Abstract
Bovine tuberculosis (bTB) is a chronic granulomatous infectious illness in cattle. The etiological agent of bTB is Mycobacterium bovis. However, other members belonging to the Mycobacterium tuberculosis complex, like M. tuberculosis, M. africanum, M. caprae, M. orygis, and M. microti are known to cause bTB in cattle. There are 303.76 million bovines in India, and it is the largest producer of milk and the second largest producer of meat worldwide. The prevalence of bTB among farm and dairy cattle in India is estimated to be around 7.3%, which makes it a country with one of the largest infected herds in the world. While bTB control programs have had considerable success in reducing the prevalence of the disease in many developed countries, they have yet to be formulated or implemented in India. Bovine TB also has a zoonotic and reverse component, which means that the disease can spread from cattle to human and from human to cattle. In a country like India, which contributes to nearly one-fourth of the global TB burden, the zoonotic aspect must be addressed so that the disease can be curbed. While cattle are the primary reservoir host to bTB, animals like goats, deer, bison, pigs, dogs, badgers, possums, and primates are also susceptible to the disease. This review talks about the burden of bTB in India and the necessity of One Health approach to combat the disease.
Keywords: Bovine tuberculosis, Tuberculosis, One Health, Zoonosis
1. Introduction
Bovine tuberculosis (bTB) is an infectious disease that has been substantially reported in cattle with significant zoonotic potential. Though the principal etiological agent is Mycobacterium bovis, other members of the Mycobacterium tuberculosis complex like M. tuberculosis, M. africanum, M. caprae, M. orygis, and M. microti are known to cause bTB in cattle [1]. Even though the primary reservoir host of bTB are cattle, animals like goats, deer, bison, pigs, dogs, badgers, possums, and primates are also susceptible to the disease [2].
India has around 303. 76 million bovines and is the largest producer of milk in the world. The revenue from milk and milk products was approximately 113 billion USD in 2020 alone [3]. Bovine TB persists and remains endemic in India due to the lack of a disease control program and associated economic costs. The prevalence of bTB in cattle in India is estimated to be 7.3%, i.e., around 21.8 million cattle are affected with bovine tuberculosis and the burden is conceived to eventually increase in the upcoming years due to several factors- like the robust intensification of the dairy industry, improved cattle rearing methods and increased focus on improving the productivity per animal [4].
India alone accounted for 26% of the TB prevalence and 34% of TB deaths globally in humans in 2020, the highest burden globally [5]. Bovine TB also has a zoonotic and reverse component, which means that the disease can spread from cattle to humans and from human to cattle. The formidable “End TB Strategy” by the World Health Organisation (WHO) and the National Tuberculosis Elimination Program (NTEP) by the Government of India will be a great success only when all routes of transmission, including zoonosis and reverse zoonosis are taken into consideration (Fig. 1). Although the risk of TB is known well in India, the public health consequences have hardly been investigated. The call for One Health Approach by the G20 Buenos Aires summit, 2018 has provided the right opportunity to evoke and put a special effort to address the effect of zoonotic and reverse zoonotic TB on the welfare of humans as well as animals, drafted to fall within the purview of the multidisciplinary United Nations Sustainable Development Goals (SDGs) and WHO's END TB Strategy [6]. This review analyses the challenges of eradicating tuberculosis in animals in India and emphasizes the need for policy changes regarding bTB.
Fig. 1.
The transmission chain of TB between humans and animals.
2. Bovine tuberculosis control programs across the globe
The incidence of bTB has been fairly restricted in developed countries with systematic bTB eradication programs, but successful eradication and maintenance of bTB-free status are still significant challenges. In addition to threatening public health, bTB is also a significant economic concern, estimated to cost $3 billion worldwide annually, accounting for losses from reduced cattle productivity, culling, and movement and trade restrictions. In most developed countries, bTB control programs have existed for a long time, where ‘test and cull’ strategy is a major approach. This resulted in exceptional benefits to human health while fetching nearly a 10-fold return on investment in animal rearing and productivity [7].
Bovine TB as a threat to public welfare was identified as earlier as the 19th century in the USA. However, by the early 20th century, it was identified that bTB could occur in humans, which led to the government's formulation of stringent eradication programs. Thus, in 1917, the ‘test and slaughter’ policy was introduced in the USA. By 1940, 232 million TB tests were administered in cattle resulting in the culling of 3.8 million cattle throughout the country. This approach, coupled with mandatory milk pasteurization, led to the prevention of 25,000 TB deaths in humans in 1940, a period when chemotherapy was not readily available for the disease [8].
Presently, in the USA, cattle herds surveillance is undertaken using ante-mortem and post-mortem methods. The United States Agricultural Department (USDA) oversees the status of bTB in the country using the methods described in the ‘Uniform Methods and Rules’ that are in effect since 2005. An ‘accredited herd’ status is offered to a herd that did not report evidence of or exposure potential to bTB for at least two consecutive official tuberculosis tests conducted in all eligible live animals at 9–15 month intervals. This accreditation status is maintained by periodic testing. The methods used for testing the cattle include tuberculin skin testing methods such as caudal fold tuberculin test, comparative cervical tuberculin test, cervical tuberculin test, and immunological tests such as interferon-gamma assay. If any of the animals show a positive reaction, the accreditation status would be suspended, pending depopulation of the herd as well as reaccreditation. [9].
Similarly, in Europe, skin testing and interferon-gamma assay are used to test herds for awarding Officially TB Free (OTF) status to herds with no reactor cattle. However, the presence of one reactor animal leads to OTF- Withdrawn (OTF—W) status, leading to quarantine and slaughter of the herd [10,11].
Post-mortem examinations of slaughtered cattle are also often carried out in these countries by trained veterinarians. Slaughterhouse surveillance is a critical component of the bTB eradication and surveillance program. In Northern Ireland, slaughterhouse surveillance is responsible for disclosing 18–28% of new bTB herd breakdowns [12]. In Great Britain, identifying at least one suspect slaughterhouse case that yielded M. bovis in culture can lead to OTF withdrawal. Abattoir examination of cattle is thus a crucial passive surveillance method for aiding field surveillance for detecting infected herds not yet disclosed by intradermal testing.
