Abstract
Introduction and importance
Mediastinal paraganglioma is a rare tumor with neuroendocrine activity, highly susceptible to misdiagnosis. Herein, we present a case misdiagnosed as Castleman disease for nearly a decade, significantly enlarged, lost the opportunity of thoracoscopic surgery, and was removed by median thoracotomy.
Case presentation
A 59-year-old woman complained of having a mediastinal mass, was diagnosed with Castleman disease for nearly a decade and was admitted to the hospital due to neck tightness. The tumor size was significantly enlarged. We proceeded to interventional angiography with gelatin sponge angioembolization, and the tumor was resected through a median sternotomy on the second day. The operation was smooth, and the tumor was gray and slightly brittle. Postoperative pathology confirmed paraganglioma; lymph node metastasis was not detected (0/3).
Clinical discussion
Mediastinal paraganglioma is a rare tumor and can be either functional or nonfunctional. It can be differentiated from many diseases. The SSTR-PET-CT labeled with 68Ga-somatostatin analog, plasma metanephrine, and normetanephrine are essential for the diagnosis. Surgical resection is the most effective form of treatment. Pre-operative embolization of the feeding artery is considered to have a low rate of intraoperative bleeding. We recommend making comprehensive preparations to ensure perioperative safety and long-term survival.
Conclusion
When a vascularized mass is discovered in the mediastinum, surgeons should consider the possibility of a paraganglioma. Multidisciplinary consultation should be involved in the formulation of treatment plans. Lifelong surveillance for residual tumor growth and recurrence is required.
Keywords: Mediastinal paraganglioma, Mistaken diagnosis, Case report
Highlights
-
•
Mediastinal paraganglioma is a rare tumor with neuroendocrine activity, and is highly susceptible to misdiagnosis. Surgeons should consider paraganglioma as a differential diagnosis for mediastinal lesions.
-
•
This mediastinal paraganglioma was misdiagnosed as Castleman disease for nearly a decade, resulting in a significantly enlarged tumor removed through the median thoracotomy.
-
•
Multidisciplinary consultation should be involved in the formulation of treatment plans. Lifelong surveillance for residual tumor growth and recurrence is required.
1. Introduction
Paragangliomas are chromaffin tumors arising from parasympathetic or sympathetic ganglia neural crest cells outside the adrenal gland. Mediastinal paraganglioma is a rare tumor with neuroendocrine activity and can be either functional with the ability to synthesize and release catecholamine or nonfunctional without the catecholamine secretion. It can develop in the anterior, middle and posterior mediastinum, originating from the paraaortic and paravertebral sympathetic ganglia. Due to its atypical imaging features, it should be differentiated from thymoma, lymphoma, schwannoma, ectopic thyroid, sarcoidosis, lymph node tuberculosis, and Castleman disease (CD). Clinical manifestations and catecholamine levels should be closely related, as most nonfunctioning tumors present with a slow-growing mass or the mass effect (dyspnea, dysphagia and chest pain). The functioning may be caused by an excess of catecholamine (headache, tachycardia, and sweating). Previous relevant clinical reports were not available. We report a mediastinal paraganglioma that was misdiagnosed as CD for nearly a decade, resulting in significant tumor enlargement, and loss of the opportunity of thoracoscopic surgery, and was removed completely by the median thoracotomy finally. It is anticipated that scholars will gain a deeper understanding of mediastinal paraganglioma. This case report was conducted following SCARE Criteria [1].
2. Presentation of case
A 59-year-old middle-aged woman was admitted to the hospital; she had complaints of mediastinal mass for >9 years and had been feeling neck tightness for the previous six months. The patient underwent a chest computed tomography (CT) scan and was found to have a mass in the anterior mediastinum due to a cough 9 years ago. The mass was located in front of the trachea, with a size of approximately 31 mm and a CT value of about 44.5HU. It is unclear concerning the surrounding vessels, and the adjacent trachea is slightly compressed and shifted to the left. In addition, a contrast-enhanced CT scan showed that the mass was significantly enlarged, and the radiological examination showed the presence of a single enlarged lymph node (lymphoma, Metastasis, or Inflammation). The 18F-FDG positron emission tomography-computed tomography (PET-CT) scan suggests mediastinal lymphadenopathy in region 2R (right upper paratracheal), the largest diameter of the tumor at diagnosis was approximately 50 mm, and SUV max was 6.27. Four hospitals had the same CD diagnosis, but there were varying expert opinions regarding the necessity of a biopsy. There was no treatment then. The patient's CT scans were reviewed annually, and in 2019, the examination revealed enlargement of the mass 2019, but she did not seek treatment due to the slow growth rate.
