Abstract
Xanthine oxidase (XO), a form of xanthine oxidoreductase, is widely distributed in various human tissues. As a major source for the generation of superoxide radicals, XO is involved in the induction of oxidative stress and inflammation during ischemic and hypoxic tissue injury. Therefore, we designed this study to identify the role of serum XO levels in acute ischemic stroke (AIS) pathogenesis. In this single-center prospective study, 328 consecutive patients with AIS for the first time were included, and 107 age- and sex-matched healthy controls from a community-based stroke screening population were also included. The serum levels of XO and several conventional stroke risk factors were assessed. Multivariate analysis was applied to evaluate the relationship between serum levels of XO and clinical outcomes, and nomogram models were developed to predict the onset, progression and prognosis of AIS. Compared with the healthy control group, the serum level of XO was significantly higher in the AIS group (P < 0.05) and was an independent risk factor for AIS (OR 8.68, 95% CI 4.62–14.33, P < 0.05). Patients with progressive stroke or a poor prognosis had a much higher serum level of XO than patients with stable stroke or a good prognosis (all P < 0.05). In addition, the serum level of XO was an independent risk factor for stroke progression (OR 1.98, 95% CI 1.12–3.50, P = 0.018) and a poor prognosis (OR 2.51, 95% CI 1.47–3.31, P = 0.001). The nomogram models including XO to predict the onset, progression and prognosis of AIS had good prediction and differentiation abilities. The findings of this study show that the serum level of XO on admission was an independent risk factor for AIS and had certain clinical predictive value for stroke progression and prognosis in patients with AIS.
Keywords: Acute ischemic stroke, Xanthine oxidase, Biomarker, Stroke progression, Stroke prognosis, Nomogram models
Highlights
-
•
The serum XO level on admission is an independent risk factor for stroke onset, progression and prognosis.
-
•
The serum XO level on admission could predict stroke progression and prognosis in patients with AIS.
-
•
Our nomogram models have a good ability to predict the onset, progression and prognosis of AIS.
1. Introduction
Acute ischemic stroke (AIS) is a devastating central nervous system (CNS) disease with limited functional recovery, high mortality and morbidity, and a poor quality of life [1]. The interruption of blood flow in AIS is usually caused by a thrombus (in situ occlusion of an artery), an embolism (thrombus originating in another location), hypoperfusion (systemic or local low blood flow), or a combination of these [2]. Therefore, in the acute phase of AIS, the most effective treatment is recanalization. Although limited by treatment time window and technical requirements, intravenous tissue-type plasminogen activator treatment (alteplase, etc.) is a proven effective drug therapy for AIS treatment, and arterial thrombectomy can recanalize the occluded large vessels in a timely manner [3]. Even if blood flow is recanalized, strong pathophysiological responses, including excitotoxicity, oxidative stress, nitrosative stress and inflammation, occur in the core area of cerebral infarction, resulting in irreversible neuronal death [4,5]. Therefore, it is particularly important to find appropriate biomarkers in the acute phase of AIS to predict the prognosis of stroke and make decisions for early intervention. In addition, these biomarkers could serve as potential therapeutic targets.
As important biomarkers, serological indicators are considered important indicators to evaluate the progression and prognosis of various diseases due to their low invasiveness and accurate results [6]. For AIS biomarkers, previous studies indicated that several indicators could predict prognosis, such as serum S100 calcium-binding protein B (S100B) [7], cellular fibronectin (c-Fn) [8], and HFABP (heart-type fatty acid binding protein) [9]. However, few of these biomarkers are largely used in routine clinical practice. Therefore, in this study, we aimed to investigate promising serological biomarkers from a real-world setting that could accurately predict the progression and prognosis of AIS.
Xanthine oxidase (XO) is one form of xanthine oxidoreductase (XOR), the forms of which include xanthine dehydrogenase (XDH) and XO. They can be reversibly converted to each other by sulfhydryl oxidation [10]. The XO system is widely present in various human tissues, especially in the liver, intestines, kidneys, lactating mammary gland and vascular endothelial cells [11]. XO is a major source for the generation of superoxide radicals [12,13]. Several studies have shown that XO plays an important role in the oxidative stress injury induced by ischemia‒reperfusion in the heart and kidneys [[14], [15], [16], [17]]. A basic study reported that in a rat model of AIS, XO expression on the infarcted side was significantly higher than that on the noninfarcted side and continued to increase over time [18]. A recent study conducted by Mateusz M et al. indicated that salivary XO may be a potential biomarker for the diagnosis of stroke, which provides a new simple strategy for early prediction in patients with AIS [19]. However, few studies have confirmed the clinical value of serum XO levels in the pathogenesis of AIS [20]. However, there are few relevant clinical studies on the role of XO in patients with AIS. Therefore, we performed this study to evaluate the prognostic significance of the serum level of XO in patients with AIS.
2. Materials and methods
2.1. Participants
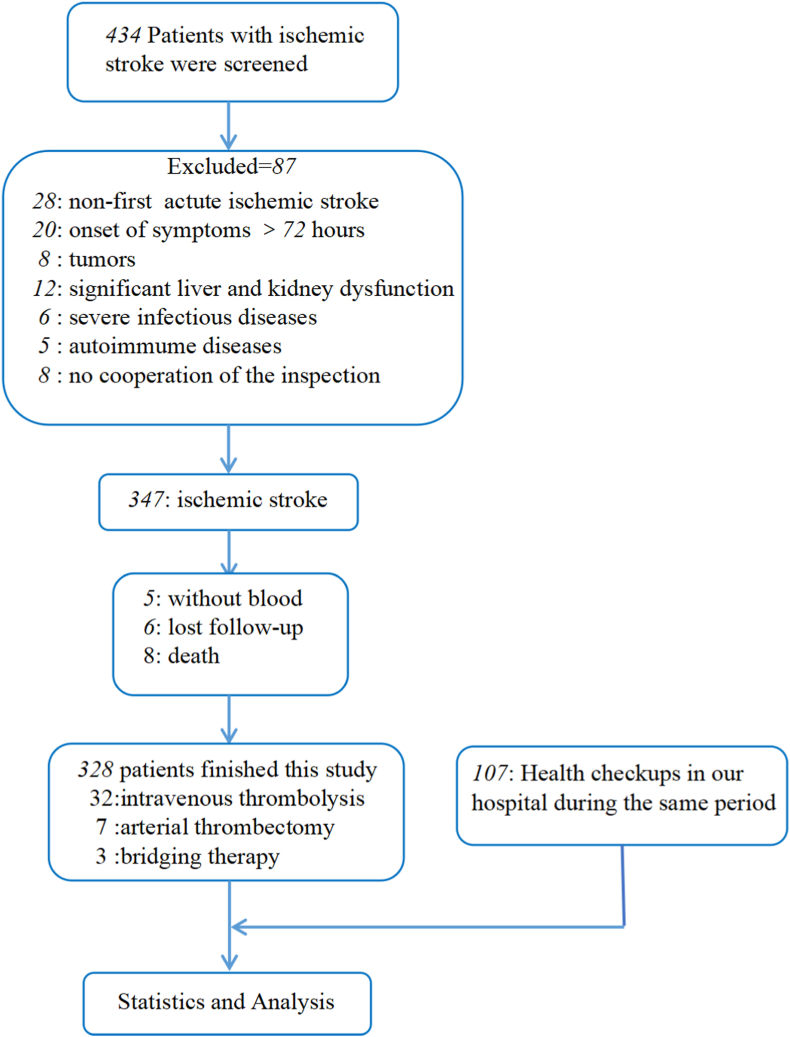
From October 2020 to September 2021, patients with AIS for the first time who were hospitalized within 3 days of onset at the Department of Neurology, North Jiangsu People's Hospital were consecutively enrolled. Patients were diagnosed by head CT or MRI within 5 days of onset. The inclusion criteria of the research participants were as follows: 1) over 18 years of age and 2) the first occurrence of AIS. The exclusion criteria were as follows: 1) A previous medical history of myocardial infarction and/or stroke. 2) A serious infection within the past six months. 3) A diagnosis of an autoimmune disease or blood disease. 4) A severe language function or hearing impairment. 5) A history of a traumatic brain injury, an intracranial mass, or brain surgery. 6) Current hemangioma, vascular malformation, or vasculitis. 7) Severe heart, lung, liver, or kidney failure, where severe heart failure was defined as a left ventricular ejection fraction (LVEF) ≤40% or Pro-BNP >1800 pg/ml; severe pulmonary failure was defined as PO2 <60 mmHg; severe liver failure was defined as ALT or AST >200 U/L; and severe renal failure was defined as urea >8 mmol/L and creatinine >106 ml/min 8) A known malignant tumor. 9) A mental or serious psychological abnormality. 10) An inability to cooperate in completing various examinations. 11) Pregnancy. 12) The use of oral XO inhibitors and dietary supplements for the past three months. 13) Malnutrition. We included age- and sex-matched participants without cerebrovascular diseases evaluated in the health examination center of our hospital during the same period as the control group. Healthy controls and patients with AIS were recruited in a 1:3 matching ratio (Fig. 1).
