a Cu catalyst for optimal C2+ production.
In this work, we present a systematic study of the impact of film structure on the electrochemical CO2RR at Cu surfaces. Inspired by the work of Sargent and co-workers35, electrochemical deposition was used to systematically manufacture electrodes containing organic films of varying thicknesses and porosities. Remarkably, a carefully prepared porous and thick 1,1′-di-p-tolyl-1,1′,4,4′-bipyridine (T-bipyridine) film deposited on a polycrystalline Cu electrode can give rise to almost an order of magnitude enhancement of the intrinsic current density of C2H4. Mechanistic studies highlight the crucial role of the physical structure of the molecular modifier for selectively converting CO2 to C2+ products. Supported by a microkinetic model, we propose the capability of porous and thick organic films to boost the C–C coupling and C2+ product generation. This occurs by establishing a *CO-rich microenvironments close to the Cu surface by retaining and extending the residence time of in situ generated CO.
Results and discussion
Electrodes preparation and surface characterization
Following a modified electrodimerization procedure published by Sargent and co-workers35, T-bipyridine films were electrodeposited on polished Cu foil electrode by applying five specific potentials in the range of −1.25 to −0.55 V versus reversible hydrogen electrode (RHE) in CO2-saturated 0.1 M KHCO3. In general, employment of a more negative potential would be anticipated to lead to a relatively larger deposit of T-bipyridine on the Cu surface, and thus a thicker film, as a greater electrochemical driving force is applied. The modified electrodes (Fig. 1a), denoted Cu-n (n = 1–5), were prepared according to the conditions summarized in Table S1.
Fig. 1. Thickness characterization of of T-bipyridine modified Cu electrodes.
a Schematic of the five T-bipyridine modified electrodes denoted Cu-n (n = 1−5). b−f AFM images and cross-sectional profiles of the edge plane (20 × 20 μm2) on Cu-n prior to electrolysis. g Average thicknesses of films on Cu-n. Error bars correspond to the standard deviation of >15 regions on each sample. Source data are provided as Source Data file.
Atomic force microscopy (AFM) measurements were performed to determine the film thickness, d, on the Cu-n plates, made possible by the fact that the electrodes had been partially covered with a piece of polyimide tape during electrochemical deposition of T-bipyridine (Fig. 1b−f). After removal of the tape, this created a distinct step between the exposed Cu substrate (dark, right side) and deposited film (bright, left side); d was determined as the mean value based on cross-sectional profiles (white rectangular box) pertaining to >15 different regions on each sample to average thickness fluctuations. Fig. 1g displays a plot of d against the applied deposition potential. A distinct correlation exists between these two parameters with d increasing from 0.10 ± 0.01 to 0.79 ± 0.08 µm as the applied potential decreases from −0.55 to −1.25 V versus RHE.
Next, scanning electron microscopy (SEM) images were captured for all electrodes to assess the film morphology (Fig. 2). Figure 2a shows that the surface of pristine Cu is flat after the initial mechanical and electrochemical polishing. Some inner degrading voids of Cu are exposed on the surface due to non-selective electrochemical corrosion by phosphoric acid, which is consistent with prior observations13,37. For the modified electrodes, the T-bipyridine film is evident, and it is becoming more porous and rougher as the deposition potential decreases to more negative values (Fig. 2b−f and S3).
Fig. 2. SEM images.
a pristine Cu and b−f T-bipyridine modified Cu-n (n = 1−5) electrodes prior to electrolysis.
Film porosity (P) was evaluated by measuring the volume of pores in each film using AFM for all the as-prepared films once they were contracted by means of drop casting acetone directly onto the organic films. In this manner, the organic film was dissolved before it was rebuilt upon acetone evaporation. Based on the assumptions that (i) the reconstructed film is a dense film without pores and (ii) the reconstructed film is distributed evenly on the electrode surface, P can be calculated according to Eq. (1).
| 1 |
Here, P is defined as the ratio of pore volume (Vpore) and total volume (Vtotal), with the latter volume determined from the thickness of the film (d) times the area of the electrode (A), and the former determined from the thickness difference of the film before and after reconstruction (drecon) times A. Table S2 collects the results, showing that P increases as the film thickness increases from Cu-1 (P = 55%) to Cu-5 (P = 83%). This substantiates our observations from SEM that the film becomes more porous as the applied potential used for the film deposition decreases.
Electrochemical CO2RR
The performance of all fabricated Cu electrodes in CO2RR was tested in bulk electrolysis experiments (at −0.96 V versus RHE in CO2-saturated 0.1 M KHCO3 for 60 min) to evaluate the extent by which the exact film structure would impact the electrocatalytic properties, both with respect to selectivity and activity (Fig. 3a and Table S3). Regarding selectivity, Cu-5 (d = 0.79 ± 0.08 µm) demonstrates the highest FEC2H4 of 46.1%, with minor CH4 production (FECH4 = 2.6%) and surprisingly no CO. The corresponding value of FEH2 is 24.7%, showing a significantly lowering of H2 production. For comparison, pristine Cu primarily produces H2 (FEH2 = 52.8%) and C1 products (FECH4 = 11.8%, FECO = 9.4%), along with minor amounts of C2+ products (FEC2H4 = 6.1%, FEC2H5OH = 1.4%). Cu-1 shows similar product distribution as pristine Cu, suggesting that introduction of a thin film (d = 0.10 ± 0.01 µm) on the surface exerts no effect on CO2RR. With Cu-2 (d = 0.16 ± 0.02 µm), FECH4 increases to 22.3%, while the improvement for C2H4 is marginal (FEC2H4 = 9.0%). In contrast, FEC2H4 increases to 16.2% on Cu-3 (d = 0.33 ± 0.05 µm) and 29.0% on Cu-4 (d = 0.62 ± 0.06 µm). It was desirable to test even thicker films−constructed by applying more negative potentials during grafting−but, unfortunately, no reliable results could be obtained because parts of the physically adsorbed film would break off during electrochemical deposition and electrolysis (Figs. S4 and S5).
