Summary
Background
Prurigo nodularis is a chronic skin disease characterised by intensely pruritic, hyperkeratotic nodules. Vixarelimab, a human monoclonal antibody, binds to the beta subunit of the oncostatin M receptor, inhibiting signalling of both interleukin 31 and oncostatin M, two cytokine pathways that contribute to pruritus and nodule formation in prurigo nodularis.
Methods
This double-blind, placebo-controlled, phase 2a trial was done at both private and academic dermatology outpatient research clinics across the United States and Canada (n = 40). Patient eligibility criteria included age 18–75 years, physician-documented diagnosis of prurigo nodularis minimum 6 months duration of prurigo nodularis, presence of at least 10 pruritic nodules approximately 0.5–2 cm in size on at least two different anatomical locations (excluding face and scalp) and involving the extremities, and presence of normal-appearing skin between nodules; atopic dermatitis as a comorbidity was exclusionary. Patients were required to have moderate-to-severe pruritus, defined as Worst Itch–Numeric Rating Scale (WI-NRS) score ≥7 at screening and LS-mean weekly WI-NRS score ≥5 for each of the 2 consecutive weeks immediately before randomisation. Participants were randomly assigned (1:1) to receive weekly subcutaneous vixarelimab 360 mg (720 mg loading dose) or placebo using stratification factors (sex and presence of atopy) and block size 4 through the IWRS system. Stratification by atopy status was based on the reported high prevalence of atopy in this population and the potential impact of atopy in the immunopathologic process in prurigo nodularis. Patients, investigators, study sponsor, and site staff were masked to study treatment. The primary efficacy endpoint was least squares (LS)-mean percent change from baseline (PCFB) at Week 8 in weekly average Worst Itch–Numeric Rating Scale (WI-NRS) score. The primary efficacy endpoint was analysed with ANCOVA including treatment as fixed effect, with baseline WI-NRS, and randomisation stratification factor as covariates. All randomised patients who had at least 1 dose of study drug or placebo were included in the Safety Analysis Set. This trial is registered at ClinicalTrials.gov, NCT03816891.
Findings
Of 50 patients randomised between March 11, 2019 and January 31, 2020, 23 vixarelimab recipients and 26 placebo recipients comprised the modified intent-to-treat analysis population (baseline LS-mean [SD] WI-NRS score, 8.3 [1.05]). Outcomes at Week 8 for vixarelimab versus placebo included LS-mean PCFB in WI-NRS score, −50.6% versus −29.4% (LS-mean difference [95% CI], −21.2% [−40.82, −1.60]; p = 0.03); ≥4-point reduction in WI-NRS score, 52.2% (12/23) versus 30.8% (8/26) (p = 0.11); PN-IGA score of 0 or 1, 30.4% (7/23) versus 7.7% (2/26) (p = 0.03); LS-mean PCFB in pruritus VAS score, −54.4% versus −32.6% (p = 0.03); and LS-mean PCFB sleep loss reduction (improvement), −56.3% versus −30.0% (p = 0.02). No deaths, serious TEAEs, or TEAEs leading to dose interruption were reported. The percentage of vixarelimab recipients reporting any TEAE was 91.3% (21/23) versus 76.9% (20/26) of placebo recipients; drug-related TEAEs generally were similar between the two groups (vixarelimab, 43.5% [10/23]; placebo, 38.5% [10/26]).
Interpretation
Vixarelimab demonstrated rapid reduction of pruritus and achievement of clear/almost clear skin in one-third of the patients by Week 8. Relief of itch and clearing of skin nodules represent two important potential therapeutic advances in the management of patients suffering from the debilitating disease Prurigo Nodularis.
Funding
Kiniksa Pharmaceuticals, Ltd.
Keywords: Interleukin-31 receptor alpha, IL-31, Nodular prurigo, Oncostatin M receptor beta, Prurigo nodularis, Pruritus, Vixarelimab
Research in context.
Evidence before this study
To assess previous research in the topic of prurigo nodularis treatment, we completed a non-systematic PubMed search using a combination of terms including, but not limited to, ‘nodular prurigo’, ‘prurigo nodularis’, ‘interleukin-31 receptor alpha’, ‘IL-31’, and ‘oncostatin M receptor beta’. The search included clinical trials, clinical observations, and treatment guidelines with a search date cut-off of June 11, 2021. Previous studies reported the characteristic hyperkeratotic, excoriated lesions of prurigo nodularis resulting from an intense, continuous itch–scratch cycle. Relentless and severe itch interfered with sleep quality, work capacity, and social/leisure aspects of life, leading to high rates of mental health comorbidities. The pathophysiology of pruritus in prurigo nodularis is attributed to nonhistaminergic mediators. Two cytokines, interleukin 31 (IL-31) and oncostatin M (OSM), play central roles in the pathophysiology of prurigo nodularis—IL-31 as a key mediator of skin pruritus and OSM as a mediator of skin inflammation, hyperkeratosis, and fibrosis. Clinical evidence supports the antipruritic effect of blocking the IL-31 pathway in patients with prurigo nodularis.
Added value of this study
This is the first published study in patients with prurigo nodularis investigating the clinical efficacy and safety of a monoclonal antibody targeting both IL-31 and OSM pathways by binding to the shared OSMRβ subunit. The data showed vixarelimab provided rapid, statistically significant, and clinically meaningful reductions in pruritus. Further, approximately one-third of patients attained clear/almost clear skin within eight weeks of starting treatment. Vixarelimab was well tolerated.
Implications of all the available evidence
Despite the challenges of comparing findings across different clinical trials, the nodule resolution observed with vixarelimab in the present study appeared to be more rapid than what would have been expected had vixarelimab affected only pruritus. This could be early evidence that the second mechanism of vixarelimab (blocking of OSM activity) contributes to lesion healing in prurigo nodularis beyond the abatement of pruritus and ensuing reduction in mechanical disruption of the skin. Further clinical studies will be needed to confirm the clinical implications of the dual mechanism of action. If validated, vixarelimab may offer a more complete treatment approach for prurigo nodularis, targeting specific pathologies implicated in both pruritus and skin lesions.