The ‘test and slaughter’ policy has proven very effective in developed industrialized countries that could afford considerably expensive tests and the loss associated with slaughtering animals that react positively to these tests [13]. Nevertheless, these measures were and still are not feasible to adopt to eliminate bTB from low and middle-income countries where TB and bTB are endemic [14,15].
3. Need for bTB control policies in India- the human front
Bovine TB has been reported in India since 1917 when Spoarkar et al. concluded that though present, the prevalence of the disease is not as high as in the west. Since then, the disease has become endemic in India and sporadic in the west [16]. The prevalence of TB in humans in India is 3120 per million population (95%CI:2860–3370) for the year 2021 as per the National Tuberculosis Prevalence Survey [17]. According to the Global Tuberculosis Report 2021, about 26% of the TB cases reported worldwide in 2020 were from India [5]. In a country with such a high TB burden in humans, it is imperative to curb the spread of the TB disease as soon as possible.
For a long time, it was surmised that M. tuberculosis is the etiological agent for TB infection in humans, and M. bovis is the etiological agent for TB in animals. According to the WHO Global TB report, zoonotic TB is defined as a disease caused by M. bovis in humans, and its mortality is 2020 in the South East Asian region alone [18]. However, recent discoveries have challenged this notion making it necessary to reassess the cause and effect of bTB in humans and animals [19]. The study by Refaya et al. in 2019 reported the isolation of M. orygis strain in comparative intradermal test (CIT)- positive cattle from a farm in Chennai using whole genome sequencing [20]. A cross-sectional surveillance study done in three farms in Chennai to test for tuberculosis among cattle and animal handlers revealed that four cattle and six animal handlers were infected with the same strain of M. tuberculosis. The authors suggest that cattle may have been infected by repeated contact with infected humans [21]. M. tuberculosis has been identified as the cause of bTB in 84.3% of MTBC-positive cultures raised from tissue and milk samples collected in Tamil Nadu, India, using multiplex PCR techniques as opposed to 15.6% of M. bovis prevalence, the classical bTB pathogen [22].
However, cases of infection caused by MTBC members other than M. tuberculosis in humans have been reported across India by research groups. In 2020, a study conducted at the Christian Medical College in Vellore, South India, reported the isolation of 7 M. orygis strains from humans using culture, molecular methods, and whole genome sequencing [19]. Mycobacterium orygis has been included in the MTBC since 2012 as a separate sub species that was initially known to cause infection in oryxes [23]. The isolation of M. orygis from cattle and the identification of the same species in humans in India has raised concerns about the broad host spectrum of the pathogen. In light of reports regarding the low susceptibility of native Indian breeds for M. bovis infection as compared to the native European breeds, Vitale hypotheses that M. bovis might have become a pathogen of Bos taurus in Europe and the Americas, while M. orygis became a pathogen infecting Bos indicus in south Asia [2,24]. This is also supported by the fact that no M. bovis whole genome sequences have been deposited in the sequence read archive (SRA) registry from the South East Asian region so far. A study conducted in 2005 at All India Institute for Medical Sciences (AIIMS) New Delhi reported the prevalence of mixed infection of M. bovis and M. tuberculosis in humans and cattle using PCR to distinguish between the species. While the study noted the prevalence of M. tuberculosis (15.7%) and M. bovis (26.8%) in humans and animals, respectively, mixed infection was observed in 8.7% of the human samples and 35.7% of cattle samples. The findings of this study demonstrate the zoonotic as well as the reverse zoonotic aspect of TB in an Indian setting [25]. Bapat et al., in 2017, reported the presence of M. bovis among three distinct groups viz. Group A: Farmers, dairy workers, and livestock keepers; Group B: Zookeepers and animal handlers; Group C: Residents of high TB endemic areas and reported a prevalence of 11.4%, 8.9%, and 12.6% in these groups, respectively, using a PCR based technique. A noteworthy observation in this study is the high prevalence of M. bovis in Group C, comprising residents who consume meat and unpasteurized milk. The study further lists the consumption of raw milk and contact with previously diagnosed TB patients as risk factors for the spread of the disease in the community [26].
Furthermore, beef and/or buffalo meat consumption in India is 42 g/capita/month [27]. India is also the second largest exporter of meat products in the world. About 1.5 million tons of buffalo meat is produced in India annually, accounting for about 30% of total meat production [28]. This mandates supply of safe meat for human consumption.
On the human front, TB caused due to M. bovis is treated the same way as TB by M. tuberculosis. The NTEP in India does not delineate between the of MTBC in human TB infection, and hence does not report the causative organism for individual TB cases. This has masked the true prevalence of TB caused by individual members of the MTBC. Moreover, the same treatment regimen of isoniazid, rifampicin, pyrazinamide, and ethambutol is advised for all patients diagnosed with TB [29]. While M. tuberculosis is susceptible to all the drugs, M. bovis is not susceptible to pyrazinamide.
4. Animal movements as a factor of bTB spread
A logical assumption reached by analyzing historical data is that bTB caused by M. bovis could have emerged from Europe, circulating from northern Italy to Western Europe, further moving along the UK, and after that, distributed throughout the world through the cattle trade from the UK and the Netherlands to their former colonies [13]. Cattle were also exported to Africa from Europe in order to improve their dairy production. This resulted in the introduction, propagation, and evolution of M. bovis strains previously restricted to only specific geographies among countries with economic and political ties [30]. A similar study in Mali used the variable number of tandem repeats (VNTR) analysis to establish a connection between the M. bovis strains isolated from the slaughterhouse and those isolated in France and Spain [31].
These pieces of evidence suggest that monitoring animal movements across borders is necessary. Testing of live animals that are exported and imported for the dairy industry, meat, etc. could prevent the transmission of bTB and hamper the introduction of new strains in the population. In the Indian context, cattle are often exported to Bangladesh and imported from Bhutan illegally due to porous international borders. Cattle are exported to Bangladesh from West Bengal and Tripura due to the rising demand for beef and other by-products like leather. Since slaughtering live animals for meat is a taboo in Bhutan, animals are imported to Arunachal Pradesh and Assam borders in India, where animals are slaughtered and cleaned. The meat is then supplied to markets across the border to Bhutan, where it is sold for human consumption [32].
