Author's summary
In recent years, there has been growing interest in the subspecialty of cardio-oncology worldwide. With the growth in cardiovascular and cancer burden in Asia, there is a greater need for cardio-oncology awareness among physicians managing this unique group of patients and need to develop country-specific cardio-oncology initiatives. In this state-of-the-art review, we sought to describe the epidemiology of cancer and cardiovascular disease burden in Asia, a region with rich cultural and socio-economic diversity. From describing the uniqueness and challenges in establishing cardio-oncology in Asia, and ways to overcome any barriers, this article aims to advance the field of cardio-oncology in this part of the world.
Keywords: Cardio-oncology, Onco-cardiology, Cardiotoxicity, Asia, Cancer
Abstract
Cardio-oncology is an emerging multi-disciplinary field, which aims to reduce morbidity and mortality of cancer patients by preventing and managing cancer treatment-related cardiovascular toxicities. With the exponential growth in cancer and cardiovascular diseases in Asia, there is an emerging need for cardio-oncology awareness among physicians and country-specific cardio-oncology initiatives. In this state-of-the-art review, we sought to describe the burden of cancer and cardiovascular disease in Asia, a region with rich cultural and socio-economic diversity. From describing the uniqueness and challenges (such as socio-economic disparity, ethnical and racial diversity, and limited training opportunities) in establishing cardio-oncology in Asia, and outlining ways to overcome any barriers, this article aims to help advance the field of cardio-oncology in Asia.
THE EMERGENCE OF CARDIO-ONCOLOGY
The precise timing of the birth of cardio-oncology is unknown, though it could have started in the 1970s with patients being admitted to the intensive care units of Memorial Sloan-Kettering Cancer Center (MSKCC) in New York for the management of cardiac failure after anthracycline therapy. Of note, doxorubicin, the most notorious cardiotoxic anthracycline, was developed as a collaborative effort by researchers at MSKCC, Farmitalia and the Instituto Nazionale di Tumori in Milano, Italy (the latter two had previously developed daunorubicin).1) Although cardiotoxicity became a dose-limiting side effect of anthracyclines and more cancer treatment-related cardiovascular toxicities (CTR-CVT) were observed with other chemotherapeutics (such as 5-fluorouracil [5-FU] and paclitaxel), a need for subspeciality care did not seem to arise. This changed paradoxically with the arrival of targeted therapies, which were expected to have less toxicities. Explicitly, it was the recognition that a significant percentage of breast cancer patients would develop heart failure, mild to life-threatening in severity, when the human epidermal growth factor receptor 2 (HER-2) directed monoclonal antibody trastuzumab was prescribed in combination with anthracyclines. This unexpected observation changed the landscape of cancer therapeutics forever, prompting engagement across various disciplines and leading to research on the previously unrecognized role of the HER-2 receptor in cardiomyocyte signaling. With the introduction of newer cancer therapeutics, a broadening spectrum of CTR-CVT emerged. The need for expertise in preventing and managing CTR-CVT ultimately led to the birth of cardio-oncology in the first decade of the 21st century.
The goals of cardio-oncology are to minimize cancer therapy disruptions and to reduce morbidity and mortality of cancer patients by preventing and managing CTR-CVT.2) To accomplish this goal, cardio-oncology has evolved into a multidisciplinary field with cardiologists collaborating closely with oncologists, hematologists, primary care physicians and allied healthcare professionals to cover the entire spectrum of prevention, early detection, and treatment of CTR-CVT in patients who may or may not have pre-existing cardiovascular disease (CVD) or are otherwise at risk of developing cardiovascular (CV) events during cancer therapy.
Over the past decade, cardio-oncology service lines have shown tremendous growth starting in the USA and Europe, and rapidly spreading across the globe including Asia. A testimony of these efforts is the exponential increase in cardio-oncology publications correlating with greater cardio-oncology awareness and research. Japan followed by China contributed the greatest number of cardio-oncology publications in Asia, ranking sixth and tenth, respectively, worldwide in a bibliometric analysis covering years 2010–2022.3) Globally, USA, Italy and England contributed the greatest number of cardio-oncology publications.3) In a concerted global effort to further advance the field of cardio-oncology, there is also robust cross-country collaboration.3)
ADVANCING THE FIELD OF CARDIO-ONCOLOGY IN ASIA
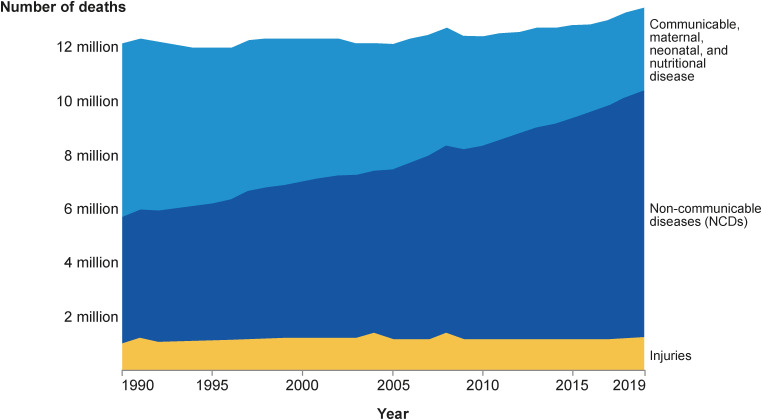
In Asia, socio-economic and healthcare developments over the past decades have led to an epidemiological shift in morbidity demographics from communicable diseases to chronic non-infectious diseases (Figure 1).4) In fact, CVD and cancer are now leading contributors to disease burden worldwide (Figure 2).4)
Figure 1. Deaths by cause, South-East Asia region (WHO), 1990–2019, showing a shift in mortality from communicable, maternal, neonatal and nutritional disease to non-communicable diseases in South-East Asia.4).
NCD = non-communicable disease; WHO = World Health Organization.
Figure 2. Burden of disease by cause, world, 2019. Total disease burden, measured in DALYs by sub-category of disease or injury. DALYs measure the total burden of disease—both from years of life lost due to premature death and years lived with a disability. One DALY equals one lost year of healthy life.4) .
DALY = disability-adjusted life year; HIV/AIDS = human immunodeficiency virus/acquired immunodeficiency syndrome; NCD = non-communicable disease; STI = sexually transmitted infection; TB = tuberculosis.
The burden of cardiovascular disease and cancer in Asia and North America/Europe
CVD accounts for approximately 35% and 21% of total deaths in Asia and the USA, respectively.5),6),7) The higher CV mortality in most parts of Asia compared to North America and Europe underscores the high burden of CVD and potential opportunities for intervention. Although Asia has a lower overall cancer incidence rate (152.2 per 100,000) compared to North America (315.6 per 100,000), and Europe (255.4 per 100,000),8) the burden of cancer in Asia accounts for over 30% of all cases worldwide9),10),11) and is projected to grow exponentially with earlier detection and improved access to newer cancer drugs.10),12) Differences in exposure to risk factors, barriers to high-quality cancer prevention and early cancer detection strategies could account for this variation in cancer incidence rates.13)
The top 5 causes of cancer among men in Asia are lung cancer (35.2 per 100,000), followed by stomach cancer (22.8 per 100,000), liver cancer (20.0 per 100,000), colorectal cancer (16.5 per 100,000) and esophageal cancer (11.4 per 100,000).8) For women in Asia, breast cancer is most common (29.1 per 100,000), followed by lung cancer (12.7 per 100,000), cervical cancer (12.7 per 100,000), colorectal cancer (11.1 per 100,000) and stomach cancer (9.3 per 100,000).8)
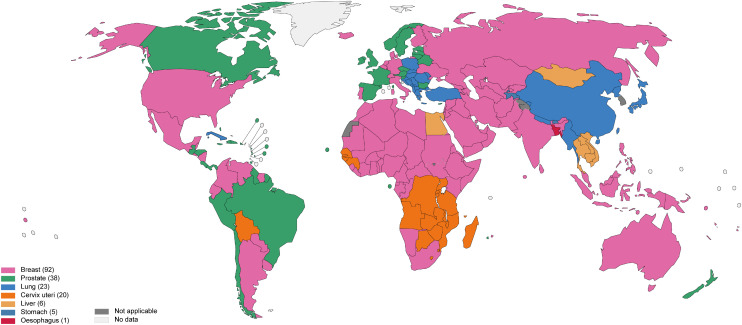
In comparison, among men in North America, prostate cancer (112.7 per 100,000), lung cancer (58.9 per 100,000), colorectal cancer (43.4 per 100,000), urinary bladder cancer (32.4 per 100,000) and skin melanoma (27.6 per 100,000) are the leading causes of cancers. Amongst females in North America, breast cancer (128.3 per 100,000), lung cancer (46.8 per 100,000), colorectal cancer (32.8 per 100,000), uterine cancer (27.8 per 100,000) and skin melanoma (25.5 per 100,000) are the most common cancers.14),15) Table 19),16),17),18) summarizes the epidemiology of leading cancers, cancer survivors and mortality in North America and Asia. Figure 319) shows a world map illustrating the most common cancers by country.