Fig. 3. Performance of pristine Cu and Cu-n (n = 1−5) electrodes in CO2RR.
a FEs of H2 (gray), CH4 (blue), CO (green) and C2H4 (orange) as function of d along with partial |j| of b CH4, c C2H4, d H2 and e H2 (under Ar atmosphere with no CO2RR) normalized with respect to the geometric surface area (=1.22 cm2, blue) and ECSA (orange), respectively, as function of d. All bulk electrolysis experiments were conducted at an applied potential of −0.96 V versus RHE for 60 min in CO2-saturated or Ar-saturated 0.1 M KHCO3, unless noted otherwise. Error bars correspond to the standard deviation of at least two independent measurements. Source data are provided as a Source Data file.
In addition to selectivity, activity is a critical performance metric38, often measured by the turnover frequency. A straightforward approach to assess the activity consists of determining the relative values of the absolute partial current densities, normalized with respect to either the geometric area (|jgeo|) or the electrochemical active surface area (|jECSA|). Thus, we determined the ECSA of each individual electrode using the double-layer capacitance method prior to performing bulk electrolysis, while the partial current density of the individual product could be calculated based on the faradaic efficiencies (FEs) determined from product analysis (Fig. S6 and Table S3). Specifically, when layer thickness increases, ECSA and thus roughness factor (RF) decreases which we attribute to blocking of active sites by the nonconductive molecular film. For example, while RF = 2.5 ± 0.2 for pristine Cu, it is 1.9 ± 0.1 for Cu-5. In this regard, comparisons of intrinsic activity with |jECSA| are therefore accurate within the variations of active site densities among samples. We also attempted to use the Pb underpotential deposition method to determine ECSAs39,40, but had to abandon it for the Cu-n electrodes because the presence of the organic films shifted the stripping curves in a positive potential direction to make it collide with the oxidation of bulk Cu itself (Fig. S7).
Figure 3b−d shows values of |jgeo| and |jECSA| obtained for CH4, C2H4, and H2 production at all Cu-n electrodes in CO2RR. Regarding CH4, the intrinsic activity in terms of |jECSA,CH4| behaves as a volcano-like curve as function of d (Fig. 3b). Starting from |jECSA,CH4| = 0.12 mA cm−2 on pristine Cu, it rises with increasing d until it passes a maximum of 0.24 mA cm−2 on Cu-2 (d = 0.16 ± 0.02 µm). From this point on, it decreases. In contrast, |jECSA,C2H4| increases as function of d (Fig. 3c), with |jECSA,C2H4| = 0.52 mA cm−2 on Cu-5 being 8.7 times higher than that on pristine Cu (|jECSA,C2H4| = 0.06 mA cm−2). This means that |jECSA,C2H4|/|jECSA,CH4| increases from 0.4 to 18.2, with d going from 0.10 ± 0.01 µm for Cu-1 to 0.79 ± 0.08 µm for Cu-5. (Fig. S8). Similarly, |jECSA,C2+| (0.68 mA cm−2) becomes 6.8 times larger than that on pristine Cu (0.10 mA cm−2) (Table S3). Thus, a thick and porous T-bipyridine film improves the activity for C2H4 while inhibiting CH4 production. Note that the total |jECSA| is, by and large, independent of film thickness and, in fact, whether the film is there or not (Table S3).
The HER is slightly suppressed by the organic film, with |jECSA,H2| declining from 0.51 mA cm−2 on pristine Cu to 0.28 mA cm−2 on Cu-5 under CO2 atmosphere (Fig. 3d). If the bulk electrolysis is performed under Ar atmosphere with no simultaneous CO2RR occurring, |jECSA,H2| is found to be considerably larger in the range from 0.74−1.07 mA cm−2, independent of the film (Fig. 3e and Table S4). This suggests that in the absence of CO2, the diffusion rate of proton carriers, and through this HER, is unaffected by the presence of the organic film. However, with CO2 present HER becomes suppressed, most likely because of active sites being blocked by generated *CO.
Previous work has reported that counter ions of the pyridinium salts, such as halide ions, could induce nanostructures on Cu surfaces29,41, potentially leading to improved performance for C2+ product formation during CO2RR. To rule out that the triflate anion in our case might corrode the Cu surface, we measured the root mean square roughness (Rq) by AFM for a Cu surface, upon removal of the physisorbed T-bipyridine film by rinsing with acetone; the surface looks flat and smooth with Rq = 1.79 nm (Fig. S9). In comparison, Rq of pristine Cu is 1.07 nm (Fig. S9). This result is consistent with the SEM observations, i.e., no nanostructured Cu is visible on the surface (Fig. S10).
Next, X-ray photoelectron spectroscopy (XPS) measurements were conducted on pristine Cu and Cu-5 (Fig. S11) to reveal the extent to which the chemical state of Cu electrode would be altered by introducing the T-bipyridine film. As seen, two binding energy peaks at 932.4 and 952.1 eV are observed on both electrodes, attributed to the Cu 2p spectra of Cu 2p3/2 and Cu 2p1/2 signals, respectively42. Lack of large shakeup satellite peaks from 938−946 eV is consistent with the absence of Cu2+ species. Usually, it is difficult to distinguish Cu0 from Cu+ because of a too-small difference of the binding energies. However, the Cu LMM Auger spectra with kinetic energy peaks at 916.8 and 918.6 eV confirm the presence of Cu+ as well as Cu0 on both pristine Cu and Cu-543. Thus, the formation of a native Cu2O layer cannot be avoided after exposure to ambient air. Still, the main conclusion is that no noteworthy valence change is found between pristine Cu and Cu-5, showing that the chemical state of the surface Cu is left unchanged by the T-bipyridine film.
Thickness
Already it has been shown that a thick and porous T-bipyridine film improves the activity of C2H4 while inhibiting CH4 production. To test the effect of d while keeping porosity intact, we prepared a variant of the Cu-5 sample (denoted Cu-5thin) using a deposition duration period of 300 s instead of 3600 s. SEM pictures validate the morphological similarity of Cu-5thin and Cu-5 (Fig. S12), while the average d of the former, as expected, is significantly lower (0.15 ± 0.03 µm versus 0.79 ± 0.08 μm) according to AFM measurements (Fig. S13). The product distribution in CO2RR shows a noticeable change (Fig. 4a), with FEC2H4 declining from 46.1 to 26.3%, FEC2+ decreasing from 61.9 to 34.7% (Table S5), and FECH4 increasing from 2.5 to 5.5% going from Cu-5 to Cu-5thin. A plausible explanation of this finding is that the residence period of in situ generated *CO on the surface decreases with the decrease of d, resulting in a poorer conversion of CO2 to C2+ products. A major aim of future work is to test this effect of d by creating thicker organic films than the already achieved 0.79 μm, if the stability issue of the physically adsorbed organic films can be solved.