They gradually emerged from the tightness of her neck and chest and shortness of breath with no obvious inducement, and she did not care in those days. Recent re-examination reveals a single mass measuring approximately 46 × 51 × 72 mm, with uniform density, CT value of about 40HU, a distinct border, and compression of the trachea and blood vessels. She panicked and arrived at our hospital for further treatment after a CT scan revealed that the size of her tumor had grown significantly since the last scan.
In 2013, she underwent breast surgery, and postoperative pathology confirmed fibromatosis. Her family history was irrelevant; she denies consuming alcohol, abusing drugs, or smoking.
On admission, a physical examination revealed that the patient had a body temperature of 36.1 °C, pulse rate of 71 beats/min, respiratory rate of 17 breaths/min, blood pressure of 133/73 mmHg, the height of 162 cm and weight of 63.5 kg. Regional superficial lymph nodes were not palpable, and the rest of the physical examination provided no additional information.
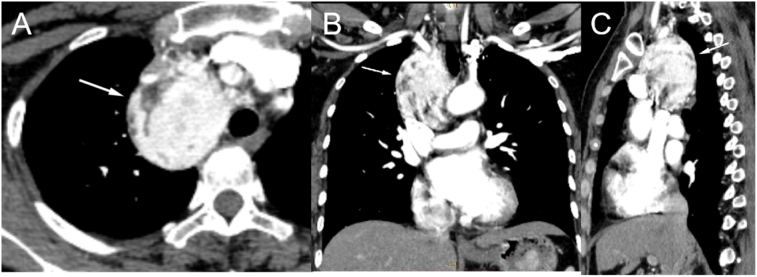
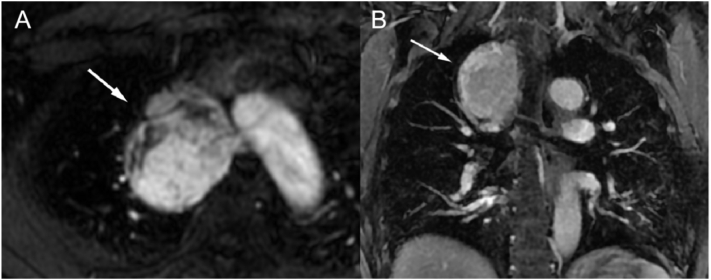
Chest CT plain scan and CT-enhanced + three-dimensional reconstruction were performed; a visible soft tissue mass with uniform density in the superior anterior mediastinum and non-uniform enhancement in enhanced scanning, patchy area of low density was observed within the lesion. The dimension was measured in maximum cross section on the transverse axis for 57 × 51 mm (Fig. 1, Fig. 3). The line of demarcation between the mass and superior vena cava and the left and right brachiocephalic veins was unclear, and blood vessel tortuosity shadows could be seen around the tumor, vascular tumor, CD, and ectopic thyroid disease must be differentiated for diagnosis. Magnetic resonance imaging (MRI) and enhancement scans were also performed, revealing an uneven mixed long T1 and T2 weighted imaging signal shadow, vascular flow void shadow, and dot sheet slightly low signal shadow. The diffusion-weighted imaging shows a high signal, whereas the apparent diffusion coefficient reveals an uneven, slightly low signal, measuring approximately 53.3 × 48 × 64 mm (Fig. 3). The enhancement was uneven, and the degree of enhancement was the same as that of adjacent large vessels, adjacent lung tissues and superior vena cava showed compression changes. We also considered the possibility of CD to be more likely. Laboratory tests (blood count, electrolytes, adrenal and hepatic function) and ultrasound of superficial lymph nodes, cardiac, hepatobiliary, and spleen revealed no additional abnormalities.
Fig. 1.
Three-dimensional reconstruction of enhanced CT scan of the mediastinal paraganglioma (A for axial, B for coronal, and C for sagittal).
Fig. 3.
MRI scan of the mediastinal paraganglioma (A for axial and B for coronal).