5. Tuberculosis in wild animals as a threat to bTB eradication
A significant hurdle many countries face in eradicating bTB is the presence of wildlife hosts. Bovine tuberculosis has a range of hosts, making the risk of spillback infections from wildlife to cattle and/or humans and establishing wildlife reservoirs more prominent [33]. Such wildlife reservoirs have been widely reported across the globe [34]. Such wildlife reservoirs make bTB control measures moot, posing the risk of spillback infections to other hosts. In India, M. tuberculosis has been identified in wild elephants post-mortem confirmed by culture and PCR methods [35]. Mycobacterium tuberculosis has also been similarly identified from one gazelle [36]. Mycobacterium orygis was isolated from free-ranging spotted deer and black buck in Guindy National Park in India, a space with extensive human animal interactions [37].
Though eradication of bTB from all fronts is necessary, identification of species that are principal drivers of bTB persistence is necessary. For instance, the feral pigs (Sus scrofa) in the Northern Territory of Australia are susceptible to bTB but do not maintain the infection [38]. On the other hand, badgers in Britain, white tailed deer in Michigan, wild boar in Northern Italy and red deer in Spain are susceptible to bTB as well as capable of maintaining and transmitting the disease [[39], [40], [41], [42], [43]]. Thus it is essential to identify which species are involved in the spectrum of bTB transmission and how they fit into the spectrum to devise bTB management programs in wildlife. Hence it is critical in the context of resource constraint settings to not only avoid wastage of resources but also to address the gap in the literature in this area.
6. Presence of MTBC organisms in the environment
The environment as a source of TB transmission has yet to be considered. Since MTBC bacteria remain in the environment long enough to pose a risk of exposure to many species cohabiting in the same habitat, it could potentially promote indirect transmission [44,45]. Some studies have shown isolation of MTBC species from soil and water samples in TB endemic regions. A total of 1500 samples (800 soil samples and 700 water samples, 47% and 53%, respectively) were collected throughout three metropolitan counties in Tehran between February 2012 and January 2014. Eleven of 800 soil samples (1%) and 71 of 700 water samples (10%) were positive for M. tuberculosis after culture and spoligotyping. T family (56 of 82, 68%) and Delhi/CAS (11/82, 13.4%) were the most common M. tuberculosis superfamilies in water and soil samples. While 27.7% of cluster isolates were related, clinical, water, and soil isolates did not share typing characteristics [46].
A similar study was conducted in South Africa. This study used polymerase chain reaction methods to compare total mycobacteria and MTBC members in both treated and untreated wastewater. Three wastewater treatment plants in Durban, a region that has a high rate of TB, were sampled. Total mycobacteria and MTBC count varied per plant in untreated wastewater samples. In addition, M. bovis and M. caprae, two other MTBC organisms, were also detected [47].
In Spain, samples were collected from Donana National Park and Ciudad Real province, with abundant and widely distributed cattle and wild ungulate populations. In this region, TB is endemic and highly prevalent. Overall, 8.9% of the water sites tested positive for MTBC in water samples, compared to 55.8% of the water points in mud samples taken from the coast. The smallest waterholes and those where cachectic animals congregate had the highest percentages of MTBC-positive samples [48].
7. Major needs and recommendations- towards a One Health approach
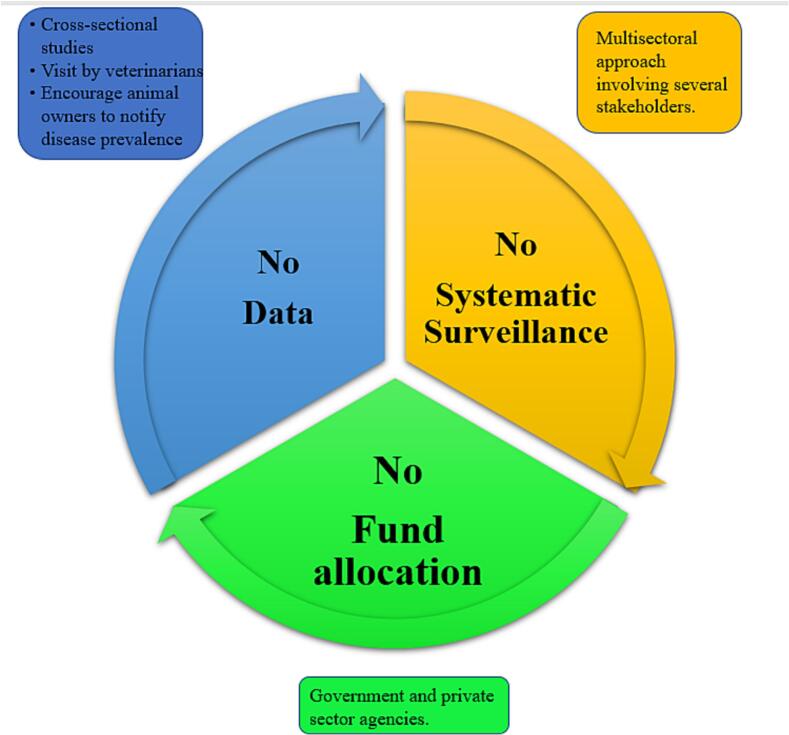
The prevalence of bTB infection in cattle and other hosts and associated risk factors have not been characterized sufficiently in India. This gap in literature needs to be addressed, and initial prevalence reports of bTB and zoonotic TB (zTB) have to be wrought. A systematic review of surveillance strategies of zTB globally categorized countries based on their income and TB surveillance methods. The review reported that out of 119 countries considered, only 12 countries had both TB surveillance and specific zTB surveillance activities and 29 countries had TB surveillance programs without zTB surveillance activities, but relevant zTB information was available. In only four of those 12 countries (all four high income countries), coordination with human and animal sectors was observed [49]. Often, the lack of data regarding prevalence leads to the inexistence of a systematic surveillance program, limiting the funds allocated for disease eradication, which again results in poor quantification of disease prevalence (Fig. 2).