Fig. 4. Effect of film thickness and porosity.
a FEs for H2 (gray), CH4 (blue), CO (green), and C2H4 (orange) production at pristine Cu, Cu-5thin, and Cu-5 electrodes. b FEs for H2 (gray), CH4 (blue), CO (green), and C2H4 (orange) production at pristine Cu, Cu-5, and Cu-5block electrodes. All bulk electrolysis experiments were conducted at an applied potential of −0.96 V versus RHE for 60 min in CO2-saturated 0.1 M KHCO3. Error bars correspond to the standard deviation of at least two independent measurements. Source data are provided as a Source Data file.
Porosity
SEM pictures demonstrate the porous structure of the T-bipyridine film once the applied potential becomes more negative than −1.0 V versus RHE (Fig. 2b–f). Formation of gaseous products on the electrode surface under these conditions, originating from both HER and CO2RR, will create cavities and voids in the organic film during the deposition process44. To shed light on the porosity effect in a direct, but also destructive way, the organic film on the best-performing electrode, Cu-5 was exposed to 50 µL acetone, drop cast directly onto the organic film. This dissolved the film which was then allowed to slowly rebuild upon acetone evaporation. As seen, the resulting organic film is characterized by a lamellar and nonporous structure, denoted Cu-5block (Fig. S14). Notably, the thickness (d = 0.13 ± 0.02 µm; Fig. S15) is lower than that of Cu-5 (d = 0.79 ± 0.08 µm) which can be converted to a value of P = 83% for Cu-5 (see Table S2). In comparison, the corresponding P´s for Cu-1, Cu-2, Cu-3, and Cu-4 are 55%, 50%, 67% and 80%, respectively.
The CO2RR performance of Cu-5block was evaluated under identical conditions as for Cu-5 (Fig. 4b and Table S6). Strikingly, the selectivity for both C2H4 (FEC2H4 = 7.8%) and C2+ products (FEC2+ < 10.0%) is considerably lower for Cu-5block, with |jECSA-C2H4| being 5.7 times smaller than that of Cu-5 (Table S6). Importantly, this cannot be attributed to the change in film thickness caused by film restructuring. Although the film on Cu-5block is small in size, becoming close to that of Cu-5thin (0.15 µm), its performance is inferior to that of the latter. This demonstrates the significance of the organic film structure in CO2RR, with the hypothesis that a high-porosity film structure can secure optimal conditions for C2+ product formation by speeding up CO2 diffusion and retaining in situ generated *CO (higher partial pressure).
Mass transport
In previous work on CO2RR, the porosity of the polymer was demonstrated to be important for mass transport of the species concerned31,45. In fact, the specific morphology of the polymer could even facilitate the supply of CO2 to the Cu-polymer interface by speeding up the diffusion of CO246. To examine the deposited films from this perspective, we selected a well-known redox probe, 1,1′-dimethyl-4,4′-bipyridinium dichloride (methyl viologen), for a cyclic voltammetric study. In addition to exhibiting a profound redox wave in a convenient potential range, the relatively large molecular size and double-charged nature of this probe can be used to put the transport capabilities of the film to the test.
Cyclic voltammetry of methyl viologen was first performed on pristine Cu using various sweep rates (v’s) from 0.5−2.0 V s−1 in Ar-saturated 0.1 M KHCO3 (Supplementary Note 1 and Fig. S16). The peak current increases with v1/2 which is in line with a diffusion-controlled process47. Next, voltammograms were recorded on the Cu-5 electrode, with the redox wave now showing an S-shaped feature and being slightly reduced in size (Fig. S17). This suggests a change in the diffusion mode, going from planar to spherical47, which, typically, is seen for ultra-microelectrodes or arrays of such. This would infer that the structure of the organic film images an ultra-microelectrode array quite well by prohibiting methyl viologen from getting through all pore sites in the film. Considering the quite large molecular size and double-charged nature of methyl viologen, this is not surprising. In a sense, it is more surprising that the film is porous to an extent that allows a significant penetration of this probe. It would suggest that the smaller CO2 and H2O molecules should be in an excellent position to gain access to the Cu surface.
Along these lines, the hydrophobicity of the organic films should be considered, also because other studies have demonstrated that submerged hydrophobic surfaces can trap gas at the nanoscale, thereby accumulating CO2 and repelling isolated H2O at the electrode-electrolyte interface48,49. To assess the hydrophobicity of the deposited T-bipyridine film, water contact angle measurements were performed (Fig. S18). As expected, the pristine Cu electrode is hydrophilic with a contact angle of 22°. In comparison, the surfaces of Cu-n (n = 1–5) are considerably more hydrophobic, with water contact angles ranging between 75° and 115°. Nevertheless, the performance of the pristine Cu and Cu-1 electrodes are almost identical, despite the different water contact angles measured for these two cases. This would imply that with thickness changes, issues related to hydrophobicity/hydrophilicity are not as important as the relative diffusion (and thus reaction rate) changes pertaining to CO2, CO, or H2O caused by the porosity effect. In our study, the HER performance in absolute terms under Ar atmosphere is unaffected by the T-bipyridine film (Fig. 3e and Table S4), showing a limited effect from film hydrophobicity. Besides the high porosity of the film, a possible reason for this is the applied electric field during electrolysis that can induce wetting of the hydrophobic film, thus making it more accessible for H2O50. In conclusion, the T-bipyridine films seem to impose no severe restrictions on the diffusion process of neither CO2 nor H2O. As a final point, the electrolyte composition (in particular, the cation) would be expected to exert a great impact on the reaction performance23. This aspect was not studied further, being outside the scope of the present study.
Mechanistic insights from operando Raman spectroscopy
To gain insight into the C–C coupling mechanism in the presence of the T-bipyridine film, operando Raman spectroscopy measurements were performed on both pristine Cu and Cu-5 electrodes. At open circuit potential, multiple bands at 427, 528, and 616 cm−1 appear (Fig. S19). These are attributed to Cu2O51 in line with formation of a native oxide layer on Cu after exposure to ambient air. The intensity of the Cu2O bands decreases (from 0 to 20 s) and finally vanishes (25 s) upon applying a negative potential of −0.2 V versus RHE, as this causes reduction of the oxide layer (Fig. S20). Accordingly, catalytic sites consist of metallic Cu rather than Cu2O, in accordance with earlier reports52,53.