Fig. 1.
A study flow diagram.
Ultimately, 328 patients with AIS were enrolled in this study, including 32 patients undergoing intravenous thrombolysis, 7 patients undergoing arterial thrombectomy, and 3 patients undergoing bridging therapy. A total of 107 healthy people were included in the control group. This study was approved by the Ethics Committee of Northern Jiangsu People's Hospital (2020ky-087-1), and informed consent was obtained from all patients included in this study.
2.2. Data collection and outcome measures
Clinical data were collected in this study, including 1) baseline information: age, sex, body mass index (BMI), history of hypertension, history of coronary heart disease (CHD), history of atrial fibrillation (AF), history of hyperlipidemia, history of diabetes, family history of cardiovascular and cerebrovascular events, history of alcoholism, history of smoking, time from symptom onset to hospitalization (≤24 h, 24 h–48 h, 48 h–72 h), TOAST (trial of ORG 10172 in acute stroke treatment) classification, and neurological deficit score on admission (National Institutes of Health Stroke Scale, NIHSS); 2) laboratory indicators: low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol (TC), triglyceride (TG), glycosylated hemoglobin (HbA1c), uric acid (UA), fasting blood glucose (FSG), neutrophil/lymphocyte ratio (NLR), etc.; and 3) imaging findings: all patients underwent head CT and MRI examination, and the cerebral infarction size was calculated according to the Pullicino formula.
During hospitalization, the NIHSS score was assessed once on admission, every 6 h within 72 h after admission, and daily after 72 h. When AIS symptoms worsened, the NIHSS score was promptly reassessed. Stroke progression is defined as an increase in the NIHSS score by more than 2 points within 7 days of stroke onset or new neurological defects [21]. All patients were followed up for 90 days by outpatient visits or telephone, and the 90-day functional outcome was assessed by using the modified Rankin Scale (mRS). An mRS score of 0–2 was defined as a good prognosis, and an mRS score of 3–6 was defined as a poor prognosis. For healthy controls, demographic information, epidemiological risk factors associated with vascular disease, and blood samples were collected.
2.3. The measurement of serum XO
Blood samples were collected from enrolled participants by trained personnel using disposable needles and syringes the morning after admission. Blood samples were collected after therapy (thrombolysis, arterial thrombectomy, or bridging therapy) for all patients. After overnight fasting (10–12 h), 5 mL of fasting blood was extracted from the preflex vein by venipuncture and stored in a serum collection tube. The blood samples were sent to the laboratory and immediately placed in a centrifuge (time: 15 min, speed: 3000 r/min), and serum samples were subsequently extracted and stored at −80 °C until assayed. The serum samples of the healthy control group were similarly collected in the morning while participants were in a fasted state. Serum XO was measured by an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions. The kit was purchased from CUSABIO (Wuhan, China).
2.4. Statistical analysis
Categorical variables are expressed as counts and percentages (%), while continuous variables are expressed as the mean ± standard deviation or median and quartile ranges. The Kolmogorov‒Smirnov test was performed to assess the distribution equality of the continuous parameters. The homogeneity of variance was tested by Levene's test. The independent sample t-test was used to compare the parameter values of the two groups, and the Mann‒Whitney U test was used to compare the nonparameter values of the two groups. Univariate logistic regression analysis was used to compare the differences between groups. The results are shown as adjusted odds ratios (ORs) and 95% confidence intervals (CIs). Multivariate logistic regression analysis was performed based on the results of univariate analysis to calculate the ORs and 95% CIs of the independent variables. Variables included in the multivariate models were selected according to their physiological correlation and statistical significance in univariate analysis, and the P value threshold was 0.25. All statistical analyses were performed using SPSS software (Version 25, IBM Corp, Armonk, NY, USA). Statistically significant differences were defined as P < 0.05.
Nomogram models were constructed by using the “rms” and “regplot” packages of R software (version 4.2.0, https://www.R-project.org). Based on the results of the multivariate analysis, nomogram models were established to better predict the onset, progression and prognosis of AIS. The research participants were randomly divided into a training set and an internal validation set at a ratio of 7:3. A receiver operating characteristic (ROC) curve was drawn, and the area under the curve (AUC) was calculated to evaluate the predictive efficacy of the nomogram model. An AUC >0.80 indicated high efficacy, an AUC >0.5–0.8 indicated moderate efficacy, and an AUC ≤0.5 was meaningless. The Youden index (the cut-off value) was calculated. The bootstrap self-sampling method was used to verify the model internally. The closer the calibration curve of the model was to the ideal curve, the higher the calibration degree of the model was.
3. Results
3.1. Comparison of clinical data between the AIS group and the healthy control group
A total of 328 patients with AIS were enrolled in our study, with an average age of 68.48 years (69.35% were male), as shown in Fig. 1. A total of 107 healthy participants were included in the control group. The serum XO level was 0.78 ± 0.51 ng/ml in the healthy control group and 1.76 ± 0.87 ng/ml in the AIS group (Table 1). When comparing demographic and clinical data between the AIS and control groups, we found that there were significant differences in the history of hypertension, history of AF, TC, HDL, LDL, FSG, HbA1c and XO level between the two groups (P < 0.05). In addition, the serum XO level on admission in the AIS group was significantly higher than that in the healthy control group (P < 0.05), as shown in Table 1. Multivariate analysis demonstrated that serum XO level (OR 8.68, 95% CI 4.62–14.33, P < 0.05), history of hypertension (OR 4.14, 95% CI 1.84–9.33, P = 0.001), history of AF (OR 5.77, 95% CI 1.28–26.11, P = 0.023), HbA1c (OR 2.28, 95% CI 1.40–3.71, P = 0.001), TC (OR 0.04, 95% CI 0.01–0.16, P < 0.05) and LDL (OR 6.55, 95% CI 1.09–39.58, P = 0.04) were independent risk factors for AIS (Table 1).
Table 1.
Demographic and clinical characteristics, Univariate and Multivariate analysis of AIS group and healthy control group.
| Characteristics | Healthy control group, n = 107 | AIS group, n = 328 | Univariate and Multivariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | SE | P value | OR (95%CI) | SE | P value | |||
| Age, y | 66.81 ± 10.39 | 68.48 ± 11.14 | 1.01 (1.00–1.03) | 0.009 | 0.173 | / | / | / |
| Men, n(%) | 64(59.81) | 227(69.21) | 1.01(0.93–1.11) | 0.05 | 0.756 | / | / | / |
| BMI(kg/m2) | 24.65 ± 2.97 | 24.18 ± 3.07 | 0.95 (0.88–1.02) | 0.033 | 0.167 | / | / | / |
| Hypertension(%) | 58(54.2) | 231(70.43) | 2.66 (1.69–4.20) | 0.62 | <0.05 | 4.14 (1.84–9.33) | 1.72 | 0.001 |
| CHD(%) | 8(7.48) | 43(13.10) | 1.86 (0.85–4.11) | 0.751 | 0.121 | / | / | / |
| AF(%) | 4(3.74) | 51(15.55) | 4.74 (1.67–13.44) | 2.52 | 0.003 | 5.77 (1.28–26.11) | 4.44 | 0.023 |
| Diabetes mellitus(%) | 22(20.56) | 105(30.01) | 1.06 (0.62–1.83) | 0.29 | 0.812 | / | / | / |
| Family history of cardiovascular and cerebrovascular events(%) | 14(13.08) | 52(15.85) | 1.25 (0.66–2.36) | 0.4 | 0.489 | / | / | / |
| Smoking history (%) | 26(24.30) | 65(19.82) | 1.16 (0.73–1.85) | 0.27 | 0.693 | / | / | / |
| TC, mmol/L | 4.85 ± 1.09 | 3.97 ± 1.05 | 0.45 (0.36–0.58) | 0.05 | <0.05 | 0.04 (0.01–0.16) | 0.02 | <0.05 |
| TG, mmol/L | 1.65 ± 0.72 | 1.55 ± 1.07 | 0.91 (0.74–1.12) | 0.09 | 0.373 | / | / | / |
| HDL, mmol/L | 1.46 ± 0.41 | 1.13 ± 0.53 | 0.30 (0.39–0.50) | 0.08 | <0.05 | / | / | / |
| LDL, mmol/L | 2.64 ± 0.92 | 2.44 ± 0.88 | 0.78 (0.62–1.00) | 0.09 | 0.049 | 6.55 (1.09–39.58) | 6.01 | 0.04 |
| FSG, mmol/L | 5.57 ± 1.22 | 6.70 ± 3.20 | 1.24 (1.10–1.42) | 0.08 | 0.001 | / | / | / |
| HbA1c | 5.67 ± 1.11 | 6.75 ± 1.69 | 2.04 (1.56–2.67) | 0.28 | <0.05 | 2.28 (1.40–3.71) | 0.56 | 0.001 |
| XO, ng/mL | 0.78 ± 0.51 | 1.76 ± 0.87 | 6.29 (4.13–9.56) | 1.34 | <0.05 | 8.68 (4.62–14.33) | 2.8 | <0.05 |
BMI, Body mass index; CHD, Coronary heart disease; AF, Atrial fibrillation; TC, Total cholesterol; TG, Triglyceride; HDL, High-density lipoprotein; LDL, Low-density lipoprotein; FSG, Fasting blood glucose; HbA1c, Glycosylated hemoglobin; XO, Xanthine oxidase.