Fig. 2.
The cycle leading to inaction against curbing bovine tuberculosis and few actionable recommendations.
Preventing the transmission of infection from bovine to humans and vice versa is the primary need. A comprehensive One Health approach could be a significant foot forward. The absence of a systematic eradication program has resulted in a poorly quantified prevalence of mycobacterial infections in Indian livestock. Good caliber surveillance information and improved reporting frameworks will help the countrys precisely screen for bTB in animal populations, helping to realize the actual burden of the disease.
The bTB control program in the USA is one of the most successful worldwide. Since its establishment, the program has relied on contributions from the states, business, and the federal government to carry out bTB control and eradication initiatives. This program's success has largely been attributed to this cooperative approach [50]. Such an approach would also benefit India, involving various central and state departments to elicit a One Health approach.
An effective One Health program requires strong political will, evidence-based policy innovations, clearly defined agency duties and tasks, coordination mechanisms at all levels, and an open information exchange culture, as demonstrated by Tamil Nadu's state-wide rabies management program in India. Key stakeholders, including the Directorate of Public Health & Preventive Medicine (DPH), Directorate of Medical Education (DME), Directorate of Rural Health & Medical Services (DHS), Municipal Administration Department (MAD), Department of Animal Husbandry (DAH), Tamil Nadu Medical Services Corporation (TNMSC), and civil society organizations were involved in documenting policy initiatives, describe the program, and comprehend their roles. To identify patterns at the district level in the state, dog bite surveillance data were triangulated with information on vaccination usage and dog population. As a result, rabies has been managed by numerous departments in Tamil Nadu. Other focused actions included waste management, animal birth control, anti-rabies vaccination, awareness campaigns, and widespread access to the vaccine at public health facilities [51].
Coordination between the veterinary, clinical, epidemiological, and public health sectors is necessary to design and structure TB eradication programs that integrate both the human and fronts. This would lead to better One Health practices that will help eradicate TB. Timely reports of infected herds and immediate steps to prevent transmission could be achieved through amicable and synergistic actions taken by these sectors. Sharing knowledge regarding the occurrence, distribution, genetic diversity, and extent and mode of interspecies transmission of bTB is very important.
In India, achieving this kind of collaboration has been a challenge. The Ministry of Health and Family Welfare's key organizations for promoting human well-being are the National Center for Disease Control (NCDC) and the Indian Council for Medical Research. The Department of Animal Husbandry and Dairying and the Animal Science division of the Indian Council for Agricultural Research operate under the Ministries of Agriculture and Farmers Welfare and Fisheries, Animal Husbandry & Dairying, focusing on animal health to increase food production and safety. The Ministry of Environment, Forests and Climate Change, which is in charge of matters connected to environmental safety and conservation, also oversees the wildlife sector. Additionally, only some representatives from the wildlife sector are found on the National Standing Committee on Zoonoses (NSCZ), a committee sponsored by the NCDC and dedicated to advancing public health. Due to this fragmentation and disparate sectoral affiliations, cross-sectoral convergence is complex, given the different objectives and power dynamics between ministries and departments [52]. Some recommendations for the eradication of bTB are as follows.
7.1. Raising awareness among animal handlers
Animal handlers come into direct contact with infected animals; as a result, it is imperative that they be informed about bTB and the effects it can have. Farmers in Guwahati, Ludhiana, and Bangalore, three important towns in India, were observed by Chauhan et al. in 2019 to assess their awareness regarding bTB in order to combat the disease. In the case of animals showing symptoms, it was noted that consultations with veterinarians were only sought out as a last resort once the disease had reached its most advanced stage. The farmers were only able to comprehend the meaning of the word “tuberculosis” in the human context [53].
The diagnosis of bTB depends upon the animal handlers' ability to identify the disease's initial symptoms. This would enable them to seek help from healthcare and veterinary service providers in both the public and private sectors. This would also help collect data regarding the diseased animals. Therefore, the need for more awareness about bTB among rural and peri-urban farmers regarding its cause and effects is the primary obstacle in thwarting bTB. Private farmers do not test their animals for tuberculin because they often do not understand zoonotic illnesses, the associated risks, or the testing methods for them. Awareness programs must be initiated at the community level and upscaled until the national level. This includes advice from veterinary and healthcare professionals following regular visits to animal enclosures, meetings at the community level, and the use of mass media. Animal handlers and people in associated professions should be made aware that they are stakeholders in eliminating bTB and their crucial role in the process.
7.2. Development of human resources
The elimination of bTB requires well-trained human resources in public health. Drawing from wisdom outside the purview of bTB, the dromedary camel project in Laikipia County, Kenya, run by the Saint Louis Zoo Institute for Conservation Medicine since 2012, is an exemplar of work at the domestic animal/wildlife/human interface. The University of Missouri- Columbia's Master of Public Health Program, the zoo, and several local partners collaborated to understand better the epidemiology of zoonotic pathogens in Kenyan dromedary camels, such as Q fever and infections caused by Brucella species. Several Kenyan and American veterinary, MSc (Master of Science), and public health students have also received training as a result of this project in order to understand better the disease risks linked to changing environmental conditions, human protein sources, and the inevitable rise in interactions at the domestic animal/wildlife/human interface. This initiative illustrates a significant transdisciplinary One Health initiative that integrates zoos and public health organizations [54]. Such initiatives could be undertaken in India regarding bTB, where the spectrum of healthcare workers could be trained in the prevention, diagnosis, and treatment of both TB and bTB.
7.3. Ante-mortem and post-mortem diagnosis of bTB in cattle
As described earlier, ante-mortem testing of cattle for bTB has played a significant role in bTB eradication programs worldwide. Once the animals are diagnosed as positive reactors, they must be segregated from the herd. This prevents the spread of disease within the animals in the respective herds. Ante-mortem testing can also be used to identify humans who are at risk of TB disease. In the initial stages of the program, cattle owners can also be given incentives for maintaining bTB-free herds to motivate them to participate in the eradication.