Introduction
Prurigo nodularis is a chronic inflammatory skin disease characterised by multiple pruritic, hyperkeratotic, firm, dome-shaped nodules and repeated scratching, picking, or rubbing.1, 2, 3, 4 Pruritus associated with prurigo nodularis is intense, painful, and relentless.5,6 In the United States, estimates of prurigo nodularis prevalence over the past decade ranged from 13 to 148 per 100,000 population, the higher estimate representing prevalence in an older (Medicare) population.7, 8, 9 A recent review article summarizes recent epidemiological studies estimating prevalence of chronic prurigo in various countries.10
The characteristic hyperkeratotic, excoriated lesions of prurigo nodularis result from an intense, continuous itch–scratch cycle. Relentless and severe itch markedly interferes with sleep quality, work capacity, and social/leisure aspects of life,11, 12, 13 leading to high rates of mental health comorbidities (e.g., anxiety, depression, suicidal ideation/self-harm, eating disorders, substance abuse).6,8,14
Management of prurigo nodularis is limited and challenging as no therapies were approved for this indication at the time this manuscript was submitted. The pathophysiology of pruritus in prurigo nodularis remains unclear but is increasingly attributed to nonhistaminergic mediators and type 2 inflammation. Two cytokines, interleukin 31 (IL-31) and oncostatin M (OSM), play central roles in the pathophysiology of prurigo nodularis—IL-31 as a key mediator of skin pruritus15 and OSM as a mediator of skin inflammation, hyperkeratosis, and fibrosis.16 In studies of patients with prurigo nodularis, itch intensity correlated with quantity of dermal IL-31 (+), IL-31RA (+), and OSM (+) cells; the number of dermal OSM receptor β cytokine receptor subunit (OSMRβ) (+) cells was also increased in these patients.17 Furthermore, clinical evidence supports the antipruritic effect of blocking the IL-31 pathway in patients with prurigo nodularis.18,19
Vixarelimab (KPL-716; Kiniksa Pharmaceuticals) is a first-in-class fully human monoclonal antibody that is under investigation for the treatment of prurigo nodularis. Vixarelimab targets OSMRβ, a cell surface receptor that differentially heterodimerizes with the IL-31 receptor α (IL-31Rα) chain to form the IL-31 receptor (IL-31RA) or with the glycoprotein 130 (gp130) chain to form the OSM type II receptor, thus mediating signalling of IL-31 or OSM, respectively.20 Consequently, vixarelimab has a dual mechanism of action, simultaneously inhibiting signalling of these two cytokine pathways implicated in pruritus, inflammation, hyperkeratosis, and fibrosis.16 Phase 1 investigations of vixarelimab in patients with atopic dermatitis indicated good tolerability, pruritus improvement, and reduced sleep loss versus placebo.21 Vixarelimab does not bind or inhibit the leukaemia inhibitor factor (LIF) receptor complex (gp130/LIFR, or OSM receptor type I) by which OSM and LIF mediate hematopoiesis.16
This randomised, placebo-controlled phase 2a study investigated vixarelimab efficacy and safety in patients with moderate-to-severe prurigo nodularis who were experiencing moderate-to-severe pruritus.
Methods
Study design
This randomised, double-blind, placebo-controlled, phase 2a trial (NCT03816891) was conducted at 27 sites in the United States and Canada. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All patients provided written informed consent. The study protocol and amendments were approved by Sterling IRB. The CONsolidated Standards of Reporting Trials (CONSORT) reporting guidelines have been utilized in the reporting of these clinical-trial results.
Patients
Patients were assessed for eligibility at a screening visit at least 14 days but no more than 28 days before treatment initiation. Patient eligibility criteria included age 18–75 years; physician-documented diagnosis of prurigo nodularis which was confirmed by central, independent review of whole-body medical photographs taken by dermatologists not involved in the study; minimum 6 months duration of prurigo nodularis; presence of at least 10 pruritic nodules approximately 0.5–2 cm in size on at least two different anatomical locations (excluding face and scalp) and involving the extremities; and presence of normal-appearing skin between nodules, with the exception of atopic dermatitis as a comorbidity. Patients were required to have moderate-to-severe pruritus, defined as Worst Itch–Numeric Rating Scale (WI-NRS) score ≥7 at screening and LS-mean weekly WI-NRS score ≥5 for each of the 2 consecutive weeks immediately before randomisation.
Exclusion criteria included past use of any investigational biologic or nonbiologic drug targeting IL-31, IL-31Rα, OSM, or OSMRβ; current use of medication known to cause pruritus; significant flare of pruritus and/or skin eruption (requiring medical intervention) during screening; presence of any inflammatory, pruritic, and/or fibrotic skin condition other than prurigo nodularis or atopic dermatitis, and systemic comorbidities that could interfere with or complicate study assessments (e.g., end stage renal failure, cholestasis, neuropathy, etc.). CBC and Chem 20 formed the basis of confirming that the laboratory results were within the acceptable ranges for exclusion criteria. A washout study design was used to establish a clear baseline and limit exposure to concomitant therapies that could affect pruritus or disease severity or potentially confound efficacy outcomes (see Supplemental Table S1 for additional details).
Randomisation and masking
In this Phase 2a study, patients were randomised between March 11, 2019 and January 31, 2020, in a 1:1 ratio to receive vixarelimab or placebo using stratification factors (sex and presence of atopy) and block size 4 through the IWRS system. Stratification by atopy status was based on the reported high prevalence of atopy in this population and the potential impact of atopy in the immunopathologic process in prurigo nodularis. Patients, investigators, study sponsor, and site staff were masked to study treatment.
Procedures
On Day 1, patients received a loading dose of vixarelimab 720 mg (2× maintenance dose) subcutaneous (SC) or matching placebo. Thereafter, study drug (vixarelimab 360 mg SC or placebo) was administered once weekly. The 360 mg dose level was fixed and was not adjusted for body weight, based upon Phase 1 PK data. Pharmacokinetic modelling predicted a maximum plasma concentration of 250 μg/mL and a trough level of approximately 150 μg/mL. The predicted trough level was comparable to a level that has demonstrated clinical efficacy in phase 1 studies and was approximately 15–30-fold greater than the efficacious trough concentration (5–10 μg/mL) identified in IL-31 intradermal challenge experiments. All study doses were administered by the investigator or qualified designee.
In December 2020, based on external data and unblinded vixarelimab phase 1 data, two protocol amendments were implemented to accelerate time to proof of concept: treatment duration was shortened from 16 to 8 weeks. These data showed that 8 weeks was a sufficient amount of time to capture the vast majority of the efficacy signal for pruritus, given that initial onset of treatment response is rapid, i.e., measurable within the first week. The additional timepoints after Week 8 were a safety follow up period as treatment washed off. The ongoing Phase 2b study includes a longer 16-week treatment period for longer-term efficacy observation followed by an open label extension. Additionally, the treatment effect magnitude supported a sample size reduction from 80 to 100 patients to 50 patients (see Statistical analysis section). Daily, patients rated the severity of their most severe itch over the prior 24 h on a Web-based eDiary using the WI-NRS scale (0 [no itch] to 10 [worst imaginable itch]). At each study visit, patients completed a pruritus visual analogue scale (VAS) assessment, rating average itch intensity (from 0 [no itch] to 10 [worst imaginable itch])22 over the prior 3 days, and the 5-D Pruritus Scale, with total scores ranging from 5 (no pruritus) to 25 (most severe pruritus) over the prior 2 weeks.23
Impact on lesional severity was assessed with two exploratory tools, the Prurigo Nodularis Investigator's Global Assessment (PN-IGA) and the Prurigo Nodularis Nodule Assessment Tool (PN-NAT), at baseline and at Weeks 2, 4, 6, and 8. The PN-IGA assesses prurigo nodularis disease severity based on presence of nodules and nodule elevation (0, clear [no nodules] to 4, severe [nodules present, most raised prominently]). PN-NAT assesses prurigo nodularis severity and factors in the estimated number of nodules over the entire body (0 [none] to 3 [more than 50 nodules]), percentage of nodules that are hard (0 [none] to 3 [more than two-thirds are hard]), extent of excoriations over the entire body (0 [none] to 3 [more than two-thirds of nodules are excoriated]), and exact nodule counts in a representative area. Kiniksa developed the PN-NAT as a supplemental tool to capture additional elements of the physical manifestations of PN. It was developed in consultation with investigators involved in the trial and was piloted during this study to inform the development of tools which could potentially be used in a Phase 3 program. Additionally, a skin area representative of patient's disease was photographed and assessed at baseline and Weeks 4 and 8.