3.2. The serum levels of XO are increased in parallel to AIS progression
We divided this cohort into a progression group (increase in NIHSS score over 2 points within 7 d after onset or any new neurological deficit) and a nonprogression group. The nonprogression group included 295 patients, and the progression group included 33 patients (Table 2). We found that the serum XO level in the progression group was significantly higher than that in the nonprogression group (P < 0.001). We also found that age, NLR, infarction area, NIHSS score, serum level of XO, family history of cardiovascular or cerebrovascular events, drinking history, and TOAST classification were significantly different between the two groups (Table 2). In addition, multivariate analysis demonstrated that serum XO level (OR 1.98, 95% CI 1.12–3.50, P = 0.018), age (OR 1.04, 95% CI 1.00–1.48, P = 0.047) and drinking history (OR 2.48, 95% CI 1.02–6.02, P = 0.044) were independent risk factors for stroke progression (Table 2).
Table 2.
Demographic and clinical characteristics, Univariate and Multivariate analysis of stroke progression group and nonprogression group.
| Characteristics | Nonprogression group, n = 295 | Progression group, n = 33 | Univariate and Multivariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | SE | P value | OR (95%CI) | SE | P value | |||
| Age, y | 67.94 ± 11.12 | 73.27 ± 10.25 | 1.05 (1.01–1.08) | 0.01 | 0.01 | 1.04 (1.00–1.48) | 0.02 | 0.047 |
| Men, n(%) | 203(89.43%) | 24(10.57%) | 0.83 (0.37–1.85) | 0.33 | 0.645 | / | / | / |
| BMI | 24.16 ± 3.10 | 24.3 ± 2.81 | 1.014 (0.90–1.14) | 0.06 | 0.804 | / | / | / |
| TC, mmol/L | 1.54 ± 1.04 | 1.58 ± 1.34 | 1.03 (0.73–1.46) | 0.18 | 0.838 | / | / | / |
| TG, mmol/L | 3.97 ± 1.05 | 4.01 ± 1.00 | 1.03 (0.75–1.42) | 0.16 | 0.857 | / | / | / |
| HDL, mmol/L | 1.13 ± 0.54 | 1.18 ± 0.50 | 1.18 (0.65–2.13) | 0.35 | 0.587 | / | / | / |
| LDL, mmol/L | 2.44 ± 0.88 | 2.40 ± 0.80 | 0.95 (0.63–1.43) | 0.19 | 0.806 | / | / | / |
| Uric acid, μmol/L | 328.85 ± 97.06 | 321.28 ± 95.51 | 1.00 (1.00–1.00) | 0.001 | 0.67 | / | / | / |
| FSG, mmol/L | 6.68 ± 3.14 | 6.88 ± 3.73 | 1.02 (0.92–1.13) | 0.05 | 0.734 | / | / | / |
| HbA1c | 6.74 ± 1.68 | 6.82 ± 1.83 | 1.03 (0.84–1.26) | 0.1 | 0.792 | / | / | / |
| NLR | 4.29 ± 3.26 | 6.28 ± 3.97 | 1.1 (1.05–1.25) | 0.05 | 0.002 | / | / | / |
| Infarction area(cm3) | 730.35(125.96, 3036.36) | 2563.18(683.59, 20474.27) | 1.00 (1.00–1.00) | <0.001 | <0.001 | / | / | / |
| NIHSS scores | 5.80 ± 4.61 | 9.33 ± 6.93 | 1.12 (1.06–1.20) | 0.03 | <0.001 | / | / | / |
| XO, ng/mL | 1.69 ± 0.85 | 2.39 ± 0.80 | 2.49 (1.62–3.83) | 0.54 | <0.001 | 1.98 (1.12–3.50) | 0.57 | 0.018 |
| Hypertension(%) | 222(89.16%) | 27(10.84%) | 1.48 (0.59–3.72) | 0.69 | 0.405 | / | / | / |
| CHD(%) | 39(90.70%) | 4(9.30%) | 0.91 (0.30–2.72) | 0.5 | 0.859 | / | / | / |
| AF(%) | 48(94.12%) | 3(5.88%) | 0.51 (0.15–1.75) | 0.32 | 0.288 | / | / | / |
| Diabetes mellitus(%) | 62(87.32%) | 9(12.68%) | 1.41(0.62–3.19) | 0.58 | 0.41 | / | / | / |
| Family history of cardiovascular and cerebrovascular events (%) | 40(76.92%) | 12(23.08%) | 3.64 (1.66–7.98) | 1.45 | 0.001 | / | / | / |
| Drinking history (%) | 78(90.70%) | 8(9.30%) | 2.62 (1.26–5.48) | 0.98 | 0.01 | 2.48 (1.02–6.02) | 1.12 | 0.044 |
| Smoking history (%) | 71(82.56%) | 15(17.44%) | 0.89 (0.39–2.06) | 0.38 | 0.785 | / | / | / |
| Onset time ≤24 h(%) | 133(90.48%) | 14(9.52%) | 1.09 (0.71–1.66) | 0.23 | 0.691 | / | / | / |
| 24 h–48 h(%) | 73(91.25%) | 7(8.75%) | / | / | / | / | / | / |
| 48 h–72 h(%) | 87(88.78%) | 11(11.22%) | / | / | / | / | / | / |
| TOAST classification, no.(%) | 1.31 (1.02–1.69) | 0.16 | 0.036 | 1.54 (1.08–21.9) | 0.27 | 0.016 | ||
| Large artery | 56(82.35%) | 12(17.65%) | / | / | / | / | / | / |
| Small artery | 127(96.21%) | 5(3.79%) | / | / | / | / | / | / |
| Cardioembolism | 64(88.89%) | 8(11.11%) | / | / | / | / | / | / |
| Other cause | 19(86.36%) | 3(13.64%) | / | / | / | / | / | / |
| Unknown | 29(85.29%) | 5(14.71%) | / | / | / | / | / | / |
BMI, Body mass index; CHD, Coronary heart disease; AF, Atrial fibrillation; TC, Total cholesterol; TG, Triglyceride; HDL, High-density lipoprotein; LDL, Low-density lipoprotein; FSG, Fasting blood glucose; HbA1c, Glycosylated hemoglobin; XO, Xanthine oxidase; NLR, Neutrophil/lymphocyte ratio; NIHSS, National Institutes of Health Stroke Scale; TOAST, Trial of ORG 10172 in acute stroke treatment.
3.3. The serum levels of XO are related to functional outcomes in patients with AIS
We divided our AIS cohort into a good prognosis group (mRS score: 0–2 points) and a poor prognosis group (mRS score: 3–6 points) based on the 90-day mRS score. The clinical data of the two groups are presented in Table 3. When matched for sex and age, we found that the serum XO level in the poor prognosis group was significantly higher than that in the good prognosis group (P < 0.05). Compared with the good prognosis group, the poor prognosis group was more inclined to have CHD, a family history of cardiovascular or cerebrovascular disease, a smoking history, a greater proportion of patients with large artery atherosclerosis, a higher NIHSS score, a larger infarct volume, a higher NLR, higher FSG, higher HbA1c and a greater proportion of patients with progressive stroke (P < 0.05), as shown in Table 3.
Table 3.
Demographic and clinical characteristics, Univariate and Multivariate analysis of good prognosis group and poor prognosis group.
