Abstract
Introduction and importance
Gastrointestinal tract (GIT) is a common site for malignant melanoma metastasis, with small bowel being the most common. It is usually difficult to diagnose at an early stage because of the anatomical location of the disease. It is also challenging for pathologists to diagnose due to the small amount of biopsy samples. Survival rates of melanoma patients with distant metastasis are very poor.
Case presentation
This study presents two males, aged 67 and 69 years old, who have metastatic melanoma within the GIT. One was metastasis to the esophagus and another with metastasis to the jejunum presenting as intraluminal masses. Their clinical history and pathologic features of the metastasis are evaluated to give an insight into this disease.
Clinical discussion
Gastrointestinal melanoma is hard to detect due to its anatomical location and limited ability to biopsy. Typically, they present at an advanced stage when diagnosed. Approximately 60 % of patients with cutaneous melanoma will have GIT metastasis at the time of autopsy. The small bowel was found to have an affinity for malignant melanoma due to the expression of the CCR9 ligand, CCL25. BRAF mutations are much less observed in GIT mucosal melanomas as compared to cutaneous melanomas. Furthermore, AKAP13-NTRK3 fusion has been reported specifically in the GIT mucosal melanomas. NTRK fusions in general can be observed in metastatic melanomas and have been reported in GIT metastatic melanomas.
Conclusion
GIT malignant melanomas are difficult to detect due to their anatomical location, with poor prognosis, and have a unique genetic profile.
Keywords: Melanoma, Metastatic malignant melanoma, Gastrointestinal system, Pathology
Highlights
-
•
Gastrointestinal tract is a common site for malignant melanoma metastasis, with small bowel being the most common.
-
•
Esophageal metastasis of malignant melanoma is extremely rare.
-
•
Gastrointestinal melanoma metastasis is hard to detect due to its anatomical location.
-
•
It is challenging for pathologists to diagnose due to the small amount of biopsy samples.
-
•
Survival rates of melanoma patients with distant metastasis are very poor.
1. Introduction
One of the most common and serious forms of skin cancer is melanoma, which is defined as malignant transformation of melanocytes. Malignant melanoma cells can spread from their original tumor site, such as skin or mucosa, and metastasize to the other parts of the body. It is estimated that if caught early on, the five-year relative survival rate of stage 0 melanoma is around 97 %, whereas the prognosis significantly deteriorates to around 5–19 % for those with advanced stage malignant melanoma [1]. Due to its poor prognosis, it is extremely important to detect the disease at its earliest form. Herein we present two interesting cases of metastatic melanoma within the gastrointestinal tract, a common site of malignant melanoma metastasis. This case report was prepared according to the SCARE Criteria [2].
2. Presentation of cases
2.1. Case 1
A 69-year-old male presented with shortness of breath, dizziness, and abnormal hemoglobin levels (6.1 g/dL). He noted having bloody stool a couple of weeks prior, for which he underwent both upper and lower endoscopy with unremarkable results. He had a remote history of shoulder skin melanoma 20 years ago which was surgically treated without subsequent treatment.
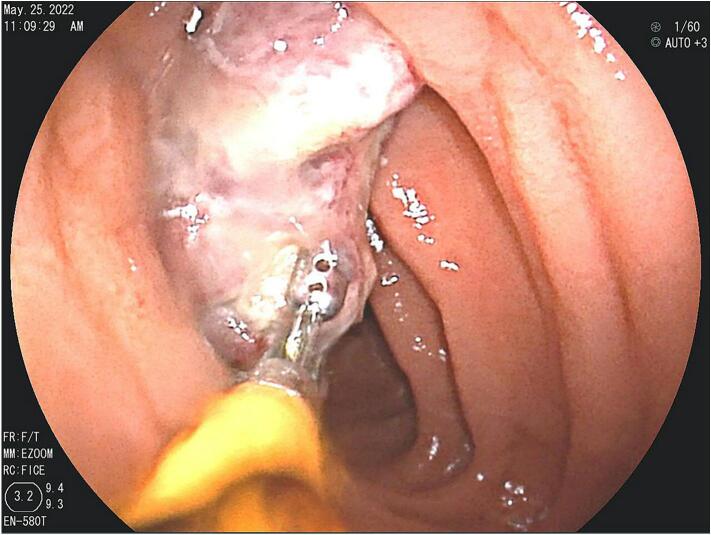
The patient was recommended a capsule endoscopy procedure to help identify the initial source of bleeding which demonstrated two rounded intraluminal masses (15–20 mm & 20 mm) within the jejunum, as well as some patchy erythema of the gastric mucosa. Both masses had surface erosions. One of the masses had an active bleeding site near the lesion which was concerning. The findings were evaluated and an agreement to follow up with double balloon enteroscopy (DBE) was made. Upper DBE showed a 3 × 4 cm polypoid, fungating mass with recent bleeding in the proximal jejunum (20 cm from the ligament of Treitz) and biopsy samples from this mass were taken. The rest of the jejunum, proximal ileum and lower DBE were normal.