Table 1. Epidemiology of leading cancers, cancer survivors and causes of death in North America and Asia9),16),17),18) .
| Country | Top cancers (by ranking) | Estimated incidence of all cancer types per year | Crude incidence of all cancer types (per 100,000) | Cancer survivors diagnosed within past 5 years (per 100,000) | Leading causes of death (by ranking)17) |
|---|---|---|---|---|---|
| USA | 1) Breast | 1,918,030 | 445.5 | 1,195.7 | 1) Ischemic heart disease |
| 2) Prostate | 2) Lung cancer | ||||
| 3) Lung | 3) COPD | ||||
| 4) Colorectal | 4) Stroke | ||||
| 5) Melanoma of the Skin | 5) Alzheimer’s disease | ||||
| China | 1) Lung | 4,568,754 table | 315.6 | 377.6 | 1) Stroke |
| 2) Colorectal | 2) Ischemic heart disease | ||||
| 3) Gastric | 3) COPD | ||||
| 4) Breast | 4) Lung cancer | ||||
| 5) Liver | 5) Stomach cancer | ||||
| India | 1) Breast | 1,324,413 | 96.0 | 173.5 | 1) Ischemic heart disease |
| 2) Lip (oral cavity) | 2) COPD | ||||
| 3) Cervix | 3) Stroke | ||||
| 4) Lung | 4) Diarrhea disease | ||||
| 5) Colorectal | 5) Neonatal disorders | ||||
| Indonesia | 1) Breast | 396,914 | 145.1 | 298 | 1) Stroke |
| 2) Cervix | 2) Ischemic heart disease | ||||
| 3) Lung | 3) Diabetes | ||||
| 4) Colorectal | 4) Cirrhosis | ||||
| 5) Liver | 5) Tuberculosis | ||||
| Japan | 1) Colorectal | 1,028,658 | 813.3 | 662.4 | 1) Alzheimer’s disease |
| 2) Lung | 2) Stroke | ||||
| 3) Stomach | 3) Ischemic heart disease | ||||
| 4) Prostate | 4) Lower respiratory infection | ||||
| 5) Breast | 5) Lung cancer | ||||
| Malaysia | 1) Breast | 48,160 | 148.8 | 337.2 | 1) Ischemic heart disease |
| 2) Colorectal | 2) Lower respiratory infection | ||||
| 3) Lung | 3) Stroke | ||||
| 4) Nasopharynx | 4) Road injuries | ||||
| 5) Liver | 5) COPD | ||||
| Philippines | 1) Breast | 153,751 | 140.0 | 196 | 1) Ischemic heart disease |
| 2) Lung | 2) Stroke | ||||
| 3) Colorectal | 3) Lower respiratory infection | ||||
| 4) Liver | 4) Chronic kidney disease | ||||
| 5) Prostate | 5) Tuberculosis | ||||
| Republic of Korea | 1) Colorectal | 230,317 | 449.2 | 880.7 | 1) Stroke |
| 2) Stomach | 2) Ischemic heart disease | ||||
| 3) Lung | 3) Lung cancer | ||||
| 4) Breast | 4) Alzheimer’s disease | ||||
| 5) Thyroid | 5) Lower respiratory infection | ||||
| Singapore | 1) Breast | 23,260 | 397.6 | 703 | 1) Ischemic heart disease |
| 2) Colorectal | 2) Lower respiratory infection | ||||
| 3) Lung | 3) Stroke | ||||
| 4) Prostate | 4) Lung cancer | ||||
| 5) Liver | 5) Alzheimer’s disease | ||||
| Taiwan | 1) Lung | 95,250 | 415 | 220 | 1) Ischemic heart disease |
| 2) Liver | 2) Stroke | ||||
| 3) Colorectal | 3) Lower respiratory infection | ||||
| 4) Breast | 4) Lung cancer | ||||
| 5) Prostate | 5) Diabetes mellitus | ||||
| Thailand | 1) Liver | 190,636 | 273.1 | 318.6 | 1) Ischemic heart disease |
| 2) Lung | 2) Stroke | ||||
| 3) Breast | 3) Lower respiratory infection | ||||
| 4) Colorectal | 4) Chronic kidney disease | ||||
| 5) Cervical | 5) Liver cancer | ||||
| Vietnam | 1) Liver | 181,333 | 186.3 | 279 | 1) Stroke |
| 2) Lung | 2) Ischemic heart disease | ||||
| 3) Breast | 3) Diabetes | ||||
| 4) Stomach | 4) COPD | ||||
| 5) Colorectal | 5) Lung cancer |
COPD = chronic obstructive pulmonary disease.
Figure 3. Top cancer per country, estimated age-standardized incidence rates in 2020, both sexes, all ages, excluding NMSC.19) .
NMSC = non-melanoma skin cancer.
Data source: GLOBOCAN 2020; Map production: International Agency for Research on Cancer, World Health Organization.
Various factors could explain the differences in cancer incidences. For instance, the higher incidence rates of breast cancer in North America could be due to a higher prevalence of lifestyle factors (such as alcohol intake, physical inactivity, overweight), a higher prevalence of reproductive and hormonal risk factors (such as early age at menarche, later age at menopause, advanced age of first birth, fewer childbirths, less breastfeeding, menopausal hormonal therapy, oral contraceptives) as well as increased detection through mammographic screening programs.13) The fact that lung cancer is the leading cause of morbidity and mortality among men in Asia could be related to higher tobacco use.13) In North America, curtailing the tobacco epidemic with smoking cessation programs and educational campaigns contributed to a decline in lung cancer rates. Following this development, prostate cancer is now the leading malignancy among men in North America13) due to lifestyle, environmental factors, and early detection through screening programs. Interestingly, the consumption of soy food which is popular in Asian culture has been associated with a 25% to 30% reduction in the risk of prostate cancer.20)
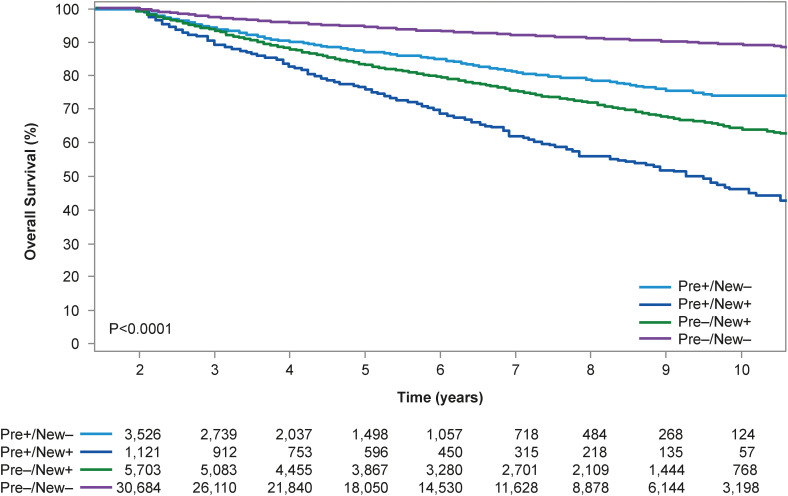
Despite having a lower cancer incidence in Asia compared to other regions, total cancer mortality is higher in Asia because of higher case fatality rates and different distribution of cancer types.13) Importantly, in both Asia and North America, CVD remains a significant cause of mortality and morbidity in cancer patients. For instance, cancer survivors living at least five years after diagnosis have a 1.3- to 3.6-fold increased risk of CVD mortality and a 1.7- to 18.5-fold increased incidence of CV risk factors such as dyslipidemia, hypertension and diabetes mellitus, compared with age-matched counterparts without a history of cancer.21),22) A study from Alberta, Canada demonstrated an 1.3-fold increased risk of CV mortality, 1.6-fold increased risk of heart failure, and 3.4-fold increased risk of pulmonary embolism after a diagnosis of cancer.23) Similarly, in a nationwide population-based cohort study of cancer patients in South Korea, 11% percent of cancer patients had pre-existing CVD at the time of cancer diagnosis, and about 16% of cancer patients were newly-diagnosed CVD, mostly within five years after the cancer diagnosis.24) Both pre-existing and new-onset CVD negatively impacted survival, with poorest survival outcomes seen in patients with both pre-existing and new-onset CVD (Figure 4).24)
Figure 4. Survival outcomes from time of cancer diagnosis stratified by pre-existing and/or newly-diagnosed cardiovascular disease in South Korea.24).
The elevated risk of CRT-CVT in cancer patients could be attributable to direct (e.g., chemotherapy, radiation, targeted therapy) and indirect (e.g., deconditioning) effects of cancer treatments against a background of physiological aging.21) Among older breast cancer survivors in the USA, a higher CV than breast cancer mortality was observed 10 to 15 years following cancer diagnosis.25) The real time risk estimation of CV morbidity and mortality is complex, and clinical decision-making is based on various cancer, patient and CV status-related factors.26) Given that CV mortality surpasses mortality from primary cancer in older cancer survivors,25) there is an urgent unmet need to manage both CVD and cancer. Even more so in Asia, cardio-oncology will become increasingly important due to shifts in demographics and disease profiles, improvement in cancer survivorship, and more widespread use of novel chemotherapeutic agents such as immune-checkpoint inhibitors, and chimeric antigen receptor T-cell (CAR T-cell) therapies. Indeed, the cancer burden in Asia is projected to grow exponentially, and a global census in 2018 already revealed that of the 43.8 million living with cancer worldwide, nearly 40% (17.3 million) reside in Asia.16) Due to aging and population growth, we can expect 29 million new cancer diagnoses per year by 2040; of which half will occur in Asia. This is not surprising as China is the top contributor of new cancer cases worldwide. Future cancer patients will be older and more likely to have pre-existing CVD.27) In cancer patients with pre-existing CVD, the ability to receive the most efficacious therapy with least CTR-CVT is desired. For cancer patients who develop new onset CTR-CVT, early recognition, and timely intervention is key to reducing unnecessary interruptions to their treatment. In cancer survivors, regular CV surveillance is geared towards achieving normal life expectancy.