| Characteristics | Good Prognosis, n = 248 | Poor Prognosis, n = 80 | Univariate and Multivariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | SE | P value | OR (95%CI) | SE | P value | |||
| Age, y | 67.96 ± 11.09 | 71.10 ± 11.19 | 1.01 (0.99–1.04) | 0.01 | 0.135 | / | / | / |
| Men, n(%) | 172(69.35) | 55(68.75) | 1.02(0.60–1.77) | 0.28 | 0.919 | / | / | / |
| BMI(kg/m2) | 24.30 ± 3.09 | 23.79 ± 2.99 | 0.94 (0.87–1.03) | 0.04 | 0.201 | / | / | / |
| Hypertension(%) | 187(75.40) | 62(77.50) | 1.12 (1.62–2.05) | 0.34 | 0.703 | / | / | / |
| CHD(%) | 29(11.69) | 14(17.50) | 1.60 (0.80–3.21) | 0.56 | 0.184 | 4.23 (1.52–11.83) | 2.22 | 0.006 |
| AF(%) | 36(14.52) | 15(18.75) | 1.35 (0.70–2.64) | 0.45 | 0.365 | / | / | / |
| Diabetes mellitus(%) | 52(20.97) | 19(23.75) | 1.17 (0.65–2.14) | 0.35 | 0.6 | / | / | / |
| Family history of cardiovascular and cerebrovascular events (%) | 26(10.48) | 26(32.50) | 4.11 (2.21–7.63) | 1.29 | <0.05 | / | / | / |
| Drinking history (%) | 63(25.40) | 23(28.75) | 1.18 (0.68–2.08) | 0.33 | 0.554 | / | / | / |
| Smoking history (%) | 48(19.35) | 32(40.0) | 3.76 (2.20–6.47) | 1.03 | <0.05 | 6.25 (2.54–11.39) | 2.87 | <0.001 |
| Onset time ≤24 h(%) | 175(70.56) | 45(56.25) | 1.30 (0.97–1.76) | 0.19 | 0.075 | 2.51 (1.47–3.31) | 0.69 | 0.001 |
| 24 h–48 h(%) | 30(12.10) | 16(20.0) | / | / | / | / | / | / |
| 48 h–72 h(%) | 43(17.34) | 19(23.75) | / | / | / | / | / | / |
| TOAST classification, no. (%) | 1.03 (0.86–1.25) | 0.09 | 0.696 | / | / | / | ||
| Large artery | 33(13.31) | 35(43.75) | / | / | / | / | / | / |
| Small artery | 116(46.77) | 16(20.0) | / | / | / | / | / | / |
| Cardioembolism | 52(20.97) | 20(25.0) | / | / | / | / | / | / |
| Other cause | 16(6.45) | 6(7.50) | / | / | / | / | / | / |
| Unknown | 31(12.5) | 3(3.75) | / | / | / | / | / | / |
| NIHSS scores | 3(2–7) | 8(5–12) | 1.2 (1.14–1.27) | 0.03 | <0.05 | 1.25 (1.12–1.41) | 0.07 | <0.001 |
| Infarction area(cm3) | 427.01(118.62, 1537.25) | 3241.51(709.58, 36182.23) | 1.00 (1.00–1.00) | <0.001 | <0.05 | 1.00 (1.00–1.00) | <0.001 | 0.035 |
| TC, mmol/L | 4.00 ± 1.07 | 3.87 ± 0.96 | 0.87 (0.69–1.12) | 0.1 | 0.296 | / | / | / |
| TG, mmol/L | 1.60 ± 1.08 | 1.38 ± 1.04 | 0.79 (0.59–1.07) | 0.12 | 0.127 | / | / | / |
| HDL, mmol/L | 1.12 ± 0.56 | 1.17 ± 0.44 | 1.17 (0.75–1.82) | 0.26 | 0.483 | / | / | / |
| LDL, mmol/L | 2.48 ± 0.90 | 2.32 ± 0.80 | 0.81 (0.61–1.09) | 0.12 | 0.174 | / | / | / |
| Uric acid, μmol/L | 330.94 ± 99.93 | 319.26 ± 86.33 | 0.99 (1.00–1.00) | 0.001 | 0.348 | / | / | / |
| FSG, mmol/L | 6.78 ± 3.18 | 6.48 ± 3.25 | 0.96 (0.89–1.05) | 0.08 | 0.001 | / | / | / |
| HbA1c | 6.75 ± 1.59 | 6.75 ± 2.00 | 1.00 (0.86–1.16) | 0.28 | <0.05 | / | / | / |
| NLR | 4.08 ± 3.17 | 5.76 ± 3.74 | 1.13 (1.06–1.22) | 0.04 | <0.05 | / | / | / |
| XO, ng/mL | 1.56 ± 0.82 | 2.38 ± 0.73 | 3.44 (2.37–4.98) | 0.64 | <0.05 | 2.11 (1.26–3.55) | 0.55 | 0.005 |
| Progressive stroke, n(%) | 4(1.61) | 29(36.25) | 34.68 (11.08–102.97) | 19.25 | <0.05 | 43.28 (9.56–195.99) | 33.35 | <0.001 |
BMI, Body mass index; CHD, Coronary heart disease; AF, Atrial fibrillation; TC, Total cholesterol; TG, Triglyceride; HDL, High-density lipoprotein; LDL, Low-density lipoprotein; FSG, Fasting blood glucose; HbA1c, Glycosylated hemoglobin; XO, Xanthine oxidase; NLR, Neutrophil/lymphocyte ratio; NIHSS, National Institutes of Health Stroke Scale; TOAST, Trial of ORG 10172 in acute stroke treatment.
The results of multivariate analysis indicated that the independent risk factors related to functional prognosis at 90 days mainly included smoking history (OR 6.25, 95% CI 2.54–11.39, P < 0.001), NIHSS score (OR 1.25, 95% CI 1.12–1.41, P < 0.001), CHD (OR 4.23, 95% CI 1.52–11.83, P = 0.006), serum XO level on admission (OR 2.51, 95% CI 1.47–3.31, P = 0.001), progressive stroke (OR 43.28, 95% CI 9.56–195.99, P < 0.001), time from symptom onset to hospitalization (OR 2.51, 95% CI 1.47–3.31, P = 0.001) and infarction area (OR 1.00, 95% CI 1.00–1.00, P = 0.035) (Table 3). A family history of cardiovascular or cerebrovascular disease, TOAST classification, and lymph node ratio were not independent risk factors for poor prognosis of stroke (P > 0.05).
3.4. The development of nomogram models for predicting the onset, progression and prognosis of AIS
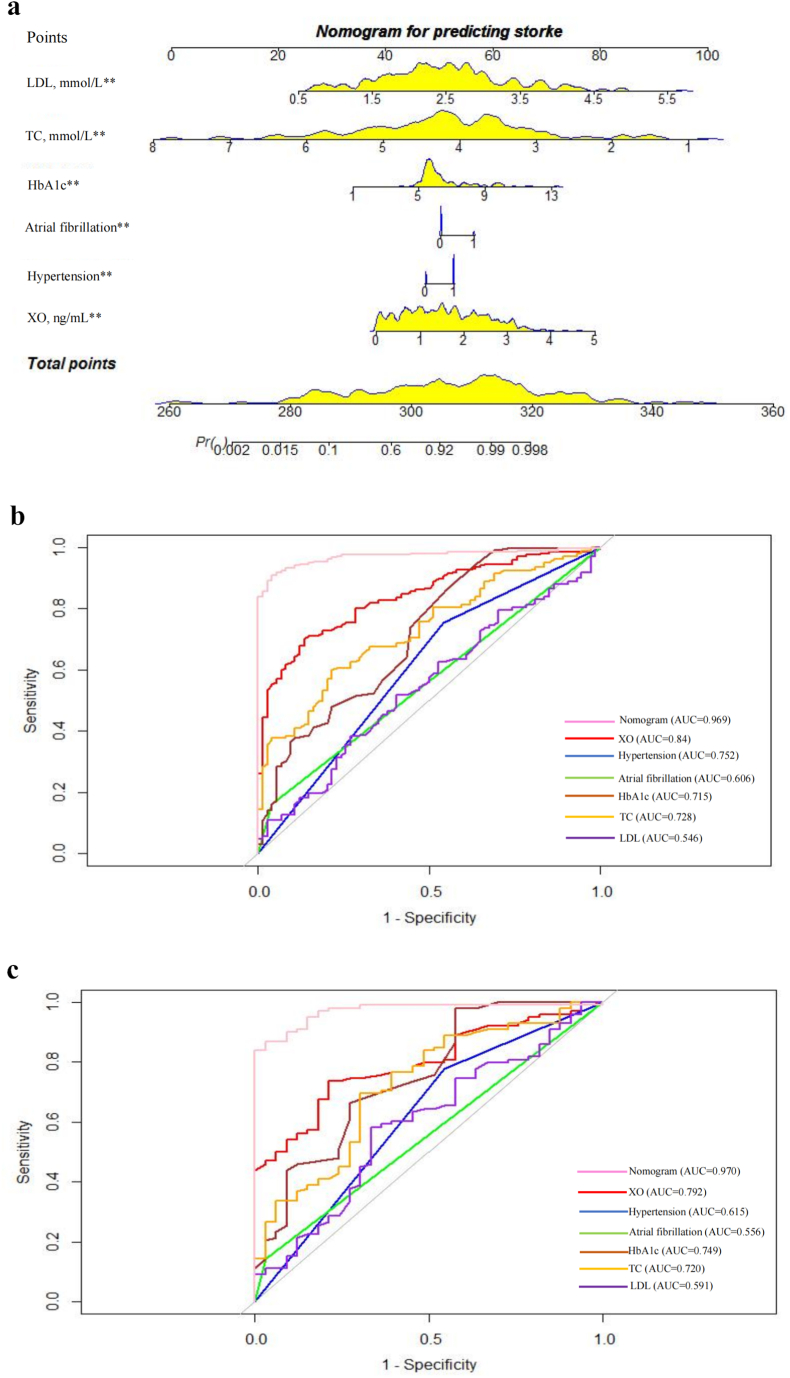
According to the above analysis, nomogram models were established to predict the onset, progression and prognosis of AIS. The nomogram models for predicting AIS onset were established with 6 variables, including serum XO level (P < 0.05), history of hypertension (P = 0.001), history of AF (P = 0.023), HbA1c (P = 0.001), TC (P < 0.05), and LDL (P = 0.04), as shown in Fig. 2a. The AUC of the training set (n = 305) was 0.969 (95% CI 0.951–0.987), and the sensitivity and specificity were 0.91 and 0.96, respectively (Fig. 2b). The AUC of the validation set (n = 130) was 0.97 (95% CI 0.943–0.996), and the sensitivity and specificity were 0.87 and 0.97, respectively (Fig. 2c).