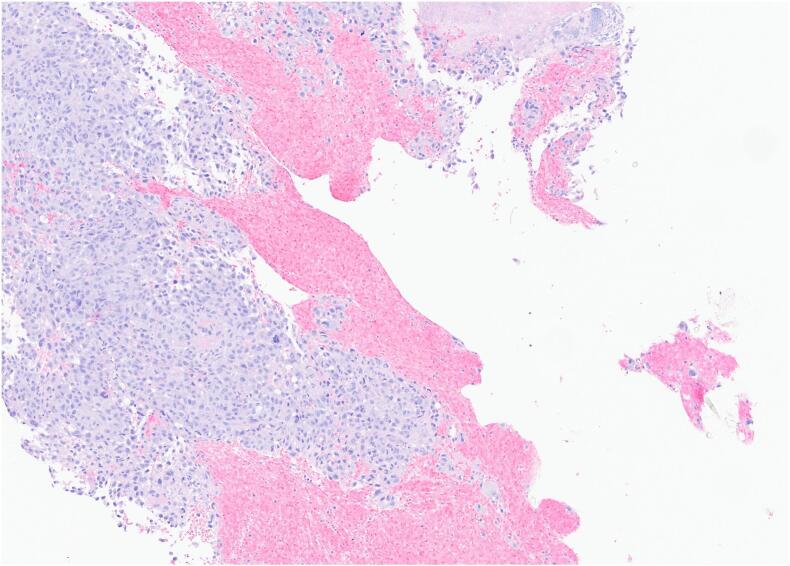
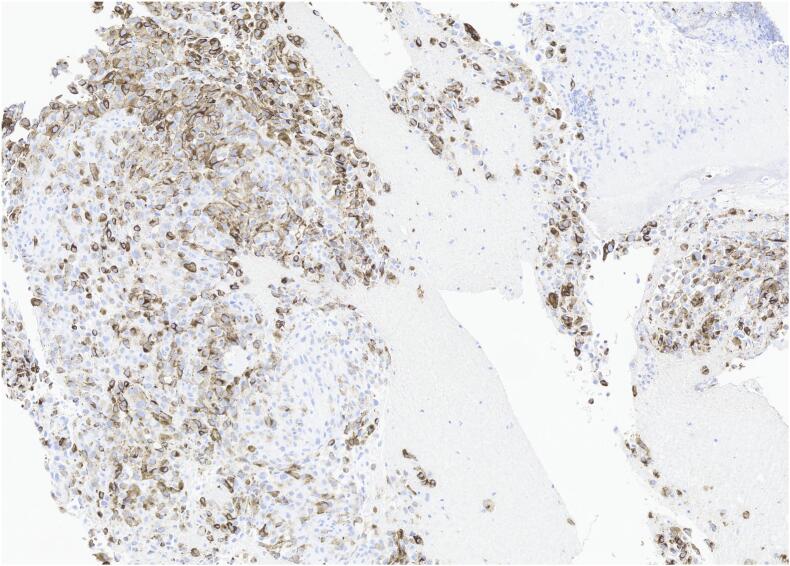
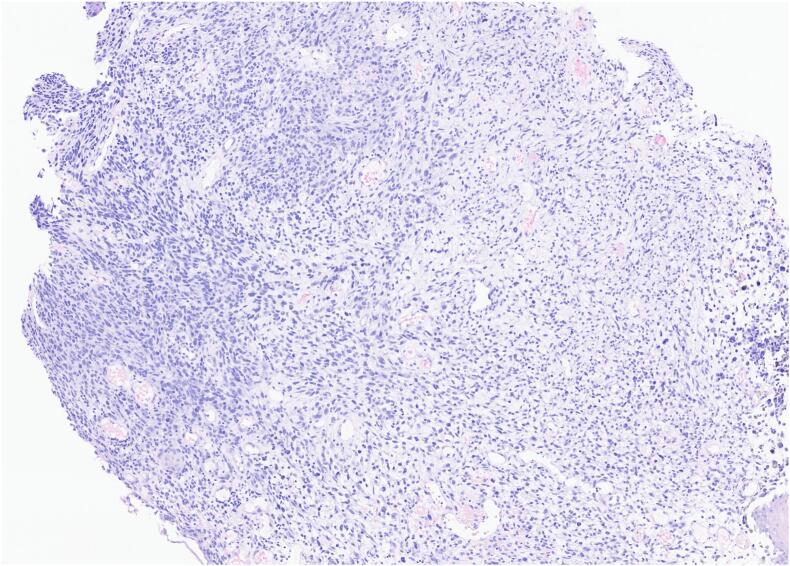
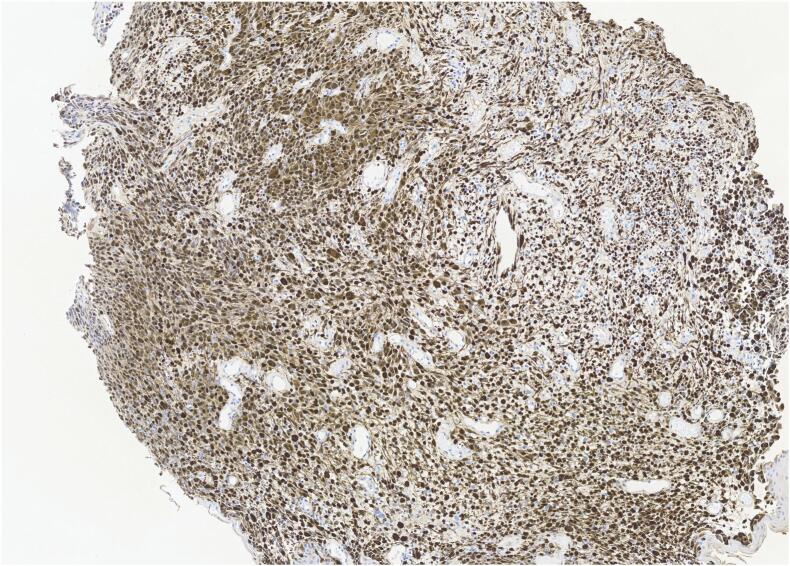
The results of the biopsy demonstrated metastatic malignant melanoma in the jejunum based on endoscopic appearance (Fig. 1), which was perceived as a possible metastasis of the skin melanoma that was excised from the patient's shoulder 20 years ago. No other suspicious findings were noted on the body/skin surveillance. Further workup with brain MRI and PET/CT imaging were scheduled in addition to molecular melanoma panels on the biopsy. The imaging showed two renal cysts (Bosniak 1 and 2) as well as pulmonary nodules in the lungs consistent with possible lung metastasis. Metastatic melanoma was confirmed via the immunohistochemical panels (Fig. 2) of the proximal jejunum biopsy, which revealed neoplastic cells positive for S100 and HMB45 (Fig. 3) and negative for cytokeratin 7, cytokeratin 20, TTF-1, and CDX-2. Genetic testing came back unremarkable for any mutations. Based on these findings, the patient was started on ipilimumab and nivolumab, as well as packed red blood cell transfusions to help treat his worsening anemia. Currently, the patient is continuing this treatment with the assistance of a right internal jugular chest port insertion and has not reported any side effects on his follow-up visits.
Fig. 1.
Endoscopy with fluoroscopy - case 1.
Fig. 2.
H&E.
Fig. 3.
HMB-45–40×.