Patient's sleep was assessed daily using two NRS scales via eDiary, one for difficulty falling asleep because of itch (0 [not difficult at all] to 10 [extremely difficult]) and one for sleep quality (0 [best possible sleep] to 10 [worst possible sleep]).24 Sleep was also assessed at each study visit using a sleep loss VAS rating of intensity of average sleeplessness experienced over the previous three nights (0 [no sleeplessness] to 10 [worst imaginable sleeplessness]).
Quality of life was assessed at baseline and every study visit using the Itchy Quality of Life (ItchyQoL), Dermatology Life Quality Index (DLQI), and Hospital Anxiety and Depression Scale (HADS). Patient Benefit Index–Pruritus (PBI–P) was used at baseline and Week 8 (Supplemental Table S2).
Routine physical examinations and laboratory surveillance were performed at prespecified intervals and as needed.
Rescue medication with topical corticosteroids and/or oral antihistamines was allowed at the investigator's discretion. To minimise confounding effects of rescue medications, efficacy assessments after use of specific rescue medications were treated as missing for predetermined time intervals (see Supplemental Methods: Statistical Analysis).
Outcomes
The primary efficacy endpoint was the least squares (LS)-mean (i.e. baseline adjusted mean from ANCOVA analysis) percent change from baseline in weekly average WI-NRS score at Week 8 for vixarelimab versus placebo. Key secondary endpoints were proportion of patients achieving ≥4-point reduction from baseline in weekly average WI-NRS and percent change from baseline in pruritus VAS at Week 8. Other secondary endpoints included change and percent change from baseline in 5-D Pruritus total score over time; proportion of PN-IGA responders (score 0 or 1); proportion of patients with PN-IGA improvement by two categories over time; change from baseline in PN-NAT over time; change and percent change from baseline in sleep loss VAS over time, in weekly average difficulty falling asleep NRS over time, and in weekly average sleep quality NRS over time; change from baseline in quality-of-life measures (ItchyQoL, DLQI, HADS) over time; and PBI-P scores at Week 8.
Safety parameters included incidence rate and severity of treatment-emergent adverse events (TEAEs).
Statistical analysis
Initial sample size calculations were based on a two-sample t-test for the primary efficacy endpoint of percentage change from baseline of WI-NRS at Week 8. Assuming a weekly average WI-NRS reduction from baseline at Week 8 of 60% for vixarelimab and 30% for placebo (1:1 randomisation and standard deviation [SD] of 50% for both), a sample size of 50 patients per group was initially considered sufficient to provide ≥90% power to detect a treatment difference (two-sided alpha, 0.20). Before enrolment completed, internal and external data supported a revised SD of 35%; thus, a total sample size of 50 was deemed sufficient.
All efficacy analyses were performed on the modified intent-to-treat (mITT) analysis population (patients who received at least one dose of study drug and had at least one postbaseline efficacy assessment); last observation carried forward (LOCF) was used to address missing data (see Supplemental Methods for additional details).
The analyses of change and percent change from baseline used an analysis of covariance (ANCOVA) model including treatment as fixed effect, with baseline WI-NRS and randomisation stratification factor as covariates. We refer to the adjusted mean in each trial arm, and the adjusted mean difference between arms, from the ANCOVA model as the least-squares (LS) mean and LS-mean difference, respectively. Multiplicity adjustment was performed for primary and key secondary endpoints (see Outcomes section above) using a hierarchical procedure at a prespecified alpha level of 0.05. If the primary endpoint had a p value <0.05, the two key secondary endpoints would be tested using the Hochberg procedure.25
Binary responder analyses used the Cochran-Mantel-Haenszel test adjusted by stratification factors (sex and atopy) for the secondary endpoints: proportions of patients with: ≥4-point reduction in weekly average WI-NRS; PN-IGA score reduction (improvement) of ≥2 points; PN-IGA score of 0–1; ≥4-point reduction in weekly average sleep quality NRS; and ≥4-point reduction in weekly average falling asleep. Statistical handling of rescue medication use is described in Supplemental Methods: Statistical Analysis. The proportion of patients achieving a 4- to <5-, 5- to <6-, 6- to <7-, 7- to <8-, 8- to <9-, and 9- to 10-point reduction in weekly average WI-NRS from baseline over time was assessed as an exploratory analysis. A post hoc analysis determined the proportion of patients who experienced both a ≥4-point reduction in weekly average WI-NRS and a PN-IGA score of 0 or 1.
All tests, except multiplicity adjustment, were at two-sided level of alpha 0.2 first and then 0.05. SAS version 9.4 was used for statistical analyses.
Role of the funding source
The funder of the study had a role in the study design, data collection, data analysis, data interpretation, and writing of the report. All investigators confirmed adherence to the protocol. LZ, GLJ, JFP, and HS had access to the dataset. HS and JFP had final responsibility for the decision to submit for publication.
Results
In total, 128 individuals were screened between March 11, 2019 and January 16, 2020; 78 were excluded (Fig. 1). Fifty patients were randomised to vixarelimab (n = 24) or placebo (n = 26). One patient assigned to vixarelimab withdrew consent before receiving treatment, leaving 49 patients in the safety and mITT population (vixarelimab, n = 23; placebo, n = 26). Baseline clinical characteristics were well balanced between the two treatment arms (Table 1). LS-mean baseline WI-NRS scores were ≥8 (indicating severe pruritus) in the vixarelimab (8.7) and placebo (8.0) treatment groups. Similar proportions of patients had a baseline PN-IGA score of 3 (moderate; vixarelimab, 56.5% [13/23]; placebo, 53.8% [14/26]) or 4 (severe; vixarelimab, 39.1% [9/23]; placebo, 46.2% [12/26]); one patient treated with vixarelimab (4.3%) had a PN-IGA score of 2. Racial distribution varied between groups and the LS-mean [SD] patient age was lower in the vixarelimab group than the placebo group (52.0 [15.56] vs 64.1 [7.86] years). This numerical disparity in age at baseline between the two groups appeared to be a play of chance due to the small sample size (23 in the vixarelimab arm and 26 in the placebo arm). This difference was found not to influence the outcome of the study, as the severity of pruritis at baseline was similar between the two groups and the magnitude of the treatment response did not appear to vary with age. Elevated immunoglobulin E (>200 IU/mL) was noted in 47.8% (11/23) of patients treated with vixarelimab and 23.1% (6/26) of patients treated with placebo. There was an apparent disparity in the history of different types of allergies/atopy and serum IgE levels at baseline between the active and placebo groups. The issue may have arisen owing to the smaller sample size in this proof-of-concept study. A history of atopy was reported in 67.3% of patients and was primarily defined as seasonal allergies.
Fig. 1.
Trial profile. ∗One patient had an unclear cause of extremely high immunoglobulin E level, and two patients were outside of the visit windows. †One patient was inadvertently randomised but never dosed after the patient was confirmed to be ineligible for the study. mITT = modified intent-to-treat.
Table 1.