Fig. 2.
The predictive model for AIS onset, a The nomogram for predicting AIS onset. Each of the independent predictors was projected upwards to the value of the “points” at the top level of the nomogram to obtain a score within the range of 0–100, and then the total score of these points was recorded to accurately predict the risk of AIS onset. ** means p < 0.05, b The receiver operating characteristic (ROC) curves of the nomogram, XO, hypertension, atrial fibrillation, HbA1c, TC and LDL for predicting AIS onset in the training set. The y-axis meant the truepositive rate of the risk prediction. The x-axis meant the false-positive rate of the risk prediction. c The ROC curves of the nomogram, XO, hypertension, atrial fibrillation, HbA1c, TC and LDL for predicting AIS onset in the testing set. AUC areas under the receiver operating characteristic curve, XO xanthine oxidase, HbA1c glycated hemoglobin, TC total cholesterol, LDL low-density lipoprotein.
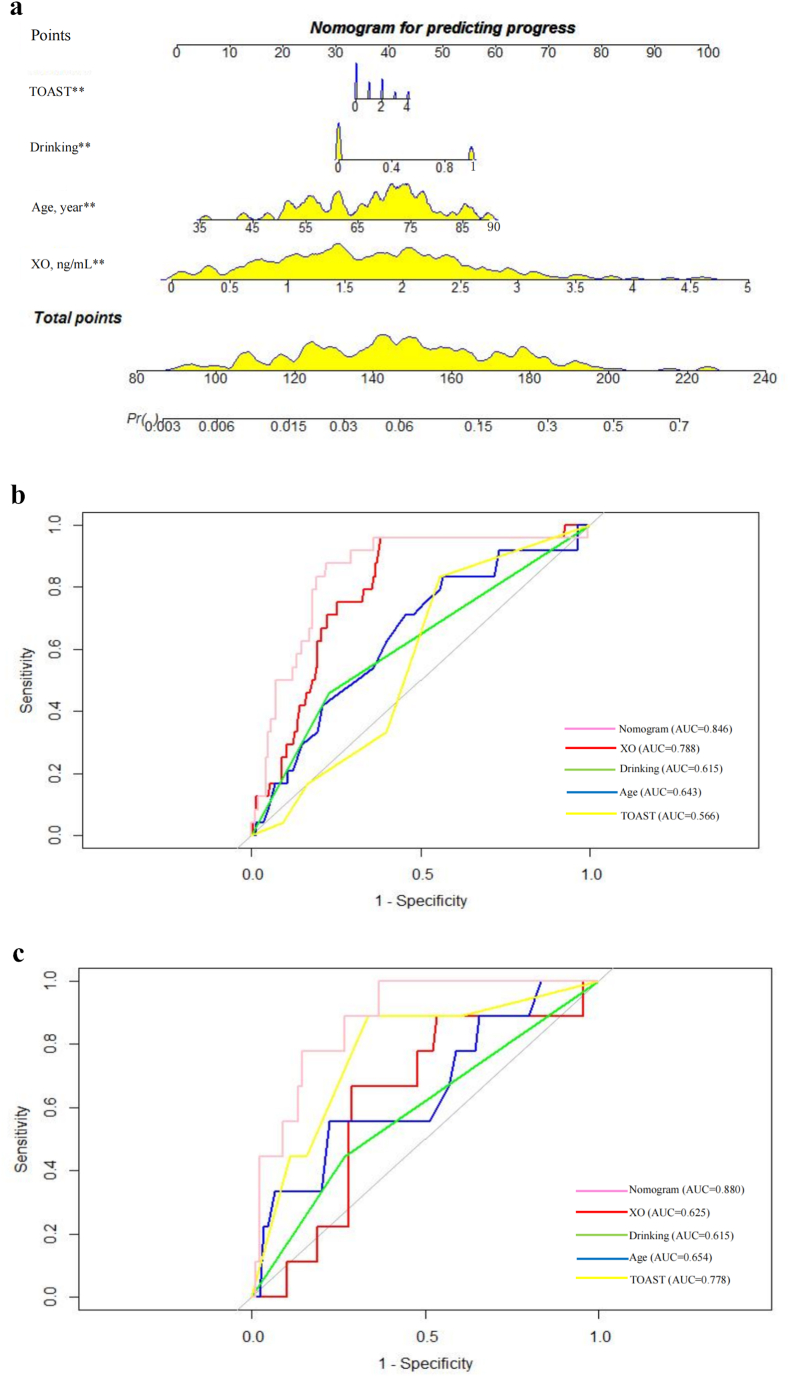
Serum XO level (P = 0.018), age (P = 0.047), drinking history (P = 0.044), and TOAST classification (P = 0.016) were used to establish a nomogram model to predict the progression of AIS (Fig. 3a). The AUC of the training set (n = 230) was 0.846 (95% CI: 0.760–0.931) and that of the validation set (n = 98) was 0.88 (95% CI: 0.788–0.973) 0.887 (95% CI: 0.808–0.966) (Fig. 3b and c).
Fig. 3.
The predictive model for AIS progression, a The nomogram for predicting the progress of AIS patients. Each of the independent predictors was projected upwards to the value of the “points” at the top level of the nomogram to obtain a score within the range of 0–100, and then the total score of these points was recorded to accurately predict the risk of progress in the AIS patients. ** means p < 0.05, b The receiver operating characteristic (ROC) curves of the nomogram, XO, drinking, age and TOAST for progress prediction of AIS patients in the training set. The y-axis meant the truepositive rate of the risk prediction. The x-axis meant the false-positive rate of the risk prediction., c The ROC curves of the nomogram, XO, drinking, age and TOAST for progress prediction of AIS patients in the testing set. AUC areas under the receiver operating characteristic curve, XO xanthine oxidase, TOAST Trial of Org 10,172 in Acute Stroke Treatment.
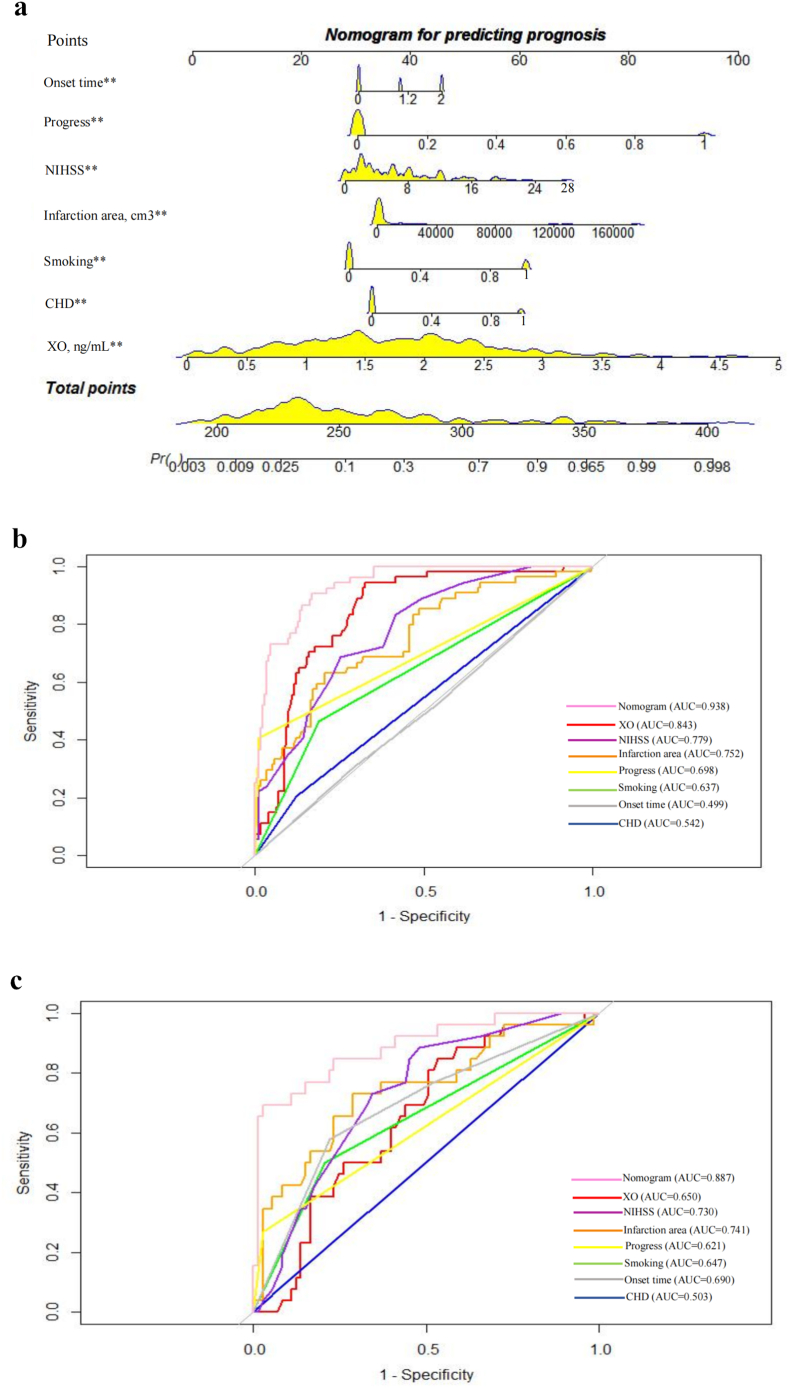
Based on the results of multivariate logistic regression analysis, smoking history (P < 0.001), NIHSS score (P < 0.001), CHD (P = 0.006), serum XO level on admission (P = 0.005), progressive stroke (P < 0.001), time from symptom onset to hospitalization (P = 0.001) and infarction area (P = 0.035) were used to establish nomogram models to predict the prognosis of AIS (Fig. 4a). The AUC of the training set was 0.938 (95% CI: 0.907–0.969) and that of the validation set was 0.887 (95% CI: 0.808–0.966) (Fig. 4b and c). The cut-off value of Serum XO levels for predicting a poor prognosis of AIS was 0.682 ng/mL
Fig. 4.