To cater to the growing demand for integrated cardio-oncology services, cardio-oncology centers have been established in various parts of Asia such as China, India, Japan, Singapore, South Korea, Taiwan, Thailand, and the Philippines. While the exact number of cardio-oncology centers in Asia is unclear, there are more than 31 cardio-oncology clinical units in China alone.11) Most of the cardio-oncology centers in Asia are situated within the cardiology units of university hospitals or academic medical centers. Considering that Asia accounts for nearly 60% of the world’s population,27) the number of cardio-oncology units remains relatively small, with ample room for growth.
CHALLENGES IN ESTABLISHING CARDIO-ONCOLOGY SERVICES IN ASIA
Disparities in socio-economic status, healthcare reimbursement systems, and access to chemotherapeutics agents and healthcare
Asia is a region with enormous socio-economic, ethnic, cultural and political diversity, resulting in disparate health statuses and healthcare systems, which are at varying stages of evolution.28) Based on the World Bank classification, countries in Asia can be classified as high income (Japan, Republic of Korea, Singapore, Taiwan), upper middle income (China, Malaysia) and lower middle income (India, Indonesia, Thailand, the Philippines and Vietnam).29) Over the last decade, the low-middle income countries have experienced a higher rate of increase in cancer cases compared to high income countries.6) By 2040, more than two-thirds of the world’s cancer patients will reside in low income and middle income countries,6) stressing the already overburdened healthcare infrastructure.
Limitations in economic resources and health care coverage adversely impact the availability and extent of medical care, and expectedly overall outcomes.30),31),32) In Asia, a lower cancer mortality-to-incidence ratio is seen in high income countries.33) For example, the age-standardized 5-year overall survival rates of breast cancer in high income countries such as South Korea, Singapore, and Hong Kong are greater than 80% compared with 60–70% in lower middle income countries such as Thailand and India.34) Without robust healthcare insurance, approximately 75% and 25% of cancer patients in India and Thailand, respectively, have to sell assets or borrow funds to pay for cancer treatment.35) These patients are at risk of so-called “financial suicide”; a topic that has gained attention globally and even in high income countries such as the USA.36) A prospective longitudinal study conducted in Southeast Asia showed that 48% of patients experienced a “financial catastrophe,” defined as out-of-pocket healthcare expenditure exceeding 30% of annual household income, within 12 months of cancer diagnosis.37) While most essential cancer drugs were available in high income and upper middle income countries for free or at subsidized rates, only 32% of essential drugs were available in lower middle income countries at full out-of-pocket cost.35) In low income countries, the restricted availability of essential cancer drugs could be due to a lack of commercial interest or budgetary restraint.33) Accordingly, economically less-privileged patients sometimes receive suboptimal or no cancer therapy at all, let alone treatment of CTR-CVT or treatment of baseline co-morbidities such as CVD. Unsurprisingly, the per capita rates of cancer treatment in high-income countries are 5 to 10 times higher than that in low-income countries.36)
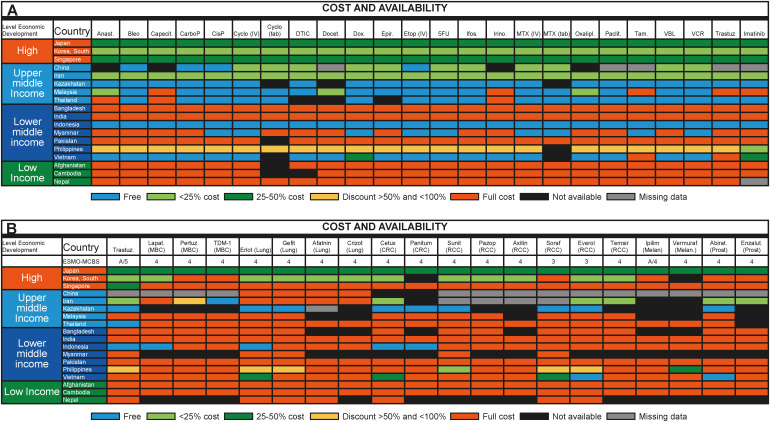
Of note, even among the high-income countries in Asia, there are diversities in the cost and availability of cancer drugs. Figure 5 summarizes the availability of World Health Organization (WHO) essential cancer drugs in Asia.33),34) Access to novel targeted cancer drugs may be further limited by cost or lack of molecular companion diagnostic tests.33) For example, in Asian patients with advanced non-small cell lung patients carrying pathogenic epidermal growth factor receptor (EGFR) mutations, the lack of molecular companion diagnostic tests could hinder the appropriate use of EGFR-targeted therapy, leading to poorer prognosis with a loss of progression-free survival of between 1.7 and 8.5 months.33) This scenario is further aggravated by the delay in regulatory approval of new cancer drugs in Asia, which often lags behind that in North America and Europe.38) Furthermore, state-of-the-art proton beam therapy which promises less bystander toxicity on the heart than traditional radiotherapy is only available in some parts of Asia such as China, Japan, South Korea, Taiwan, and Singapore.39)
Figure 5. (A) Data on from 2015 ESMO International Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in countries outside of Europe: Asian data for medications on the WHO Model List of Essential Medicines: formulary availability and out-of-pocket costs. (B) Data from the 2015 ESMO International Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in countries outside of Europe: Asian data of recently approved medications not on the WHO Model List of Essential Medicines, with an ESMO-MCBS score greater than 2: formulary availability and out-of-pocket costs.33).
Abirat = abiraterone; Aftatin = atafinib; Anast = anastrozole; Axitin = axitinib; Bleo = bleomycin; Capecit = capecitabine; CarboP = carboplatin; Cetux = cetuximab; CisP = cisplatin; Crizot = crizotinib; CRC = colorectal cancer; Cyclo = cyclophosphamide; DTIC = dacarbazine; Docet = docetaxel; Enzalut = enzalutamide; Epir = eprirubicin; Erlot = erlotinib; ESMO = European Society for Medical Oncology; Etop = etoposide; Everol = everolimus; Gefit = gefitinib; Ifos = ifosfamide; Ipilim = ipilimumab; Irino = irinotecan; Lapat = lapatinib; MBC = metastatic breast cancer; MCBS = Magnitude of Clinical Benefit Scale; Melan = melanoma; MTX = methotrexate; Oxalipl = oxaliplatin; Paclit = paclitaxel; Panitum = panitumumab; Pazop = pazopanib; Pertuz = pertuzumab; Prost = prostate; RCC = renal cell cancer; Soraf = sorafenib; Suni = sunitinib; Tam = tamoxifen; Temsir = temsirolimus; Trastuz = trastuzumab; VBL = vinblastine; VCR = vincristine; Vemuraf = vemurafenib; WHO = World Health Organization.
Ethnic and racial diversity in Asia
There is a diverse mix of races and ethnicities in Asia, with important dietary, cultural and lifestyle differences, as well as differences in disease burden and outcomes. South Asians, for instance, have a 3- to 5-fold increased risk of CV deaths compared with other ethnic groups.40) As another example, due to gene polymorphisms of the renin-angiotensin system, Asian patients with hypertension are more predisposed to CVD than white patients.41)
To add to this complexity, Asians also have different genetic susceptibilities to cancer. Compared to white women, Korean, Filipina, Vietnamese, and Chinese women have a significantly higher risk of being diagnosed with HER-2-positive breast cancer; which carries a poorer prognosis and higher treatment costs.42) In the non-small cell lung cancer cohort, Asian patients have a higher prevalence of EGFR mutation and a lower prevalence of KRAS mutation; hence more patients respond to EGFR tyrosine kinase inhibitors, resulting in more favorable outcomes compared with Caucasian patients.43) The efficacy of cancer treatment and related drug toxicities may also differ across different ethnicities. For instance, Asians are inherently more prone to develop coronary vasospasm,44) but how this impacts the frequency and severity of coronary vasospasm associated with 5-FU therapy is not well described.45)
CV drug responses also demonstrate ethnic differences. For instance, Asian patients on warfarin for thromboembolic protection require a lower international normalized ratio (INR) target due to increased risk of bleeding. Even at lower INR values, they experience more bleeding events compared with white Caucasian patients. This could be due to genetic polymorphisms of the functional VKORC1 allele, which is more commonly found in Asians than in white Caucasians (90–95% compared to 45%).42) Another example is the high prevalence of CYP2C19 polymorphisms in Asians, which impacts clinical management as CYP2C19 poor metabolizers may not achieve adequate platelet inhibition with the CYP219-dependent prodrugs such as clopidogrel.43) The use of anthracyclines in African American cancer patients was independently associated with a 1.7-fold greater relative risk of CTR-CVT; this has been thought to be influenced by genetic polymorphism in the CBR1 and CRB3 genes, which converts doxorubicin into doxorubicinol, a potentially cardiotoxic metabolite.44) Lastly, differences in response to beta-blockers and angiotensin converting enzyme-inhibitors have also been observed in various ethnicities.9)
Cardioprotective strategies are generally not well-defined in Asian patients because most of the cardio-oncology trials were conducted in North America/Europe with only a small number of Asian subjects. It is for this fact and the lack of evidence in the Japanese population that dexrazoxane was not approved for the prevention of anthracycline toxicity in Japan.12) Interestingly, the use of traditional Chinese medicine has been shown to reduce anthracycline-related CTR-CVT.45) For the reasons outlined above, dedicated studies are needed to define the scope and management of CV toxicities with cancer treatment in various Asian populations.