Patient demographics and baseline characteristics.
| Vixarelimab (n = 23) | Placebo (n = 26) | |
|---|---|---|
| Age, years | ||
| Mean (SD) | 52.0 (15.56) | 64.1 (7.86) |
| Min, max | 20, 72 | 41, 75 |
| Sex, n (%) | ||
| Male | 10 (43.5%) | 10 (38.5%) |
| Female | 13 (56.5%) | 16 (61.5%) |
| Race, n (%) | ||
| White | 15 (65.2%) | 21 (80.8%) |
| Black or African American | 5 (21.7%) | 3 (11.5%) |
| Asian | 2 (8.7%) | 0 |
| American Indian or Alaska Native | 0 | 1 (3.8%) |
| Multiple | 1 (4.3%) | 0 |
| Other | 0 | 1 (3.8%) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 2 (8.7%) | 0 |
| Not Hispanic or Latino | 21 (91.3%) | 26 (100.0%) |
| Presence of atopy,a n (%) | 15 (65.2%) | 18 (69.2%) |
| Elevated immunoglobulin E (>200 IU/mL), n (%) | 11 (47.8%) | 6 (23.1%) |
| Historical allergy, n (%) | ||
| Any historical allergy | 10 (43.5%) | 14 (53.8%) |
| Asthma | 1 (4.3%) | 1 (3.8%) |
| Atopic dermatitis | 1 (4.3%) | 2 (7.7%) |
| Allergic rhinitis | 4 (17.4%) | 2 (7.7%) |
| Otherb | 9 (39.1%) | 14 (53.8%) |
| Years since first prurigo nodularis nodules | ||
| Mean (SD) | 12.2 (13.32) | 17.2 (14.89) |
| Min, max | 0.8, 45.5 | 1.1, 53.5 |
| Weekly average WI-NRS (0–10 scale), mean (SD) | 8.7 (0.88) | 8.0 (1.09) |
| Pruritus VAS (0–10 scale), mean (SD) | 8.9 (1.11) | 8.1 (1.41) |
| 5-D Pruritus total score (5–25 scale), mean (SD) | 19.9 (2.70) | 17.6 (4.05) |
| PN-NAT (0–9 scale), mean (SD)c | 6.9 (1.36) | 7.6 (1.42) |
| PN-IGA score (0–4 scale), n (%) | ||
| 2 | 1 (4.3) | 0 |
| 3 | 13 (56.5) | 14 (53.8) |
| 4 | 9 (39.1) | 12 (46.2) |
| Weekly average difficulty falling asleep NRS (0–10 scale), mean (SD) | 7.4 (2.52) | 7.2 (1.36) |
| Weekly average sleep quality NRS (0–10 scale), mean (SD) | 7.6 (2.21) | 7.0 (1.52) |
| Sleep loss VAS (0–10 scale), mean (SD) | 8.1 (2.29) | 7.0 (2.35) |
| ItchyQoL (0–110 scale), mean (SD) | 91.4 (16.49) | 86.6 (16.27) |
| DLQI (0–30 scale), mean (SD) | 16.1 (7.99) | 14.8 (7.07) |
| HADS total score (0–42 scale), mean (SD) | 15.1 (11.40) | 14.9 (6.38) |
DLQI = Dermatology Life Quality Index. HADS = Hospital Anxiety and Depression Scale. NRS = numeric rating scale. PN-IGA = Prurigo Nodularis Investigator's Global Assessment. PN-NAT = Prurigo Nodularis Nodule Assessment Tool. SD = standard deviation. VAS = visual analogue scale. WI-NRS = Worst Itch–Numeric Rating Scale.
Primarily defined as seasonal allergies.
Food, drug, bee sting, environmental, etc.
Subtotal of number of nodules, hardness of nodules, and extent of excoriations.
Twenty vixarelimab patients (87.0%) and 18 placebo patients (69.2%) received all eight study doses; three (13.0%) vixarelimab and seven (26.9%) placebo patients missed one or two doses, and one (3.8%) placebo patient missed four doses. Treatment was discontinued in one placebo patient because of scheduling conflicts.
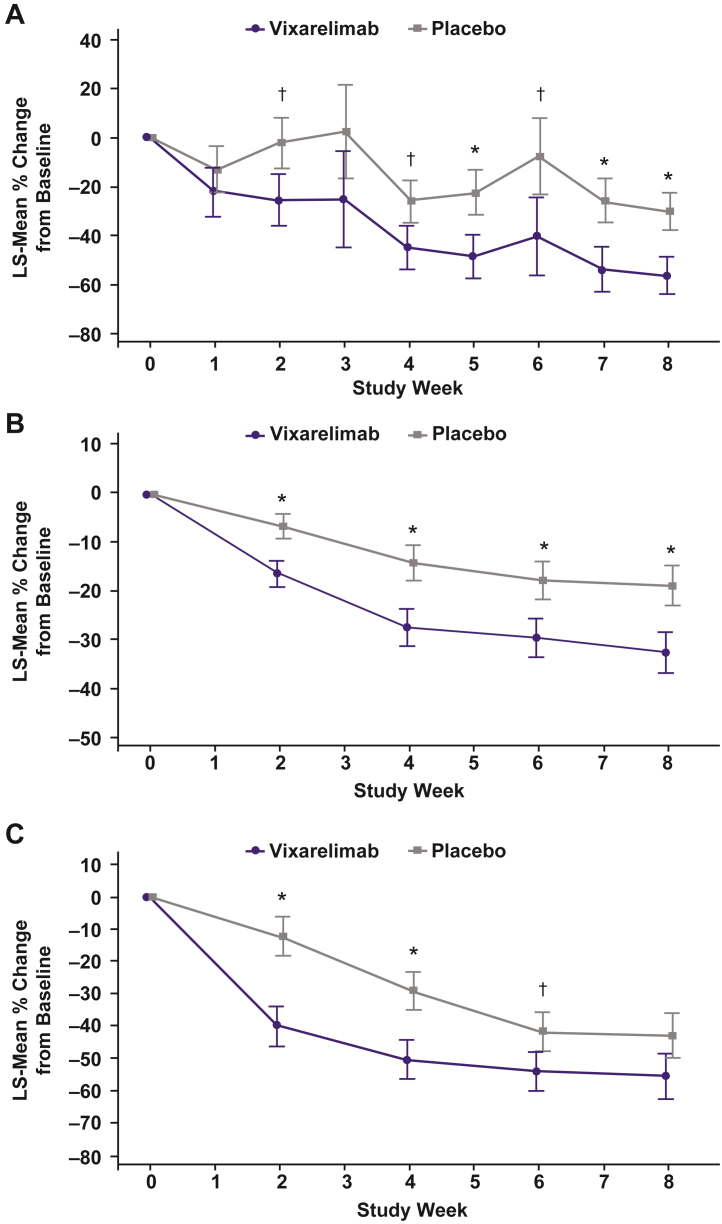
For the primary efficacy outcome, LS-mean percent change from baseline in weekly average WI-NRS score at Week 8 was −50.6% in vixarelimab recipients and −29.4% in placebo recipients (LS-mean difference [95% CI], −21.2% [−40.82, −1.60]; p = 0.03) (Fig. 2). Highly statistically significant differences (p < 0.05) were observed at Week 3 and at each subsequent visit through Week 8. LS-mean (SD) absolute change from baseline to Week 8 in the 0- to 10-point WI-NRS score was −4.5 (3.24) and −2.7 (2.19) points in the vixarelimab and placebo groups, respectively. Median (interquartile range [IQR]) percent reduction from baseline in WI-NRS to Week 8 was 69.8% (83.1%, 12.8%) for vixarelimab and 36.1% (55.0%, 11.9%) for placebo (Fig. 2).
Fig. 2.