The predictive model for the prognosis of AIS, a The nomogram for predicting the prognosis of AIS patients. Each of the independent predictors was projected upwards to the value of the “points” at the top level of the nomogram to obtain a score within the range of 0–100, and then the total score of these points was recorded to accurately predict the risk of prognosis in the AIS patients. ** means p < 0.05, b The receiver operating characteristic (ROC) curves of the nomogram, XO, NIHSS, infarction area, progress, smoking, onset time and CHD for prognosis prediction of AIS patients in the training set. The y-axis meant the truepositive rate of the risk prediction. The x-axis meant the false-positive rate of the risk prediction. c The ROC curves of the nomogram, XO, NIHSS, infarction area, progress, smoking, onset time and CHD for prognosis prediction of AIS patients in the testing set. AUC areas under the receiver operating characteristic curve, XO xanthine oxidase, NIHSS National Institutes of Health Stroke Scale scores, CHD coronary heart disease.
The results show that the nomogram models had high predictive efficacy in both the training set and the internal validation set. The AUC of the nomogram model established in this study was better than the AUC of any single risk factor. The bootstrap self-sampling method was used to verify the model. The results showed that after 1000 repeated samplings, in the nomogram models for predicting the onset, progression and prognosis of AIS, the mean absolute errors between the predicted risk and the actual risk of the training set were 0.023, 0.034 and 0.032, respectively. The results indicate that the predicted value was basically consistent with the measured value, and it had good prediction and differentiation abilities.
4. Discussion
This study shows that the serum XO level in patients with AIS was significantly higher than that in healthy people. The serum XO level on admission was an independent risk factor for the onset, progression and prognosis of AIS and had a certain clinical predictive value for clinical outcomes.
Finding useful blood biomarkers for predicting the clinical prognosis of AIS has important implications for the precise treatment of this condition [6]. XOD, including XO and XDH, is not only an important catalytic enzyme for purine metabolism but also an important source of reactive oxygen species [22]. In the presence of ischemia and hypoxia, XDH is irreversibly converted to XO by proteolysis. XO utilizes O2 as an electron acceptor to generate superoxide during the biosynthesis of uric acid products. The superoxide produced is involved in oxidative stress and inflammation induction after tissue damage [23].
XOR is expressed in a variety of human tissues. The XOR is mainly expressed in hepatocytes and Kupfer cells in the liver, and also exited in jejunal cells and goblet cells of the proximal intestine. In addition, in the skeletal muscle, kidney, and jejunum, previous research reported that XOR presented in capillary endothelial cells of those organs [11]. The presence of circulating XOR in mammals has been demonstrated, but previous studies have shown that for humans XOR enzyme activity is relatively high only in the liver and intestine. However, several studies indicated that the level of XO enzyme activity detected in the circulation is particularly low or even undetectable [[24], [25], [26]]; in addition, enzyme activity of XO may be inactivated in serum or during the activity-assay [27]. Therefore, in our study, we applied the ELISA method to determine the XO concentration in human serum in a more sensitive and direct way, to assess the diagnostic value of XO in ischemic injury in terms of changes in the serum XO levels, and to discover the importance of changes in the XO concentration in the diagnosis of ischemic stroke. A study by Aygul R et al. showed that the level of plasma XO in patients with AIS was significantly higher than that in healthy controls. Their result is similar to ours. Although the sample size of their clinical study was only 39 patients and the level of XO was measured by the method of measuring uric acid, their finding is still a good indication of a role for blood XO in AIS [12]. In a recent study, the utility of salivary XO in the differential diagnosis of stroke and in assessing the functional status of patients was demonstrated [19]. Since salivary XO may penetrate into saliva through the capillaries of the salivary glands [28,29]. As a non-invasive, simple and inexpensive monitoring method, the determination of XO activity in saliva has been shown to be useful in the identification of patients with ischemic stroke and in assessing the functional status of patients. The value of salivary XO in the diagnosis of neurovascular diseases deserves further attention.
The main research direction of this study was to explore whether the serum XO level on admission in patients with AIS was correlated with stroke onset, clinical progression during hospitalization and clinical prognosis three months later. In this study, we found that serum XO level on admission, smoking history, NIHSS score, CHD, stroke progression, time from onset to hospitalization and infarct area were independent risk factors for the prognosis of AIS. The infarct area and NIHSS score are indicators routinely used to evaluate the prognosis of cerebral infarction. Generally, a larger infarct area indicates more severe brain injury, and patients usually have higher NIHSS scores and poorer prognoses [30]. We found that serum XO level, NIHSS score and infarct area had similar predictive effects on prognosis. The AUC of the nomogram model established in this study was better than the AUC of any single risk factor. In addition, serum XO level on admission, age, and drinking history were independent risk factors for stroke progression. This may be related to the important role of XO in cerebral ischemic injury. When AIS occurs, XDH in the brain will be irreversibly converted to XO, which will further aggravate the brain damage of AIS [31,32]. In the ischemic state, due to the low ATP level, XO induces hypoxanthine to form xanthine and uric acid and releases superoxide and hydrogen peroxide during this process, causing more oxidative stress. Superoxide passes through the cell membrane through negatively charged channels, and hydrogen peroxide enters the cytoplasm directly, causing irreversible cell damage and death [33]. Due to the importance of XO in the pathophysiology of AIS, serum XO levels have the potential to be a predictor of stroke progression and prognosis.
In addition, we also found that the serum XO level on admission was an independent risk factor for AIS. This is an interesting finding because the conventional view is that in the process of AIS, nerve cell injury leads to an increase in XO, which in turn triggers a series of oxidative and inflammatory reactions that further aggravate nerve damage [23]. However, whether there is a reverse causality, that is, an increase in XO is the cause of AIS, has not yet been studied. Recent studies have shown that XO in the blood can promote the development of atherosclerosis. XO in the blood is related to the formation of carotid atherosclerotic plaques in patients with ischemic stroke. The serum XO level in patients with ischemic carotid plaques is significantly higher than that in patients with asymptomatic carotid plaques [34]. XO mainly exists in blood macrophages, which play a pivotal role in the progression of atherosclerosis. These cells develop into foam cells in chronic atherosclerotic plaques and promote the degradation of the extracellular matrix, the release of inflammatory mediators, the formation of necrotic cores, and the eventual destruction of atherosclerotic plaques, leading to atherosclerotic plaque rupture. The rupture of an atherosclerotic plaque will lead to the occlusion of blood vessels in the brain along with the interruption of blood flow, resulting in the occurrence of AIS [[35], [36], [37]]. This may be the underlying mechanism by which XO causes AIS, but more favorable evidence is needed for verification. As the serum XO level in this study was measured after the onset of AIS, data on the serum concentration of XO before the onset of stroke were lacking, and the causal relationship between XO and the onset of stroke could not be determined. Therefore, large-scale epidemiological studies are needed to confirm the relationship between XO and AIS. In the next phase of the study, we will collect blood samples from healthy people to measure the baseline XO levels and conduct long-term follow-up to further confirm the relationship between XO and AIS.