Lack of awareness in cancer treatment-related cardiovascular toxicities among physicians and limited cardio-oncology training opportunities in Asia
Many cardiologists have acknowledged that they are uncomfortable managing CTR-CVT in cancer patients.46) Similarly, not all hematologists and oncologists know the full spectrum of CTR-CVT. Significant knowledge gaps regarding the identification, prevention, and treatment of CTR-CVT exist among cardiologists, hematologists, and oncologists. Direct comparisons of countries have not been conducted regarding the awareness of chemotherapeutic agents and the potential for or interplay with CV outcomes; thus, no comparative statement can be made.
More objective data are available regarding cardio-oncology training. While the topic of cardio-oncology is being increasingly introduced into the general cardiology and oncology/hematology fellowship curricula and dedicated cardio-oncology fellowship programs are emerging in Western countries, this is much less the case in Asia.47) A virtual cardio-oncology training program tapping on mentors, structured educational series, case logbooks, and online assessment can be explored for interested physicians who cannot pursue overseas fellowship training. Conceivably, there are ample opportunities for education in cardio-oncology across the globe but especially in Asia.
Patients’ awareness and perception of cancer care
The lack of physicians’ awareness of potential CTR-CVT has downstream effects on patients. Only 40% of cancer patients recall having been counselled on CTR-CVT, and of those who were counselled, 30–40% still had questions.47) Only 25% of cancer patients were offered screening for CV toxicities during treatment.47),48) Patients may not fully understand that CTR-CVT can occur during or years after completion of cancer treatment. Given that the concept of cancer survivorships is relatively new in Asia, there would be opportunities to improve CV surveillance of cancer survivors.
Upon being diagnosed with cancer, patients are often overwhelmed emotionally and financially. Non-cancer health problems such as pre-existing or new onset CVD are frequently neglected under these circumstances. In addition, due to cultural beliefs and/or lack of accessible healthcare, some cancer patients in Asia may choose alternative medicines such as ayurvedic medicine or traditional herbal remedies over evidence-based treatment.
OVERCOMING BARRIERS TO CARDIO-ONCOLOGY IN ASIA
Cardio-oncology practice models that can be customized according to socio-economic circumstances
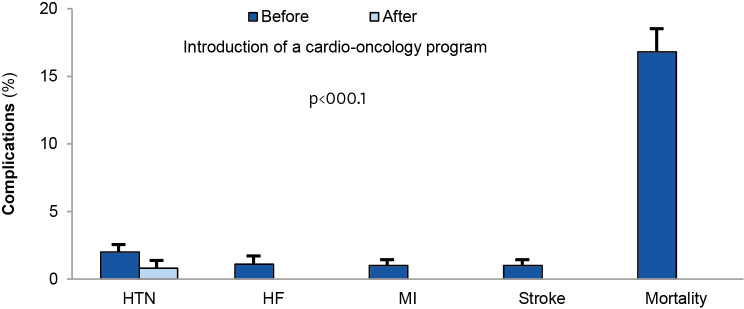
A cardio-oncology program should demonstrate benefit from both, a clinical and a fiscal perspective by monitoring patient and physician satisfaction, outpatient clinic visit and CV testing volumes, as well as outcomes.46) For example, establishing cardio-oncology clinics in Taiwan led to a reduction in adverse CV events and improvement in outcomes.49) In this real-world prospective study of a cardio-oncology program in Taiwan, none of the patients in the cardio-oncology program developed heart failure symptoms during follow-up, compared to the 1% incidence of new-onset heart failure before program initiation.6) Other reductions included new-onset hypertension from 1.7% to 0.6%, ischemic stroke from 0.9% to 0% and most importantly a reduction in mortality from 16.8% to 0% (Figure 6).49) In the United Kingdom, establishing a cardio-oncology service line led to improvements in patients' left ventricular ejection fraction and New York Heart Association (NYHA) class, and reductions in cancer treatment interruptions.50)
Figure 6. Outcomes of a cardio-oncology in Taiwan.49).
HF = heart failure; HTN = hypertension; MI = myocardial infarction.
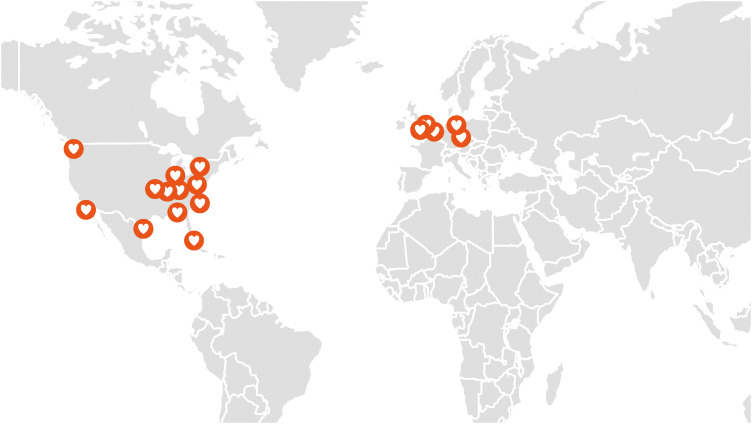
We propose a model of cardio-oncology proficiencies (Table 2), which can be customized according to the country’s socio-economic status and healthcare infrastructure. Table 3 summarizes some commonly used chemotherapeutic agents and a suggested management approach. A basic approach is recommended in all patients undergoing chemotherapy with potential CV toxicity. An ideal approach is suggested for countries with sufficient resources to set up dedicated cardo-oncology services. Countries with ample resources and established clinical cardio-oncology services are well suited to contribute to the advancement of knowledge in cardio-oncology in Asia through research and education. These centers can also apply for the certification by the International Cardio-Oncology Society (ICOS) as a Center of Excellence. This certification program recognizes institutions for their clinical cardio-oncology expertise, for their contribution to the field via research and education, and for quality improvement programs to further improve their program. Currently, most of the ICOS Centers of Excellence are located in USA and Europe (Figure 7).51)
Table 2. Proposed level of proficiencies.
| Model | Proposed proficiencies |
|---|---|
| Basic | - Multidisciplinary collaboration amongst cardiologists, oncologists and other stakeholders |
| - Incorporation of cardio-oncology module in medical education, and basic knowledge of cardiac toxicities of various therapies | |
| Ideal | In addition to above, |
| - Regular multidisciplinary discussions about challenging patients | |
| - Dedicated cardio-oncology clinics for baseline assessment and follow-up during therapy | |
| - Survivorship program for surveillance of late onset cardiac toxicities | |
| Optimal | In addition to above, |
| - Research on strategies to detect early cardiotoxicity, and manage them without unnecessary interruption of the chemotherapeutic regime | |
| - Formal cardio-oncology fellowship training program |
Table 3. Current approach for managing and monitoring cardiotoxicity of common classes of chemotherapeutic agents.
| Class of chemotherapeutic agent | Cancer Indication | Commonly associated treatment related cardiotoxicity | Current approach | Suggested approach | |
|---|---|---|---|---|---|
| Anthracycline (doxorubicin/daunorubicin, epirubicin) | Breast cancer, lymphoma, acute leukemia | Heart failure, left ventricular dysfunction | - Baseline LVEF assessment and monitoring during therapy. | Basic: Baseline assessment of LVEF (±NT-proBNP) prior to initiation of chemotherapy. Follow up as required, guided by symptoms. | |
| - Use of biomarkers such as NT-proBNP/troponin during routine follow up. | Ideal: Risk stratification prior to cancer therapy. Serial follow up to assess LVEF. Early initiation of adjunctive cardioprotective strategies in patients with subclinical cardiotoxicity or at-risk patients. | ||||
| - Early initiation of adjunctive cardioprotective strategies such as ACE-inhibitors or betablockers upon detection of subclinical cardiac toxicity. | Optimal: Cancer survivorship program to review patients who may develop late-onset cardiomyopathy | ||||
| Antimetabolites | |||||
| 5-Flurouracil | Breast cancer, colorectal cancer, pancreatic cancer, gastric cancer | Vasospasm, myocardial ischemia, arrhythmias, heart failure | - Consider pre-medication with calcium channel blockers. Use of intravenous bolus regimen. | Basic: In patients with pre-existing vascular disease, clinical follow up as needed. | |
| - ECG monitoring during intravenous infusion. | Ideal: Risk stratification prior to cancer therapy to guide further testing as necessary. | ||||
| Optimal: Consider stress test with peripheral vaso-functional study, or even coronary angiogram with full microvascular assessment | |||||
| HER-2-targeted therapies (trastuzumab, pertuzumab, trastuzumab ematansine T-DM1) | HER-2+ breast cancer | Heart failure, Left ventricular dysfunction, Hypertension | - Serial monitoring of LVEF | Basic: Basement evaluation of LVEF | |
| HER-2+ gastric cancer | - Consider suspending therapy and initiation of cardioprotective agents (ACE-inhibitors/β-blockers) when there is a significant drop in LVEF | Ideal: Regular serial monitoring of LVEF during therapy. If significant drop in LVEF to <40%, consider suspending treatment and initiating cardioprotective agents. | |||
| Optimal: Routine monitoring of LVEF. Cancer survivorship surveillance program. | |||||
| VEGF inhibitors (sunitinib, sorafenib, vandetanib) | Renal cell carcinoma, Hepatocellular cancer, Medullary thyroid cancer | Hypertension, heart failure, left ventricular dysfunction, arterial thromboembolism, QTc prolongation | - Early recognition and treatment of hypertension. Dose up-titration of antihypertensives for existing patients | Basic: Monitoring of blood pressure, and optimization of anti-hypertensive therapy in patients with pre-existing hypertension. | |
| - ECG to assess QTc duration | Ideal: Risk stratification prior to starting chemotherapy. Monitoring of blood pressure during follow-up visit. | ||||
| Optimal: Echocardiogram after first month of therapy and every 3 monthly | |||||
| Androgen deprivation therapies | Prostate cancer | Atherosclerosis, myocardial ischemia and infarction, diabetes mellitus, hypertension | - Baseline assessment of 10-year atherosclerotic CV risk. | Basic: Screening of CV risk factors prior to initiation | |
| 1. GnRH agonists (gosrelin, leuprorelin) | - Control of CV risk factors such as diabetes mellitus, dyslipidemia as per existing guidelines. | ||||
| 2. GnRH antagonists (degarelix) | Ideal: Baseline assessment of 10-year atherosclerotic CV risk, and optimizing CV risk factors such as diabetes mellitus and dyslipidemia at baseline and follow-up | ||||
| 3. Antiandrogens (abiraterone) | Optimal: In patients with pre-existing ischemic heart disease, consider stress testing or even coronary angiogram depending on signs and symptoms | ||||
ACE = angiotensin-converting-enzyme; CV = cardiovascular; ECG = electrocardiogram; GnRH = gonadotropin-releasing hormone antagonist; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal prohormone B-type natriuretic peptide.