Figure 5a shows two bands appearing at 280 and 366 cm−1 at a potential of −0.9 V versus RHE in CO2-saturated 0.1 M KHCO3, corresponding to the frustrated ρ (Cu–CO) rotational mode and v (Cu–CO) stretching mode, respectively54. The band between 1950 and 2100 cm−1 arises from CO stretching of the *CO adsorbed on metal sites (Fig. 5b)55,56. Notably, the intensity of these bands is greater on Cu-5 than pristine Cu, suggesting that the presence of the T-bipyridine film enhances *CO coverage which will better enable C2+ product formation through C–C coupling. Moreover, a blue shift of the CO stretching band would be anticipated if CO is bound strongly to Cu22, but we note that the Cu–CO stretching band is located in the same position on Cu-5 as on pristine Cu, implying that the Cu–CO bond strength is identical in the two cases. This also shows that the film itself is incapable of stabilizing the *CO intermediate to an extent where this comes through as a decrease of the adsorption energy. Thus, the enhanced CO2RR activity cannot be attributed to changed energetics of *CO, but instead to a larger coverage resulting from higher local CO partial pressure.
Fig. 5. Operando Raman spectroscopy.
Spectra were collected for pristine Cu (gray) and Cu-5 (green) during CO2 reduction at two regions, i.e., a low wavenumber range at 200–700 cm−1 and b high wavenumber range at 1700–2250 cm−1 at an applied potential of −0.9 V versus RHE in CO2-saturated 0.1 M KHCO3. Source data are provided as a Source Data file.
Microkinetic model
To corroborate our working hypothesis on the key role of the partial pressure of CO in the organic films, we developed a microkinetic model to depict the alterations in selectivity on the Cu surface (see Supplementary Note 2 in the Supporting Information). The goal is to build a basic model with as few assumptions as possible that would still be capable of qualitatively characterizing the change in product distribution with varying amounts of carbon reactants (e.g., CO) and hydrogen reactants (e.g., protons) at the surface. This microkinetic model is derived from the Langmuir adsorption expressions as shown in Eqs. (2)−(4).
| 2 |
| 3 |
| 4 |
Here, A and B represent two different adsorbates, corresponding to hydrogen (H) and carbon (C) in our system; , , and are the coverages on the surface of free sites, adsorbate A, and adsorbate B, respectively; and are the equilibrium constants of adsorbates A and B, respectively; and is the partial pressure of the given adsorbate.
The Langmuir adsorption model has the following inherent assumptions: 1) equilibrium is ensured, i.e., the chemical potential of the adsorbate is the same in gas phase as on the surface, 2) one monolayer of adsorbate at most is attainable, 3) adsorbate–adsorbate interactions are ignored, and 4) surface sites are all equivalent. The FEs for the various product are calculated from the rate equations for production of H2, C1 (CH4, CO, and HCOO−), C2 (CH3COO−, C2H4, and C2H5OH), and C3 (C3H7OH) products.
The rate equations are given by Eqs. (5)−(8).
| 5 |
| 6 |
| 7 |
| 8 |
Here, r is the reaction rate, k is the forward rate constant, P is the partial pressure, is the coverage of free sites, and O is the reaction order for either H or , with a, b, c, and d indicating the corresponding reaction order for the formation of (a) C1, (b) C2, (c) C3, and (d) H2.
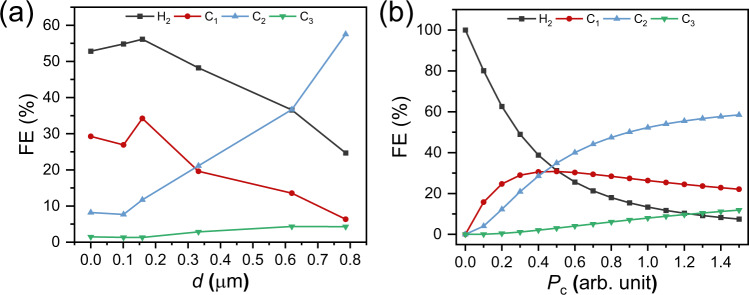
Figure 6 shows FEs for H2, C1, C2, and C3 products for modified Cu as function of d (i.e., thickness) for the experimental results (Fig. 6a), and as function of carbon partial pressure (PC) at the surface for the microkinetic model (Fig. 6b). Experimentally, both FEC2 and FEC3 increase while FEH2 decreases with d. Interestingly, FEC1 exhibits volcano-like behavior with an increase of d until 0.16 ± 0.02 μm, followed by a steady decrease. Encouragingly, the microkinetic model shows the exact same trend for all products, with PC as the primary descriptor. While we have not established a direct relationship between d and PC on the Cu surface, we see similar qualitative trend in the experimental d´s at 0–0.79 µm and the model PC data at 0.3–1.0. This backs up the findings from the experiments, corroborating the hypothesis that the molecular film enhances *CO coverage at the surface.
Fig. 6. Microkinetic model.
Comparison of FEs of H2 (black), C1 (red), C2 (blue), and C3 (green) from a experimental results as function of d and b model results as function of carbon partial pressure PC (arb. unit) at the surface. Source data are provided as a Source Data file.
Previously, analyzes of CO2 and H2O diffusion coefficients in water and various polymeric systems pointed in the same direction32,33,57. Christensen et al. discovered that the molecular modifier facilitated the diffusion of CO2 relative to H2O57. It was hypothesized that this would alter the chemical potential (and concentration) at the surface in favor of CO2 and CO, increasing the C–C coupling required for C2+ products, while lowering HER selectivity. Fig. S21 displays the model findings for greater values of PC and proton partial pressure (PH), demonstrating that the model has the expected asymptotic behavior at high PH, with H2 generation dominating when PH >> PC.