Least squares mean (SE) percent change from baseline and median percent change from baseline in weekly average WI-NRS scores. ∗p < 0.05. †p < 0.20. LS = least squares. SE = standard error. WI-NRS = Worst Itch–Numeric Rating Scale.
At Week 8, 52.2% (12/23) of vixarelimab recipients achieved a ≥4-point reduction in WI-NRS versus 30.8% (8/26) of placebo recipients (p = 0.11) (Fig. 3). The percentages of patients who achieved a ≥4-point reduction in weekly average WI-NRS were significantly higher for vixarelimab recipients than placebo recipients from Week 2 through Week 8 (p < 0.20); the differences were highly statistically significant (p < 0.05) at Weeks 3, 4, and 5. An analysis of ≥4-point reduction in WI-NRS from baseline to Week 8 in patient subgroups by baseline characteristics is summarised in Supplemental Table S3.
Fig. 3.
Percentages of patients in vixarelimab and placebo groups with clinically meaningful (≥4-point) reductions from baseline in weekly average WI-NRS scores over 8 weeks, by categorical reduction in WI-NRS. ∗p < 0.05. †p < 0.20. Vixarelimab vs placebo at time point for total number of patients with WI-NRS reductions ≥4 points. WI-NRS = Worst Itch–Numeric Rating Scale.
Large-magnitude reductions from baseline in WI-NRS score (e.g., 6, 7, and ≥8 points) were observed among only vixarelimab recipients, and as early as Week 3 (Fig. 3). Individual patient responses for percent change in weekly average WI-NRS revealed that vixarelimab recipients ‘clustered’ into two subgroups based on degree of magnitude of response: 52.2% (12/23) experienced a WI-NRS reduction greater than the clinically meaningful change of ≥4 points, while 47.8% (11/23) had a reduction of <4 points (Supplemental Fig. S1). This pattern was less apparent in placebo recipients.
Based on regulatory feedback, a post hoc sensitivity analysis of LOCF was performed with data imputed for continuous variables with the LOCF method and data assessments set to nonresponder for categorical variables on or after rescue medication use and for missing values. This analysis found statistically significant differences (p < 0.05) in favour of vixarelimab for all primary and key secondary efficacy endpoints: percent change from baseline in WI-NRS at Week 8 (p = 0.01); proportion of patients with ≥4-point reduction in WI-NRS at Week 8 (p = 0.02); and percent change from baseline in pruritus VAS at Week 8 (p = 0.01) (data not shown). Post hoc analysis of Week 8 data found that 85.7% (6/7) of vixarelimab recipients who achieved a PN-IGA rating of 0 or 1 also experienced a ≥4-point reduction in WI-NRS; no placebo recipient achieved this benchmark.
Consistent with the other results at Week 8, LS-mean percent change (95% CI) from baseline in pruritus VAS was significantly greater in vixarelimab recipients than placebo recipients at Week 8 (−54.4% [−68.55, −40.21] and −32.6% [−46.64, −18.60], respectively; p = 0.03) (Supplemental Fig. S2). At Weeks 3 through 8, this outcome reached the prespecified phase 2 standard of statistical significance (p < 0.20). At each post-baseline study visit through Week 8, LS-mean percent change from baseline in 5-D Pruritus total score was statistically significantly greater for vixarelimab versus placebo (p < 0.20) (Supplemental Fig. S3).
At Week 8, a statistically significantly greater proportion of vixarelimab recipients (30.4% [7/23]) achieved a PN-IGA score of 0 or 1 (skin lesions clear or almost clear) versus placebo recipients (7.7% [2/26]; p = 0.03). For this outcome, the difference between groups reached significance at Week 6 (vixarelimab, 30.4% [7/23], vs placebo, 3.8% [1/26]; p = 0.01) with separation from placebo apparent as early as Week 4 (Fig. 4). The proportion of vixarelimab vs placebo recipients who achieved ≥2-point reduction in PN-IGA total score was significantly greater at Week 8 (43.5% [10/23] vs 15.4% [4/26]; p = 0.02) and also at Week 6 (43.5% [10/23] vs 11.5% [3/26]; p = 0.01), with separation from placebo observed as early as Week 4 (Fig. 4). Representative visual images show the degree of nodule resolution in two vixarelimab recipients and one placebo recipient from Day 1 to Week 8 (Fig. 5).
Fig. 4.
Percentage of PN-IGA responders (score of 0 or 1) and patients with a reduction in PN-IGA score ≥2 points from baseline. ∗p < 0.05. PN-IGA = Prurigo Nodularis Investigator's Global Assessment.
Fig. 5.
Representative images of nodule resolution from Day 1 to Week 8 in vixarelimab and placebo recipients. PN-IGA = Prurigo Nodularis Investigator's Global Assessment. WI-NRS = Worst Itch–Numeric Rating Scale.
Reductions from baseline in PN-NAT scores (Supplemental Table S4) were numerically improved for vixarelimab versus placebo at almost every visit; significant differences (p < 0.20) were observed between treatments for distribution of nodules over the entire body (Week 4) and exact nodule count in a representative area (Weeks 4 and 6).
LS-mean percent reduction from baseline in sleep loss VAS at Week 8 was significantly greater with vixarelimab versus placebo (−56.3% vs −30.0%; p = 0.02). Statistically significant improvements were observed at Weeks 5, 7, and 8 (p < 0.05) and Weeks 2, 4, and 6 (p < 0.20) (Fig. 6A). LS-mean percent change from baseline in weekly average sleep quality NRS at Week 8 was numerically greater (reduced score = improved sleep) with vixarelimab versus placebo (−46.1% vs −33.5%; p = 0.22) and statistically significantly greater at Week 7 (−45.3% vs −31.1%; p = 0.16), with curve separation apparent at Week 3 (Supplemental Fig. S4). The proportion of vixarelimab recipients who achieved ≥4-point improvement in weekly average sleep quality NRS was statistically greater than that of placebo recipients at Week 2 through Week 8 (p < 0.20); a higher standard for statistical significance (p < 0.05) was observed for differences between groups at Week 3 (33.3% [7/21] vs 7.7% [2/26]), Week 5 (47.6% [10/21] vs 15.4% [4/26]), and Week 6 (52.4% [11/21] vs 23.1% [6/26]) (vixarelimab vs placebo). At each study visit from Weeks 1 through 8, LS-mean percent change from baseline in weekly average difficulty falling asleep NRS scores was numerically greater (improved) with vixarelimab versus placebo, but the differences were not statistically significant (Supplemental Table S5). The percentage of responders (≥4-point reduction [improvement]) for weekly average difficulty falling asleep NRS at Week 8 was significantly greater in the vixarelimab versus the placebo group (60.0% vs 30.8%; p = 0.04) and achieved statistical significance at Weeks 2 through 6 (p < 0.20).
Fig. 6.
Least squares mean (SE) percent change from baseline in A) sleep loss VAS ratings, B) ItchyQoL total score, and C) DLQI total score. ∗p < 0.05. †p < 0.20. DLQI = Dermatology Life Quality Index. ItchyQoL = Itchy Quality of Life. LS = least squares. SE = standard error. VAS = visual analogue scale.
LS-mean improvement from baseline in ItchyQoL total score was statistically significantly greater with vixarelimab versus placebo from Week 2 through Week 8 (p < 0.05) (Fig. 6B). ItchyQoL Symptom subscores showed improvements similar to that for total score (Supplemental Table S6).