Routine slaughterhouse surveillance in abattoirs in India is not a norm. The global prevalence of bTB caused by MTBC organisms estimated by slaughterhouse surveillance alone is 426 per 1000 slaughtered cattle showing visible TB like lesions [1]. Slaughterhouse surveillance can thus play a pivotal role in bTB control, especially in endemic areas, through proper meat inspection protocols. So far in India, only two studies have reported the prevalence of bTB through slaughterhouse surveillance [55,56].
7.4. Mandatory pasteurization of milk
Nearly 46% of the milk produced in India is either consumed by the producers, most often in the unpasteurized form, or sold to non-producers in rural areas. In contrast, the remaining 54% is available for sale to organized and unorganized sectors. The unorganized/informal sector involves local milkmen, contractors, etc. [57]. Several studies from India have documented the isolation of either M. tuberculosis or M. bovis from milk samples collected from infected animals [36,58,59]. In the context of these results and the evidence of the drastic decline in the prevalence of TB in Britain after enacting compulsory laws regarding the pasteurization of milk in Great Britain [7], stringent laws in India could significantly lower of the prevalence of the disease.
7.5. Contact tracing of infected cattle
Following the isolation of a reactor cattle in the herd, it is imperative to identify those animals that are currently in contact with the infected animal or has been previously exposed to it and test them for bTB. A disturbing trend observed in Africa that could apply to the Indian context is that animal handlers usually sell the reactor cattle in the market because culling them would lead to economic losses since no compensation from the government or other stakeholders is provided. This could lead to the spread of the disease from one herd to another [60].
A study in the United States reported that contact tracing has helped trace back 70% of TB-infected adult bovines and 50% of TB-infected calves (less than two years) to epidemiologically linked herds [61].
In India, about 80 million rural households engage themselves in the production of milk. A large proportion of milk producers (about 95%) in the country hold 1 to 5 milch animals per household as a part of the subsistence farming system [57]. This means a significant population of the country share living quarters with animals and is in close contact with them. This results in a greater zoonotic and reverse zoonotic risk. In a study conducted in Chennai, 18.5% of animal handlers who rear cattle showed symptoms of TB disease. When their sputum samples were tested, 12% of those who showed symptoms were found to be culture positive for TB [21]. Contact tracing of humans and cattle in close proximity to the infected animals will help identify diseased herds and humans to begin preventive steps to deter transmission at the earliest.
7.6. Vaccination of cattle
Vaccination of cattle and other reservoirs of bTB is hypothetically the cornerstone of a bTB eradication program. The BCG (Bacille de Calmette et Guérin) vaccine has been used in countries like the United Kingdom, USA, Africa, Canada, and Australia but with no apparent success. A systematic review and meta-analysis by Srinivasan et al., in 2021 reports an overall vaccine efficacy of 25% (95% CI: 18, 32) as measured by the presence of visible lesions and/or culture in experimentally infected cattle as well as naturally infected cattle across the globe. The study further speculates that in high disease-burden countries (prevalence between 20 and 40%), 50–95% of cumulative cases may be averted over the next 50 years, and in the case of low to moderate (prevalence <15%) settings, officially TB free status can be achieved in the next ten years upon immediate implementation of BCG vaccine [62].
However, this strategy suffers a distinct disadvantage- the test sensitizes cattle to tuberculin skin tests. If the test and slaughter policy is administered in India, a positive reaction to the tuberculin skin test will result in culling productive disease-free cattle [2]. Recently, vaccine research has focused on antigens that do not react with tuberculin skin tests i.e., those antigens that are absent in BCG but present in M. bovis. The ESAT-6, CFP10, and Rv3615c antigens have shown a possibility for prospects in this area [[63], [64], [65]]. In addition, other developments, including the innovation of DIVA (differentiate diseased animals from vaccinated animals) tests, might enable the BCG vaccine to be a part of routine testing as well [66].
8. Conclusion
This review has presented information about the current state of bTB in India, where the importance and necessity of bTB surveillance programs is increasing at the human-animal-environment front. It is imperative to form new policies that would systematically eradicate the disease in humans, animals and the environment. Incorporating the One Health approach to attain this goal will ensure the eradication of origin and disease transmission from all fronts.
Ethical approval
The authors have declared that ethical statement is not applicable for this manuscript.
Funding
The project was funded by ICMR (Grant IDs: ZON/41/2019/ECD-II and Fellowship/97/2022/ECD-II).The funding agency has no role in planning this study.
Declaration of Competing Interest
Authors declare no conflict of interest.
Acknowledgements
Harini Ramanujam thanks ICMR for the ICMR-Senior Research Fellow (SRF) fellowship (Fellowship/97/2022/ECD-II).
Data availability
Data will be made available on request.