2.2. Case 2
A 67-year-old male presented with acute encephalopathy after being found unconscious at home. A CT scan of the head was performed revealing multiple hemorrhagic foci within brain parenchyma in multi vascular distribution in the juxtacortical regions, concerning for hemorrhagic metastatic lesions. CT of the chest, abdomen, and pelvis were ordered along with repeat CTs of the head.
Repeated CT scan of the head showed new subarachnoid blood products along the right parietal convexity and multiple hemorrhagic parenchymal foci, otherwise unchanged with associated vasogenic edema. Abdominal and pelvic CT scan demonstrated bilobar hypodense hepatic lesions measuring up to 2.5 cm, a 1.5 cm hypodense lesion in the spleen, some heterogeneous soft tissue along the anterior aspect of proximal stomach, and some enlarged periceliac & peripancreatic lymph nodes. CT scan of the chest showed an isolated right paraoesophageal node/mass with possible metastatic foci in the liver. After consulting gastroenterology, it was decided to proceed with an esophagogastroduodenoscopy (EGD) with endoscopic ultrasound to further elucidate the esophageal mass and gastric lesions. Neurology was also consulted for continued workup of the brain.
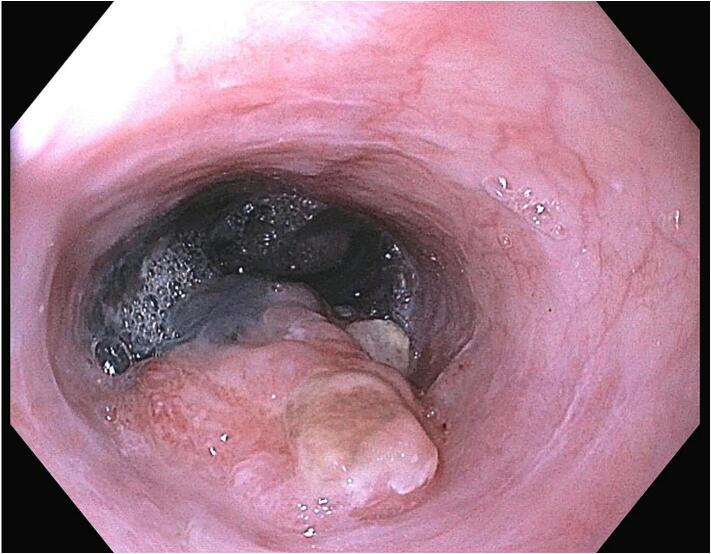
Subsequent EGD showed a 6-cm circumferential mass with necrotic features in the middle-third of the esophagus (Fig. 4), as well as several non-bleeding ulcers and gastropathy. Biopsies were taken and gastrointestinal bleeding was monitored. Neurosurgery recommended avoiding neurosurgical intervention based on the patient's multi-focal hemorrhaging and disseminated systemic metastasis.
Fig. 4.
Upper GI endoscopy - case 2.
Esophageal biopsies demonstrated stage IV metastatic malignant melanoma (Fig. 5) with metastasis to the brain. Immunohistochemical analysis of the biopsy revealed neoplastic cells that are positive for S100 (Fig. 6) and SOX10 and negative for pancytokeratin (AE1/3). Genetic testing revealed a BRAF mutation at c.1781A > G in DNA and p.D594G (Asp594Gly) in amino acid. The patient declined any cancer-directed therapy, and he is currently at a short-term rehabilitation facility.
Fig. 5.
H&E.
Fig. 6.
S100-medium magnification.
3. Discussion
Primary gastrointestinal melanoma is a major type of mucosal melanoma. The prognosis for mucosal melanoma is extremely poor compared to the more common skin or cutaneous melanoma, with the 5-year survival rate for mucosal melanoma being 25 % compared to skin melanoma's 81 % [3]. This poor prognosis can be linked to many factors, one of the most prominent being how difficult gastrointestinal malignant melanoma is to diagnose given its anatomical location within the body and limited ability to biopsy [3]. Gastrointestinal melanoma is typically at an advanced stage when diagnosed with metastasis to other organs is evident and thus harder to treat. Additionally, it is believed that mitotic rate is higher in gastrointestinal melanoma than in cutaneous melanoma inducing a more treacherous disease and poorer long-term prognosis. A study by Simons et al. revealed that up to 60 % of those with cutaneous melanoma will have gastrointestinal tract metastases at the time of autopsy and only 2 % of the gastrointestinal tract melanoma cases are diagnosed before death. [4]. In this article, we present unique presentations of small bowel and esophageal metastatic gastrointestinal melanoma. In both cases, it is believed that the gastrointestinal tract metastases are secondary to a primary cutaneous or other bodily metastasis.