At Week 8, there were numerical differences favouring vixarelimab versus placebo with regard to LS-mean changes from baseline in DLQI total score (Fig. 6C); statistically significant differences favouring vixarelimab were achieved at Week 2 (p < 0.05), Week 4 (p < 0.05), and Week 6 (p < 0.20). There were numerical differences favouring vixarelimab versus placebo in LS-mean HADS total score (Supplemental Table S7), although the differences did not reach statistical significance. At Week 8, LS-mean (standard error) PBI-P scores were 2.0 (0.29) in the vixarelimab group and 1.5 (0.21) in the placebo group.
Fewer vixarelimab recipients used rescue medication versus placebo recipients (21.7% [5/23] vs 30.8% [8/26]). Time to first rescue medication use was notably sooner in the placebo group (median [IQR] 5.6 [2.6, 8.6] weeks vs 13.9 [5.3, 14.1] weeks), and placebo recipients used rescue medication for twice the number of days vs vixarelimab recipients (median [IQR] 22.0 [3.0, 50.0] days vs 41.5 [19.5, 53.5] days).
No deaths, serious TEAEs, or TEAEs leading to dose interruption were reported. The percentage of vixarelimab recipients reporting any TEAE was 91.3% (21/23) versus 76.9% (20/26) of placebo recipients; drug-related TEAEs generally were similar between the two groups (vixarelimab, 43.5% [10/23]; placebo, 38.5% [10/26]) (Table 2).
Table 2.
Treatment-emergent adverse events—overall incidence and events occurring in >5% of patients in either treatment group.
|
TEAE, n (%) |
Vixarelimab (n = 23) | Placebo (n = 26) |
|---|---|---|
| Any event | 21 (91.3%) | 20 (76.9%) |
| Drug related | 10 (43.5%) | 10 (38.5%) |
| Infections and infestations | 10 (43.5%) | 16 (61.5%) |
| Upper respiratory tract infection | 5 (21.7%) | 2 (7.7%) |
| Nasopharyngitis | 3 (13.0%) | 4 (15.4%) |
| Urinary tract infection | 0 | 4 (15.4%) |
| Skin and subcutaneous tissue disorders | 7 (30.4%) | 4 (15.4%) |
| Nummular eczema | 2 (8.7%) | 1 (3.8%) |
| Urticaria | 2a (8.7%) | 0 |
| Skin burning sensation | 0 | 2 (7.7%) |
| Injury, poisoning, procedural complications | 5 (21.7%) | 6 (23.1%) |
| Procedural headache | 2 (8.7%) | 1 (3.8%) |
| Nervous system disorders | 5 (21.7%) | 5 (19.2%) |
| Headache | 3 (13.0%) | 3 (11.5%) |
TEAE = treatment-emergent adverse event.
One acute, mild, self-limiting event considered possibly related to treatment and one acute, moderate-severity event in a patient with long-standing idiopathic angioedema considered unlikely related to treatment.
The most common TEAEs (>5%) in the vixarelimab group were upper respiratory tract infection, nasopharyngitis, headache, nummular eczema, urticaria (acute onset, short lived), and procedural headache, most of which were reported with generally similar frequency in the placebo group (Table 2). Upper respiratory infections were reported in five (21.7%) vixarelimab patients and two (7.7%) placebo patients. There were no atopic dermatitis or asthma flares. Injection-site reactions were experienced by 4.3% (1/23) of vixarelimab recipients (injection-site hematoma) and 7.7% (2/26) of placebo recipients (mild erythema and pruritus; mild swelling); all events were mild or moderate in severity. None of the reported TEAEs was assessed as severe.
Discussion
This phase 2a trial is the first published report of an investigational therapeutic strategy in patients with prurigo nodularis of simultaneously targeting IL-31 and oncostatin M using a first-in-class monoclonal antibody that binds the OSM receptor beta subunit. This novel mechanism blocks the activity of two pathologically relevant cytokines, IL-31 (pruritus) and OSM (skin inflammation, hyperkeratosis, and fibrosis), with a single epitope.20 In adults with moderate-to-severe prurigo nodularis, vixarelimab improved pruritus and lesion burden and a number of other clinically meaningful endpoints. Breakthrough Therapy Designation for vixarelimab to reduce pruritus associated with prurigo nodularis was granted by the FDA in November 2020.
Rapid onset and sustained reduction in pruritus (through Week 8) were evidenced by significantly greater improvement from baseline in WI-NRS scores with vixarelimab versus placebo. Curve separation was significant as early as Week 3. Further, more vixarelimab recipients than placebo recipients achieved a clinically meaningful ≥4-point reduction in WI-NRS. Some vixarelimab recipients experienced an 8+ point reduction in weekly average WI-NRS scores, occurring as early as Week 3. Notably, this level of improvement was not observed in any placebo recipients at any timepoint. As further support for anti-pruritic efficacy, not only were rescue medications used by fewer vixarelimab recipients, placebo recipients used rescue medications substantially sooner and for twice the duration versus vixarelimab recipients.
Although much of the focus in treating prurigo nodularis is on relieving the intense itch, equally important for effective treatment are the clearing of lesions/improving physical appearance and improving sleep and quality of life. All these disease factors cause psychological distress and hinder social participation.3,26 In this study, clear or almost clear skin was attained in almost one-third of vixarelimab recipients by Week 8 based on PN-IGA scores, while significantly fewer placebo recipients achieved this level of lesion improvement. This magnitude of attainment of clear or almost clear skin (PN-IGA scores of 0 or 1) was significant at Week 6, with separation from placebo as early as Week 4, indicating a rate of skin improvement concordant with improvements in pruritus. Similarly, more vixarelimab recipients experienced improvement in overall disease severity, including reduction of the number of nodules and disease activity, as indicated by PN-IGA score reductions ≥2 points.
The reductions in pruritus and skin lesion severity led to improvements in various quality-of-life dimensions. Vixarelimab treatment improved sleep, as evidenced by significantly greater changes from baseline in sleep loss VAS compared with placebo, and by more patients achieving ≥4-point reduction in weekly average sleep quality NRS at various time points. Furthermore, significant ItchyQoL improvements over 8 weeks compared with placebo suggest an impact of vixarelimab on relieving pruritus-related effects on daily activities and emotional distress. Consistent with improvements noted in symptoms, QoL, and skin lesions, depression and anxiety scores also improved numerically by 35.1% in vixarelimab recipients vs 27.7% in placebo recipients.
For most efficacy outcomes evaluated, including the primary study outcome, LS-mean percent change from baseline in the weekly WI-NRS score, vixarelimab treatment was statistically significantly superior to placebo. For the endpoints that did not reach statistical significance, numerical differences between treatments favoured vixarelimab in almost every instance. The relatively small sample size, powered for the primary efficacy endpoint, could have contributed to lack of statistical differences for outcomes that were less sensitive. Further, some assessment tools, including the PN-NAT, are new and not yet validated in prurigo nodularis. PN-NAT was not formally compared to a different outcomes measure instrument, Prurigo Activity Score,27 which may limit the generalisability of this outcomes measure instrument.