References
- 1.Ramanujam H., Thiruvengadam K., Singaraj R., Palaniyandi K. Role of abattoir monitoring in determining the prevalence of bovine tuberculosis: a systematic review and meta-analysis, Transbound. Emerg. Dis. 2021:1–16. doi: 10.1111/tbed.14118. [DOI] [PubMed] [Google Scholar]
- 2.Refaya A.K., Bhargavi G., Mathew N.C., Rajendran A., Krishnamoorthy R., Swaminathan S., Palaniyandi K. A review on bovine tuberculosis in India. Tuberculosis. 2020;122 doi: 10.1016/j.tube.2020.101923. [DOI] [PubMed] [Google Scholar]
- 3.NDDB Annual Report National Dairy Development Board. 2021. http://www.nddb.org/sites/default/files/NDDB_AR_2015-16Eng.pdf
- 4.Srinivasan S., Easterling L., Rimal B., Niu X.M., Conlan A.J.K., Dudas P., Kapur V. Prevalence of Bovine Tuberculosis in India: a systematic review and meta-analysis. Transbound. Emerg. Dis. 2018;65:1627–1640. doi: 10.1111/tbed.12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Global Tuberculosis Report 2021. 2021. https://www.who.int/publications/i/item/9789240037021
- 6.OIE Roadmap for zoonotic Tuberculosis. 2018. www.who.int (accessed December 7, 2021) [DOI] [PubMed]
- 7.Waters W.R., Palmer M.V., Buddle B.M., Vordermeier H.M. Bovine tuberculosis vaccine research: historical perspectives and recent advances. Vaccine. 2012;30:2611–2622. doi: 10.1016/j.vaccine.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Olmstead A.L., Rhode P.W. An impossible undertaking: the eradication of bovine tuberculosis in the United States. J. Econ. Hist. 2004;64:734–772. doi: 10.1017/s0022050704002955. [DOI] [Google Scholar]
- 9.USDA . 2005. Bovine Tuberculosis Eradication Uniform Methods and Rules. [Google Scholar]
- 10.Lawes J.R. Bovine TB surveillance in Great Britain in 2014. Vet. Rec. 2016;178:310–315. doi: 10.1136/vr.i1616. [DOI] [PubMed] [Google Scholar]
- 11.European Commission . 2013. Health & Consumer Protection Directorate-General. [Google Scholar]
- 12.Pascual-Linaza A.V., Gordon A.W., Stringer L.A., Menzies F.D. Efficiency of slaughterhouse surveillance for the detection of bovine tuberculosis in cattle in Northern Ireland. Epidemiol. Infect. 2017;145:995–1005. doi: 10.1017/S0950268816003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michel A.L., Müller B., Van Helden P. David. Mycobacterium bovis at the animal human interface: a problem, or not? Vet. Microbiol. 2010;140:371–381. doi: 10.1016/j.vetmic.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 14.Islam S.K.S., Rumi T.B., Kabir S.M.L., van der Zanden A.G.M., Kapur V., Rahman A.K.M.A., Ward M.P., Bakker D., Ross A.G., Rahim Z. Bovine tuberculosis prevalence and risk factors in selected districts of Bangladesh. PLoS One. 2020;15 doi: 10.1371/JOURNAL.PONE.0241717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnot L.F., Michel A. Challenges for controlling bovine tuberculosis in South Africa. Onderstepoort J. Vet. Res. 2020;87 doi: 10.4102/OJVR.V87I1.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SM D.R. Mallick, Aggarwal H. An investigation into the incidence and type of tuberculous infection in cattle at Amritsar with special reference to human infections. Indian Meddical Gaz. 1942;77:668–672. [PMC free article] [PubMed] [Google Scholar]
- 17.National tuberculosis Prevalence Survey 2019–2021. 2021. [Google Scholar]
- 18.WHO Global Tuberculosis Report 2020, Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO. 2020. http://apps.who.int/bookorders (accessed November 29, 2020)
- 19.Duffy S.C., Srinivasan S., Schilling M.A., Stuber T., Danchuk S.N., Michael J.S., Venkatesan M., Bansal N. Reconsidering Mycobacterium bovis as a proxy for zoonotic tuberculosis : a molecular epidemiological surveillance study. The Lancet Microbe. 2020;1:e66–e73. doi: 10.1016/S2666-5247(20)30038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Refaya A.K., Kumar N., Raj D., Veerasamy M., Balaji S., Shanmugam S., Rajendran A., Tripathy S.P., Swaminathan S., Peacock S.J., Palaniyandi K. Whole-genome sequencing of a Mycobacterium orygis strain isolated from cattle in Chennai, India. Microbiol. Resour. Announc. 2019;8:1–3. doi: 10.1128/mra.01080-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palaniyandi K., Kumar N., Veerasamy M., Kabir Refaya A., Dolla C., Balaji S., Baskaran D., Thiruvengadam K., Rajendran A., Narayanan S., Raj D., Swaminathan S., Peacock S.J. Isolation and comparative genomics of Mycobacterium tuberculosis isolates from cattle and their attendants in South India. Sci. Rep. 2019;9:1–7. doi: 10.1038/s41598-019-54268-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sweetline Anne N., Ronald B.S.M., Kumar T.M.A.S., Kannan P., Thangavelu A. Molecular identification of Mycobacterium tuberculosis in cattle. Vet. Microbiol. 2017;198:81–87. doi: 10.1016/j.vetmic.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 23.van Ingen J., Rahim Z., Mulder A., Boeree M.J., Simeone R., Brosch R., van Soolingen D. Characterization of Mycobacterium orygis as M. tuberculosis complex subspecies. Emerg. Infect. Dis. 2012;18:653–655. doi: 10.3201/eid1804.110888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vitale M. Zoonotic tuberculosis: a complex issue of the Mycobacterium tuberculosis complex. The Lancet Microbe. 2020;1:e45–e46. doi: 10.1016/S2666-5247(20)30032-X. [DOI] [PubMed] [Google Scholar]
- 25.Prasad H.K., Singhal A., Mishra A., Shah N.P., Katoch V.M., Thakral S.S., Singh D.V., Chumber S., Bal S., Aggarwal S., Padma M.V., Kumar S., Singh M.K., Acharya S.K. Bovine tuberculosis in India: potential basis for zoonosis. Tuberculosis. 2005;85:421–428. doi: 10.1016/j.tube.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Bapat P.R., Dodkey R.S., Shekhawat S.D., Husain A.A., Nayak A.R., Kawle A.P., Daginawala H.F., Singh L.K., Kashyap R.S. Prevalence of zoonotic tuberculosis and associated risk factors in Central Indian populations. J. Epidemiol. Glob. Health. 2017;7:277. doi: 10.1016/J.JEGH.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NSSO . Vol. 558. 2014. Household Consumer Expenditure, NSS 68th Round Sch1.0 Type 1: July 2011—June 2012; p. 457.http://mospi.nic.in/sites/default/files/publication_reports/Report_no558_rou68_30june14.pdf [Google Scholar]