Gastrointestinal tract is the second most common site of melanoma metastasis, after lungs [5]. Approximately 51–71 % of malignant melanomas that spread to the gastrointestinal tract are being found within the small bowel, which indicates a clear affinity for this location. This has been linked to the expression of CCR9 ligand, CCL25, by the small bowel having an affinity for the CCR9 chemokine receptor on the cell surface of melanoma cells [6]. Our first patient with small bowel metastasis had some common symptoms of the disease such as previous hematochezia without remarkable endoscopies. Additional endoscopies were performed, which found fungating masses in the jejunum consistent with prior reports of gastrointestinal melanoma [6]. It was only after we discussed these findings with the patient, that he revealed he had a remote history of cutaneous melanoma localized to his shoulder which had been excised almost two decades ago. As such, we would like to emphasize the importance of utilizing endoscopic procedures to elucidate the diagnoses of these rare pathologies.
Overall, esophageal metastasis is an extremely rare finding in gastrointestinal metastatic melanoma. Multiple studies indicate that primary or secondary metastasis in the esophagus is around 5 % [5], [6]. The prognosis of this type of melanoma is dismal, nonetheless. Jora et al. found the overall 5-year survival rate of primary esophageal metastasis is between 0 and 4 % [7]. The most common symptoms of esophageal metastasis include dysphagia or epigastric pain-like symptoms [7], which we did not observe in our second case. He was unable to describe such symptoms due to his encephalitic encounter, which made it even more challenging to diagnose. Initial imaging of the head returned multiple hemorrhagic foci within brain parenchyma concerning for hemorrhagic metastatic lesions. Further imaging of the entire body revealed gastric lesions, enlarged gastric lymph nodes, and a paraoesophageal mass. Immunohistochemical analysis of the esophageal mass biopsy demonstrated stage IV metastatic melanoma with metastasis to the brain. It is unclear whether the patient had a history of cutaneous melanoma or whether the primary tumor was arising from the gastrointestinal tract with brain metastasis for that matter. What is known is that genetic testing revealed the patient retained a genetic mutation in the BRAFV600E (BRAF) gene, specifically a DNA change of c.1781A > G, which is a remarkable finding as it may help act as a good indicator for melanoma and possible metastasis. Malignant melanoma has a specific mutational profile based on the ultraviolet radiation damage and the most common mutations such as the BRAFV600E can be observed even in the earliest stages of melanoma [8]. Akiyama et al. demonstrated that BRAF mutations are typically expressed at lower levels in gastrointestinal metastasis than skin melanoma [3].
Mucosal melanomas, such as primary gastrointestinal melanomas, genetically differ from skin melanomas. It has been demonstrated that they have distinct genetic alterations. Primary mucosal melanomas often carry mutations and/or increased copy number of KIT [9]. Primary gastrointestinal tract malignant melanomas exhibit mutations involving KIT, NRAS and BRAF, but BRAF mutation is much less observed in these tumors compared to cutaneous melanomas [10]. It has been also reported that NF1, SF3B1, KRAS, KIT, BRCA2, TP53 and CDKN2A are some of the most common mutated genes in primary malignant melanoma of the esophagus [11]. Furthermore, AKAP13-NTRK3 fusion has also been reported in the primary mucosal malignant melanomas, especially in the gastrointestinal tract mucosa [12]. Detection of NTRK fusions may carry a clinical importance due to high response rates to TRK inhibitors in solid tumors carrying NTRK gene fusions. TRIM63- NTRK1, DDR2- NTRK1, GON4L- NTRK1, LMNA-NTRK1, TP53-NTRK1, TRAF2- NTRK2, ETV6-NTRK3, MYO5A-NTRK3 and MYH9-NTRK3 are some of the other NTRK fusions that have been detected in malignant melanoma so far [13], [14]. Metastatic malignant melanomas of the gastrointestinal tract genetically differ from the primary gastrointestinal malignant melanomas. In general, MITF amplification, TERT promoter mutations, and CDKN2A loss are observed more commonly in metastatic melanomas compared to primary melanomas [15]. NTRK fusions can also be observed in metastatic melanoma. A study by Kim et al. [13] demonstrated that 4 patients that had metastatic malignant melanoma (2 of them were in colon and duodenum) was carrying the NTRK gene fusion in their cohort. In addition, CAT-TRIM44, COMMD9-C11ORF91, FRMD4A-APBB1IP, GRID1-HECTD2, HMGXB4-MGMT, INPP5F-GRK5, MAP3K8-LYZL2, OSBP2-MRPL43, PDZD8-ENO4, PLXDC2-THNSL1, PRMT3-MALRD1, SLC25A16-DRGX, SSH2-VSIR, and TAF3-SVIL gene fusions have been reported in metastatic gastrointestinal melanomas [16], [17].