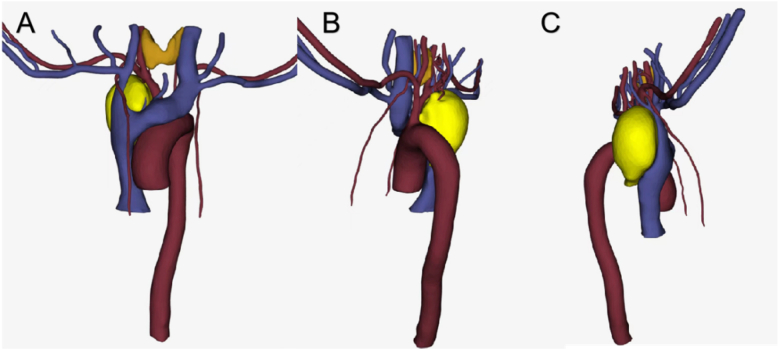
Fig. 2.
Three-dimensional visualization of the lesions and the adjacent veins and arteries.
On July 25, 2022, we performed interventional angiography with gelatin sponge angioembolization and found that the primary blood supply vessel was a branch of the right subclavian artery. Patients with pre-operative embolization of blood supply vessels are considered to have a low incidence of intraoperative bleeding. On the second day, the tumor was resected through a median sternotomy. The operation was conducted without massive hemorrhage or hypertension crisis, and the tumor was found to be gray and slightly tough. Red blood cells (RBCs) (4 U) and fresh frozen plasma (300 ml) were transfused intraoperatively, followed by the transfusion of 2 U RBCs postoperatively. The patient's postoperative recovery was uneventful, and the hospital stay was eight days. Postoperative pathology confirmed paraganglioma, and pathological examination revealed no metastasis in the mediastinal lymph node (0/3). Immunohistochemistry results were: AE1/AE3(−), CD31(vessels +), CgA(+), EMA(−), Ki-67(index 3 %), Syn(+) TdT(−), S-100(+), SMA(−).
In the one-month postoperative follow-up, she continued to cough occasionally. Two months after the operation, the metanephrines (MNs) in the blood were normal.
3. Discussion
3.1. Origin and clinical manifestations of mediastinal paraganglioma
Paraganglioma is a rare neuroendocrine tumor derived from neural crest cells. World Health Organization (WHO) defines it as a tumor that synthesizes, store and secrete catecholamine, originating outside the adrenal glands and can arise anywhere in the body. Paragangliomas in the mediastinum are rare, accounting for only 1 %–2 % of all paragangliomas and <0.3 % of all mediastinal tumors [2], [3] associated with the sympathetic ganglia. Most mediastinal paragangliomas originate from the posterior region's paraaortic or paravertebral sympathetic ganglia. In the present case, the mass was thought to originate from the aortic sinus sympathetic ganglion, which was located in the superior anterior mediastinum and was. Its etiology remains unclear but is likely associated with the chemoreceptors' origin, function and the occurrence of genetic clustering [4]. Most paragangliomas manifest between 30 and 60 years of age, are sporadic, and are more prevalent in females than males. In addition, paraganglioma's benign and malignant classification was based on clinical distant metastasis judgment, not pathology.
The patients were classified as functioning or nonfunctioning based on the endocrine function of the paraganglioma. In the present case, the mass was considered nonfunctional. Clinical presentation is related to tumor location and levels of catecholamines. Most mediastinal paragangliomas are nonfunctional, asymptomatic, and typically discovered incidentally; however, some patients may develop Hornor symptoms, dyspnea, dysphagia, and chest pain due to the compression of the mass. In this case, the patient's neck stiffness may be relevant. Functional tumors are often associated with the symptoms related to catecholamine hypersecretion (hypertension, tachycardia, headache, and diaphoresis). The metastases of mA mass effect and symptoms, including pathological fracture, accompanied the metastases of malignant paraganglioma and differential diagnosis of mediastinal paraganglioma.