Vixarelimab was well tolerated. The frequency of TEAEs, including injection-site reactions, generally was similar for vixarelimab and placebo. There were no atopic dermatitis flares. In this study, there were no adverse drug-related signals for infections overall, immunologic reactions, abnormal liver function, hematologic changes, malignancies, injection-site reactions, or for cardiac toxicity. The overall rate of infections was lower in the vixarelimab group than in the placebo group, although upper respiratory tract infections occurred more frequently in the vixarelimab group. The absence of hematologic parameter changes with vixarelimab likely reflects its specificity of action on the type II OSM receptor by binding OSMRß only. In contrast, the investigational anti-OSM monoclonal antibody GSK2330811, which non-specifically blocks all OSM signalling including that mediated by the LIF (type I OSM) receptor, produced dose-dependent thrombocytopenia in healthy volunteers.28
The efficacy of inhibiting only the IL-31 receptor was previously tested in prurigo nodularis with nemolizumab, an investigational humanised antihuman IL-31 receptor A monoclonal antibody that inhibits IL-31 signal transduction by disrupting binding of IL-31 to IL-31Rα.19 In a phase 2 trial in patients with moderate-to-severe prurigo nodularis, Week 8 data demonstrated nemolizumab treatment to be significantly better than placebo in reducing pruritus, with a similar mean effect size; however, the proportion of ‘responders’ by WI-NRS was not shown. Attainment of IGA score of 0/1 plus improvement of 2 points from baseline was 10.6% at Week 8.19 In the present study, a threefold higher percentage of patients (30.4%; 7/23) receiving vixarelimab achieved a PN-IGA score of 0 or 1 at the same time point. Different IGA tools were used in these two studies, but the descriptions for clear (score 0) and almost clear skin lesions (score 1) are similar between both tools. While comparisons between different studies should always be made with caution, the seemingly more rapid resolution of nodules and higher proportion of patients achieving PN-IGA 0 or 1 observed in the current vixarelimab study could be early evidence that the second mechanism of vixarelimab (blocking of OSM activity) contributes to healing beyond the abatement of pruritus and the ensuing reduction in mechanical disruption of the skin. The OSM pathway contributes fibrosis pathogenesis, and blocking this pathway may have an effect on skin fibrosis and an additive effect beyond the attenuation of itch.
In conclusion, targeted therapy for prurigo nodularis is a major unmet need. This phase 2a clinical trial with vixarelimab, a fully human monoclonal antibody with a novel mechanism of action targeting two primary drivers of pruritus and nodule formation (IL-31 and OSM type II receptor signalling), is the first reported clinical experience with an OSM beta receptor blocker in this disease. The study demonstrated rapid and clinically meaningful improvements in both pruritus and skin nodules, and treatment was well tolerated. Studies with larger populations and longer treatment durations are needed to confirm the benefits of vixarelimab in this burdensome and difficult-to-treat disease.
Contributors
Study design: JFP; Literature search: GLJ; Study investigator: HS, ST, WJL; Enrolled patients: HS, ST, WJL; Collection and assembly of data: GLJ, LZ; Data analysis: LZ, GLJ, JFP; Data verification: HS, GLJ, JFP; Data interpretation: HS, RB, GY, JI, ST, WJL, MZ, ML, LZ, GLJ, JFP; Manuscript preparation: GLJ, JFP; Manuscript review and revisions: HS, RB, GY, JI, ST, WJL, MZ, ML, LZ, GLJ, JFP; Final approval of manuscript: HS, RB, GY, JI, ST, WJL, MZ, ML, LZ, GLJ, JFP.
Data sharing statement
The individual anonymised data supporting the analyses contained in the manuscript will be made available upon reasonable written request from researchers whose proposed use of the data for a specific purpose was approved. Data will not be provided to requesters with potential or actual conflicts of interest, including individuals requesting access for commercial, competitive, or legal purposes. Data access may be precluded for studies in which clinical data were collected subject to legal, contractual or consent provisions that prohibit transfer to third parties. All those receiving access to data will be required to enter into a Data Use Agreement (DUA), which shall contain terms and conditions that are customary for similar agreements and similar companies in the industry. For requests, please email Dr. John F. Paolini, Chief Medical Officer, Kiniksa Pharmaceuticals, jpaolini@kiniksa.com.
Declaration of interests
HS has received honoraria, grants, and/or research funding as a speaker, investigator, and advisory board member for AbbVie, Amgen, BMS, Dermavant, Eli Lilly, Galderma, LEO Pharma, Kiniksa, and UCB.
RB is an advisory board member, consultant, speaker, and/or investigator for, and receives honoraria and/or grants from, AbbVie, Arcutis, Arena Pharma, Asana Biosciences, Bellus Health, Bluefin Biomedicine, Boehringer-Ingelheim, Boston, CARA Therapeutic, Dermavant, Eli Lilly, EMD Serono, Evidera, Galderma, GlaxoSmithKline, Incyte, Inmagene Bio, Janssen, Kiniksa, Kyowa Kirin, LEO Pharma, Novan, Pfizer, Ralexar, RAPT Therapeutic, Regeneron, Respivant, Sanofi, Sienna, Target RWE, and Vyne Therapeutics. He is also an employee and shareholder of Innovaderm Research.
GY is an advisory board member for Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Sanofi, Trevi Therapeutics, Abbvie, Aslan and Arcutis; has received grants/research funding from Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Escient and Eli Lilly; and is an investigator for Regeneron Pharmaceuticals, Inc. and Sanofi.
JS has received honoraria as a consultant and/or advisory board member for Abbvie, Afyx, Aobiome, Arena, Asana, Aslan, BioMX, Bluefin, Bodewell, Boehringer-Ingelheim, Celgene, Connect Biopharma, Dermavant, Dermira, Eli Lilly, Galderma, GlaxoSmithKline, Incyte, Kiniksa, LEO Pharma, Luna, Menlo, Novartis, Pfizer, RAPT, Regeneron, and Sanofi-Genzyme and as a speaker for Abbvie, Eli Lilly, LEO Pharma, Pfizer, Regeneron, and Sanofi-Genzyme; his institution has received grants from Galderma and Pfizer.
ST has received grant support from Kiniksa for clinical research.
WJL serves on speakers bureaus for, and receives honoraria from, Pfizer, Novartis, Amgen, Galderma Canada Inc., Janssen-Ortho, Leo Pharma, Abbvie, Hoffmann-La Roche Limited, Celgene, Pediapharm, and Bausch Health, and serves on advisory boards for Novartis, Janssen-Ortho, Amgen, Abbvie, Galderma, Leo Pharma, Sanofi Genzyme, Pfizer, Sun Pharma, Bausch Health, and Eli Lilly.
MZ has received grants and research support from Pfizer, Novartis, Sienna, Janssen, Endo International, Lilly, Dermira, Moberg Pharma, Soligenix, Allergan, Asana BioSciences, Athenex, Foamix, Incyte, Sun Pharma and Verrice.
ML has received grants and research support from AbbVie, Boehringer Ingelheim, Novartis, Lilly, Janssen, Leo Pharmaceuticals, Dermira, UCB, Aclaris, Valeant and Asana BioSciences.
JFP is an employee of Kiniksa and owns stock in the company.
GLJ and LZ are former employees of Kiniksa and were employed by the company at the time of the study.
Acknowledgments
The authors thank the patients for their participation, and for making the trial possible. Medical writing assistance was provided by JoAnn Clair, PhD, of Kiniksa Pharmaceuticals, Ltd and Sandra Westra, PharmD, of Peloton Advantage, LLC, an OPEN Health company, and funded by Kiniksa Pharmaceuticals, Ltd.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101826.