Currently, there are a few treatment options that have been shown to have a positive impact on the symptoms and survival outcomes of gastrointestinal metastasis, one being the surgical resection of metastatic lesion. This has been shown to be a palliative treatment for gastrointestinal metastasis, often reducing pain and the other symptoms and increasing survival rates [18]. Chemotherapy is also another possible treatment route which should be carefully considered with gastrointestinal tract metastasis, as it may have serious complications such as neutropenia and thrombocytopenia [5], [19]. Immunotherapies are also effective for improving patient outcomes, especially with melanomas. Nivolumab, Ipilimumab, and Pembrolizumab have all been shown to be effective against melanomas [20]. They are often preferred because of their non-invasive nature. Our first case is currently receiving ipilimumab and nivolumab which he tolerates well.
4. Conclusions
We describe two unique presentations of metastatic melanoma to gastrointestinal tract to broaden the perception of how this rare disease may present. While we have identified the pathology antemortem through various endoscopic and surgical biopsy procedures, the overall prognosis for this disease is still very poor. As such, further research must be conducted into understanding the mechanisms behind gastrointestinal tract metastasis as well as therapies to improve patient life expectancies beyond a couple of years at best.
Consent
The patients have provided written informed consent to publish the data obtained in the study.
CRediT authorship contribution statement
1- Momin Ahmed - data collection and analysis, writing initial and final draft.
2- Aziza Nassar - study concept and design, data interpretation and analysis, writing daft and final manuscript.
3- Gokce Deniz Ardor - data collection and analysis, writing initial draft and completed final manuscript.
4- Helena Hanna - data review and data analysis, draft revisions and review final manuscript.
5- Ahmed Alhaj - data collection and review, data interpretation and analysis, manuscript review and final draft.
Ethical approval
Ethical approval is exempt at our institution.
Funding
Not applicable.
Guarantor
Aziza Nassar, MD, MPH.
Research registration number
The registration number is 23-001009.
Declaration of competing interest
Not applicable.
Acknowledgements
The authors express our gratitude to all the staff of the pathology and laboratory medicine department of our hospital.
Contributor Information
Momin Ahmed, Email: momin.ahmed@ufl.edu.
Gokce Deniz Ardor, Email: ardor.gokce@mayo.edu.
Helena Hanna, Email: Helena151@usf.edu.
Ahmed M. Alhaj, Email: alhaj.ahmed@mayo.edu.
Aziza Nassar, Email: nassar.aziza@mayo.edu.