Figure 7. Worldwide distribution of International Cardio-Oncology Society Centers of cardio-oncology excellence.51) .
ICOS = International Cardio-Oncology Society.
As a basic requirement, cardiologists should possess knowledge of identifying and screening for CTR-CVT. Clinical practice guidelines issued by international bodies or local authorities allow clinicians to keep abreast with the latest developments in the cardio-oncology field. To increase awareness of cardio-oncology among future physicians, a dedicated cardio-oncology core curriculum can be developed and integrated into medical school education and residency training. Multidisciplinary collaboration amongst cardiologists, hematologists, oncologists, and other stakeholders is essential so that cancer patients with CV issues can be promptly reviewed to minimize delay in their cancer treatment. To facilitate this and to ensure the alignment of therapeutic goals, efforts should be made to establish foundational relationships between cardiologists and hematologists/oncologists. Engaging allied healthcare workers such as nurses, pharmacists and physiotherapists will furthermore facilitate more holistic CV care of cancer patients.
Ideally, a tertiary oncology or CV center should have a dedicated cardio-oncology clinic to review patients who develop CTR-CVT during cancer treatment. Baseline risk assessment protocols can be used to optimize the health of cancer patients with high CV toxicity risk prior to cancer treatment.52) Early detection of subclinical CTR-CVT would allow for timely initiation of cardioprotective agents such as beta-blockers and angiotensin-converting enzyme inhibitors, potentially minimizing CV toxicity. Survivorship programs incorporating surveillance for delayed CTR-CVT such as radiation-induced coronary artery disease or valvular heart disease may reduce the risk of cancer survivors succumbing to CV events.
In an optimal setting, there should also be ongoing research to evaluate the mechanisms of CTR-CVT, which will guide preventive and treatment strategies. A formal cardio-oncology fellowship program will allow further subspecialized training for interested physicians.
Improving awareness of cardio-oncology in Asia through education and research
Education
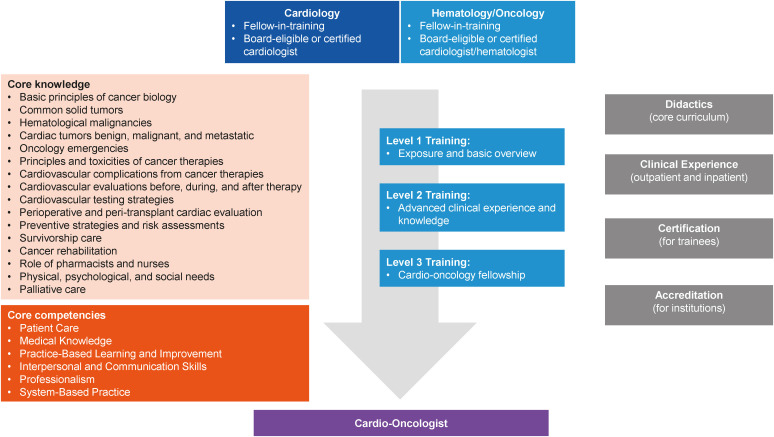
A formalized training for cardiology or oncology fellows interested in cardio-oncology was proposed by the American College of Cardiology Cardio-Oncology council, with different training goals depending on the availability of resources.2) In the USA, just one-half of cardiology fellows receive cardio-oncology exposure based on a survey in 2018.53) This percentage is expected to have increased since then. A dedicated training curriculum for cardiology and cardio-oncology fellows provides an environment for structured knowledge acquisition.2) At present, specialized cardio-oncology training opportunities are limited worldwide, with only a few hospitals in the USA, Europe and South America offering fellowship.2) Training ranges from basic level 1 training whereby trainees obtain sufficient knowledge to perform essential evaluation and management of cardio-oncology patients to advanced level 3 training for full-fledged cardio-oncologists to start their own cardio-oncology programs.2) Figure 8 illustrates the central elements of a cardio-oncology training program.2) Board-type certification exams administered by organizations such as ICOS will ensure that accredited cardio-oncologists possess the required expert knowledge and skill set.
Figure 8. Central elements of cardio-oncology training.2).
A concerted movement to transition knowledge from cardio-oncology guidelines into actual practice is crucial to improve CV outcomes of cancer patients.54) Patient education is also essential to raise awareness on the potential CTR-CVT; hence facilitating early identification of CV toxicity.
With the coronavirus disease 2019 (COVID-19) pandemic, there has been accelerated availability of online resources such as journal club discussions, lectures, and conferences, which transcend geographical boundaries. Societies such as ICOS provide a platform for collaboration among researchers, clinicians, allied health, and educators worldwide to advance the CV care of cancer patients. In Asia, countries such as China, India, Japan, Singapore, South Korea and the Philippines have their local ICOS chapters or local cardio-oncology societies to promote awareness and facilitate collaboration.11),12),49),55),56)
Research
Research is essential for advancing the field of cardio-oncology.57) Given that most CV trials excluded cancer patients, there is a void of data for this unique group of patients.57) Currently, most management strategies are translated from clinical trials in CV patients without cancer. For instance, strategies from heart failure guidelines are adopted when managing patients with chemotherapeutics-related cardiomyopathy. Despite this, not all cancer patients with chemotherapeutics-related cardiomyopathy receive treatment consistent with heart failure guidelines.58) Likewise, most oncology pharmaceutical trials exclude patients with CVD despite the high prevalence of CVD in cancer patients.
Given the shifting demographic and epidemiologic landscape (aging population with higher rates of CV disease, longer treatment durations, potential drug-drug interactions, new mechanisms of CV toxicity), oncology trials should broaden eligibility criteria to include patients with CV diseases to accurately capture the risks and benefits of therapy in real-world populations.59) Dual assessment of both CV and cancer outcomes has been proposed to evaluate the aggregate health effects of an investigated drug. Active post-marketing surveillance of cancer drugs can provide timely insights into any potential CTR-CVT.59)
Considering the different genetic make-up and pharmacogenetics of the Asian population, more data from registry and longitudinal studies would be required to study strategies to best identify cancer patients at risk of CTR-CVT and the efficacy of cardioprotective strategies.
Improving access to healthcare and chemotherapeutic treatment in Asia
While most Asian countries such as China, Japan, South Korea, Taiwan, Thailand, and Singapore have universal healthcare covering more than 95% of their population, this is not the case for India, Indonesia, Bangladesh, and Nepal.60),61) However, these countries are working hard to expand cost-effective healthcare to their population by investing in government health insurance, thereby reducing patients’ out-of-pocket payments.62) There are also ongoing efforts to improve accessibility to cancer drugs by both governments and pharmaceutical companies. In China, oncology spending grew by 8.2 billion dollars since 2016, due to increased access to novel therapeutics and existing cancer drugs including generics, as well as investment in home-grown research.36) In fact, citacabtagene autoleucal (Carvykti) became the first Chinese-developed CAR T-cell therapy to be approved by the US Food and Drug Administration (FDA).36) These initiatives allow more cancer patients to access state-of-the-art cancer treatment with less financial concerns and healthcare diversity.