This research demonstrates that the activity and selectivity of Cu for CO2RR can be modulated by adjusting the physical structure of a deposited organic film, with emphasis on porosity and thickness. With a total FE of 61.2%, the best-performing electrode shows strong CO2RR activity toward C2+ products. Although essentially no changes are observed to the total activity, once normalizing the current density with the ECSA of each electrode, the intrinsic activity of C2H4 improves 8.7-fold compared with an analogous polycrystalline Cu electrode with no active layer. AFM, SEM, and XPS studies rule out noticeable contributions from surface nanostructuring or oxidation state changes. Instead, mechanistic insights from operando Raman measurements and a microkinetic model indicate that the greater local CO partial pressure and *CO surface coverage are responsible for the activity and selectivity changes. On a *CO intermediate-rich Cu surface, C–C coupling would be enhanced, thus altering the reaction pathway from C1 to C2+ products. Overall, this study proposes an approach for enhancing the CO2-to-C2+ conversion by customizing the physical structure of the modifier and the microenvironment at the electrode–electrolyte interface.
Methods
Materials
All solvents and reagents were obtained from commercial sources (Aldrich and Merck) and used as received, unless stated otherwise. Cu foil (99.99%) was purchased from Goodfellow. Deuterium dioxide (D 99.96%), d-chloroform (D 99.8%), and d6-dimethylsulfoxide (D 99.8%) were purchased from Cambridge Isotope Laboratories. Water was purified by a Nanopure Analytical Ultrapure Water System (Thermo Scientific) or a Milli-Q Advantage A10 Water Purification System (Millipore) with specific resistance of 18.2 MΩ cm at 25 °C. 1H nuclear magnetic resonance (NMR) spectra were recorded on a Bruker 400 MHz instrument with a prodigy broadband cryoprobe. Shifts were reported relative to the residual solvent peak.
Characterization of electrodes
XPS spectra were recorded on a Kratos Axis Ultra-DLD instrument using monochromatic Al Kα X-ray source at 150 W. Measurements were performed while keeping pressure below 5 × 10−12 bar. Full survey spectra were acquired with pass energy of 160 eV and high-resolution spectra of C 1s, N 1s, Cu 2p and Cu LMM with 20 eV. CasaXPS software was employed for deconvolution and analysis of spectra. SEM images were acquired on a FEI Magellan 400 SEM instrument equipped with a through-the-lens detector at 5 kV accelerating voltage. All AFM images were captured by commercial Dimension Icon AFM (Bruker, Santa Barbara, USA). Topographies of samples were imaged in the tapping mode with ultrasharp probes (RTESP-37) in ambient pressure condition. The water contact angle was measured by the sessile drop method using an FTA1000 contact angle system (First Ten Angstroms, Inc.) at ambient temperature. The volume of the water droplets was 2.0 µL.
Electrochemical analysis
Linear sweep voltammetry and chronoamperometry measurements were carried out in a custom-made PEEK flow cell setup similar to the one reported by ref. 6, with two chambers separated by Sustainion® X37–50 exchange membrane. The three-electrode system consisted of pristine Cu or T-bipyridine modified Cu foil as working electrode, a platinum foil as counter electrode, and leak-free Ag/AgCl (Innovative Instruments Inc.) as reference electrode. O-rings were used to secure the working and counter electrodes, with exposed surface area of 1.22 cm2 on each. Supporting electrolyte (2 mL for each chamber) was either Ar-saturated 0.1 M KHCO3 (pH = 8.4) or CO2-saturated 0.1 M KHCO3 (pH = 6.8). All electrochemical measurements were carried out using an Autolab potentiostat (PGSTAT204). Potentiostatic electrochemical impedance spectroscopy (PEIS) using frequencies from 0.1 to 100 kHz was carried out prior to each electrolysis experiment to determine ohmic resistance of the flow cell. Typically, resistances were in a range from 65 to 75 Ω.
The applied potential measured against Ag/AgCl was converted to the RHE with iR correction (i is the current and R is the resistance measured by PEIS) using Eq. (9).
| 9 |
Bulk electrolysis was performed at an applied potential of −0.96 V versus RHE with CO2 or Ar gas stream flowing into the cell (2.3 mL min−1) from the bottom for 60 min. Gaseous products (H2, CO, CH4, and C2H4) were quantified by an Agilent Technologies 7890B gas chromatography system equipped with a thermal conductivity detector and flame ionization detector. Every 15 min, 250 µL of gas was sampled to determine the concentration of gaseous products. FEs of products were calculated as the average of four separate injections. After electrolysis, 1 mL of catholyte was mixed with 200 µL deuteroxide and 70 µL of 10 mM DMSO/D2O (internal standard) in a glass sample tube. Half of the mixture (635 µL) was transferred to a NMR tube and analyzed by 1H NMR spectroscopy to quantify liquid products by comparing the 1H peak area of each product with that of DMSO. The signal of water was suppressed using a presaturation sequence.
The partial current density of a specific product (|j|) normalized with respect to the geometric surface area (Ageo = 1.22 cm2) and ECSA (AECSA) of the electrode was obtained by Eqs. (10) and (11).
| 10 |
| 11 |
In the equation, I represents the absolute average current during electrolysis.
Electrode preparation
Polycrystalline Cu foil (99.99% metal basis, 1 mm thickness, Goodfellow) was cut into 2.5 × 2.5 cm2 square pieces and polished with sandpaper and diamond slurries (9, 3, 1, and 0.25 µm). Subsequently, these cut-out electrodes were electrochemically polished in 85 wt% phosphoric acid by applying a potential of 2.1 V versus a graphite rod counter electrode for 5 min to remove residuals from the diamond slurries. Finally, they were rinsed with Milli-Q water and dried in a stream of Ar.
T-bipyridine film was obtained from electroreductive dimerization of 10 mM 1-(4-tolyl)pyridinium triflate (T-Pyr), with synthesis details of T-Pyr provided in Supplementary Notes 3 and 4, and Figs. S22 and S23. Electrochemical film assembling took place on the polycrystalline Cu electrodes in CO2-saturated 0.1 M KHCO3 (pH = 6.8) using the same setup as for bulk electrolysis. Film thickness and porosity could be controlled by applying a potential ranging from −1.25 to −0.55 V versus RHE, with the latter potential determined from cyclic voltammetry recording (Fig. S24). Deposition conditions are summarized in Table S1.