Considering early and timely treatment has a great impact on the prognosis of AIS patients, some patients in our cohort received intravenous thrombolysis and arterial thrombectomy based on the guideline [38] (the strict treatment time window). In addition, all enrolled patients were required to be informed of the risks and benefits in detail, so an informed consent form was usually signed after the completion of thrombolysis and arterial thrombectomy. Therefore, blood samples for the measurement of XO levels were taken in the early morning of the second day after admission while participants were in a fasted state. Since the serum XO level is directly related to the stage of ischemia damage, different treatment methods do not affect the measurement of serum XO levels in AIS patients with stroke prognosis. The TOAST classification is the commonly used etiological classification of AIS. Although there are different causes of AIS, there is no difference in the oxidative stress response and inflammatory response mechanism when cerebral infarction occurs [39], so this study did not analyze different stroke subtypes.
XO is an important catalytic enzyme for purine metabolism and uric acid production. Interestingly, we found that the levels of XO were higher in patients with poor prognosis, while circulating levels of uric acid, which represents the main product of the enzymatic activity of XO, were similar. The possible reason for this finding is that uric acid production is a complex biochemical process, and although XO is elevated during cerebral ischemia‒reperfusion, XO is not the only determinant of uric acid production [40]. Over the past few decades, numerous studies have analyzed the relationship between serum uric acid levels and stroke, but the results have been contradictory [41]. XO is involved in the production of reactive oxygen species in damaged tissues and is one of the main sources of oxidative stress. However, uric acid itself has an antioxidant capacity that protects nerve cells from excitotoxicity and metabolic damage and plays a protective role in reactive oxygen species-induced brain damage [42,43]. On the other hand, hyperuricemia is associated with increased oxidative stress, an increased systemic inflammatory response, and thrombosis [44,45]. The dual effects of uric acid suggest that the role of uric acid in the pathogenesis of AIS remains controversial.
In conclusion, this study found that the serum XO level was an independent risk factor for the onset, progression and prognosis of AIS. Based on the serum XO level and selected risk factors, we established nomogram models to predict the onset, progression and prognosis of AIS. It was shown that this prediction model has good accuracy, differentiation ability and prediction ability for AIS. This model is helpful for the early assessment of AIS patients to administer early intervention.
This study has certain limitations. First, this study was a prospective study performed at a single center with a small sample size, especially in the progression group. Second, we measured the level of serum XO only once, that is, in a blood sample obtained in the morning on the day after admission while participants were in a fasted state, and multiple dynamic measurements should be performed according to the different states of the patient to obtain more reliable results. Third, we did not measure the serum XO level in stroke patients before AIS onset. Therefore, our study lacks a comparison of XO levels before and after onset of stroke in the same patients, and we were thus unable to determine the causal relationship between elevated serum XO levels and the onset of stroke. Fourth, we did not measure the level of salivary XO, which is also a good diagnostic indicator. Fifth, we did not investigate the alterations in XO levels before and after therapy, which may provide more information about the influence of brain reperfusion on the stage of oxidative stress. Finally, some parameters, including platelet, UA, AST and ALT levels, were not collected and incorporated in the analysis, which could be considered in future studies. Therefore, more cases and further pathological mechanism studies are needed to explore the potential value of serum XO levels in AIS.
5. Conclusions
The serum XO level of patients with AIS on admission was significantly higher than that of the healthy control group. The serum XO level on admission had certain clinical predictive value in predicting stroke progression and prognosis in patients with AIS. Nomogram models established by the serum XO level combined with other high-risk factors have a good ability to predict the onset, progression and prognosis of AIS. The serum XO level was an independent risk factor also associated with a poor prognosis in AIS patients. The pathophysiological mechanism of XO affecting the clinical prognosis of AIS and the clinical value of serum XO levels as a potential biomarker need to be further studied.
Author contribution
The study was conceived and planned by Hailong Yu, Yinzhu Chen, Yuping Li. The manuscript was written and revised by Yuping Li and Xin Chen, Hailong Yu, Guangyu Lu. Xin Chen, Xin Guo, Danni Chen participated in the data curation, formal analysis, methodology, writing–original draft,. The partial experiments were performed by Li Jiang, Yajie Qi, Jun Shao, Luhang Tao, Jing Hang. All authors have seen the manuscript and approved to submit to your journal.
Funding
This work was funded by the Natural Science Foundation of Jiangsu Province (BK20221280), the Special Fund for Social Key Research and Development Plan of Yangzhou City (YZ2022097), the National Natural Science Foundation of China (No. 82172603), the Chinese Postdoctoral Science Foundation (2022M711426), the Jiangsu Provincial Health Commission New Technology Introduction and Evaluation Project (M2022044) and the Fifteenth “Six Talent Peaks” Project of Jiangsu Province (No. WSW-246). The funding sources had no involvement in the collection, analysis and interpretation of data, the writing of the report or in the decision to submit the article for publication.
Declaration of competing interest
None of the authors have any conflicts of interest to disclose.
Acknowledgments
NONE.
Contributor Information
Yingzhu Chen, Email: 18051063159@yzu.edu.cn.
Yuping Li, Email: yupingli@yzu.edu.cn.
Data availability
Data will be made available on request.
References
- 1.Krishnamurthi R.V., Ikeda T., Feigin V.L. Global, regional and country-specific burden of ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage: a systematic analysis of the global burden of disease study 2017. Neuroepidemiology. 2020;54(2):171–179. doi: 10.1159/000506396. [DOI] [PubMed] [Google Scholar]
- 2.Sloane K.L., Camargo E.C. Antithrombotic management of ischemic stroke. Curr. Treat. Options Cardiovasc. Med. 2019;21(11):78. doi: 10.1007/s11936-019-0778-4. [DOI] [PubMed] [Google Scholar]
- 3.Jadhav A.P., Desai S.M., Jovin T.G. Indications for mechanical thrombectomy for acute ischemic stroke: current guidelines and beyond. Neurology. 2021;97(20 Suppl 2):S126–s136. doi: 10.1212/WNL.0000000000012801. [DOI] [PubMed] [Google Scholar]
- 4.Chamorro Á., Dirnagl U., Urra X., Planas A.M. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15(8):869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 5.Lo E.H., Moskowitz M.A., Jacobs T.P. Exciting, radical, suicidal: how brain cells die after stroke. Stroke. 2005;36(2):189–192. doi: 10.1161/01.STR.0000153069.96296.fd. [DOI] [PubMed] [Google Scholar]
- 6.Alhazzani A., Venkatachalapathy P., Padhilahouse S., Sellappan M., Munisamy M., Sekaran M., Kumar A. Biomarkers for antiplatelet therapies in acute ischemic stroke: a clinical review. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.667234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foerch C., Otto B., Singer O.C., Neumann-Haefelin T., Yan B., Berkefeld J., Steinmetz H., Sitzer M. Serum S100B predicts a malignant course of infarction in patients with acute middle cerebral artery occlusion. Stroke. 2004;35(9):2160–2164. doi: 10.1161/01.STR.0000138730.03264.ac. [DOI] [PubMed] [Google Scholar]
- 8.Serena J., Blanco M., Castellanos M., Silva Y., Vivancos J., Moro M.A., Leira R., Lizasoain I., Castillo J., Dávalos A. The prediction of malignant cerebral infarction by molecular brain barrier disruption markers. Stroke. 2005;36(9):1921–1926. doi: 10.1161/01.STR.0000177870.14967.94. [DOI] [PubMed] [Google Scholar]
- 9.Park S.Y., Kim M.H., Kim O.J., Ahn H.J., Song J.Y., Jeong J.Y., Oh S.H. Plasma heart-type fatty acid binding protein level in acute ischemic stroke: comparative analysis with plasma S100B level for diagnosis of stroke and prediction of long-term clinical outcome. Clin. Neurol. Neurosurg. 2013;115(4):405–410. doi: 10.1016/j.clineuro.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Battelli M.G., Polito L., Bortolotti M., Bolognesi A. Xanthine oxidoreductase in cancer: more than a differentiation marker. Cancer Med. 2016;5(3):546–557. doi: 10.1002/cam4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linder N., Rapola J., Raivio K.O. Cellular expression of xanthine oxidoreductase protein in normal human tissues. Lab. Invest. 1999;79(8):967–974. [PubMed] [Google Scholar]