To improve the availability of novel chemotherapeutic agents, regulatory bodies can look at expediting the time needed for the approval and registration of cancer drugs. Some regulatory bodies such as the Pharmaceutical and Medical Devices Agency in Japan require submission of clinical safety and efficacy data for Japanese patients, inevitably resulting in delays. Having a global clinical trial strategy for drug development that includes Asia will reduce the lag time for registration of new medicines.38) For instance, of the 1,577 CAR T-cell therapy trial sites globally, 322 were located in China, signalling immense interest in the field.36) A mechanism for fast-tracked review and accelerated approval by the country’s drug regulatory body will reduce the lag time for the safe introduction of new efficacious cancer drugs. In addition, compulsory licensing, pharmaceutical company-sponsored patient assistance programs, price negotiation by the country’s healthcare body, using biosimilars or generic medications, and having tax incentives can improve the affordability of cancer drugs.33)
The model of healthcare delivery in many parts of the world has been disrupted by the COVID-19 pandemic, with a dramatic shift from in-person to virtual consultations. Virtual care using telephone or video-consultations instead of in-person visits can be considered for stable patients, thus overcoming geographical barriers, and reducing the burden of hospital visits. Not only that, the use of telemedicine could be integrated into oncology care by having internal-consults.63) Nonetheless, triaging is important to ensure that unstable cancer patients who require cardiologists’ care can be expeditiously seen.
Strengthen collaboration between stakeholders
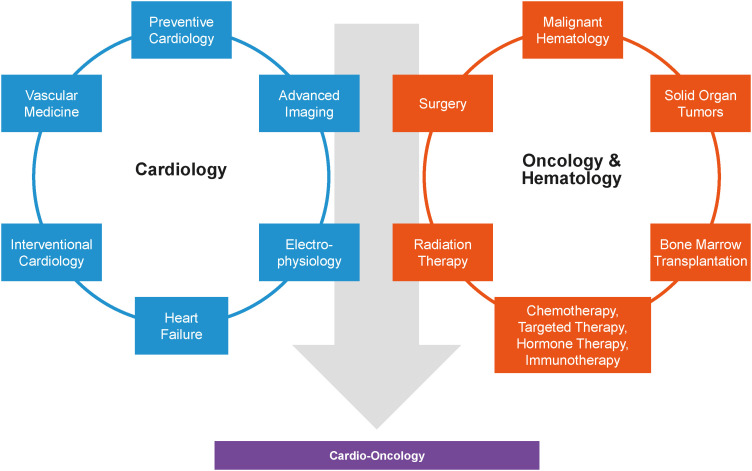
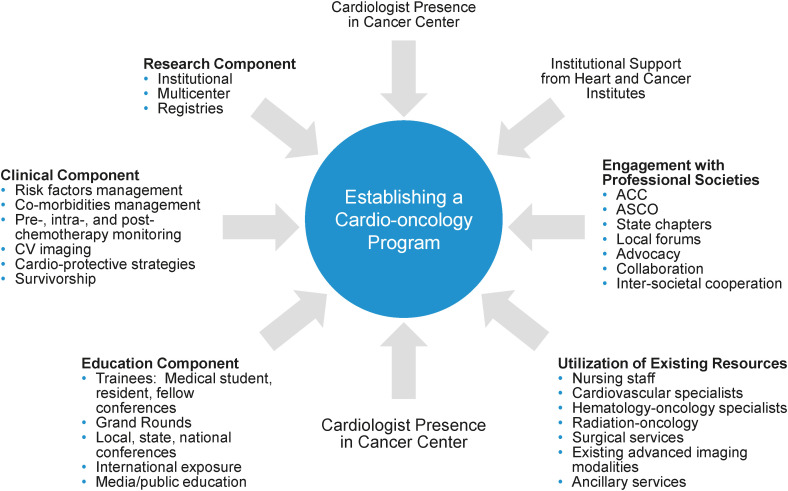
Clinicians interested in cardio-oncology should work together to advance the field, by strengthening awareness and education amongst physicians, patients, and allied health. The establishment of a cardio-oncology program must start with clear leadership and vision.48) Notably, there must be concrete data and feasible objectives to assess the program’s success. The first step often starts with building strong relationships between the cardiologists and referring oncologists or hematologists. It must be emphasized that cardio-oncology practice requires intra-specialty (involving electrophysiologists, interventionists, and heart failure cardiologists) and cross-specialty collaboration (involving oncologists and hematologists) given the complexities of patients (Figure 9).2) In some lower income countries utilizing paper-based medical record systems, progressive implementation of electronic records can facilitate more efficient multi-disciplinary sharing of patient information.64) Once concrete data for a cardio-oncology program are available, institutional support can be obtained for the formal establishment of cardio-oncology services.48) Regular audits will provide valuable insights regarding the patient cohort and effectiveness of treatment interventions, allowing for ongoing program improvement. Figure 10 illustrates the key components of a cardio-oncology program.65)
Figure 9. Intradisciplinary and cross-specialty collaboration among cardiologists, hematologist and oncologists in cardio-oncology.2).
Figure 10. Key components of a cardio-oncology program.65).
ACC = American College of Cardiology; ASCO = American Society of Clinical Oncology; CV = cardiovascular.
With the ever-growing number of chemotherapeutic agents, physicians as well as cancer patients should be aware of potential CTR-CVT. Given the broad spectrum of CTR-CVT ranging from cardiac dysfunction, arrhythmias, valvular heart disease, hypertension and vascular toxicities, consensus statements on standardized definitions of the various cardiac side effects provide a uniform platform for effective communication across different clinical subspecialties and help facilitate research.66)
CHANGING FACE OF CANCER CARE: THE NEXT FRONTIER OF CARDIO-ONCOLOGY
The increasing prevalence of cancer patients and recognition of CTR-CVT with various chemotherapeutic agents has translated into a growing need for cardio-oncology expertise ultimately to prevent or mitigate immediate or long-term CV complications and minimize unnecessary interruption to cancer treatment.
A need for more precise CTR-CVT risk predictions at baseline and risk mitigation strategies remains. Thoughts have been raised for precision cardio-oncology, which takes into account the patient’s CV risks, type of cancer, treatment regimen and ethnicity, allowing for an individualized treatment approach to reduce CTR-CVT.67) By using a larger “-omics”-based approach incorporating radiomics, cell biomics, genomics, metabolomics, and transcriptomics, one can potentially improve cardiac risk stratification, formulate personalized preventative and targeted surveillance strategies. Biomarkers such as N-terminal prohormone B-type natriuretic peptide (NT-proBNP) and troponin used for CV surveillance lack specificity for drug-induced toxicity. Multi-level omics data can help narrow the search for novel biomarker candidates, which can accurately identify at-risk populations, diagnose CTR-CVT and help guide therapies.68)
Genomics could be leveraged to individualize cardioprotective strategies. For instance, a significant number of cancer patients who developed anthracycline-related CTR-CVT had truncating mutations in the titin gene; and animal models showed TTN-variant mice exposed to doxorubicin are more susceptible to developing CV toxicity compared to wild-type mice. This findings provided proof for the concept that patients may have predisposing genetic risks which are triggered by the “second-hit” of chemotherapy.69) As another example, genomics-adjusted radiation therapy, which individualises radiation dose based on genetic polymorphisms in the cancer patient, can potentially reduce radiation-induced CV toxicity.70)
With electronic medical records, there is a valuable source of “Big Data”, which can be processed and incorporated into machine learning algorithms. In recent years, artificial intelligence applied to imaging studies or electrocardiograms has gained momentum.71) Machine learning employs advanced computational analytics to recognize complex patterns. For instance, machine learning has been used to determine which patients are predisposed to developing heart failure, atrial fibrillation, coronary artery disease, myocardial infarction, stroke and de novo chemotherapeutics-related cardiac dysfunction (and thereby advancing risk stratification).72) In paediatric cancer survivors specifically, machine learning can recognize clinical and genetic factors associated with anthracycline-related CTR-CVT, hence identifying patients who may benefit from intensified CV surveillance.73)
As with any emerging field, registries capturing patient-centric data can help to better understand the burden of CTR-CVT, disease course and outcome with cardioprotective strategies, and provide quality metrics to justify and improve cardio-cardiology programs. Participating in an international registry such as the Global Cardio-Oncology Registry (G-COR) not only fosters collaborative research, but also aims to improve understanding of risk factors impacting CTR-CVT in different geographical locations and to devise mitigating strategies.74)
To inspire the confidence of patients and stakeholders, and to establish cardio-oncology as an essential clinical service, there should be ongoing efforts to improve the standard of clinical care. In this regard, ICOS has been instrumental in advancing the field of cardio-oncology. Regular virtual journal club discussions and webinars provide educational opportunities for interested physicians and allied health, regardless of geographical location. By having an international database of members, ICOS provides a valuable platform for collaboration across the globe. More recently, ICOS-administered board-style exams ensure that practicing cardio-oncologists possess a high level of knowledge and skillset. Designated centers of cardio-oncology excellence provide a valuable reference for patients and referring physicians. More importantly, they serve as models for institutions aspiring to set up cardio-oncology services. To encourage research in the field of cardio-oncology, dedicated journals such as JACC: Cardio-Oncology and Cardio-Oncology are now available. In response to physicians’ feedback, JACC: Cardio-Oncology has a dedicated “How To” series, which contains succinct yet practical guidance on the diagnosis and treatment of clinical scenarios encountered in the treatment of cancer patients.
In Asia, the top priority is to boost cardio-oncology services to cope with the upcoming cancer and CVD pandemic. The timely and appropriate intervention of cancer patients who experience CV toxicity can potentially help to reduce both CVD and cancer mortality. Concurrently, establishing a regional registry or joining an international registry such as G-COR would facilitate the collection of country-specific data to better understand the disease prevalence and outcomes of cardioprotective strategies, and provide feedback for improving existing cardio-oncology programs. Based on the epidemiology of CTR-CVT in Asian patients, as well as ongoing research into the pharmacogenetics/pharmacokinetics of cancer and cardio-protective agents, customized guidelines for the Asian population can be developed. Given that there is significant healthcare and socio-economic disparity in Asia, countries with established cardio-oncology services should take the lead in mentoring upcoming centers by sharing their experiences and knowledge.