On imaging, the shape was tentatively classified as round, oval, fusiform, or irregular, and the boundary always appeared clear. When the tumor is small, its density may appear uniform, whereas as it grows, it needs to become heterogeneous with areas of cystic change, hemorrhage, and necrosis. The tumor is growing as an isolated nodule pressing the adjacent tissues. MRI can detect the empty flow sign, the thickened blood supply artery sign, the pepper salt sign, and the bulb sign. In dynamic enhancement examination, T2 weighted imaging showed uniform or non-uniform high signal intensity, specific obvious early enhancement, a distinct delay period, and few progressive enhancements. When differential diagnoses are difficult to exclude, the labeling of 68Ga-DOTA-SSA, 18F-FDOPA, 18F-FDG, 131I/123I-MIGB is frequently used for imaging and diagnosis. The somatostatin receptor (SSTR) is overexpressed in many neuroendocrine tumors (NETs), including paraganglioma; the SSTR-PET-CT is currently the preferred method for precision diagnostics by using 68Ga-somatostatin analog labeling.
In addition, blood chemistry and urine analysis were used to exclude the differential diagnosis. The catecholamine level and its metabolites in blood and urine are essential diagnostic indicators due to its paroxysmal or continuous catecholamine secretion. Plasma metanephrine (MN) and normetanephrine (NMN) are the preferred diagnostic tests for paraganglioma (Clinical Practice Guidelines NCCN Guideline in 2021 [5]).
CD is a chronic lymphoproliferative disorder characterized by unexplained lymph node enlargement, frequently occurs in hyaline-vascular type and may also arise from the mediastinum, constituting one of the major differential diagnoses for mediastinal paraganglioma. The patient was initially misdiagnosed with CD. However, the CD cannot cause hypertension; necrosis and cystic degeneration are rare, and the degree of enhancement is marginally less than that of paraganglioma. Due to the ribbon-like fiber scar, the tumor's center can develop a low density when it is sufficiently large. After enhancement, it is evident that there is no enhancement focus in the fissure shape, despite the obvious enhancement of the mass. Some individuals can observe branching and spotty or annular calcification. The enhancement mode is transparent and continuous. The patient was diagnosed with paraganglioma rather than CD before postoperative pathology.
In this case, the tumor's location must also be distinguished from the ectopic thyroid. Intrathoracic ectopic thyroid is a rare location of ectopic thyroid, accounting for approximately 1 % of all mediastinal tumors [6]. It typically exhibits a smoothly marginated, well-circumscribed, lobulated architecture. A classic finding on a CT scan is the continuity of the cervical and mediastinal components of the thyroid. In this case, the lump grows from the mediastinum into the neck, and the patient experiences neck tightness. During CT plain scan, the density of iodine in the ectopic thyroid was higher than that of adjacent soft tissues. The enhancement scan showed obvious enhancement, and the enhancement time was longer.
The final diagnosis of paraganglioma was based on pathology results. Still, the majority are regarded as benign tumors based on imaging characteristics, and almost all patients underwent immediate surgery, as preoperative biopsy is infrequently used for diagnosis in general. In addition, the need for benign or malignant treatment depends on metastasis (lymph node or distant metastasis) and postoperative recurrence.
In conclusion, if mediastinal paraganglioma is difficult to differentiate clinically, it can be further distinguished by its image characteristics, functional imaging, and body fluid testing. We should evaluate its benign and malignant functions over time.
3.2. Treatment of mediastinal paraganglioma
The only treatment option is surgical resection, as chemotherapy and radiotherapy are ineffective [2], [7]. Complete resection is the preferred treatment for early-stage mediastinal paraganglioma, where the tumor is larger and surgical risks during resection are high, as well as when it comes to local recurrence following surgery.
Although mediastinal paragangliomas are typically well-differentiated, slow-growing, benign tumors, the patient, in this case, was followed up for nearly a decade before exhibiting clinical symptoms. However, the authors suggest early surgical resection should be the first option for diagnosing mediastinal paraganglioma. In addition, with the advancement of thoracoscopic surgery, thoracoscopic surgery is performed at an earlier stage, resulting in less trauma and faster recovery. In this case, the tumor was significantly enlarged after long-term follow-up and was finally completely removed by the median thoracotomy. Regarding postoperative recurrence, familial paraganglioma has a higher recurrence than sporadic paraganglioma. Larger tumor size (≥5 cm) and younger age at primary tumor diagnosis are independent risk factors for recurrence; therefore, lifelong follow-up is necessary to determine recurrence [8].
When the tumor is larger, and the surgical risks during resection are high, pre-operative embolization of the feeding artery is advised [9] as in this case. It is a prerequisite that the patient's physical condition be suitable for surgery. When the patient's clinical course and physical condition are intolerable, radionuclide therapy alone, a combination approach with pre-operative embolization or stereotactic body radiotherapy may be used [10], [11], [12]. Some novel molecular-targeted drugs include cabozantinib, selpercatinib, everolimus, sunitinib, alpelisib, trametinib, niraparib, and entinostat could be effective treatment options [13], [14].