Electrochemical active surface area (ECSA) and roughness factor (RF)
ECSA and RF of each single electrode were determined by measuring the double-layer capacitance (CDL) of the system prior to bulk electrolysis. First, a non-Faradaic potential range was identified to be from −0.05 to 0.05 V versus RHE from cyclic voltammetry in quiescent solution. Then cyclic voltammetry was performed at v’s from 20 to 120 mV s−1 in CO2-saturated 0.1 M KHCO3 (pH = 6.8). The absolute electrochemical double-layer capacitive current density, |jc|, was calculated by averaging the cathodic and anodic current densities at 0 V versus RHE from the cyclic voltammograms. The value of CDL was calculated by plotting |jc| against v, noting that ECSA is proportional to CDL (Fig. S6)58,59. With this, RF of the Cu-based catalyst could be calculated as CDL/CS where CS is the specific capacitance of the material; we use CS = 29 µF cm−2 for electropolished polycrystalline Cu foil60. Current densities recorded were normalized with respect to either geometric area (|jgeo| using Ageo = 1.22 cm2) or ECSA (|jECSA| using the measured RF × Ageo as AECSA).
Operando Raman spectroscopy
A three-electrode electrochemical cell with a quartz window by E-CATA (Shen Zhen) Instrument Equipment Company was used for operando Raman measurements (Fig. S25). Graphite and leak-free Ag/AgCl served as counter and reference electrode, respectively. Pristine Cu foil or Cu-5 electrode was used as working electrode. The counter electrode was separated from the working electrode by a Sustainion® X37–50 exchange membrane to avoid cross-contamination. Electrolyte (CO2-saturated 0.1 M KHCO3) was forced to flow into the cell by a peristaltic pump to replenish CO2 and remove generated gas bubbles, which would interfere with the Raman signal collection. All electrochemical tests were conducted using a CHI potentiostat. Operando Raman spectra were recorded versus time employing a Renishaw inVia Raman spectrometer and a near-infrared laser (785 nm) excitation source. Each spectrum was the representative of multiple measurements at different time scale of electrolysis. Signal acquisition time for each Raman spectrum was ~50 s (20 accumulations).
Supplementary information
Acknowledgements
This research was supported by the Novo Nordisk Foundation CO2 Research Center (Grant no. NNF21SA0072700). S.Z. (CSC no.201906920080) acknowledges the China Scholarship Council. H.L. is grateful for a Humboldt Research Fellowship (Alexander von Humboldt Foundation). O.C., A.B., and J.R. acknowledge the Danish National Research Foundation Centers of Excellence, the Center for High Entropy Alloy Catalysis (Project DNRF149), and the Independent Research Fund Denmark (Grant no. 0217-00014B).
Source data
Author contributions
S.Z. performed catalyst preparation, electrochemical experiments, characterization, and data interpretation. O.C. performed the microkinetic model. Z.S. performed X-ray photoemission spectroscopy analysis and data interpretation. S.Z., O.C., and K.D. wrote the manuscript. H.L., A.B., K.T., P.N., J.V.L., S.U.P., and J.R. added to the discussion and contributed to the preparation of the manuscript. J.R. and K.D. conceived and supervised the project. All authors have given approval to the final version of the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Data availability
Synthetic procedures, NMR spectra as well as electrochemical and characterization data are available in the Supplementary Information. Source data are provided with this paper.
Code availability
The script for the microkinetic model is available at https://sid.erda.dk/cgi-sid/ls.py?share_id=e3OC2vAbe1.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jan Rossmeisl, Email: jan.rossmeisl@chem.ku.dk.
Kim Daasbjerg, Email: kdaa@chem.au.dk.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-36530-z.
References
- 1.De Luna P, et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science. 2019;364:eaav3506. doi: 10.1126/science.aav3506. [DOI] [PubMed] [Google Scholar]
- 2.Verma S, Kim B, Jhong H-R“Molly”, Ma S, Kenis PJA. A gross-margin model for defining technoeconomic benchmarks in the electroreduction of CO2. ChemSusChem. 2016;9:1972–1979. doi: 10.1002/cssc.201600394. [DOI] [PubMed] [Google Scholar]
- 3.Ma W, et al. Electrocatalytic reduction of CO2 and CO to multi-carbon compounds over Cu-based catalysts. Chem. Soc. Rev. 2021;50:12897–12914. doi: 10.1039/D1CS00535A. [DOI] [PubMed] [Google Scholar]
- 4.Nitopi S, et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 2019;119:7610–7672. doi: 10.1021/acs.chemrev.8b00705. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, et al. Long-chain hydrocarbons by CO2 electroreduction using polarized nickel catalysts. Nat. Catal. 2022;5:545–554. doi: 10.1038/s41929-022-00803-5. [DOI] [Google Scholar]
- 6.Kuhl KP, Cave ER, Abram DN, Jaramillo TF. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012;5:7050. doi: 10.1039/c2ee21234j. [DOI] [Google Scholar]
- 7.Gao D, Arán-Ais RM, Jeon HS, Roldan Cuenya B. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2019;2:198–210. doi: 10.1038/s41929-019-0235-5. [DOI] [Google Scholar]
- 8.Qiao J, Liu Y, Hong F, Zhang J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 2014;43:631–675. doi: 10.1039/C3CS60323G. [DOI] [PubMed] [Google Scholar]
- 9.Reske R, Mistry H, Behafarid F, Roldan Cuenya B, Strasser P. Particle size effects in the catalytic electroreduction of CO2 on Cu nanoparticles. J. Am. Chem. Soc. 2014;136:6978–6986. doi: 10.1021/ja500328k. [DOI] [PubMed] [Google Scholar]
- 10.Mariano RG, McKelvey K, White HS, Kanan MW. Selective increase in CO2 electroreduction activity at grain-boundary surface terminations. Science. 2017;358:1187–1192. doi: 10.1126/science.aao3691. [DOI] [PubMed] [Google Scholar]