References
- 1.Sandru A., Voinea S., Panaitescu E., Blidaru A. Survival rates of patients with metastatic malignant melanoma. J. Med. Life. 2014;7(4):572–576. [PMC free article] [PubMed] [Google Scholar]
- 2.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama M., Matsuda Y., Arai T., Saeki H. Clinicopathological characteristics of malignant melanomas of the skin and gastrointestinal tract. Oncol. Lett. 2018;16(2):2675–2681. doi: 10.3892/ol.2018.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simons M., Ferreira J., Meunier R., Moss S. Primary versus metastatic gastrointestinal melanoma: a rare case and review of current literature. Case Rep. Gastrointest. Med. 2016;2016 doi: 10.1155/2016/2306180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang K.V., Sanderson S.O., Nowakowski G.S., Arora A.S. Metastatic malignant melanoma of the gastrointestinal tract. Mayo Clin. Proc. 2006;81(4):511–516. doi: 10.4065/81.4.511. [DOI] [PubMed] [Google Scholar]
- 6.Kohoutova D., Worku D., Aziz H., Teare J., Weir J., Larkin J. Malignant melanoma of the gastrointestinal tract: symptoms, diagnosis, and current treatment options. Cells. 2021;10(2):327. doi: 10.3390/cells10020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jora C., Pankaj P., Verma R., Jain A., Belho E.S. Primary malignant melanoma of the esophagus. Indian J. Nucl. Med. 2015;30(2):162–164. doi: 10.4103/0972-3919.152983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moran B., Silva R., Perry A.S., Gallagher W.M. Epigenetics of malignant melanoma. Semin. Cancer Biol. 2018;51:80–88. doi: 10.1016/j.semcancer.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Curtin J.A., Busam K., Pinkel D., Bastian B.C. Somatic activation of KIT in distinct subtypes of melanoma. J. Clin. Oncol. 2006;24(26):4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 10.Sivasubramaniam P., Tiegs-Heiden C.A., Sturgis C.D., Hagen C.E., Hartley C.P., Thangaiah J.J. Malignant gastrointestinal neuroectodermal tumor: cytologic, histologic, immunohistochemical, and molecular pitfalls. Ann. Diagn. Pathol. 2021;55 doi: 10.1016/j.anndiagpath.2021.151813. [DOI] [PubMed] [Google Scholar]
- 11.Tsuyama S., Kohsaka S., Hayashi T., et al. Comprehensive clinicopathological and molecular analysis of primary malignant melanoma of the oesophagus. Histopathology. 2021;78(2):240–251. doi: 10.1111/his.14210. [DOI] [PubMed] [Google Scholar]
- 12.Wei D., Qi C., Wu Y., Zhang X., Ren G. AKAP13-NTRK3: a novel NTRK3 oncogenic fusion variant in a patient with melanoma. Oral Oncol. 2020;111 doi: 10.1016/j.oraloncology.2020.104891. [DOI] [PubMed] [Google Scholar]
- 13.Kim J., Lee Y., Cho H.J., Lee Y.E., An J., Cho G.H. NTRK1 fusion in glioblastoma multiforme. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0091940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lezcano C., Shoushtari A.N., Ariyan C., Hollmann T.J., Busam K.J. Primary and metastatic melanoma with NTRK fusions. Am. J. Surg. Pathol. 2018;42(8):1052–1058. doi: 10.1097/PAS.0000000000001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner N., Ware O., Bosenberg M. Genetics of metastasis: melanoma and other cancers. Clin. Exp. Metastasis. 2018;35(5–6):379–391. doi: 10.1007/s10585-018-9893-y. [DOI] [PubMed] [Google Scholar]
- 16.Newman S., Fan L., Pribnow A., et al. Clinical genome sequencing uncovers potentially targetable truncations and fusions of MAP3K8 in spitzoid and other melanomas. Nat. Med. 2019;25(4):597–602. doi: 10.1038/s41591-019-0373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancer Genome Atlas Network Genomic classification of cutaneous melanoma. Cell. 2015;161(7):1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ollila D.W., Essner R., Wanek L.A., Morton D.L. Surgical resection for melanoma metastatic to the gastrointestinal tract. Arch. Surg. 1996;131(9):975–980. doi: 10.1001/archsurg.1996.01430210073013. [DOI] [PubMed] [Google Scholar]
- 19.Schuchter L.M., Green R., Fraker D. Primary and metastatic diseases in malignant melanoma of the gastrointestinal tract. Curr. Opin. Oncol. 2000;12(2):181–185. doi: 10.1097/00001622-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Ralli M., Botticelli A., Visconti I.C., Angeletti D., Fiore M., Marchetti P. Immunotherapy in the treatment of metastatic melanoma: current knowledge and future directions. J. Immunol. Res. 2020;2020 doi: 10.1155/2020/9235638. [DOI] [PMC free article] [PubMed] [Google Scholar]