It should be noted that the catecholamine levels in patients with functional paraganglioma are elevated for an extended period, the peripheral blood vessels of the whole body are highly contracted, and the circulating blood volume is reduced by 20–50 % compared to normal individuals. During the operation, it is essential to minimize the extrusion of the tumor body and prevent a large amount of catecholamine from entering the blood to cause a hypertension crisis. After tumor resection, catecholamine in the blood drops precipitously, resulting in the expansion of blood vessels, peripheral resistance reduction, and a steep drop in blood pressure. Anesthesiologists must pay close attention to the administration of vasopressors and fluid infusion rate. Therefore, when preoperative misdiagnosis or its function is unknown, anesthesiologists and thoracic surgeons face a major challenge.
We recommend the preparations to ensure perioperative safety and long-term survival as follows [15], [16], [17]. Physicians in the endocrinology department assess their functional status through consultation. When necessary, physicians in the interventional department consult to evaluate the blood supply and embolize the blood vessels before surgery to prevent intraoperative bleeding and large release of catecholamine during surgery. Cardiovascular surgeons can confer to determine whether to replace blood vessels and cardiopulmonary bypass. The thoracic surgeon must administer a continuous high-volume fluid infusion to supplement blood volume before surgery. The anesthesiologist should prepare sufficient drugs for use and monitor them continuously throughout the operation. Thoracic surgeons should avoid squeezing tumors during surgery and clamp the blood supply artery as soon as possible. When necessary, oncologists and radiologists should consult with each other to provide comprehensive postoperative care. Generally, relevant symptoms can be relieved after surgery [18], [19], [20].
4. Conclusions
In conclusion, mediastinal paragangliomas are rare tumors and, therefore, not typically included in the differential diagnosis of mediastinal tumors. Surgeons should consider paraganglioma as a differential diagnosis for mediastinal lesions when they discover a mass with a rich blood supply in the mediastinum, especially when the mediastinal tumor is accompanied by hypertension symptoms or young patients with unexplained hypertension. Further examination combined with MNs in blood and urine and 68Ga-DOTA-SSA PET-CT scan imaging for deeper differentiation when necessary.
When clinically considering a diagnosis of mediastinal paraganglioma, it is necessary to avoid a hypertensive crisis caused by pathological biopsy. The author suggests multidisciplinary consultation for the formulation of treatment plans. Long-term, life-long monitoring for residual tumor growth and recurrence is necessary.
Abbreviations
Patient consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Sources of funding
This study was supported by grants from the National Natural Science Foundation of China (32070623) and Science and Technology Project of Zhengzhou University (XKZDQY202006).
Ethical approval
Ethical Approval was provided by the authors' institution.
Author contribution
Wensong Shi, Yuzhui Hu, Guotao Chang, Huiyu Zheng performed experiments and generated the data. Wensong Shi and Yuzhui Hu wrote the manuscript under the guidance of Yulun Yang, Xiangnan Li. All authors reviewed the final version of the manuscript.
Guarantor
Wensong Shi.
Research registration
N/A.
Declaration of competing interest
N/A.
Contributor Information
Yulun Yang, Email: hhyyl@126.com.
Xiangnan Li, Email: lxn-2000@163.com.