- 11.Feng X, Jiang K, Fan S, Kanan MW. A direct grain-boundary-activity correlation for CO electroreduction on Cu nanoparticles. ACS Cent. Sci. 2016;2:169–174. doi: 10.1021/acscentsci.6b00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, Handoko AD, Hirunsit P, Yeo BS. Electrochemical reduction of CO2 using copper single-crystal surfaces: effects of CO* coverage on the selective formation of ethylene. ACS Catal. 2017;7:1749–1756. doi: 10.1021/acscatal.6b03147. [DOI] [Google Scholar]
- 13.Roldán Cuenya B, Scholten F, Nguyen K-LC, Bruce JP, Heyde M. Identifying structure‐selectivity correlations in the electrochemical reduction of CO2: comparison of well‐ordered atomically‐clean and chemically‐etched Cu single crystal surfaces. Angew. Chem. Int. Ed. 2021;60:19318–19324. doi: 10.1002/anie.202103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn C, et al. Engineering Cu surfaces for the electrocatalytic conversion of CO2: Controlling selectivity toward oxygenates and hydrocarbons. Proc. Natl Acad. Sci. USA. 2017;114:5918–5923. doi: 10.1073/pnas.1618935114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z-Z, et al. Identification of Cu(100)/Cu(111) interfaces as superior active sites for CO dimerization during CO2 electroreduction. J. Am. Chem. Soc. 2022;144:259–269. doi: 10.1021/jacs.1c09508. [DOI] [PubMed] [Google Scholar]
- 16.Li CW, Ciston J, Kanan MW. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature. 2014;508:504–507. doi: 10.1038/nature13249. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, et al. Branched copper oxide nanoparticles induce highly selective ethylene production by electrochemical carbon dioxide reduction. J. Am. Chem. Soc. 2019;141:6986–6994. doi: 10.1021/jacs.9b00911. [DOI] [PubMed] [Google Scholar]
- 18.Li CW, Kanan MW. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 2012;134:7231–7234. doi: 10.1021/ja3010978. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, et al. Controlling speciation during CO2 reduction on Cu-alloy electrodes. ACS Catal. 2020;10:672–682. doi: 10.1021/acscatal.9b04368. [DOI] [Google Scholar]
- 20.Li Q, et al. Tuning Sn-catalysis for electrochemical reduction of CO2 to CO via the core/shell Cu/SnO2 structure. J. Am. Chem. Soc. 2017;139:4290–4293. doi: 10.1021/jacs.7b00261. [DOI] [PubMed] [Google Scholar]
- 21.Hoang TTH, et al. Nanoporous copper–silver alloys by additive-controlled electrodeposition for the selective electroreduction of CO2 to ethylene and ethanol. J. Am. Chem. Soc. 2018;140:5791–5797. doi: 10.1021/jacs.8b01868. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, et al. Efficient electrosynthesis of n-propanol from carbon monoxide using a Ag–Ru–Cu catalyst. Nat. Energy. 2022;7:170–176. doi: 10.1038/s41560-021-00967-7. [DOI] [Google Scholar]
- 23.Monteiro MCO, et al. Absence of CO2 electroreduction on copper, gold and silver electrodes without metal cations in solution. Nat. Catal. 2021;4:654–662. doi: 10.1038/s41929-021-00655-5. [DOI] [Google Scholar]
- 24.Singh MR, Kwon Y, Lum Y, Ager JW, Bell AT. Hydrolysis of electrolyte cations enhances the electrochemical reduction of CO2 over Ag and Cu. J. Am. Chem. Soc. 2016;138:13006–13012. doi: 10.1021/jacs.6b07612. [DOI] [PubMed] [Google Scholar]
- 25.Huang Y, Ong CW, Yeo BS. Effects of electrolyte anions on the reduction of carbon dioxide to ethylene and ethanol on copper (100) and (111) surfaces. ChemSusChem. 2018;11:3299–3306. doi: 10.1002/cssc.201801078. [DOI] [PubMed] [Google Scholar]
- 26.Nam D-H, et al. Molecular enhancement of heterogeneous CO2 reduction. Nat. Mater. 2020;19:266–276. doi: 10.1038/s41563-020-0610-2. [DOI] [PubMed] [Google Scholar]
- 27.Li F, et al. Cooperative CO2-to-ethanol conversion via enriched intermediates at molecule–metal catalyst interfaces. Nat. Catal. 2020;3:75–82. doi: 10.1038/s41929-019-0383-7. [DOI] [Google Scholar]
- 28.Kim C, et al. Tailored catalyst microenvironments for CO2 electroreduction to multicarbon products on copper using bilayer ionomer coatings. Nat. Energy. 2021;6:1026–1034. doi: 10.1038/s41560-021-00920-8. [DOI] [Google Scholar]
- 29.Thevenon A, Rosas‐Hernández A, Peters JC, Agapie T. In‐situ nanostructuring and stabilization of polycrystalline copper by an organic salt additive promotes electrocatalytic CO2 reduction to ethylene. Angew. Chem. Int. Ed. 2019;58:16952–16958. doi: 10.1002/anie.201907935. [DOI] [PubMed] [Google Scholar]
- 30.Xie MS, et al. Amino acid modified copper electrodes for the enhanced selective electroreduction of carbon dioxide towards hydrocarbons. Energy Environ. Sci. 2016;9:1687–1695. doi: 10.1039/C5EE03694A. [DOI] [Google Scholar]
- 31.Wang J, et al. Selective CO2 electrochemical reduction enabled by a tricomponent copolymer modifier on a copper surface. J. Am. Chem. Soc. 2021;143:2857–2865. doi: 10.1021/jacs.0c12478. [DOI] [PubMed] [Google Scholar]
- 32.Liang H-Q, et al. Hydrophobic copper interfaces boost electroreduction of carbon dioxide to ethylene in water. ACS Catal. 2021;11:958–966. doi: 10.1021/acscatal.0c03766. [DOI] [Google Scholar]
- 33.Wakerley D, et al. Bio-inspired hydrophobicity promotes CO2 reduction on a Cu surface. Nat. Mater. 2019;18:1222–1227. doi: 10.1038/s41563-019-0445-x. [DOI] [PubMed] [Google Scholar]
- 34.Chen X, et al. Electrochemical CO2-to-ethylene conversion on polyamine-incorporated Cu electrodes. Nat. Catal. 2020;4:20–27. doi: 10.1038/s41929-020-00547-0. [DOI] [Google Scholar]
- 35.Li F, et al. Molecular tuning of CO2-to-ethylene conversion. Nature. 2020;577:509–513. doi: 10.1038/s41586-019-1782-2. [DOI] [PubMed] [Google Scholar]