- 28.Central Pollution Control Board Revised Comprehensive Industry Document on Slaughter Houses Central. 2017. https://cpcb.nic.in/openpdffile.php?id=TGF0ZXN0RmlsZS8xNzVfMTUxMTI2NDE0MV9tZWRpYXBob3RvODkzOS5wZGY
- 29.Revised National Tuberculosis Control Programme 2017. https://www.nhp.gov.in/revised-national-tuberculosis-control-programme_pg (accessed April 19, 2021)
- 30.Müller B., Hilty M., Berg S., Garcia-Pelayo M.C., Dale J., Boschiroli M.L., Cadmus S., Ngandolo B.N.R., Godreuil S., Diguimbaye-Djaibé C., Kazwala R., Bonfoh B., Njanpop-Lafourcade B.M., Sahraoui N., Guetarni D., Aseffa A., Mekonnen M.H., Razanamparany V.R., Ramarokoto H., Djønne B., Oloya J., Machado A., Mucavele C., Skjerve E., Portaels F., Rigouts L., Michel A., Müller A., Källenius G., Van Helden P.D., Hewinson R.G., Zinsstag J., Gordon S.V., Smith N.H. African 1, an epidemiologically important clonal complex of Mycobacterium bovis dominant in Mali, Nigeria, Cameroon, and Chad. J. Bacteriol. 2009;191:1951–1960. doi: 10.1128/JB.01590-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller B., Steiner B., Bonfoh B., Fané A., Smith N.H., Zinsstag J. Molecular characterisation of Mycobacterium bovis isolated from cattle slaughtered at the Bamako abattoir in Mali. BMC Vet. Res. 2008;4:2–7. doi: 10.1186/1746-6148-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.To define and analyse cross- border and in-country livestock and livestock- products market systems in India for control of Transboundary Animal Diseases. 2013. [Google Scholar]
- 33.Nugent G. Maintenance, spillover and spillback transmission of bovine tuberculosis in multi-host wildlife complexes: a New Zealand case study. Vet. Microbiol. 2011;151:34–42. doi: 10.1016/j.vetmic.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 34.Allen A.R., Skuce R.A., Byrne A.W. Bovine tuberculosis in Britain and Ireland - a perfect storm? The confluence of potential ecological and epidemiological impediments to controlling a chronic infectious disease. Front. Vet. Sci. 2018;5:109. doi: 10.3389/FVETS.2018.00109/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zachariah A., Pandiyan J., Madhavilatha G.K., Mundayoor S., Chandramohan B., Sajesh P.K., Santhosh S., Mikota S.K. Mycobacterium tuberculosis in wild Asian elephants, southern India. Emerg. Infect. Dis. 2017;23:504–506. doi: 10.3201/EID2303.161741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukherjee F., Kanani A.N., Sharma G.K., Srinivasan V.A., Bahekar V.S., Pasha S.Y., Kannan P., Prasad A., Rana S.K., Premalatha D. Isolation and analysis of the molecular epidemiology and zoonotic significance of Mycobacterium tuberculosis in domestic and wildlife ruminants from three states in India. Rev. Sci. Tech. 2018;37:999–1012. doi: 10.20506/rst.37.3.2902. [DOI] [PubMed] [Google Scholar]
- 37.Refaya A.K., Ramanujam H., Ramalingam M., Rao G.V.S., Ravikumar D., Sangamithrai D., Shanmugam S., Palaniyandi K. Tuberculosis caused by Mycobacterium orygis in wild ungulates in Chennai, South India, Transbound. Emerg. Dis. 2022:1–7. doi: 10.1111/tbed.14613. [DOI] [PubMed] [Google Scholar]
- 38.Mcinerney J., Small K., Caley P. Prevalence of Mycobacterium bovis infection in feral pigs in the Northern Territory. Aust. Vet. J. 1995;72:448–451. doi: 10.1111/j.1751-0813.1995.tb03486.x. [DOI] [PubMed] [Google Scholar]
- 39.Schmitt S.M., Fitzgerald S.D., Cooley T.M., Bruning-Fann C.S., Sullivan L., Berry D., Carlson T., Minnis R.B., Payeur J.B., Sikarskie J. Bovine tuberculosis in free-ranging white-tailed deer from Michigan. J. Wildl. Dis. 1997;33:749–758. doi: 10.7589/0090-3558-33.4.749. [DOI] [PubMed] [Google Scholar]
- 40.Morris R.S., Pfeiffer D.U., Jackson R. The epidemiology of Mycobacterium bovis infections. Vet. Microbiol. 1994;40:153–177. doi: 10.1016/0378-1135(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 41.Dorn-In S., Körner T., Büttner M., Hafner-Marx A., Müller M., Heurich M., Varadharajan A., Blum H., Gareis M., Schwaiger K. Shedding of Mycobacterium caprae by wild red deer (Cervus elaphus) in the Bavarian alpine regions, Germany. Transbound. Emerg. Dis. 2020;67:308–317. doi: 10.1111/tbed.13353. [DOI] [PubMed] [Google Scholar]
- 42.Tagliapietra V., Boniotti M.B., Mangeli A., Karaman I., Alborali G., Chiari M., D’incau M., Zanoni M., Rizzoli A., Pacciarini M.L. Mycobacterium microti at the environment and wildlife interface. Microorganisms. 2021;9 doi: 10.3390/MICROORGANISMS9102084/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghielmetti G., Kupca A.M., Hanczaruk M., Friedel U., Weinberger H., Revilla-Fernández S., Hofer E., Riehm J.M., Stephan R., Glawischnig W. Mycobacterium microti infections in free-ranging red deer (Cervus elaphus) Emerg. Infect. Dis. 2021;27:2025–2032. doi: 10.3201/eid2708.210634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghodbane R., Medie F.M., Lepidi H., Nappez C., Drancourt M. Long-term survival of tuberculosis complex mycobacteria in soil. Microbiol. (United Kingdom). 2014;160:496–501. doi: 10.1099/mic.0.073379-0. [DOI] [PubMed] [Google Scholar]
- 45.Palmer M.V., Waters W.R., Whipple D.L. Investigation of the transmission of Mycobacterium bovis from deer to cattle through indirect contact. Am. J. Vet. Res. 2004;65:1483–1489. doi: 10.2460/ajvr.2004.65.1483. [DOI] [PubMed] [Google Scholar]