CONCLUSIONS
In conclusion, the field of cardio-oncology is transforming the way cardiologists, oncologists, and hematologists screen for and manage CTR-CVT. While it is still in the developing phase, the various cardio-oncology communities in Asia and across the world are working closely together to advance the field by optimizing clinical care, education, and research, with a united mission of allowing cancer patients to complete their treatment and lead quality lives with minimal CVD burden thereafter.
Footnotes
Funding: Joerg Herrmann received funding from the NIH/NCI (CA233601) and the Miami Heart Research Institute and the Mayo Cardiovascular Research Center and Center for Biomedical Discovery. The other authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest: The authors have no relevant conflicts of interest.
Data Sharing Statement: The data generated in this study is available from the corresponding authors upon reasonable request.
- Conceptualization: Ng CT, Tan LL, Oka T, Chang WT, Tan MKC, Herrmann J.
- Data curation: Ng CT, Tan MKC, Cruz RR, Astuti A, Do VC, Herrmann J.
- Methodology: Herrmann J.
- Project administration: Herrmann J.
- Supervision: Herrmann J.
- Writing - original draft: Ng CT, Herrmann J.
- Writing - review & editing: Ng CT, Tan LL, Sohn IS, Gonzalez Bonilla H, Oka T, Yinchoncharoen T, Chang WT, Chong JH, Tan MKC, Cruz RR, Astuti A, Agarwala V, Do VC, Youn JC, Tong J, Herrmann J.
References
- 1.Falzone L, Salomone S, Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol. 2018;9:1300. doi: 10.3389/fphar.2018.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Cardona JA, Ray J, Carver J, et al. Cardio-oncology education and training: JACC Council perspectives. J Am Coll Cardiol. 2020;76:2267–2281. doi: 10.1016/j.jacc.2020.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi S, Lv J, Chai R, et al. Opportunities and challenges in cardio-oncology: a bibliometric analysis from 2010 to 2022. Curr Probl Cardiol. 2022:101227. doi: 10.1016/j.cpcardiol.2022.101227. [DOI] [PubMed] [Google Scholar]
- 4.Ritchie H, Spooner F, Roser M. Causes of death [Internet] place unknown: Our World in Data; 2018. [cited 2022 August 5]. Available from: https://ourworldindata.org/causes-of-death. [Google Scholar]
- 5.Zhao D. Epidemiological features of cardiovascular disease in Asia. JACC Asia. 2021;1:1–13. doi: 10.1016/j.jacasi.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global Burden of Disease 2019 Cancer Collaboration. Kocarnik JM, Compton K, et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol. 2022;8:420–444. doi: 10.1001/jamaoncol.2021.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention, National Center for Health Statistics. About multiple cause of death, 1999–2020. CDC WONDER Online Database website [Internet] Atlanta (GA): Centers for Disease Control and Prevention; 2022. [cited 2022 February 21]. Available from: https://wonder.cdc.gov/ [Google Scholar]
- 8.Ng CJ, Teo CH, Abdullah N, Tan WP, Tan HM. Relationships between cancer pattern, country income and geographical region in Asia. BMC Cancer. 2015;15:613. doi: 10.1186/s12885-015-1615-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Global health estimates: life expectancy and leading causes of death and disability [Internet] Geneva: World Health Organization; 2022. [cited 2022 February 8]. Available from: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates. [Google Scholar]
- 10.Cao M, Li H, Sun D, Chen W. Cancer burden of major cancers in China: a need for sustainable actions. Cancer Commun (Lond) 2020;40:205–210. doi: 10.1002/cac2.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Zhang YL, Liu JW, Fang FQ, Li JM, Xia YL. Emergence, development, and future of cardio-oncology in China. Chin Med J (Engl) 2018;131:2640–2644. doi: 10.4103/0366-6999.244101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oka T, Akazawa H, Sase K, Hatake K, Komuro I. Cardio-oncology in Japan. JACC CardioOncol. 2020;2:815–818. doi: 10.1016/j.jaccao.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 14.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts [Internet] Bethesda (MD): National Cancer Institute; 2022. [cited 2022 July 21]. Available from: https://seer.cancer.gov. [Google Scholar]
- 16.American Cancer Society. The Cancer Atlas [Internet] Atlanta (GA): American Cancer Society; 2022. [cited 2022 July 11]. Available from: https://canceratlas.cancer.org. [Google Scholar]
- 17.Institute for Health Metrics and Evaluation (IHME) Website of IHME [Internet] Seattle (WA): Institute for Health Metrics and Evaluation; 2022. [cited 2022 July 7]. Available from: https://www.healthdata.org. [Google Scholar]
- 18.Centers for Disease Control and Prevention, National Center for Health Statistics. Leading causes of death [Internet] Atlanta (GA): Centers for Disease Control and Prevention; 2022. [cited 2022 July 29]. Available from: https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. [Google Scholar]
- 19.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global Cancer Observatory: Cancer Today [Internet] Lyon: International Agency for Research on Cancer; 2020. [cited 2022 August 10]. Available from: https://gco.iarc.fr/today. [Google Scholar]
- 20.Kimura T. East meets West: ethnic differences in prostate cancer epidemiology between East Asians and Caucasians. Chin J Cancer. 2012;31:421–429. doi: 10.5732/cjc.011.10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilchrist SC, Barac A, Ades PA, et al. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation. 2019;139:e997–1012. doi: 10.1161/CIR.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwan ML, Cheng RK, Iribarren C, et al. Risk of heart failure with preserved versus reduced ejection fraction in women with breast cancer. Breast Cancer Res Treat. 2022;193:669–675. doi: 10.1007/s10549-022-06586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paterson DI, Wiebe N, Cheung WY, et al. Incident cardiovascular disease among adults with cancer: a population-based cohort study. JACC CardioOncol. 2022;4:85–94. doi: 10.1016/j.jaccao.2022.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Youn JC, Chung WB, Ezekowitz JA, et al. Cardiovascular disease burden in adult patients with cancer: an 11-year nationwide population-based cohort study. Int J Cardiol. 2020;317:167–173. doi: 10.1016/j.ijcard.2020.04.080. [DOI] [PubMed] [Google Scholar]
- 25.Strongman H, Gadd S, Matthews AA, et al. Does cardiovascular mortality overtake cancer mortality during cancer survivorship?: an English retrospective cohort study. JACC CardioOncol. 2022;4:113–123. doi: 10.1016/j.jaccao.2022.01.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwala V, Choudhary N, Gupta S. A risk-benefit assessment approach to selection of adjuvant chemotherapy in elderly patients with early breast cancer: a mini review. Indian J Med Paediatr Oncol. 2017;38:526–534. doi: 10.4103/ijmpo.ijmpo_96_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okura Y, Takayama T, Ozaki K, et al. Future projection of cancer patients with cardiovascular disease in Japan by the year 2039: a pilot study. Int J Clin Oncol. 2019;24:983–994. doi: 10.1007/s10147-019-01426-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chongsuvivatwong V, Phua KH, Yap MT, et al. Health and health-care systems in southeast Asia: diversity and transitions. Lancet. 2011;377:429–437. doi: 10.1016/S0140-6736(10)61507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The World Bank. World Bank open data [Internet] Washington, D.C.: The World Bank; 2022. [cited 2022 July 29]. Available from: https://data.worldbank.org/ [Google Scholar]
- 30.Rahman MM, Karan A, Rahman MS, et al. Progress toward universal health coverage: a comparative analysis in 5 South Asian countries. JAMA Intern Med. 2017;177:1297–1305. doi: 10.1001/jamainternmed.2017.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohman RE, Yang EH, Abel ML. Inequity in cardio-oncology: identifying disparities in cardiotoxicity and links to cardiac and cancer outcomes. J Am Heart Assoc. 2021;10:e023852. doi: 10.1161/JAHA.121.023852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Economic and Social Commission for Asia and the Pacific. Inequality in Asia and the Pacific [Internet] Bangkok: Economic and Social Commission for Asia and the Pacific; 2022. [cited 2022 July 11]. https://www.unescap.org/sites/default/d8files/01ExecutiveSummary.pdf . [Google Scholar]
- 33.Eniu A, Cherny NI, Bertram M, et al. Cancer medicines in Asia and Asia-Pacific: what is available, and is it effective enough? ESMO Open. 2019;4:e000483. doi: 10.1136/esmoopen-2018-000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goh BC, Lim JF. Cancer drugs in Asian populations: availability, accessibility, and affordability. Cancer J. 2020;26:323–329. doi: 10.1097/PPO.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 35.Leighl NB, Nirmalakumar S, Ezeife DA, Gyawali B. An arm and a leg: the rising cost of cancer drugs and impact on access. Am Soc Clin Oncol Educ Book. 2021;41:1–12. doi: 10.1200/EDBK_100028. [DOI] [PubMed] [Google Scholar]
- 36.IQVIA Institute. Global Oncology Trends 2022. Durham (NC): IQVIA Institute; 2022. [Google Scholar]