- 36.Pham THM, et al. Enhanced electrocatalytic CO2 reduction to C2+ products by adjusting the local reaction environment with polymer binders. Adv. Energy Mater. 2022;12:2103663. doi: 10.1002/aenm.202103663. [DOI] [Google Scholar]
- 37.Moser S, Kleinbichler M, Kubicek S, Zechner J, Cordill MJ. Electropolishing—a practical method for accessing voids in metal films for analyses. Int. J. Appl. Sci. 2021;11:7009. [Google Scholar]
- 38.Chan K. A few basic concepts in electrochemical carbon dioxide reduction. Nat. Commun. 2020;11:5954. doi: 10.1038/s41467-020-19369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plaza-Mayoral E, et al. Preparation of high surface area Cu‐Au bimetallic nanostructured materials by co‐electrodeposition in a deep eutectic solvent. Electrochim. Acta. 2021;398:139309. doi: 10.1016/j.electacta.2021.139309. [DOI] [Google Scholar]
- 40.Baturina OA, et al. CO2 electroreduction to hydrocarbons on carbon-supported Cu nanoparticles. ACS Catal. 2014;4:3682–3695. doi: 10.1021/cs500537y. [DOI] [Google Scholar]
- 41.Ogata S, et al. Nanoscale corrosion behavior of polycrystalline copper fine wires in dilute NaCl solution investigated by in-situ atomic force microscopy. Corros. Sci. 2016;105:177–182. doi: 10.1016/j.corsci.2016.01.015. [DOI] [Google Scholar]
- 42.Li D, Wang G, Cheng L, Wang C, Mei X. Engineering the self-assembly induced emission of copper nanoclusters as 3d nanomaterials with mesoporous sphere structures by the crosslinking of Ce3+ ACS Omega. 2018;3:14755–14765. doi: 10.1021/acsomega.8b02204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scholten F, Sinev I, Bernal M, Roldan Cuenya B. Plasma-modified dendritic Cu catalyst for CO2 electroreduction. ACS Catal. 2019;9:5496–5502. doi: 10.1021/acscatal.9b00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao J, Wang H. Zeolitic imidazolate framework composite membranes and thin films: synthesis and applications. Chem. Soc. Rev. 2014;43:4470–4493. doi: 10.1039/C3CS60480B. [DOI] [PubMed] [Google Scholar]
- 45.Soniat M, et al. Predictive simulation of non-steady-state transport of gases through rubbery polymer membranes. Polymer. 2018;134:125–142. doi: 10.1016/j.polymer.2017.11.055. [DOI] [Google Scholar]
- 46.Duan G, et al. Highly efficient electrocatalytic CO2 reduction to C2+ products on a poly(ionic liquid)‐based Cu0–CuI tandem catalyst. Angew. Chem. Int. Ed. 2022;61:e202110657. doi: 10.1002/anie.202110657. [DOI] [PubMed] [Google Scholar]
- 47.Arrigan, D. W. M. Nanoelectrode arrays for electroanalysis. Fronti. Nanosci.18, 49–86 (2021).
- 48.Pan H, Barile CJ. Electrochemical CO2 reduction on polycrystalline copper by modulating proton transfer with fluoropolymer composites. ACS Appl. Energy Mater. 2022;5:4712–4721. doi: 10.1021/acsaem.2c00136. [DOI] [Google Scholar]
- 49.Checco A, Hofmann T, DiMasi E, Black CT, Ocko BM. Morphology of air nanobubbles trapped at hydrophobic nanopatterned surfaces. Nano Lett. 2010;10:1354–1358. doi: 10.1021/nl9042246. [DOI] [PubMed] [Google Scholar]
- 50.Klesse G, Tucker SJ, Sansom MSP. Electric field induced wetting of a hydrophobic gate in a model nanopore based on the 5-HT3 receptor channel. ACS Nano. 2020;14:10480–10491. doi: 10.1021/acsnano.0c04387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ren D, et al. Selective electrochemical reduction of carbon dioxide to ethylene and ethanol on copper(i) oxide catalysts. ACS Catal. 2015;5:2814–2821. doi: 10.1021/cs502128q. [DOI] [Google Scholar]
- 52.Mandal L, et al. Investigating the role of copper oxide in electrochemical CO2 reduction in real time. ACS Appl. Mater. Interfaces. 2018;10:8574–8584. doi: 10.1021/acsami.7b15418. [DOI] [PubMed] [Google Scholar]
- 53.Li J, et al. Electrokinetic and in situ spectroscopic investigations of CO electrochemical reduction on copper. Nat. Commun. 2021;12:3264. doi: 10.1038/s41467-021-23582-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao Y, et al. Speciation of Cu surfaces during the electrochemical CO reduction reaction. J. Am. Chem. Soc. 2020;142:9735–9743. doi: 10.1021/jacs.0c02354. [DOI] [PubMed] [Google Scholar]
- 55.Gunathunge CM, Ovalle VJ, Li Y, Janik MJ, Waegele MM. Existence of an electrochemically inert CO population on Cu electrodes in alkaline pH. ACS Catal. 2018;8:7507–7516. doi: 10.1021/acscatal.8b01552. [DOI] [Google Scholar]
- 56.Chang X, Zhao Y, Xu B. pH dependence of Cu surface speciation in the electrochemical CO reduction reaction. ACS Catal. 2020;10:13737–13747. doi: 10.1021/acscatal.0c03108. [DOI] [Google Scholar]
- 57.Christensen O, et al. Can the CO2 reduction reaction be improved on Cu: selectivity and intrinsic activity of functionalized Cu surfaces. ACS Catal. 2022;12:15737–15749. doi: 10.1021/acscatal.2c04200. [DOI] [Google Scholar]
- 58.McCrory CCL, et al. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 2015;137:4347–4357. doi: 10.1021/ja510442p. [DOI] [PubMed] [Google Scholar]
- 59.Benck JD, Chen Z, Kuritzky LY, Forman AJ, Jaramillo TF. Amorphous molybdenum sulfide catalysts for electrochemical hydrogen production: insights into the origin of their catalytic activity. ACS Catal. 2012;2:1916–1923. doi: 10.1021/cs300451q. [DOI] [Google Scholar]
- 60.Verdaguer-Casadevall A, et al. Probing the active surface sites for CO reduction on oxide-derived copper electrocatalysts. J. Am. Chem. Soc. 2015;137:9808–9811. doi: 10.1021/jacs.5b06227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Synthetic procedures, NMR spectra as well as electrochemical and characterization data are available in the Supplementary Information. Source data are provided with this paper.
The script for the microkinetic model is available at https://sid.erda.dk/cgi-sid/ls.py?share_id=e3OC2vAbe1.