- 46.Velayati A.A., Farnia P., Mozafari M., Malekshahian D., Farahbod A.M., Seif S., Rahideh S., Mirsaeidi M. Identification and genotyping of Mycobacterium tuberculosis isolated from water and soil samples of a Metropolitan City. Chest. 2015;147:1094. doi: 10.1378/CHEST.14-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mtetwa H.N., Amoah I.D., Kumari S., Bux F., Reddy P. Molecular surveillance of tuberculosis-causing mycobacteria in wastewater. Heliyon. 2022;8 doi: 10.1016/J.HELIYON.2022.E08910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barasona J.A., Vicente J., Díez-Delgado I., Aznar J., Gortázar C., Torres M.J. Environmental presence of Mycobacterium tuberculosis complex in aggregation points at the wildlife/livestock interface. Transbound. Emerg. Dis. 2017;64:1148–1158. doi: 10.1111/tbed.12480. [DOI] [PubMed] [Google Scholar]
- 49.de Macedo Couto R., Santana G.O., Ranzani O.T., Waldman E.A. One Health and surveillance of zoonotic tuberculosis in selected low-income, middle-income and high-income countries: a systematic review. PLoS Negl. Trop. Dis. 2022;16 doi: 10.1371/JOURNAL.PNTD.0010428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thoen C.O., Steele J.H., Kaneene J.B. third edition. Wiley Blackwell; 2014. Zoonotic Tuberculosis. [Google Scholar]
- 51.Abbas S.S., Venkataramanan V., Pathak G., Kakkar M. Rabies control initiative in Tamil Nadu, India: a test case for the “One Health” approach. Int. Health. 2011;3:231–239. doi: 10.1016/j.inhe.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Asaaga F.A., Young J.C., Oommen M.A., Chandarana R., August J., Joshi J., Chanda M.M., Vanak A.T., Srinivas P.N., Hoti S.L., Seshadri T., Purse B.V. Operationalising the “One Health” approach in India: facilitators of and barriers to effective cross-sector convergence for zoonoses prevention and control. BMC Public Health. 2021;21:1–21. doi: 10.1186/s12889-021-11545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chauhan A.S., George M.S., Lindahl J., Grace D., Kakkar M. Community, system and policy level drivers of bovine tuberculosis in smallholder periurban dairy farms in India: a qualitative enquiry. BMC Public Health. 2019;19:301. doi: 10.1186/s12889-019-6634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robinette C., Saffran L., Ruple A., Deem S.L. Zoos and public health: a partnership on the one health frontier. One Heal. 2017;3:1. doi: 10.1016/J.ONEHLT.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barua A.G., Raj H., Goswami C. Slaughter house surveillance for tuberculosis among cattle in Ri-Bhoi district of Meghalaya. Int. J. Vet. Sci. Anim. Husb. 2016;1:14–15. www.veterinarypaper.com [Google Scholar]
- 56.Barua A., Raj H., Goswami C., Konwar P., Chutia J. Bovine tuberculosis- abattoir prevalence, species identification and its economic impact assessment of Assam and Meghalaya. Int. J. Livest. Res. 2018;8:204. doi: 10.5455/ijlr.20180209095048. [DOI] [Google Scholar]
- 57.Department of Animal Husbandry and Dairying Government of India Annual Report 2018–2019. 2019. http://dahd.gov.in/sites/default/filess/AnnualReport 2019-20.pdf
- 58.Srivastava K., Chauhan D.S., Gupta P., Singh H.B., Sharma V.D., Yadav V.S., Sreekumaran S.S., Thakral J.S., Dharamdheeran P., Nigam H.K., Prasad V.M. Katoch. Isolation of Mycobacterium bovis & M. tuberculosis from cattle of some farms in north India - Possible relevance in human health. Indian J. Med. Res. 2008;128:26–31. [PubMed] [Google Scholar]
- 59.Amy S.A., Batish V.K., Parkash O.M., Ranganathan B. Prevalence of Mycobacteria in Raw Milk Sampled in Karnal, India. 1980. http://meridian.allenpress.com/jfp/article-pdf/43/10/778/1649996/0362-028x-43_10_778.pdf (accessed July 29, 2020) [DOI] [PubMed]
- 60.Benkirane A. Bovine tuberculosis in Africa. World Anim. Rev. 1998 http://www.fao.org/ag/aga/AGAP/FRG/FEEDback/War/W8600t/w8600t09.htm (accessed January 1, 2021) [Google Scholar]
- 61.Humphrey H.M., Orloski K.A., Olea-Popelka F.J. Bovine tuberculosis slaughter surveillance in the United States 2001-2010: assessment of its traceback investigation function. BMC Vet. Res. 2014;10:182. doi: 10.1186/s12917-014-0182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Srinivasan S., Conlan A.J.K., Easterling L.A., Herrera C., Dandapat P., Veerasami M., Ameni G., Jindal N., Raj G.D., Wood J., Juleff N., Bakker D., Vordermeier M., Kapur V. A meta-analysis of the effect of Bacillus Calmette-Guérin vaccination against bovine tuberculosis: is perfect the enemy of good? Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.637580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whelan A.O., Clifford D., Upadhyay B., Breadon E.L., McNair J., Hewinson G.R., Vordermeier M.H. Development of a skin test for bovine tuberculosis for differentiating infected from vaccinated animals. J. Clin. Microbiol. 2010;48:3176–3181. doi: 10.1128/JCM.00420-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vordermeier H.M., Jones G.J., Buddle B.M., Hewinson R.G., Villarreal-Ramos B. Bovine tuberculosis in cattle: vaccines, DIVA tests, and host biomarker discovery. Annu. Rev. Anim. Biosci. 2016;4:87–109. doi: 10.1146/annurev-animal-021815-111311. [DOI] [PubMed] [Google Scholar]
- 65.Sidders B., Pirson C., Hogarth P.J., Hewinson R.G., Stoker N.G., Vordermeier H.M., Ewer K. Screening of highly expressed mycobacterial genes identifies Rv3615c as a useful differential diagnostic antigen for the Mycobacterium tuberculosis complex. Infect. Immun. 2008;76:3932–3939. doi: 10.1128/IAI.00150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chambers M.A., Graham S.P., La Ragione R.M. Methods Mol. Biol. Humana Press Inc.; 2016. Challenges in veterinary vaccine development and immunization; pp. 3–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.