- 37.Kimman M, Jan S, Yip CH, et al. ACTION Study Group. Catastrophic health expenditure and 12-month mortality associated with cancer in Southeast Asia: results from a longitudinal study in eight countries. BMC Med. 2015;13:190. doi: 10.1186/s12916-015-0433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venkatakrishnan K, Burgess C, Gupta N, et al. Toward optimum benefit-risk and reduced access lag for cancer drugs in Asia: a global development framework guided by clinical pharmacology principles. Clin Transl Sci. 2016;9:9–22. doi: 10.1111/cts.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frankart AJ, Nagarajan R, Pater L. The impact of proton therapy on cardiotoxicity following radiation treatment. J Thromb Thrombolysis. 2021;51:877–883. doi: 10.1007/s11239-020-02303-4. [DOI] [PubMed] [Google Scholar]
- 40.Gupta M, Singh N, Verma S. South Asians and cardiovascular risk: what clinicians should know. Circulation. 2006;113:e924–e929. doi: 10.1161/CIRCULATIONAHA.105.583815. [DOI] [PubMed] [Google Scholar]
- 41.Kario K, Chen CH, Park S, et al. Consensus document on improving hypertension management in Asian patients, taking into account Asian characteristics. Hypertension. 2018;71:375–382. doi: 10.1161/HYPERTENSIONAHA.117.10238. [DOI] [PubMed] [Google Scholar]
- 42.Telli ML, Chang ET, Kurian AW, et al. Asian ethnicity and breast cancer subtypes: a study from the California Cancer Registry. Breast Cancer Res Treat. 2011;127:471–478. doi: 10.1007/s10549-010-1173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou W, Christiani DC. East meets West: ethnic differences in epidemiology and clinical behaviors of lung cancer between East Asians and Caucasians. Chin J Cancer. 2011;30:287–292. doi: 10.5732/cjc.011.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Donnell PH, Dolan ME. Cancer pharmacoethnicity: ethnic differences in susceptibility to the effects of chemotherapy. Clin Cancer Res. 2009;15:4806–4814. doi: 10.1158/1078-0432.CCR-09-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hao W, Shi YY, Qin YN, et al. Cardioprotective effect of Chinese herbal medicine for anthracycline-induced cardiotoxicity in cancer patients: a meta-analysis of prospective studies. Medicine (Baltimore) 2022;101:e29691. doi: 10.1097/MD.0000000000029691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng RK. Developing a cardio-oncology program from an early career perspective: challenges faced and lessons learned [Internet] Washington, D.C.: American College of Cardiology; 2018. [cited 2022 September 1]. Available from: https://www.acc.org/membership/sections-and-councils/imaging-section/section-updates/2018/01/03/22/05/developing-a-cardio-oncology-program-from-an-early-career-perspective-challenges-faced-and-lessons-learned. [Google Scholar]
- 47.Bhatia N, Lenihan D, Sawyer DB, Lenneman CG. Getting the SCOOP-survey of cardiovascular outcomes from oncology patients during survivorship. Am J Med Sci. 2016;351:570–575. doi: 10.1016/j.amjms.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 48.Herrmann J, Loprinzi C, Ruddy K. Building a cardio-onco-hematology program. Curr Oncol Rep. 2018;20:81. doi: 10.1007/s11912-018-0725-7. [DOI] [PubMed] [Google Scholar]
- 49.Chang WT, Feng YH, Kuo YH, et al. The impact of a multidisciplinary cardio-oncology programme on cardiovascular outcomes in Taiwan. ESC Heart Fail. 2020;7:2135–2139. doi: 10.1002/ehf2.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pareek N, Cevallos J, Moliner P, et al. Activity and outcomes of a cardio-oncology service in the United Kingdom-a five-year experience. Eur J Heart Fail. 2018;20:1721–1731. doi: 10.1002/ejhf.1292. [DOI] [PubMed] [Google Scholar]
- 51.International Cardio-Oncology Society. IC-OS Centers of excellence certification [Internet] Tampa (FL): International Cardio-Oncology Society; 2022. [cited 2022 August 20]. Available from: https://ic-os.org/excellence-certification/ [Google Scholar]
- 52.Lyon AR, Dent S, Stanway S, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020;22:1945–1960. doi: 10.1002/ejhf.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayek SS, Ganatra S, Lenneman C, et al. Preparing the cardiovascular workforce to care for oncology patients: JACC review topic of the week. J Am Coll Cardiol. 2019;73:2226–2235. doi: 10.1016/j.jacc.2019.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng J, Rushton M, Johnson C, et al. An international survey of healthcare providers’ knowledge of cardiac complications of cancer treatments. Cardiooncology. 2019;5:12. doi: 10.1186/s40959-019-0049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xia Y, Liu J. Working together to advance cardio-oncology in China. JACC CardioOncol. 2020;2:144–145. doi: 10.1016/j.jaccao.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim H, Chung WB, Cho KI, et al. Diagnosis, treatment, and prevention of cardiovascular toxicity related to anti-cancer treatment in clinical practice: an opinion paper from the Working Group on Cardio-Oncology of the Korean Society of Echocardiography. J Cardiovasc Ultrasound. 2018;26:1–25. doi: 10.4250/jcu.2018.26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhatt DL. Birth and maturation of cardio-oncology. JACC CardioOncol. 2019;1:114–116. doi: 10.1016/j.jaccao.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoon GJ, Telli ML, Kao DP, Matsuda KY, Carlson RW, Witteles RM. Left ventricular dysfunction in patients receiving cardiotoxic cancer therapies are clinicians responding optimally? J Am Coll Cardiol. 2010;56:1644–1650. doi: 10.1016/j.jacc.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oren O, Neilan TG, Fradley MG, Bhatt DL. Cardiovascular safety assessment in cancer drug development. J Am Heart Assoc. 2021;10:e024033. doi: 10.1161/JAHA.121.024033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCain K, Hardesty C, Wong A. Charting a course to remodel universal healthcare in the Asia-Pacific [Internet] Cologny: World Economic Forum; 2021. [cited 2022 July 16]. Available from: https://www.weforum.org/agenda/2021/11/how-to-remodel-universal-healthcare-in-the-asia-pacific/ [Google Scholar]
- 61.Khalid A. Expanding universal health coverage in Asia [Internet] Tacoma (WA): The Borgen Project; 2015. [cited 2022 August 25]. Available from: https://borgenproject.org/universal-health-care-in-asia/ [Google Scholar]
- 62.Lagomarsino G, Garabrant A, Adyas A, Muga R, Otoo N. Moving towards universal health coverage: health insurance reforms in nine developing countries in Africa and Asia. Lancet. 2012;380:933–943. doi: 10.1016/S0140-6736(12)61147-7. [DOI] [PubMed] [Google Scholar]
- 63.Addison D, Campbell CM, Guha A, Ghosh AK, Dent SF, Jneid H. Cardio-oncology in the era of the COVID-19 pandemic and beyond. J Am Heart Assoc. 2020;9:e017787. doi: 10.1161/JAHA.120.017787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Evans WK, Ashbury FD, Hogue GL, Smith A, Pun J. Implementing a regional oncology information system: approach and lessons learned. Curr Oncol. 2014;21:224–233. doi: 10.3747/co.21.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sadler D, Chaulagain C, Alvarado B, et al. Practical and cost-effective model to build and sustain a cardio-oncology program. Cardiooncology. 2020;6:9. doi: 10.1186/s40959-020-00063-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herrmann J, Lenihan D, Armenian S, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. 2022;43:280–299. doi: 10.1093/eurheartj/ehab674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jahangir E. The need for precision cardio-oncology [Internet] Washington, D.C.: American College of Cardiology; 2020. [cited 2022 August 2]. Available from: https://www.acc.org/latest-in-cardiology/articles/2020/09/21/19/13/the-need-for-precision-cardio-oncology. [Google Scholar]
- 68.Rhee JW, Ky B, Armenian SH, Yancy CW, Wu JC. Primer on biomarker discovery in cardio-oncology: application of omics technologies. JACC CardioOncol. 2020;2:379–384. doi: 10.1016/j.jaccao.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gerik L. Cardio-oncology, then and now: an interview with Barry Trachtenberg. Methodist DeBakey Cardiovasc J. 2019;15:e1–e3. [Google Scholar]
- 70.Dreyfuss AD, Bravo PE, Koumenis C, Ky B. Precision cardio-oncology. J Nucl Med. 2019;60:443–450. doi: 10.2967/jnumed.118.220137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Attia ZI, Kapa S, Lopez-Jimenez F, et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat Med. 2019;25:70–74. doi: 10.1038/s41591-018-0240-2. [DOI] [PubMed] [Google Scholar]
- 72.Zhou Y, Hou Y, Hussain M, et al. Machine learning-based risk assessment for cancer therapy-related cardiac dysfunction in 4300 longitudinal oncology patients. J Am Heart Assoc. 2020;9:e019628. doi: 10.1161/JAHA.120.019628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chaix MA, Parmar N, Kinnear C, et al. Machine learning identifies clinical and genetic factors associated with anthracycline cardiotoxicity in pediatric cancer survivors. JACC CardioOncol. 2020;2:690–706. doi: 10.1016/j.jaccao.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sadler DB, Moudgil R, Arco T, et al. Abstract 11675: Global cardio oncology registry: structure for a multinational collaboration. Circulation. 2021;144(Suppl 1):11675 [Google Scholar]