Appendix A. Supplementary data
References
- 1.Zeidler C., Tsianakas A., Pereira M., Ständer H., Yosipovitch G., Ständer S. Chronic prurigo of nodular type: a review. Acta Derm Venereol. 2018;98(2):173–179. doi: 10.2340/00015555-2774. [DOI] [PubMed] [Google Scholar]
- 2.Kwon C.D., Khanna R., Williams K.A., Kwatra M.M., Kwatra S.G. Diagnostic workup and evaluation of patients with prurigo nodularis. Medicines (Basel, Switzerland) 2019;6(4) doi: 10.3390/medicines6040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams K.A., Roh Y.S., Brown I., et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14(1):67–77. doi: 10.1080/17512433.2021.1852080. [DOI] [PubMed] [Google Scholar]
- 4.Elmariah S., Kim B., Berger T., et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84(3):747–760. doi: 10.1016/j.jaad.2020.07.025. [DOI] [PubMed] [Google Scholar]
- 5.Steinke S., Zeidler C., Riepe C., et al. Humanistic burden of chronic pruritus in patients with inflammatory dermatoses: results of the European academy of dermatology and venereology network on assessment of severity and burden of pruritus (PruNet) cross-sectional trial. J Am Acad Dermatol. 2018;79(3):457–463. doi: 10.1016/j.jaad.2018.04.044. e5. [DOI] [PubMed] [Google Scholar]
- 6.Brenaut E., Halvorsen J.A., Dalgard F.J., et al. The self-assessed psychological comorbidities of prurigo in European patients: a multicentre study in 13 countries. J Eur Acad Dermatol Venereol. 2019;33(1):157–162. doi: 10.1111/jdv.15145. [DOI] [PubMed] [Google Scholar]
- 7.Ständer S., Augustin M., Berger T., et al. Prevalence of prurigo nodularis in the United States of America: a retrospective database analysis. JAAD Int. 2021;2:28–30. doi: 10.1016/j.jdin.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang A.H., Canner J.K., Khanna R., Kang S., Kwatra S.G. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol. 2020;140(2):480–483. doi: 10.1016/j.jid.2019.07.697. e4. [DOI] [PubMed] [Google Scholar]
- 9.Whang K.A., Mahadevan V., Bakhshi P.R., et al. Prevalence of prurigo nodularis in the United States. J Allergy Clin Immunol Pract. 2020;8:3240–3241. doi: 10.1016/j.jaip.2020.05.051. [DOI] [PubMed] [Google Scholar]
- 10.Misery L. Chronic prurigo. Br J Dermatol. 2022;187:464–471. doi: 10.1111/bjd.21698. [DOI] [PubMed] [Google Scholar]
- 11.Garcovich S., Maurelli M., Gisondi P., Peris K., Yosipovitch G., Girolomoni G. Pruritus as a distinctive feature of type 2 inflammation. Vaccines. 2021;9(3):303. doi: 10.3390/vaccines9030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gwillim E.C., Nattkemper L., Yosipovitch G. Impact of itch on sleep disturbance in patients with prurigo nodularis. Acta Derm Venereol. 2021;101(3) doi: 10.2340/00015555-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janmohamed S.R., Gwillim E.C., Yousaf M., Patel K.R., Silverberg J.I. The impact of prurigo nodularis on quality of life: a systematic review and meta-analysis. Arch Dermatol Res. 2021;313(8):669–677. doi: 10.1007/s00403-020-02148-0. [DOI] [PubMed] [Google Scholar]
- 14.Jørgensen K.M., Egeberg A., Gislason G.H., Skov L., Thyssen J.P. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol. 2017;31(2):e106–e107. doi: 10.1111/jdv.13827. [DOI] [PubMed] [Google Scholar]
- 15.Bagci I.S., Ruzicka T. IL-31: a new key player in dermatology and beyond. J Allergy Clin Immunol. 2018;141(3):858–866. doi: 10.1016/j.jaci.2017.10.045. [DOI] [PubMed] [Google Scholar]
- 16.Richards C.D., Gandhi R., Botelho F., Ho L., Paolini J.F. Oncostatin M induction of monocyte chemoattractant protein 1 is inhibited by anti-oncostatin M receptor beta monoclonal antibody KPL-716. Acta Derm Venereol. 2020;100(14) doi: 10.2340/00015555-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto T., Nattkemper L.A., Kim H.S., et al. Itch intensity in prurigo nodularis is closely related to dermal interleukin-31, oncostatin M, IL-31 receptor alpha and oncostatin M receptor beta. Exp Dermatol. 2021;30(6):804–810. doi: 10.1111/exd.14279. [DOI] [PubMed] [Google Scholar]
- 18.Silverberg J.I., Pinter A., Pulka G., et al. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J Allergy Clin Immunol. 2020;145(1):173–182. doi: 10.1016/j.jaci.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Ständer S., Yosipovitch G., Legat F.J., et al. Trial of nemolizumab in moderate-to-severe prurigo nodularis. N Engl J Med. 2020;382(8):706–716. doi: 10.1056/NEJMoa1908316. [DOI] [PubMed] [Google Scholar]
- 20.Mikhak Z., Ständer S., Metze D., et al. The oncostatin M receptor beta axis identified in prurigo nodularis. J Invest Dermatol. 2019;139(5):S35. [Google Scholar]
- 21.Mikhak Z., Bissonnette R., Siri D., et al. KPL-716, anti-Oncostatin M receptor beta antibody, reduced pruritus in atopic dermatitis. J Invest Dermatol. 2019;139:S96. [Google Scholar]
- 22.Naegeli A.N., Flood E., Tucker J., Devlen J., Edson-Heredia E. The Worst Itch Numeric Rating Scale for patients with moderate to severe plaque psoriasis or psoriatic arthritis. Int J Dermatol. 2015;54(6):715–722. doi: 10.1111/ijd.12645. [DOI] [PubMed] [Google Scholar]
- 23.Elman S., Hynan L.S., Gabriel V., Mayo M.J. The 5-D itch scale: a new measure of pruritus. Br J Dermatol. 2010;162(3):587–593. doi: 10.1111/j.1365-2133.2009.09586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin S., Chandran A., Zografos L., Zlateva G. Evaluation of the impact of fibromyalgia on patients' sleep and the content validity of two sleep scales. Health Qual Life Outcomes. 2009;7:64. doi: 10.1186/1477-7525-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75(4):800–802. [Google Scholar]
- 26.Pereira M.P., Basta S., Moore J., Ständer S. Prurigo nodularis: a physician survey to evaluate current perceptions of its classification, clinical experience and unmet need. J Eur Acad Dermatol Venereol. 2018;32(12):2224–2229. doi: 10.1111/jdv.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polking J., Zeidler C., Schedel F., et al. Prurigo Activity Score (PAS): validity and reliability of a new instrument to monitor chronic prurigo. J Eur Acad Dermatol Venereol. 2018;32:1754–1760. doi: 10.1111/jdv.15040. [DOI] [PubMed] [Google Scholar]
- 28.Reid J., Zamuner S., Edwards K., et al. In vivo affinity and target engagement in skin and blood in a first-time-in-human study of an anti-oncostatin M monoclonal antibody. Br J Clin Pharmacol. 2018;84(10):2280–2291. doi: 10.1111/bcp.13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.