- 12.Aygul R., Kotan D., Demirbas F., Ulvi H., Deniz O. Plasma oxidants and antioxidants in acute ischaemic stroke. J. Int. Med. Res. 2006;34(4):413–418. doi: 10.1177/147323000603400411. [DOI] [PubMed] [Google Scholar]
- 13.Saugstad O.D. Role of xanthine oxidase and its inhibitor in hypoxia: reoxygenation injury. Pediatrics. 1996;98(1):103–107. [PubMed] [Google Scholar]
- 14.Berry C.E., Hare J.M. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J. Physiol. 2004;555(3):589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farag M.M., Ahmed S.M., Elhadidy W.F., Rashad R.M. Superior protective effects of febuxostat plus alpha-lipoic acid on renal ischemia/reperfusion-induced hepatorenal injury in rats. Saudi J. Kidney Dis. Transpl. 2019;30(6):1364–1374. doi: 10.4103/1319-2442.275480. [DOI] [PubMed] [Google Scholar]
- 16.Fujii K., Kubo A., Miyashita K., Sato M., Hagiwara A., Inoue H., Ryuzaki M., Tamaki M., Hishiki T., Hayakawa N., Kabe Y., Itoh H., Suematsu M. Xanthine oxidase inhibitor ameliorates postischemic renal injury in mice by promoting resynthesis of adenine nucleotides. JCI insight. 2019;4(22) doi: 10.1172/jci.insight.124816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moens A.L., Champion H.C., Claeys M.J., Tavazzi B., Kaminski P.M., Wolin M.S., Borgonjon D.J., Van Nassauw L., Haile A., Zviman M., Bedja D., Wuyts F.L., Elsaesser R.S., Cos P., Gabrielson K.L., Lazzarino G., Paolocci N., Timmermans J.P., Vrints C.J., Kass D.A. High-dose folic acid pretreatment blunts cardiac dysfunction during ischemia coupled to maintenance of high-energy phosphates and reduces postreperfusion injury. Circulation. 2008;117(14):1810–1819. doi: 10.1161/CIRCULATIONAHA.107.725481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindsay S., Liu T.H., Xu J.A., Marshall P.A., Thompson J.K., Parks D.A., Freeman B.A., Hsu C.Y., Beckman J.S. Role of xanthine dehydrogenase and oxidase in focal cerebral ischemic injury to rat. Am. J. Physiol. 1991;261(6 Pt 2):H2051–H2057. doi: 10.1152/ajpheart.1991.261.6.H2051. [DOI] [PubMed] [Google Scholar]
- 19.Maciejczyk M., Nesterowicz M., Zalewska A., Biedrzycki G., Gerreth P., Hojan K., Gerreth K. Salivary xanthine oxidase as a potential biomarker in stroke diagnostics. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.897413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamtchum-Tatuene J., Jickling G.C. Blood biomarkers for stroke diagnosis and management. NeuroMolecular Med. 2019;21(4):344–368. doi: 10.1007/s12017-019-08530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryu W.S., Schellingerhout D., Jeong S.W., Nahrendorf M., Kim D.E. Association between serum lipid profiles and early neurological deterioration in acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2016;25(8):2024–2030. doi: 10.1016/j.jstrokecerebrovasdis.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Fujimura Y., Yamauchi Y., Murase T., Nakamura T., Fujita S.I., Fujisaka T., Ito T., Sohmiya K., Hoshiga M., Ishizaka N. Relationship between plasma xanthine oxidoreductase activity and left ventricular ejection fraction and hypertrophy among cardiac patients. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0182699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ives A., Nomura J., Martinon F., Roger T., LeRoy D., Miner J.N., Simon G., Busso N., So A. Xanthine oxidoreductase regulates macrophage IL1β secretion upon NLRP3 inflammasome activation. Nat. Commun. 2015;6:6555. doi: 10.1038/ncomms7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarnesto A., Linder N., Raivio K.O. Organ distribution and molecular forms of human xanthine dehydrogenase/xanthine oxidase protein. Lab. Invest. 1996;74(1):48–56. [PubMed] [Google Scholar]
- 25.Kooij A., Schijns M., Frederiks W.M., Van Noorden C.J., James J. Distribution of xanthine oxidoreductase activity in human tissues--a histochemical and biochemical study. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1992;63(1):17–23. doi: 10.1007/BF02899240. [DOI] [PubMed] [Google Scholar]
- 26.Lewis S.E., Rosencrance C.B., De Vallance E., Giromini A., Williams X.M., Oh J.Y., Schmidt H., Straub A.C., Chantler P.D., Patel R.P., Kelley E.E. Human and rodent red blood cells do not demonstrate xanthine oxidase activity or XO-catalyzed nitrite reduction to NO. Free Radic. Biol. Med. 2021;174:84–88. doi: 10.1016/j.freeradbiomed.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison R. Structure and function of xanthine oxidoreductase: where are we now? Free Radic. Biol. Med. 2002;33(6):774–797. doi: 10.1016/s0891-5849(02)00956-5. [DOI] [PubMed] [Google Scholar]
- 28.Porcheri C., Mitsiadis T.A. Physiology, pathology and regeneration of salivary glands. Cells. 2019;8(9) doi: 10.3390/cells8090976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nam Y., Kim Y.Y., Chang J.Y., Kho H.S. Salivary biomarkers of inflammation and oxidative stress in healthy adults. Arch. Oral Biol. 2019;97:215–222. doi: 10.1016/j.archoralbio.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Payabvash S., Benson J.C., Tyan A.E., Taleb S., McKinney A.M. Multivariate prognostic model of acute stroke combining admission infarct location and symptom severity: a proof-of-concept study. J. Stroke Cerebrovasc. Dis. 2018;27(4):936–944. doi: 10.1016/j.jstrokecerebrovasdis.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 31.Förstermann U., Xia N., Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017;120(4):713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 32.Kinuta Y., Kikuchi H., Ishikawa M. Ischaemic brain oedema and xanthine-xanthine oxidase system. Acta Neurochir. Suppl. 1990;51:192–194. doi: 10.1007/978-3-7091-9115-6_65. [DOI] [PubMed] [Google Scholar]
- 33.Wu M.Y., Yiang G.T., Liao W.T., Tsai A.P., Cheng Y.L., Cheng P.W., Li C.Y., Li C.J. Current mechanistic concepts in ischemia and reperfusion injury. Cell. Physiol. Biochem. 2018;46(4):1650–1667. doi: 10.1159/000489241. [DOI] [PubMed] [Google Scholar]
- 34.Ganji M., Nardi V., Prasad M., Jordan K.L., Bois M.C., Franchi F., Zhu X.Y., Tang H., Young M.D., Lerman L.O., Lerman A. Carotid plaques from symptomatic patients are characterized by local increase in xanthine oxidase expression. Stroke. 2021;52(9):2792–2801. doi: 10.1161/STROKEAHA.120.032964. [DOI] [PubMed] [Google Scholar]
- 35.Cho K.Y., Miyoshi H., Kuroda S., Yasuda H., Kamiyama K., Nakagawara J., Takigami M., Kondo T., Atsumi T. The phenotype of infiltrating macrophages influences arteriosclerotic plaque vulnerability in the carotid artery. J. Stroke Cerebrovasc. Dis. 2013;22(7):910–918. doi: 10.1016/j.jstrokecerebrovasdis.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Shaikh S., Brittenden J., Lahiri R., Brown P.A., Thies F., Wilson H.M. Macrophage subtypes in symptomatic carotid artery and femoral artery plaques. Eur. J. Vasc. Endovasc. Surg. 2012;44(5):491–497. doi: 10.1016/j.ejvs.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Stöger J.L., Gijbels M.J., van der Velden S., Manca M., van der Loos C.M., Biessen E.A., Daemen M.J., Lutgens E., de Winther M.P. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 2012;225(2):461–468. doi: 10.1016/j.atherosclerosis.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 38.Powers W.J., Rabinstein A.A., Ackerson T., Adeoye O.M., Bambakidis N.C., Becker K., Biller J., Brown M., Demaerschalk B.M., Hoh B., Jauch E.C., Kidwell C.S., Leslie-Mazwi T.M., Ovbiagele B., Scott P.A., Sheth K.N., Southerland A.M., Summers D.V., Tirschwell D.L. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2018;49(3):e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 39.Hui C., Tadi P., Patti L. StatPearls Publishing LLC.; Treasure Island (FL): 2022. Ischemic Stroke, StatPearls. StatPearls Publishing Copyright © 2022. [PubMed] [Google Scholar]
- 40.Skoczyńska M., Chowaniec M., Szymczak A., Langner-Hetmańczuk A., Maciążek-Chyra B., Wiland P. Pathophysiology of hyperuricemia and its clinical significance - a narrative review. Reumatologia. 2020;58(5):312–323. doi: 10.5114/reum.2020.100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tariq M.A., Shamim S.A., Rana K.F., Saeed A., Malik B.H. Serum uric acid - risk factor for acute ischemic stroke and poor outcomes. Cureus. 2019;11(10) doi: 10.7759/cureus.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seet R.C., Kasiman K., Gruber J., Tang S.Y., Wong M.C., Chang H.M., Chan Y.H., Halliwell B., Chen C.P. Is uric acid protective or deleterious in acute ischemic stroke? A prospective cohort study. Atherosclerosis. 2010;209(1):215–219. doi: 10.1016/j.atherosclerosis.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Yu Z.F., Bruce-Keller A.J., Goodman Y., Mattson M.P. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J. Neurosci. Res. 1998;53(5):613–625. doi: 10.1002/(SICI)1097-4547(19980901)53:5<613::AID-JNR11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 44.Glantzounis G.K., Tsimoyiannis E.C., Kappas A.M., Galaris D.A. Uric acid and oxidative stress. Curr. Pharmaceut. Des. 2005;11(32):4145–4151. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 45.Nieto F.J., Iribarren C., Gross M.D., Comstock G.W., Cutler R.G. Uric acid and serum antioxidant capacity: a reaction to atherosclerosis? Atherosclerosis. 2000;148(1):131–139. doi: 10.1016/s0021-9150(99)00214-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.