References
- 1.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan S.N., Radecki K.M., Chambers L.W. Mediastinal paraganglioma. Ann. Thorac. Surg. 2017;103:e413–e414. doi: 10.1016/j.athoracsur.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 3.Yin Y.Y., Yang B., Ahmed Y.A., Xin H. Thoracotomy of an asymptomatic, functional, posterior mediastinal paraganglioma: a case report. World J. Clin. Cases. 2019;7:1529–1534. doi: 10.12998/wjcc.v7.i12.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samuel N., Ejaz R., Silver J., Ezzat S., Cusimano R.J., Kim R.H. Primary mediastinal paraganglioma is associated with a familial variant in the succinate dehydrogenase B subunit gene. J. Surg. Oncol. 2018;117:160–162. doi: 10.1002/jso.24818. [DOI] [PubMed] [Google Scholar]
- 5.Shah M.H., Goldner W.S., Benson A.B., et al. Neuroendocrine and adrenal tumors, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021;19:839–868. doi: 10.6004/jnccn.2021.0032. [DOI] [PubMed] [Google Scholar]
- 6.Noussios G., Anagnostis P., Goulis D.G., Lappas D., Natsis K. Ectopic thyroid tissue: anatomical, clinical, and surgical implications of a rare entity. Eur. J. Endocrinol. 2011;165:375–382. doi: 10.1530/EJE-11-0461. [DOI] [PubMed] [Google Scholar]
- 7.Lin F., Liu C., Ma L., et al. Unusual cause of massive hemothorax: spontaneous rupture of nonfunctioning mediastinal paraganglioma. J. Thorac. Dis. 2016;8:E1572–e1575. doi: 10.21037/jtd.2016.12.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M., Prodanov T., Meuter L., et al. Recurrent disease in patients with sporadic pheochromocytoma and paraganglioma. J. Clin. Endocrinol. Metab. 2023;108(2):397–404. doi: 10.1210/clinem/dgac563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rakovich G., Ferraro P., Therasse E., Duranceau A. Preoperative embolization in the management of a mediastinal paraganglioma. Ann. Thorac. Surg. 2001;72:601–603. doi: 10.1016/s0003-4975(00)02293-1. [DOI] [PubMed] [Google Scholar]
- 10.Trêpa M., Silveira I., Amaral C., Luz A. Innovative approach to a functional mediastinal paraganglioma with anomalous coronary supply: a case report. Eur. Heart J. Case Rep. 2020;4:1–6. doi: 10.1093/ehjcr/ytaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashwathanarayana A.G., Biswal C.K., Sood A., Parihar A.S., Kapoor R., Mittal B.R. Imaging-guided use of combined (177)Lu-DOTATATE and capecitabine therapy in metastatic mediastinal paraganglioma. J. Nucl. Med. Technol. 2017;45:314–316. doi: 10.2967/jnmt.117.197400. [DOI] [PubMed] [Google Scholar]
- 12.Gigliotti M.J., Hasan S., Liang Y., Chen D., Fuhrer R., Wegner R.E. A 10-year experience of linear accelerator-based stereotactic radiosurgery/radiotherapy (SRS/SRT) for paraganglioma: a single institution experience and review of the literature. J. Radiosurg. SBRT. 2018;5:183–190. [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Y., Li Q., Huang W., et al. Successful treatment of paraganglioma with sorafenib: a case report and brief review of the literature. Onco Targets Ther. 2013;6:1559–1562. doi: 10.2147/OTT.S53813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang K., Schütze I., Gulde S., et al. Personalized drug testing in human pheochromocytoma/paraganglioma primary cultures. Endocr. Relat. Cancer. 2022;29:285–306. doi: 10.1530/ERC-21-0355. [DOI] [PubMed] [Google Scholar]
- 15.Yang Z., Shi Q., Bao F. A case of an unexpected posterior mediastinal functional paraganglioma: case report and literature review. BMC Anesthesiol. 2020;20:109. doi: 10.1186/s12871-020-01026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumura Y., Fukuhara M., Tanabe H., et al. Thoracoscopic resection of posterior mediastinal paraganglioma: perioperative management and surgical tips. J. Cardiothorac. Surg. 2022;17:143. doi: 10.1186/s13019-022-01892-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muñoz-Largacha J.A., Glocker R.J., Moalem J., Singh M.J., Litle V.R. Incidental posterior mediastinal paraganglioma: the safe approach to management, case report. Int. J. Surg. Case Rep. 2017;35:25–28. doi: 10.1016/j.ijscr.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nam J.H., Park J.S., Choi J.H. Paraganglioma in the posterior mediastinum: a case report. BMC Cardiovasc. Disord. 2020;20:492. doi: 10.1186/s12872-020-01752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.SenthilKumar G., Rokkas C.K., Sheinin Y.M., Linsky P.L. Rare and complicated functional posterior mediastinal paraganglioma. BMJ Case Rep. 2022:15. doi: 10.1136/bcr-2022-250500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li B., Yan Z., Huang H. Case report: an unusual case of ectopic ACTH syndrome caused by mediastinal paraganglioma. Front. Endocrinol. (Lausanne) 2021;12 doi: 10.3389/fendo.2021.790975. [DOI] [PMC free article] [PubMed] [Google Scholar]